Abstract
Objective
To report an 18-month follow-up on creatine and minocycline futility study, the Neuroprotective Exploratory Trials in Parkinson Disease, Futility Study 1 (NET-PD FS-1).
Background
The NET-PD FS-1 futility study on creatine and minocycline found neither agent futile in slowing down the progression of disability in Parkinson disease (PD) at 12 months using the prespecified futility threshold. An additional 6 months of follow-up aimed to assess safety and potential interactions of the study interventions with anti-parkinsonian therapy.
Methods
Additional 6 months of follow-up in randomized, blinded phase II trial of creatine (dosage, 10 g/d) and minocycline (dosage, 200 mg/d) in subjects with early PD.
Results
By 18 months, symptomatic treatment of PD symptoms was required in 61% of creatine, 62% of minocycline, and 60% of placebo-treated subjects. Study treatment was prematurely discontinued in 9%, 23%, and 6% of subjects in the creatine, minocycline, and placebo arms, respectively. Creatine and minocycline did not seem to adversely influence the response to symptomatic therapy nor increase adverse events.
Conclusions
Data from this small, 18-month phase II trial of creatine and minocycline do not demonstrate safety concerns that would preclude a large, phase III efficacy trial, although the decreased tolerability of minocycline is a concern.
Keywords: clinical trial, creatine, futility, minocycline, Parkinson disease
Parkinson disease (PD) is a common neurodegenerative disorder that affects about 1 million Americans,1 with annual direct medical care costs attributable to PD of more than US $10,000 per patient.2,3 Although available medications improve some of the most disabling symptoms of PD, patients accrue substantial disability over time and face a future of dependency. Thus, there is a compelling need to develop treatments that might slow down the functional decline in PD.
The National Institute of Neurological Disorders and Stroke has sponsored efforts to identify agents with the potential to favorably modify disease progression in PD. Through the Neuroprotective Exploratory Trials in Parkinson Disease (NET-PD) program, 4 agents have been evaluated, and those agents not found to be futile may be carried forward into a phase III efficacy trial.4–6 Creatine and minocycline were prioritized for the first study, the NET-PD Futility Study 1 (NET-PD FS-1).4,7 Creatine is a nutritional supplement that enhances cellular energy function and has antioxidant properties,8 and the antibiotic minocycline has anti-inflammatory and antiapoptotic actions.9 There is evidence that both agents can protect against the dopaminergic neuronal loss associated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration in a mouse model of PD,10 although the results with minocycline are not consistent.11–14
In the initial futility study, 200 subjects with early untreated PD were randomly assigned in a 1:1:1 ratio to creatine (dosage, 10 g/d), minocycline (dosage, 200 mg/d), or placebo, respectively. Change in total Unified Parkinson Disease Rating Scale (UPDRS) score between baseline and 12 months or the time at which symptomatic treatment was required in each single arm was compared with a predetermined futility threshold.15 The primary analysis at 12 months showed that the mean changes in UPDRS in the creatine and minocycline groups did not exceed the prespecified futility threshold,4 although subsequent exploratory analyses gave more support to creatine than minocycline.6 Although the primary futility assessment was planned for 12 months, we designed the study so that each subject would receive the treatment assignment for 18 months. The purpose of this design was to obtain additional information about the safety of the treatment interventions in subjects not yet on symptomatic therapy and in those started on symptomatic therapy. Such data would be important to take into account when considering long-term use and would be useful for the design of a later phase III trial.
METHODS
Detailed information on study organization and conduct is published in the primary article.4 The protocol and the consent were approved by the institutional review boards of each of the participating sites, and each subject gave written informed consent. Forty-two clinical sites enrolled 200 male and female research participants with PD within 5 years of diagnosis and not yet requiring symptomatic therapy. The subjects were randomly assigned to creatine (dosage, 10 g/d), minocycline (dosage, 200 mg/d), or placebo in a 1:1:1 ratio, respectively. During the course of the study, symptomatic therapy could be initiated according to predetermined symptom criteria as previously described.4 Such treatment included levodopa or dopamine agonists and other agents such as amantadine or anticholinergic agents.
In-person assessments of PD at baseline, 1, 3, 6, 9, 12, and 18 months included the UPDRS, Hoehn and Yahr Stage, Schwab and England Disability Scale, and systematic solicitation of any potential adverse events by open-ended questioning. An interim telephone visit at 15 months assessed safety, tolerability, and compliance. On the basis of the known tolerability profiles of creatine16 and minocycline,17,18 we prespecified a number of adverse events, including falls, dizziness, nausea/vomiting/dyspepsia, skin reaction, headache, infection, and tooth discoloration. The relationship of an adverse event to study drug was assessed by the site investigator and the independent medical monitor and was defined as definite, probable, possible, unlikely, or not related. Laboratory assessments, including blood cell count and serum chemistry, were collected at baseline, 6, 12, and 18 months.
For the 18-month assessments described in this article, UPDRS data were analyzed using descriptive statistics because the study was not designed nor powered to detect differences between treatment arms. Worst-change score for the group was used to impute missing clinical outcome values. Kaplan-Meier curves were used to estimate the time to need for symptomatic therapy. For the evaluation of the prespecified adverse events, incidence densities were computed as events per 100 person-years during the time when the subjects were receiving and were not receiving antiparkinsonian therapy. These values were adjusted for the length of time at risk (ie, time on symptomatic treatment).
RESULTS
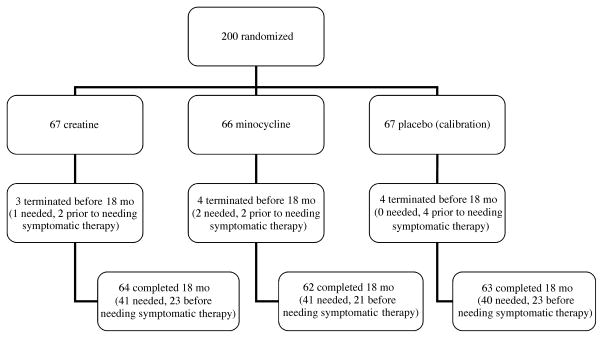
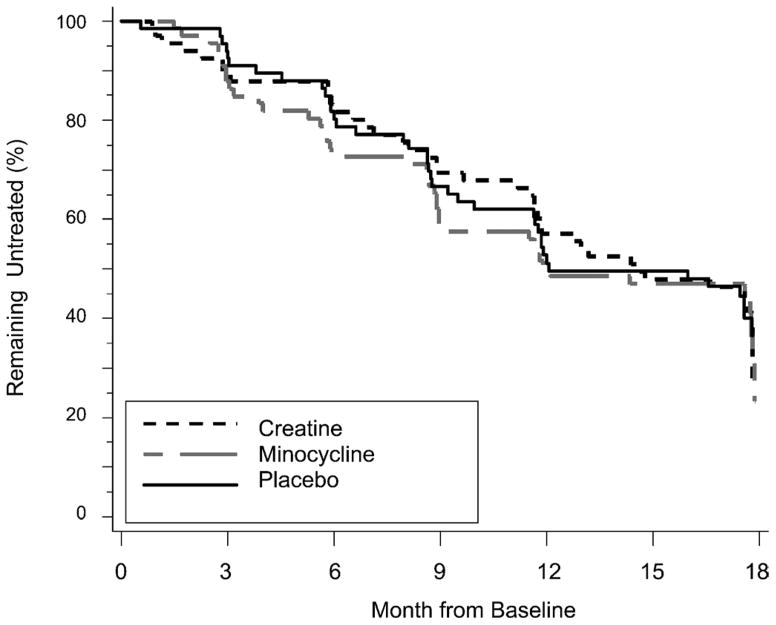
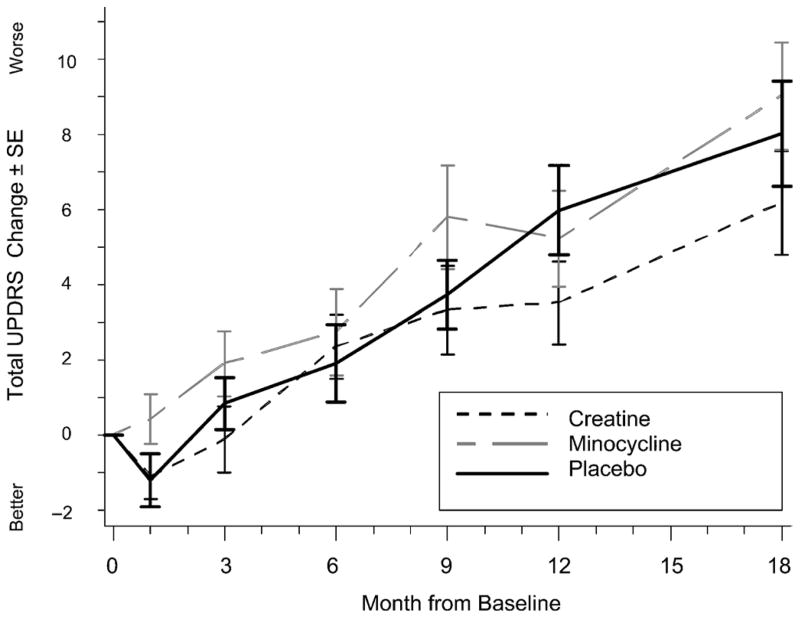
During the 18 months of the study, 41 (61%) of 67 creatine-treated, 41 (62%) of 66 minocycline-treated, and 40 (60%) of 67 placebo-treated subjects achieved a level of disability sufficient to warrant the initiation of symptomatic treatment (Fig. 1). The survival curves show a similar course of development for the need of symptomatic therapy in all groups (Fig. 2). The actual change in total UPDRS from baseline to 18 months was evaluated. Including values obtained while on symptomatic therapy, the change was 6.18 ± 11.4 in the creatine group, 9.03 ± 11.6 in the minocycline group, and 8.03 ± 11.5 in the placebo group (Fig. 3).
FIGURE 1.
Flow diagram from randomization to study completion (duration, 18 months). The number of subjects terminating the study before 18 months, the number of subjects completing the study, and the number of subjects requiring symptomatic therapy are indicated.
FIGURE 2.
Kaplan-Meier estimate of time to need for symptomatic therapy.
FIGURE 3.
Change in total UPDRS over time. Subject may be receiving symptomatic therapy at the time of visit. The UPDRS value just before initiating symptomatic therapy is not carried forward. The worst observation for the group is used to impute missing values. At 18 months, missing values were imputed for 2 creatine, 4 minocycline, and 4 placebo subjects. Bars represent SEM at 1, 3, 6, 9, 12, and 18 months.
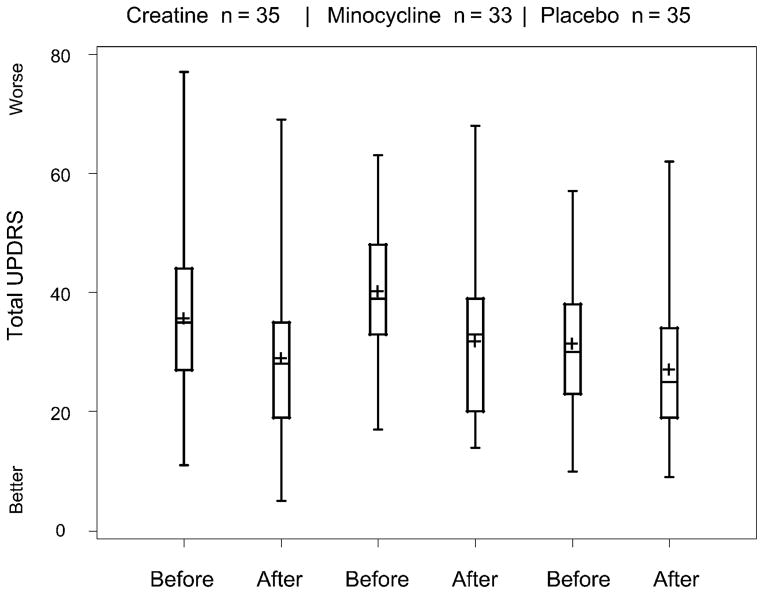
The impact of the study treatments on the clinical improvement associated with symptomatic therapy was considered by examining total UPDRS values for those subjects who required symptomatic therapy. A Box-and-Whisker plot (Fig. 4) shows the UPDRS values before initiating symptomatic therapy (at the need for symptomatic therapy visit) and at the first available visit after initiating symptomatic therapy (for those with available visits). Neither creatine nor minocycline seems to adversely affect the beneficial impact of symptomatic therapy.
FIGURE 4.
Box-and-Whisker plot of improvement in total UPDRS after initiating symptomatic therapy. Before is the value at the need for symptomatic therapy visit. After is the value at the first visit after initiating symptomatic therapy. Only subjects requiring symptomatic therapy with a subsequent UPDRS evaluation are included (creatine, 35; minocycline, 33; placebo, 35). The length of the box represents the interquartile range. The plus sign represents the mean; the horizontal line in the box represents the median. Whiskers extend to minimum and maximum values.
There were 27 serious adverse events (SAEs) during the 18-month course of the study, 14 of which occurred during the first 12 months of treatment and have been reported previously.4 For the 12-month data, 1 SAE (squamous cell carcinoma in a minocycline-treated subject) was viewed as possibly related to the study intervention by the blinded clinical site investigator. After further review by the independent medical monitor, none of the SAEs reported in the active treatment arms were thought to be definitely or probably related to the study interventions. During the subsequent 6 months, 2 SAEs occurred in the creatine group (chest pain, 2), 5 in the minocycline group (arthritis, 2; squamous cell carcinoma, 1; bladder prolapse, 1; renal calculus, 1), and 6 in the placebo group (back pain, 2; chest pain, 1; abdominal adhesions, 1; male genital neoplasm, 1; and thrombophlebitis, 1). Of the SAEs in the 2 active treatment arms occurring during this additional 6 months of follow-up, none was determined to be possibly, probably, or definitely related to the study intervention by the blinded site investigator or the independent medical monitors.
Throughout the 18-month course of the study, the most commonly reported adverse events across the treatment groups were nausea (19%), joint pain (25%), and upper respiratory symptoms (bronchitis, coughing, upper respiratory tract infection, sinusitis; 34%). All the nausea events occurred before 12 months, whereas joint pain and upper respiratory symptoms occurred throughout the study period. Eight cases of tooth discoloration were reported; 7 cases were in the minocycline group and occurred during the initial 12-month treatment period, and 1 case was reported in creatine group during the additional 6-month follow-up period.
To address whether adverse events were more frequent in the study participants after the initiation of symptomatic therapy, we calculated the incidence densities of adverse events during the period before symptomatic therapy and also while on symptomatic therapy. The average follow-up period was 1 year without antiparkinsonian treatment and 0.8 year with antiparkinsonian treatment. Table 1 lists the incidence densities and 95% confidence intervals (CIs) for the prespecified and for the 3 most frequently occurring adverse events. The prespecified adverse events were those adverse events known from earlier studies to be associated with at least 1 of the treatments. In general, the incidence densities are similar before and after the initiation of symptomatic treatment. The incidence density of joint pain increased from 18 to 28 (events per 100 person-years) in the creatine arm, although there was a large overlap of the 95% CIs. A similar incidence density for joint pain was present in the minocycline and placebo arms before the initiation of symptomatic therapy.
TABLE 1.
Incidence Density (Events per 100 Person-Years) and 95% CI for Prespecified and Most Frequently Occurring Adverse Events*
| Adverse Event | Creatine
|
Minocycline
|
Placebo
|
|||
|---|---|---|---|---|---|---|
| Not Requiring Symptomatic Therapy | Requiring Symptomatic Therapy | Not Requiring Symptomatic Therapy | Requiring Symptomatic Therapy | Not Requiring Symptomatic Therapy | Requiring Symptomatic Therapy | |
| Falls | 4 (1, 13) | 0 (0, 0) | 13 (6, 26) | 7 (1, 25) | 8 (2, 18) | 0 (0, 0) |
| Dizziness | 12 (5, 23) | 8 (1, 29) | 18 (9, 33) | 19 (6, 45) | 6 (2, 15) | 4 (0, 20) |
| Nausea/dyspepsia/vomit | 16 (8, 30) | 16 (4, 42) | 34 (20, 53) | 24 (9, 52) | 16 (8, 30) | 16 (4, 41) |
| Skin reaction | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| Headache | 9 (3, 19) | 4 (0, 22) | 15 (7, 29) | 7 (1, 26) | 8 (3, 18) | 4 (3, 18) |
| Infection | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 4 (1, 13) | 0 (0, 0) |
| Tooth discoloration | 1 (0, 8) | 0 (0, 0) | 10 (4, 21) | 4 (0, 20) | 0 (0, 0) | 0 (0, 0) |
| Joint pain | 18 (9, 32) | 28 (10, 61) | 33 (20, 52) | 14 (4, 37) | 28 (16, 46) | 4 (0, 20) |
| Upper respiratory symptoms | 15 (7, 28) | 4 (0, 22) | 10 (4, 21) | 7 (1, 25) | 18 (9, 32) | 16 (4, 41) |
Incidence densities reported while subjects were and were not receiving symptomatic therapy. Numbers in boldface indicate increased incidence density.
A total of 25 subjects prematurely discontinued study treatment (6 [9%] in the creatine arm, 15 [23%] in the minocycline arm, and 4 [6%] in the placebo arm). Of those receiving creatine, 5 discontinued the treatment before the initiation of symptomatic treatment and 1 after. Of those receiving minocycline, 14 discontinued the treatment before receiving symptomatic therapy, and 1 after. The 4 subjects receiving placebo who prematurely discontinued the study treatment did so before beginning symptomatic therapy.
Laboratory values were unremarkable across treatment arms except for clinically significant elevations in glucose in 5 subjects (creatine, 1; minocycline, 2; placebo, 2), alanine aminotransferase (ALT) in 3 subjects (creatine, 1; placebo, 2), and serum creatinine levels in 5 subjects (all creatine). Of these 5 subjects with elevated creatinine levels, 3 subjects had baseline creatinine values at the high range of reference value, and 1 subject had an elevated baseline creatinine level. Three of the 5 subjects permanently discontinued the administration of study drug, and the creatinine values returned to baseline. Creatinine values were elevated at the final visit in 2 subjects, and no study data were collected thereafter.
DISCUSSION
This report extends the experience of subjects in the double-blind futility trial of minocycline and creatine in early PD.4 The present data from these small phase II studies do not raise concerns regarding the safety of either agent alone or in conjunction with medications used to treat the symptoms of PD. Moreover, neither agent seems to interfere with the beneficial effects of symptomatic therapy. A concern remains regarding the high proportion of subjects prematurely discontinuing minocycline (23%); this trend was evident early on with the use of minocycline because all but 1 subject stopped this medication during the first year. These findings are important determinants when considering these agents for future phase III efficacy trials.
Although the study treatments were generally well tolerated and seem safe when taken during an 18-month period, it should, however, be noted that the numbers of subjects on a particular symptomatic treatment are small, and the period of follow-up on the combined treatment is variable. Additional safety concerns may arise when a larger sample is evaluated or if a sample is followed up for a longer duration. When viewing the occurrence of adverse events across treatment arms in the absence and in the presence of symptomatic therapy, there is no increase in the occurrence of adverse events associated with the introduction of symptomatic treatments, except for an increase in joint pain in those subjects on creatine. The significance of this finding is difficult to interpret given the small sample size, short follow-up period, and overlap of the CIs between the 2 groups. Although there is no evidence to suggest that creatine, in combination with therapies commonly used to treat PD, would be expected to exacerbate joint pain, the increased incidence density in this small sample suggests that monitoring for this adverse event is warranted if future studies are conducted. The finding that the adverse event profile is not significantly altered after the introduction of symptomatic therapy also suggests that subjects with more severe PD tolerate the study agents as well as those earlier in their disease course not yet requiring symptomatic therapy. However, it must be noted that those subjects on symptomatic therapy could not, by definition, have as much follow-up and, thus, as much chance to experience an adverse event. Although incidence densities were determined to adjust for this unequal follow-up, this computation does not adjust for the difference in disease severity and likelihood that those with more advanced disease may be more likely to experience an adverse event. Yet, such a difference in adverse events was not noted despite the difference in disease severity. It is also possible that symptomatic therapy may improve quality of life to the extent that fewer adverse events were reported. In addition, only 1 subject each in the creatine and minocycline groups prematurely discontinued study treatment after the introduction of symptomatic therapy, suggesting that the addition of symptomatic therapy is well tolerated. However, the ability to detect such differences is limited by the shorter exposure times with symptomatic treatment. It is thus possible that we may have missed adverse effects that would be detected in a larger, longer-term study. Although creatinine levels were found to be elevated in a subset of subjects in the creatine arm, the use of creatine has not been associated with renal dysfunction,19 and levels returned to baseline in the 3 subjects who discontinued creatine treatment in the present study.
Estimates of the time to need symptomatic therapy do not differ between the groups. By the end of 18 months, 60% of all patients needed symptomatic therapy. On the other hand, looking at the change from baseline in total UPDRS score (actual change; Fig. 3), there seems to be the beginnings of a separation between the groups, although the study was not powered to detect such differences. These data suggest that a longer period of follow-up may be required to determine treatment differences. The reduction in total UPDRS score after initiation of symptomatic therapy is similar to that observed in earlier studies.20
The results of this study suggest that both creatine and minocycline seem safe when taken over an 18-month period. These agents do not seem to influence the response to symptomatic therapy, and the introduction of symptomatic therapy is not associated with a worrisome increase in adverse events. However, the tolerability of minocycline may be of concern when considering a long-term study where even moderate withdrawal occurring early on could dilute the ability to detect a treatment effect. In addition, the sample size in the present study was small, and it cannot be concluded that safety concerns may not arise in a larger sample studied for a longer duration.
Acknowledgments
Supported by the National Institutes of Health (National Institute of Neurological Disorders and Stroke) grants U01NS043127, U01NS043128, and U10NS44415 through U10NS44555.
Data Safety Monitoring Board: Cynthia R. Gross, PhD (Chair), University of Minnesota, Minneapolis; Donna T. Chen, MD, MPH, University of Virginia Health System, Charlottesville; David E. Levy, MD, Neurobiological Technologies, Inc, Edge-water, NJ; Karen Marder, MD, MPH, Columbia University, New York, NY; Robert L. Rodnitzky, MD, University of Iowa College of Medicine, Iowa City.
Oversight Board: K. Michael Welch, MD (Chair), Rosalind Franklin University of Medicine and Science, North Chicago, Ill; M. Flint Beal, MD, Weill Medical College of Cornell University, New York, NY; Jeffrey L. Cummings, MD, University of California, Los Angeles; David J. Edwards, PharmD, Wayne State University, Detroit, Mich; Stanley Fahn, MD, Bruce Levin, PhD, Columbia University, New York, NY; Russell G. Katz, MD, Food and Drug Administration, Rockville, Md; William McDonald, MD, Emory University, Atlanta, Ga; Deborah B. Marin, MD, C. Warren Olanow, MD, Mount Sinai School of Medicine, New York, NY; Jeffrey C. Martin, Esq, Shea & Gardner, Washington, DC; Diane Murphy, PhD, Eugene Oliver, PhD, National Institute of Neurological Disorders and Stroke, Bethesda, Md; Steven Piantadosi, MD, PhD, Johns Hopkins University, Baltimore, Md; William J. Powers, MD, Washington University School of Medicine, St Louis, Mo; Leon J. Thal, MD, University of California, San Diego; Alison Wichman, MD, National Institutes of Health, Bethesda, Md.
National Institutes of Health: John Marler, MD, Joanne Odenkirchen, MPH, National Institute of Neurological Disorders and Stroke, Bethesda, Md.
We thank the patients and families who participated in this study.
APPENDIX (AUTHORS)
NET-PD Steering Committee: Karl Kieburtz, MD, MPH (principal investigator, Coordination Center), University of Rochester, NY; Barbara Tilley, PhD (principal investigator, Statistical Center), Medical University of South Carolina, Charleston; Bernard Ravina, MD, MSCE, National Institutes of Health, Bethesda, Md (current affiliation: University of Rochester, NY); Wendy Galpern, MD, PhD, National Institutes of Health, Bethesda, Md; Kathleen Shannon, MD, Rush University Medical Center, Chicago, Ill; Caroline Tanner, MD, PhD, The Parkinson Institute, Sunnyvale, Calif; G. Frederick Wooten, MD, University of Virginia, Charlottesville.
Participating Investigators and Coordinators: Brad Racette, MD, Patricia Deppen, Washington University School of Medicine, St Louis, Mo; Richard B. Dewey Jr, MD, University of Texas Southwestern Medical School, Dallas; Burton Scott, MD, Joanne Field, BSN, RN, Duke University, Durham, NC; Julie Carter, RN, MN, ANP, Matthew Brodsky, MD, Pamela Andrews, Oregon Health and Science University, Portland, Ore; Bala Manyam, MD, Jacqueline Whetteckey, Scott & White Clinic/Texas A&M University, Temple, Tex; Jayaraman Rao, MD, Maureen Cook, RN, BSN, Louisiana State University Health Sciences Center, New Orleans, La; Michael J. Aminoff, MD, FRCP, Chadwick Christine, MD, Jessie Roth, RN, University of California, San Francisco; Martha Nance, MD, Sotirios Parashos, MD, Susan Peterson, RN, Struthers Parkinson Center, Golden Valley, Minn; Kathleen Shannon, MD, Jeana Jaglin, RN, CCRC, Rush University Medical Center, Chicago, Ill; Carlos Singer, MD, Marian A. Perez, AA, Anita Blenke, PA-C, MS, University of Miami, Fla; Robert Hauser, MD, Theresa McClain, ARNP, Summer Wolfrath, MSPH, University of South Florida, Tampa; Ted Dawson, MD, PhD, Becky Dunlop, RN, Johns Hopkins University, Baltimore, Md; Rajesh Pahwa, MD, Kelly Lyons, PhD, Amy Parsons, RN, BSN, University of Kansas Medical Center, Kansas City; Maureen Leehey, MD, Jacci Bainbridge, Pharm D, University of Colorado Health Sciences Center, Aurora; Lisa Shulman, MD, William Weiner, MD, Katharine Pabst, CRNP, MS, MPH, University of Maryland, Baltimore; Rodger Elble, MD, PhD, Charlene Young, RN, MSN, CFNP, Southern Illinois University, Springfield, Ill; Kapil Sethi, MD, Buff Dill, BS, ED, Medical College of Georgia, Augusta; Wayne Martin, MD, Germaine McInnes, RN, University of Alberta Glenrose Rehab Hospital, Edmonton; Vincent P. Calabrese, MD, Peggy Roberge, RN, Hunter Holmes McGuire Veterans Medical Center, Richmond, Va; Robert Hamill, MD, University of Vermont, Burlington; Ivan G. Bodis-Wollner, MD, Elizabeth Hayes, RN, State University of New York Downstate Medical Center, Brooklyn, NY; G. Webster Ross, MD, Stephanie Terashita, RN, Pacific Health Research Institute, Honolulu, Hawaii; G. Frederick Wooten, MD, Joel Trugman, MD, Margaret F. Keller, RN, MS, CCRC, University of Virginia, Charlottesville; Jacob I. Sage, MD, Debbie Caputo, RN, MSN, UMDNJ Robert Wood Johnson Medical School, New Brunswick, NJ; John Fang, MD, Dorothy Shearon, RN, Vanderbilt University Medical Center, Nashville, Tenn; Danna Jennings, MD, Institute for Neurodegenerative Disorders, New Haven, Conn; Joanne Wojcieszek, MD, Joann Belden, RN, Indiana University School of Medicine, Indianapolis, Ind; Andrew Feigin, MD, Margaret Marie Cox, RN, BSN, North Shore University Hospital, Manhasset, NY; Ray L. Watts, MD, Natividad Stover, MD, Rebecca McMurray, RN, MSN, University of Alabama, Birmingham; Roger Albin, MD, Kristine Wernette, RN, MS, University of Michigan, Ann Arbor; Andrew Siderowf, MD, Sue Reichwein, CCRC, University of Pennsylvania, Philadelphia; David Simon, MD, Daniel Tarsy, MD, Lisa Scollins, NP, Beth Israel Deaconess Medical Center, Boston, Mass; Ron Tintner, MD, Christine Hunter, RN, CCRC, Baylor College of Medicine, Houston, Tex; Peter LeWitt, MD, Maryan Deangelis, RN, William Beaumont Hospital, Southfield, Mich; Richard S. Burns, MD, Lynn Marlor, RN, Barrow Neurological Institute, Phoenix, Ariz; Charles Adler, MD, PhD, Marlene Lind, RN, Mayo Clinic Scottsdale, Ariz; Jay Gorell, MD, Shana Krstevska, MD, Marilyn Flewellen, RN, Henry Ford Health System, Detroit, Mich; Jay Schneider, PhD, Stephanie Sendek, Thomas Jefferson University, Philadelphia, Pa; Stephen Gollomp, MD, Gwyn Vernon, MSN, CRNP, Thomas Jefferson University/Lankenau Hospital, Wynnewood, Pa; David Coffey, MD, Pauline LeBlanc, BS, Dartmouth Hitchcock Medical Center, Lebanon, NH; Mark F. Lew, MD, Allan Wu, MD, Connie Kawai, RN, BSN, CCRC, University of Southern California, Los Angeles; Ryan Uitti, MD, Margaret Turk, RN, Mayo Clinic Jacksonville, Fla; James Bower, MD, Susan Torgrimson, RN, Mayo Clinic Rochester, Minn; Joseph Handler, MD, Neurology Associates PA, Wilmington, Del; Marwan Sabbagh, MD, Zoran Obradov, CRC, Sun Health Research Institute, Sun City, Ariz.
NET-PD Statistical Center: Jordan Elm, MA, Paulo Guimaraes, PhD, Peng Huang, PhD, Yuko Palesch, PhD, Medical University of South Carolina, Charleston.
NET-PD Clinical Trials Coordination Center Staff: Irenita Gardiner, RN, CCRC, Cornelia Kamp, MBA, CCRC, Aileen Shinaman, JD, Debbie Johnsen, BS, Alicia Brocht, BA, Susan Bennett, AAS, Chris Weaver, CCRP, University of Rochester, NY.
NET-PD Consultants: Christopher Goetz, MD, Rush University Medical Center, Chicago, Ill; Janis Miyasaki, MD, FRCPC, Toronto Western Hospital, Toronto, Ontario; Susan Fagan, Pharm D, BCPS, FCCP, University of Georgia.
References
- 1.Schapira AH, Olanow CW. Neuroprotection in Parkinson disease: mysteries, myths, and misconceptions. JAMA. 2004;291:358–364. doi: 10.1001/jama.291.3.358. [DOI] [PubMed] [Google Scholar]
- 2.Huse DM, Schulman K, Orsini L, et al. Burden of illness in Parkinson’s disease. Mov Disord. 2005;20:1449–1454. doi: 10.1002/mds.20609. [DOI] [PubMed] [Google Scholar]
- 3.Noyes K, Liu H, Li Y, et al. Economic burden associated with Parkinson’s disease on elderly Medicare beneficiaries. Mov Disord. 2006;21:362–372. doi: 10.1002/mds.20727. [DOI] [PubMed] [Google Scholar]
- 4.The NINDS NET-PD Investigators. A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology. 2006;66:664–671. doi: 10.1212/01.wnl.0000201252.57661.e1. [DOI] [PubMed] [Google Scholar]
- 5.Tilley BC, Palesch YY, Kieburtz K, et al. Optimizing the ongoing search for new treatments for Parkinson disease: using futility designs. Neurology. 2006;66:628–633. doi: 10.1212/01.wnl.0000201251.33253.fb. [DOI] [PubMed] [Google Scholar]
- 6.The NINDS NET-PD Investigators. A randomized, double blind, futility clinical trial of coenzyme Q10 and GPI-1485 in early Parkinson’s disease. Neurology. 2007;68:20–28. doi: 10.1212/01.wnl.0000250355.28474.8e. [DOI] [PubMed] [Google Scholar]
- 7.Ravina BM, Fagan SC, Hart RG, et al. Neuroprotective agents for clinical trials in Parkinson’s disease: a systematic assessment. Neurology. 2003;60:1234–1240. doi: 10.1212/01.wnl.0000058760.13152.1a. [DOI] [PubMed] [Google Scholar]
- 8.Tarnopolsky MA, Beal MF. Potential for creatine and other therapies targeting cellular energy dysfunction in neurological disorders. Ann Neurol. 2001;49:561–574. [PubMed] [Google Scholar]
- 9.Elewa HF, Hilali H, Hess DC, et al. Minocycline for short-term neuroprotection. Pharmacotherapy. 2006;26:515–521. doi: 10.1592/phco.26.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthews RT, Ferrante RJ, Klivenyi P, et al. Creatine and cyclocreatine attenuate MPTP neurotoxicity. Exp Neurol. 1999;157:142–149. doi: 10.1006/exnr.1999.7049. [DOI] [PubMed] [Google Scholar]
- 11.Du Y, Ma Z, Lin S, et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2001;98:14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu DC, Jackson-Lewis V, Vila M, et al. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang L, Sugama S, Chirichigno JW, et al. Minocycline enhances MPTP toxicity to dopaminergic neurons. J Neurosci Res. 2003;74:278–285. doi: 10.1002/jnr.10709. [DOI] [PubMed] [Google Scholar]
- 14.Diguet E, Fernagut PO, Wei X, et al. Deleterious effects of minocycline in animal models of Parkinson’s disease and Huntington’s disease. Eur J Neurosci. 2004;19:3266–3276. doi: 10.1111/j.0953-816X.2004.03372.x. [DOI] [PubMed] [Google Scholar]
- 15.Elm JJ, Goetz CG, Ravina B, et al. A responsive outcome for Parkinson’s disease neuroprotection futility studies. Ann Neurol. 2005;57:197–203. doi: 10.1002/ana.20361. [DOI] [PubMed] [Google Scholar]
- 16.Brudnak MA. Creatine: are the benefits worth the risk? Toxicol Lett. 2004;150:123–130. doi: 10.1016/j.toxlet.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Somech R, Arav-Boger R, Assia A, et al. Complications of minocycline therapy for acne vulgaris: case reports and review of the literature. Pediatr Dermatol. 1999;16:469–472. doi: 10.1046/j.1525-1470.1999.00106.x. [DOI] [PubMed] [Google Scholar]
- 18.Gordon PH, Moore DH, Gelinas DF, et al. Placebo-controlled phase I/II studies of minocycline in amyotrophic lateral sclerosis. Neurology. 2004;62:1845–1847. doi: 10.1212/01.wnl.0000125321.92112.7e. [DOI] [PubMed] [Google Scholar]
- 19.Groeneveld GJ, Beijer C, Veldink JH, et al. Few adverse effects of long-term creatine supplementation in a placebo-controlled trial. Int J Sports Med. 2005;26:307–313. doi: 10.1055/s-2004-817917. [DOI] [PubMed] [Google Scholar]
- 20.Holloway RG, Shoulson I, Fahn S, et al. Pramipexole vs levodopa as initial treatment for Parkinson disease: a 4-year randomized controlled trial. Arch Neurol. 2004;61:1044–1053. doi: 10.1001/archneur.61.7.1044. [DOI] [PubMed] [Google Scholar]