Abstract
Socioeconomic status (SES) differences in attitudes towards cancer have been implicated in the differential screening uptake and the timeliness of symptomatic presentation. However, the predominant emphasis of this work has been on cancer fatalism, and many studies focus on specific community subgroups. This study aimed to assess SES differences in positive and negative attitudes towards cancer in UK adults. A population-based sample of UK adults (n=6965, age≥50 years) completed the Awareness and Beliefs about Cancer scale, including six belief items: three positively framed (e.g. ‘Cancer can often be cured’) and three negatively framed (e.g. ‘A cancer diagnosis is a death sentence’). SES was indexed by education. Analyses controlled for sex, ethnicity, marital status, age, self-rated health, and cancer experience. There were few education-level differences for the positive statements, and overall agreement was high (all>90%). In contrast, there were strong differences for negative statements (all Ps<0.001). Among respondents with lower education levels, 57% agreed that ‘treatment is worse than cancer’, 27% that cancer is ‘a death sentence’ and 16% ‘would not want to know if I have cancer’. Among those with university education, the respective proportions were 34, 17 and 6%. Differences were not explained by cancer experience or health status. In conclusion, positive statements about cancer outcomes attract near-universal agreement. However, this optimistic perspective coexists alongside widespread fears about survival and treatment, especially among less-educated groups. Health education campaigns targeting socioeconomically disadvantaged groups might benefit from a focus on reducing negative attitudes, which is not necessarily achieved by promoting positive attitudes.
Keywords: attitude, cancer, education, fear, hope, social class
Introduction
Inequalities in cancer survival by socioeconomic status (SES) are seen even in countries whose medical systems provide care without cost at the point of delivery (Jeffreys et al., 2009; Booth et al., 2010; Rachet et al., 2010). Part of the survival gradient is explained by later-stage disease at diagnosis among lower SES groups (Woods et al., 2006; Rutherford et al., 2013).
Analyses of UK data suggest that SES differences in the stage at diagnosis tend to be highest for cancers with a clear ‘symptom signature’ (e.g. breast cancer) (Lyratzopoulos et al., 2013). In these cancers, there is little or no SES difference in the number of medical contacts before diagnosis (Lyratzopoulos et al., 2012), but there are differences in the time interval between the patient noticing the symptom and seeking medical help (Macleod et al., 2009). Although this could be due to a lack of awareness of the implication of the symptom, SES differences in help-seeking intervals are seen for cancers for which public awareness is high. This suggests that other factors, which could include attitudes towards a cancer diagnosis, play a role in delayed help-seeking.
Fatalistic attitudes (the belief that cancer risk is predetermined and invariably fatal) have been reported to be more common in lower SES groups (Niederdeppe and Levy, 2007; Beeken et al., 2011; Espinosa de los Monteros and Gallo, 2011; Miles et al., 2011). Qualitative analyses implicate a more general pessimism about cancer outcomes (Balshem, 1991; Peek et al., 2008), and some quantitative studies support this idea. For example, levels of cancer worry have been found to be higher in lower SES groups (Wardle et al., 2004; Byrne et al., 2008) and the value of early detection lower (Beeken et al., 2011). One US survey found educational differences in endorsing myths about cancer surgery (Gansler et al., 2005), and in a sample of British women, those in manual (vs. professional) occupations were more concerned that breast cancer surgery would lead to disfigurement (Grunfeld et al., 2002). However, in the same sample, there were no differences in attitudes towards breast cancer patients’ quality of life, and women from manual backgrounds were more likely to believe that treatment would be beneficial, indicating that attitudes of women from lower SES backgrounds are not entirely negative.
Qualitative studies have also found some evidence of positive and negative attitudes coexisting. Adults from deprived areas in Scotland expressed despair about cancer, but also acknowledged the benefits of early detection (Rowa-Dewar et al., 2007). A similar observation was made within a socioeconomically diverse sample, suggesting that coexisting positive and negative cancer beliefs could characterize public discourse more generally (Robb et al., 2014). These findings suggest that SES inequalities in cancer attitudes may be more nuanced; perhaps they are overlooked owing to the predominant focus on negative attitudes in previous research. There is a need to understand the balance of both positive and negative cancer beliefs across socioeconomic groups to direct campaigns that engage hard-to-reach groups effectively. We provide the first population-based quantitative study to examine this issue specifically.
Materials and methods
Data were collected in 2011 as part of the International Cancer Benchmarking Partnership (ICBP; CR-UK, 2010). The present analyses use respondents from the UK (England, Wales and Northern Ireland). Landline telephone numbers were sampled from electronic listings using random probability sampling methods. The final two digits of each telephone number were exchanged for two random digits, to include unlisted numbers. For households with two or more eligible adults, the ‘Rizzo’ method was used to select one adult at random (Rizzo et al., 2004). Ethical approval was sought within each jurisdiction.
Measures
Telephone interviewers administered the Awareness and Beliefs about Cancer Measure (ABC: Simon et al., 2012). Cancer beliefs were assessed with six items: three were positively framed items (P1: These days, many people with cancer can expect to continue with normal activities and responsibilities; P2: Cancer can often be cured; P3: Going to the doctor as quickly as possible after noticing a symptom of cancer could increase the chances of surviving) and three were negatively framed items (N1: A cancer diagnosis is a death sentence; N2: I would not want to know if I have cancer; N3: Most cancer treatment is worse than cancer itself). The item order was rotated to minimize response bias. Respondents were asked: ‘Can you tell me how much you agree or disagree with each item’, with response categories of strongly disagree, tend to disagree, tend to agree, strongly agree and don’t know. Responses were combined into strongly disagree/agree versus disagree/strongly disagree/don’t know because we were specifically interested in the predictors of agreement, and excluding cases who responded ‘don’t know’ would have inflated the apparent percentage agreeing. However, we also carried out a sensitivity analysis, repeating the analyses after excluding cases with ‘don’t know’ responses for any item.
Information was collected on age, sex, marital status and ethnicity. Marital status was grouped into ‘single, divorced or widowed’ and ‘married or cohabiting’. Because of the low number of respondents in any one ethnic subgroup, ethnicity was grouped into ‘White’ and ‘non-White’. As a marker of SES, respondents reported their highest level of education (left school at or before the age of 15 years; Certificate of Secondary Education, O-levels or equivalent, A-levels or equivalent, university degree). We used a single item measure of self-rated health (very good, good, fair, poor, very poor), common in previous studies (e.g. DeSalvo et al., 2006). Cancer experience was assessed by asking respondents, ‘Have you, or any friends or family members that are close to you, ever been diagnosed with cancer?’ For analyses, responses were dichotomised as yes (self, someone close, both or prefer not to say who) or no.
Analyses
To correct for over-representation and under-representation of particular demographic groups, cases were weighted to reflect the distribution of demographic characteristics of adults over 50 years of age in the UK. A design weight was also applied that adjusted for the number of eligible adults in each household and the relative sizes of each country’s population. For further information regarding data sources and weighting methods, see the online supplementary information in the ICBP report (Forbes et al., 2013).
Associations between demographic variables and beliefs were explored using χ2 analyses. Multivariable logistic regression analyses were used to assess the independent effects of age, sex, marital status, ethnicity and education, adjusted for UK region, self-rated health and cancer experience. For the main analyses, six regression models were computed, predicting agreement with each belief.
Results
The target sample was 6000 adults aged at least 50 years across England, Wales and Northern Ireland. A total of 24 231 households were successfully contacted and assessed for eligibility, from which 10 977 individuals aged at least 50 years were identified, and 6965 completed the interview, giving a response rate of 19.4% (response rate type 3; The American Association of Public Opinion Research, 2011). This type of response rate was used because the denominator of eligible individuals was unknown (see the ICBP report: Forbes et al., 2013).
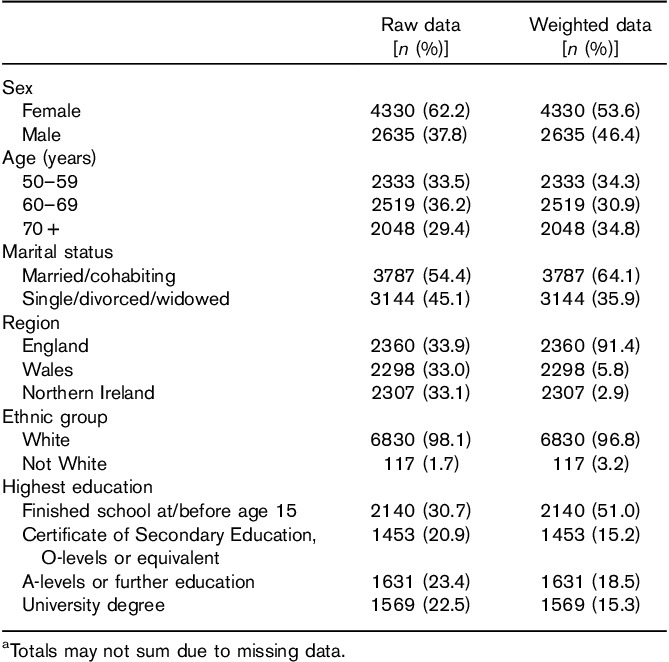
Table 1 shows raw and weighted sample characteristics. Respondents’ average age was 63 years (SD=18.3). The majority were White (98.1% in the unweighted sample and 96.8% after weighting) and female (62.2 and 53.2%, respectively). A minority had university level education (22.5 and 15.2%). The majority (69%) rated their health as good or very good, and most (80.1%) had experienced cancer personally or in close others.
Table 1.
Participant characteristics (n=6965)a
Positive beliefs
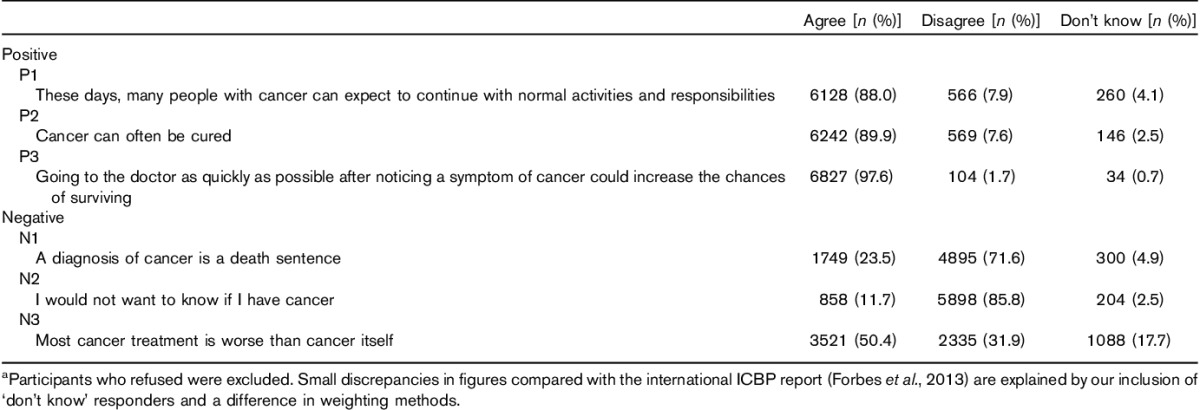
On the basis of endorsement of the positive beliefs, attitudes towards cancer were strongly optimistic, with 90% agreeing that ‘cancer can often be cured’, 98% that ‘going to the doctors quickly can increase the chance of surviving’, and 88% that you can ‘continue with normal activities and responsibilities’ after a cancer diagnosis (Table 2).
Table 2.
Frequencies describing the pattern of responses to each belief item (n=6965)a
Demographic differences were small (Table 3). There were some differences by education, with 97% of those with basic education agreeing that going to the doctor early increases the chance of surviving, as compared with 99% of those with a university education, 85 versus 92% for ‘continue with normal activities’, and 88 versus 92% for ‘cancer can often be cured’. There were no significant differences by sex or country. Older respondents were slightly less positive about the value of early presentation (95% in ≥70 years vs. 99% in 50–59 years), cure (87 vs. 93%) and continuing with normal activities (84 vs. 91%), with all effects significant in multivariable analyses. Unmarried individuals were slightly less positive than married individuals (87 vs. 92% for cure; 84 vs. 90% for ‘continue with normal activities’), which was also significant in multivariable analyses. The only significant ethnic difference was a slightly lower endorsement of the value of early diagnosis in ethnic minority respondents (93 vs. 98%).
Table 3.
Frequencies and multivariable logistic regression models predicting agreement (agree or strongly agree) with positively framed cancer beliefsa
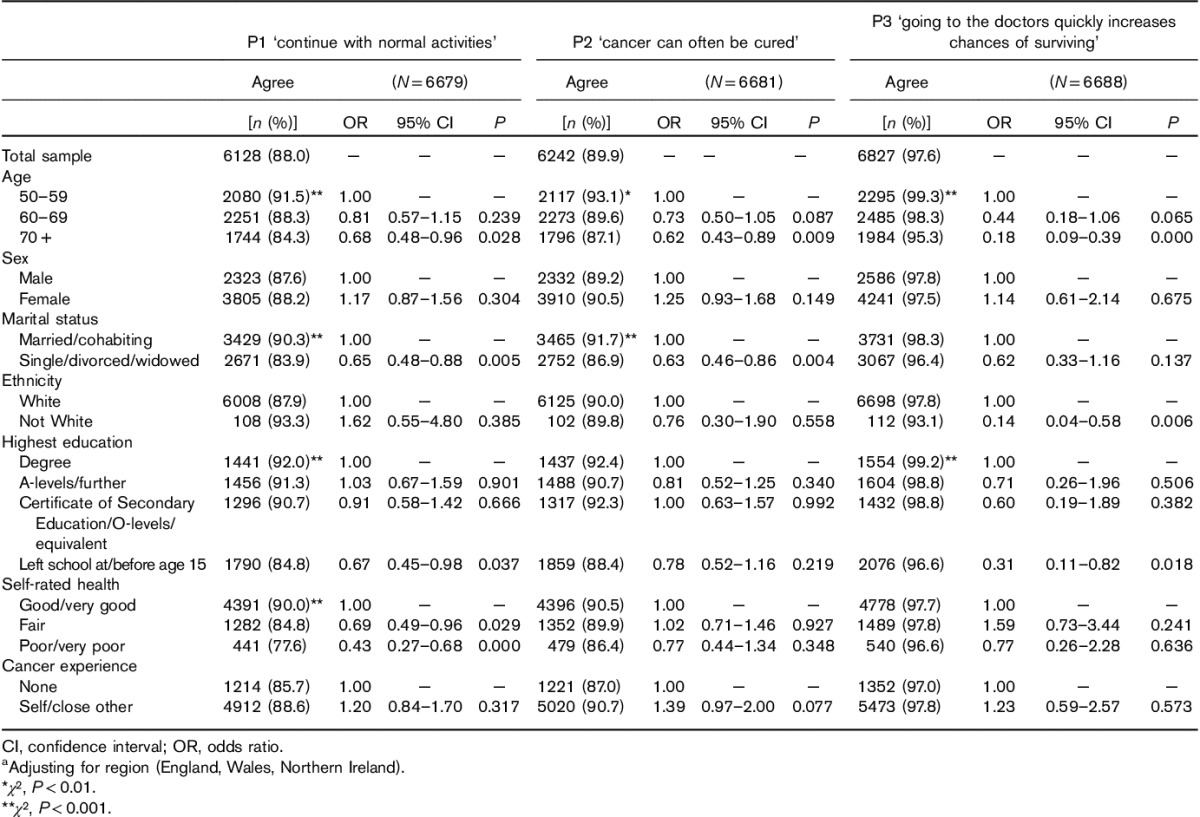
Respondents who rated their health as poor or very poor were less likely to believe that someone with cancer can continue as normal (78 vs. 90%), but there were no significant differences by health status for other positive items. Cancer experience was not significantly associated with any of the positive statements.
Analyses were repeated excluding all cases with ‘don’t know’ responses for any of the belief items (n=5139). The pattern of results remained the same although the absolute differences were smaller and some of the associations were no longer significant.
Negative beliefs
The picture that emerged from the negative beliefs was far from the mirror image of the positive beliefs, either in terms of the numbers endorsing each item (Table 2) or the demographic associations (Table 4). Almost a quarter (24%) of the participants thought that a ‘cancer diagnosis is a death sentence’, half of them thought that ‘most cancer treatment is worse than cancer’ and 12% ‘would not want to know if I have cancer’.
Table 4.
Frequencies and multivariable logistic regression models predicting agreement (agree or strongly agree) with negatively framed cancer beliefsa
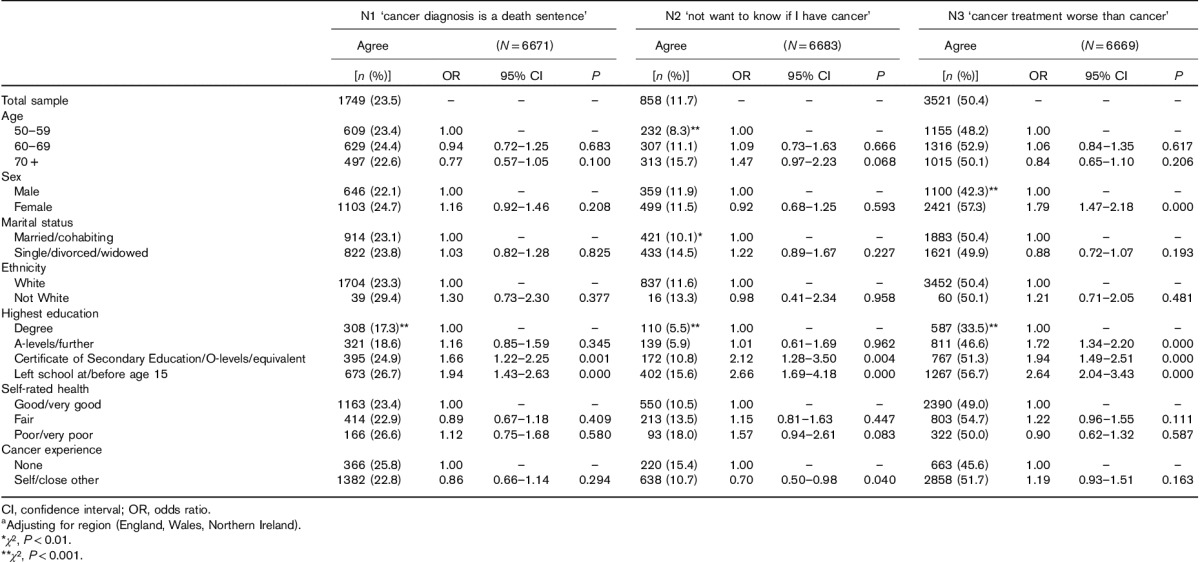
Respondents with basic education were substantially more likely to endorse each of the negative beliefs than those with a university education: 27 versus 17% for ‘cancer is a death sentence’, 57 versus 34% for most ‘cancer treatment is worse than cancer’ and 16 versus 6% for ‘would not want to know if I have cancer’, with all effects graded across levels of education. More women than men felt negative about treatment (57 vs. 42%), but there were no other sex differences. There were no significant differences by marital status, age, ethnicity, UK nation or self-rated health.
In terms of cancer experience, those with experience were less likely to say that they wouldn’t want to know (11 vs. 15%), which was significant in the multiple regression (P=0.04). There were no other significant associations.
The analyses were repeated excluding cases responding ‘don’t know’ to any item (n=5139). The pattern of associations was unchanged, and most effects remained significant (data not shown).
Coexisting beliefs
As is apparent from the percentages, many respondents simultaneously held opposing positive and negative cancer beliefs. For example, a fifth agreed that ‘a cancer diagnosis is a death sentence’, but also that ‘cancer can often be cured’. The demographic characteristics associated with endorsing both beliefs were inevitably largely the same as for the negative beliefs, because endorsement of positive beliefs was consistently high. More respondents from the lowest education group therefore held both positive and negative beliefs than those with a university education (23 vs. 15%).
Discussion
This is the first large-scale study to explore the sociodemographic patterning of attitudes towards different cancer outcomes using both negatively and positively framed belief items. On the basis of the responses to the positively framed items, attitudes towards cancer were almost universally optimistic, with between 88 and 98% of the respondents endorsing the value of early diagnosis, the chance of cure and the prospect of a normal life after a cancer diagnosis. The value attached to early detection has been observed previously, with these studies also reporting high percentages (e.g. 91%, Beeken et al., 2011; 85%, National Cancer Institute, 2007). Combined with the widespread belief that cure and a normal life are possible after cancer, this suggests that the public recognizes that cancer outcomes are improving. There was slightly lower agreement by people with less education, but absolute differences were very small.
A completely different perspective emerged from the negatively framed items. Notwithstanding the near-unanimity on positive items, almost a quarter of respondents saw cancer as a death sentence, 50% thought that treatment is worse than cancer and 12% wouldn’t want to know if they had cancer. Those with basic education were substantially more likely to endorse each item. This effect was graded across levels of education and not explained by differences in health status or cancer experience.
This finding is consistent with evidence for a pessimistic outlook about cancer among lower SES groups (Grunfeld et al., 2002; McCaffery et al., 2003; Wardle et al., 2004) and a higher prevalence of fatalistic beliefs (Ramirez et al., 2000; Niederdeppe and Levy, 2007; Peek et al., 2008; Beeken et al., 2011; Espinosa de los Monteros and Gallo, 2011). We had speculated that because the more fatal cancers (e.g. lung, head and neck, pancreas) are more common in lower SES groups (Clegg et al., 2009), this could cause more pessimistic cultural narratives (Balshem, 1991; Shahid and Thompson, 2009; von Wagner et al., 2011). However, there were few associations between cancer experience and beliefs, and controlling for cancer experience did not diminish the educational differences. Interestingly, people with experience of cancer were less likely to ‘not want to know’; perhaps contact with the disease alerts people to the value of a proactive approach. Nevertheless, we did not collect data on the type and the valence of these experiences, which may better predict attitudes, or on the experience and knowledge garnered through wider social networks. Previous experience of interactions with healthcare may also help inform expectations of cancer outcomes, as these have been implicated in engagement with cancer screening (Ekberg et al., 2014). Research examining the influence of particular aspects of cancer experience on attitudes is needed.
Clearly, respondents with less education were more likely to be simultaneously hopeful and fearful about cancer outcomes. This largely reflects their greater likelihood of holding negative beliefs. Taking the two most opposing beliefs as an example, believing that cancer is both a ‘death sentence’ and ‘can often be cured,’ was most common among individuals with lower education. We cannot infer that holding both beliefs is contradictory. Respondents may have drawn on different scenarios when responding to each statement in light of the diversity of the disease and their experiences. Previously, we showed that the public are aware that survival varies by cancer type (Whitaker et al., 2012). Furthermore, because cancer outcomes are worse in lower SES groups, a mismatch between widely promoted positive cancer messages and negative experiences of cancer is more likely. Perhaps a more conflicting opinion is to be expected from lower SES groups; although they hear wider evidence that cancer outcomes are improving, this may not be borne out within their own social networks. A recent qualitative study suggested that the public discourse around cancer more generally is mixed (Robb et al., 2014), and our present findings extend these results by suggesting that the likelihood of holding conflicting beliefs increases with socioeconomic deprivation. This mirrors the observations of a qualitative study which found that people from disadvantaged neighbourhoods recognize the potential of modern medicine, but also express pessimism (Rowa-Dewar et al., 2007). It also supports the finding that while negatively framed (pessimistic) attitudes in general are strongly graded by SES, the SES gradient for positively framed (optimistic) attitudes is marginal (Robb et al., 2009); hence, this phenomenon may extend beyond cancer-specific attitudes. Together, these findings support our approach of measuring positive and negative beliefs simultaneously.
That one in two respondents perceived cancer treatment to be worse than cancer echoes results from qualitative studies highlighting fear of cancer treatments (Smith et al., 2005). The absolute difference between the numbers of participants with basic education compared with university education agreeing with this belief was very high (57 and 34%), suggesting that fear of cancer treatment is a particular issue in socioeconomically disadvantaged communities, an observation reported by Gansler et al. (2005). It is also of note that more women (57%) than men (42%) held this view, which could help explain their higher levels of cancer worry (Wardle et al., 2005; Sach and Whynes, 2009; Keeney et al., 2010). One contributing factor may be the association with disfigurement, which could be more threatening to a woman’s identity. Public views of cancer treatment deserve further exploration.
The relative importance of positive and negative beliefs in individuals’ decisions to seek medical help or engage in cancer prevention behaviours has not been explored directly. However, studies assessing negative beliefs alone have found higher healthcare avoidance (Moser et al., 2013), lower cancer screening uptake (Miles et al., 2011; Wardle et al., 2004) and fear of help-seeking (Beeken et al., 2011). One implication of these findings is that pessimistic beliefs could maintain social inequalities in cancer outcomes. Despite near-universal recognition that early detection can, in principle, save lives, this belief may not be powerful enough to counter deep-seated fatalistic beliefs in groups who experience poorer cancer outcomes as their reality. More generally, Nettle (2010) proposes that lower SES individuals perceive less control over their risk of mortality; this results in a more pessimistic outlook, less invested effort in prevention and, consequently, even poorer health outcomes. A concerted effort is needed to address this self-perpetuating and cyclic pessimistic cultural narrative, of which changing attitudes will be a fundamental part.
The study had several limitations. Interviewing by telephone and using only ‘landline’ numbers excluded individuals without landline telephones. Just 6% of the older adults in the UK are in ‘mobile-only’ households, but this figure is likely to be higher among lower SES groups (Ofcom, 2013), resulting in their under-representation. Ethnic minority groups were also under-represented and so the null findings should be treated cautiously. We used single items to keep the participant burden low, which reduces reliability, but this was likely to be offset by the large sample size. Again, to reduce participant burden, the only individual-level marker of SES was education. This was selected as appropriate for an older population for whom current income and employment status may be less valid, but alternative markers could produce different results.
Conclusion
We found that older adults in the UK almost unanimously endorse positive statements about improving cancer outcomes and the value of early detection, but many, particularly those with lower levels of education, simultaneously hold negative beliefs. If negative beliefs play an important role in decisions about screening and early presentation, this needs to be considered in designing targeted educational materials about early detection. In particular, health education campaigns targeting socioeconomically deprived groups might benefit from a focus on reducing negative attitudes, not necessarily achieved by promoting positive attitudes. A better understanding of attitudes towards cancer and its associations with cancer control behaviours will help to ensure that cancer control programmes are not only effective but equitable.
Acknowledgements
The authors thank Anna Carluccio, Colin Gardiner, Julia Pye, Laura Thomas and Chris Marshall of IPSOS Mori for coordinating the fieldwork, and Kate Aldersey, Martine Bomb, Catherine Foot, Donia Sadik and Emily Fulleylove of Cancer Research UK for managing the programme and monitoring the media.
ICBP Programme Board: Ole Andersen, Søren Brostrøm, Heather Bryant, David Currow, Anna Gavin, Gunilla Gunnarsson, Jane Hanson, Todd Harper, Stein Kaasa, Nicola Quin, Linda Rabeneck, Michael A Richards, Michael Sherar and Bob Thomas.
Academic Reference Group: Neil Aaronson, David Cella, Henrik Møller, Keith Petrie and Liesbeth Van Osch.
ICBP Module 2 Working Group: Michael Donnelly, David Donnelly, Anette Fischer Pedersen, Line Hvidberg, Christian Wulff, Deb Keen, Chris Roberts, James Kite, Blythe O’Hara, Donna Perez, Lisa Petermann, Chris Roberts and Melanie Wakefield.
This study was supported by the ICBP Programme Board and supporting Module 2 committees and advisers in the UK. Northern Ireland study and Northern Ireland Cancer Registry funded by Public Health Agency for Northern Ireland. Funding for the Welsh arm of this study was provided by Tenovus and the Welsh Government. Professor Jane Wardle is supported by Cancer Research UK. These analyses were carried out with funding from the Department of Health Policy Research Unit in Cancer Awareness, Screening and Early Diagnosis. The Policy Research Unit in Cancer Awareness, Screening, and Early Diagnosis receives funding for a research programme from the Department of Health Policy Research Programme. It is a collaboration between researchers from seven institutions (Queen Mary University of London, University College London, King’s College London, London School of Hygiene and Tropical Medicine, Hull York Medical School, Durham University and Peninsula Medical School). The views expressed are those of the authors and not necessarily those of the NHS, or the Department of Health.
Conflicts of interest
There are no conflicts of interest.
References
- Balshem M. (1991). Cancer, control, and causality: talking about cancer in a working-class community. Am Ethnol 18:152–172. [Google Scholar]
- Beeken RJ, Simon AE, von Wagner C, Whitaker KL, Wardle J. (2011). Cancer fatalism: deterring early presentation and increasing social inequalities? Cancer Epidemiol Biomarkers Prev 20:2127–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth CM, Li G, Zhang-Salomons J, Mackillop WJ. (2010). The impact of socioeconomic status on stage of cancer at diagnosis and survival: a population-based study in Ontario, Canada. Cancer 116:4160–4167. [DOI] [PubMed] [Google Scholar]
- Byrne MM, Weissfeld J, Roberts MS. (2008). Anxiety, fear of cancer, and perceived risk of cancer following lung cancer screening. Med Decis Making 28:917–925. [DOI] [PubMed] [Google Scholar]
- Clegg LX, Reichman ME, Miller BA, Hankey BF, Singh GK, Lin YD, et al. (2009). Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control 20:417–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CR-UK (2010). International Cancer Benchmarking Partnership. Available at: http://www.cancerresearchuk.org/cancer-info/spotcancerearly/ICBP/. [Accessed 13 September 2014].
- DeSalvo KB, Bloser N, Reynolds K, He J, Muntner P. (2006). Mortality prediction with a single general self-rated health question. A meta-analysis. J Gen Intern Med 21:267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekberg M, Callender M, Hamer H, Rogers S. (2014). Exploring the decision to participate in the National Health Service Bowel Cancer Screening Programme. Eur J Cancer Prev 23:391–397. [DOI] [PubMed] [Google Scholar]
- Espinosa de los Monteros K, Gallo LC. (2011). The relevance of fatalism in the study of Latinas’ cancer screening behavior: a systematic review of the literature. Int J Behav Med 18:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes LJ, Simon AE, Warburton F, Boniface D, Brain KE, Dessaix A, et al. , International Cancer Benchmarking Partnership Module 2 Working Group (2013). Differences in cancer awareness and beliefs between Australia, Canada, Denmark, Norway, Sweden and the UK (the International Cancer Benchmarking Partnership): do they contribute to differences in cancer survival? Br J Cancer 108:292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gansler T, Henley SJ, Stein K, Nehl EJ, Smigal C, Slaughter E. (2005). Sociodemographic determinants of cancer treatment health literacy. Cancer 104:653–660. [DOI] [PubMed] [Google Scholar]
- Grunfeld EA, Ramirez AJ, Hunter MS, Richards MA. (2002). Women’s knowledge and beliefs regarding breast cancer. Br J Cancer 86:1373–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys M, Sarfati D, Stevanovic V, Tobias M, Lewis C, Pearce N, Blakely T. (2009). Socioeconomic inequalities in cancer survival in New Zealand: the role of extent of disease at diagnosis. Cancer Epidemiol Biomarkers Prev 18:915–921. [DOI] [PubMed] [Google Scholar]
- Keeney S, McKenna H, Fleming P, McIlfatrick S. (2010). Attitudes to cancer and cancer prevention: what do people aged 35–54 years think? Eur J Cancer Care (Engl) 19:769–777. [DOI] [PubMed] [Google Scholar]
- Lyratzopoulos G, Neal RD, Barbiere JM, Rubin GP, Abel GA. (2012). Variation in number of general practitioner consultations before hospital referral for cancer: findings from the 2010 National Cancer Patient Experience Survey in England. Lancet Oncol 13:353–365. [DOI] [PubMed] [Google Scholar]
- Lyratzopoulos G, Abel GA, Brown CH, Rous BA, Vernon SA, Roland M, Greenberg DC. (2013). Socio-demographic inequalities in stage of cancer diagnosis: evidence from patients with female breast, lung, colon, rectal, prostate, renal, bladder, melanoma, ovarian and endometrial cancer. Ann Oncol 24:843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod U, Mitchell ED, Burgess C, Macdonald S, Ramirez AJ. (2009). Risk factors for delayed presentation and referral of symptomatic cancer: evidence for common cancers. Br J Cancer 101 (Suppl 2):S92–S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffery K, Wardle J, Waller J. (2003). Knowledge, attitudes, and behavioral intentions in relation to the early detection of colorectal cancer in the United Kingdom. Prev Med 36:525–535. [DOI] [PubMed] [Google Scholar]
- Miles A, Rainbow S, von Wagner C. (2011). Cancer fatalism and poor self-rated health mediate the association between socioeconomic status and uptake of colorectal cancer screening in England. Cancer Epidemiol Biomarkers Prev 20:2132–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser RP, Arndt J, Han PK, Waters EA, Amsellem M, Hesse BW. (2014). Perceptions of cancer as a death sentence: prevalence and consequences. J Health Psychol 19:1518–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute (2007). Health Information National Trends Survey. Available at: http://hints.cancer.gov/question-details.aspx?dataset=2007&qid=513. [Accessed 13 September 2014].
- Nettle D. (2010). Why are there social gradients in preventative health behavior? A perspective from behavioral ecology. PLoS One 5:e13371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederdeppe J, Levy AG. (2007). Fatalistic beliefs about cancer prevention and three prevention behaviors. Cancer Epidemiol Biomarkers Prev 16:998–1003. [DOI] [PubMed] [Google Scholar]
- Ofcom (2013). The communications market. Available at: http://stakeholders.ofcom.org.uk/binaries/research/cmr/cmr13/UK_5.pdf. [Accessed 13 September 2014].
- Peek ME, Sayad JV, Markwardt R. (2008). Fear, fatalism and breast cancer screening in low-income African–American women: the role of clinicians and the health care system. J Gen Intern Med 23:1847–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachet B, Ellis L, Maringe C, Chu T, Nur U, Quaresma M, et al. (2010). Socioeconomic inequalities in cancer survival in England after the NHS cancer plan. Br J Cancer 103:446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez AG, Suarez L, Laufman L, Barroso C, Chalela P. (2000). Hispanic women’s breast and cervical cancer knowledge, attitudes, and screening behaviors. Am J Health Promot 14:292–300. [DOI] [PubMed] [Google Scholar]
- Rizzo L, Brick JM, Park I. (2004). A minimally intrusive method for sampling persons in random digit dial surveys. Public Opin Q 68:267–274. [Google Scholar]
- Robb KA, Simon AE, Wardle J. (2009). Socioeconomic disparities in optimism and pessimism. Int J Behav Med 16:331–338. [DOI] [PubMed] [Google Scholar]
- Robb KA, Simon AE, Miles A, Wardle J. (2014). Public perceptions of cancer: a qualitative study of the balance of positive and negative beliefs. BMJ Open 4:e005434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowa-Dewar N, Ager W, Kearney N, Seaman P. (2007). Glasgow public involvement in cancer. Available at: http://www.gcph.co.uk/publications/123_glasgow_public_involvement_in_cancer. [Accessed 13 September 2014].
- Rutherford MJ, Hinchliffe SR, Abel GA, Lyratzopoulos G, Lambert PC, Greenberg DC. (2013). How much of the deprivation gap in cancer survival can be explained by variation in stage at diagnosis: an example from breast cancer in the East of England. Int J Cancer 133:2192–2200. [DOI] [PubMed] [Google Scholar]
- Sach TH, Whynes DK. (2009). Men and women: beliefs about cancer and about screening. BMC Public Health 9:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid S, Thompson SC. (2009). An overview of cancer and beliefs about the disease in Indigenous people of Australia, Canada, New Zealand and the US. Aust N Z J Public Health 33:109–118. [DOI] [PubMed] [Google Scholar]
- Simon AE, Forbes LJ, Boniface D, Warburton F, Brain KE, Dessaix A, et al. (2012). An international measure of awareness and beliefs about cancer: development and testing of the ABC. BMJ Open 2:e001758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LK, Pope C, Botha JL. (2005). Patients’ help-seeking experiences and delay in cancer presentation: a qualitative synthesis. Lancet 366:825–831. [DOI] [PubMed] [Google Scholar]
- The American Association of Public Opinion Research (2011). Standard definitions: final dispositions of case codes and outcome rates for surveys. 7th ed. Available at: http://www.aapor.org/AAPORKentico/AAPOR_Main/media/MainSiteFiles/StandardDefinitions2011_1.pdf. [Accessed 13 September 2014].
- von Wagner C, Good A, Whitaker KL, Wardle J. (2011). Psychosocial determinants of socioeconomic inequalities in cancer screening participation: a conceptual framework. Epidemiol Rev 33:135–147. [DOI] [PubMed] [Google Scholar]
- Wardle J, McCaffery K, Nadel M, Atkin W. (2004). Socioeconomic differences in cancer screening participation: comparing cognitive and psychosocial explanations. Soc Sci Med 59:249–261. [DOI] [PubMed] [Google Scholar]
- Wardle J, Miles A, Atkin W. (2005). Gender differences in utilization of colorectal cancer screening. J Med Screen 12:20–27. [DOI] [PubMed] [Google Scholar]
- Whitaker KL, Simon AE, Beeken RJ, Wardle J. (2012). Do the British public recognise differences in survival between three common cancers? Br J Cancer 106:1907–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods LM, Rachet B, Coleman MP. (2006). Origins of socio-economic inequalities in cancer survival: a review. Ann Oncol 17:5–19. [DOI] [PubMed] [Google Scholar]


