Abstract
Purpose
To evaluate the risk of a subsequent primary thyroid cancer (SPTC) in patients with common invasive cancers, with attention to latency trends and histology associations.
Methods
Patients with one of 10 common invasive cancers were followed from 1975 to 2008 in 9 registries participating in the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database. Standardized incidence ratios (SIRs) for SPTC were determined by the multiple primary-SIR program in SEER*Stat.
Results
A total of 2502 SPTCs were observed. Greatly elevated SIRs for SPTC were noted for 9 of 10 evaluated cancers in the 12 months after initial diagnosis. The SIRs remained elevated 12–59 months after diagnosis for all cancers except leukemia, uterine, and bladder cancers. Increased risks persisted 60–119 months beyond diagnosis for renal (SIR 2.56) and breast cancer (SIR 1.16); and 120+ months for renal cancer (SIR 2.46). Increased SPTC risk after renal and female breast cancers was mostly seen in nonirradiated patients. The principal histology association was between papillary thyroid cancer and renal cell carcinomas.
Conclusions
Many common cancers are associated with increased risk of SPTC beyond 12 months of initial diagnosis. Although this can be explained partly by continued surveillance bias, radiation effects, and known rare familial associations for some tumors, these factors alone are unlikely to explain the persistent, significant two-way association with renal and breast cancers. Additional research is needed to further define the biological and environmental mechanisms underlying these associations.
Thyroid cancer is the most common malignancy affecting the endocrine glands, with 2004–2008 age-adjusted incidence rates of 16.3 per 100,000 women and 5.6 per 100,000 men.1 Exposure to ionizing radiation and family history of cancer syndromes are well-known risk factors for thyroid cancer development; however, much remains to be learned regarding its etiology. Existing literature supports an increased risk of second primary malignancies in patients treated for thyroid cancers with various reports showing a 30–42% increased risk.2–8 In some studies, the elevated risk was attributed to I-131 exposure in the treatment of thyroid cancer, whereas in others there did not appear to be any correlation with exposure to this radioisotope.2,4,5,8,9
The converse is also true in that there appears to be an increased risk of subsequent primary thyroid cancers (SPTC) in patients with other malignancies.7 Ronckers et al. reported a 29% increased risk of thyroid cancer after any primary malignancy, when compared to the general population.8 For thyroid cancer, this increased risk may be related to treatment effects, particularly external beam radiation. The British Childhood Cancer Survivor Study showed that risk of thyroid cancer as a second primary neoplasm was highest after Hodgkin disease and non-Hodgkin lymphoma. Survivors treated with radiotherapy had a relative risk of 4.6 compared to those not treated with radiotherapy.10 However, other authors note increased risks of SPTC even in patients who did not undergo any radiation treatment.8,11,12
Although these cohort studies provide valuable information, most report overall risks of cancer development, which can be misleading as the risk of diagnosing a second primary is most pronounced in the first year of diagnosis, possibly as a result of initial cancer staging studies or increased surveillance. As such, some of these second primaries may be synchronous rather than metachronous and lead to overestimates of risk. In addition, most studies on SPTC include all histologic subtypes of thyroid cancers together in the analyses. Inclusion of medullary thyroid cancers in particular, which have a different cellular origin and a known strong familial association has the potential to confound risk estimates.8,10 Given the above, we sought to systematically evaluate the risk of a SPTC in patients with common invasive cancers, with a particular emphasis on examining the variation of risk trends over latency periods, especially beyond the first year of diagnosis of the initial primary. We also evaluated whether these risks were associated with particular thyroid tumor histologies.
METHODS
The Surveillance, Epidemiology and End Results (SEER) program of the National Cancer Institute was used to identify our study population. The SEER program collects data on all individuals diagnosed with cancer residing in several geographically defined regions of the United States.13 To allow sufficient time for follow-up, our study included patients from the original 9 SEER Registries (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco–Oakland, Seattle–Puget Sound, and Utah). Data were available for cases diagnosed from 1975 through 2008 and collectively cover about 10% of the U.S. population.
We chose to focus on the 10 most commonly diagnosed cancers. These included first primary cancers only of the uterus, colorectum, female breast, renal, lung, prostate and bladder cancers, lymphoma, leukemia and skin melanoma. Inception cohorts of individuals each with one of these 10 most common cancers were identified and observed for the development of SPTC from 1975–2008. To measure relative risk for SPTC compared to the general population in the 9 SEER areas, we calculated a standardized incidence ratio (SIR) for each type of second and higher primary cancer (i.e. observed/expected) along with its exact 95% confidence interval (CI). The SEER*Stat Multiple Primary-SIR program (version 7.0.4) was used to calculate the SIRs. P values of <0.05 were considered statistically significant. Person-years at risk in the cohort were accumulated by 5-year age groups (0–4, 5–9,…85+), latency periods (2–11 months, 12–59 months, 60–119 months, 120+ months) and 5-year calendar periods (1975–79, 1980–84,…2005–2008) after the date of diagnosis of the first cancer to the date of either their diagnosis of the targeted second or higher cancer, last known follow-up, death or December 31, 2008, whichever occurred first. Cancer incidence rates specific for the strata in which person-years were distributed were multiplied by the corresponding person-years at risk to estimate the number of cancer cases expected in each stratum.
Thyroid cancers were included when they occurred after the specified common cancer as the second or higher malignant cancer. Histologic subtypes of thyroid cancer included were based on the ICD-O-3 morphology codes and were classified into groups on the basis of the World Health Organization classification of thyroid cancers.14 For the purposes of these groupings, follicular variant of papillary thyroid cancer was included with the papillary thyroid carcinoma group as a result of their similar clinical behavior. Cases diagnosed within 2 months of the initial primary tumor were excluded as these were likely to represent synchronous cancers. Lastly, death certificate or autopsy only cases were also excluded to limit the analyses to clinically relevant tumors. Renal tumors were also classified into groups (Renal cell tumors, transitional cell carcinoma, other adenocarcinomas, anaplastic tumors, mesenchymal tumors, nephroblastic tumors, lymphomas and other) by using the World Health Organization classification as a guideline.15
RESULTS
SIRs for SPTC of Various Histologic Subtypes
The study cohort included 2,152,303 patients with one of the 10 most common cancers with a total of 2502 SPTCs included in the final analyses. Eight of the 10 studied malignancies were associated with a significantly increased overall SIR for SPTC (Table 1). The only exceptions were uterine and bladder cancers.
TABLE 1.
Risk of SPTC after 10 common cancers, stratified by histology of thyroid cancer
| Cancer | 2–11 months
|
12–59 months
|
60–119 months
|
120+ months
|
Total
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. observed cases |
SIR (95% CI) | No. observed cases |
SIR (95% CI) | No. observed cases |
SIR (95% CI) | No. observed cases |
SIR (95% CI) | No. observed cases |
SIR (95% CI) | |
| Lymphoma | 43 | 4.45 (3.22–5.99)* | 49 | 1.47 (1.09–1.94)* | 32 | 1.25 (0.85–1.76) | 94 | 2.86 (2.31–3.50)* | 218 | 2.15 (1.87–2.45)* |
| Papillary SPTC | 41 | 5.83 (4.18–7.91)* | 40 | 1.62 (1.16–2.21)* | 29 | 1.50 (1.0–2.15)* | 81 | 3.13 (2.49–3.89)* | 191 | 2.48 (2.14–2.86)* |
| Follicular SPTC | 1 | 1.06 (0.03–5.92) | 2 | 0.65 (0.08–2.36) | 1 | 0.46 (0.01–2.54) | 7 | 2.89 (1.16–5.95)* | 11 | 1.28 (0.64–2.28) |
| Other SPTC | 0 | 0 (0–9.53) | 6 | 4.95 (1.82–10.8)* | 2 | 2.35 (0.28–8.47) | 2 | 2.22 (0.27–8.03) | 10 | 2.99 (1.43–5.49)* |
| Colorectum | 42 | 1.62 (1.17–2.19)* | 106 | 1.25 (1.02–1.51)* | 74 | 1.19 (0.94–1.50) | 67 | 0.98 (0.76–1.25) | 289 | 1.20 (1.06–1.34)* |
| Papillary SPTC | 29 | 1.72 (1.15–2.47)* | 79 | 1.42 (1.13–1.78)* | 43 | 1.08 (0.78–1.45) | 42 | 0.97 (0.7–1.30) | 193 | 1.24 (1.07–1.43)* |
| Follicular SPTC | 4 | 1.32 (0.36–3.39) | 8 | 0.84 (0.36–1.66) | 11 | 1.67 (0.84–3.00) | 3 | 0.47 (0.1–1.38) | 26 | 1.02 (0.67–1.50) |
| Renal | 28 | 6.13 (4.07–8.85)* | 39 | 2.61 (1.86–3.57)* | 27 | 2.56 (1.68–3.72)* | 27 | 2.46 (1.62–3.58)* | 121 | 2.95 (2.45–3.52)* |
| Papillary SPTC | 26 | 7.84 (5.12–11.5)* | 33 | 3.04 (2.09–4.27)* | 22 | 2.91 (1.82–4.4)* | 22 | 2.81 (1.76–4.26)* | 103 | 3.49 (2.85–4.23)* |
| Follicular SPTC | 2 | 4.50 (0.55–16.3) | 3 | 2.12 (0.44–6.2) | 4 | 4.10 (1.12–10.5) | 1 | 1.07 (0.03–5.97) | 10 | 2.65 (1.27–4.88)* |
| Skin Melanoma | 50 | 5.28 (3.92–6.96)* | 60 | 1.59 (1.21–2.05)* | 44 | 1.31 (0.95–1.75) | 69 | 1.35 (1.05–1.71)* | 223 | 1.69 (1.47–1.93)* |
| Papillary SPTC | 42 | 5.73 (4.13–7.75)* | 53 | 1.81 (1.35–2.36)* | 37 | 1.41 (0.99–1.94) | 61 | 1.53 (1.17–1.97)* | 193 | 1.88 (1.62–2.16)* |
| Follicular SPTC | 7 | 8.59 (3.45–17.7)* | 3 | 0.95 (0.2–2.78) | 2 | 0.74 (0.09–2.69) | 2 | 0.55 (0.07–1.99) | 14 | 1.36 (0.74–2.28) |
| Lung | 74 | 3.82 (3.00–4.79)* | 47 | 1.46 (1.07–1.94)* | 23 | 1.40 (0.89–2.10) | 19 | 1.37 (0.82–2.13) | 163 | 1.99 (1.70–2.32)* |
| Papillary SPTC | 58 | 4.36 (3.31–5.63)* | 27 | 1.21 (0.8–1.76) | 16 | 1.41 (0.81–2.29) | 16 | 1.68 (0.96–2.72) | 117 | 2.07 (1.71–2.48)* |
| Follicular SPTC | 8 | 3.77 (1.63–7.43)* | 8 | 2.40 (1.04–4.74)* | 4 | 2.47 (0.67–6.32) | 0 | 0 (0–2.96) | 20 | 2.41 (1.47–3.72)* |
| Other SPTC | 5 | 5.42 (1.76–12.7)* | 7 | 4.85 (1.95–9.99)* | 1 | 1.34 (0.03–7.48) | 1 | 1.5 (0.04–8.34) | 14 | 3.70 (2.03–6.22* |
| Bladder | 15 | 1.62 (0.91–2.68) | 31 | 0.93 (0.63–1.32) | 25 | 0.94 (0.61–1.39) | 31 | 1.04 (0.71–1.48) | 102 | 1.03 (0.84–1.25) |
| Papillary SPTC | 12 | 2.02 (1.04–3.53)* | 19 | 0.88 (0.53–1.38) | 17 | 0.99 (0.58–1.59) | 21 | 1.08 (0.67–1.66) | 69 | 1.08 (0.84–1.37) |
| Follicular SPTC | 3 | 2.81 (0.58–8.23) | 5 | 1.34 (0.44–3.13) | 1 | 0.36 (0.01–1.98) | 3 | 1.09 (0.22–3.17) | 12 | 1.16 (0.60–2.02) |
| Prostate | 49 | 1.54 (1.14–2.03)* | 150 | 1.23 (1.04–1.45)* | 97 | 1.08 (0.87–1.31) | 62 | 1.15 (0.88–1.48) | 358 | 1.20 (1.08–1.34)* |
| Papillary SPTC | 32 | 1.54 (1.05–2.17)* | 105 | 1.33 (1.09–1.61)* | 78 | 1.34 (1.06–1.67)* | 45 | 1.38 (1.01–1.85)* | 260 | 1.36 (1.20–1.54)* |
| Follicular SPTC | 4 | 1.06 (0.29–2.71) | 19 | 1.38 (0.83–2.15) | 4 | 0.42 (0.12–1.08) | 2 | 0.39 (0.05–1.41) | 29 | 0.90 (0.60–1.30) |
| Medullary SPTC | 5 | 3.69 (1.2–8.62)* | 7 | 1.39 (0.56–2.87) | 4 | 1.09 (0.3–2.79) | 2 | 0.87 (0.11–3.16) | 18 | 1.46 (0.87–2.31) |
| Leukemia | 22 | 4.83 (3.03–7.31)* | 19 | 1.38 (0.83–2.15) | 12 | 1.34 (0.69–2.33) | 29 | 3.14 (2.10–4.50)* | 82 | 2.24 (1.78–2.78)* |
| Papillary SPTC | 17 | 5.44 (3.17–8.71)* | 16 | 1.68 (0.96–2.73) | 10 | 1.59 (0.76–2.92) | 24 | 3.47 (2.22–5.16)* | 67 | 2.59 (2.01–3.29)* |
| Follicular SPTC | 3 | 6.11 (1.26–17.86)* | 1 | 0.70 (0.02–2.7) | 0 | 0 (0–4.3) | 1 | 1.34 (0.03–7.48) | 5 | 1.42 (0.46–3.32) |
| Uterus | 26 | 2.31 (1.51–3.38)* | 55 | 1.27 (0.95–1.65) | 25 | 0.63 (0.41–0.94)* | 49 | 0.80 (0.59–1.05) | 155 | 0.99 (0.84–1.16) |
| Papillary SPTC | 21 | 2.55 (1.58–3.9)* | 43 | 1.37 (0.99–1.84) | 19 | 0.68 (0.41–1.07) | 27 | 0.66 (0.43–0.96) | 110 | 1.01 (0.83–1.22) |
| Follicular SPTC | 1 | 0.89 (0.02–4.94) | 6 | 1.41 (0.52–3.07) | 1 | 0.27 (0.01–1.49) | 1 | 0.19 (0–1.03) | 9 | 0.62 (0.28–1.18) |
| Female breast | 114 | 2.13 (1.76–2.56)* | 257 | 1.22 (1.07–1.38)* | 204 | 1.16 (1.00–1.33)* | 216 | 1.05 (0.92–1.2) | 791 | 1.22 (1.14–1.31)* |
| Papillary SPTC | 96 | 2.39 (1.94–2.92)* | 204 | 1.29 (1.12–1.48)* | 161 | 1.23 (1.04–1.43)* | 155 | 1.03 (0.88–1.21) | 616 | 1.28 (1.19–1.39)* |
| Follicular SPTC | 6 | 1.21 (0.44–2.62) | 17 | 0.91 (0.53–1.45) | 13 | 0.90 (0.48–1.53) | 24 | 1.58 (1.01–2.35)* | 60 | 1.12 (0.86–1.45) |
Includes risks associated with only the most common histologies of thyroid cancer and any other thyroid histologic groups with significantly elevated SIRs. The SPTC tumors were divided into the following groups: (1) papillary thyroid cancer, (2) follicular cancer, (3) Hürthle cell cancer, (4) anaplastic cancer, (5) medullary thyroid cancer, (6) thyroid lymphoma, and (7) other, including carcinomas NOS, sarcomas, and squamous cell and neuroendocrine tumors
P <0.05
Examination of the latency trends revealed that 9 of 10 evaluated cancer types (with the exception of bladder cancer) had significantly elevated SIRs for SPTC in the first year after initial diagnosis. SIRs for SPTC were also elevated at 12–59 months after initial diagnosis for most of the evaluated cancers. The exceptions were leukemia, uterine and bladder cancers.
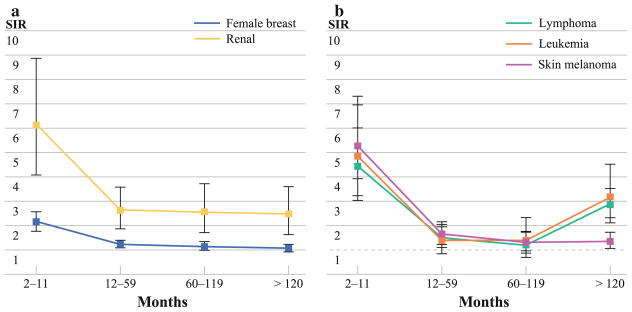
Beyond this latency period, the increased risk of SPTC persisted at 60–119 months for renal and female breast cancer (Fig. 1a). However, only renal cancers were associated with a persistently elevated SIR after 120+ months of diagnosis of the initial primary tumor.
FIG. 1.
a Latency course of risk of SPTC for tumors associated with persistently elevated SIRs >1 beyond 60 months of diagnosis of the initial primary. The error bars indicate the 95% CIs. b Latency course of risk of SPTC for tumors associated with delayed elevated SIRs of >1 beyond 60 months of diagnosis of the initial primary. The error bars indicate the 95% CIs. Note that the SIRs cross 1 after 59 months of diagnosis, then show a later increase beyond 120 months of diagnosis of the initial primary tumor
In contrast to the above primaries, leukemias, lymphoma and melanoma were associated with initial overall elevated SIRs, followed by a delayed, rather than persistent, increased risk of SPTC 120+ months after diagnosis of the initial tumor (Fig. 1b). Interestingly, uterine cancer was associated with a significantly elevated risk of SPTC only in the first year of diagnosis. This risk did not persist and in fact this was the only malignancy for which SIRs were significantly lower during prolonged follow-up.
Most of the elevated SIRs were associated with the papillary group (including the follicular variant of papillary thyroid cancer) or follicular carcinoma, except for prostate cancer which was associated with second primary medullary thyroid cancers, albeit only in the first year of diagnosis. Lymphomas and lung tumors also demonstrated elevated SIRs for other rare thyroid tumor types up to 60 months.
Tumors with Persistently Elevated SIRs
We decided to focus on the cancers with persistently elevated risks noted beyond 60 months and throughout the latency periods (renal and female breast cancers) as this trend was not noted in other malignancies. To explore the effects of radiation treatment of the primary malignancy on the development of SPTC, the SIRs were stratified by whether or not patients received or planned to receive radiation as part of the first course of therapy. As shown in Table 2, the significantly increased risk of SPTC after renal cancers was primarily observed in nonirradiated patients. Moreover, the latency and histology trends remained similar to those observed in Table 1—that is, the risk of SPTC remained elevated throughout the latency periods and this was accompanied by a significant SIR for follicular thyroid cancers in the period of 60–119 months. Very few patients received radiotherapy, and although the overall SIR for this group was elevated, most of the risk was attributable to the first year of diagnosis. Significantly increased overall risks of SPTC after breast cancers were observed in both irradiated and nonirradiated patients. In terms of the latency trends, elevated SIRs were noted in radiated patients up to 60 months of diagnosis, but the long-term elevated risks noted in Table 1 (i.e., up to 119 months after diagnosis) were seen only in the nonirradiated group.
TABLE 2.
Risk of SPTC after renal and female breast cancer, stratified by radiation treatment
| Therapy | Cancer | 2–11 months
|
12–59 months
|
60–119 months
|
120+ months
|
Total
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. observed cases |
SIR (95% CI) | No. observed cases |
SIR (95% CI) | No. observed cases |
SIR (95% CI) | No. observed cases |
SIR (95% CI) | No. observed cases |
SIR (95% CI) | ||
| Renal | |||||||||||
| Radiotherapy | SPTC | 28 | 6.13 (4.07–8.85)* | 39 | 2.61 (1.86–3.57)* | 27 | 2.56 (1.68–3.72)* | 27 | 2.46 (1.62–3.58)* | 121 | 2.95 (2.45–3.52)* |
| No | Papillary | 24 | 7.66 (4.91–11.4)* | 32 | 3.02 (2.07–4.26)* | 22 | 2.96 (1.85–4.48)* | 20 | 2.71(1.66–4.19)* | 98 | 3.43 (2.79–4.19)* |
| Follicular | 2 | 4.86 (0.59–17.55) | 3 | 2.2 (0.45–6.44) | 4 | 4.22 (1.15–0.8)* | 1 | 1.13 (0.03–6.32) | 10 | 2.78 (1.33–5.1)* | |
| Yes | Papillary | 2 | 11.19 (1.36–40.4)* | 1 | 3.94 (0.1–21.95) | 0 | 0 (0–27.1) | 1 | 2.25 (0.06–12.53) | 4 | 3.95 (1.08–10.11)* |
| Othera | Papillary | 0 | 0 (0–257.94) | 0 | 0 (0–117.36) | 0 | 0 (0–187.88) | 1 | 25.87 (0.66–144.1) | 1 | 9.61 (0.24–53.56) |
| Breast | |||||||||||
| Radiotherapy | SPTC | 114 | 2.13 (1.76–2.56)* | 257 | 1.22 (1.07–1.38)* | 204 | 1.16 (1.00–1.33)* | 216 | 1.05 (0.92–1.2) | 791 | 1.22 (1.14–1.31)* |
| No | Papillary | 45 | 2.24 (1.64–3.0)* | 107 | 1.35 (1.1–1.63)* | 93 | 1.32 (1.06–1.61)* | 106 | 1.04 (0.85–1.26) | 351 | 1.29 (1.16–1.43)* |
| Follicular | 3 | 1 (0.21–2.92) | 12 | 1.05 (0.54–1.84) | 7 | 0.77 (0.31–1.58) | 13 | 1.19 (0.63–2.03) | 35 | 1.01 (0.71–1.41) | |
| Other | 5 | 3.99 (1.29–9.31)* | 7 | 1.45 (0.58–2.98) | 3 | 0.72 (0.15–2.1) | 4 | 0.66 (0.18–1.69) | 19 | 1.16 (0.7–1.82) | |
| Yes | Papillary | 46 | 2.44 (1.79–3.25)* | 94 | 1.27 (1.02–1.55)* | 67 | 1.16 (0.9–1.47) | 47 | 1.02 (0.75–1.35) | 254 | 1.29 (1.14–1.46)* |
| Follicular | 2 | 1.08 (0.13–3.92) | 5 | 0.72 (0.24–1.69) | 6 | 1.18 (0.43–2.57) | 9 | 2.24 (1.03–4.26)* | 22 | 1.23 (0.77–1.87) | |
| Othera | Papillary | 5 | 4.02 (1.3–9.37)* | 3 | 0.7 (0.14–2.05) | 1 | 0.32 (0.01–1.78) | 2 | 0.8 (0.1–2.9) | 11 | 0.99 (0.49–1.77) |
| Follicular | 1 | 7.63 (0.19–42.51 | 0 | 0 (0–8.53) | 0 | 0 (0–12.7) | 2 | 8.98 (1.09–32.43)* | 3 | 2.79 (0.57–8.14) | |
Includes risks associated with only the most common histologies of thyroid cancer and any other thyroid histologic groups with significantly elevated SIRs. The SPTC tumors were divided into the following groups: (1) papillary thyroid cancer, (2) follicular cancer, (3) Hürthle cell cancer, (4) anaplastic cancer, (5) medullary thyroid cancer, (6) thyroid lymphoma, and (7) other, including carcinomas NOS, sarcomas, and squamous cell and neuroendocrine tumors
Refused, recommended, unknown
P <0.05
In order to further elucidate long-term risks related to these two tumors, SIRs were calculated for the reciprocal situations (i.e., thyroid cancer presenting first). Significantly increased reciprocal overall SIRs were noted for the development of both renal and female breast cancer after initial primary thyroid cancer (Table 3). For renal cancers after a thyroid primary, the SIRs followed similar latency trends as in Table 1. In addition, the risks of developing breast cancer appeared to be elevated only through the initial 119-month periods. The elevated risks did not persist 120+ months after thyroid cancer. With respect to tumor histology, while the increased risks were primarily associated with papillary and follicular thyroid cancers, there were increased SIRs associated with anaplastic and other morphologies, albeit the observed number of cases in these situations was small. Similar to the trend noted in Table 1, follicular thyroid cancers were more prominent in the later latency periods, particularly after 60 months.
TABLE 3.
Risk of renal and female breast cancer after a primary thyroid cancer, stratified by histology of thyroid cancer
| Cancer | 2–11 months
|
12–59 months
|
60–119 months
|
120+ months
|
Total
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. observed cases |
SIR (95% CI) | No. observed cases |
SIR (95% CI) | No. observed cases |
SIR (95% CI) | No. observed cases |
SIR (95% CI) | No. observed cases |
SIR | |
| Renal | 16 | 3.12 (1.78–5.06)* | 47 | 2.21 (1.63–2.94)* | 43 | 2.03 (1.47–2.74)* | 100 | 2.13 (1.73–2.59)* | 206 | 2.18 (1.89–2.5)* |
| Papillary | 12 | 3.17 (1.64–5.55)* | 40 | 2.50 (1.79–3.41)* | 29 | 1.82 (1.22–2.62)* | 74 | 2.08 (1.63–2.61)* | 155 | 2.17 (1.85–2.54) |
| Follicular | 3 | 5.48 (1.13–16.02)* | 3 | 1.27 (0.26–3.68) | 8 | 3.02 (1.3–5.95)* | 18 | 2.56 (1.52–4.04)* | 32 | 2.54 (1.73–3.58)* |
| Anaplastic | 0 | 0 (0–66.13) | 2 | 26.23 (3.18–94.75)* | 1 | 17.62 (0.45–98.2) | 0 | 0 (0–48.6) | 3 | 11.1 (2.29–32.45)* |
| Other | 1 | 7.72 (0.2–43.04) | 1 | 2.49 (0.06–13.9) | 2 | 5.55 (0.67–20.03) | 5 | 4.89 (1.59–11.4)* | 9 | 4.70 (2.15–8.92)* |
| Breast | 56 | 1.08 (0.82–1.4) | 254 | 1.18 (1.04–1.33)* | 254 | 1.19 (1.05–1.35)* | 449 | 1.02(0.92–1.12) | 1008 | 1.10 (1.03–1.17)* |
| Papillary | 50 | 1.25 (0.93–1.65) | 202 | 1.20 (1.04–1.38)* | 200 | 1.21 (1.04–1.38)* | 339 | 1.0 (0.89–1.11) | 791 | 1.11 (1.03–1.19)* |
Includes risks associated with only the most common histologies of thyroid cancer and any other thyroid histologic groups with significantly elevated SIRs. The SPTC tumors were divided into the following groups: (1) papillary thyroid cancer, (2) follicular cancer, (3) Hürthle cell cancer, (4) anaplastic cancer, (5) medullary thyroid cancer, (6) thyroid lymphoma, and (7) other, including carcinomas NOS, sarcomas, and squamous cell and neuroendocrine tumors
P <0.05
Persistent Two-way Association with Renal Cancer
The strong, long-term persistent two-way association between renal and thyroid cancer observed throughout the latency periods (and seemingly unrelated to radiation treatment) was further explored to determine if any particular histology of renal cancers was contributing to the elevated SIRs. The histologies were stratified for the reciprocal situation (i.e., renal cancer after thyroid cancer, as thyroid cancers have a better prognosis and this strategy would maximize number of cases to discern any significant associations). Most of the elevated SIRs were associated with the renal cell carcinoma histology group (Table 4), rather than other tumor types. The SIRs were also stratified by treatment received for thyroid cancer. Significantly elevated SIRs for renal cancer were seen in not only patients who underwent treatment of thyroid cancer with radio-isotopes, but also in patients who did not receive any radiation/radio-isotope therapy (Table 5). Moreover, the SIRs remained elevated throughout the latency periods in the no radiation group.
TABLE 4.
Risk of renal cancers after thyroid cancer, stratified by histology of renal tumors
| Cancer | 2–11 months
|
12–59 months
|
60–119 months
|
120+ months
|
Total
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. observed cases |
SIR (95% CI) | No. observed cases |
SIR (95% CI) | No. observed cases |
SIR (95% CI) | No. observed cases |
SIR (95% CI) | No. observed cases |
SIR (95% CI) | |
| Total | 16 | 3.12 (1.78–5.06)* | 47 | 2.21 (1.63–2.94)* | 43 | 2.03 (1.47–2.74)* | 100 | 2.13 (1.73–2.59)* | 206 | 2.18 (1.89–2.5)* |
| RCC | 14 | 3.01 (1.65–5.06)* | 46 | 2.39 (1.75–3.19)* | 42 | 2.19 (1.58–2.96)* | 95 | 2.22 (1.8–2.71)* | 197 | 2.29 (1.99–2.64)* |
Other renal cancer histologies examined include transitional cell carcinoma, other adenocarcinomas, anaplastic tumors, mesenchymal tumors, nephroblastic tumors, lymphomas, and other rare tumor types. Includes risks associated with only the histologic group with significantly elevated SIRs
RCC renal cell tumors
P <0.05
TABLE 5.
Risk of renal cancers after primary thyroid cancer, stratified by treatment for thyroid cancer
| Treatment | 2–11 months
|
12–59 months
|
60–119 months
|
120+ months
|
Total
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. observed cases |
SIR (95% CI) | No. observed cases |
SIR (95% CI) | No. observed cases |
SIR (95% CI) | No. observed cases |
SIR (95% CI) | No. observed cases |
SIR (95% CI) | |
| None | 13 | 4.75 (2.53–8.13)* | 22 | 1.86 (1.17–2.82)* | 23 | 1.84 (1.16–2.75)* | 65 | 1.89 (1.46–2.42)* | 123 | 2.0 (1.67–2.39)* |
| Radioisotopes | 1 | 0.58 (0.01–3.25) | 19 | 2.81 (1.69–4.38)* | 11 | 1.92 (0.96–3.44) | 12 | 2.66 (1.38–4.65)* | 43 | 2.30 (1.66–3.1)* |
| Recommended, unknown whether administered | 0 | 0 (0–61.91) | 0 | 0 (0–16.06) | 1 | 4.62 (0.12–25.74) | 1 | 2.76 (0.07–15.36) | 2 | 2.3 (0.28–8.32) |
| Other radiation | 2 | 3.29 (0.4–11.9) | 6 | 2.54(0.93–5.53) | 8 | 3.09 (1.33–6.08)* | 22 | 2.90 (1.82–4.39)* | 38 | 2.89 (2.05–3.97)* |
P <0.05
DISCUSSION
There have been several studies evaluating the risk of SPTCs in patients with other malignancies.3,7,8 In contrast to the published literature, our study is novel in that it focuses on the examination of the short and long-latency course of the risks rather than overall risks to examine the role of diagnosis and surveillance bias in these associated cancers. In addition, our study is the first to examine the risks as they pertain to different tumor histologies.
Our evaluation shows that many commonly diagnosed cancers are associated with an increased overall risk of SPTC, similar to the observations of other authors.3,7,8 Furthermore, in keeping with other studies, most of the elevated overall SIRs could be attributed to the increased risks noted in the first year after diagnosis of the initial malignancy. This trend was present even after excluding SPTCs diagnosed within 2 months of the primary and would thus be considered synchronous cancers. Despite this, it is still possible that some of the SPTCs observed in the first year could still be considered synchronous. Increased surveillance of these patients in the first year of diagnosis could also account for the observed elevated SIRs.
Interestingly, many malignancies continued to have significantly elevated SIRs up to 5 years after initial diagnosis. This can be explained partly by continued surveillance bias and possibly, known rare familial associations for some tumors. With respect to the latter, Malchoff et al. described an association between papillary thyroid cancer and papillary renal neoplasia in a large kindred.16 Thyroid cancers are also included in the spectrum of Familial Adenomatosis Polyposis, Cowden syndrome and Werner syndrome.17 Mutations in the CHEK2 gene, which is involved in DNA damage repair, lead to an increased predilection for multiple cancers including colon, breast, thyroid, prostate and kidney tumors.18 These rare syndromes, however, are likely to account for only a minor part of the increased risks observed in most subsequent primary studies. Although radiotherapy may be used in the treatment of several of these malignancies and the fields could involve the thyroid gland (lung, melanoma, breast), radiation effects are less likely to be seen in this latency period. Typically reported latency periods of thyroid cancer after radiation exposure are 20–30 years.19 Radiation would especially be an unlikely risk factor in tumors where the thyroid is not in the field of radiation (such as renal, prostate, uterine and colorectal cancers).
When long-term increased risks (>10 years after diagnosis) are examined, the delayed risks seen with lymphoma and leukemia are not surprising, and are most likely related to radiation treatment (whole body radiation or mantle radiation which involves the thyroid) for these malignancies.10 Similar effects may also account for the smaller delayed increases seen in the risk for skin melanoma, many of which are treated with adjuvant radiotherapy, especially in the head and neck region.
More intriguing is the persistent, rather than delayed elevated risk of SPTC in patients with primary breast and renal cancers up to and beyond 10 years after diagnosis. Although a small component of this may be accounted for by genetic syndromes, certain observations warrant consideration. Because treatment effects can have potential impacts in this latency period, we examined our data set further by evaluating the reciprocal risks of developing breast or renal cancer in patients with an initial thyroid cancer and stratifying patients by whether radiation treatment was received or planned as part of the first course of therapy. Interestingly, the risks of developing both malignancies after an initial thyroid cancer were elevated and moreover, the SIRs followed a latency course similar to that of SPTC after initial renal or breast cancer. In the case of renal cancers, few patients received external beam radiation and the majority of the increased risk for SPTC was observed in nonirradiated patients. The fact that the overall risk was elevated in the radiation group and was largely confined to the initial few months after diagnosis further speaks to the importance of examining latency trends rather than overall risks. In case of breast cancers, while increased risks of SPTC were noted in both nonirradiated and irradiated patients, the delayed risks >60 months after diagnosis were noted only in the nonirradiated group. Our results from these analyses would suggest that radiation treatment effect alone is unlikely to be the sole factor responsible for the associations seen between these malignancies.
A review of the existing studies shows that other investigators have also reported reciprocal associations of thyroid with breast and renal cancers. Ronckers et al. noted an association between thyroid cancer and cancers of the breast and kidney irrespective of which cancer occurred first.8 They also observed a significantly increased risk of a SPTC in patients with renal cancer who had received some radiation during the course of the treatment (SIR 5.51). However, similar to our findings, significantly more patients who developed a SPTC in this setting had not received any radiotherapy. In contrast to our findings, however, patients in their study who had received radiotherapy for breast cancer did not show a statistically significant overall increase in the risk of SPTC. Significantly more SPTCs were observed in patients who did not undergo radiation for breast cancer, but similar to renal cancers, the risk did not remain elevated ≥10 years after diagnosis. Although their study also used data from the 9 SEER registries, the discrepancies in our findings may be related to our longer period of follow-up. Another reciprocal study that used data from the California Cancer Registry, noted an overall SIR of 3.9 for the development of renal cancer after an initial thyroid cancer, with the elevated risks noted in the latency periods>1 year and also ≥5 years after diagnosis.3 Again, the increased risks were noted in nonirradiated patients. Sandeep et al. noted persistently elevated risks for the development of SPTC after kidney cancer or female breast for the 1–9 years and >10 years after diagnosis and vice versa.7 This study did not look at radiation effects. Brown et al. noted an SIR of 2.4 for the development of cancers of the kidney and renal pelvis after a primary thyroid cancer.2 The increased risk was noted in patients not exposed to radiotherapy. Liu et al. also noted reciprocal increased risks of renal cancers after thyroid, breast, prostate and other malignancies in their study of the Swedish Family-Cancer database.20 In all of the above studies, the association between thyroid and renal cancers seems to be the strongest. A recent meta-analysis reported that prior radioactive iodine therapy for thyroid cancer does not appear to increase the risk of subsequent renal cancers.21 Our examination of the effects of prior radioactive iodine therapy on risk of subsequent renal cancers does show delayed increased risks. However, risks for second renal cancers seems to be elevated throughout the study period in patients who did not undergo any radiation or radioisotope therapy, indicating that this factor alone is not responsible for the marked associations we have observed. Although there were no data available in SEER*Stat regarding chemotherapy, immuno-, or hormonal therapies, the observations described above, combined with our data, suggest that there are likely as yet undefined etiologic risk factors and/or treatment effect associations between thyroid, renal, and breast cancers.
To the best of our knowledge, our study is the first to examine population-based data in this setting with specific attention to tumor histology. We found that, most of the elevated SPTC risk is attributable to well-differentiated tumors of the papillary, the follicular variant of papillary thyroid cancer, or follicular types. Increased SPTC risk for medullary thyroid cancer was noted only for prostate cancer, and it was confined to the first year after diagnosis, indicating a surveillance bias effect. An increase of “other” thyroid histologies was noted for lung and lymphoma in the 12–59 month latency period; however, because the number of observed cases is relatively small, the clinical significance of these is not known. Follicular thyroid cancers, rather than papillary cancers, seem to feature more prominently in the delayed and persistent risks associated with renal cancers, however, the mechanisms underlying this association remain to be determined. With respect to renal cancers, most of the elevated risks are attributable to primary renal cell carcinomas (the most common subtype), rather than other histologic types of renal cancer.
Although our study is limited by the treatment effects that can be examined by using the SEER database and by its retrospective nature, its strengths include a large patient population with extensive follow-up and in-depth evaluation of the latency course of risks rather than only an assessment of overall risks. Our findings are important in that they may have potential implications for thyroid cancer screening in patients with certain types of malignancies. Although our data provide insights into particular cancer associations, additional research is needed to further define the possible biological and environmental mechanisms underlying them.
Footnotes
Presented in part as a poster at the annual AAES meeting, Houston, TX, April 2011.
References
- 1.Howlader N, Krapcho M, Neyman N, et al., editors. SEER cancer statistics review, 1975–2008. Bethesda, MD: National Cancer Institute; 2010. http://seer.cancer.gov/csr/1975_2008/ [Google Scholar]
- 2.Brown P, Chen J, Hitchcock YJ, Szabo A, Shrieve DC, Tward JD. The risk of second primary malignancies up to three decades after the treatment of differentiated thyroid cancer. J Clin Endocrinol Metab. 2008;93:504–15. doi: 10.1210/jc.2007-1154. [DOI] [PubMed] [Google Scholar]
- 3.Canchola AJ, Horn-Ross PL, Purdie DM. Risk of second primary malignancies in women with papillary thyroid cancer. Am J Epidemiol. 2006;163:521–7. doi: 10.1093/aje/kwj072. [DOI] [PubMed] [Google Scholar]
- 4.Rubino C, de Vathaire F, Dottorini ME, et al. Second primary malignancies in thyroid cancer patients. Br J Cancer. 2003;89:1638–44. doi: 10.1038/sj.bjc.6601319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berthe E, Henry-Amar M, Michels JJ, et al. Risk of second primary cancer following differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2004;31:685–91. doi: 10.1007/s00259-003-1448-y. [DOI] [PubMed] [Google Scholar]
- 6.Subramanian S, Goldstein DP, Parlea L, et al. Second primary malignancy risk in thyroid cancer survivors: a systematic review and meta-analysis. Thyroid. 2007;17:1277–88. doi: 10.1089/thy.2007.0171. [DOI] [PubMed] [Google Scholar]
- 7.Sandeep TC, Strachan MW, Reynolds RM, et al. Second primary cancers in thyroid cancer patients: a multinational record linkage study. J Clin Endocrinol Metab. 2006;91:1819–25. doi: 10.1210/jc.2005-2009. [DOI] [PubMed] [Google Scholar]
- 8.Ronckers CM, McCarron P, Ron E. Thyroid cancer and multiple primary tumors in the SEER cancer registries. Int J Cancer. 2005;117:281–8. doi: 10.1002/ijc.21064. [DOI] [PubMed] [Google Scholar]
- 9.Chuang SC, Hashibe M, Yu GP, et al. Radiotherapy for primary thyroid cancer as a risk factor for second primary cancers. Cancer Lett. 2006;238:42–52. doi: 10.1016/j.canlet.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Taylor AJ, Croft AP, Palace AM, et al. Risk of thyroid cancer in survivors of childhood cancer: results from the British Childhood Cancer Survivor Study. Int J Cancer. 2009;125:2400–5. doi: 10.1002/ijc.24581. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong GT, Stovall M, Robison LL. Long-term effects of radiation exposure among adult survivors of childhood cancer: results from the Childhood Cancer Survivor Study. Radiat Res. 2010;174:840–50. doi: 10.1667/RR1903.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatti P, Veiga LH, Ronckers CM, et al. Risk of second primary thyroid cancer after radiotherapy for a childhood cancer in a large cohort study: an update from the Childhood Cancer Survivor Study. Radiat Res. 2010;174:741–52. doi: 10.1667/RR2240.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Cancer Institute. Surveillance, Epidemiology, and End Results. SEER registry groupings for analyses. http://www.seer.cancer.gov/registries/terms.html.
- 14.Hedinger C, Williams ED, Sobin LH. The WHO histological classification of thyroid tumors: a commentary on the second edition. Cancer. 1989;63:908–11. doi: 10.1002/1097-0142(19890301)63:5<908::aid-cncr2820630520>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Beltran A, Scarpelli M, Montironi R, Kirkali Z. 2004 WHO classification of the renal tumors of the adults. Eur Urol. 2006;49:798–805. doi: 10.1016/j.eururo.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 16.Malchoff CD, Sarfarazi M, Tendler B, et al. Papillary thyroid carcinoma associated with papillary renal neoplasia: genetic linkage analysis of a distinct heritable tumor syndrome. J Clin Endocrinol Metab. 2000;85:1758–64. doi: 10.1210/jcem.85.5.6557. [DOI] [PubMed] [Google Scholar]
- 17.Richards ML. Familial syndromes associated with thyroid cancer in the era of personalized medicine. Thyroid. 2010;20:707–13. doi: 10.1089/thy.2010.1641. [DOI] [PubMed] [Google Scholar]
- 18.Cybulski C, Gorski B, Huzarski T, et al. CHEK2 is a multiorgan cancer susceptibility gene. Am J Hum Genet. 2004;75:1131–5. doi: 10.1086/426403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kikuchi S, Perrier ND, Ituarte P, Siperstein AE, Duh QY, Clark OH. Latency period of thyroid neoplasia after radiation exposure. Ann Surg. 2004;239:536–43. doi: 10.1097/01.sla.0000118752.34052.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H, Hemminki K, Sundquist J. Renal cell carcinoma as first and second primary cancer: etiological clues from the Swedish Family-Cancer Database. J Urol. 2011;185:2045–9. doi: 10.1016/j.juro.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Sawka AM, Thabane L, Parlea L, et al. Second primary malignancy risk after radioactive iodine treatment for thyroid cancer: a systematic review and meta-analysis. Thyroid. 2009;19:451–7. doi: 10.1089/thy.2008.0392. [DOI] [PubMed] [Google Scholar]