Introduction
Sleep disordered breathing includes obstructive and central sleep apnea (CSA), obesity hypoventilatory syndrome, snoring and Cheyne-Stokes breathing. Obstructive sleep apnea (OSA) is usually diagnosed via polysomnography (PSG) and quantified by the number of episodes of cessation (apnea) or reduction (hypopneas) of airflow, lasting ≥ 10 seconds per hour of sleep [1]. An apnea-hypopnea index (AHI) of ≥ 5 per hour is considered significant for OSA. A diagnosis of OSA syndrome (OSAS) is made when patients have OSA and symptoms. CSA has similar clinical features, but is due to reduced central drive vs OSA which is usually due to anatomical obstruction. PSG testing distinguishes between CSA and OSA.
Epidemiology
OSA prevalence varies across populations. In middle-aged adults in the Midwest, 25% of men and 10% of women have OSA. [2, 3] The prevalence in the general US population is approximately 4% (18 million) and predicted to increase in line with the rising obesity epidemic. Prevalence of hypertension (HTN) in those with OSA is 50–60% and is related to severity. 30–40% of hypertensives also have OSA. OSA is more prevalent in obese, young to middle-aged men and in those with resistant hypertension. Indeed, 70% or more of those with resistant hypertension will have OSA and secondary hyperaldosteronism. [4, 5]
Symptoms
These include daytime somnolence, fatigue, nocturia, and disruptive snoring. Witnessed apneas are highly predictive of OSA, snoring is suggestive, and daytime somnolence may be less evident in patients with cardiovascular co-morbidities such as heart failure and atrial fibrillation.
Signs
High BMI, short thick neck, macroglossia with crowded oropharynx, nocturnal hypertension or nondipping profile, and nocturnal bradyarrhythmias. Note that some populations, such as Southeast Asians, may have sleep apnea even in the context of what by Western standards is considered a normal BMI.
Diagnosing OSA
The gold standard for diagnosis of OSA is considered to be attended PSG, although home overnight oximetry and polygraphy (measures of oxygen saturation and indices of breathing and airflow), are increasingly being used for screening purposes. Screening questionnaires (STOPBANG, Berlin and Epworth) are also utilized; however, the sensitivity and specificity of these tests vary in different populations. Resistant hypertension (unachieved target BP despite 3 or more medications) and symptoms of OSA should prompt further investigation.
Sleep apnea and hypertension
OSA is accepted as an important independent risk factor for cardiovascular diseases in general, and in particular for hypertension. It is prominent in European and American guidelines as an identifiable and treatable cause of secondary hypertension.[6–8] It is difficult to tease out confounding variables and infer a direct causal relationship for OSA and hypertension. Nevertheless, a substantive body of evidence suggests a link, after adjusting for confounders. Importantly, treating OSA may improve hypertension and may translate into improved CV risk profile and patient outcomes. In a prospective cohort study, patients with BMI <27 and severe OSA had 3 fold higher odds of hypertension. [9] OSA is seen in 71% of those with resistant hypertension vs 38% of those with controlled hypertension. [10] The mechanisms by which OSA is thought to elicit hypertension include sympathetic activation, [11] endothelial dysfunction, increased endothelin, reduced nitric oxide, and systemic inflammation. [1] Note that an increased likelihood of hypertension has also been associated with restless legs syndrome[12] and reduced sleep duration, [13]independent of the presence of OSA. Furthermore, OSA has also been linked to diabetes, metabolic syndrome, heart failure, arrhythmia, depression, and erectile dysfunction.
Using ambulatory BP monitoring to diagnose hypertension in OSA
Making a diagnosis of hypertension in OSA may be more accurate using ambulatory blood pressure monitoring (ABPM) especially if ‘white-coat’ or pseudo-resistance (eg inappropriately sized cuff ) is suspected. [6, 14] Nocturnal systolic BP may predict CV mortality and morbidity better than daytime BP.
Management of OSA
These include postural measures, such as encouraging patients to sleep in a lateral position, since apnea is often worse sleeping supine, presumably due to the gravitational effects on the tongue and upper airway, promoting occlusion. Weight loss is very helpful, and avoidance of alcohol and other neural-depressant medications before bedtime may be effective. Mandibular devices are used for milder OSA, and keep the lower jaw and tongue from “falling” backwards during sleep. The standard therapy is continuous positive airway pressure (CPAP). In those who cannot tolerate CPAP, pilot studies suggest a potential future role for hypoglossal nerve stimulation.[15] Severe and potentially life threatening OSA can be treated, at least in the short term, with tracheostomy. At present, there are no standard pharmacologic treatments for OSA.
Treatment of OSA – effects on blood pressure
Several meta-analyses and randomized trials demonstrate improved blood pressure with the use of positive airway pressure (continuous or bilevel). [16–18] A recent meta-analysis of 30 randomized trials pooled 1900 patients and showed a mean net reduction in BP of 2.5 mm Hg [16]. BP lowering effects are especially evident in those with more severe OSA. Even a modest BP reduction is of relevance when considered in the context of the 1 to 5 mmHg reduction in BP achieved by ACE inhibitors, which may reduce risk of stroke, major cardiovascular events and death between 20% to 25%. [19] Randomized trials suggest that treatment of OSA with mandibular devices can also lower BP. [20–22].
Whether other beneficial effects of OSA therapy, such as attenuation of nocturnal hypoxemia, sympathetic activation, and systemic inflammation, confer further benefit beyond blood pressure reduction remains to be determined. Currently, no class of antihypertensive is best suited to treat OSA-related hypertension. Aldosterone antagonists may be beneficial in those with resistant hypertension and OSA, but randomized data are unavailable. A recent meta-analysis suggests renal denervation may prove to be beneficial as an adjunct for patients with OSA and hypertension.[23]
Figure 1.
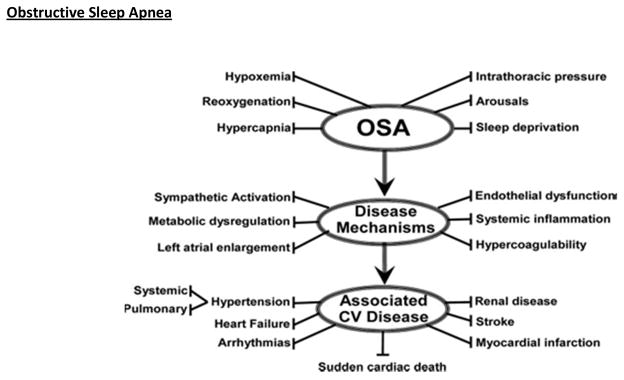
Schematic outlining proposed pathophysiological components of OSA, activation of cardiovascular disease mechanisms, and consequent development of established cardiovascular disease. Reprinted from Somers VK et al 1 with permission from Lippincot Williams & Wilkins. Copyright 2008, American heart Association.
Table 1.
Obstructive sleep apnea
| Signs, symptoms, and risk factors | Disruptive snoring |
| Witnessed apnea or gasping | |
| Obesity and/or enlarged neck size | |
| Hypersomnolence (not common in children or in heart failure) | |
| Other signs and symptoms include male gender, crowded-appearing pharyngeal airway, increased blood pressure, morning headache, sexual dysfunction, behavioral changes (especially in children) | |
| Screening and diagnostic tests | Questionnaires (STOPBANG, Berlin, Epworth) |
| Holter (24h ECG) monitoring | |
| Overnight oximetry | |
| Home-based/ambulatory unattended polysomnography | |
| In-hospital attended overnight polysomnography | |
| Treatment Options | Positional therapy |
| Weight loss | |
| Avoidance of alcohol and sedatives | |
| Positive airway pressure | |
| Oral appliances | |
| Surgery | |
| Radiofrequency ablation of the soft palate | |
| Uvulopalatopharyngoplasty | |
| Tonsillectomy | |
| Tracheostomy |
Modified from Somers VK et al 1 with permission from Lippincott Williams & Wilkins. Copyright 2008, American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
C. Anwar A. Chahal, Fellow, Cardiovascular Diseases, Mayo Clinic, Specialty Registrar in Cardiology, London Deanery, UK; Mayo Graduate School, Rochester, MN.
Virend K. Somers, Professor of Medicine, Division of Cardiovascular Diseases, Department of Internal Medicine, Mayo Clinic, Rochester, MN.
References
- 1.Somers VK, et al. Sleep apnea and cardiovascular disease: an American Heart Association/american College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118(10):1080–111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 2.Young T, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Peppard PE, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dudenbostel T, Calhoun DA. Resistant hypertension, obstructive sleep apnoea and aldosterone. J Hum Hypertens. 2012;26(5):281–7. doi: 10.1038/jhh.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Svatikova A, et al. Obstructive sleep apnea and aldosterone. Sleep. 2009;32(12):1589–92. doi: 10.1093/sleep/32.12.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parati G, et al. Position paper on the management of patients with obstructive sleep apnea and hypertension: joint recommendations by the European Society of Hypertension, by the European Respiratory Society and by the members of European COST (COoperation in Scientific and Technological research) ACTION B26 on obstructive sleep apnea. J Hypertens. 2012;30(4):633–46. doi: 10.1097/HJH.0b013e328350e53b. [DOI] [PubMed] [Google Scholar]
- 7.The Seventh Report of the Joint National Committee on Prevention. Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda MD: 2004. [DOI] [PubMed] [Google Scholar]
- 8.Grote L, Hedner J, Peter JH. Sleep-related breathing disorder is an independent risk factor for uncontrolled hypertension. J Hypertens. 2000;18(6):679–85. doi: 10.1097/00004872-200018060-00004. [DOI] [PubMed] [Google Scholar]
- 9.Marin JM, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307(20):2169–76. doi: 10.1001/jama.2012.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goncalves SC, et al. Obstructive sleep apnea and resistant hypertension: a case-control study. Chest. 2007;132(6):1858–62. doi: 10.1378/chest.07-1170. [DOI] [PubMed] [Google Scholar]
- 11.Somers VK, et al. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batool-Anwar S, et al. Restless legs syndrome and hypertension in middle-aged women. Hypertension. 2011;58(5):791–6. doi: 10.1161/HYPERTENSIONAHA.111.174037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gangwisch JE, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47(5):833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 14.James PA, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 15.Strollo PJ, Jr, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139–49. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 16.Sundstrom J, et al. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384(9943):591–8. doi: 10.1016/S0140-6736(14)61212-5. [DOI] [PubMed] [Google Scholar]
- 17.Haentjens P, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167(8):757–64. doi: 10.1001/archinte.167.8.757. [DOI] [PubMed] [Google Scholar]
- 18.Bazzano LA, et al. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50(2):417–23. doi: 10.1161/HYPERTENSIONAHA.106.085175. [DOI] [PubMed] [Google Scholar]
- 19.Turnbull F. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362(9395):1527–35. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 20.Otsuka R, et al. The effect of oral appliance therapy on blood pressure in patients with obstructive sleep apnea. Sleep Breath. 2006;10(1):29–36. doi: 10.1007/s11325-005-0038-6. Obstructive Sleep Apnea. [DOI] [PubMed] [Google Scholar]
- 21.Gotsopoulos H, Kelly JJ, Cistulli PA. Oral appliance therapy reduces blood pressure in obstructive sleep apnea: a randomized, controlled trial. Sleep. 2004;27(5):934–41. doi: 10.1093/sleep/27.5.934. [DOI] [PubMed] [Google Scholar]
- 22.Sharples L, et al. Clinical effectiveness and cost-effectiveness results from the randomised controlled Trial of Oral Mandibular Advancement Devices for Obstructive sleep apnoea-hypopnoea (TOMADO) and long-term economic analysis of oral devices and continuous positive airway pressure. Health Technol Assess. 2014;18(67):1–296. doi: 10.3310/hta18670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shantha GP, Pancholy SB. Effect of renal sympathetic denervation on apnea-hypopnea index in patients with obstructive sleep apnea: a systematic review and meta-analysis. Sleep Breath. 2014 doi: 10.1007/s11325-014-0991-z. [DOI] [PubMed] [Google Scholar]


