Abstract
Topically applied microbicides potently inhibit HIV in vitro but have largely failed to exert protective effects in clinical trials. One possible reason for this discrepancy is that the preclinical testing of microbicides does not faithfully reflect the conditions of HIV sexual transmission. Here, we report that candidate microbicides that target HIV components show greatly reduced antiviral efficacy in the presence of semen, the main vector for HIV transmission. This diminished antiviral activity was dependent on the ability of amyloid fibrils in semen to enhance the infectivity of HIV. Thus, the anti-HIV efficacy of microbicides determined in the absence of semen greatly underestimated the drug concentrations needed to block semen-exposed virus. One notable exception was Maraviroc. This HIV entry inhibitor targets the host cell CCR5 coreceptor and was highly active against both untreated and semen-exposed HIV. These data help explain why microbicides have failed to protect against HIV in clinical trials and suggest that antiviral compounds targeting host factors hold promise for further development. These findings also suggest that the in vitro efficacy of candidate microbicides should be determined in the presence of semen to identify the best candidates for the prevention of HIV sexual transmission.
Introduction
With no effective HIV vaccine available (1), considerable efforts have been made to develop microbicides as a strategy to curb sexual transmission of HIV. Unfortunately, many of the topical microbicides investigated to date have proved inactive or even increased the risk of HIV acquisition in clinical trials (2–5). One putative exception is the use of a vaginal gel containing Tenofovir that has shown a 54% protection rate in the CAPRISA 004 trial (6). Unfortunately, however, a larger trial testing the same formula was stopped due to lack of efficacy (7). The failure of topical microbicides has been attributed to lack of adherence, as well as the induction of inflammation and cytotoxic effects (3, 4). Here, we explore the possibility that the HIV enhancing activity of semen (8–13) may diminish the efficacy of anti-HIV microbicides.
Results
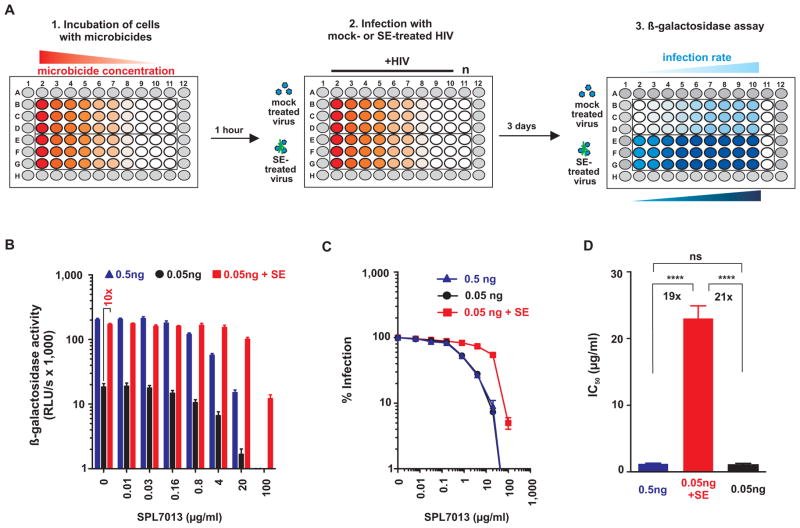
We previously established protocols that permit analysis of the infectivity-enhancing activity of human semen by minimizing its cytotoxic effects (8, 12, 13). To examine the ability of semen to enhance HIV infection of microbicide-treated cells, we modified this assay. As shown in Fig. 1A, either semen-treated or mock-treated CCR5-tropic HIV was added to TZM-bl reporter cells containing serial dilutions of the microbicides or antiviral drugs of interest. After 2 hours, the semen-containing inoculum was removed to prevent cytotoxic effects (8, 12, 13), and fresh medium supplemented with antiviral drugs or microbicides was added. Infection rates were determined 3 days later by quantifying β-galactosidase activities in cellular lysates (Fig. 1A).
Fig. 1. Effect of semen on the antiviral activity of the microbicide SPL7013.
(A) TZM-bl cells containing increasing concentrations of microbicides were inoculated with 0.05 ng p24 antigen of mock-treated (upper half of microtiter plate) or semen-treated (bottom half of microtiter plate) R5-tropic HIV. Two hours later, the inocula were removed to prevent semen-mediated cytotoxic effects, and replaced with fresh medium containing microbicides. Infection rates were determined by measuring β-galactosidase activities in cellular lysates 3 days post-infection. The protease inhibitor was analyzed the same way except that CEMx-M7 cells were used and infection rates were quantified 4 days post-infection by measuring luciferase activities. (B–D) SPL7013 loses antiviral activity in the presence of semen. (B) Infection rates of TZM-bl cells containing increasing concentrations of SPL7013 that were inoculated with 0.05 ng mock-exposed and semen-exposed HIV, and 0.5 ng HIV as infectivity-matched control. Shown are mean β-galactosidase activities ± standard deviation derived from triplicate infections. RLU/s: relative light units per second. (C) Normalized infection rates. β-galactosidase activities obtained from infected cells in the absence of inhibitor were set at 100%. (D) Calculated IC50 values derived from experiments shown in B and C. The number above the bar represents the fold-change in the IC50 derived from 0.05 ng semen-exposed relative to 0.05 or 0.5 ng mock-exposed virus infection. Ns, no statistically significant difference; **** p<0.0001 (unpaired t-test).
We first analyzed the effect of semen on the antiviral activity of the microbicide SPL7013 (14,15). Development of this negatively-charged dendrimer as a microbicide was terminated just recently due to adverse events (16). As previously observed (8, 13), HIV virion exposure to 10% semen increased low-dose HIV infectivity (0.05 ng p24 antigen) by approximately 10-fold (Fig. 1B). Thus, we used a 10-fold higher amount of mock-treated HIV (0.5 ng p24 antigen) as an “infectivity-matched” control (Fig. 1B). SPL7013 blocked infection at both doses of HIV with IC50 values of 1.2±0.1 and 1.1±0.2 μg/ml, respectively (Fig. 1B, D; Table 1). In contrast, SPL7013 was about 20-fold less effective against semen-exposed virus (IC50 = 23±1.9 μg/ml) (Fig. 1C, D; Table 1). Notably, semen-treated HIV still efficiently infected cells in the presence of 100 μg/ml of the SPL7013 dendrimer, a concentration that prevented mock-treated HIV infection entirely (Fig. 1B, C). Higher concentrations of the dendrimer were cytotoxic and could thus not be tested (fig. S1A). Next, we examined seven transmitter/founder (T/F) HIV-1 strains that are particularly relevant for HIV/AIDS transmission studies (17). We found that 10% semen enhanced T/F virus infectivity by 7-fold to 16-fold (fig. S1B), and on average impaired the antiviral efficacy of SLP7013 by 60-fold (IC50 increased from 0.9±0.3 μg/ml to 53.9±16.3 μg/ml) (fig. S1C, S1D).
Table 1.
Antiviral activity of microbicides against mock-exposed and semen-exposed HIV-1 in TZM-bl cells or CEMx-M7 cells.
| Compound | Class | Inhibitory Concentration 50% (IC50) | Fold increase in IC50 of SE-exposed relative to non- exposed HIV | |||
|---|---|---|---|---|---|---|
| 0.5 ng HIV | 0.05 ng HIV | 0.05 ng HIV + 10% SE | 0.5 ng HIV | 0.05 ng HIV | ||
| SPL7013 | Polyanion | 1.2±0.1 μg/ml | 1.1±0.2 μg/ml | 23.0±1.9 μg/ml | 19 | 21 |
| PSA | Polyanion | 1.0±0.1 μg/ml | 1.0±0.1 μg/ml | 20.1±0.5 μg/ml | 20 | 20 |
| PNS | Polyanion | 1.0±0.2 μg/ml | 1.2±0.3 μg/ml | 21.0±1.2 μg/ml | 21 | 18 |
| CS | Polyanion | 1.1±0.1 μg/ml | 1.2±0.4 μg/ml | 20.3±1.3 μg/ml | 19 | 17 |
| 2F5 | Neutralizing antibody | 2.6±0.3 μg/ml | 2.7±0.1 μg/ml | 22.2±1.2 μg/ml | 9 | 8 |
| 2G12 | Neutralizing antibody | 0.7±0.1 μg/ml | 0.7±0.1 μg/ml | 6.0±0.5 μg/ml | 9 | 8 |
| TDF | NtRTI | 1.7±0.3 nM | 1.4±0.2 nM | 17.9±0.6 nM | 10 | 13 |
| NVP | NNRTI | 0.18±0.03 μM | 0.18±0.06 μM | 2.1±0.1 μM | 12 | 11 |
| EVG | Integrase inhibitor | 0.60±0.07 nM | 0.72±0.05 nM | 5.6±0.5 nM | 9 | 8 |
| IDV (CEMx-M7 cells) | Protease inhibitor | 1.9±0.2 nM | 2.3±0.2 nM | 23.2±3.0 nM | 12 | 10 |
| MVC | CCR5 antagonist | 3.7±0.7 nM | 3.9±0.2 nM | 4.3±0.3 nM | 1.2 | 1.1 |
To examine whether other anionic polymers are also less effective against semen-exposed virus, we analyzed polystyrene acid, polynaphthalene sulfonate and cellulose sulfate. These compounds were among the first to be considered for microbicide development because of their potent in vitro activity (18–21) but failed to prevent HIV transmission in people (2, 5, 22, 23). Similar to the results described above for SPL7013, polystyrene acid (fig. S2A), polynaphthalene sulfonate (fig. S2B) and cellulose sulfate (fig. S2C) were 19-fold to 21-fold less active against semen-treated virus (Table 1), and generally failed to prevent semen-exposed virus infection. Again, concentrations ≥100 μg/ml could not be tested because of cytotoxic effects (fig. S2D). Thus, these three former microbicide candidates were poorly effective against HIV in the presence of semen.
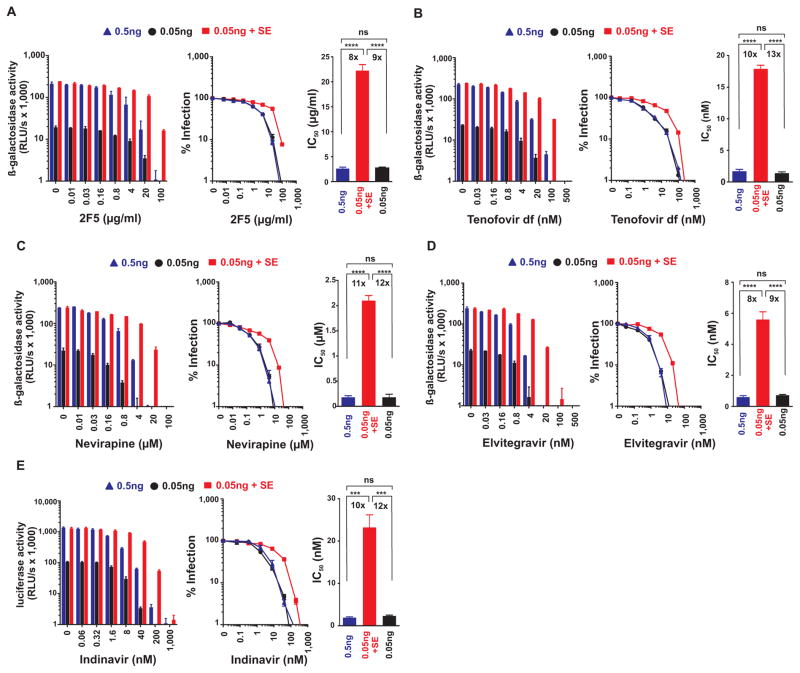
Another class of candidate microbicides are neutralizing antibodies (nAbs). The gp41-specific 2F5 nAb (Fig. 2A) efficiently blocked infection with HIV stocks containing 0.5 and 0.05 ng p24 capsid antigen in the absence of semen with IC50 values of 2.6±0.3 and 2.7±0.1 μg/ml, respectively (Fig. 2A, Table 1). However, pretreatment of 0.05 ng HIV with semen increased the IC50 to 22.2±1.2 μg/ml (Fig. 2A, Table 1). Similar results were obtained for the 2G12 nAb targeting gp120 (fig. S3A, Table 1). Thus, exposure of HIV to 10% semen decreased the antiviral efficacy of neutralizing antibodies by almost 10-fold.
Fig. 2. Antiviral drugs targeting various steps in the viral life cycle lose efficacy against semen-exposed HIV.
The neutralizing antibody 2F5 (A), the Nucleotide analog reverse-transcriptase inhibitor Tenofovir disoproxil fumarate (B), the Non-nucleoside reverse-transcriptase inhibitor Nevirapine (C), and the integrase inhibitor Elvitegravir (D) were added to TZM-bl cells, and the protease inhibitor Indinavir (E) to CEMx-M7 cells. Cells were inoculated with 0.05 ng mock-exposed and semen-exposed HIV, and 0.5 ng HIV as infectivity-matched control. Infection rates were measured 3 days post infection by measuring β-galactosidase (A–D) or 4 days post infection by measuring luciferase activities. The left panels show the mean enzyme activities ± standard deviation derived from triplicate infections. RLU/s: relative light units per second. Middle panels show normalized infection rates in which reporter enzyme activities obtained from infected cells in the absence of inhibitor were set at 100%. The right panels depict the calculated IC50 values. The number above the bar represents the fold-change in the IC50 derived from 0.05 ng semen-exposed relative to 0.05 or 0.5 ng mock-exposed virus infection. Ns, no statistically significant difference; **** p<0.0001; *** p<0.001 (unpaired t-test).
Polyanions and nAbs target HIV outside host cells. Remarkably, we found that antiviral drugs acting on intracellular targets, such as the HIV nucleotide reverse transcriptase inhibitor (NtRTI) Tenofovir disoproxil fumarate (Fig. 2B), the non-nucleoside reverse transcriptase inhibitor (NNRTI) Nevirapine (Fig. 2C), and the integrase inhibitor (INI) Elvitegravir (Fig. 2D), were also about 8-fold to 13-fold less effective against semen-exposed virus (Table 1). For example, Elvitegravir inhibited infection with both doses of HIV-1 at IC50 values of 0.60±0.07 and 0.72±0.05 nM, respectively, whereas the IC50 was increased to 5.6±0.5 nM in the presence of semen (Fig. 2D, Table 1). Finally, we analyzed the HIV protease inhibitor Indinavir, which acts at a very late stage of the viral replication cycle. Similar to all other drugs tested so far, semen exposure reduced antiretroviral efficacy by 10-fold to 12-fold (Fig. 2E, Table 1). In contrast to polyanions (fig. S1A, S2D), none of the nAbs or inhibitors against the viral enzymes were cytotoxic (fig. S3B, S3C). These results indicate that semen reduces the sensitivity of HIV to multiple classes of antiretroviral therapeutics.
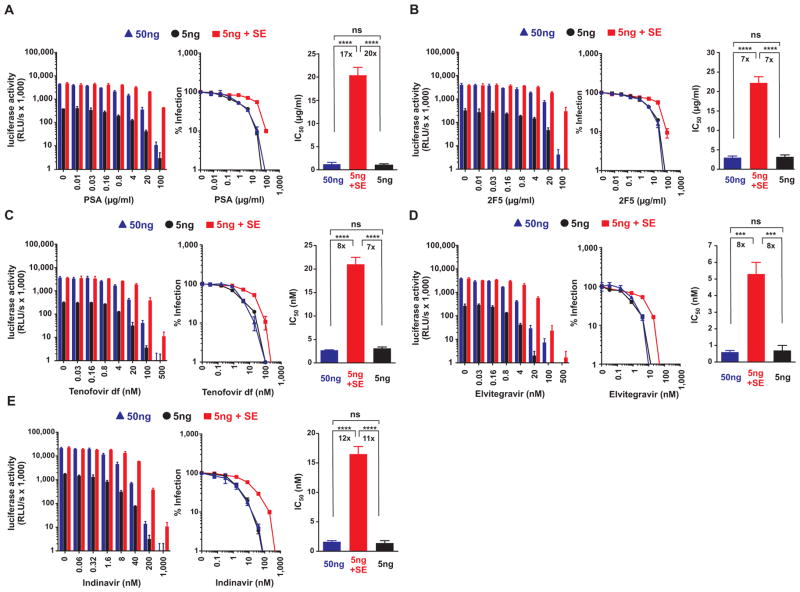
The findings described above were obtained from experiments performed in vitro in immortalized TZM-bl cells, or CEMx-M7 cells in the case of Indinavir (Table 1). To examine the effects of semen on the antiviral efficacy of microbicides in the primary target cells of HIV, human peripheral blood mononuclear cells (PBMCs) were supplemented with respective antiretroviral drugs and inoculated with either 5 ng mock-exposed or semen-exposed virus encoding a luciferase (and 50 ng mock-treated virus as an infectivity-matched control). As previously reported (8, 13), 10% semen enhanced HIV-1 infection of PBMCs by ~9-fold (Fig. 3A–E; left panels, absence of drugs). Similar to the results obtained in the cell lines (Table 1), the IC50 values of polystyrene acid (Fig. 3A), the 2F5 neutralizing antibody (Fig. 3B), Tenofovir disoproxil fumarate (Fig. 3C), Elvitegravir (Fig. 3D) and Indinavir (Fig. 3E) increased by 7-fold to 20-fold if cells were inoculated with semen-exposed virus (Table 2). Thus, semen reduced the antiviral efficacy of antiretroviral drugs in primary HIV target cells.
Fig. 3. Semen reduces the antiviral efficacy of microbicides to block HIV infection of human PBMCs.
Human PBMCs containing indicated concentrations of the polyanion polystyrene acid (A), the nAb 2F5 (B), the Nucleotide analog reverse-transcriptase inhibitor Tenofovir disoproxil fumarate (C), the integrase inhibitor Elvitegravir (D), and the protease inhibitor Indinavir (E) were inoculated with 5 ng mock-exposed or semen-exposed R5-HIV-luciferase, or 50 ng R5-HIV-luciferase as infectivity matched control. Infection rates were determined 3 days post inoculation (A–D) or 4 days post inoculation (E) by quantifying luciferase activities in cellular supernatants. The left panels depict the mean luciferase activities ± standard deviation derived from triplicate infections. RLU/s: relative light units per second. Middle panels show normalized infection rates in which luciferase activities obtained from infected cells in the absence of inhibitor were set at 100%. The right panels depict the calculated IC50 values. The number above the bar represents the fold-change in the IC50. Ns, no statistically significant difference; **** p<0.0001; *** p<0.001 (unpaired t-test).
Table 2.
Antiviral activity of microbicides against mock-exposed and semen-exposed HIV-1 in human PBMCs.
| Compound | Class | Inhibitory Concentration 50% (IC50) | Fold increase in IC50 of SE-exposed relative to non- exposed HIV | |||
|---|---|---|---|---|---|---|
| 50 ng HIV | 5 ng HIV | 5 ng HIV + 10% SE | 50 ng HIV | 5 ng HIV | ||
| PSA | Polyanion | 1.2±0.4 μg/ml | 1.0±0.3 μg/ml | 20.4±1.7 μg/ml | 17 | 20 |
| 2F5 | Neutralizing antibody | 3.0±0.4 μg/ml | 3.2±0.5 μg/ml | 22.2±1.6 μg/ml | 7 | 7 |
| TDF | NtRTI | 2.7±0.1 nM | 3.1±0.3 nM | 21.0±1.5 nM | 8 | 7 |
| EVG | Integrase inhibitor | 0.67±0.08 nM | 0.64±0.28 nM | 5.3±0.7 nM | 8 | 8 |
| IDV (CEMx-M7 cells) | Protease inhibitor | 1.6±0.2 nM | 1.4±0.4 nM | 16.5±0.3 nM | 11 | 12 |
| MVC | CCR5 antagonist | 3.5±0.1 nM | 3.6±0.4 nM | 4.3±0.1 nM | 1.2 | 1.2 |
In the absence of semen the antiviral efficacy of the analyzed compounds was not markedly affected by the viral inocula (Table 1, compare IC50 values obtained by infection with 0.05 vs. 0.5 ng p24 virus). However, the IC50 values after infection with 0.05 ng of semen-exposed virus were between 8-fold to 21-fold higher than those for the “p24-matched” (0.05 ng) and the “infectivity-matched” control (0.5 ng) (Table 1). Thus, a specific property of semen rather than the increase in viral infection rates was responsible for the diminished antiviral efficacy. To examine whether the infectivity-enhancing activity of amyloid in semen may play a role, we tested the effect of a semen-derived amyloid (8) (semen-derived enhancer of virus infection or SEVI) under the same experimental conditions. We found that the microbicides described above were all less active against SEVI-exposed HIV (fig. S4, Table 3). The differences in the IC50 values (19-fold to 22-fold for polyanions; 7-fold to 17-fold for remaining classes) were similar to those obtained after treatment with semen (compare Tables 1 and 3) suggesting the same underlying mechanism.
Table 3.
Antiviral activity of microbicides against mock-exposed and SEVI-exposed HIV-1 in TZM-bl cells or CEMx-M7 cells.
| Compound | Class | Inhibitory Concentration 50% (IC50) | Fold increase in IC50 of SEVI-exposed relative to non- exposed HIV | |||
|---|---|---|---|---|---|---|
| 0.5 ng HIV | 0.05 ng HIV | 0.05 ng HIV + SEVI | 0.5 ng HIV | 0.05 ng HIV | ||
| SPL7013 | Polyanion | 1.1±0.1 μg/ml | 0.9±0.1 μg/ml | 20.1±1.5 μg/ml | 19 | 22 |
| PSA | Polyanion | 1.1±0.2 μg/ml | 1.2±0.1 μg/ml | 21.4±2.5 μg/ml | 20 | 19 |
| 2F5 | Neutralizing antibody | 3.4±0.2 μg/ml | 3.5±0.4 μg/ml | 23.1±1.0 μg/ml | 7 | 7 |
| TDF | NtRTI | 1.7±0.1 nM | 2.0±0.3 nM | 16.1±1.4 nM | 10 | 8 |
| EVG | Integrase inhibitor | 0.63±0.08 nM | 0.66±0.11 nM | 4.3±0.6 nM | 7 | 6 |
| IDV (CEMx-M7 cells) | Protease inhibitor | 2.0±0.3 nM | 3.1±1.7 nM | 28.4±3.1 nM | 14 | 17 |
| MVC | CCR5 antagonist | 3.1±0.4 nM | 3.4±0.5 nM | 4.4±0.5 nM | 1.4 | 1.3 |
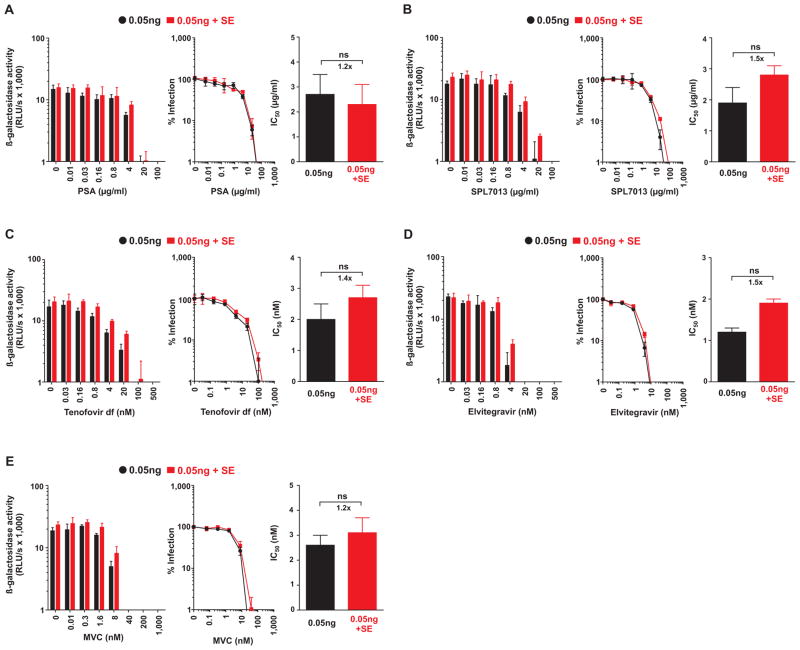
If seminal amyloid accounts for the reduced antiviral efficacies of microbicides, then semen lacking amyloid should not show this effect. To test whether this was indeed the case, we took advantage of the fact that human semen incubated for extended periods of time (≥ 24 h) loses the ability to enhance HIV infection (24). In contrast to regular semen (Table 1), 48 h-incubated semen increased the IC50 values of various microbicides only marginally (Fig. 4; Table 4). To further examine whether semen lacking HIV-enhancing activity does not diminish the antiviral activity of the microbicides, we obtained semen from patients with ejaculatory duct obstruction. Semen from these patients does not enhance HIV infection due to a lack of amyloid (12). Consistent with the results obtained using the 48 h-incubated semen samples, semen from patients with ejaculatory duct obstruction did not impair the antiviral activity of cellulose sulfate (fig. S5). Unfortunately, the effect on other classes of microbicides could not be determined because only very limited quantities of semen from these patients were available.
Fig. 4. Semen lacking HIV-enhancing activity does not markedly diminish the antiviral activity of microbicides.
In order to eliminate HIV-enhancing activity, human semen samples were incubated at 37°C for 48 hours prior to use in infection assays. Mock or 10 % semen treated HIV was added to TZM-bl cells supplemented with the indicated concentrations of the polyanion polystyrene acid (A), the dendrimer SPL7013 (B), the Nucleotide analog reverse-transcriptase inhibitor Tenofovir disoproxil fumarate (C), the integrase inhibitor Elvitegravir (D), and the CCR5-antagonist Maraviroc (E). Infection rates were measured 3 days post infection by measuring β-galactosidase activities. The left panels show the mean enzyme activities ± standard deviation derived from triplicate infections. RLU/s: relative light units per second. Middle panels show normalized infection rates in which reporter enzyme activities obtained from infected cells in the absence of inhibitor were set at 100%. The right panels depict the calculated IC50 values. The number above the bar represents the fold-change in the IC50 derived from 0.05 ng semen-exposed relative to 0.05 ng mock-exposed virus infection. Ns, no statistically significant difference (unpaired t-test).
Table 4.
Antiviral activity of microbicides against mock-exposed and semen-exposed HIV-1 in the presence of semen lacking HIV-enhancing activity due to extended incubation.
| Compound | Class | Inhibitory Concentration 50% (IC50) | Fold increase in IC50 of SE- exposed relative to non- exposed HIV | |
|---|---|---|---|---|
| 0.05 ng HIV | 0.05 ng HIV + SE | 0.05 ng HIV | ||
| SPL7013 | Polyanion | 1.9±0.5 μg/ml | 2.8±0.3 μg/ml | 1.5 |
| PSA | Polyanion | 2.7±0.8 μg/ml | 2.3±0.8 μg/ml | 1.2 |
| TDF | NtRTI | 2.0±0.5 nM | 2.7±0.4 nM | 1.4 |
| EVG | Integrase inhibitor | 1.2±0.1 nM | 1.9±0.1 nM | 1.5 |
| MVC | CCR5 antagonist | 2.6±0.4 nM | 3.1±0.6 nM | 1.2 |
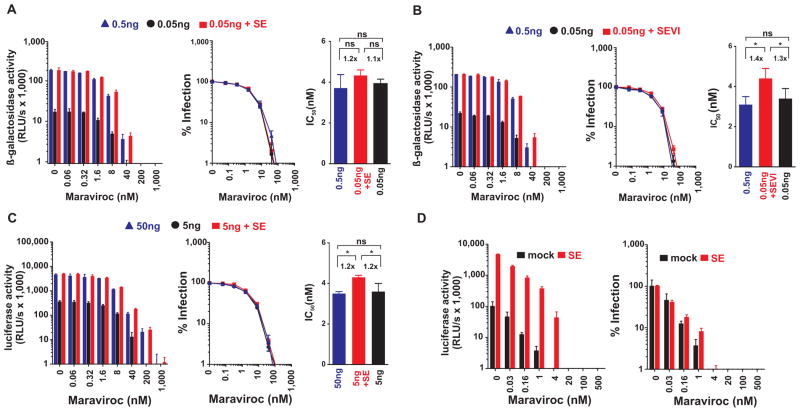
In marked contrast to the microbicides discussed thus far, the only anti-HIV compound that was almost equally active against mock-exposed and semen-exposed virus was the CCR5 antagonist Maraviroc, which is currently under consideration for microbicide development (25, 26). Maraviroc inhibited infection of TZM-bl cells by untreated HIV with IC50 values of 3.7±0.7 nM (0.5 ng of p24) and 3.9±0.2 nM (0.05 ng of p24), respectively, and these values only marginally increased to 4.3±0.3 nM in the presence of semen (Fig. 5A, Table 1). Similarly, exposure of virions to SEVI amyloid fibrils increased the IC50 values only marginally (Fig. 5B, Table 3). Maraviroc prevented semen-treated HIV infection of TZM-bl cells (Fig. 5A) and of human PBMCs (Fig. 5C) at concentrations ranging from 200 to 1,000 nM. These concentrations of Maraviroc are lower than those detected in the human genital tract (~2.4 μM in cervicovaginal fluid and ~1.7 μM in vaginal tissue) following oral administration (27). Potent inhibition of HIV by Maraviroc in the presence of semen was confirmed in a transmission assay examining transmission from cervical epithelial cells (CaSki) to CEMx-M7 indicator cells (Fig. 5D). Thus, unlike the antiretroviral drugs targeting viral components, Maraviroc, an antagonist of the host cell receptor CCR5, maintains potent anti-HIV activity in the presence of semen.
Fig. 5. Maraviroc retains antiviral activity against semen-exposed HIV.
Maraviroc inhibits (A) semen- and (B) SEVI-treated HIV infection of TZM-bl cells. Cells were inoculated with 0.05 ng p24 antigen of mock-exposed, semen-exposed or SEVI-exposed HIV, and 0.5 ng HIV as infectivity-matched control. Infection rates were measured 3 days post infection by measuring β-galactosidase activities. (C) Maraviroc blocks semen-treated HIV infection of PBMCs. Human PBMCs containing indicated concentrations of the CCR5 antagonist were inoculated with 5 ng mock-exposed or semen-exposed R5-HIV-luciferase, or 50 ng R5-HIV-luciferase as infectivity matched control. Infection rates were determined 3 days post inoculation by quantifying luciferase activities in cellular supernatants. The left panels in A–C show the mean enzyme activities ± standard deviation derived from triplicate infections. RLU/s: relative light units per second. Middle panels show normalized infection rates in which reporter enzyme activities obtained from infected cells in the absence of inhibitor were set at 100%. The right panels depict the calculated IC50 values. The number above the bar represents the fold-change in the IC50 values. Ns, no statistically significant difference; *p>0.01 and <0.05 (unpaired t-test). (D) Maraviroc inhibits trans-infection of semen-treated virus from cervical epithelial cells to target cells. Cervical epithelial cells supplemented with Maraviroc were inoculated with mock-treated or semen-treated HIV. After 2 hours inocula were removed, and CEMx-M7 reporter cells were added onto cervical epithelial cells in the presence of Maraviroc. 3 days later, infection rates were determined by quantifying luciferase activities in cellular lysates. The left panel shows infection rates as mean luciferase activities obtained from triplicate infections ± standard deviation, whereas the right panel shows the normalized values as % infection.
Discussion
Here, we examined whether the HIV infection-promoting effects of semen may explain why many microbicides efficiently inhibit HIV in cell culture but fail to exert protective effects against sexual acquisition of HIV in clinical trials (2–5, 16, 22, 23, 28). We have shown that brief exposure of HIV to semen reduced the antiviral efficacy of most microbicides by 7-fold to 22-fold. Importantly, this was not simply due to different infection rates because the same phenomenon was also observed when mock-treated and semen-treated HIV was normalized for the same infectivity. Unexpectedly, semen treatment not only reduced the susceptibility of HIV to extracellular microbicides but also conferred significant resistance to reverse transcriptase, integrase, and protease inhibitors that act inside the cell. The only microbicide candidate that remained fully active against semen-treated HIV was Maraviroc. This inhibitor of HIV entry was also the only compound that targeted a cellular factor instead of the virus itself. These findings suggest that microbicides targeting viral components are much less effective in the presence of semen, and help to explain the discrepancy between in vitro studies and the outcomes of HIV microbicide clinical trials. Our results do not rule out the possibility that in vivo drug concentrations in the genital tract or rectum that are effective against HIV even in the presence of semen can be reached. However, our data demonstrate that antiviral efficacies of microbicides determined in standard infection assays in the absence of semen may result in misleading results that do not reflect the antiviral potency of the compounds during sexual transmission. Given that semen is the major vector fuelling the global spread of HIV, we recommend preclinical testing of microbicide candidates in vitro with semen-exposed HIV to identify the best candidates for the prevention of HIV transmission by sexual intercourse.
The mechanisms underlying the impaired activity of microbicides against semen-treated HIV need further investigation. It has previously been shown that semen components or the alkaline pH of this body fluid reduces the anti-HIV activity of polyanions by neutralizing their negative charge (29–33) or by competitive binding to the viral envelope (34). Even though these findings provide conceivable explanations for why semen abrogates the antiviral activity of polyanions, they are unlikely to be the only mechanisms. First, the final semen concentrations in our cell culture studies were below 0.7%, which is too low to directly neutralize the anionic polymers (29); and second, semen also undermines the efficacy of agents blocking HIV replication through envelope-independent mechanisms such as Reverse transcriptase, Integrase and Protease inhibitors. Our results clearly show that seminal amyloid is critical for the decreased antiviral efficacy of the various classes of microbicides because semen lacking HIV enhancing activity did not impair drug efficacy, whereas synthetic SEVI amyloid fibrils reduced drug efficacy as effectively as semen itself. Because seminal amyloids bind to HIV virions and efficiently enhance and accelerate their attachment to target cells (8, 12, 35, 36), they shorten the time period of virion exposure to microbicides acting outside the cell and may impede the accessibility of the viral membrane and glycoproteins to polyanions or neutralizing antibodies.
It is less obvious why amyloid fibrils also impair the anti-HIV efficacy of intracellular drugs, particularly as this effect was also observed when mock-treated and semen-treated HIV were normalized to the same infectivity. It is tempting to speculate that altered infection kinetics may play a key role. Usually virion attachment is the rate-limiting step during infection (37). In the presence of amyloid fibrils that capture dozens of virions, all viral particles are attached and concentrated onto the cell surface (fig. S6) (12, 35, 36, 38). Thus, only semen-exposed virus may cause simultaneous infection of individual cells, which results in increased viral enzyme production inside the cell. Consequently, higher intracellular levels of antiretroviral drugs are required for effective inhibition of HIV replication. Notably, local increases in the number of virions at cellular entry sites have also recently been suggested to decrease the effectiveness of reverse transcriptase inhibitors during cell-to-cell transmission (39). In contrast to drugs that target viral components, Maraviroc inhibits viral entry via binding to the cellular CCR5 receptor. Maraviroc acts as an allosteric inhibitor (40) that renders CCR5 more rigid, and thereby prevents conformational changes that allow HIV to enter cells (41). Thus, it is conceivable that a saturating drug concentration that occupies all CCR5 molecules at the cell surface prevents HIV entry in a manner that is largely independent of the number of viral particles or the presence of amyloid. Thus, agents targeting cellular factors may be particularly promising for the development of effective microbicides.
To ensure that the impact of semen on the efficacy of microbicides was also relevant for the infection of primary HIV target cells, we performed experiments using human PBMCs. We observed similar effects as in the TZM-bl reporter cells. This was predicted from previous studies demonstrating that semen not only enhances HIV infection of a large variety of cell lines (8, 12, 13, 35) but also of PBMCs (8, 13), macrophages (13, 35) and endometrial CD4 cells (13), and also boosts viral trans-infection of T cells by dendritic cells or endothelial cells (13).
We also performed pilot studies in cervicovaginal tissues. However, this experimental system was not suitable to examine the effects of semen on the antiviral efficacy of microbicides because productive HIV infection was variable and required exceedingly high viral doses (42–46), whereas enhancing effects of semen are most pronounced at low viral doses (8, 12, 13, 35). Thus, a limitation of the present study is that it could not be tested whether the infection enhancing activity of semen undermines the antiviral efficacy of microbicides at the primary entry portals of HIV, and studies in animal models are required to determine whether semen also undermines the antiretroviral efficacy of microbicides in vivo.
A recent pilot study in macaques suggested that human semen and SEVI did not significantly enhance the efficiency of vaginal SIV transmission (47), but a major confounding factor in this study was the low number of animals analyzed and their high variability in susceptibility to vaginal SIV infection. This study did, however, provide preliminary evidence that semen and seminal amyloids may facilitate SIV transmission after exposure to low viral doses that most closely approximate the in vivo situation (47). Thus, further in vivo studies on the effects of semen on virus transmission and the efficacy of microbicides using T/F SHIVs (48) are warranted.
In summary, the reduced activity of microbicides that target HIV components against semen-exposed HIV may explain why many compounds that show high efficacy in cell culture infection assays failed in subsequent clinical trials. Maraviroc, however, maintained antiretroviral potency in the presence of semen suggesting that agents targeting cellular factors may be particularly promising for the development of effective microbicides. Our data also suggest that agents that antagonize seminal amyloid may exert dual beneficial effects because they would (i) prevent enhancement of HIV attachment and infection, and (ii) restore the antiviral activity of other microbicides in the presence of semen. Several agents that abrogate fibril formation or antagonize their HIV enhancing activity have been described (9–11, 49–52). Thus, combinations of antiretroviral microbicides and agents disrupting the infection-enhancing activity of semen should be more effective than single-component formulations and represent a new strategy for preventing sexual transmission of HIV.
Materials and Methods
Study design
We aimed to study the effect of human semen on the antiviral efficacy of antiretroviral drugs and microbicides. De-identified semen samples were obtained, following informed consent, from adult donors presenting at the “Kinderwunschzentrum Ulm”, a fertility centre in Ulm, Germany. After ejaculation, samples were allowed to liquefy for approximately 30 min, pooled, aliquoted, and frozen at −20°C. Experiments were performed with freshly thawed aliquots and were approved by the local ethics committee at Ulm university (proposal numbers 351/10 and 156/13). Serial dilutions of antiviral compounds targeting different steps in the viral life cycle were added to TZM-bl and CEMx-M7 reporter cells which were subsequently inoculated with HIV that was treated with buffer, semen or synthetic seminal amyloid. Infection rates were determined by measuring β-galactosidase (TZM-bl) and luciferase (CEMx-M7) activities in luminescence-based assays 3 to 4 days post infection. Similar experiments were performed in primary peripheral blood mononuclear cells (PBMCs) that were inoculated with a luciferase-encoding virus. All experiments were performed in triplicates and the results were confirmed in at least one independent experiment. Data were evaluated by a statistician.
Reagents
CS, PSA, and PNS were obtained from Acros Organics (Morris Plains, NJ), PolyScience, Inc. (Warrington, PA), and BASF (Parsippany, NJ), respectively. SPL7013, the active ingredient of VivaGel®, was obtained from Starpharma and dissolved in PBS (100 mg/ml). Monoclonal Antibodies (2F5, 2G12) were purchased from Polymun. Maraviroc (MVC) was obtained from Pfizer. Nevirapine, Tenofovir disoproxil fumarate and Elvitegravir were purchased from Selleckchem. Indinavir was provided from the NIH AIDS Research and Reference Reagent Program (ARRRP). SEVI was generated as described (8).
Cell culture
Adherent TZM-bl reporter cells (ARRRP) containing lacZ reporter genes under the control of the HIV LTR promoter and suspension CEMx-M7 cells (ARRRP) containing the gene encoding firefly luciferase under the control of the HIV LTR promoter were cultured as described (12). Human peripheral blood mononuclear cells (PBMCs) were prepared as described (8). Unless noted otherwise, all media contained 10% FBS, Penicillin/Streptomycin, L-glutamine, and 50 μg/ml Gentamicin (Gibco).
Infection of TZM-bl cells by mock- or semen-treated HIV in the presence of microbicides
Briefly, liquefied semen derived from > 20 de-identified donors was pooled. Semen samples from de-identified EDO patients were kindly provided by Ole Sorensen (Lund University, Sweden). To examine the activity of candidate microbicides in the presence of semen, 5 × 103 TZM-bl cells were seeded into 96 well flat-bottom plates (100 μl). The next day, when cells were ~70 % confluent, the medium was replaced by fresh medium (250 μl) in the presence of antivirals (30 μl). After 1 hour incubation, semen was mixed 1:1 (volume/volume) with virus in order to obtain semen concentrations of 10% during virion treatment. Viral stocks of R5-tropic HIV-1 NL4-3_92TH014.12 or T/F viruses was obtained by transient transfection of 293T cells with proviral DNA and were titrated on TZM-bl cells (8, 17). After incubating the semen/virus mixture for 5 min, 20 μl of the semen-virus (or PBS-virus control) were then added to compound-treated cells (volume 280 μl). Final cell culture concentrations of semen were 0.67 or 0 %. We used a viral multiplicity of infection (MOI) of ~ 0.05 (corresponding to 0.05 ng p24 antigen of HIV-1 NL4-3_92TH014.12), which gives a low, but consistently detectable level of infection in the absence of semen. This MOI allowed us to detect an enhancing effect of semen, as assessed by an increase in the numbers of infected cells. Furthermore, we used a MOI ~ 0.5 (corresponding to 0.5 ng p24 antigen of HIV-1 NL4-3_92TH014.12) which gives the same detectable level of background infection as a low MOI in the presence of semen. This high MOI is necessary to compare the inhibitory effect of the microbicides in presence and absence of semen, as assessed by a decrease in the numbers of infected cells. After 2 hours of incubation, cells were washed and further cultivated in 200 μl fresh media supplemented with the microbicides. The addition of gentamicin was crucial to prevent bacterial growth. 3 days later, infection rates were measured using the Gal-Screen system (Applied Biosystems).
Infection of PBMCs by mock- or semen-treated HIV in the presence of microbicides
PBMCs were isolated and prepared as described (8). An R5-tropic HIV NL4-3 construct expressing a luciferase was generated by transient transfection of 293T cells as described (8). 2 × 105 activated PBMCs were preincubated with compounds in 280 μl RPMI medium for 1 hour. Cells were then infected with 20 μl of 10% semen or mock-exposed virus containing either 5 or 50 ng of p24 antigen. To remove the cytotoxic semen inoculums, cells were transferred to V-shaped microtiter plates after 2 hours, and pelleted at 1,000 rpm for 5 min. Supernatants were removed, and cells were resuspended in 180 μl of fresh medium with 20 μl of the candidate microbicides. Gaussia-luciferase activities were determined 3 days later. A 1 mg Coelenterazine substrate solution was prepared by adding Coelenterazine to acidified methanol. The substrate was then diluted 1:170 in dilution buffer (0.1 M Tris, 0.3 M sodium ascorbate, pH 7.4). 20 μl of PBMC supernatants were then mixed with 80 μl of Gaussia substrate and luminescence was recorded on an Orion microplate luminometer as relative light units per second (RLU/s). To determine the antiviral activity of Indinavir, cells were pelleted 2 days post infection, and further cultivated in 200 μl fresh media supplemented with the microbicides. Supernatants obtained at day 4 post infection where then assayed for Gaussia-luciferase activity as described above.
Infection of CEMx-M7 cells by mock- or semen-treated HIV in the presence of a protease inhibitor
To examine the activity of the protease inhibitor Indinavir, 2 × 105 CEMx-M7 cells were seeded into 96 well, v-shape plates (250 μl). Then, 30 μl of antivirals were added and incubated for 1 hour. Semen was mixed 1:1 (volume/volume) with virus in order to obtain semen concentrations of 10% during virion treatment. After incubating the Ssemen/virus mixture for 5 min, 20 μl of the semen-virus (or PBS-virus control) were added to compound-treated cells (volume 280 μl). Final cell culture concentrations of semen were 0.67 or 0 %. After 2 hours of incubation, cells were washed and further cultivated in 200 μl fresh media supplemented with the microbicides. 2 days post infection, cells were pelleted and further cultivated in 200 μl fresh media supplemented with the microbicides. 4 days later, infection rates were measured using the Luciferase assay system (Promega).
Infection of TZM-bl cells by mock- or SEVI-treated HIV in the presence of microbicides
SEVI was generated as described (8). To analyze the activity of candidate microbicides in the presence of SEVI, 5 × 103 TZM-bl cells were seeded into 96-well flat-bottom plates (100 μl). The next day, when cells were ~70 % confluent, the medium was replaced by fresh medium (160 μl) in the presence of antivirals (20 μl). After 1 hour incubation, SEVI was mixed 1:1 (volume/volume) with virus in order to obtain SEVI concentrations of 35 μg/ml during virion treatment. After incubating the SEVI/virus mixture for 5 min, 20 μl of the SEVI-virus (or PBS-virus control) were then added to compound-treated cells (volume 180 μl). 3 days later, infection rates were measured using the Gal-Screen system (Applied Biosystems). The effect of SEVI on the antiviral activity of the protease inhibitor was analyzed in CEMx-M7 cells essentially as described above for the analysis of semen, except that virions were treated with 35 μg/ml of SEVI instead of semen.
Infection of TZM-bl cells by HIV treated with semen devoid of its HIV-enhancing activity in the presence of microbicides
Semen samples from de-identified individuals were incubated at 37°C for 48 h resulting in a loss of infectivity enhancing activity (24). 5 × 103 TZM-bl cells were seeded into 96 well flat-bottom plates (100 μl) and the next day, when cells were ~70 % confluent, media were replaced with fresh media (250 μl) in the presence of antivirals (30 μl). After 1 h incubation, incubated semen was mixed 1:1 (volume/volume) with virus in order to obtain semen concentrations of 10% during virion treatment. After incubating the Ssemen/virus mixture for 5 min, 20 μl of the semen-virus (or PBS-virus control) were added to compound-treated cells (volume 280 μl). Final cell culture concentrations of semen were 0.67 or 0 %. After 2 hours of incubation, cells were washed and further cultivated in 200 μl fresh media supplemented with the microbicides. 3 days later, infection rates were measured using the Gal-Screen system (Applied Biosystems).
Cell-to-cell transmission
1 × 104 CaSki cells (epithelial cervical carcinoma cell line), were seeded in 250 μl medium and incubated overnight. The next day, the cells were preincubated with the indicated concentrations of candidate microbicides (30 μl) for 1 hour and then inoculated with 20 μl of semen-treated HIV (92TH014.12) for 2 hours. Inoculum was then removed, and 5 × 104 CEMx-M7 cells were added onto the CaSki cells in the presence of the candidate microbicides. The antiviral activity of candidate microbicides was determined by measuring luciferase activity as described above.
Cell Viability
The effect of semen on metabolic activity of cells was analyzed using the MTT assay under experimental conditions corresponding to those used in the respective infection assays. To assess cell viability for each compound, 1 × 104 TZM-bl cells (in 180 μl) were incubated with 20 μl of the indicated compounds to achieve the indicated final concentration. After 3 days of incubation, 20 μl of a 5 mg/ml MTT (3-(4,5-dimethylthiazole-2-yl)-2,5 diphenyltetrazolium bromide, Sigma #M2003) solution was added to cells. After 3 hours, the cell-free supernatant was discarded, formazan crystals were dissolved in 100 μl DMSO-ethanol (1:1) and OD was detected at 490/650 nM. The % viability rates were calculated relative to NAD(P)H-dependent cellular oxidoreductase activity in PBS-treated cells (100%).
IC50 calculation and statistical analysis
For each compound, IC50s were calculated using the dose-response inhibition model in GraphPad Prism (Graph Pad prism software, Version 5.03). All statistical analyses were calculated in the R software using an unpaired t-test which compared the mean IC50 values derived from three replicate experiments. All results are represented as mean ± standard deviation. Ns: no statistically significant, p>0.05; *, p< 0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001.
Supplementary Material
Decreased antiviral efficacy of SPL7013 against semen-treated transmitted/founder HIV variants.
Polyanions lose antiviral efficacy in the presence of semen and are cytotoxic at elevated concentrations.
Decreased antiviral activity of the 2G12 antibody against semen-treated HIV and lack of cytotoxicity of non-polyanionic compounds.
Decreased antiviral activity of candidate microbicides against SEVI-exposed R5-tropic HIV.
Semen from patients with ejaculatory duct obstruction that lacks amyloid and viral enhancing activity does not antagonize the antiviral activity of a polyanionic microbicide.
Schematic of in vitro assay and results.
Accessible Summary.
Naturally occurring semen factors undermine the effectiveness of HIV microbicides
HIV microbicides potently inhibit the virus in vitro but failed in clinical trials. Semen is the main vector mediating HIV transmission and naturally contains amyloid fibrils that boost HIV infectivity. Here, we show that semen impairs the antiviral efficacy of microbicides that target viral components. Only Maraviroc, which binds to the host CCR5 coreceptor of HIV entry, retains full antiviral activity in the presence of semen. Our results help explain why current microbicides have fallen short in performance when tested in vivo. Our findings further suggest that future in vitro testing of microbicides should be performed in the presence of semen to better predict the antiretroviral efficacy in vivo.
Acknowledgments
We thank O. Sorensen for semen samples from patients with ejaculatory duct obstruction, and A.L. Lucido for editorial assistance.
Funding: Janis A. Müller is funded by a fellowship from the International Graduate School in Molecular Medicine Ulm. This work was further supported, in whole or in part, by DFG grant MU 3115/2-1 - AO 585882 (to J.M.), the Ministerium für Wissenschaft, Forschung und Kunst, Baden-Württemberg D.3268 (to J.M.), the 5K12DK083021-04 and the K99AI104262 (to N.R.R.), the DFG Leibniz award, ERC advanced investigator grant and KFO167 (to F.K.), and the PO1 AI083050-01 (to W. C. G.) and U19 AI076964 (to S.J.).
Footnotes
Competing interests: The authors declare that they have no competing interests.
Author contributions: O.Z. performed experiments shown in Figs. 1–4 and Fig. 5A–C; J.A.M. and S.F.K. supported O.Z. in generation of virus stocks, cell culture and infectivity assays; K.K. established the assays and performed experiments shown in Fig. 5D and Fig. S1; N.R.R. supervised experiments shown in Fig. 4 and Fig. S5F. B.M. performed statistics, S. J. and W.C.G. planned experiments, F.K. and J.M. planned experiments and evaluated results, and F.K., N.R.R. and J.M. wrote the manuscript.
References
- 1.Koff WC, Burton DR, Johnson PR, Walker BD, King CR, Nabel GJ, Ahmed R, Bhan MK, Plotkin SA. Accelerating next-generation vaccine development for global disease prevention. Science. 2013;340:1232910. doi: 10.1126/science.1232910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant RM, Hamer D, Hope T, Johnston R, Lange J, Lederman MM, Lieberman J, Miller CJ, Moore JP, Mosier DE, Richman DD, Schooley RT, Springer MS, Veazey RS, Wainberg MA. Whither or wither microbicides? Science. 2008;321:532–534. doi: 10.1126/science.1160355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanpouille C, Arakelyan A, Margolis L. Microbicides: still a long road to success. Trends Microbiol. 2012;20:369–75. doi: 10.1016/j.tim.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fichorova RN, Tucker LD, Anderson DJ. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J Infect Dis. 2001;184:418–428. doi: 10.1086/322047. [DOI] [PubMed] [Google Scholar]
- 5.McCormack S, Ramjee G, Kamali A, Rees H, Crook AM, Gafos M, Jentsch U, Pool R, Chisembele M, Kapiga S, Mutemwa R, Vallely A, Palanee T, Sookrajh Y, Lacey CJ, Darbyshire J, Grosskurth H, Profy A, Nunn A, Hayes R, Weber J. PRO2000 vaginal gel for prevention of HIV-1 infection (Microbicides Development Programme 301): a phase 3, randomised, double-blind, parallel-group trial. Lancet. 2010;376:1329–1337. doi: 10.1016/S0140-6736(10)61086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D. CAPRISA 004 Trial Group, Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marrazzo J, Ramjee G, Nair G, et al. Pre--exposure prophylaxis for HIV in Women: daily oral tenofovir, oral tenofovir/emtricitabine, or vaginal tenofovir gel in the VOICE Study (MTN 003). 20th Conference on Retroviruses and Opportunistic Infections; March 3–6, 2013; Atlanta. p. Abstract 26LB. http://www.natap.org/2013/CROI/croi_09.htm. [Google Scholar]
- 8.Münch J, Rücker E, Ständker L, Adermann K, Goffinet C, Schindler M, Wildum S, Chinnadurai R, Rajan D, Specht A, Giménez-Gallego G, Sánchez PC, Fowler DM, Koulov A, Kelly JW, Mothes W, Grivel JC, Margolis L, Keppler OT, Forssmann WG, Kirchhoff F. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell. 2007;131:1059–1071. doi: 10.1016/j.cell.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Hauber I, Hohenberg H, Holstermann B, Hunstein W, Hauber J. The main green tea polyphenol epigallocatechin-3-gallate counteracts semen-mediated enhancement of HIV infection. Proc Natl Acad Sci U S A. 2009;106:9033–9038. doi: 10.1073/pnas.0811827106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roan NR, Sowinski S, Münch J, Kirchhoff F, Greene WC. Aminoquinoline surfen inhibits the action of SEVI (semen-derived enhancer of viral infection) J Biol Chem. 2010;285:1861–1869. doi: 10.1074/jbc.M109.066167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsen JS, Brown C, Capule CC, Rubinshtein M, Doran TM, Srivastava RK, Feng C, Nilsson BL, Yang J, Dewhurst S. Amyloid-binding small molecules efficiently block SEVI (semen-derived enhancer of virus infection)- and semen-mediated enhancement of HIV-1 infection. J Biol Chem. 2010;285:35488–35496. doi: 10.1074/jbc.M110.163659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roan NR, Müller JA, Liu H, Chu S, Arnold F, Stürzel CM, Walther P, Dong M, Witkowska HE, Kirchhoff F, Münch J, Greene WC. Peptides released by physiological cleavage of semen coagulum proteins form amyloids that enhance HIV infection. Cell Host Microbe. 2011;10:541–550. doi: 10.1016/j.chom.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim KA, Yolamanova M, Zirafi O, Roan NR, Staendker L, Forssmann WG, Burgener A, Dejucq-Rainsford N, Hahn BH, Shaw GM, Greene WC, Kirchhoff F, Münch J. Semen-mediated enhancement of HIV infection is donor-dependent and correlates with the levels of SEVI. Retrovirology. 2010;7:5. doi: 10.1186/1742-4690-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernstein DI, Stanberry LR, Sacks S, Ayisi NK, Gong YH, Ireland J, Mumper RJ, Holan G, Matthews B, McCarthy T, Bourne N. Evaluations of unformulated and formulated dendrimer-based microbicide candidates in mouse and guinea pig models of genital herpes. Antimicrob Agents Chemother. 2003;47:3784–3788. doi: 10.1128/AAC.47.12.3784-3788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price CF, Tyssen D, Sonza S, Davie A, Evans S, Lewis GR, Xia S, Spelman T, Hodsman P, Moench TR, Humberstone A, Paull JR, Tachedjian G. SPL7013 Gel (VivaGel®) retains potent HIV-1 and HSV-2 inhibitory activity following vaginal administration in humans. PLoS One. 2011;6:e24095. doi: 10.1371/journal.pone.0024095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGowan I, Gomez K, Bruder K, Febo I, Chen BA, Richardson BA, Husnik M, Livant E, Price C, Jacobson C. AIDS, Phase 1 randomized trial of the vaginal safety and acceptability of SPL7013 gel (VivaGel) in sexually active young women (MTN-004)) AIDS. 2011;25:1057–1064. doi: 10.1097/QAD.0b013e328346bd3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochsenbauer C, Edmonds TG, Ding H, Keele BF, Decker J, Salazar MG, Salazar-Gonzalez JF, Shattock R, Haynes BF, Shaw GM, Hahn BH, Kappes JC. Generation of transmitted/founder HIV-1 infectious molecular clones and characterization of their replication capacity in CD4 T lymphocytes and monocyte-derived macrophages. J Virol. 2012;86:2715–28. doi: 10.1128/JVI.06157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohan P, Schols D, Baba M, De Clercq E. Sulfonic acid polymers as a new class of human immunodeficiency virus inhibitors. Antiviral Res. 1992;18:139–50. doi: 10.1016/0166-3542(92)90034-3. [DOI] [PubMed] [Google Scholar]
- 19.Rusconi S, Moonis M, Merrill DP, Pallai PV, Neidhardt EA, Singh SK, Willis KJ, Osburne MS, Profy AT, Jenson JC, Hirsch MS. Naphthalene sulfonate polymers with CD4-blocking and anti-human immunodeficiency virus type 1 activities. Antimicrob Agents Chemother. 1996;40:234–236. doi: 10.1128/aac.40.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huskens D, Vermeire K, Profy AT, Schols D. The candidate sulfonated microbicide, PRO 2000, has potential multiple mechanisms of action against HIV-1. Antiviral Res. 2009;84:38–47. doi: 10.1016/j.antiviral.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Scordi-Bello IA, Mosoian A, He C, Chen Y, Cheng Y, Jarvis GA, Keller MJ, Hogarty K, Waller DP, Profy AT, Herold BC, Klotman ME. Candidate sulfonated and sulfated topical microbicides: comparison of anti-human immunodeficiency virus activities and mechanisms of action. Antimicrob Agents Chemother. 2005;49:3607–3615. doi: 10.1128/AAC.49.9.3607-3615.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Damme L, Govinden R, Mirembe FM, Guédou F, Solomon S, Becker ML, Pradeep BS, Krishnan AK, Alary M, Pande B, Ramjee G, Deese J, Crucitti T, Taylor D. CS Study Group, Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl J Med. 2008;359:463–472. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 23.Adool Karim SS, Richardson BA, Ramjee G, Hoffman IF, Chirenje ZM, Taha T, Kapina M, Maslankowski L, Coletti A, Profy A, Moench TR, Piwowar-Manning E, Mâsse B, Hillier SL, Soto-Torres L HIV Prevention Trials Network (HPTN) 035 Study Team. Safety and effectiveness of BufferGel and 0.5% PRO2000 gel for the prevention of HIV infection in women. AIDS. 2011;25:957–966. doi: 10.1097/QAD.0b013e32834541d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roan NR, Liu H, Usmani SM, Neidleman J, Müller JA, Avila-Herrera A, Gawanbacht A, Zirafi O, Chu S, Dong M, Kumar ST, Smith JF, Pollard KS, Fändrich M, Kirchhoff F, Münch J, Witkowska HE, Greene WC. Liquefaction of Semen Generates and Later Degrades a Conserved Semenogelin Peptide That Enhances HIV Infection. J Virol. 2014;88:7221–7234. doi: 10.1128/JVI.00269-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fätkenheuer G, Pozniak AL, Johnson MA, Plettenberg A, Staszewski S, Hoepelman AI, Saag MS, Goebel FD, Rockstroh JK, Dezube BJ, Jenkins TM, Medhurst C, Sullivan JF, Ridgway C, Abel S, James IT, Youle M, van der Ryst E. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat Med. 2005;11:1170–1172. doi: 10.1038/nm1319. [DOI] [PubMed] [Google Scholar]
- 26.Veazey RS, Ketas TJ, Dufour J, Moroney-Rasmussen T, Green LC, Klasse PJ, Moore JP. Protection of rhesus macaques from vaginal infection by vaginally delivered maraviroc, an inhibitor of HIV-1 entry via the CCR5 co-receptor. J Infect Dis. 2010;202:739–744. doi: 10.1086/655661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumond JB, Patterson KB, Pecha AL, Werner RE, Andrews E, Damle B, Tressler R, Worsley J, Kashuba AD. Maraviroc concentrates in the cervicovaginal fluid and vaginal tissue of HIV-negative women. J Acquir Immune Defic Syndr. 2009;51:546–553. doi: 10.1097/QAI.0b013e3181ae69c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karim QA, Baxter C. Microbicides for the prevention of sexually transmitted HIV infection. Expert Rev Anti Infect Ther. 2013;11:13–23. doi: 10.1586/eri.12.153. [DOI] [PubMed] [Google Scholar]
- 29.Neurath AR, Strick N, Li YY. Role of seminal plasma in the anti-HIV-1 activity of candidate microbicides. BMC Infect Dis. 2006;6:150. doi: 10.1186/1471-2334-6-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lackman-Smith C, Osterling C, Luckenbaugh K, Mankowski M, Snyder B, Lewis G, Paull J, Profy A, Ptak RG, Buckheit RW, Jr, Watson KM, Cummins JE, Jr, Sanders-Beer BE. Development of a comprehensive human immunodeficiency virus type 1 screening algorithm for discovery and preclinical testing of topical microbicides. Antimicrob Agents Chemother. 2008;52:1768–1781. doi: 10.1128/AAC.01328-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan S, Lu L, Li L, Liu J, Oksov Y, Lu H, Jiang S, Liu S. Polyanionic candidate microbicides accelerate the formation of semen-derived amyloid fibrils to enhance HIV-1 infection. PLoS One. 2013;8:e59777. doi: 10.1371/journal.pone.0059777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keller MJ, Mesquita PM, Torres NM, Cho S, Shust G, Madan RP, Cohen HW, Petrie J, Ford T, Soto-Torres L, Profy AT, Herold BC. Postcoital bioavailability and antiviral activity of 0.5% PRO 2000 gel: implications for future microbicide clinical trials. PLoS One. 2010;5:e87812010. doi: 10.1371/journal.pone.0008781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herold BC, Mesquita PM, Madan RP, Keller MJ. Female genital tract secretions and semen impact the development of microbicides for the prevention of HIV and other sexually transmitted infections. Am J Reprod Immunol. 2011;65:325–33. doi: 10.1111/j.1600-0897.2010.00932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel S, Hazrati E, Cheshenko N, Galen B, Yang H, Guzman E, Wang R, Herold BC, Keller MJ. Seminal plasma reduces the effectiveness of topical polyanionic microbicides. J Infect Dis. 2007;196:1394–1402. doi: 10.1086/522606. [DOI] [PubMed] [Google Scholar]
- 35.Arnold F, Schnell J, Zirafi O, Stürzel C, Meier C, Weil T, Ständker L, Forssmann WG, Roan NR, Greene WC, Kirchhoff F, Münch J. Naturally occurring fragments from two distinct regions of the prostatic acid phosphatase form amyloidogenic enhancers of HIV infection. J Virol. 2012;86:1244–1249. doi: 10.1128/JVI.06121-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Usmani SM, Zirafi O, Müller JA, Sandi-Monroy NL, Yadav JK, Meier C, Weil T, Roan NR, Greene WC, Walther P, Nilsson KP, Hammarström P, Wetzel R, Pilcher CD, Gagsteiger F, Fändrich M, Kirchhoff F, Münch J. Direct visualization of HIV-enhancing endogenous amyloid fibrils in human semen. Nat Commun. 2014;5:3508. doi: 10.1038/ncomms4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mondor I, Ugolini S, Sattentau QJ. Human immunodeficiency virus type 1 attachment to HeLa CD4 cells is CD4 independent and gp120 dependent and requires cell surface heparans. J Virol. 1998;72:3623–3634. doi: 10.1128/jvi.72.5.3623-3634.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yolamanova M, Meier C, Shaytan AK, Vas V, Bertoncini CW, Arnold F, Zirafi O, Usmani SM, Müller JA, Sauter D, Goffinet C, Palesch D, Walther P, Roan NR, Geiger H, Lunov O, Simmet T, Bohne J, Schrezenmeier H, Schwarz K, Ständker L, Forssmann WG, Salvatella X, Khalatur PG, Khokhlov AR, Knowles TP, Weil T, Kirchhoff F, Münch J. Peptide nanofibrils boost retroviral gene transfer and provide a rapid means for concentrating viruses. Nat Nanotechnol. 2013;8:130–136. doi: 10.1038/nnano.2012.248. [DOI] [PubMed] [Google Scholar]
- 39.Sigal A, Kim JT, Balazs AB, Dekel E, Mayo A, Milo R, Baltimore D. Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature. 2011;477:95–98. doi: 10.1038/nature10347. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Perez J, Rueda P, Alcami J, Rognan D, Arenzana-Seisdedos F, Lagane B, Kellenberger E. Allosteric model of maraviroc binding to CC chemokine receptor 5 (CCR5) J Biol Chem. 2011;286:33409–33421. doi: 10.1074/jbc.M111.279596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan Q, Zhu Y, Li J, Chen Z, Han GW, Kufareva I, Li T, Ma L, Fenalti G, Li J, Zhang W, Xie X, Yang H, Jiang H, Cherezov V, Liu H, Stevens RC, Zhao Q, Wu B. Structure of the CCR5 chemokine receptor-HIV entry inhibitor maraviroc complex. Science. 2013;341:1387–1390. doi: 10.1126/science.1241475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins KB, Patterson BK, Naus GJ, Landers DV, Gupta P. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat Med. 2000;6:475–479. doi: 10.1038/74743. [DOI] [PubMed] [Google Scholar]
- 43.Fox-Canale AM, Hope TJ, Martinson J, Lurain JR, Rademaker AW, Bremer JW, Landay A, Spear GT, Lurain NS. Human cytomegalovirus and human immunodeficiency virus type-1 co-infection in human cervical tissue. Virology. 2007;369:55–68. doi: 10.1016/j.virol.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenhead P, Hayes P, Watts PS, Laing KG, Griffin GE, Shattock RJ. Parameters of Human Immunodeficiency Virus Infection of Human Cervical Tissue and Inhibition by Vaginal Virucides. J Virol. 2000;74:5577–5586. doi: 10.1128/jvi.74.12.5577-5586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fletcher P, Kiselyeva Y, Wallace G, Romano J, Griffin G, Margolis L, Shattock R. The Nonnucleoside Reverse Transcriptase Inhibitor UC-781 Inhibits Human Immunodeficiency Virus Type 1 Infection of Human Cervical Tissue and Dissemination by Migratory Cells. J Virol. 2005;79:11179–11186. doi: 10.1128/JVI.79.17.11179-11186.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cummins JE, Jr, Guarner J, Flowers L, Guenthner PC, Bartlett J, Morken T, Grohskopf LA, Paxton L, Dezzutti CS. Preclinical Testing of Candidate Topical Microbicides for Anti-Human Immunodeficiency Virus Type 1 Activity and Tissue Toxicity in a Human Cervical Explant Culture. Antimicrob Agents Chemother. 2007;51:1770–1779. doi: 10.1128/AAC.01129-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Münch J, Sauermann U, Yolamanova M, Raue K, Stahl-Hennig C, Kirchhoff F. Effect of semen and seminal amyloid on vaginal transmission of simian immunodeficiency virus. Retrovirology. 2013;10:148. doi: 10.1186/1742-4690-10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Del Prete GQ, Ailers B, Moldt B, Keele BF, Estes JD, Rodriguez A, Sampias M, Oswald K, Fast R, Trubey CM, Chertova E, Smedley J, LaBranche CC, Montefiori DC, Burton DR, Shaw GM, Markowitz M, Piatak M, Jr, KewalRamani VN, Bieniasz PD, Lifson JD, Hatziioannou T. Selection of Unadapted Pathogenic SHIVs Encoding Newly Transmitted HIV-1 Envelope Proteins. Cell Host Microbe. 2014;16:412–418. doi: 10.1016/j.chom.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Capule CC, Brown C, Olsen JS, Dewhurst S, Yang J. Oligovalent amyloid-binding agents reduce SEVI-mediated enhancement of HIV-1 infection. J Am Chem Soc. 2012;134:905–908. doi: 10.1021/ja210931b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang J, Li L, Jin H, Tan S, Qiu J, Yang L, Ding Y, Jiang ZH, Jiang S, Liu S. Vaginal gel formulation based on theaflavin derivatives as a microbicide to prevent HIV sexual transmission. AIDS Res Hum Retroviruses. 2012;28:1498–1508. doi: 10.1089/AID.2012.0084. [DOI] [PubMed] [Google Scholar]
- 51.Sheftic SR, Snell JM, Jha S, Alexandrescu AT. Inhibition of semen-derived enhancer of virus infection (SEVI) fibrillogenesis by zinc and copper. Eur Biophys J. 2012;41:695–704. doi: 10.1007/s00249-012-0846-0. [DOI] [PubMed] [Google Scholar]
- 52.Sievers SA, Karanicolas J, Chang HW, Zhao A, Jiang L, Zirafi O, Stevens JT, Münch J, Baker D, Eisenberg D. Structure-based design of non-natural amino-acid inhibitors of amyloid fibril formation. Nature. 2011;475:96–100. doi: 10.1038/nature10154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Decreased antiviral efficacy of SPL7013 against semen-treated transmitted/founder HIV variants.
Polyanions lose antiviral efficacy in the presence of semen and are cytotoxic at elevated concentrations.
Decreased antiviral activity of the 2G12 antibody against semen-treated HIV and lack of cytotoxicity of non-polyanionic compounds.
Decreased antiviral activity of candidate microbicides against SEVI-exposed R5-tropic HIV.
Semen from patients with ejaculatory duct obstruction that lacks amyloid and viral enhancing activity does not antagonize the antiviral activity of a polyanionic microbicide.
Schematic of in vitro assay and results.