Abstract
Background
Smoking in pregnancy is known to be associated with a range of adverse pregnancy outcomes, yet there is a high prevalence of smoking among pregnant women in many countries, and it remains a major public health concern. We have conducted a systematic review and meta-analysis to provide contemporary estimates of the association between maternal smoking in pregnancy and the risk of stillbirth.
Methods
We searched four databases namely MEDLINE, EMBASE, Psych Info and Web of Science for all relevant original studies published until 31st December 2012. We included observational studies that measured the association between maternal smoking during pregnancy and the risk of stillbirth.
Results
1766 studies were screened for title analysis, of which 34 papers (21 cohorts, 8 case controls and 5 cross sectional studies) met the inclusion criteria. In meta-analysis smoking during pregnancy was significantly associated with a 47% increase in the odds of stillbirth (OR 1.47, 95% CI 1.37, 1.57, p < 0.0001). In subgroup analysis, smoking 1-9 cig/day and ≥10 cig/day was associated with an 9% and 52% increase in the odds of stillbirth respectively. Subsequently, studies defining stillbirth at ≥ 20 weeks demonstrated a 43% increase in odds for smoking mothers compared to mothers who do not smoke, (OR 1.43, 95% CI 1.32, 1.54, p < 0.0001), whereas studies with stillbirth defined at ≥ 24 weeks and ≥ 28 weeks showed 58% and 33% increase in the odds of stillbirth respectively.
Conclusion
Our review confirms a dose-response effect of maternal smoking in pregnancy on risk of stillbirth. To minimise the risk of stillbirth, reducing current smoking prevalence in pregnancy should continue to be a key public health high priority.
Electronic supplementary material
The online version of this article (doi:10.1186/s12889-015-1552-5) contains supplementary material, which is available to authorized users.
Background
Smoking in pregnancy is a major public health problem in the developed countries [1]. The World Health Organisation (WHO) predicts that this will reach ‘epidemic’ proportions in developing countries in the near future [1]. Within the current challenging economic climate in many countries, smoking in pregnancy imposes a significant burden on population health and resources, and is associated with a range of poor outcomes for both mother and child, such as ectopic pregnancy, miscarriage, placental abruption, preterm birth and low birth weight [2,3]. The harmful effects of tobacco smoke exposure in pregnancy can be avoided [4] and it is one of the most prevalent modifiable risk factors for adverse pregnancy outcomes [5,6].
In many developed countries, the rates of smoking in pregnancy have been declining over recent decades [7] with current prevalence estimates between 10% – 19% [8], and data from the UK suggesting that one out of eight women smoke throughout the pregnancy [9]. Moreover, this decline has not been consistent across all social classes; lower rates of decline have been noted across less advantaged socioeconomic groups [10]. Smoking in pregnancy in developed countries tends to be higher among women who have low income and poor education [11].
Stillbirth rates widely vary across low, middle and high-income countries [12-14]. The lowest rates have been observed in Finland with 2.0 per 1000 live births and in the developing world rates are much higher at more than 40 per 1000 live births in countries like Nigeria and Pakistan [6]. Two previous systematic reviews and meta-analyses have estimated the size of effect of smoking during pregnancy and the risk of stillbirth. A report by the United States Surgeon General [15] showed a relative risk of stillbirth of 1.2-1.8 within smokers versus non-smokers. The analysis was based on three studies [16-18] conducted in two countries, the United States and Sweden. Recent stillbirth statistics by Flenady et al [8] was based on a systematic review from high-income countries; and four studies were included in the meta-analysis of the association between any smoking in pregnancy and the risk of stillbirth yielding an odds ratio of 1.36 (95% CI 1.27, 1.46). Results from these published reviews were limited to literature from developed countries; yet stillbirth rates are much higher in developing countries, and it is therefore imperative to conduct a comprehensive review, which reflects the impact of smoking in pregnancy on stillbirth. We have conducted a systematic review and meta-analysis to provide contemporary estimates of the association between maternal smoking in pregnancy and the risk of stillbirth.
Methods
A detailed electronic search was performed through four databases namely MEDLINE, EMBASE, Psych Info and Web of Science. All relevant published studies with sufficient data on maternal cigarette or tobacco smoking during pregnancy with the outcome of stillbirth were included. Due to the nature of the research objective, there were no randomised control trials (RCTs) identified, and observational studies (such as cohort design, case control studies and cross sectional surveys) were considered using the standard guidelines of Meta-analysis of Observational Studies in Epidemiology (MOOSE) [19]. All relevant studies published in the English language, up to 31st December 2012 were included in the review. Case reports, non-English publications and those, which only involved passive or environmental smoke information, were excluded. We carefully considered studies where the study populations were similar to avoid duplication of cases; where studies were based on exactly the same population during the same time period, but there were differences in sample sizes, we selected the results from the publication with the larger sample size for inclusion in the meta-analysis, though those excluded from quantitative analysis were included in a narrative synthesis.
Search strategy
We developed search terms based on medical subject headings (MeSH), free text words and words in the title or abstract. We combined search terms of exposure (smoking during pregnancy) with the outcome of the study (stillbirth). The MeSH terms used included combinations of the terms: stillbirth, maternal smoking, and pregnancy, also applied with special characters ($, *), wherever required. The study protocol was agreed within the team, however it was not published prior to commencement of the review. We also checked the reference list of all identified papers and the most recent similar reviews for any additional studies. A complete online search strategy along with search terms is attached (Additional file 1).
Study selection and outcome definition
The electronic search was performed with a three-stage approach; first titles were screened by TM and AA, then abstracts were screened by two authors independently (TM and SL). For studies, which appeared to be eligible, full texts were obtained and reviewed independently by two authors who further performed data extraction independently on pre-piloted forms, and discrepancies were resolved by consensus. We also obtained full texts for those studies where a decision could not be made based on title and abstract. Data extraction forms included followings; study design, exposure validation, definition of exposure (smoking) and outcome (stillbirth), confounders adjustments, sample size and study location.
Since stillbirth is defined differently in different countries and different studies, stillbirth for this review was defined as fetal loss or death at 20 weeks gestation and above [20]; this included both early (20-28 weeks gestation) and late (after 28 weeks) stillbirth enabling inclusion of a wider range of international studies. Sensitivity analysis was done using alternative definitions of stillbirth:
Stillbirth at ≥ 20 weeks of gestation
Stillbirth at ≥ 24 weeks of gestation
Stillbirth at ≥ 28 weeks of gestation
Where both unadjusted and adjusted measures were reported, the latter were extracted. Quality assessment was based on a Newcastle Ottawa Scale (NOS). Cohort and case control studies were awarded up to a maximum quality score of nine and cross sectional studies were given up to a maximum score of seven [21]. A definition of ‘high quality’, was given to cohort and case control studies with a score of 7 or above or cross-sectional studies with a score of 5 or above; the rest were deemed ‘low quality’ [21]. Each quality assessment (NOS) was conducted by at least by two authors independently and then findings were verified.
Statistical analysis
Meta-analysis was performed using Rev-Man 5.3 [22] with a random effects model. The I2 statistic was applied to calculate heterogeneity (I2 more than 75% was considered high heterogeneity, more than 50% moderate and 25% was considered as low heterogeneity) [22]. The odds ratio for the overall effect of smoking in pregnancy from each study was used, which was the main outcome reported in included literature. The ratio was calculated from the available data, wherever feasible. Studies included in the meta-analysis were further considered for subgroup analysis based on study quality, definition of stillbirth and cigarette consumption (1-9 cig/day and ≥10 cig/day). The funnel plot method was used to assess publication bias. Studies without statistical data presentation, and those where cases were potentially overlapping between publications, were considered in a narrative synthesis.
Results
The initial database search produced 2,934 papers. We found 1,168 duplicate studies; meaning the same study was obtained from more than one of the four databases used in the review. After removal of duplicates, 1,766 study titles were screened, 94 were found eligible for abstract screening and 35 papers were considered for full text analysis. Out of 35 full texts sought, 34 were obtained within the available research timelines, and of these, 29 were included in the systematic review [12-14,16-18,23-45]. Eight full text studies, which did not have sufficient qualitative or quantitative data relevant to this review, were excluded [46-53]. An additional five relevant studies [54-58] were identified from references listed in the full text eligible studies. Details of the electronic search are explained in Figure 1. Out of the 34 included studies, 24 were included in the meta-analysis [12-14,17,23,24,26-29,31-41,54-56] and the other remaining ten in the qualitative synthesis [16,18,25,30,42-45,57,58]. One of the studies, [23] used two separate study designs, case control and bidirectional crossover methodology; therefore the study was considered in the meta-analysis separately as two observations resulting in 25 studies included in the quantitative synthesis. Two studies references [30,31] were derived from the same dataset (Missouri, USA) conducted in the same timeframe (1978-1997). Study [31] with a higher sample size was included in the meta-analysis and the other [30] was considered in the narrative synthesis. Four studies [16,17,25,29] used the same study population (Swedish National registry data) with differences in sample size and study methodology. Studies [17,25] had the same time frame, with study [17] having a higher sample size. Studies [16,17,29] had overlapping but not the exact dataset time frames. Study [16] time period (1983 – 1985) was exceeded by study 17 (1983-1989) which also had a higher sample size. Study [29] used the data for 1984 and 1991 for comparative purposes. To avoid having any woman’s data being counted twice in the meta-analysis, only the estimates obtained for 1991 data [29] were used in the meta-analysis. Two studies [17,29] were included in the meta-analysis whilst the other two studies [16,25] were included in the narrative synthesis only.
Figure 1.
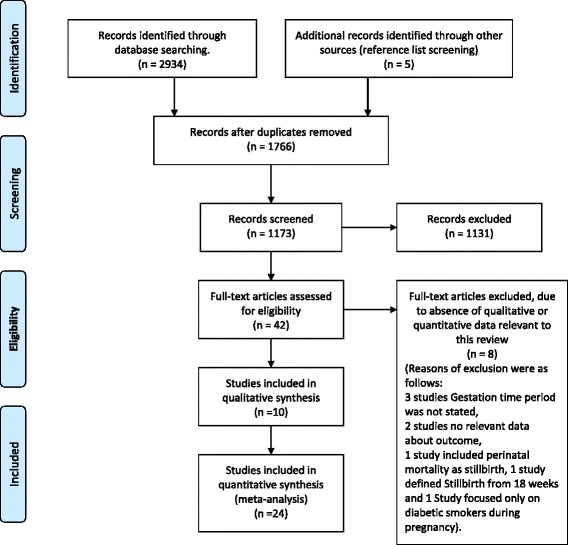
Flow diagram of included and excluded studies.
Included studies are further detailed in Table 1.
Table 1.
Table of included studies
| Reference | Author year | Study type | Population | Location | Exposure: smoking during pregnancy and validity | Definition of stillbirth (outcome) and gestational period | Estimates | Factors adjusted for in individual studies if available |
|---|---|---|---|---|---|---|---|---|
| Studies included in meta-analysis | ||||||||
| 12 | Wood et al (2009) | Cohort | 158,502 | Alberta Perinatal Health Program, 1991-2004 Canada | Smokers: Yes | Fetal death after at least 24 completed weeks of gestation. | Adjusted OR 1.04 (0.74-1.47) | Maternal age, diabetes, hypertension, previous caesarean section. |
| Not validated | ||||||||
| 13 | Smith et al (2007) | Cohort | 84,769 | Scotland morbidity records 1991-2001, UK | Smokers: Yes | Fetal death after at least 24 completed weeks of gestation. | OR for smoking was calculated 2.15 (95% CI 1.74-2.65) | Age, deprivation, height, BMI, Marital Status. |
| Not validated | ||||||||
| 14 | Hogberg and Cnattingius (2007) | Cohort | 526,691 | Sweden – Swedish National study 1983-2001- Participants with two consecutive pregnancies. | Smokers: Yes | Fetal death after at least 28 completed weeks of gestation. | OR for smoking in both Pregnancies 1.35 (1.15-1.58). 1-9cig/day OR 1.16 (0.92-1.46) ≥10 cig/day 0R 1.55 (1.17-2.04). OR for smoking in the 1st pregnancy only OR 1.02 (0.79-1.30), 1-9 cig/day OR 1.13 (0.9-1.39), ≥10 cig/day OR 1.14 (0.87-1.51). OR for smoking in 2nd pregnancy only 0.84 (0.55-1.26), 1-9cig/day OR 1.10 (0.89-1.35), ≥ 10 cig/day OR 1.45 (1.16-1.81). | Maternal age, education, cohabiting with infant’s father, mother’s country of birth, year of second delivery, inter-pregnancy interval and stillbirth in first pregnancy. |
| 1-9 cig/day, ≥10 cig/day. | ||||||||
| Not validated | ||||||||
| 26 | Salihu et al (2008) | Case control | 1,444,378 | USA – Missouri 1978-1997. | Smokers: Yes | In-utero fetal death at least 20 week into gestation. | a) Bidirectional case cross-over OR 1.20 (1.03-1.39) 1-9 cig/day OR 1.16 (0.88-1.53) 10-19 cig/day OR 1.10 (0.90-1.24) ≥ 20 cig/day OR 1.21 (1.01-1.50), | Maternal race and age. |
| 1-9 cig/day, | ||||||||
| 10-19 cig/day, | b) Case Control OR 1.34 (1.26-1.43) 1-9 cig/day OR 0.94 (0.76-1.70) 10-19 cig/day OR 1.31(1.22-1.41) ≥20 cig/day OR 1.43 (1.31-1.54). | |||||||
| ≥20 cig/day. | ||||||||
| Not validated | ||||||||
| 34*** | Ahlenius and Thomassen (1999) | Cohort | 94,270 in 1984 and 124,201 in 1991. | Sweden - Using the Swedish medical birth register in 1984 and 1991 | Smokers: Yes. | Death of a fetus before or during delivery with a gestational duration of at least 28 weeks. | Combined (1984 and 1991) OR 1.37 (1.17 to 1.61) | Maternal age and parity. |
| 1-9 cig/day, ≥10 cig/day. | 1-9 Cig/day OR 1.22 | |||||||
| (1.00-1.48) | ||||||||
| Not validated | ≥10 Cig/day OR 1.62 (1.31-2.00) | |||||||
| 1991 OR | ||||||||
| 1.47 (1.19 to 1.82) | ||||||||
| >10 Cig/day OR 1.97 (1.51-2.59) | ||||||||
| 36*** | Aliyu et al (2007) | Cohort | 1,436, 725 | USA Missouri – Using Missouri maternally linked cohort data 1978 – 1997 | Smokers: Yes | In utero fetal death at < = 20 weeks gestation | OR(originally presented as HRs) 1.43 (1.36-1.51) | N/A* |
| 1-9 cig/day, 10-19 cig/day, ≥ 20 cig/day. | ||||||||
| Not validated | ||||||||
| 37 | Gray et al (2009) | Cohort | 532,016 | UK- Scotland, National study 1994-2003. | Smokers: Yes. | 24-44 weeks’ gestation | OR 1.75 (1.61-1.91) | N/A* |
| Not validated | ||||||||
| 38 | McCowan et al (2007) | Cohort | 69,173 | New Zealand - National Women’s | Smokers: Yes | Birth of a baby with no signs of life delivered at ≥ | OR 1.33 (0.99-1.79) | Infant sex, maternal ethnicity, parity, marital status, maternal age at delivery, history of previous miscarriage, history of previous abortion, previous low-birth weight infant, previous C-section. |
| Hospital, Auckland, New Zealand from 1993 to 2000 | Not validated | 20 weeks or with a birth weight of > 400 g if gestation was not known. | ||||||
| 39 | Miller et al (2010) | Cohort | 359,747 | Canada – Ontario state 2004-2006 | Smokers: Yes | Death of fetus at 20 weeks of gestation | Overall OR 1.58 (1.38-1.81) | Age, parity, multiple gestation |
| Not validated | ||||||||
| 40 | Moshin et al (2005) | Cohort | 433,227 | Australia – New South Wales 1998-2002 | Smokers: Yes | Fetal death at ≥ 20 weeks gestation or of 400 g or more birth weight. | Overall adjusted OR 1.17 (1.05–1.31) Unadjusted OR 1.05 (0.94–1.17). | Maternal age, maternal country of birth, socioeconomic status, maternal diabetes mellitus, and first antenatal care visit. |
| Not validated | ||||||||
| 17*** | Raymond et al (1994) | Cohort | 638,242 | Sweden National study 1983-1989 | Smokers: Yes | Fetus death at 28 weeks of gestation or more. | OR 1.4 (1.2-1.4) | Age, parity, pregnancy complications, hypertensive diseases, diabetes, placental complications and intrauterine growth retardation (IUGR). |
| Not validated | ||||||||
| 42 | Reddy et al (2006) | Cohort | 174,809 | USA – 12 clinic centres and 19 hospital sites. 2002-2008. | Smokers: Yes | Fetal death at 23 weeks gestation and beyond. | OR (originally presented as HRs) 1.57 (1.21-2.02) | N/A* |
| Not validated | ||||||||
| 43 | Sutan et al (2010) | Cohort | 541,811 | UK- Scotland, National study (NHS Hospital) | Smokers: Yes | Late fetal death from 20 weeks gestation. | OR 1.64 (1.46-1.84) | Non stated |
| Not validated | ||||||||
| 44 | Tuthill et al (1999) | Cohort | 16,047 | Wales – All Wales Perinatal Survey 1993-1995 | Smokers: Yes | Mortality from 20 completed weeks of gestation | OR 1.72(1.38-2.13) | Social class, infection, placental abruption, sudden infant death syndrome. |
| Not validated | ||||||||
| 45 | Wisborg et al (2001) | Cohort | 25,102 | Denmark – Aarhust University Hospital 1989-1996. | Smokers: Yes | Delivery of a dead fetus occurring at 28 weeks or more gestation. | OR 1.9 (1.3-2.9), | Sex of child, parity, maternal age, education, alcohol, caffeine intake. |
| 1-9 cig/day, ≥10 cig/day. | 1-9 cig/day OR 1.5 (0.9-2.4) ≥10 cig/day 1.8 (1.2-2.8) | |||||||
| Not validated | ||||||||
| 46 | Winbo et al (2001) | Cohort | 1,412,747 | Sweden – National study 1983 - 1995 | Smokers: Yes | Death of fetus after competing 28 weeks of gestation. | OR stillbirths ≥37 weeks compared with live births 1.08 (0.96-1.22) | Non stated |
| Not validated | ||||||||
| 27 | Ferraz and Gray (1991) | Case control | 11,483 | Brazil – Natal city and state of Rio Grande do Norte 1984-1986 | Smokers: Yes | Stillborn cases weighing 500 g or more | OR 1.4 (1.0-2.0) | Non stated |
| Not validated | ||||||||
| 29 | Dodds et al (2006) | Case control | 494 | Canada – Nova Scotia and Eastern Ontario 1999-2001 | Smokers: Yes | Not specified - Death before delivery of a fetus weighing 500 g or more | OR (originally presented in HRs) Smoking during 1st trimester 2.13 (1.23-3.64) | N/A* |
| Not validated | ||||||||
| 30 | Efkarpidis et al (2004) | Case control | 599 | UK – Nottingham City Hospital 1991-1997. | Smokers: Yes | Definition of stillbirth not stated- pregnancies considered from ≥ 24 weeks | OR 1.2 (0.64-1.6) | Non stated |
| Not validated | ||||||||
| 31 | Froen et al (2002) | Case control | 598 | Norway – Oslo 1986-1995. | Smokers: Yes | Intrauterine death before onset of labour of a fetus > =22 weeks gestation or > = 500 g body mass | OR calculated Using Stats 1.81 (1.02-3.15) 1-9 cig/day OR 1.0 (0.43-2.12), ≥10 cig/day 0R 3.04 (1.13-8.19) | N/A* |
| 1-9 cig/day, ≥10 cig/day. | ||||||||
| Not validated | ||||||||
| 32 | Goy et al (2008) | Case control | 510 | Canada – Nova Scotia and Eastern Ontario 1999-2001. | Smokers: Yes | Not specified - Death before delivery of a fetus weighing 500 g or more | OR 2.06 (1.22-3.47) | Province of origin, age, inactivity during pregnancy, stillbirth in previous pregnancy, used fertility treatment |
| Not validated | ||||||||
| 33 | Little and Weiberg (1993) | Case control | 4667 | USA | Smokers: Yes | Fetal death at 28 weeks or more gestation | OR calculated using Stata 1.29 (1.09-1.53) | N/A* |
| 1-19 cig/day, | OR 1-19 cig/day 1.3 (1.09-1.57), 20-29 cig/day OR 1.39 (1.06-1.82), ≥30 cig/day OR 0.67 (0.41-1.12). | |||||||
| 20-29 cig/day, | ||||||||
| ≥ 30 cig/day. | ||||||||
| Not validated | ||||||||
| 47 | Mishra et al (2005) | Cross-sectional | 16,802 | India - Survey conducted 98-99 | Smokers: Yes | Delivery of a dead baby at > 28 weeks of pregnancy | OR 1.23 (0.92-1.64) | Cooking smoke, anaemia, BMI, education, standard of living, house type. |
| Not validated | Unadjusted OR 1.60 (1.23-2.08). | |||||||
| 48 | Robson et al (2006) | Cross-sectional | 21, 880 | Australia – New South Wales 1998-2003. | Smokers: Yes | Not stated | OR 1.65 (1.15-2.38) | Age, parity, medical/obstetric complications |
| 1-10 cig/day >10 cig/day. | 20 weeks | 1- 10 cig/day OR 1.16 (0.80-1.69) >10 cig/day OR 1.68 (1.17-2.41). | ||||||
| Not validated | ||||||||
| Studies included in narrative synthesis | ||||||||
| 16*** | Cnattingius et al (1988) | Cross-sectional | 262,582 | Sweden National Study – Using Swedish medical birth registry covering more than 99% of all births in Sweden. 1983-1985 | Smokers: Yes | Late fetal death – still birth occurring at 28 weeks of gestation or later | OR (originally presented as RR) 1.37 (1.20-1.57) | N/A* |
| 1-9 cig/day, | 1-9 cig/day 1.32 (1.12-1.55) | |||||||
| ≥10 cig/day. | ≥10 cig/day 1.45 (1.21-1.75) | |||||||
| Not validated | ||||||||
| 28*** | Walles (1994) | Case control | 202 | Sweden – 5 centres (Boras, Helsingborg, Karlskrona, Kristianstastad and Lund) 1983-1989. | Smokers: Yes | Definition of stillbirth not stated, pregnancies considered | OR calculated using Stata. OR 1.18 (0.62-2.26) | N/A* |
| 1-10 cig/day, | 1-10 cig/day OR 1.33 (0.58-3.09) >10 cig/day 1. 04 (0.43-2.50) | |||||||
| ≥10 cig/day. | ≥ 28 weeks | |||||||
| Not validated | ||||||||
| 35*** | Aliyu et al (2008) | Cohort | 1,436,628 | USA Missouri – Using Missouri maternally linked cohort data 1978 – 1997. | Smokers: Yes. | In utero fetal death at < = 20 weeks gestation. | OR (originally presented as HRs) 1.48 (1.40-1.56) | N/A** |
| < 35 years old, ≥ 35yers old. Not validated | < 35 years smokers1.43 (1.35-1.51) | |||||||
| ≥35 years Smokers OR 2.4 (2.06-2.80) | ||||||||
| 49 | Kleinman et al (1988) | Cohort | 362,261 | USA Missouri 1978-1983 | Smokers: Yes | (Fetal mortality) Fetal death at 20 weeks or more. | OR Primiparas | N/A** |
| < 1 pack/day ≥1 pack/day. | <1 pack/day 1.36(1.16-1.59) ≥1 pack/day 1.62 (1.34-1.97) | |||||||
| Not validated | Multiparas | |||||||
| <1 pack/day 1.21 (1.06-1.38) ≥1pack/day 1.15(0.99-1.34) | ||||||||
| 50 | Raatikainen et al (2006) | Cohort | 25,591 | Finland-Kuopio University Hospital 1989-2001. | Smokers: Yes. | Fetal death was defined as intrauterine death before | No estimates | N/A** |
| Not validated | ||||||||
| 22 weeks of pregnancy or 500 g fetal weight. | ||||||||
| 51 | Aliyu et al (2010) | Cohort | 633,849 | US Missouri – Using Missouri maternally-linked cohort data files 1978 – 1997. | Smokers: Yes | In utero fetal death at > = 20 weeks gestation. | Results HRs, | N/A** |
| Not validated | <15 years 3.3 (1.4-7.8), 15-19 years 1.7 (1.5-1.9), 20-24 years 1.5 (1.4-1.6) | |||||||
| No sufficient data to calculate Odds ratios. | ||||||||
| 52 | Bai et al (2000) | Cohort | 7138 | Australia- Liverpool hospital Sydney March 1996 to June 1998 | Smokers: Yes | Not Clear (births occurring at less than 20 weeks gestation age exclusion criteria). | Results presented in Percentages. No odds ratios and no sufficient data to calculate OR. | N/A** |
| Not validated | ||||||||
| 53 | Huang et al (2000) | Cohort | 84,294 | Canada – The Royal Victoria Hospital in Montreal 1961-1974 and 1978-1996. | Smokers: Yes | Fetal deaths occurring before labour without evidence of significant fetal, maternal or placental pathology weighing 500 g or more. | No estimates | N/A** |
| Not validated | ||||||||
| 18 | Schramm (1997) | Cross-sectional | 176,843 | USA – Missouri 1978-1990 | Smokers: Yes Not validated | Fetal death greater or equal to 20 weeks gestation. | Results in RR no sufficient data to calculate OR | N/A** |
| Smoking in 1st and not in 2nd pregnancy RR 1.11, Not smoking in 1st but in 2nd pregnancy RR 1.6.smoking in both pregnancies RR 1.19. | ||||||||
| 54 | Salihu et al (2004) | Cross-sectional | 7,792,990 | USA – 1995-1997. | Smokers: Yes | Intrauterine fetal demise after 20 weeks of gestation. | Estimates in Hazard ratios not enough data to calculate OR. | N/A** |
| Not validated | ≥ 40 years 2.71 (1.88-3.91), 30-39 years 1.58 (0.96-2.60), 20-29 years 1.41 (1.16-1.71) | |||||||
OR – Odds Ratio, HR – Hazard Ratio, RR – Risk Ratio.
N/A* - Original results were presented in HR/RR, OR was calculated using original data.
N/A** - Studies included in descriptive synthesis only.
*** - Studies using same dataset.
Swedish National registry data set ref 16: 1933-1985, 17 and 28 : 1983-1989, ref 34: 1984 and1991.
US – Missouri data set ref 35 and 36.
Out of the 34 eligible studies (Table 1), eight were case control studies [23-28,54,55], five cross sectional [16,18,40,41,45] and twenty-one were cohort studies [12-14,17,29-39,42-44,56-58]. Fourteen were conducted in Europe, [13,14,16,17,25,27,29,32,36-39,43,54], four in Australia [34,41,56,58], fourteen in North America [12,18,23,26,28,30,31,33,35,42,44,45,55,57], one in Asia [40] and one in South America [24]. The largest sample size observed was 7,792,990 [45] with the smallest being 202 [25]. Ten studies [13,16,25,26,30-32,35,54,55] did not present their results with odds ratios but had sufficient data to calculate the ratio using STATA 12, which was further used in the meta-analysis. Seven studies [18,42-45,57,58] did not present estimates or sufficient data to calculate an odds ratio; therefore they were included in the narrative synthesis. Based on NOS scale, overall study quality was moderately satisfactory. Ten cohort studies [12,14,17,32,35-39,42] and four case control studies [23,24,54,55] were considered of high quality with seven or more points, and only two cross sectional studies [16,45] were of high quality having five or more points. Scores ranged from 2 to 9 and the median score was 6.
The type of exposure
Eleven studies clearly defined the level of exposure (smoking) [14,16,17,25,29,31,33,34,38,54,56]. Most of the studies used categories of 1-9cig/day and ≥10 cig/day to categorise participants according to the number of cigarettes or packs consumed daily, but the Robson study [41] used slightly different categories. Seventeen studies did not define smoking according to level of cigarette consumption [12,13,24,26-28,30,32,35,36,40,41,43,45,55,57,58] but according to the smoking status (yes/no). Fifteen studies collected exposure information during pregnancy [12-14,16,17,27,29,30,32,33,38,42,43,54,56] and the rest of the studies [18,23-26,31,34,35,39-41,44,45,55,57,58] obtained the information after delivery. None of the studies reported biochemical validation of smoking status such as salivary cotinine assessments.
Data collection on outcome
The definition of stillbirth varied across studies. Twenty-one out of thirty-four studies included stillbirth from early gestational age ≥ 20 weeks [18,23,30,31,33,34,36,37,41-43,45,56-58], ≥22 weeks [54], ≥23 weeks [35], and four studies used birth weight to estimate the gestational week of stillbirth (Birth weight ≥ 500 g, [24,26,28,44]. The rest of the studies used late gestational ages as the cut off values for stillbirth, ≥24 weeks in four studies [12,13,27,32], ≥28 weeks in nine studies [14,16,17,25,29,38-40,55]. In all studies, outcome data was either obtained from medical or clinical records, medical data sets or birth registry records.
Fourteen studies included in the quantitative synthesis adjusted for one or more confounders (Table 1). Maternal age was the most common adjusted factor. Five studies adjusted for socioeconomic status (SES) including education [13,14,37,38,40]. Other factors that were adjusted for include parity, BMI, ethnicity/race, infant sex, perinatal care and marital status, alcohol, caffeine intake and cohabitation. Four studies [24,27,36,39] did not adjust for any confounders and six studies [26,31,32,35,54,55] had OR calculated therefore considered as not adjusted for any confounders. Factors adjusted in cohort studies varied widely. Several studies [12,17,28,34,37,41,56] considered one or more medical conditions such as pregnancy complications, diabetes mellitus and hypertension as possible confounders.
Meta-analysis of maternal smoking and risk of stillbirth
In meta-analysis of all 25 studies (Figure 2), smoking during pregnancy was associated with a 47% increase in the odds of stillbirth (OR 1.47, 95% CI 1.37, 1.57, p < 0.0001) with an overall moderate heterogeneity (I2 = 79%). There was no significant difference in the size of this estimate between study designs (p = 0.11); the odds of stillbirth were increased by 34% in relation to smoking in pregnancy in case-control studies (OR 1.34, 95% CI 1.23, 1.45, p < 0.0001, 8 studies), 49% in cohort studies (OR 1.49, 95% CI 1.35, 1.64, p < 0.0001, 15 studies) and 62% in cross-sectional studies (OR 1.62, 95% CI 1.31, 2.00, p < 0.0001, 2 studies). Results were more heterogeneous for cohort studies than other study types.
Figure 2.
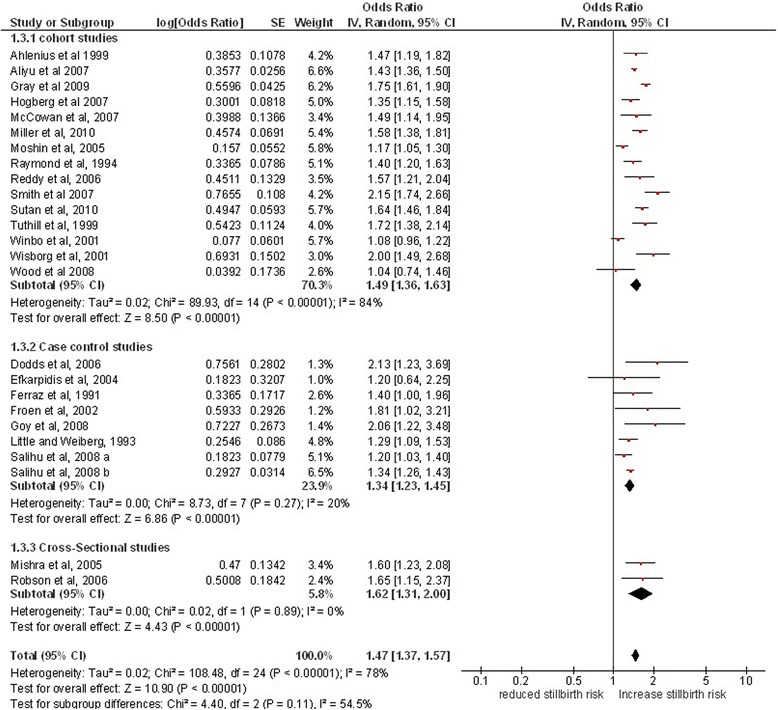
Maternal smoking in pregnancy and the risk of stillbirth.
Subgroup analysis by study quality
Results from high quality score studies showed that women who smoke during pregnancy, are 41% more likely to have a stillbirth compared to women who do not smoke during pregnancy (OR 1.41, 95% CI 1.28, 1.55, p <0.0001, 14 studies). Low quality score studies showed that women who smoke during pregnancy were at 49% increased odds of stillbirth compared to women who do not smoke during pregnancy (OR 1.49, 95% CI 1.33, 1.67, p <0.0001, 11 studies). There was no significant difference between the two subgroups (p = 0.44).
Subgroup analysis by categories of cigarette consumption
Seven studies (14, 26 a-b,31,34,45,48) were included in this subgroup analysis, with consumption categorised as 1-9 cig/day and ≥10 cig/day (Figure 3). One study [29] presented OR values for the ≥10 cig/day category only. A study by Robson et al [41] used slightly different categories; 1-10 cig/day and >10 cig/day. This study was included in this analysis with odds ratios for the 1-10 cig/day, and > 10 cig/day categories used for the 1-9 cig/day and ≥ 10 cig/day groups respectively. Further two studies by Salihu et al (2008a) [23] and Salihu et al (2008b) [23] had a classification of 1-9 cig/day, 10-19 cig/day and ≥ 20 cig/day. There was no sufficient raw data to calculate the OR for ≥10 cig/day. Therefore these studies have been included in this analysis conservatively using the ORs from the 10-19 cig/day categories (which is a lower value than the ≥ 20 cig/day value) for the ≥ 10 cig/day subgroup. Most of the studies included in this subgroup analysis (4 out of 7) used this categorisation (1-9 cig/day and ≥10 cig/day), thus the same was applied in the subgroup analysis. Meta-analysis showed that smoking 1-9 cig/day was associated with an 9% increased odds of having a stillbirth compared to women who do not smoke in pregnancy (OR 1.09, 95% CI 1.09, 1.24, p = 0.55, 6 studies), whilst smoking ≥10 cig/day was associated with a 52% increase in odds of stillbirth (OR 1.52, 95% CI 1.30, 1.78, p < 0.0001, 7studies); the results for these two subgroups differed significantly (p = 0.001).
Figure 3.
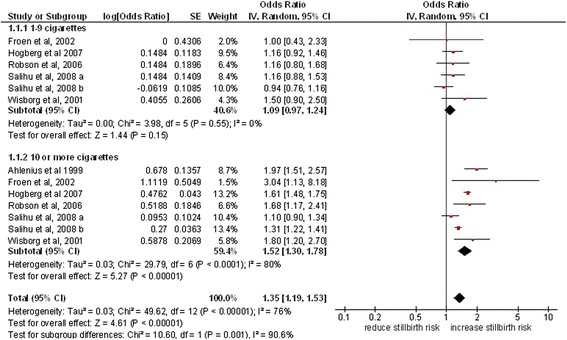
Stillbirth risk according to the amount of daily cigarette consumption.
Subgroup analysis by gestation of stillbirth
Twenty-two studies were included in this subgroup analysis (Figure 4). The meta-analysis of studies defining stillbirth at ≥ 20 weeks demonstrated a 43% increase in odds for smoking mothers compared to mothers who do not smoke, (OR 1.43, 95% CI 1.32, 1.54, p < 0.0001, 11 studies), whereas studies with stillbirth defined at ≥ 24 weeks showed 58% increase in odds (OR 1.58, 95% CI 1.21, 1.2.06, p < 0.0003, 4 studies) and in pooled estimates of studies with stillbirth defined at ≥ 28 weeks, the odds was increased to 33% (OR 1.33, 95% CI 1.18, 1.49, p < 0.02, 7 studies). There was no significant difference in the odds ratios for these different subgroups (p = 0.39).
Figure 4.
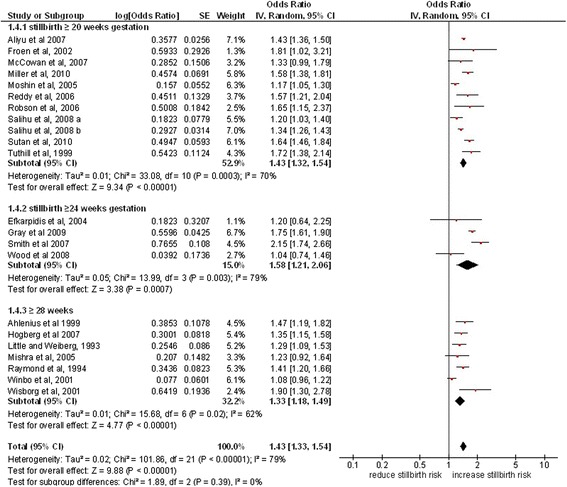
Maternal smoking and the risk of stillbirth according to the gestation period.
Subgroup analysis by study location
Only two studies were conducted in developing countries [24,40]. Both studies support evidence from research conducted in developed countries concluding that women who smoke during pregnancy are at a risk of stillbirth. A study [40] demonstrated a very marginal difference showing those women who smoke during pregnancy are at least 40% at risk of experiencing a stillbirth (OR 1.40, 95% CI 1.0, 1.96), similar to the pooled odds ratio of studies conducted in North America (OR 1.39, 95% CI 1.29, 1.50) P value < 0.0001, 9 studies) and Europe (OR 1.55, 95% CI 1.36, 1.78, P value < 0.0001,11 studies).
Narrative synthesis
Seven studies [18,42-45,57,58] presented their results in other than odds ratio and there was no sufficient data to calculate the odds ratio, and three studies [16,25,30] were derived from same study population as [17,31] respectively. Six of the studies [16,18,30,42,45,57] observed an association between maternal smoking in pregnancy and the risk of stillbirth but the other four [25,43,44,58] observed no significant differences. Two studies [16,42] observed a dose related response with high risk ratio of stillbirth in mothers who smoke ≥10 cig/day compared to 1-9 cig/day and those who smoke ≥1 pack/day in contrast to <1 pack/day respectively. Three studies (51, 35 54) showed the risk of stillbirth to be higher in young mothers <15 years old (HR 3.3, 95% CI 1.4, 7.8) and also in older mothers (≥35 years, HR 3.2, 95% CI 2.2, 4.5) (≥40 years, HR 2.71, 95% CI 1.88, 3.91) respectively. A study from Sweden suggested that placental abruption is likely to be common in smokers [25].
Publication bias
The publication bias was assessed visually using a funnel plot developed by Rev Man 5.3 [22]. The plot was symmetrical indicating a low risk of publication bias (Additional file 2).
Discussion
This study provides a comprehensive review of the current evidence and estimates of the effect of maternal smoking on the risk of stillbirth. It suggests, women who smoke during pregnancy have a 47% increased risk of stillbirth and that the risk of stillbirth is more at higher levels of cigarette consumption (Table 2). This effect does not vary according to the gestation at which still birth occurs, however, comparisons between studies would be easier if future work adopted the definition of stillbirth recommended by the WHO [20].
Table 2.
Result summary: maternal smoking in pregnancy and the risk of stillbirth
| Assessment criteria | Odds ratio | 95% CI |
|---|---|---|
| i) Overall pooled Results from main meta-analysis (25 studies) | 1.47 | 1.37-1.57 |
| ii) Subgroup analysis by daily amount of cigarettes consumption | ||
| 1-9 cig/day (6 studies) | 1.09 | 0.97-1.24 |
| ≥ 10 cig/day (7 studies) | 1.52 | 1.30-1.78 |
| iii) Subgroup analysis by gestation period at which stillbirth occurred | ||
| Stillbirth ≥ 20 weeks (11 studies) | 1.43 | 1.32-1.54 |
| Stillbirth ≥ 24 week (4 studies) | 1.58 | 1.21-2.06 |
| Stillbirth ≥ 28 weeks (7 studies) | 1.33 | 1.18-1.49 |
| iv) Subgroup analysis by geographic location | ||
| North America (9 studies) | 1.39 | 1.29-1.50 |
| Europe (11 studies) | 1.55 | 1.36-1.78 |
| Asia (1 study) | 1.23 | 0.92-1.64 |
| South America (1 study) | 1.40 | 1.00-1.96 |
| Australia (3 studies) | 1.29 | 1.07-1.55 |
| v) Subgroup analysis by quality | ||
| High score (14 studies) | 1.41 | 1.28-1.55 |
| Low score (11 studies) | 1.49 | 1.33-1.67 |
Strengths and limitations
We implemented a comprehensive search strategy with strict adherence to the protocol, and results were presented in accordance with MOOSE guidelines [19]. Search strategy, data extraction, analysis and quality assessment was performed independently by authors and findings were confirmed within the team. There was no evidence of publication bias. However, the review has some limitations. We could not obtain one study within available timelines [59], and due to limited data presentation, seven studies [18,42-45,57,58] were considered in the narrative synthesis. Secondly, only English language studies were considered mainly for practical purposes and the available timeframe.
Heterogeneity was explored using a variety of subgroup analyses; a high level of heterogeneity was found amongst cohort studies and the pooled estimate derived from these should be treated with some caution. This could be partially attributable to the different factors controlled for in the cohort studies as explained previously. The overall heterogeneity (I2 = 77%, Figure 2) was higher mainly due to cohort studies, however in case of case control studies (I2 = 20%) and cross-sectional studies (I2 = 0%), very low levels of heterogeneity was observed. However, this estimate was consistent with synthesised estimates derived from studies with other designs and this consistency suggests it is likely to be valid. It appears that the effect could differ by age [24,30,38,45,57], but data on maternal age was not available in included studies. Study quality was found to be satisfactory.
Synthesis
The most recent systematic review by Flenady et al [8] on maternal smoking and the risk of stillbirth, focusing on high-income countries, reported a 36% increase in the odds of stillbirth (OR 1.36). The review considered cohort and case control studies published between 1998 and 2009 and excluded those which did not control for confounding factors, hospital based studies and took a slightly different approach to dealing with duplicate studies. Acknowledging the difference in the approach, our study is in agreement with the results from Flenady’s et al [8] review, and showed a 46% increased risk of stillbirth (OR 1.46 95% CI 1.36, 1.55). We included 25 studies in our main meta-analysis, conducted in both developing and developed countries making our findings generalisable. Our review has also analysed the available data according to the definition of stillbirth in gestational weeks, number of cigarettes and study quality, which were not reported in the previous published literature. The review also provides strong evidence that the risk is higher at higher levels of cigarette consumption, indicating a dose related response relationship, outlined in some of the included studies [15,38,42].
The consistency in the size of the effect observed in this study throughout different subgroup analyses and across different study designs, each with their own biases and strengths and limitations, as well as the consistency across studies of differing quality, suggests that this is a true estimate of the size of effect. Further support is obtained from studies where mothers change in smoking behaviour from one pregnancy to another. Hogberg and Cnattingius [14] suggested that mothers who quit smoking from first to second pregnancy reduced their risk of stillbirth to the same level as non-smokers in both pregnancies, while those who smoked in both pregnancies had a 35% increased risk of stillbirth (OR 1.35) compared to non-smokers.
Our results also provide evidence that the effect of maternal smoking in pregnancy on risk of stillbirth is not dependent on the definition of stillbirth or on the stage of pregnancy at which it occurs. Moreover we did not ascertain, at what point during pregnancy the effect of the exposure is occurring as most studies evaluated the exposure at one point in time only. Previous studies [26,38] suggested that the timing of exposure may influence the risk of stillbirth and in particular [38] suggested that those who quite early in pregnancy may have similar risk to non-smokers. Future studies of this association should measure exposure at different stages of gestation. There are important potential confounders for the effect of maternal smoking on risk of stillbirth, the most important ones are SES, maternal age, maternal weight and medical comorbidities, majority of studies have adjusted for these. Only two studies did not adjust for any confounders [36,43] with no further information.
The exact pathophysiology of fetal exposure to tobacco smoke is not entirely understood, however based on available evidence, possible mechanisms have been conceptualised [30]. Nicotine has been known to cause narrowing of the placental vessels [60,61], which coupled with reduced prostacycline synthesis [62], resulting in increased vascular resistance and consequently impairing blood supply to the fetus [63]. Carbon monoxide in tobacco smoke also reduces fetal oxygenation by forming carboxy-haemoglobin in turn interfering with oxygenation transfer [64]. The correlation between smoking and stillbirth is likely to be explained through increased prevalence of placental complications and fetal growth restriction [14]. The resultant physiological effect of these changes increases the risk of fetal morbidity (small for gestation age and preterm birth) and subsequently may lead to fetal death [30]. Therefore, there is strong biological plausibility for smoking in pregnancy causing stillbirth [46,65].
Recent Cochrane reviews have concluded that behavioural interventions can be effective in helping women to stop smoking in pregnancy [66] even though there remains no evidence for the effectiveness of pharmacotherapy such as NRT in pregnancy [67]. The result of our study suggests that such interventions are important to reduce the risk of stillbirth.
Conclusion
Our findings support that smoking greatly increases the risk of stillbirth. Smoking in pregnancy is an established cause of a range of pregnancy complications and poor pregnancy outcomes. Every opportunity must be utilised to record smoking status during pregnancy, to give advice and support, including necessary facilities to help women stop smoking. Although we have conducted a comprehensive review, most of the available evidence on the risk from smoking is from developed countries. Smoking is rapidly increasing in the developing world where stillbirth is a major problem; therefore along with focusing on research initiatives, it is important to invest efforts on educating women on the risks of smoking to their unborn child and providing smoking cessation support for pregnant women in these parts of world.
Acknowledgments
We acknowledge the support of the Greenfield Medical Library staff of University of Nottingham, UK.
Abbreviations
- NOS
New castle Ottawa scale
- SES
Socioeconomic status
Additional files
Supplementary 1. Sample search strategy used for identification of studies.
Funnel plot.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
The study was conceptualised by SL, TC, TM and AA. All authors participated in electronic search, title screening, study selection, data extraction, analysis and manuscript preparation. AA and TM contributed equally in the manuscript preparation, and thus shared the first authorship equally. All four authors provided intellectual content and approved the manuscript for publication.
Contributor Information
Takawira C Marufu, Email: msxtm1@nottingham.ac.uk.
Anand Ahankari, Email: mcxaa31@nottingham.ac.uk.
Tim Coleman, Email: Tim.coleman@nottingham.ac.uk.
Sarah Lewis, Email: Sarah.lewis@nottingham.ac.uk.
References
- 1.World Health Organisation (WHO) Recommendations for the prevention and management of tobacco use and second- hand smoke exposure in pregnancy. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 2.Royal College of Physicians . Passive smoking and children: a report of the Tobacco Advisory Group of the Royal College of Physicians. London: Royal College of Physicians; 2013. [Google Scholar]
- 3.Royal College of Physicians . Smoking and the young. London: Royal college of Physicians; 1992. [Google Scholar]
- 4.Cnattingius S, Bergstrom R, Lipworth L, Kramer MS. Pre-pregnancy weight and the risk of adverse pregnancy outcomes. N Eng J Med. 1998;338:147–52. doi: 10.1056/NEJM199801153380302. [DOI] [PubMed] [Google Scholar]
- 5.Fretts RC. Etiology and prevention of stillbirth. Am J Obstet Gynecol. 2005;193:1923–35. doi: 10.1016/j.ajog.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 6.Lawn JE, Blencowe H, Pattinson R, Cousens S, Kumar R, Ibiebele I, et al. Stillbirths: Where? When? Why? How to make the data count? Lancet. 2011;377:1448–63. doi: 10.1016/S0140-6736(10)62187-3. [DOI] [PubMed] [Google Scholar]
- 7.Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res. 2004;6:S125–40. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- 8.Flenady V, Koopmans L, Middleton P, Froen JF, Smith GC, Gibbons K, et al. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet. 2011;377:1331–40. doi: 10.1016/S0140-6736(10)62233-7. [DOI] [PubMed] [Google Scholar]
- 9.NHS Information centre. The Infant Feeding Survey 2010. Available at http://www.hscic.gov.uk [Accessed January 2013].
- 10.Scollo MM, Winstanley MH. Tobacco in Australia: Facts and issues. 4. Melbourne: Cancer Council Victoria; 2012. [Google Scholar]
- 11.Ebert LM, Fahy K. Why do women continue to smoke in pregnancy? Women Birth. 2007;20:161–8. doi: 10.1016/j.wombi.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Wood SL, Chen S, Ross S, Sauve R. The risk of unexplained antepartum stillbirth in second pregnancies following caesarean section in the first pregnancy. BJOG. 2008;115:726–31. doi: 10.1111/j.1471-0528.2008.01705.x. [DOI] [PubMed] [Google Scholar]
- 13.Smith GCS, Shah I, White IR, Pell JP, Crossley JA, Dobbie R. Maternal and biochemical predictors of antepartum stillbirth among nulliparous women in relation to gestational age of fetal death. BJOG. 2007;114:705–14. doi: 10.1111/j.1471-0528.2007.01343.x. [DOI] [PubMed] [Google Scholar]
- 14.Hogberg L, Cnattingius S. The influence of maternal smoking habits on the risk of subsequent stillbirth: Is there a causal relation? BJOG. 2007;114:699–704. doi: 10.1111/j.1471-0528.2007.01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.United States Department of Health and Human Services (USDHHS) Women and smoking: a report of the surgeon general. Rockville MD: U.S: Department of Health and Human Services, Centre for Disease Control and Prevention, National Centre for Chronic Disease Prevention and Health Promotion, Office of Smoking and Health; 2001. Health Consequences of Tobacco use among women; pp. 177–450. [Google Scholar]
- 16.Cnattingius S, Haglund B, Meirik O. Cigarette smoking as risk factor for late fetal and early neonatal death. BMJ. 1988;297:258–61. doi: 10.1136/bmj.297.6643.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raymond EG, Cnattingius S, Kiely JL. Effects of maternal age, parity and smoking on the risk of stillbirth. BJOG. 1994;101:301–6. doi: 10.1111/j.1471-0528.1994.tb13614.x. [DOI] [PubMed] [Google Scholar]
- 18.Schramm WF. Smoking during pregnancy: Missouri longitudinal study. Paediatr Perinat Epidemiol. 1997;11:73–83. doi: 10.1046/j.1365-3016.11.s1.10.x. [DOI] [PubMed] [Google Scholar]
- 19.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:1–5. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 20.Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, et al. The International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) Revised Glossary on ART Terminology. Hum Reprod. 2009;24:2683–7. doi: 10.1093/humrep/dep343. [DOI] [PubMed] [Google Scholar]
- 21.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. Newcastle-Ottawa scale (NOS) for assessing the quality of non-randomised studies in meta-analysis. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed March 10, 2012).
- 22.Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011. Available from http://www.cochrane-handbook.org.
- 23.Salihu HM, Sharma PP, Getahun D, Hedaytzadeh M, Peters S, Kirby RS, et al. Prenatal tobacco use and risk of stillbirth: a case-control and bidirectional case-crossover study. Nicotine Tob Res. 2008;10:159–66. doi: 10.1080/14622200701705431. [DOI] [PubMed] [Google Scholar]
- 24.Ferraz EM, Gray RH. A case-control study of stillbirths in Northeast Brazil. Int J Gynaecol Obstet. 1991;34:3–19. doi: 10.1016/0020-7292(91)90532-a. [DOI] [PubMed] [Google Scholar]
- 25.Walles B, Tyden T, Herbst A, Ljungblad U, Rydhstrom H. Maternal health-care program and makers for late fetal death. Acta Obstet Gynecol Scand. 1994;73:773–8. doi: 10.3109/00016349409072503. [DOI] [PubMed] [Google Scholar]
- 26.Dodds L, King WD, Fell DB, Armson BA, Allen A, Nimrod C. Stillbirth risk factors according to timing of exposure. Ann Epidemiol. 2006;16:607–13. doi: 10.1016/j.annepidem.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Efkarpidis S, Alexopoulos E, Kean L, Liu D, Fay T. Case-control study of factors associated with intrauterine fetal deaths. MedGenMed. 2004;6:53. [PMC free article] [PubMed] [Google Scholar]
- 28.Goy J, Dodds L, Rosenberg MW, King W. Health-risk behaviours: examining social disparities in the occurrence of stillbirth. Paediatr Perinat Epidemiol. 2008;22:314–20. doi: 10.1111/j.1365-3016.2008.00947.x. [DOI] [PubMed] [Google Scholar]
- 29.Ahlenius I, Thomassen P. The changing panorama of late fetal death in Sweden between 1984 and 1991. Acta Obstet Gynecol Scand. 1999;78:408–14. doi: 10.1080/j.1600-0412.1999.780512.x. [DOI] [PubMed] [Google Scholar]
- 30.Aliyu MH, Salihu HM, Wilson RE, Alio AP, Kirby RS. The risk of intrapartum stillbirth among smokers of advanced maternal age. Arch Gynecol Obstet. 2008;278:39–45. doi: 10.1007/s00404-007-0529-8. [DOI] [PubMed] [Google Scholar]
- 31.Aliyu MH, Salihu HM, Wilson RE, Kirby RS. Prenatal smoking and risk of intrapartum stillbirth. Arch Environ Occup Health. 2007;62:87–92. doi: 10.3200/AEOH.62.2.87-92. [DOI] [PubMed] [Google Scholar]
- 32.Gray R, Bonellie SR, Chalmers J, Greer I, Jarvis S, Kurinczuk JJ, et al. Contribution of smoking during pregnancy to inequalities in stillbirth and infant death in Scotland 1994-2003: retrospective population based study using hospital maternity records. BMJ. 2009;339:b3754. doi: 10.1136/bmj.b3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller EC, Cao HL, Wen SW, Yang Q, Lafleche J, Walker M. The risk of adverse pregnancy outcomes is increased in preeclamptic women who smoke compared with nonpreeclamptic women who do not smoke. Am J Obstet Gynecol. 2010;203:1–8. doi: 10.1016/j.ajog.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 34.Mohsin M, Bauman AE, Jalaludin B. The influence of antenatal and maternal factors on stillbirths and neonatal deaths in New South Wales. J Biosoc Sci. 2006;38:643–57. doi: 10.1017/S002193200502701X. [DOI] [PubMed] [Google Scholar]
- 35.Reddy UM, Laughon SK, Sun L, Troendle J, Willinger M, Zhang J. Prepregnancy risk factors for antepartum stillbirth in the United States. Obstet Gynecol. 2010;116:1119–26. doi: 10.1097/AOG.0b013e3181f903f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sutan R, Campbell D, Prescott GJ, Smith WCS. The risk factors for unexplained antepartum stillbirths in Scotland, 1994 to 2003. J Perinatol. 2010;30:311–8. doi: 10.1038/jp.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tuthill DP, Stewart AH, Coles EC, Andrews J, Cartidge PH. Maternal cigarette smoking and pregnancy outcome. Paediatr Perinat Epidemiol. 1999;13:245–53. doi: 10.1046/j.1365-3016.1999.00187.x. [DOI] [PubMed] [Google Scholar]
- 38.Wisborg K, Kesmodel U, Henriksen TB, Olsen SF, Secher NJ. Exposure to tobacco smoke in utero and the risk of stillbirth and death in the first year of life. Am J Epidemiol. 2001;154:322–7. doi: 10.1093/aje/154.4.322. [DOI] [PubMed] [Google Scholar]
- 39.Winbo I, Serenius F, Dahlquist G, Kallen B. Maternal risk factors for cause-specific stillbirth and neonatal death. Acta Obstet Gynecol Scand. 2001;80:235–44. doi: 10.1034/j.1600-0412.2001.080003235.x. [DOI] [PubMed] [Google Scholar]
- 40.Mishra V, Retherford RD, Smith KR. Cooking smoke and tobacco smoke as risk factors for stillbirth. Int J Environ Health Res. 2005;15:397–410. doi: 10.1080/09603120500288913. [DOI] [PubMed] [Google Scholar]
- 41.Robson S, Cameron CA, Roberts CL. Birth outcomes for teenage women in New South Wales, 1998-2003. Aust N Z J Obstet Gynaecol. 2006;46:305–10. doi: 10.1111/j.1479-828X.2006.00597.x. [DOI] [PubMed] [Google Scholar]
- 42.Kleinman JC, Pierre MB, Madans JH, Jr, Land GH, Schramm WF. The effects of maternal smoking on fetal and infant mortality. Am J Epidemiol. 1988;127:274–82. doi: 10.1093/oxfordjournals.aje.a114803. [DOI] [PubMed] [Google Scholar]
- 43.Raatikainen K, Huurinainen P, Heinonen S. Smoking in early gestation or through pregnancy: a decision crucial to pregnancy outcome. Prev Med. 2007;44:59–63. doi: 10.1016/j.ypmed.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 44.Huang DY, Usher RH, Kramer MS, Yang H, Morin L, Fretts RC. Determinants of unexplained antepartum fetal deaths. Obstet Gynecol. 2000;95:215–21. doi: 10.1016/S0029-7844(99)00536-0. [DOI] [PubMed] [Google Scholar]
- 45.Salihu HM, Shumpert MN, Aliyu MH, Alexander MR, Kirby RS, Alexander GR. Stillbirths and infant deaths associated with maternal smoking among mothers aged not greater than or equal to 40 years: a population study. Am J Perinatol. 2004;21:121–9. doi: 10.1055/s-2004-823776. [DOI] [PubMed] [Google Scholar]
- 46.Moga M, Preda GH. Smoking and pregnancy. J Environ Prot Ecol. 2008;9:566–73. [Google Scholar]
- 47.Bukowski R, Carpenter M, Conway D, Coustan D, Dudley DJ, Goldenberg RL, et al. Association between stillbirth and risk factors known at pregnancy confirmation. JAMA. 2011;306:2469–79. doi: 10.1001/jama.2011.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kunzel W, Misselwitz B. Unexpected fetal death during pregnancy – a problem of unrecognised fetal disorders during antenatal care? Eur J Obstet Gynecol Reprod Biol. 2003;110:S86–92. doi: 10.1016/S0301-2115(03)00177-5. [DOI] [PubMed] [Google Scholar]
- 49.Xu B, Jarvelin MR, Rantakallio P. Maternal smoking in pregnancy and sex differences in perinatal death between boys and girls. Soc Biol. 1998;45:273–7. doi: 10.1080/19485565.1998.9988978. [DOI] [PubMed] [Google Scholar]
- 50.Kallen K. The impact of maternal smoking during pregnancy on delivery outcome. Eur J Public Health. 2001;11:329–33. doi: 10.1093/eurpub/11.3.329. [DOI] [PubMed] [Google Scholar]
- 51.Verkerk PH, Buitendijk SE, Verloove-Vanhorick SP. Differential misclassification of alcohol and cigarette consumption by pregnancy outcome. Int J Epidemiol. 1994;23:1218–25. doi: 10.1093/ije/23.6.1218. [DOI] [PubMed] [Google Scholar]
- 52.Maleckiene L, Nadisauskiene R, Bergstrom S. Scio-economic demographic and obstetric risk factors for late death of unknown etiology in Luthuania: a case—referent study. Acta Obstet Gynecol Scand. 2001;80:321–5. [PubMed] [Google Scholar]
- 53.Beyerleinl A, Von Kriesl R, Hummel M, Lack N, Schiessl B, Giani G. Improvement in pregnancy-related outcomes in the offspring of diabetic mothers in Bavaria, Germany, during 1987–2007. Diabet Med. 2010;27:1379–84. doi: 10.1111/j.1464-5491.2010.03109.x. [DOI] [PubMed] [Google Scholar]
- 54.Froen JF, Arnestad M, Vege A, Irgens, Rognum T, Saugstad O. Comparative epidemiology of sudden infant death syndrome and sudden intrauterine unexplained death. Arch Dis Child. 2002;87:118–21. doi: 10.1136/fn.87.2.F118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Little RE, Weinberg CR. Risk-factors for antepartum and intrapartum still-birth. Am J Epidemiol. 1993;137:1177–89. doi: 10.1093/oxfordjournals.aje.a116620. [DOI] [PubMed] [Google Scholar]
- 56.McCowan LM, George-Haddad M, Stacey T, Thompson JM. Fetal growth restriction and other risk factors for stillbirth in a New Zealand setting. Aust N Z J Obstet Gynaecol. 2007;47:450–6. doi: 10.1111/j.1479-828X.2007.00778.x. [DOI] [PubMed] [Google Scholar]
- 57.Aliyu MH, Salihu HM, Alio AP, Wilson RE, Charkrabarty S, Clayton HB. Prenatal smoking among adolescents and risk of fetal demise before and during labor. J Pediatr Adolesc Gynecol. 2010;23:129–35. doi: 10.1016/j.jpag.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 58.Bai J, Wong FW, Gyneshwar R, Stewart HC. Profile of maternal smokers and their pregnancy outcomes in south western Sydney. J Obstet Gynaecol Res. 2000;26:127–32. doi: 10.1111/j.1447-0756.2000.tb01295.x. [DOI] [PubMed] [Google Scholar]
- 59.Flenady V. Causes and risk factors of stillbirth in Australia. Perinatal Society of Austarlia and New Zealand Congress 2008. Gold Coast. Paediatr Child Health. 2008:2-10.
- 60.Marrow RJ, Ritchie JW, Bull SB. Maternal cigarette smoking: the effects on umbilical and uterine blood flow velocity. Am J Obstet Gynecol. 1988;159:1069–71. doi: 10.1016/0002-9378(88)90415-2. [DOI] [PubMed] [Google Scholar]
- 61.Lambers DS, Clark KE. The maternal and fetal physiologic effects of nicotine. Semin Perinatol. 1996;20:115–26. doi: 10.1016/S0146-0005(96)80079-6. [DOI] [PubMed] [Google Scholar]
- 62.Ylikorkala O, Viinikka L, Lehtovirta P. Effect of nicotine on fetal prostacyclin and thromboxane in humans. Obstet Gynecol. 1985;66:102–5. [PubMed] [Google Scholar]
- 63.Bruner JP, Forouzan I. Smoking and Bucally administered nicotine. Acute effect on uterine and umbilical artery Doppler flow velocity wave forms. J Reprod Med. 1991;36:435–40. [PubMed] [Google Scholar]
- 64.Salihu HM, Aliyu HM, Kirby RS. In utero nicotine exposure and fetal growth inhibition among twins. Am J Perinatol. 2005;22:421–7. doi: 10.1055/s-2005-915219. [DOI] [PubMed] [Google Scholar]
- 65.Hammoud AO, Bujold E, Sorokin Y, Schild C, Krapp M, Baumann P. Smoking in pregnancy revisited: findings from a large population-based study. Am J Obstet Gynecol. 2005;192:1856–63. doi: 10.1016/j.ajog.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 66.Hajek P, Stead LF, West R, Jarvis M, Hartmann-Boyce J, Lancaster T. Relapse prevention interventions for smoking cessation. Cochrane Database Syst Rev 2013, Issue 8. Art. No.: CD003999. doi:10.1002/14651858.CD003999.pub4. [DOI] [PubMed]
- 67.Coleman T, Chamberlain C, Davey MA, Cooper SE, Leonardi-Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev 2012, Issue 9. Art. No.: CD010078. doi:10.1002/14651858.CD010078 [DOI] [PubMed]


