Introduction
Since its first description over 20 years ago, ureteroscopy has progressed from an awkward diagnostic procedure with limited visualisation to a precise, complex surgical intervention allowing access to the entire collecting system. With advances in flexible ureterorenoscopy (fURS), retrograde intrarenal surgery has become a reality. However, acceptance and widespread use of this new technology has been and still is hampered by the fragility of instruments and high initial and maintenance/repair costs [1]. Despite these set-backs, tertiary and subspecialised stone centres have adopted this new technology, and its use is steadily increasing, competing with ESWL and percutaneous nephrolithotomy in the process [2]. In this review we highlight the new technologies of fURS.
Digital flexible technologies
Since 1988, it has been stated that the development of fURS should include improvement of the fibre-optic resolution, of the angulation capabilities, and of the working tools [3]. Current flexible ureteroscopes have a tip diameter of 6.9–9.8 F with 7.5 F most commonly used, a working channel of 3.6 F, and visualisation by fibre-optic bundles with 3400–5000 fibres [4]. The actively deflecting ureteroscopes offer increased lower-pole access by either dual-level primary and secondary deflection or by increased bidirectional primary deflection. One of the drawbacks of fURS is limited visualisation imposed by fibre-optic technology in an endoscope, as well as fragility of fibre-based optics. With use, water can leak into the lens and the fibres can burn out or fracture, resulting in a grainy image. Novel digital flexible ureteroscopes have been developed to overcome these problems.
High-definition imaging (HD)
There are two established international standards enjoying trademark protection, i.e. ‘HD ready’ and ‘full HD’. ‘HD ready’ is technically defined by a minimum image requirement of 720 horizontal lines, an image format of 16:9, and a clock rate of 50/60 Hz. ‘Full HD’ implies 1920 vertical image lines to 1280 horizontal lines, at the same format, 16:9. For comparison, a usual PC monitor has 1024 vertical to 768 horizontal lines when put on highest resolution. Only since around 2006 has HD been increasingly used in medicine [5]. HD technology is consumer-driven and was mainly used in entertainment electronics. Its transfer into medical imaging technology encountered various technical problems. Special chips had to be developed to fit onto endoscopic instruments and create an image inside the patient. Another problem was the transformation of a round (endoscopic) image into the rectangular 16:9 format. This was only possible by zooming, which lost 25–45% of the peripheral image. Initial clinical studies showed that comparing standard definition (SD) and HD endoscopes, operations were indeed performed faster and equally safe using HD. This was mainly attributed to the improvement in the depth and enhancement of the illumination of the operating field. Having established a clinical advantage of HD over SD through these studies, the industry finally, in 2007, developed a third standard, the Medical-HD. It uses an image resolution of 1280 vertical to 1024 horizontal image lines at a format of 5:4. This enables an endoscopic picture generated inside the patient to be visualised on a rectangular monitor without loss of image.
In comparison with current fibre-optic image acquisition, digital fURS improves image quality and zooming capability by up to 150%. In addition, minor image interference was noted with laser activation [4]. It is advisable to minimise the laser localisation beam because its interference with digital image capture is more marked than with conventional fibre-optic image capture.
Chip-on-the-tip technology
Together with HD imaging, distal tip sensors (‘chip-on-the-tip’) provide high-resolution images. There are two types of sensors, the charge-coupled device (CCD) and complementary metal oxide semiconductor (CMOS). CCD and CMOS image sensors are two different technologies for capturing images digitally. Each 1-mm chip has unique strengths and weaknesses, giving advantages in different applications. A CCD consists of several hundred thousand individual picture elements (pixels) on a tiny chip. Each pixel responds to light falling on it by storing a tiny charge of electricity. The pixels are arranged on a precise grid, with vertical and horizontal transfer registers carrying the signals to the camera’s video processing circuitry. This transfer of signals occurs at 60 Hz. The CCD chip provides narrow band imaging (NBI), which is thought to be able to better identify cancerous tissue [5]. The CMOS chip is a miniature device able to provide digital colour images. A CMOS chip is a type of active pixel sensor made using the CMOS semiconductor process. Extra circuitry next to each photosensor converts the light energy to a voltage. Additional circuitry on the chip can be included to convert the voltage to digital data. CMOS can potentially be implemented with fewer components, use less power and/or provide faster readout than CCDs. CCD is a more mature technology and is in most respects the equal of CMOS. CMOS sensors are less expensive to manufacture than CCD sensors [6]. CCD and CMOS imaging technologies can be applied to thin-tissue autoradiography as potential imaging alternatives to using conventional film. Compared to film, silicon-based imaging technologies have enhanced sensitivity, dynamic range and linearity [7].
The chip-on-the-tip technology eliminates the need for a camera head on the endoscope handle. Endoscopes have become about 50% lighter, which greatly increases their handling properties [8].
Light-emitting diodes (LEDs)
LEDs are so-called semiconductors that operate by electroluminescence, a phenomenon in which the emission of photons is caused by electronic excitation of a material charged with an electric current. Thus electric charge is transferred into visible light. LEDs are used in many electronic devices as indicator lamps, in automobiles as rear-window and brake lights, and on state-of-the-art endoscopic devices. LED chips are typically 250 × 250 × 250 μm in size. LEDs can be used as a light source for a short-range fibre-optic transmission system [9].
The tip of the digital flexible ureteroscope houses dual LED-driven light carriers, which obviates the need for an external light source and therefore there is no risk of drape fires or patient burns [10]. The LED light lasts up to 10,000 h, which is 10–20 times longer than xenon lights [10]. The existence of a digital camera at the tip eliminates the need for fragile low-resolution fibre-optics, and as there are no external cameras or light cables, the flexible ureteroscope is much lighter. The digital ureteroscope image is bright, with high resolution. Higher resolution allows fURS to become more safe and efficient. Thus, gone are the days of increasing black dots in the picture, reminding the surgeon of progressing fibre breakages and impending costly scope repairs.
Endoscopic protection system (EPS)
Laser damage is thought to be the main factor for the short lifespan of the ureterorenoscopes [1]. EPS is an optional feature offered in the DUR-D (ACMI-Gyrus, Southborough, MA, USA) endoscope (Fig. 1). EPS uses optical feedback from a digital sensor to terminate laser energy on retraction of the laser fibre towards the tip of the scope. The laser shut down occurs before the actively firing laser fibre enters into the endoscope, usually at 0–2 mm distance and in all the tested cases. In no case was laser damage to the endoscope reported; it was therefore deemed reliable and efficient [11]. EPS could help to prevent endoscope damage and extend the life of the endoscope, thus preventing costly repairs.
Figure 1.

The DUR-D endoscope.
A study assessed the EPS in 20 patients, where the laser was retracted into the ureteroscope four times during active firing using a fast pull (5 cm/s) and a slow pull (2 cm/s) with the ureteroscope straight and flexed [11]. The endoscope protection system was completely effective in shutting down the laser before entry into the ureteroscope in all trials. The mean length of fibre showing from the tip of the ureteroscope at shutdown was 1.5 mm when the ureteroscope was straight and 1.2 mm when it was flexed. Also, a flexible protective sheath has been evaluated, but despite reducing the amount of force required inserting the laser fibre through the working channel, it did not protect the ureteroscope from laser energy damage [12].
NBI
NBI is an optical filter technology that radically improves the visibility of capillaries, veins and other subtle tissue structures, by optimizing the absorbance and scattering characteristics of light. NBI uses two discrete bands of light: one blue, at 415 nm, and one green, at 540 nm. NB blue light shows superficial capillary networks, while green light shows subepithelial vessels, and when combined they offer an extremely high-contrast image of the tissue surface [13]. NBI can be used when CCD chip technology is employed. NBI should be particularly useful when fURS is used for tumour work, such as biopsies or laser ablation of suspicious areas within the pelvicalyceal system. However, in other areas of endoscopy NBI has not yielded the expected results [14].
Recently, Traxer et al. [15] reported that NBI significantly improved the endoscopic visualisation of tumours, providing a detailed description of their limits and vascular architecture. They performed NBI and white-light fURS in 27 patients, and concluded that NBI technology is a valuable diagnostic method, because it considerably improves tumour detection rate by 23% compared with white light.
Working tools in fURS
Influence of working tools
Instrumentation is one of the most important key issues for success in fURS [16]. The introduction of the laser fibre and other working tools such as the basket and the biopsy forceps influences the deflection angle and the irrigation flow in the flexible ureteroscope. Bach et al. [17] used a laser fibre (273 μm), biopsy forceps (2.4–3 F), and tipless nitinol baskets (1.5–2.4 F) in five flexible ureteroscopes of the latest generation. They measured the deflection angle, the lowest diameter of the bent tip, and the flow rates in the ureteroscopes. The authors found that deflection had no influence on flow rate, while the size of the basket had no influence on the maximum angle of deflection. Introduction of the laser fibre or the biopsy forceps resulted in relevant loss of deflection (laser fibre 4.4–10.2% and biopsy forceps 30.7–57.8%). The flow rates were dependent on the size of the tool used. The loss of irrigation volume varied; 53.7% for the laser fibre, 62.2% for 1.5 F tools and 99% for 3 F tools.
Stone basket
A novel nitinol stone basket prevents stone migration and facilitates simultaneous laser lithotripsy in situ. In a prospective study this basket was used in 23 patients with renal or ureteric stones [18]. The mean stone diameter was 1.4 cm, the mean fragmentation time was 44 min and no complications were encountered. All but three patients were rendered stone-free; of the three patients with residual stones, two had residual fragments of <3 mm in diameter.
Access sheath
The ureteric access sheath is commonly used to facilitate the insertion and straight alignment of the flexible ureteroscope in the upper urinary tract. Its use has been reported to facilitate ureteric re-entry and efflux of irrigation fluid, decrease operative time and cost, as well as minimise morbidity [19]. However, difficulties can be experienced when inserting the access sheath and therefore novel devices have been designed. A prospective study in 98 patients compared novel reinforced sheaths with the nonreinforced ones [20]. There was no significant difference in the overall success rates between the use of these sheaths. The sheath-specific limitations included kinking and sheath angulation/deformity. A pre-existing stent was significantly associated with statistically significant successful deployment.
Traditional ureteric access sheaths rely on tapered dilators and the principle of axial force to gain access into the ureter. Harper et al. [21] compared the performance of a novel balloon-expandable ureteric access sheath using radial dilatation (Fig. 2) with that of a conventional one. Ten pigs had the novel sheath placed (randomised) in one ureter and a conventional ureteric access sheath in the contralateral ureter, followed by videotaped URS. The novel ureteric access sheath was inserted with less maximum and average force. The flow rate during 5 min was higher in the new sheath, while withdrawal forces were not statistically different. Furthermore, the novel sheath had a lower subjective trauma scale rating.
Figure 2.
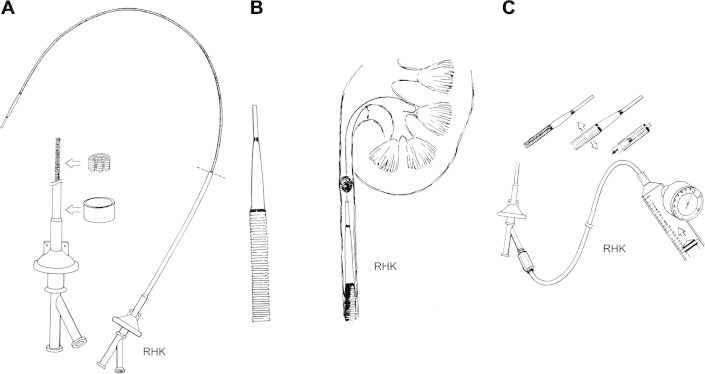
Expandable ureteric access sheath with radial dilatation: (A) the access sheath with its distal position (inset) in folded 9.5 Fr configuration (B), unexpanded sheath inserted over guide wire just below the stone (C), high pressure insufflators used to expand sheath to 2.0 MPa, allowing tip to retract into expanded sheath (inset).
Robotic fURS
A novel robotic catheter system has been developed for performing retrograde ureterorenoscopy (Fig. 3). Desai et al. [22] used remote robotic fURS bilaterally in five acute pigs. The authors used a 14 F robotic catheter system, which manipulated a passive optical fibre-scope mounted on a remote catheter manipulator. The robotic flexible ureteroscope could be successfully manipulated into 83 of the 85 (98%) calyces. The time required to inspect all calyces decreased from 15 min in the first kidney to 49 s in the last. On a visual analogue scale, the reproducibility of calyceal access was rated at 8, and instrument tip stability was rated at 10. The potential advantages of robotic fURS compared with conventional manual fURS include an increased range of motion, instrument stability, and improved ergonomics. However, relevant comparative studies are warranted, as well as refinement of this technology.
Figure 3.
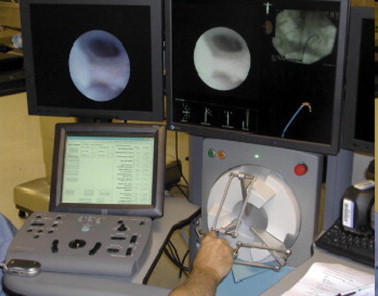
Robotic fURS system.
Semidisposable fURS
Despite improvements in instrumentation and technology in fURS, the issue of procedural and off-procedural damage remains a problem. The modular design of the semidisposable PolyScope system (Fig. 4; Lumenis, Santa Clara, CA, USA) makes it a more cost-effective option [23]. This 8 F scope has a deflexion angle of 180° and a working channel of 1.2 mm. Relatively cheap and disposable multilumen catheters preclude the need for sterilisation of the optic cable, thus decreasing the chances of handling-related damages. The chance of instrument-related infection is minimal. Besides, it can be used as a semirigid ureteroscope should the need arise [23]. Clinical studies show that the PolyScope was simple to use, effective and reliable [24]. It overcomes the inherent fragility of comparable devices, which renders the need for maintenance unnecessary.
Figure 4.

The semidisposable PolyScope.
Epilogue
Flexible ureteroscopes were developed from the limited deflectable first-generation ones, to the present digital very manoeuvrable models. The ancillary instruments and the energy sources underwent a similar development. fURS is a very useful investigative method, especially in patients with equivocal data provided by the imaging [25]. Independent, noncorporate-sponsored research will help urologists using fURS in instrumentation selection and reduce reliance on uncontrolled clinical evaluations of new products in theatres.
Footnotes
Editorial comment: In this report Papatsoris et al. review the newest technical developments in flexible ureterorenoscopy. Technological advances such as miniaturisation of ureteroscopes and improved video imaging clearly have expanded the indications for ureteroscopy . The entire upper urinary tract can now be accessed for diagnosis and treatment of many common urological conditions, and technological research and development undoubtedly will continue to drive future improvements in the technique and applications of ureterorenoscopy [26]. These advances have resulted in the developing indications for ureteroscopy. Flexible ureteroscopy with laser vaporisation has appeared to be a promising solution for local nephron-sparing treatment of low-grade smaller TCC of the upper urinary tract, and newer technical developments such as NBI have shown considerable improvements in tumour visibility and detection rate [27]. Further studies are warranted to clarify the effects of this technology on the diagnosis, recurrence rate, tumour-free survival period, and overall management of these patients. Future directions include combining NBI with molecular markers in those at high risk of recurrence and progression [27]. In many challenging patients, such as kidney stones in prepubertal children, in pregnancy, urinary diversions (antegrade approach), morbidly obese patients, pelvic kidneys, polycystic kidney disease, horseshoe kidneys, calyceal diverticula and lower-pole stones, fURS is now considered by many endourologists as the first-line treatment [26,28–30]. Although fURS is considered to have a limited role in the treatment of intrarenal calculi of >2 cm, an important transition is occurring in the treatment of proximal ureteric and selected intrarenal calculi. Larger stones are increasingly being addressed with a retrograde ureteroscopic approach. In a series of 30 patients with kidney calculi of >2 cm and treated with retrograde ureterorenoscopic holmium laser lithotripsy, 77% of patients were successfully rendered stone-free in a single procedure [31]. Other authors have reported similar results. Indeed, some groups are routinely performing ureterorenoscopy even for staghorn calculi. This indication is probably especially valid in patients who have significant comorbidities [26], including those on anticoagulants, in whom fURS can be performed safely and effectively without discontinuing the medication [32]. Thus, fURS is emerging as one of the mainstays of treatment of upper urinary calculi and other upper tract pathologies, rather than as a technique for exclusive use of the enthusiast [33]. Papatsoris et al. highlight that technological advances have been made in several areas, especially in the areas of ureteroscope design, video and imaging, endoscopic protection systems, intracorporeal lithotripters, and accessory devices. Collectively, these advances have resulted in higher stone-free rates, lower morbidity, and the ability to access and treat virtually any area of the intrarenal collecting system in most patients, including those who have anomalous or reconstructed urinary tract anatomy. However, despite the widespread use of fURS for the treatment of upper urinary tract calculi, the procedure still does not appear as first-line treatment in, for instance, the ‘Guidelines on Urolithiasis’ of the European Association of Urology [34]. This is due to the lack of well-designed controlled trials, which is highly desirable in this area. On the other hand, rapid technological developments continuously set new standards, making it difficult design meaningful trials. Improved updated knowledge of the new technologies as offered in this report by Papatsoris et al., therefore, is very important in guiding future research and clinical performance.
Palle J. Osther, MD, PhD, Professor of Urology, Chairman, EAU Section of Urolithiasis Urological Research Center, Department of Urology, University of Southern Denmark Fredericia, Denmark.
References
- 1.Sooriakumaran P., Kaba R., Andrews H.O., Buchholz N.P.N. Evaluation of the mechanism of damage of flexible ureteroscopes and suggestions for ureteroscope preservation. Asian J Androl. 2005;7:433–438. doi: 10.1111/j.1745-7262.2005.00077.x. [DOI] [PubMed] [Google Scholar]
- 2.Skolarikos A.A., Papatsoris A.G., Mitsogiannis I.C., Chatzidarellis L., Liakouras C., Deliveliotis C. Current status of ureteroscopic treatment for urolithiasis. Int J Urol. 2009;16:713–717. doi: 10.1111/j.1442-2042.2009.02364.x. [DOI] [PubMed] [Google Scholar]
- 3.Aso Y., Takayasu H., Ohta N., Tajima A. Flexible ureterorenoscopy. Urol Clin North Am. 1988;15:329–338. [PubMed] [Google Scholar]
- 4.Mitchell S., Havranek E., Patel A. First digital flexible ureterorenoscope: initial experience. J Endourol. 2008;22:47–50. doi: 10.1089/end.2007.0046. [DOI] [PubMed] [Google Scholar]
- 5.http://www.sico-med.de/cms/fileadmin/downloads/Medical%20HD-Leitartikel-final%2008407-engl.pdf [retrieved 18.12.2010].
- 6.http://www.cctvconsult.com/pages/ccd.htm [retrieved 18.12.2010].
- 7.Cabello J., Bailey A., Kitchen I., Prydderch M., Clark A., Turchetta R. Digital autoradiography using room temperature CCD and CMOS imaging technology. Phys Med Biol. 2007;52:4993–5011. doi: 10.1088/0031-9155/52/16/019. [DOI] [PubMed] [Google Scholar]
- 8.http://www.dalsa.com/corp/markets/CCD_vs_CMOS.aspx [retrieved 19.2.2011].
- 9.http://www.britannica.com/EBchecked/topic/340594/light-emitting-diode [retrieved 18.12.2010].
- 10.Andonian S., Okeke Z., Smith A.D. Digital ureteroscopy: the next step. J Endourol. 2008;22:603–606. doi: 10.1089/end.2008.0017. [DOI] [PubMed] [Google Scholar]
- 11.Xavier K., Hruby G.W., Kelly C.R., Landman J., Gupta M. Clinical evaluation of efficacy of novel optically activated digital protection system against laser energy damage. Urology. 2009;73:37–40. doi: 10.1016/j.urology.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 12.Durak E., Hruby G., Mitchell R., Marruffo F., Abundez J.O., Landman J. Evaluation of a protective laser sheath for application in flexible ureteroscopy. J Endourol. 2008;22:57–60. doi: 10.1089/end.2006.0394. [DOI] [PubMed] [Google Scholar]
- 13.http://www.keymed.co.uk/index.cfm/page/products.index.cfm/id/869/navid/869/parentid/207 [retrieved 18.12.2010].
- 14.van den Broek F.J., Fockens P., van Eeden S., Stokkers P.C., Ponsioen C.Y., Reitsma J.B. Narrow-band imaging versus high-definition endoscopy for the diagnosis of neoplasia in ulcerative colitis. Endoscopy. 2011;43:108–115. doi: 10.1055/s-0030-1255956. [DOI] [PubMed] [Google Scholar]
- 15.Traxer O., Geavlete B., de Medina S.G., Sibony M., Al-Qahtani S.M. Narrow-band imaging digital flexible ureteroscopy in detection of upper urinary tract transitional-cell carcinoma: initial experience. J Endourol. 2011;25:19–23. doi: 10.1089/end.2009.0593. [DOI] [PubMed] [Google Scholar]
- 16.Canes D., Desai M.M. New technology in the treatment of nephrolithiasis. The individual patient. Curr Opin Urol. 2008;18:235–240. doi: 10.1097/MOU.0b013e3282f51949. [DOI] [PubMed] [Google Scholar]
- 17.Bach T., Geavlete B., Herrmann T.R., Gross A.J. Working tools in flexible ureterorenoscopy – influence on flow and deflection: what does matter? J Endourol. 2008;22:1639–1643. doi: 10.1089/end.2008.0184. [DOI] [PubMed] [Google Scholar]
- 18.Kesler S.S., Pierre S.A., Brison D.I., Preminger G.M., Munver R. Use of the Escape nitinol stone retrieval basket facilitates fragmentation and extraction of ureteral and renal calculi: a pilot study. J Endourol. 2008;22:1213–1217. doi: 10.1089/end.2008.0070. [DOI] [PubMed] [Google Scholar]
- 19.Holden T., Pedro R.N., Hendlin K., Durfee W., Monga M. Evidence-based instrumentation for flexible ureteroscopy: a review. J Endourol. 2008;22:1423–1426. doi: 10.1089/end.2007.0327. [DOI] [PubMed] [Google Scholar]
- 20.Shields J.M., Tunuguntla H.S., Bhalani V.K., Ayyathurai R., Bird V.G. Construction-related differences seen in ureteral access sheaths. Comparison of reinforced versus nonreinforced ureteral access sheaths. Urology. 2009;73:241–244. doi: 10.1016/j.urology.2008.07.055. [DOI] [PubMed] [Google Scholar]
- 21.Harper J.D., Ebrahimi K.Y., Auge B.K., Lamberton G.R., Pham A.K., Zuppan C. Comparison of a novel radially dilating balloon ureteral access sheath to a conventional sheath in the porcine model. J Urol. 2008;179:2042–2045. doi: 10.1016/j.juro.2007.12.042. [DOI] [PubMed] [Google Scholar]
- 22.Desai M.M., Aron M., Gill I.S. Flexible robotic retrograde renoscopy. Description of novel robotic device and preliminary laboratory experience. Urology. 2008;72:42–46. doi: 10.1016/j.urology.2008.01.076. [DOI] [PubMed] [Google Scholar]
- 23.Bansal H., Swain S., Sharma G.K., Mathanya M., Trivedi S., Dwivedi U.S. Polyscope. A new era in flexible ureterorenoscopy. J Endourol. 2010 doi: 10.1089/end.2009.0584. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Bader M.J., Gratzke C., Walther S., Schlenker B., Tilki D., Hocaoglu Y. The PolyScope: a modular design, semidisposable flexible ureterorenoscope system. J Endourol. 2011;24:1061–1066. doi: 10.1089/end.2010.0077. [DOI] [PubMed] [Google Scholar]
- 25.Geavlete P., Multescu R., Geavlete B. Retrograde flexible ureteroscopy: reshaping the upper urinary tract endourology. Arch Esp Urol. 2011;64:3–13. [PubMed] [Google Scholar]
- 26.Beiko D.T., Denstedt J.D. Advances in ureterorenoscopy. Urol Clin North Am. 2007;34:397–408. doi: 10.1016/j.ucl.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Traxer O., Geavlete B., Gil-Diez de Medina S., Sibony M., Al-Qahtani S.M. Narrow-band imaging digital flexible ureteroscopy in detection of upper urinary tract transitional-cell carcinoma: initial experience. J Endourol. 2011;23:19–23. doi: 10.1089/end.2009.0593. [DOI] [PubMed] [Google Scholar]
- 28.Mufti U.B., Nalagatla S.K. Nephrolithiasis in autosomal dominant polycystic kidney disease. J Endourol. 2010;24:1557–1561. doi: 10.1089/end.2010.0093. [DOI] [PubMed] [Google Scholar]
- 29.Molimard B., Al-qahtani S., Lakmichi A., Sajiny M., Gil-Diez de Medina S., Carpentier X. Flexible ureterorenoscopy with holmium laser in horseshoe kidneys. Urology. 2010;76:1334–1337. doi: 10.1016/j.urology.2010.02.072. [DOI] [PubMed] [Google Scholar]
- 30.Sejiny M., Al-Qahtani S., Elhaous A., Molimard B., Traxer O. Efficacy of flexible ureterorenoscopy with holmium laser in the management of stone-bearing caliceal diverticula. J Endourol. 2010;24:961–967. doi: 10.1089/end.2009.0437. [DOI] [PubMed] [Google Scholar]
- 31.El-Anany F.G., Hammouda H.A., Elakkad M.A. Retrograde ureteropyeloscopic holmium laser lithotripsy for large renal calculi. BJU Int. 2001;88:850–853. doi: 10.1046/j.1464-4096.2001.01248.x. [DOI] [PubMed] [Google Scholar]
- 32.Turna B., Stein R.J., Smaldone M.C., Santos B.R., Kefer J.C., Jackman S.V. Safety and efficacy of flexible ureterorenoscopy and holmium:YAG lithotripsy for intrarenal stones in anticoagulated cases. J Urol. 2008;179:1415–1419. doi: 10.1016/j.juro.2007.11.076. [DOI] [PubMed] [Google Scholar]
- 33.Smith R.D., Patel A. Impact of flexible ureterorenoscopy in current management of nephrolithiasis. Curr Opin Urol. 2007;17:114–119. doi: 10.1097/MOU.0b013e328028fe0c. [DOI] [PubMed] [Google Scholar]
- 34.Türk C, Knoll T, Petrik A, Sarica K, Seitz C, Straub M, et al. Guidelines on urolithiasis. European Association of Urology; 2010. http://www.uroweb.org/publications/eau-guidelines/.


