Abstract
Objective To compare the benefit and harm of restrictive versus liberal transfusion strategies to guide red blood cell transfusions.
Design Systematic review with meta-analyses and trial sequential analyses of randomised clinical trials.
Data sources Cochrane central register of controlled trials, SilverPlatter Medline (1950 to date), SilverPlatter Embase (1980 to date), and Science Citation Index Expanded (1900 to present). Reference lists of identified trials and other systematic reviews were assessed, and authors and experts in transfusion were contacted to identify additional trials.
Trial selection Published and unpublished randomised clinical trials that evaluated a restrictive compared with a liberal transfusion strategy in adults or children, irrespective of language, blinding procedure, publication status, or sample size.
Data extraction Two authors independently screened titles and abstracts of trials identified, and relevant trials were evaluated in full text for eligibility. Two reviewers then independently extracted data on methods, interventions, outcomes, and risk of bias from included trials. random effects models were used to estimate risk ratios and mean differences with 95% confidence intervals.
Results 31 trials totalling 9813 randomised patients were included. The proportion of patients receiving red blood cells (relative risk 0.54, 95% confidence interval 0.47 to 0.63, 8923 patients, 24 trials) and the number of red blood cell units transfused (mean difference −1.43, 95% confidence interval −2.01 to −0.86) were lower with the restrictive compared with liberal transfusion strategies. Restrictive compared with liberal transfusion strategies were not associated with risk of death (0.86, 0.74 to 1.01, 5707 patients, nine lower risk of bias trials), overall morbidity (0.98, 0.85 to 1.12, 4517 patients, six lower risk of bias trials), or fatal or non-fatal myocardial infarction (1.28, 0.66 to 2.49, 4730 patients, seven lower risk of bias trials). Results were not affected by the inclusion of trials with unclear or high risk of bias. Using trial sequential analyses on mortality and myocardial infarction, the required information size was not reached, but a 15% relative risk reduction or increase in overall morbidity with restrictive transfusion strategies could be excluded.
Conclusions Compared with liberal strategies, restrictive transfusion strategies were associated with a reduction in the number of red blood cell units transfused and number of patients being transfused, but mortality, overall morbidity, and myocardial infarction seemed to be unaltered. Restrictive transfusion strategies are safe in most clinical settings. Liberal transfusion strategies have not been shown to convey any benefit to patients.
Trial registration PROSPERO CRD42013004272.
Introduction
Transfusion of red blood cells are often used to treat anaemia or bleeding in a variety of patient groups.1 2 3 Recent results of randomised clinical trials4 5 6 7 8 have favoured restrictive transfusion strategies and elucidated potential harm with liberal transfusion strategies. Data from several newly published randomised controlled trials9 10 11 12 13 warrant an up to date review of the available evidence comparing the effects of different transfusion thresholds to inform on the benefits and harms of transfusion strategies guiding red blood cell transfusion. A Cochrane review identified 19 randomised controlled trials including 6264 patients.14 Most of the data on mortality were from the Transfusion Requirements in Critical Care (TRICC) trial4 (52%) and Transfusion Trigger Trial for Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair (FOCUS) trial (23%),15 underlining the somewhat limited evidence base for guiding the use of red blood cells.16
We carried out a systematic review including data from the latest published randomised controlled trials and used conventional meta-analysis to compare the effects of different transfusion strategies on important outcomes in various patient groups. We were particularly interested to examine whether the evidence supported a restrictive strategy without harm to patients.
Methods
Our systematic review was conducted according to the protocol previously published in the PROSPERO register (www.crd.york.ac.uk/PROSPERO). The methodology and reporting were based on recommendations from the Cochrane Collaboration17 and the preferred reporting items for systematic reviews and meta-analyses statement,18 and evaluated according to the GRADE (grading of recommendations assessment, development, and evaluation) guidelines.19
Eligibility criteria
We considered prospective randomised controlled trials to be eligible for inclusion if red blood cell transfusions were administered on the basis of a clear transfusion “trigger” or “threshold,” defined as a specific haemoglobin or haematocrit level. Comparator group patients were required to be either transfused at higher haemoglobin or haematocrit levels than the intervention group or transfused in accordance with current transfusion practices. We considered for inclusion trials that included surgical or medical patients and adults or children, but excluded trials conducted on neonates and children with low birth weight.
All randomised controlled trials were eligible irrespective of language, blinding, publication status or date, or sample size. We excluded quasirandomised trials for assessment of benefit but considered them for inclusion for assessment of harm.
Search strategy
We identified relevant randomised controlled trials through an up to date systematic search strategy used in a published Cochrane review14; in the Cochrane central register of controlled trials, SilverPlatter Medline (1950 to October 2014), SilverPlatter Embase (1980 to October 2014), and Science Citation Index Expanded (1900 to October 2014). To identify any planned, unreported, or ongoing trials we contacted the main authors of included trials and experts in this discipline. We reviewed the references of included trials to identify additional trials. Moreover, we identified ongoing clinical trials and unpublished trials through Current Controlled Trials, ClinicalTrials.gov, and www.centerwatch.com (see supplementary appendix 1 for detailed information on the search strategy).
Trial selection
Authors (LB, MWP, and NH) independently reviewed all titles and abstracts identified through the systematic search. They excluded trials that did not fulfil the eligibility criteria and evaluated the remaining trials in full text. Disagreements were resolved with JW.
Data extraction
The researchers were not masked to the author, institution, and publication source of trials at any time. Using preprepared extraction forms the researchers (LBH, NH, or MWP) independently extracted the characteristics of the trials (single or multicentre, country), baseline characteristics of the patients (age, sex, disease severity), inclusion and exclusion criteria, the description of intervention (thresholds, duration), and outcomes. When information was unclear or missing we contacted the corresponding authors of the relevant trials.
Predefined primary outcomes were mortality and overall morbidity, defined by authors as one or more complications, overall complications, or any adverse event (if not reported, we included the most common complication). Secondary outcomes were adverse events (transfusion reactions, cardiac events—for example, myocardial infarction, cardiac arrest, acute arrhythmia, angina), renal failure, thromboembolic events, infections, haemorrhagic events, stroke, or transitory cerebral ischaemia. We also registered the proportion of patients transfused with allogeneic or autologous red blood cells, and the number of allogeneic and autologous blood units transfused. Haemoglobin or haematocrit levels during intervention and length of hospital stay were regarded as process variables and thus reported as trial characteristics.
Risk of bias assessment
According to recommendations from the Cochrane Collaboration17 we reviewed the major domains of bias (random sequence generation, allocation concealment, blinding of participants and staff, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, baseline imbalance, sponsor bias (bias related to funding source), and academic (whether authors had published other trials in the same field of research) in all trials. We categorised trials with low risk of bias as those with a lower risk of bias in all domains except blinding because blinding of trigger guided transfusion is generally not feasible. All other trials were categorised as unclear or at high risk of bias.
Grading quality of evidence
We assessed the quality of evidence for mortality, overall morbidity, and fatal and non-fatal myocardial infarction according to GRADE methodology19 for risk of bias, inconsistency, indirectness, imprecision, and publication bias; classified as very low, low, moderate, or high.
Statistical analysis
All statistical analyses were performed using Review Manager (RevMan) version 5.3.3 (Nordic Cochrane Centre, Cochrane Collaboration) and trial sequential analysis program version 0.9 beta (www.ctu.dk/tsa).20 For all included trials we report relative risks (95% confidence intervals) for dichotomous outcomes and mean differences (95% confidence intervals) for continuous outcomes. We pooled these measures in meta-analyses.
If data from two or more trials were included in analysis of an outcome, we used random effects20 and fixed effect models21 for meta-analyses. We report the results from both models if there was discrepancy between the two; otherwise we report results from the random effects model. Heterogeneity among trials was quantified with inconsistency factor (I2) or (D2) statistics22 and by χ2 test, with significance set at a P value of 0.10. We did sensitivity analyses by applying continuity adjustment in trials with zero events.17
For risk of bias we performed predefined subgroup analyses (lower versus high or unclear risk) and we emphasise the results from the trials with lower risk of bias,17 patient populations (adults versus children; surgical versus medical), length of follow-up (≤90 days versus >90 days), and transfusion product (leucocyte reduced versus non-leucocyte reduced red blood cell suspensions). Only subgroup analyses showing a statistically significant test of interaction (P<0.05) were considered to provide evidence of an intervention effect. We preplanned exploration of moderate to high heterogeneity using metaregression, including mean age and fraction of men as covariates if possible. However this was not feasible owing to missing values of the covariates in the included trials, but we performed a post hoc subgroup analysis, stratifying trials according to clinical setting. There were no data to support the predefined subgroup analysis of randomised trials of patients with sepsis compared with patients without sepsis.
Meta-analyses may result in type I errors owing to an increased risk of random error when sparse data are analysed23 and due to repeated significance testing when a cumulative meta-analysis is updated with new trials.20 24 To assess the risk of type I errors we applied trial sequential analysis to cumulative meta-analysis. Trial sequential analysis combines an estimation of information size (cumulated sample size of included trials) with an adjusted threshold for statistical significance20 25 in the cumulative meta-analyses.26 The latter, termed trial sequential monitoring boundaries, adjusts the confidence intervals and reduces type I errors. When the cumulative z curve crosses the trial sequential monitoring boundary, a sufficient level of evidence for the anticipated intervention effect may have been reached and no further trials are needed. If the z curve does not cross any of the boundaries and the required information size has not been reached, evidence to reach a conclusion is insufficient. We calculated information size as a diversity adjusted required information size,27 suggested by the diversity of the intervention effect estimates among the included trials.
The required information size was calculated based on a relative risk reduction of 15% in mortality and overall morbidity and a relative risk reduction of 50% in myocardial infarction. We appropriately adjusted all trial sequential analyses for heterogeneity (diversity adjustment) according to an overall type I error of 5% and a power of 80%, considering early and repetitive testing.
Results
Trial selection
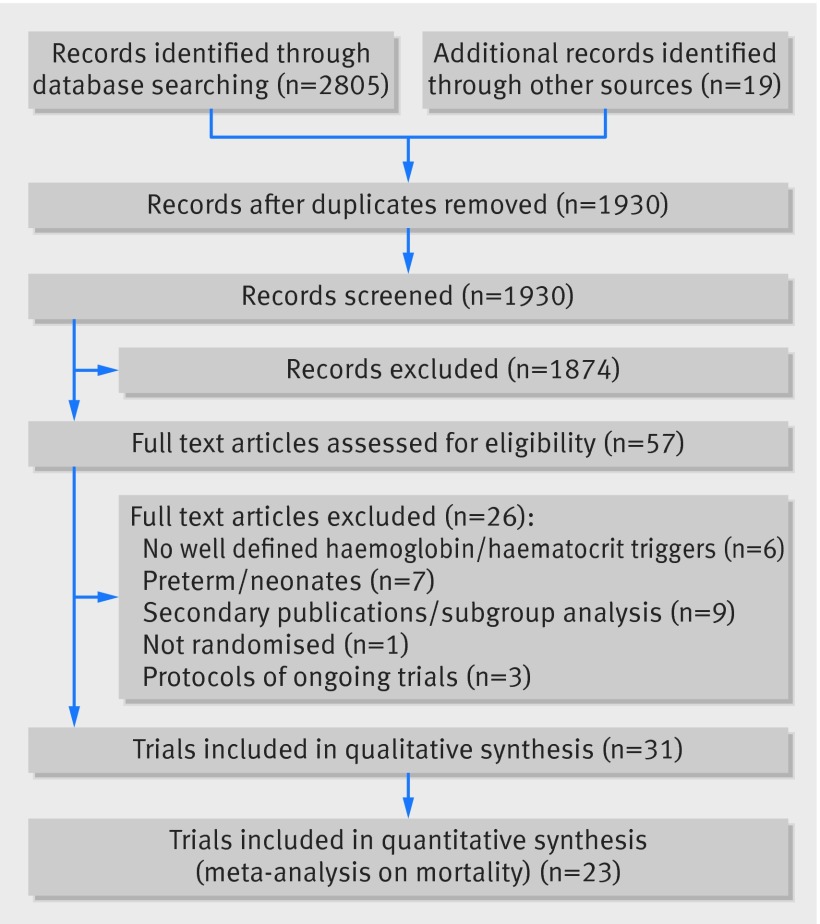
In the updated systematic search strategy we identified an additional 1930 records, of which 38 were assessed in full text for eligibility to supplement the former 19 published randomised controlled trials. In total we found 33 eligible records published, all in English, between October 1986 and October 2014, describing 31 trials of 9813 patients.4 5 6 7 8 9 10 11 12 13 15 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 72 73 Three identified records provided data from the same trial.45 46 47 We excluded a total of 26 records,46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 the primary reasons being a lack of well defined haemoglobin or haematocrit levels guiding the intervention (six records),48 49 50 51 52 53 the inclusion of preterm or very low birth weight neonates (seven records),54 55 56 57 58 59 60 71 and secondary publications or subgroup analyses (nine records).46 47 60 61 62 63 64 65 66 Three records related to ongoing trials.67 68 69 Figure 1 summarises the results of the search strategy.
Fig 1 Flow of trials through study
Characteristics of trials
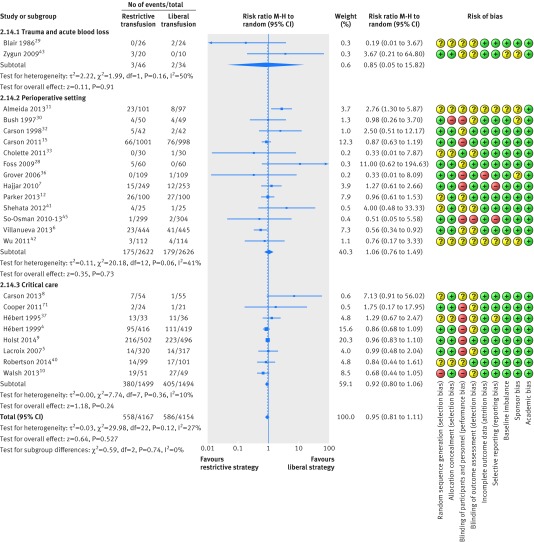
We included both single (17 trials)6 7 11 12 28 29 30 31 33 34 35 38 39 41 42 43 72 and multicentre (14 trials)4 5 8 9 10 13 15 32 36 37 40 44 45 73 randomised controlled trials. Population sizes ranged from 2534 to 2016,15 and eight trials included more than 500 patients.4 5 6 7 9 13 15 45 The clinical settings of most of the randomised controlled trials were perioperative and acute blood loss (20 trials),6 7 11 12 13 15 30 31 32 33 34 35 36 38 39 41 42 45 72 critical care (eight trials),4 5 8 9 10 37 40 73 and trauma (two trials),29 43 and one trial included patients with leukaemia undergoing stem cell transplantation.44 Table 1 summarises the characteristics of the included trials.
Table 1.
Characteristics of included trials
| Reference | Source | Country | No of patients/No of trial sites | Inclusion period | Clinical setting | RBCs (type/suspension/leucocyte reduced) | Storage age* | Protocol violations† | Intervention trigger value‡ |
|---|---|---|---|---|---|---|---|---|---|
| Almeida 201311 | Critical Care | Brazil | 198/1 | Jan 2012-Dec 2012 | Patients with cancer undergoing major abdominal surgery requiring postoperative ICU care | NA/NA/NA | NA | NA | R: 7, L: 9 |
| Blair 198629 | Brirish Journal of Surgery | UK | 50/1 | NA | Surgical patients with gastrointestinal bleeding | Allogen/citrate/NA | NA | NA | R: 8 or persistent shock, L: 2 units |
| Bracey 199931 | Transfusion | USA | 428/1 | Feb 1997-Nov 1997 | First time elective CABG surgery | Allogen/NA/NA | NA | NA | R: 8 or predefined clinical condition, L: 9 |
| Bush 199730 | American Journal of Surgery | USA | 99/1 | Aug 1995-Nov 1996 | Elective aortic or infrainguinal arterial reconstruction | Allogen/NA/NA | NA | R: 3, L: 2 | R: 9, L: 10 |
| Carson 199832 | Transfusion | USA/Scotland | 84/4 | Mar 1996-Mar 1997 | Patients with primary hip fracture | Allogen/NA/NA | NA | R: 4/42, L: 1/42 | R: 8 or symptoms of anaemia, L: 10 |
| Carson 201115 | New England Journal of Medicine | USA/Canada | 2016/47 | May 2003-Oct 2009 | Primary with hip fracture and CVD or risk of CVD | Allogen/NA/leucocyte reduced (R: 88.6%, L: 90.2%) | R: 22.1 (9.9), L: 22.0 (9.5) | R: 56/1007, L: 91/1006 | R: 8 or symptoms of anaemia, L: 10 |
| Carson 20138 | American Heart Journal | USA | 110/8 | Mar 2010-May 2012 | Patients with coronary syndrome or stable coronary artery disease undergoing catheterisation | Allogen/NA/leucocyte reduced (R: 92%, L: 95%) | R: 24.6 (9.1), L: 23.4 (10.9) | R: 1/55, L: 5/55 | R: 8 or symptoms of anaemia, L: 10 |
| Cholette 201133 | Pediatric Critical Care | USA | 60/1 | Aug 2006-Sep 2009 | Infants and children with single ventricle physiology undergoing cavapulmonary bypass | Allogen/NA/leucocyte reduced | NA | R: 0/30, L: 0/30 | R: 9 with symptoms, L: 13 |
| Cooper 201171 | American Journal of Cardiology | USA | 45/2 | May 2003-Oct 2009 | Acute myocardial infarction | Allogen/NA/leucocyte reduced | NA | NA | R: haematocrit <24 (24-27)%, L: 30 (30-33)% |
| De Gast-Bakker 201335 | Intensive Care Medicine | Netherlands | 107/1 | Apr 2009-Jan 2012 | Infants and children undergoing elective heart surgery for congenital heart defect | Allogen/NA/leucocyte reduced | R: 9.8 (6.8), L: 9.8 (7.2) | R: 3/53, L: 4/54 | R: 8.0, L: 10.8 |
| Gregersen 201372 | European Geriatric Medicine | Denmark | 160/1 | NA | Patients with hip fracture | NA/NA/NA | NA | NA | R: 9.7, L: 11.3 |
| Fortune 198734 | Journal of Trauma | USA | 25/1 | NA | Traumatic pts with haemorrhagic shock (class 3-4) | Allogen/NA/NA | NA | NA | R: haematocrit <30%, L: <40% |
| Foss 200928 | Transfusion | Denmark | 120/1 | Feb 2004-Jul 2006 | Patients with primary hip fracture | Allogen/NA/leucocyte reduced | NA | NA | R: 8.0, L: 10.0 |
| Grover 200636 | Vox Sanguinis | UK | 260/3 | NA | Elective total knee or hip arthroplasty | Allogen/NA/leucocyte reduced | NA | NA | R: 8 and maintained between 8.0 and 9.5, L: <10 and maintained between 10.0 and 12.0 |
| Hajjar 20107 | Journal of the American Medical Association | Brazil | 502/1 | Feb 2009-Feb 2010 | Elective CABG and/or valve replacement | Allogen/citrate/non-leucocyte reduced | NA | R: 0/255, L: 1/257 | R: haemoatocrit <24%, L: <30% |
| Hébert 199537 | Journal of the American Medical Association | Canada | 69/25 | Mar 1993-Jan 1994 | Euvolaemic, critically ill patients | Allogen/NA/non-leucocyte reduced | NA | R: 2/33, L: 2/36 | R: 7, L: 9 |
| Hebert 19994 | New England Journal of Medicine | Canada | 838/25 | Nov 1994-Nov 1997 | Euvolaemic, critically ill patients | Allogen/citrate/non-leucocyte reduced | NA | R: 6/418 (crossover: 4/418), L: 18/420, (crossover: 11/420) | R: 7.0 (7.0-9.0), L: 10.0 (10.0-12.0) |
| Holst 20149 | New England Journal of Medicine | Scandinavia | 1005/32 | Dec 2011-Dec 2013 | Septic shock | Allogen/SAGM/leucocyte reduced | NA | R: 45/463, L: 16/470 | R: 7, L: 9 |
| Johnson 199238 | Journal of Thoracic and Cardiovascular Surgery | USA | 39/1 | NA | Elective revascularisation | Allogen and autologous/NA/non-leucocyte reduced | NA | R: 1/21, L: 0/18 | R: haematocrit <25%, L: <32% |
| Lacroix 20075 | New England Journal of Medicine | Canada/USA/UK/Belgium | 648/19 | Nov 2001-Aug 2005 | Stable, critically ill infants and children | Allogen/NA/leucocyte reduced | R: 16.0 (10.5), L: 15.7 (10.3) | R: 1/320, L: 10/317 | R: 7 (8.5-9.5), L: 9.5 (11.0-12.0) |
| Lotke 199939 | Journal of Arthroplasty | USA | 152/1 | NA | Total knee arthroplasty | Allogen and autologous/NA/non-leucocyte reduced | NA | NA | R: 9.0, L: 2 units |
| Parker 201312 | Injury | England | 200/1 | NA | Patients with primary hip fracture | NA/NA/NA | NA | NA | R: symptoms of anaemia,§ L: 10 |
| Prick 201313 | British Journal of Obstetrics and Gynaecology | Netherlands | 521/37 | May 2004-Feb 2011 | Patients with sustained postpartum haemorrhage | Allogen/NA/NA | NA | R: 33, L: 7 | R: symptoms of anaemia¶, L: 8.9 |
| Robertson 201440 | Journal of the American Medical Association | USA | 200/2 | May 2006-Aug 2012 | Patients with closed head injury | NA/NA/leucocyte reduced | NA | R: 4/99, L: 0/101 | R: 7, L: 10 |
| Shehata 201241 | Transfusion | Canada | 50/1 | Jan 2007-Jun 2010 | Patients with elective cardiac surgery | Allogen/NA/NA | NA | R: 16%, L: 59% | R: 70 g/L perioperatively and 75 g/L postoperatively, L: 95 g/L perioperatively and 100 g/L postoperatively |
| So-Osman 201045 | Vox Sanguinis | Netherlands | 619/3 | 2001-2003 | Primary elective hip or knee replacement | Allogen/NA/leucocyte reduced | NA | NA | R: new restrictive transfusion policy related to cardiopulmonary comorbidity, time since surgery, and including centre L: standard of care |
| Villanueva 20136 | New England Journal of Medicine | Spain | 921/1 | NA | Patients with upper gastrointestinal bleeding | Allogen/NA/leucocyte reduced | NA | R: 39/444, L: 15/445 | R: 7, L: 9 |
| Walsh 201310 | Critical Care Medicine | England | 100/6 | Aug 2009-Dec 2010 | Mechanically ventilated patients in ICU | Allogen/SAGM/leucocyte reduced | NA | R: 2/51, L: 3/49 | R: 7, L: 9 |
| Webert 200844 | Transfusion | Canada | 60/4 | Mar 2003-Oct 2004 | Patients with acute leukaemia undergoing stem cell transplantation | Allogen/AS-3/leucocyte reduced | NA | R: 24/29, L: 28/31 | R: 80 g/L, L: 120 g/L |
| Wu 201142 | ESICM 24th annual congress 2011 | China | 226/1 | NA | Orthotopic liver transplantation | NA/NA/NA | NA | NA | R: 7 (7-9), L: 10 (10-12) |
| Zygun 200943 | Neurological Critical Care | England | 30/1 | Jan 2003-Jul 2005 | Severe traumatic brain injury | Allogen/NA/NA | NA | NA | R: 8.0 (2 units transfused), L1: 9.0 (2 units transfused), L2: 10.0 (2 units transfused) |
RBC=red blood cells; ICU=intensive care unit; NA=not available; R=restrictive; L=liberal; CABG=coronary artery bypass graft; CVD=cardiovascular disease; SAGM=erythrocyte storage in hypertonic conservation medium; ESCIM=European Society of Intensive Care Medicine.
*Values are mean (standard deviation) days unless otherwise specified.
†Values are proportions of patients unless otherwise specified.
‡Haemoglobin levels are reported in g/dL unless stated otherwise.
§Symptoms of anaemia included recurrent vasovagal episodes on mobilisation, chest pain of cardiac origin, congestive cardiac failure, unexplained tachycardia, hypotension or dyspnoea due to anaemia, decreased urine output unresponsive to fluid replacement, and any other symptoms believed appropriate by the medical staff looking after the patient.
¶Symptoms of anaemia defined as dyspnoea or syncope.
Intervention
In 24 trials,4 5 [8-7] 9 10 12 13 28 30 31 32 33 34 35 36 37 40 41 43 44 45 73 patients received allogeneic red blood cells, and among these two trials also allowed the use of autologous transfusion.38 39 For the remaining five trials there was no information on the type of red blood cells used.11 12 40 42 72 Leucocyte reduced red blood cells were transfused in 12 trials,5 6 9 10 28 33 35 36 40 45 46 73 and partially leucocyte reduced red blood cells were administered in two trials.8 15 Non-leucocyte reduced red blood cells were used in five trials,4 7 37 38 39 and information was not provided for the remaining 12 trials.11 12 13 29 30 31 32 34 42 43 72
The intervention trigger value varied between trials. The triggers for restrictive transfusion ranged from haemoglobin 7.0 to 9.7 g/dL, haematocrit 24% to 30%, or symptoms of anaemia as defined by the authors. The triggers for liberal transfusion ranged from haemoglobin 9 to 13 g/dL and haematocrit 30% to 40%.
Risk of bias assessment
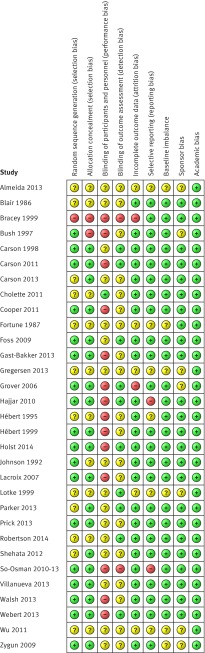
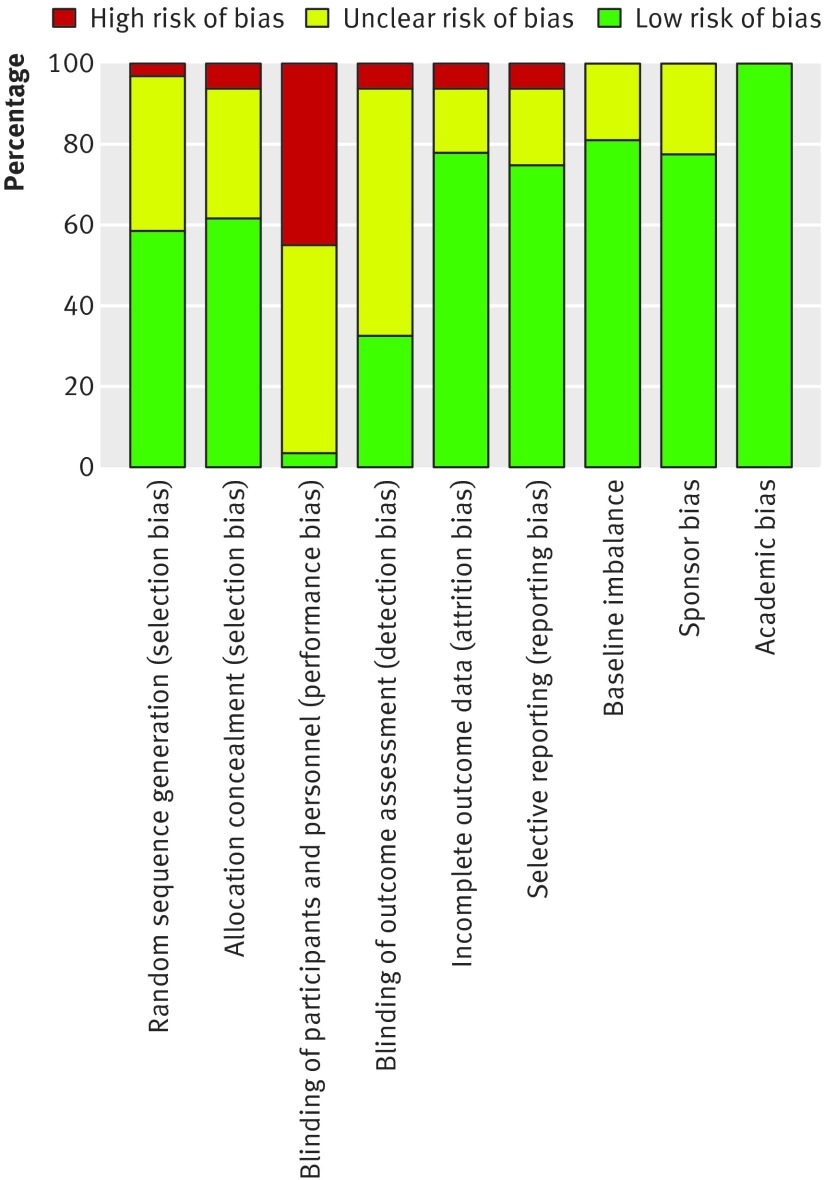
Table 2 provides detailed information on blinding. Overall, 12 randomised controlled trials were categorised as at lower risk of bias,4 5 6 9 10 13 14 15 28 32 35 73 14 as unclear,8 11 12 29 33 34 37 38 39 40 41 42 43 72 and five as at high risk of bias.7 30 31 36 45 Figures 2 and 3 summarise the risks of bias.
Table 2.
Summary of reported blinding procedure in included trials to supplement ROB table (figure 2.) on blinding procedure, not assessed in overall evaluation of trial bias domains owing to feasibility issues
| Reference | Patient | Clinical/trial staff | Outcome assessor |
|---|---|---|---|
| Almeida 201311 | Not available | Not available | Not available |
| Blair 198629 | Not available | Not available | Not available |
| Bracey 199931 | Not blinded | Not blinded | Not blinded |
| Bush 199730 | Not available | Surgeons/anaesthesiologists not blinded; clinical staff not available | Not available |
| Carson 199832 | Not available | Not available | Study nurses obtaining subjective (functional status, place of residence) and objective outcomes (60 day survival status) were blinded for intervention during follow-up by telephone |
| Carson 201115 | Not blinded | Not blinded | Study nurses obtaining subjective (functional status, place of residence) and objective outcomes (60 day survival status) were blinded for intervention during follow-up by telephone |
| Carson 20138 | Not available | Not available | Composite outcome of death and myocardial infarction; study nurses obtaining subjective (functional status, place of residence) and objective outcomes (myocardial infarction, unstable angina, 60 day survival status) were blinded for intervention during follow-up by telephone |
| Cholette 201133 | Not blinded | Operation staff blinded perioperatively; clinical staff not blinded postoperatively | Outcome assessor not available; data and safety monitoring committee blinded |
| Cooper 201171 | Not blinded | Not blinded | Not available |
| Fortune 198734 | Not available | Not available | Not available |
| Foss 200928 | Blinded | Not available | Physiotherapist assessing ambulation blinded |
| De Gast-Bakker 201335 | Not blinded | Not blinded | Not blinded |
| Gregersen 201372 | Not available | Not available | Not available |
| Grover 200636 | Blinded | Surgeons/anaesthesiologists not blinded; clinical staff not available | Holter monitor assessor blinded |
| Hajjar 20107 | Blinded | Anaesthesiologists/intensive care unit clinicians blinded; surgeons not available | Outcome assessor blinded; data and safety monitoring committee not available |
| Hébert 199537 | Not blinded | Not blinded | Not available |
| Hébert 19994 | Not blinded | Not blinded | Outcome assessor not available; data and safety monitoring committee blinded |
| Holst 20149 | Not blinded | Not blinded | Outcome assessor; statisticians and data and safety monitoring committee blinded |
| Johnson 199238 | Not available | Surgeons/anaesthesiologists not blinded; clinical staff not available | Not available |
| Lacroix 20075 | Not blinded | Not blinded | Outcome assessor not available; statisticians and data and safety monitoring committee blinded |
| Lotke 199939 | Not available | Not available | Blinded |
| Parker 201312 | Not available | Not available | Study nurses assessing mobility score blinded |
| Prick 201313 | Not available | Not available | Not available |
| Robertson 201440 | Not available | Not blinded | Trial investigators not blinded; outcome assessors blinded |
| Shehata 201241 | Not available | Not available | Not available |
| So-Osman 201045 | Not blinded | Surgeons/clinicians not blinded | Assessor and study investigators blinded; study nurses not blinded |
| Villanueuva 20136 | Not blinded | Not blinded | Not blinded |
| Walsh 201310 | Blinded | Not blinded | Not available |
| Webert 200844 | Not blinded | Not blinded | Study staff assessing bleeding and adjudication committee blinded |
| Wu 201142 | Not available | Not available | Not available |
| Zygun 200943 | Not available | Not available | Not available |
Fig 2 Risk of bias summary for all included records
Fig 3 Risk of bias graph
Clinical outcomes
Mortality
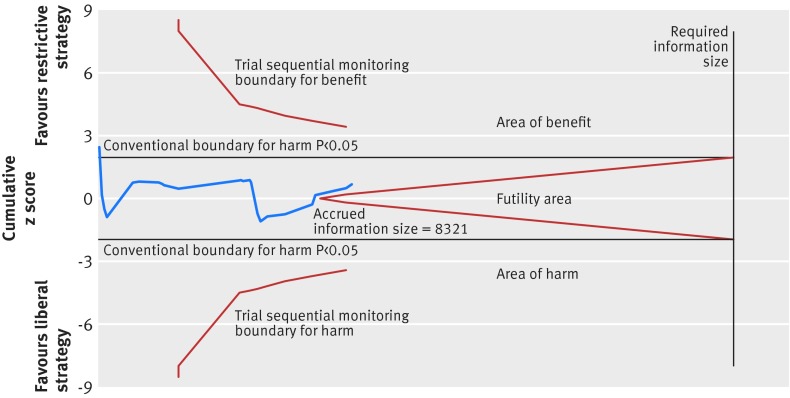
Data on mortality were provided in 23 trials (8321 patients),4 5 6 7 8 9 10 11 12 15 28 29 30 32 33 36 37 40 41 42 43 73 but few trials followed the patients for 90 days or more.8 9 10 12 37 40
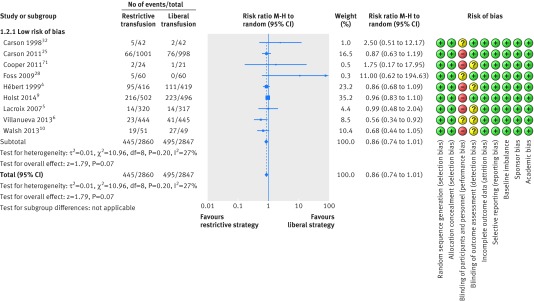
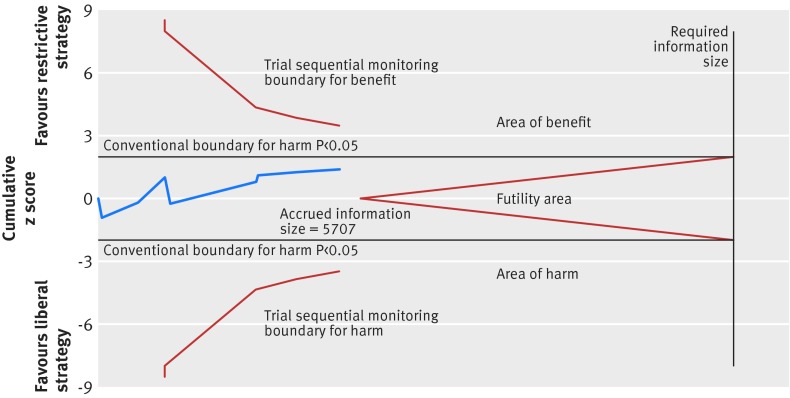
A total of nine trials with 5707 randomised patients were included in the analysis of mortality in trials with lower risk of bias (fig 4),4 5 6 9 10 15 28 32 73 showing a relative risk of 0.86 (95% confidence interval 0.74 to 1.01; P=0.07; I2=27%) for restrictive versus liberal transfusion; the GRADE quality was judged to be low (table 3). The trial sequential analysis adjusted 95% confidence interval was 0.67 to 1.12 (fig 5).
Fig 4 Forest plot of mortality in lower risk of bias trials. Size of squares for risk ratio reflects weight of trial in pooled analysis. Horizontal bars represent 95% confidence intervals
Table 3.
Summary of findings including GRADE quality assessment of evidence trials with lower risk of bias
| Variables | No of participants (No of studies) | No with event/No in group (%) | Relative risk (95% CI) | Absolute effect | Quality of the evidence (GRADE) | Quality assessment domains | |
|---|---|---|---|---|---|---|---|
| Restrictive transfusion group | Liberal transfusion group | ||||||
| All cause mortality, longest follow-up, low risk of bias trials | 5707 (9) | 445/2860 (15.6) | 495/2847 (17.4) | Random effects 0.86 (0.74 to 1.01); I2=27%; trial sequential analysis adjusted 95% CI 0.67 to 1.12 | 24 fewer per 1000 (from 45 fewer to 2 more) | Low; critical importance | Inconsistency: not serious*; indirectness: not serious; imprecision: serious†; reporting bias: reporting bias‡ |
| Overall morbidity, lower risk of bias trials | 4517 (6) | 858/2261 (37.9) | 897/2256 (39.8) | Random effects 0.98 (0.85 to 1.12); I2=60%; trial sequential analysis adjusted 95% CI 0.81 to 1.19 | 8 fewer per 1000 (from 60 fewer to 48 more) | Very low; critical importance | Inconsistency: serious§; indirectness: not serious; imprecision: serious¶; reporting bias: reporting bias‡ |
| Fatal and non-fatal myocardial infarction in lower risk of bias trials | 4730 (7) | 59/2369 (2.5) | 43/2361 (1.8) | Random effects 1.28 (0.66 to 2.49); I2=34%; trial sequential analysis adjusted 95% CI 0.40 to 4.13 | 6 more per 1000 (from 6 fewer to 27 more) | Very low; critical importance | Inconsistency: serious**; indirectness: not serious; imprecision: very serious††; reporting bias: reporting bias‡ |
GRADE Working Group grades of evidence: low quality=further research is likely to have an important impact on confidence in estimate of effect and is likely to change the estimate; very low quality=very uncertain about the estimate.
Quality assessment domains: inconsistency=unexplained heterogeneity of results; indirectness=differences in population, intervention, comparator, and outcome measures; imprecision=relatively few patients and few events resulting in wide confidence intervals; reporting bias=publication bias is a systematic under-estimation or over-estimation of underlying beneficial or harmful effect owing to selective publication of trial results.
*I2=27%, P=0.20for heterogeneity, overlap of confidence intervals.
†Anticipation of 15% relative risk reduction results in trial sequential analysis adjusted confidence intervals, including >25% relative risk reduction or >25% relative risk increase. However, <15% relative risk reduction or relative risk increase may also be considered clinically relevant and these are apparently not excluded in any analyses.
‡Possibility for publication bias according to funnel plot owing to smaller trials showing benefit for restrictive transfusion strategy.
§I2=60%, P=0.03 for heterogeneity, overlap of confidence intervals.
¶Two trials showed no effect and appreciable harm with restrictive transfusion strategy.
**I2=34%, P=0.11 for heterogeneity, variance in point estimates, from 0.25 to 2.97.
††6 of 7 trials showed no effect and appreciable harm with restrictive transfusion strategy.
Fig 5 Trial sequential analysis of nine trials with lower risk of bias reporting all cause mortality, control event proportion of 17.4%, diversity of 56%, α of 5%, power of 80%, and relative risk reduction of 15%. The required information size of 14 217 has not been reached and none of the boundaries for benefit, harm, or futility has been crossed, leaving the meta-analysis inconclusive of a 15% relative risk reduction. The trial sequential analysis adjusted 95% confidence interval for a relative risk of 0.86 is 0.67 to 1.12
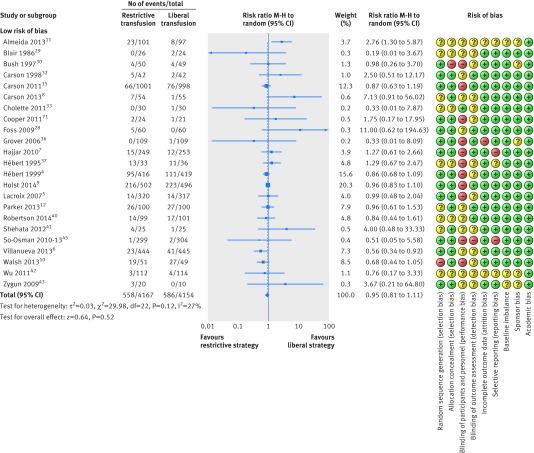
Restrictive versus liberal transfusion strategies did not affect the relative risk of death (0.95, 95% confidence interval 0.81 to 1.11; P=0.52; I2=27%), including all trials despite risk of bias (fig 6); the GRADE quality was judged to be low (table 4). The trial sequential analysis adjusted 95% confidence interval was 0.74 to 1.21 (fig 7). Figure 8 shows the results of meta-analysis on mortality in trials stratified by clinical setting.
Fig 6 Forest plot of mortality despite risk of bias. Size of squares for risk ratio reflects weight of trial in pooled analysis. Horizontal bars represent 95% confidence intervals
Table 4.
Summary of findings including GRADE quality assessment of evidence, all trials
| Variables | No of Participants (studies) | No of events/No in group (%) | Relative risk (95% CI) | Absolute effect | Quality of the evidence (GRADE) | Quality assessment domains | |
|---|---|---|---|---|---|---|---|
| Restrictive transfusion group | Liberal transfusion group | ||||||
| All cause mortality, longest follow up, all trials | 8321 (23) | 558/4167 (13.4) | 586/4154 (14.1) | Random effects 0.95 (0.81 to 1.11); I2=27%); trial sequential analysis adjusted 95% CI 0.74 to 1.21 | 7 fewer per 1000 (from 27 fewer to 16 more) | Very low; critical importance | Risk of bias: very serious*; inconsistency: not serious†; indirectness: not serious; imprecision: serious‡; reporting bias |
| Overall morbidity, all trials | 5975 (12) | 1070/2982 (35.9) | 1084/2993 (36.2) | 1.06 (0.93 to 1.21); I2=58% | 22 more per 1000 (from 25 fewer to 76 more) | Very low; critical importance | Risk of bias: very serious§; inconsistency: not serious¶; indirectness: not serious; imprecision: serious**; reporting bias: reporting bias†† |
| Fatal and non-fatal myocardial infarction, all trials | 6501 (16) | 145/3259 (4.4) | 137/3248 (4.2) | 1.05 (0.82 to 1.36); I2=6% | 2 more per 1000 (from 8 fewer to 15 more) | Low; critical importance | Risk of bias: very serious‡‡; inconsistency: not serious§§; indirectness: not serious; imprecision: serious¶¶; reporting bias: none |
GRADE Working Group grades of evidence: low quality=further research is likely to have an important impact on confidence in estimate of effect and is likely to change the estimate; very low quality=very uncertain about the estimate.
Quality assessment domains: inconsistency=unexplained heterogeneity of results; indirectness=differences in population, intervention, comparator, and outcome measures; imprecision=relatively few patients and few events resulting in wide confidence intervals; reporting bias=publication bias is a systematic under-estimation or over-estimation of underlying beneficial or harmful effect owing to selective publication of trial results.
*Overall 8 lower, 10 unclear, and 5 high risk of bias trials; limitations for more than one criterion; no blinded trials; assessor outcome not important for all cause mortality so only one level downgrade.
†I2=27% , P=0.12 for heterogeneity, overlap of confidence intervals.
‡Anticipation of 15% relative risk reduction results in trial sequential analysis adjusted confidence intervals including >25% relative risk reduction or >25% relative risk increase. However, <15% relative risk reduction or relative risk increase may also be considered clinically relevant and these are apparently not excluded in any analyses.
§Overall 6 lower, 4 unclear and 2 high risk of bias trials; limitations for more than one criterion; possible assessment bias as all trials are unblended.
¶I2=58% and P=0.006 for heterogeneity.
**Five of 12 trials showing no effect and appreciable harm with restrictive transfusion strategy.
††Possibility for publication bias according to funnel plot owing to smaller trials showing benefit for restrictive transfusion strategy.
‡‡Overall 5 lower, 5 unclear, and 5 high risk of bias trials.
§§ I2=11% and P=0.33 for heterogeneity.
¶¶10 trials showing no effect and appreciable benefit or harm with restrictive transfusion strategy.
Fig 7 Trial sequential analysis of 23 trials (despite risk of bias) reporting mortality, with control event proportion of 13.7%, diversity of 62%, α of 5%, power of 80%, and relative risk reduction of 15%. The required information size of 20 799 is far from reached and none of the boundaries for benefit, harm, or futility has been crossed, leaving the meta-analysis inconclusive of a 15% relative risk reduction. The trial sequential analysis adjusted 95% confidence interval for a relative risk of 0.95 is 0.74 to 1.21
Fig 8 Forest plot of mortality in trials stratified by clinical setting. Size of squares for risk ratio reflects weight of trial in pooled analysis. Horizontal bars represent 95% confidence intervals
Overall morbidity
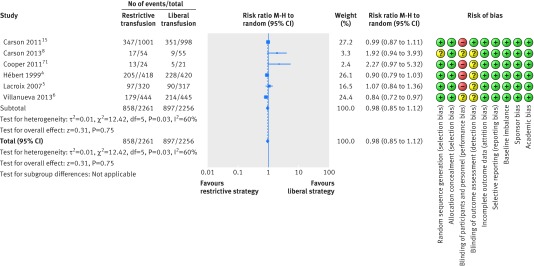
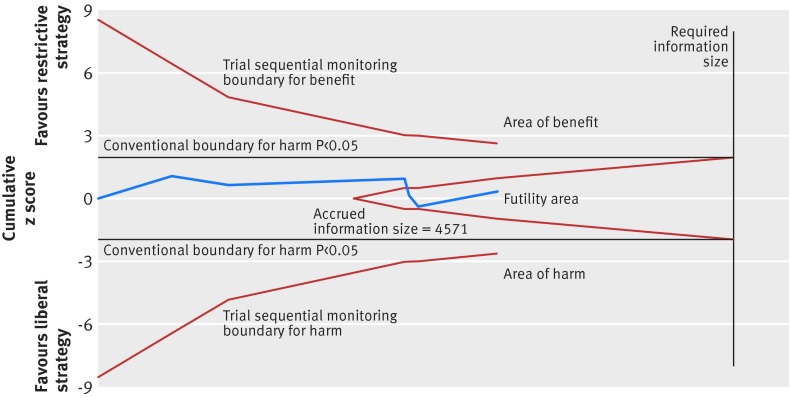
A total of six trials with lower risk of bias including 4517 patients were included in the meta-analysis of overall morbidity (fig 9).4 5 6 8 15 73 Overall morbidity did not differ between the restrictive and liberal transfusion strategies (relative risk 0.98, 95% confidence interval 0.85 to 1.12; P=0.75; I2=60%) and the trial sequential analysis adjusted 95% confidence interval was 0.81 to 1.19. Future trials are unlikely to show a 15% relative risk reduction in favour of restrictive or liberal strategies as the boundary for futility was crossed (fig 10). The GRADE quality of evidence was judged to be very low (table 3).
Fig 9 Forest plot of overall morbidity in low risk of bias trials. Size of squares for risk ratio reflects weight of trial in pooled analysis. Horizontal bars represent 95% confidence intervals
Fig 10 Trial sequential analysis of six trials reporting overall morbidity, a control event proportion of 40%, diversity of 75%, α of 5%, power of 80%, and relative risk reduction of 15%. The required information size of 7188 has not been reached, but the boundaries for futility are crossed, leaving out the possibility of a 15% relative risk reduction. The trial sequential analysis adjusted 95% confidence interval for a relative risk of 0.98 is 0.81 to 1.19
A total of 12 trials with 5975 randomised patients were included in the meta-analysis of overall morbidity regardless of risk of bias (relative risk 1.06, 95% confidence interval 0.93 to 1.21; P=0.36; I2=58%).4 5 6 7 8 15 35 37 39 41 46 73
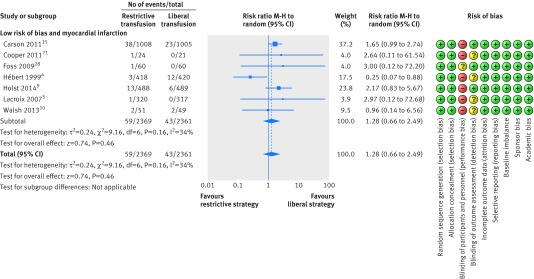
Fatal or non-fatal myocardial infarction
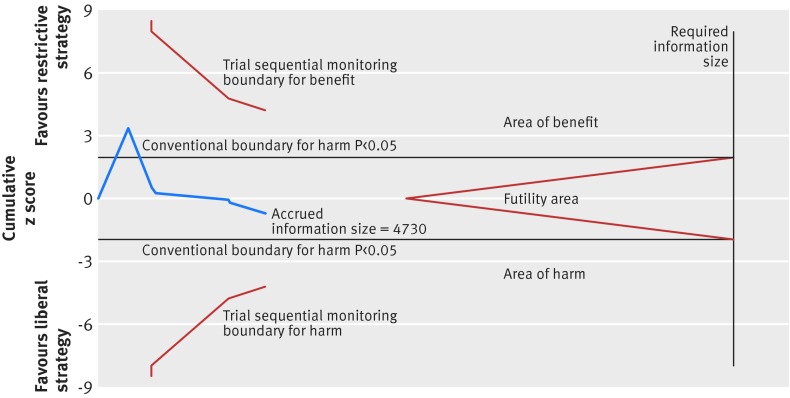
Seven trials assessing fatal or non-fatal myocardial infarction including 4730 patients were defined as trials with lower risk of bias.4 5 9 10 15 28 73 Restrictive transfusion strategies were not associated with a relative risk reduction or relative risk increase in fatal or non-fatal myocardial infarction (relative risk 1.28, 95% confidence interval 0.66 to 2.49; P=0.46; I2=34%) (fig 11) and the trial sequential analysis adjusted 95% confidence interval was 0.40 to 4.31 (fig 12). The GRADE quality of evidence was judged to be very low (table 3). A total of 16 trials with 6501 randomised patients were included in the meta-analysis of fatal or non-fatal myocardial infarction regardless of risk of bias (1.05, 0.82 to 1.36; P=0.70; I2=6%); the GRADE quality of evidence was judged to be low (table 4).4 5 7 8 9 10 15 28 30 31 36 38 39 40 41 73
Fig 11 Forest plot of myocardial infarctions in low risk of bias trials. Size of squares for risk ratio reflects weight of trial in pooled analysis. Horizontal bars represent 95% confidence intervals
Fig 12 Trial sequential analysis of seven trials reporting myocardial infarction, with a control event proportion of 1.8%, diversity of 62.3%, α of 5%, power of 80%, and relative risk reduction of 50%. The diversity adjusted required information size of 13 686 is far from reached and none of the boundaries for benefit, harm, or futility has been crossed, leaving the meta-analysis inconclusive of even a 50% relative risk reduction. The trial sequential analysis adjusted 95% confidence interval for a relative risk of 1.28 is 0.40 to 4.13
Other adverse events
A total of eight trials defined as lower risk of bias with 5107 patients were included in the meta-analysis on infectious complications. Our analysis showed an association in favour of using a restrictive transfusion strategy (relative risk 0.73, 95% confidence interval 0.55 to 0.98, P=0.03, I2=53%) (see supplementary appendix 2).4 5 6 13 15 28 32 35 The inclusion of all 15 trials (7217 patients) regardless of risk of bias did not alter the result (0.79, 0.64 to 0.97, P=0.03, I2=40%).4 5 6 7 8 13 15 28 31 32 35 36 40 41 46 Our analysis showed no association of restrictive versus liberal transfusion with other adverse events (cardiac complications, renal failure, thromboembolic stroke or transitory ischaemic insult, or haemorrhage) (see supplementary appendices 3 and 4).
Number of patients and units transfused
A total of 24 trials with 8923 patients were included in the meta-analysis of the proportion of patients receiving red blood cells (relative risk 0.54, 95% confidence interval 0.47 to 0.63; P<0.001; I2=95%), and a total of 12 trials with 4022 patients were included in the meta-analysis of the number of units transfused (mean difference −1.43, 95% confidence interval −2.01 to −0.86; P<0.001; I2=96%) both showing lower numbers associated with restrictive versus liberal transfusion strategies (see supplementary appendices 5 and 6).
Discussion
We did not find any association with mortality, overall morbidity, or myocardial infarction when comparing restrictive transfusion strategies with liberal transfusion strategies; however, the overall quality of evidence was low. We performed trial sequential analysis to account for sparse data and repetitive testing on accumulating data and found that the 95% confidence intervals of the point estimates widened and the results supported those obtained in the conventional meta-analyses. In our analysis of all cause mortality, the cumulative z curve did not cross any boundaries, with only 40% of the required information size being reached (5707 of 14 217 patients), indicating that further trials are needed to establish firm evidence. In our analysis of all trials, the trial sequential analysis indicated that it is unlikely that future trials will show overall harm with restrictive transfusion strategies. Regarding overall morbidity, we found no association with benefit or harm between groups, but the trial sequential analysis indicated it would be futile to obtain more trial data on this outcome. We found that the trial sequential analysis of pooled risk of fatal or non-fatal myocardial infarction was inconclusive, because only 26% of the required information size was obtained. Regarding infectious complications, our analysis indicated a possible association between a restrictive transfusion strategy and reduced rates of infection across the different clinical settings.
Relation to other reviews
Well conducted systematic reviews with meta-analysis on red blood cell transfusion have been published. A Cochrane review indicated that restrictive transfusion strategies were not associated with the rate of adverse events (that is, mortality, cardiac events, stroke, pneumonia, and thromboembolism) compared with liberal transfusion strategies. Restrictive transfusion strategies were associated with a reduction in hospital mortality (relative risk 0.77, 95% confidence interval 0.62 to 0.95) but not in 30 day mortality (0.85, 0.70 to 1.03).14
A review was published in 2014 including 6936 patients from 19 trials assessing the impact of red blood cell transfusion.74 Pooled data from three trials (2364 patients) using restrictive haemoglobin transfusion triggers of 7 g/dL showed reductions in in-hospital mortality (relative risk 0.74, 95% confidence interval 0.60 to 0.92), total mortality (0.80, 0.65 to 0.98), rebleeding (0.64, 0.45 to 0.90), acute coronary syndrome (0.44, 0.22 to 0.89), pulmonary oedema (0.48, 0.33 to 0.72), and bacterial infections (0.86, 0.73 to 1.00) compared with liberal transfusion. In contrast, pooled data including trials with less restrictive transfusion thresholds did not show associations with any of the predefined outcomes.
A recent systematic review with meta-analysis included 18 randomised controlled trials reporting data on in-hospital infections.75 Restrictive transfusion strategies were associated with a reduced risk of infections among patients admitted to hospital compared with liberal transfusion strategies (0.88, 0.78 to 0.99). Our analysis showed comparable results, with a possibility of lowering the rate of infections using restrictive transfusion strategies. We also included data on non-healthcare associated infections, but our results may be influenced by multiple testing and sparse data.
We included data from the recent Transfusion Requirements In Septic Shock (TRISS) trial, which randomised 1005 patients with septic shock in the intensive care unit, in which there was no difference in mortality or morbidity with the use of pre-stored leucocyte reduced red blood cells at a transfusion trigger of 7 versus 9 g/dL.9 In accordance with the Cochrane review we did not find evidence of harm with the use of restrictive transfusion strategies compared with liberal transfusion strategies. However, our trial sequential analyses were inconclusive for the assessment of mortality and myocardial infarction owing to insufficient information sizes.
Strengths and limitations of this review
Applying Cochrane methodology is a major strength of this systematic review, comprising a prepublished protocol, a non-restricted up to date literature search, independent data extraction by at least two authors, and risk of bias assessment leading to GRADE evaluations of important outcomes. Trial sequential analysis was performed to explore the risk of random error as a result of sparse data and repetitive testing in order to increase the robustness of the meta-analyses and distinguish the current information size from the required information size.
Our systematic review also has limitations. The randomised controlled trials included in the primary analysis dealt with different indications for transfusion by randomising a variety of patient groups (for example, children and adults) in different clinical settings (for example, elective surgery and critical illness). Thus, the risk of introducing potentially important heterogeneity is imminent. To get a clinical applicable result, we excluded trials of neonates and infants with very low birth weight. None of the included trials was blinded, as this is not feasible. This may introduce both performance and detection bias. However, the primary outcome of all cause mortality is less prone to be influenced by lack of blinding.76 Transfusion triggers varied between trials, with some using a liberal transfusion threshold equal to the restrictive one in other trials, introducing clinical heterogeneity. Both clinical heterogeneity and inadequate follow-up increase the risk of type II error. Bias in the included trials, losses to follow-up, and incomplete reporting of outcome measures are additional limitations in this review. The definitions of overall morbidity and adverse events were heterogeneous and should be taken into account when interpreting these data. Finally, for some of the predefined outcomes, limited trial data could be included in the meta-analyses resulting in wide confidence intervals and less certain point estimates.
Unanswered questions
Whether the overall use of red blood cells should be guided by a restrictive or a liberal transfusion strategy is still debatable. Patients with coronary artery disease, and in particular patients with ongoing cardiac ischaemia, might require a higher haemoglobin level to sustain oxygen delivery to the myocardial cells and to reduce the sympathetically mediated compensatory mechanisms of anaemia and reduce myocardial oxygen demand. However, red blood cell transfusion could worsen patient outcome as a result of an increased risk of circulatory overload and increased thrombogenicity with higher haematocrit levels. Results from the FOCUS trial showed no association with the primary composite outcome of morbidity and mortality 60 days postoperatively or the incidence of coronary syndrome when comparing two transfusion strategies (8 g/dL (or symptoms of anaemia) versus 10 g/dL).15 Two small randomised controlled trials evaluating a restrictive transfusion trigger of haemoglobin <8 g/dL in patients with symptomatic coronary artery disease have been published8 73; pooled data from these two trials randomising a total of 155 patients did not show an association between a restrictive transfusion strategy and cardiac events or mortality compared with a liberal transfusion strategy. A meta-analysis including observational studies on transfusion in patients with myocardial infarction indicates that the rates of subsequent myocardial infarction and all cause mortality may be associated with blood transfusions compared with standard supportive interventions, after adjustment for possible confounding variables.77 Large randomised controlled trials of restrictive compared with liberal transfusion are warranted in patients with myocardial infarction.
Patients with traumatic brain injury may require more liberal transfusion strategies to prevent secondary cerebral ischaemic insults because the injured brain may not compensate for decreased oxygen delivery associated with anaemia.78 One randomised controlled trial using a factorial design compared the effects of erythropoietin and two different haemoglobin thresholds for red blood cell transfusion (7 versus 10 g/dL) in 200 patients with a closed head injury and showed no difference in neurological outcome at six months.40 Also in patients with acute brain injury data from high quality randomised controlled trials are needed to guide transfusion practice.
Conclusions
In conventional meta-analyses restrictive transfusion strategies compared with liberal transfusion strategies were associated with a reduction in the number of red blood cells used and the number of patients being transfused but were not associated with benefit or harm regarding mortality, overall morbidity, and fatal or non-fatal myocardial infarction in various clinical settings. However, the required information sizes were not reached except for overall morbidity, where a 15% relative risk reduction or increase with restrictive transfusion strategies may be refuted. Analyses of all trials, regardless of risk of bias, showed similar findings. We found possible associations between restrictive transfusion strategies and a reduced number of infectious complications.
Restrictive transfusion strategies are safe in most clinical settings. Liberal transfusion strategies have not been shown to confer any benefit to patients but have the potential for harm.
What is already known on this topic
Red blood cells are commonly used in the treatment of haemorrhage and anaemia, but recent trials have shown potential harm with this intervention
Recent meta-analysis indicates no harm with the use of a restrictive transfusion strategy
What this study adds
This review includes new data from five recently published randomised trials of restrictive versus liberal transfusion strategies and includes data from more than 9000 patients
Pooled analyses did not show harm with restrictive transfusion strategies (no increased risk of mortality, overall morbidity, or acute myocardial infarction) but the number of units and number of patients transfused were reduced compared with liberal strategies
Liberal strategies have possible associations with harm (risk of infectious complications)
Further large trials with lower risk of bias are needed to establish firm evidence to guide transfusion in subgroups of patients
We thank Sarah Klingenberg, search coordinator for the Cochrane Hepato-Biliary Group, for performing the literature search.
Contributors: LBH developed the protocol, was responsible for the searches, selected trials, extracted data, assessed the risk of bias of trials, did the data analysis, and developed the final review. MWP developed the protocol, selected trials, extracted data, assessed the risk of bias of trials, and developed the final review. NH developed the protocol, selected trials, extracted data, assessed the risk of bias of trials, and developed the final review. AP developed the protocol, analysed data, and developed the final review. JW developed the initial idea for the review, developed the protocol, selected trials, advised on statistical methods, analysed data and resolved any disagreements during data extraction and bias assessment, and developed the final review. All authors read and approved the final manuscript. LBH and JW are the guarantors and affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Funding: This review was funded by the Danish Strategic Research Council (09-066938), supported by Copenhagen University Hospital, Rigshospitalet. The funders had no influence on the protocol, data analyses, or reporting.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: AP was principal investigator for Transfusion Requirements In Septic Shock (TRISS) trial and LBH, NH, and JW were members of the steering committee. AP is head of research in his intensive care unit, which receives research grants from CSL Behring, Fresenius Kabi, and Cosmed.
Ethical approval: Not required.
Data sharing: No additional data available.
Transparency: The lead authors (LBH and JW) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Cite this as: BMJ 2015;350:h1354
Web Extra. Extra material supplied by the author
Supplementary material
References
- 1.Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Abraham E, et al. The CRIT Study: anemia and blood transfusion in the critically ill-current clinical practice in the United States. Crit Care Med 2004;32:39-52. [DOI] [PubMed] [Google Scholar]
- 2.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 34:344-53. [DOI] [PubMed]
- 3.Rosland RG, Hagen MU, Haase N, Holst LB, Plambech M, Madsen KR, et al. Red blood cell transfusion in septic shock—clinical characteristics and outcome of unselected patients in a prospective, multicentre cohort. Scand J Trauma Resusc Emerg Med 2014;22:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hébert P, Wells G, Blajchman MA, Marshall JC. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med 1999;340:409-17. [DOI] [PubMed] [Google Scholar]
- 5.Lacroix J, Hébert PC, Hutchison JS, Hume HA. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med 2007;356:1609-19. [DOI] [PubMed] [Google Scholar]
- 6.Villanueva C, Colomo A, Bosch A, Concepción M, Hernandez-Gea V, Aracil C, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013;368:11-21. [DOI] [PubMed] [Google Scholar]
- 7.Hajjar LA, Vincent J, Galas FR, Nakamura RE. Transfusion Requirements After Cardiac Surgery: the TRACS Randomized Controlled Trial. JAMA 2010;304:1559-67. [DOI] [PubMed] [Google Scholar]
- 8.Carson JL, Brooks MM, Abbott JD, Chaitman B, Kelsey SF, Triulzi DJ, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J 2013;165:964-71.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holst LB, Haase N, Wetterslev J, Wernerman J, Guttormsen AB, Karlsson S, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med 2014;371:1381-91. [DOI] [PubMed] [Google Scholar]
- 10.Walsh TS, Boyd JA, Watson D, Hope D, Lewis S, Krishan A, et al. Restrictive versus liberal transfusion strategies for older mechanically ventilated critically ill patients: a randomized pilot trial. Crit Care Med 2013;41:1-10. [DOI] [PubMed] [Google Scholar]
- 11.Almeida J, Galas F, Osawa E, Fukisama J. Transfusion Requirements in Surgical Oncology Patients (TRISOP): a randomized, controlled trial. Crit Care 2013;17:s137. [Google Scholar]
- 12.Parker MJ. Randomised trial of blood transfusion versus a restrictive transfusion policy after hip fracture surgery. Injury 2013;44:1916-8. [DOI] [PubMed] [Google Scholar]
- 13.Prick BW, Jansen AJG, Steegers EAP, Hop WCJ. Transfusion policy after severe postpartum haemorrhage: a randomised non-inferiority trial. Br J Obstetr Gynaecol 2014;121:1005-14. [DOI] [PubMed] [Google Scholar]
- 14.Carson JL, Carless PA, Hébert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst 2012;18:CD002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011;365:2453-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Napolitano LM, Kurek S, Luchette FA, Corwin HL, Barie PS, Tisherman SA, et al. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Crit Care Med 2009;37:3124-57. [DOI] [PubMed] [Google Scholar]
- 17.Higgins J, Green S, eds. Cochrane handbook for systematic reviews of interventions, version 5.1.0 [updated March 2011]. Cochrane Collaboration 2011. www.cochrane-handbook.org.
- 18.Moher D, Liberati A, Tetzlaff J, Altman D, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;399:b2700. [PMC free article] [PubMed] [Google Scholar]
- 19.Guyatt GH. GRADE: what is “ quality of evidence ” and why is it important to clinicians? BMJ 2008;336:995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol 2008;61:64-75. [DOI] [PubMed] [Google Scholar]
- 21.DeMets DL. Methods of combining randomized clinical trials: strengths and limitations. Stat Med 1987;6:341-50. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [DOI] [PubMed] [Google Scholar]
- 23.Turner RM, Bird SM, Higgins JPT. The impact of study size on meta-analyses: examination of underpowered studies in Cochrane reviews. PLoS One 2013;8:e59202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analyses may be inconclusive—trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol 2009;38:287-98. [DOI] [PubMed] [Google Scholar]
- 25.Bangalore S, Kumar S, Wetterslev J, Messerli FH. Angiotensin receptor blockers and risk of myocardial infarction: meta-analyses and trial sequential analyses of 147 020 patients from randomised trials. BMJ 2011;342:d2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int J Epidemiol 2009;38:276-86. [DOI] [PubMed] [Google Scholar]
- 27.Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Med Res Methodol 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foss NB, Kristensen MT, Jensen PS, Palm H. The effects of liberal versus restrictive transfusion thresholds on ambulation after hip fracture surgery. Transfusion 2009;49:227-34. [DOI] [PubMed] [Google Scholar]
- 29.Blair SD, Janvrin SB, McCollum CN, Greenhalgh RM. Effect of early blood transfusion on gastrointestinal haemorrhage. Br J Surg 1986;73:783-5. [DOI] [PubMed] [Google Scholar]
- 30.Bush RL, Pevec WC, Holcroft JW. A prospective, randomized trial limiting perioperative red blood cell transfusions in vascular patients. Am J Surg 1997;174:143-8. [DOI] [PubMed] [Google Scholar]
- 31.Bracey AW, Radovancevic R, Riggs SA, Houston S, Cozart H, Vaughn WK, et al. Lowering the hemoglobin threshold for transfusion in coronary artery bypass procedures: effect on patient outcome. Transfusion 1999;39:1070-7. [DOI] [PubMed] [Google Scholar]
- 32.Carson JL, Terrin ML, Barton FB, Aaron R, Greenburg AG, Heck DA, et al. A pilot randomized trial comparing symptomatic vs. hemoglobin- level-driven red blood cell transfusions following hip fracture. Transfusion 1998;38:522-9. [DOI] [PubMed] [Google Scholar]
- 33.Cholette JM, Rubenstein JS, Alfieris GM, Powers KS, Eaton M, Lerner NB. Children with single-ventricle physiology do not benefit from higher hemoglobin levels post cavopulmonary connection: results of a prospective, randomized, controlled trial of a restrictive versus liberal red-cell transfusion strategy. Pediatr Crit care Med 2011;12:39-45. [DOI] [PubMed] [Google Scholar]
- 34.Fortune JB, Feustel PJ, Saifi J, Stratton HH, Newell JC, Shah DM. Influence of hematocrit on cardiopulmonary function after acute hemorrhage. J Trauma 1987;27:243-9. [DOI] [PubMed] [Google Scholar]
- 35.De Gast-Bakker DH, de Wilde RBP, Hazekamp MG, Sojak V, Zwaginga JJ, Wolterbeek R, et al. Safety and effects of two red blood cell transfusion strategies in pediatric cardiac surgery patients: a randomized controlled trial. Intensive Care Med 2013;39:2011-9. [DOI] [PubMed] [Google Scholar]
- 36.Grover M, Talwalkar S, Casbard A, Boralessa H, Contreras M, Brett S, et al. Silent myocardial ischaemia and haemoglobin concentration: a randomized controlled trial of transfusion strategy in lower limb arthroplasty. Vox Sang 2006;90:105-12. [DOI] [PubMed] [Google Scholar]
- 37.Hébert PC. Transfusion requirements in critical care, a pilot study. JAMA 1995;273:1439-48. [DOI] [PubMed] [Google Scholar]
- 38.Johnson RG, Thurer RL, Kruskall MS, Sirois C, Gervino E V, Critchlow J, et al. Comparison of two transfusion strategies after elective operations for myocardial revascularization. J Thorac Cardiovasc Surg 1992;104:307-14. [PubMed] [Google Scholar]
- 39.Lotke PA, Barth P, Garino JP, Cook EF. Predonated autologous blood transfusions after total knee arthroplasty: immediate versus delayed administration. J Arthroplasty 1999;14:647-50. [DOI] [PubMed] [Google Scholar]
- 40.Robertson CS, Hannay HJ, Yamal J-M, Gopinath S, Goodman JC, Tilley BC, et al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury. JAMA 2014;312:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shehata N, Burns LA, Nathan H, Hébert P, Hare GMT, Fergusson D, et al. A randomized controlled pilot study of adherence to transfusion strategies in cardiac surgery. Transfusion 2012;52:91-9. [DOI] [PubMed] [Google Scholar]
- 42.Wu JF, Guan XD, Chen J, Ou-Yang B. A randomized, controlled clinical trial of transfusion of requirement in patients after orthotopicliver transplantation. ESICM LIVES 2011, 24th annual congress 2011;37:1-314. [Google Scholar]
- 43.Zygun DA, Nortje J, Hutchinson PJ, Timofeev I, Menon DK, Gupta AK. The effect of red blood cell transfusion on cerebral oxygenation and metabolism after severe traumatic brain injury. Neurologic Crit Care 2009;37:1074-8. [DOI] [PubMed] [Google Scholar]
- 44.Webert KE, Cook RJ, Couban S, Carruthers J, Lee KA, Blajchman MA, et al. A multicenter pilot-randomized controlled trial of the feasibility of an augmented red blood cell transfusion strategy for patients treated with induction chemotherapy for acute leukemia or stem cell transplantation. Transfusion 2008;48(1):81-91. [DOI] [PubMed] [Google Scholar]
- 45.So-Osman C, Nelissen R, Te Slaa R, Coene L, Brand R, Brand A. A randomized comparison of transfusion triggers in elective orthopaedic surgery using leucocyte-depleted red blood cells. Vox Sang 2010;98:56-64. [DOI] [PubMed] [Google Scholar]
- 46.So-Osman C, Hout WB Van Den, Brand R, Brand A. Patient blood management in elective total hip- and knee-replacement surgery (part 2): a randomized controlled trial on blood salvage as transfusion alternative using a restrictive transfusion policy in patients with a preoperative hemoglobin above 13 g/dl. Anesthesiology 2014;120:852-60. [DOI] [PubMed] [Google Scholar]
- 47.So-Osman C, Nelissen RGHH, Koopman-van Gemert AWMM, Kluyver E, Pöll RG, Onstenk R, et al. Patient blood management in elective total hip- and knee-replacement surgery (Part 1): a randomized controlled trial on erythropoietin and blood salvage as transfusion alternatives using a restrictive transfusion policy in erythropoietin-eligible patients. Anesthesiology 2014;120:839-51. [DOI] [PubMed] [Google Scholar]
- 48.Topley E, Fisher MR. The illness of trauma. Br J Clin Pract 1956;10:770-6. [PubMed] [Google Scholar]
- 49.Vichinsky EP, Haberkern CM, Neumayr L, Earles AN, Black D, Koshy M, et al. A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. The Preoperative Transfusion in Sickle Cell Disease Study Group. N Engl J Med 1995;333:206-13. [DOI] [PubMed] [Google Scholar]
- 50.Olgun H, Buyukavci M, Sepetcigil O, Yildirim ZK, Karacan M, Ceviz N. Comparison of safety and effectiveness of two different transfusion rates in children with severe anemia. J Pediatr Hematol Oncol 2009;31:843-6. [DOI] [PubMed] [Google Scholar]
- 51.Atilla E, Topcuoglu P, Yavasoglu S, Karakaya A, Gencturk C, Bozdag S, et al. A randomized comparison of hemoglobin content-based vs standard (Unit-Based) RBC transfusion policy efficiencies. Vox Sang 2011;101(suppl 2):39-134. [Google Scholar]
- 52.Karkouti K, Wijeysundera DN, Yau TM, McCluskey SA, Chan CT, Wong PY, et al. Advance targeted transfusion in anemic cardiac surgical patients for kidney protection: an unblinded randomized pilot clinical trial. Anesthesiology 2012;116:613-21. [DOI] [PubMed] [Google Scholar]
- 53.Zheng H, Wu JJ, Wang J. Evaluation of effectiveness and analysis of goal-directed blood transfusion in peri-operation of major orthopedic surgery in elderly patients. Exp Ther Med 2013;5:511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCoy TE, Conrad AL, Richman LC, Lindgren SD, Nopoulos PC, Bell EF. Neurocognitive profiles of preterm infants randomly assigned to lower or higher hematocrit thresholds for transfusion. Child Neuropsychol 2011;17:347-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen H-L, Tseng H-I, Lu C-C, Yang S-N, Fan H-C, Yang R-C. Effect of blood transfusions on the outcome of very low body weight preterm infants under two different transfusion criteria. Pediatr Neonatol 2009;50:110-6. [DOI] [PubMed] [Google Scholar]
- 56.Bell 2005
- 57.Whyte RK, Kirpalani H, Asztalos EV, Andersen C, Blajchman M, Heddle N, et al. Neurodevelopmental outcome of extremely low birth weight infants randomly assigned to restrictive or liberal hemoglobin thresholds for blood transfusion. Pediatrics 2009;123:207-13. [DOI] [PubMed] [Google Scholar]
- 58.Nopoulos PC, Conrad AL, Bell EF, Strauss RG, Widness JA, Magnotta VA. Long-term outcome of brain structure in premature infants: effects of liberal vs restricted red blood cell transfusions. Arch Pediatr Adolesc Med 2011;165:443-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fredrickson LK, Bell EF, Cress GA, Johnson KJ, Zimmerman MB, Mahoney LT, et al. Acute physiological effects of packed red blood cell transfusion in preterm infants with different degrees of anaemia. Arch Dis Child Fetal Neonatal Ed 2011;96:249-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elterman J, Brasel K, Brown S, Bulger E, Kerby JD, Kannas D, et al. Transfusion of red blood cells in patients with a pre-hospital gcs ≤8 and no evidence of shock is associated with worse outcomes. J Trauma Acute Care Sur 2013;75:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Colomo A, Hernandez-Gea V, Muniz-Diaz E, Madoz P, Araci LC, C. Alvarez-Urturi C, et al. Transfusion strategies in patients with cirrhosis and acute gastrointestinal bleeding. Hepatology 2008;48:413A. [Google Scholar]
- 62.Karam O, Tucci M, Ducruet T, Hume HA, Lacroix J, Gauvin F. Red blood cell transfusion thresholds in pediatric patients with sepsis. Pediatr Crit Care Med 2011;12:512-8. [DOI] [PubMed] [Google Scholar]
- 63.Rouette J, Trottier H, Ducruet T, Beaunoyer M, Lacroix J, Tucci M. Red blood cell transfusion threshold in postsurgical pediatric intensive care patients: a randomized clinical trial. Ann Surg 2010;251:421-7. [DOI] [PubMed] [Google Scholar]
- 64.Willems A, Harrington K, Lacroix J, Biarent D, Joffe AR, Wensley D, et al. Comparison of two red-cell transfusion strategies after pediatric cardiac surgery: a subgroup analysis. Pediatr Crit care 2010;38:649-56. [DOI] [PubMed] [Google Scholar]
- 65.Nakamura R, Sundin M, Fukushima J, Almeida J, Gergamin F. A liberal strategy of red blood cell transfusion reduces cardiovascular complications in older patients undergoing cardiac surgery. Crit Care 2014;18:P107. [Google Scholar]
- 66.Bergamin F, Almeida J, Park C, Osawa E, Silva J. Transfusion requirements in septic shock patients: a randomized controlled trial. Crit Care 2014;18:112.24602370 [Google Scholar]
- 67.Pike K, Brierley R, Rogers CA, Murphy GJ, Reeves BC. Adherence in a randomised controlled trial comparing liberal and restrictive red blood cell (RBC) transfusion protocols after cardiac surgery (TITRe2). Trials 2011;12(suppl 1):A121. [Google Scholar]
- 68.Gray A, Jairath V, Kahan B, Dore C, Palmer K, Travis S, et al. Restrictive versus liberal blood transfusion for acute upper gastrointestinal bleeding (trigger): pragmatic, cluster randomised, feasibility trial. Emerg Med J 2014;31:780. [Google Scholar]
- 69.Franz AR, Maier RF, Thome UH, Rudiger M, Kron M, Bassler D, et al. The “effects of transfusion thresholds on neurocognitive outcome of extremely low birth-weight infants (ETTNO)” study: background, aims, and study protocol. Neonatology 2012;101:301-5. [DOI] [PubMed] [Google Scholar]
- 70.Brown CH, Grega M, Selnes OA, McKhann GM, Shah AS, LaFlam A, et al. Length of red cell unit storage and risk for delirium after cardiac surgery. Anesth Analg 2014;119:242-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kirpalani H, Whyte RK, Andersen C, Asztalos EV, Heddle N, Blajchman MA, et al. The premature infants in need of transfusion(pint) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr 2006;149:301-7.e3. [DOI] [PubMed] [Google Scholar]
- 72.Gregersen M, Borris LC, Damsgaard EM. A liberal blood transfusion strategy after hip fracture surgery does not increase the risk of infection in frail elderly. Eur Geriatr Med 2012;32:S74. [Google Scholar]
- 73.Cooper HA, Rao SV, Greenberg MD, Rumsey MP, McKenzie M, Alcorn KW, et al. Conservative versus liberal red cell transfusion in acute myocardial infarction (the CRIT Randomized Pilot Study). Am J Cardiol 2011;108:1108-11. [DOI] [PubMed] [Google Scholar]
- 74.Salpeter SR, Buckley JS, Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a meta-analysis and systematic review. Am J Med 2013;127:124-31.e3. [DOI] [PubMed] [Google Scholar]
- 75.Rohde JM, Dimcheff DE, Blumberg N, Saint S, Langa KM, Kuhn L, et al. Health care-associated infection after red blood cell transfusion. JAMA 2014;311:1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Savovic J, Jones HE, Altman DG, Harris RJ. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Ann Intern Med 2012;157:429-38. [DOI] [PubMed] [Google Scholar]
- 77.Chatterjee S, Wetterslev J, Sharma A, Lichstein E, Mukherjee D. Association of blood transfusion with increased mortality in myocardial infarction: a meta-analysis and diversity-adjusted study sequential analysis. JAMA Intern Med 2013;173:132-9. [DOI] [PubMed] [Google Scholar]
- 78.LeRoux P. Haemoglobin management in acute brain injury. Curr Opin Crit Care 2013;19:83-91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material