Abstract
Background
Recently, an Indian-origin macaque was found dead and Chromobacterium violaceum was isolated from the skin wound, and hepatic and pulmonary abscesses.
Methods
By searching the database, a total of thirteen cases of C. violaceum infection in pigtail macaques (n = 8), rhesus macaques (n = 4), and one baboon were identified from 2001 to 2010 at Tulane National Primate Research Center. Medical records were reviewed for breed, sex, age, clinical findings, treatment, outcome, bacteriology, and gross and histological findings.
Results
Seven pigtail macaques and one Indian-origin rhesus macaque died of chromobacterial septicemia. All chromobacterial septicemic pigtail macaques were adult with higher incidence in female. Hepatic abscess and thrombosis were typical findings along with pulmonary abscess and thrombosis, renal venous thromboembolism, and necrosuppurative pleuritis, peritonitis, splenitis, myocarditis, pericarditis, and meningoencephalitis. Skin wound, uterine infection, and oral and respiratory exposure were considered the points of entry for these animals.
Conclusions
This represents the first report of chromobacteriosis in pigtail, rhesus macaque, and baboon. Our experience suggests that chromobacterial infections may be more common in non-human primates than previously recognized.
Keywords: Chromobacterium violaceum, Macaca mulatta, Macaca nemestrina, Papio papio
Introduction
Chromobacterium violaceum is a Gram-negative saprophytic bacterium of soil and water in tropical and sub-tropical regions of the world [1–4]. Although the infections of humans and animals are rare, the outcomes are often fatal because of rapid septicemia with multiple abscesses, predominantly in lung, liver, and spleen [1–3]. Since C. violaceum was identified as the cause of the death in a water buffalo in the Philippines in 1905 [5] and in humans in Malaysia in 1927 [6], there have been approximately 150 cases reported in humans [7] and fewer cases reported in animals [1, 8–11].
In non-human primates, Groves first reported gibbons died because of chromobacterial infection at Malaysia National Zoo in 1969 [2]. Three more cases, thereafter, have been reported, including a Macaca assamensis transported to Atlanta from an outdoor facility in Florida [12], colobus monkeys at Great Baton Rouge Zoo in Louisiana [13], and a wild adult male howler monkey in Costa Rica [14]. In these cases, abscesses were the common finding in liver, lung, and occasionally in spleen, lymph node, and brain. This report summarizes 13 cases of chromobacterial infection from 2001 to 2010 at the Tulane National Primate Research Center (TNPRC). To the best of our knowledge, this is also the first report of chromobacteriosis in pigtail macaque (Macaca nemestrina), Chinese- and Indian-origin rhesus macaque (Macaca mulatta), and baboon (Papio papio).
Methods
A database search of the records at TNPRC for the cases of C. violaceum infection from 2001 to 2010 indentified a total of thirteen animals that included eight pigtail macaques, three Indian-origin and one Chinese-origin rhesus macaques, and one baboon. All animals were born and kept in an outdoor facility with chlorinated well-water supply at TNPRC. Medical records were reviewed for breed, sex, age, clinical findings, treatment, outcome, gross and histological findings, and bacteriology. All animals were used in this study, which received prior approval from the institutional animal care and use committee (IACUC) of the TNPRC in Covington, LA. This study was conducted within the guidelines for ethical use of animals in United States Public Health Service policy as outlined in the Guide for the Care and Use of Laboratory Animals.
Most animals had complete necropsy, and the representative tissues from major organs and lesions were collected, fixed in 10% neutral buffered formalin, embedded in paraffin blocks, sectioned at 4 lm, and stained with hematoxylin and eosin using routine techniques. Selected sections were also stained with Gram's stain. Culture swabs and aspirated samples from clinical lesions and tissue sites at necropsy were submitted for bacterial identification. Bacterial isolation was performed on Trypticase Soy Agar, Modified (TSA II) with 5% sheep blood and MacConkey II agar of BBL prepared plated media (BD Diagnostic, Sparks, MD, USA). The biochemical tests and characterizations of isolated bacteria were assayed by API-20E kit (BioMerieux, Durham, NC, USA).
Results
Clinical histories
Five pigtail macaques (Case Nos. 1, 3–5, 8) were found dead unexpectedly without histories of illnesses and evidence of external trauma. Pigtail macaque (Case No. 2) presented with bloody vaginal discharge and moderately neutrophilic leukocytosis (total WBC = 15,360 and 75.4% of neutrophils). She was treated with benzylpenicillin and found dead 2 days later. Six years prior to death, pigtail macaque (Case No. 6) had a history of traumatic laceration on dorsal head and treatment with ampicillin, but no bacterial isolation was performed at that time. She was found dead 6 years later, and pure C. violaceum was isolated from the liver at necropsy. Pigtail macaque (Case No. 7) was brought to the clinic because of hypothermia, dehydration, and pale mucous membranes. This animal was found dead 1 day later, although she was treated with benzylpenicillin. Indian-origin macaque (Case No. 9) had a focal, 1- to 2-cm-diameter skin ulcer on the left inguinal area and severe neutrophilic leukocytosis (total WBC = 31,430 and 91.5% of neutrophils). Chromobacterium violaceum and Enterococcus faecalis were isolated from the skin wound. This animal was treated with chloromycelin and cefazolin, but found dead 11 days later. A neonatal Indian-origin macaque (Case No. 10) had edema with multifocal erythematous rashes on the skin. This animal was found dead 4 days later. An adult male Indian-origin macaque (Case No. 11) had skin lacerations and ulcers on the whole body. Chromobacterium violaceum, Streptococcus faecalis, and Pseudomonas aeruginosa were isolated from the wounds. This macaque was treated with benzylpenicillin and cefazolin, but euthanized because of the poor prognosis 9 days later. A Chinese-origin macaque (Case No. 12) had multiple subcutaneous abscesses at left axillary region, and pure C. violaceum was isolated from the abscesses. This animal completely recovered after treatment with benzylpenicillin and enrofloxacin, but euthanized for unrelated reason 3 years later. A young baboon (Case No. 13) presented with multiple finger wounds containing purulent exudate. Chromobacterium violaceum, Escherichia coli, and Proteus spp. were isolated from the wounds. The animal recovered with benzylpenicillin and enrofloxacin treatment and is still alive. (Table 1) All 13 infections occurred from June to October with 77% of the incidents in July to September. A higher proportion of female pigtail macaques (six of seven) died of chromobacterial septicemia compared to the average female-to-male ratio of 2.84 for the same period of time in the colony. All septicemic pigtail macaques were adult, although chromobacterial infected animals had a wide age range from 0.1 to 13.69 years). (Table 1) The age and sex of other species were non-significant because of the small number of cases.
Table 1. Summary of C. violaceum infections in 13 non-human primates.
| Case No. | Species | Sex | Age (years) | Clinical history | Major necropsy and microscopic findings | Bacteriology | Diagnosis and outcome |
|---|---|---|---|---|---|---|---|
| 1 | Pigtail m | F | 8.51 | Found dead | Hepatic abscesses; Fibrinous peritonitis; Necrohemorrhagic splenitis | Liver: C. v; Ent. f | Septicemia |
| 2 | Pigtail m | F | 10.46 | Bloody vaginal discharge (2 days before FD) | Hepatic and pulmonary abscesses and thromboses; Fibrinous pleuritis; hemorrhagic splenitis, myocarditis, and pericarditis; Renal thromboembolism; Neutrophilic meningitis and cerebellar microabscesses | Liver: C. v Lung aspirate: C. v; E. coli; Kle. p; Str. f; Cor | Septicemia |
| 3 | Pigtail m | F | 2.64 | Found dead | Hepatic abscesses; Hepatic and pulmonary thromboses; Fibrinous pleuritis; Hemorrhagic splenitis, myocarditis and pericarditis, neutrophilic meningitis | Liver: C. v | Septicemia |
| 4 | Pigtail m | M | 10.28 | Found dead | Hepatic abscesses and thromboses; Suppurative peritonitis | Liver: C. v | Septicemia |
| 5 | Pigtail m | F | 13.64 | Found dead | Hepatic abscesses; Pulmonary thromboses; Suppurative peritonitis; Necrohemorrhagic splenitis, myocarditis, and pericarditis; Renal thromboembolism | Liver: C. v | Septicemia |
| 6 | Pigtail m | F | 13.69 | Laceration to dorsal head (6 years before FD) | Hepatic abscesses; Hepatic and pulmonary thromboses; Fibrinous pleuritis and peritonitis; Necrosuppurative splenitis, myocarditis, and pericarditis; Renal thromboses | Liver: C. v | Septicemia |
| 7 | Pigtail m | F | 5.62 | Hypothermia, dehydration (1 day before FD) | Hepatic abscesses; Pulmonary abscesses; Suppurative peritonitis & splenitis; Necrohemorrhagic myocarditis and pericarditis | Liver: C. v | Septicemia |
| 8 | Pigtail m | F | 0.02 | Found dead | Firm with red-tan patches; Necrohemorrhagic bronchopneumonia with numerous cocci; Focal hemorrhagic meningitis. | Lung: C. v; Str. p | Streptococcal pneumonia |
| 9 | Ind. m | F | 9.95 | Focal skin ulcer at left inguinal area (11 days before FD) | Hepatic and pulmonary abscesses; Pulmonary thromboses; Suppurative pleuritis; Focal skin ulcer | Liver: C. v; Lung: C. v; Ent. f Skin ulcer: C. v. | Septicemia |
| 10 | Ind. m | M | 0.01 | Erythematous rashes and edema of skin (4 days to FD) | Red, firm patches of lungs; Necrohemorrhagic bronchopneumonia with numerous cocci. | Lung: C. v; Str. p | Streptococcal pneumonia |
| 11 | Ind. m | M | 10.30 | Skin lacerations and ulcers to entire body1 (9 days to EDPG) | Purulent skin ulcers on of entire body; No septicemic findings; No tissues for histopathology. | Skin wounds: C. v.; Str. f; Pse. a | Self-trauma/Non-septicemi disease |
| 12 | Chi. m | F | 10.831 | Axillary abscesses (3 years to EDES) | No significant findings | Skin wounds: C. v. | Recovered |
| 13 | Baboon | M | 0.501 | Finger wounds with purulent exudates (6 years ago) | Not available (still alive) | Finger wounds: C. v.; E. Coli; Pro | Recovered |
Pigtail m: pigtail macaque (Macaca nemestrina); Ind. M: Indian-origin macaque (Macaca mulatta); Chi. m: Chinese-origin macaque (Macaca mulatta); Baboon (Papio papio); F: female; M: male; FD: found dead; EDPG: euthanasia because of poor prognosis; EDES: euthanasia because of end of the study; C. v: Chromobacterium violaceum; Ent. f: Enterococcus faecalis; Str p: Streptococcus pyogenes group A; Kle. p: Klebsiella pneumoniae; Str. f: Streptococcus faecalis; Cor: Corynebacterium spp; Pro: Proteus spp; Pse. a: Pseudomonas aeruginosa.
The age when C. violaceum isolated from wounds.
Gross findings
Grossly, hepatic abscess was consistently found in all septicemic macaques (Case Nos. 1–7, 9). The livers were moderately to severely friable and enlarged with round lobular edges. Hepatic surfaces were spotted with many variable-sized (2–20 mm in diameter), bulging, well-demarcated, pale-white nodules. When sectioned, similar nodules were also scattered in hepatic parenchyma, and all nodules contained abundant yellow-white thick exudate (Fig. 1). Pulmonary abscesses were noted in one pigtail macaque and one Indian-origin macaque. In addition to the abscesses, the lungs were uncollapsed, firm and had multifocal mottled dark-red patches (Fig. 2). Other common findings were multifocal to coalescing fibrinohemorrhagic suppurative pleuritis, peritonitis, and pericarditis, usually with variable amount of exudate in the cavities. Some animals (Case Nos. 8, 10–13) had no septicemic gross lesions. The lungs of two neonatal macaques (Case Nos. 8 and 10) were firm with multifocal red-tan patches. One of them had focal meningeal hemorrhage. Indian-origin macaque (Case No. 11) had multifocal purulent skin ulcers, but all other organs were grossly unremarkable. Recovered Chinese-origin macaque (Case No. 12) had no significant gross findings when presented 3 years after infection. The recovered baboon (Case No. 13) is still alive. (Table 1).
Fig. 1.
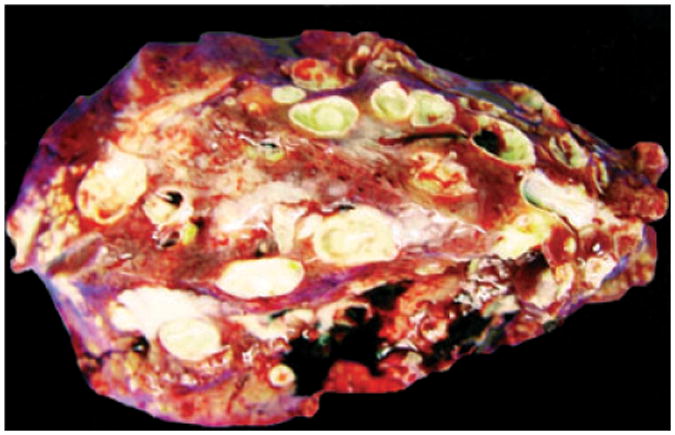
Pigtail macaque (Case No. 6). Cut surface of the liver. Multifocal variable-sized abscesses throughout liver.
Fig. 2.
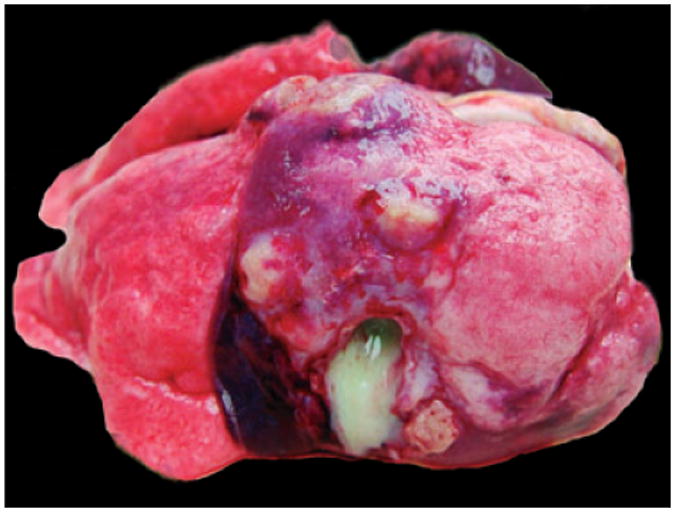
Indian-origin macaque (Case No. 8). Surface of the lung. Un-collapsed lung lobes with multifocal abscesses.
Histopathological findings
Major histopathological changes are summarized in Table 1. Seven pigtail macaques (Case Nos. 1–7) and one Indian-origin macaque (Case No. 9) died suddenly of septicemia. Hepatic abscesses were presented in all of these animals. The liver was disrupted by multifocal to coalescing, random, variable-sized, discrete areas of lytic necrosis characterized by the loss of normal hepatic architecture and replaced by characteristic abscesses, with numerous degenerated neutrophils, eosinophilic cellular and karyorrhectic debris, and admixed with numerous bacilli. Adjacent hepatic parenchyma showed variable degrees of coagulation necrosis, dilated sinusoids, edema, fibrin, and hemorrhage. Multifocal organized fibrin and bacterial thrombosis were also the common findings in the hepatic central veins (Fig. 3). Pulmonary bacterial thrombosis in the small to medium sized arteries was noted in six adult pigtail macaques and the Indian-origin macaque, although only two animals had multifocal pulmonary abscess. Moderate to severe perivascular edema, alveolar fibrin and edema, and diffuse fibrinosuppurative pleuritis and/or peritonitis were the common pulmonary findings among these animals (Fig. 4). Six animals had either multifocal hemorrhagic or necrosuppurative splenitis (Fig. 5). Two pigtail macaques showed multifocal neutrophilic meningoencephalitis with distinct cerebellar microabscess in one of them (Fig. 6). Five animals presented with multifocal, mild myocardial degeneration and necrosis admixed with neutrophils, mononuclear cells, and hemorrhage. Fibrinosuppurative pericarditis was also a common finding among these animals. Thromboembolism of renal pelvic vessels was noted in three pigtail macaques. Two neonatal macaques (Case Nos. 8 and 10) had multifocal to coalescing, severe necrosuppurative bronchopneumonia with numerous intralesional Gram-positive cocci. Indian-origin rhesus macaque (Case No. 11) had no tissues available for histopathological evaluation. There were no significant microscopic findings from Chinese-origin macaque, and the baboon is still alive. (Table 1).
Fig. 3.
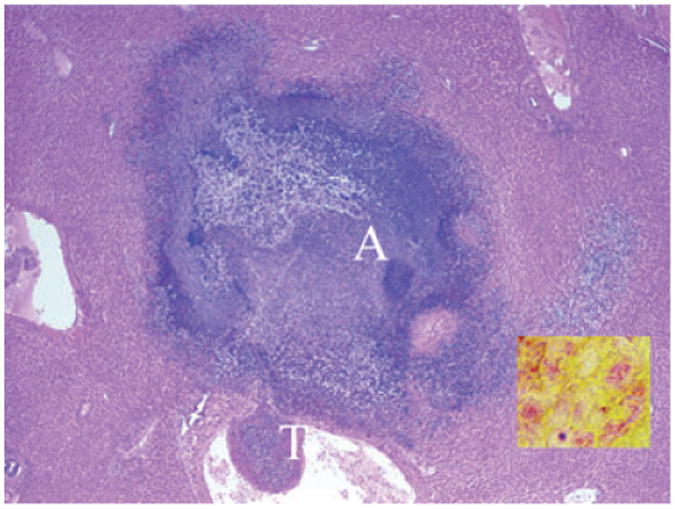
Pigtail macaque (Case No. 6). Liver. Hepatic abscess (A) and thrombosis (T). 20×, H & E stained section. Insert: Myriad intralesional Gram-negative bacilli (red-staining rods). 1000×.
Fig. 4.
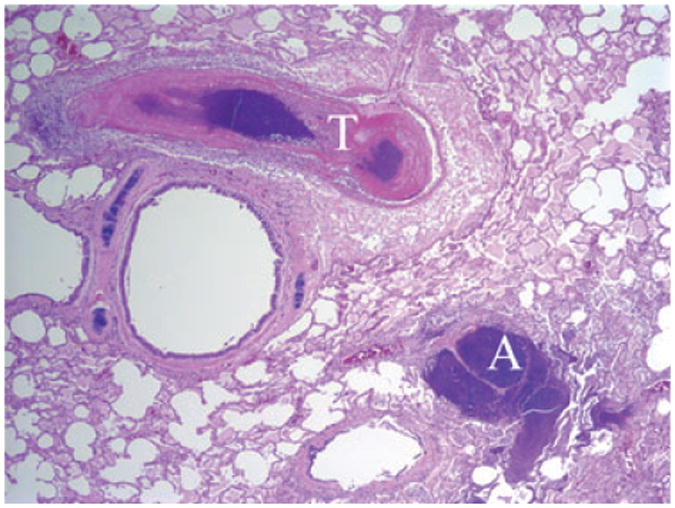
Indian-origin macaque (Case No. 8). Lung, pulmonary abscess (A) and thrombosis (T). 20×, H & E stained section.
Fig. 5.
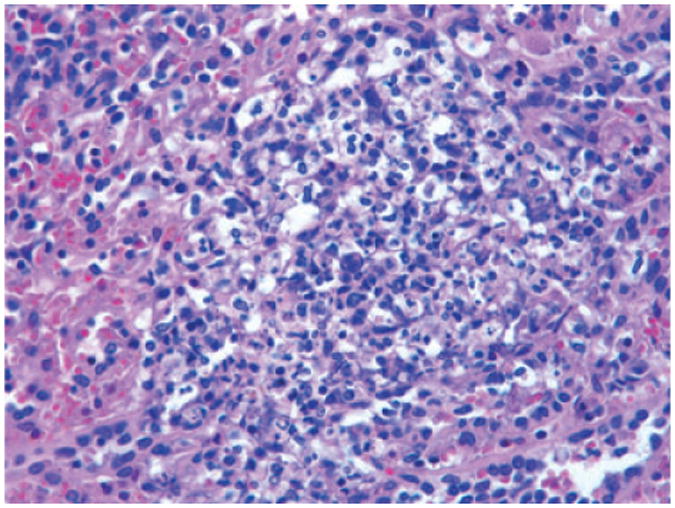
Pigtail macaque (Case No. 7). Spleen. Splenic microabscess. 400×, H & E stained section.
Fig. 6.
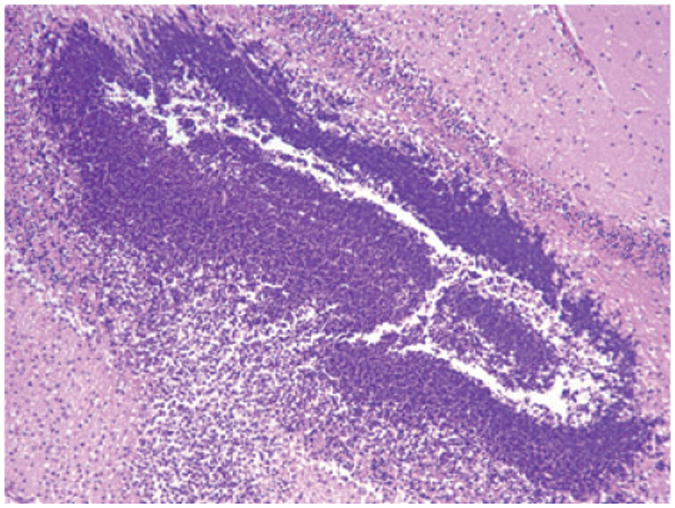
Pigtail macaque (Case No. 2). Cerebellum. Cerebellar microabscess. 100×, H & E stained section.
Bacteriology
Bacterial cultures from the samples of skin wounds, livers, and/or lungs at necropsy yielded distinctively brilliant violet pigmented bacterial colonies on TSA II (with narrow zone of beta-hemolysis) (Fig. 7) and MAC media after anaerobic incubation for 24 hours. The bacterium was 95.8% identical to C. violaceum by biochemical tests. Chromobacterium violaceum was isolated from the liver abscesses of all eight septicemic animals. Enterococcus faecalis was also isolated from the hepatic and pulmonary abscess of two animals (Case Nos. 1 and 9, respectively). Chromobacterium violaceum, S. faecalis, Escherichia coli, Klebsiella pneumoniae, and Corynebacterium spp. were isolated from the lung aspirate of a pigtail macaque (Case No. 2). Chromobacterium violaceum and Streptococcus pyogenes group A were isolated from the lungs of two neonatal animals (Case Nos. 8 and 10). Chromobacterium violaceum was isolated from head and elbow wounds 9 days before necropsy (Case No. 11). C. violaceum, S. faecalis, and P. aeruginosa were isolated from the skin wounds of Indian-origin macaque (Case No. 11). Pure C. violaceum was isolated from multiple subcutaneous abscesses of Chinese-origin macaque (Case No. 12). Chromobacterium violaceum, E. coli, and Proteus spp. were isolated from the finger wounds of the baboon (Case No. 13). (Table 1) Direct Gram stain of livers and/or lungs from all the septicemic animals demonstrated that the intralesional bacilli were Gram-negative bacteria (Insert at Fig. 3).
Fig. 7.
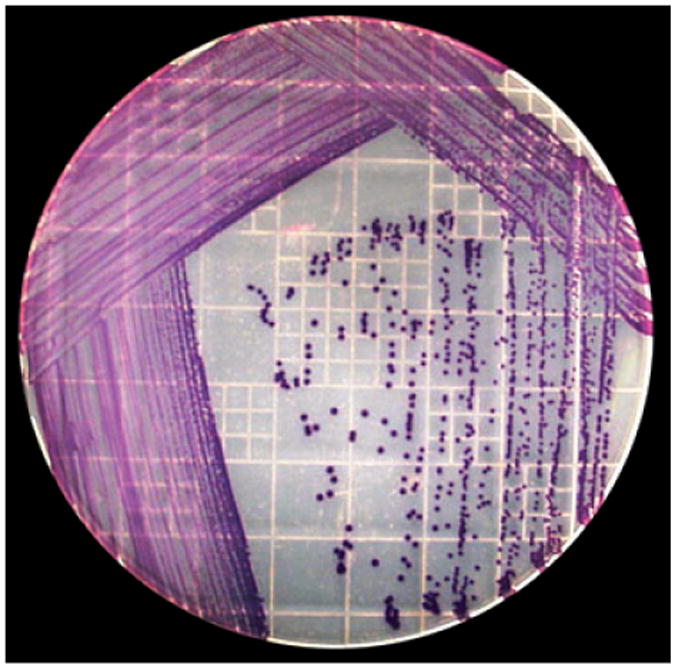
Chromobacterium violaceum isolation (From the liver of Case No. 3). Distinctive violet pigmented colonies were developed on Trypticase Soy Agar, Modified (TSA II) with 5% sheep blood media after anaerobic incubation for 24 hours at 37°C.
Discussion
Chromobacterium violaceum is a facultative, anaerobic, catalse-positive, Gram-negative motile rod-shaped bacterium. The bacteria are not acid-fast, no capsule or metachromatic granules, and non-sporulating [15]. The bacteria grow rapidly on sheep blood agar and MacConkey II agar and produce brilliant violet pigment, violacein, after anaerobic incubation for 24 hours [3, 4]. All isolates in this report were violet pigmented and 95.8% identical to C. violaceum by biochemical assays. Although E. faecalis and other bacteria were also isolated from a liver and two lungs of septicemic animals, C. violaceum was considered the primary etiology of septicemia based on the gross, histopathological finding, bacterial culture and biochemical test, and Gram stain. Bacterial contaminations and secondary involvement are also reported in animals infected by C. violaceum [1, 13]. Although both C. violaceum and Streptococcus pyogenes group A were isolated from the lungs of two neonatal animals, they likely died of streptococcal pneumonia. The diffuse fibrinohemorrhagic and necrotizing lesions with numerous Gram-positive cocci in the lungs of these two animals were consistent with the changes caused by Streptococcus in non-human primates [7].
Chromobacterium violaceum can only survive above 4°C preferring a temperature of 20–37°C [16]. Most cases have been documented to tropical and subtropical regions around the world [1, 3, 4, 12]. In the United States, chromobacteriosis had been mainly reported from the southeastern states, like Florida, Louisiana, and Texas [1, 10, 13, 17]. The main season of chromobacteriosis in humans runs from June to September in tropical and subtropical regions in the world [18]. Our data show that July to September had the highest chromobacterial incidents in our facility. Chromobacterium violaceum contains not only multiple drug and antibiotic resistance genes, like beta-lactam genes and drug efflux pump genes, but also there are many genes that enable C. violaceum to compete with other bacteria in the environment [19]. Chromobacterium violaceum has been showed to resist penicillin, ampicillin, cephalosporins, ceftazidime rifampin, vancomycin, and ciprofloxacin [19, 20]. The combination of co-trimoxazole, chloramphenicol, quinolones, or carbapenems is likely to be effective in treatment [20]. Our experience suggested a combination of benzylpenicillin and enrofloxacin was effective because a Chinese-origin adult female macaque and a young male baboon completely recovered using this combined therapy.
Common clinical presentations in most reported human cases are fever, anorexia, and often with evidence of skin trauma [3, 20]. Similar clinical signs were also noted in some animals in this study. The skin wounds were likely the route of entry for most animals in this study. A septemic pigtail macaque with 2-day history of bloody vaginal discharge suggested that the uterus was another possible portal of entry. Oral exposure to contaminated soil and water was also suspected in some animals, because systemic infections can occur following the aspiration or ingestion of contaminated water [2, 3, 13, 18]. In addition, insect transmission cannot be ruled out because C. violaceum can be isolated from the Ixodes ricinus tick [21].
In humans, most cases of chromobacteriosis worldwide are in young male patients [15, 18, 20, 21]. However, there are no clear age and sex predilection from published literatures in animals and non-human primates [2, 12–14]. Our data showed all septicemic pigtail macaques were adult. Also, the number of infected female pigtail macaques was higher than that of males with a female/male ratio of 6, which was much higher than the total average of female/male ratio (2.84) from year 2001 to 2010 in our colony.
Many important factors in the pathogeneses of chromobacteriosis have been reported in the human literature. The patients with neutrophil dysfunction in chronic granulomatous disease or severe polymorphonuclear G-6-PD deficiency have a higher susceptibility to chromobacterial infections because polymorphonuclear leukocytes and monocytes are unable to convert oxygen into oxygen metabolites that are essential for the digestion of phagocytized Gram-negative bacteria, like C. violaceum [17, 18]. No granulomatous lesions were noted in our animals, and polymorphonuclear G-6-PD deficiency was not tested in this study. Chromobacterium violaceum septicemia in patients with low CD4 T-cells and evidence in dogs with a primary parvovirus infection suggests that an immunocompromised immune system may be involved in chromobacteriosis [4, 9]. Moreover, many virulence factors of C. violaceum also determine the potential for pathogenesis. Lipopolysaccharide is a large component of the outer cell wall of Gram-negative bacteria. It activates host immune responses, but often causes septic shock by inducing host cells to produce excessive levels of inflammatory cytokines, like TNF, IL-1, and IL-12 [22]. Chromobacterium violaceum uses type IV pili for motility to aggregate bacteria, and adhere to host cells [23]. A type III secretion system in C. violaceum works like a syringe to inject effector proteins from bacteria into host cells and results in severe morphologic changes in affected host cells. Some genes of this secretion system are either homologous to Salmonella spp. or similar to Yersinia spp. [24]. Chromobacterium violaceum also produces hemolysins, colicins, and detoxification enzymes that block destruction by host defense system [24].
In summary, a total of thirteen infections of C. violaceum in non-human primates were documented in this report. Fatal septicemia of C. violaceum was observed in seven pigtail macaques and one Indian-origin macaque. The disease was typical of chromobacteriosis with hepatic abscesses in all animals. Pulmonary abscess, fibrinosuppurative pleuritis and peritonitis, and thrombosis were also common findings. All infections occurred in summer. All septicemic pigtail macaques were adult with higher incidence in females. Traumatized skin and uterine infection were considered the points of entry for some animals. Oral exposure to contaminated soil and water was also suspected. Neutrophilic leukocytosis was noted in three animals with clinical signs. However, most animals were found dead without clinical signs. Although the infections caused by C. violaceum in non-human primates are rare, the increasing incidences at our facility suggest that the infections may be more common in non-human primates than previously recognized in tropical and subtropical areas.
Acknowledgments
We thank the bacteriology, histology, pathology laboratories at TNPRC, the technicians and animal care staff at TNPRC, especially Maurice Duplantis for the necropsy, Robin Rodriguez for the image support, Kathrine Falkenstein for providing sex distribution data in our colony, and Carol Coyne for the histology. This work was supported by the TNPRC base grant P51 RR000164 from the National Institutes of Health.
This work was supported by the TNPRC base grant P51 RR000164 from the National Institutes of Health.
References
- 1.Ajithdoss DK, Porter BF, Calise DV, Libal MC, Edwards JF. Septicemia in a neonatal calf associated with Chromobacterium violaceum. Vet Pathol. 2009;46:71–4. doi: 10.1354/vp.46-1-71. [DOI] [PubMed] [Google Scholar]
- 2.Groves MG, Strauss JM, Abbas J, Davis CE. Natural infections of gibbons with a bacterium producing violet pigment (Chromobacterium violaceum) J Infect Dis. 1969;120:605–10. doi: 10.1093/infdis/120.5.605. [DOI] [PubMed] [Google Scholar]
- 3.Martinez P, Mattar S. Fatal septicemia caused by Chromobacterium violaceum in a child from Colombia. Rev Inst Med Trop Sao Paulo. 2007;49:391–3. doi: 10.1590/s0036-46652007000600011. [DOI] [PubMed] [Google Scholar]
- 4.Teoh AY, Hui M, Ngo KY, Wong J, Lee KF, Lai PB. Fatal septicaemia from Chromobacterium violaceum: case reports and review of the literature. Hong Kong Med J. 2006;12:228–31. [PubMed] [Google Scholar]
- 5.Wooley PG. Bacillus violaceum manilae (a pathogenic organism) Bull Johns Hopkins Hosp. 1905;16:89–93. [Google Scholar]
- 6.Sneath PH, Whelan JP, Bhagwan Singh R, Edwards D. Fatal infection by Chromobacterium violaceum. Lancet. 1953;265:276–8. doi: 10.1016/s0140-6736(53)91132-5. [DOI] [PubMed] [Google Scholar]
- 7.Bennett BT, Abee CR, Henrickson R. Nonhuman Primates in Biomedical Research Diseases. 2. Vol. 60. San Diego, CA: Academic Press; 1998. pp. 289–293. [Google Scholar]
- 8.Carrasco L, Astorga R, Méndez A, Maldonado A, Barazona J, Perea A. Acute pleuropneumonia in Barbary sheep (Amnotragus lervia) associated with Chromobacterium violaceum. Vet Rec. 1956;138:499–500. doi: 10.1136/vr.138.20.499. [DOI] [PubMed] [Google Scholar]
- 9.Crosse PA, Soares K, Wheeler JL, Cooke KL, Adin CA, O'Kelley JJ, Levy JK. Chromobacterium violaceum infection in two dogs. J Am Anim Hosp Assoc. 2006;42:154–9. doi: 10.5326/0420154. [DOI] [PubMed] [Google Scholar]
- 10.Dyer NW, Krogh DF, DeVold R, Wilson SL, White DG. Chromobacteriosis in a Chinese red panda (Ailurus fulgens styani) J Vet Diagn Invest. 2000;12:177–9. doi: 10.1177/104063870001200217. [DOI] [PubMed] [Google Scholar]
- 11.Sippel WL, Medina G, Atwood MB. Outbreaks of disease in animals associated with Chromobacterium violaceum. I. The disease in swine. J Am Vet Med Assoc. 1954;124:467–71. [PubMed] [Google Scholar]
- 12.McClure HM, Chang CJ. Chromobacterium violaceum infection in a nonhuman primate (Macaca assamensis) Lab Anim Sci. 1976;26:807–10. [PubMed] [Google Scholar]
- 13.Kornegay RW, Pirie G, Brown CC, Newton JC. Chromobacteriosis (Chromobacterium violaceum) in three colobus monkeys (Colobus polykomos) J Zoo Wildl Med. 1991;22:476–84. [Google Scholar]
- 14.Baldi M, Morales JA, Hernández G, Jiménez M, Alfaro A, BarqueroCalvo E. Chromobacterium violaceum Infection in a free-ranging howler monkey in Costa Rica. J Wildl Dis. 2010;46:306–10. doi: 10.7589/0090-3558-46.1.306. [DOI] [PubMed] [Google Scholar]
- 15.Sneath PH. Cultural and biochemical characteristics of the genus Chromobacterium. J Gen Microbiol. 1956;15:70–8. doi: 10.1099/00221287-15-1-70. [DOI] [PubMed] [Google Scholar]
- 16.Efthimion MH, Corpe WA. Effect of Cold Temperatures on the Viability of Chromobacterium violaceum. Appl Microbiol. 1969;17:169–75. doi: 10.1128/am.17.1.169-175.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macher AM, Casale TB, Fauci AS. Chronic granulomatous disease of childhood and Chromobacterium violaceum infections in the southeastern United States. Ann Intern Med J. 1982;97:51–5. doi: 10.7326/0003-4819-97-1-51. [DOI] [PubMed] [Google Scholar]
- 18.Ponte R, Jenkins SG. Fatal Chromobacterium violaceum infections associated with exposure to stagnant waters. Pediatr Infect Dis J. 1992;11:583–6. doi: 10.1097/00006454-199207000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Fantinatti-Garboggini F, Almeida R, Portillo Vdo A, Barbosa TA, Trevilato PB, Neto CE, Coêlho RD, Silva DW, Bartoleti LA, Hanna ES, Brocchi M, Manfio GP. Drug resistance in Chromobacterium violaceum. Genet Mol Res. 2004;3:134–47. [PubMed] [Google Scholar]
- 20.Jitmuang A. Human Chromobacterium violaceum infection in southeast Asia: case reports and literature review. Southeast Asian J Trop Med Public Health. 2008;39:452–60. [PubMed] [Google Scholar]
- 21.Stojek NM, Dutkiewicz J. Studies on the occurrence of Gram-negative bacteria in ticks: ixodes ricinus as a potential vector of Pasteurella. Ann Agric Environ Med. 2004;11:319–22. [PubMed] [Google Scholar]
- 22.McAdam AJ, Sharpe AH. Infectious Diseases. In: Kummar, Robbinss, Cotran, editors. Pathologic Basis of Disease. 7th. Vol. 2005. Philadelphia, PA: Elsevier Saunders; 2004. p. 358. [Google Scholar]
- 23.Thanassi DG, Hultgren SJ. Assembly of complex organelles: pilus biogenesis in Gram-negative bacteria as a model system. Methods. 2000;20:111–28. doi: 10.1006/meth.1999.0910. [DOI] [PubMed] [Google Scholar]
- 24.Brito CF, Carvalho CB, Santos F, Gazzinelli RT, Oliveira SC, Azevedo V, Teixeira SM. Chromobacterium violaceum genome: molecular mechanisms associated with pathogenicity. Genet Mol Res. 2004;3:148–61. [PubMed] [Google Scholar]


