Abstract
It has been approximately 50 years since neurologists were introduced to the entities progressive supranuclear palsy and corticobasal degeneration. Since the two seminal publications, there have been significant advancements in our understanding of these two neurodegenerative diseases, particularly the fact that both are associated with tau. Recent advances over the past 3 years that are notable to the field are discussed in this review that covers clinical diagnosis, pathological features, neuroimaging and CSF biomarkers, genetic associations and clinical trials related to progressive supranuclear palsy and corticobasal degeneration.
Keywords: PSP, corticobasal, tau, update, MRI, GWAS, Davunetide, longitudinal, Tideglusib, CSF
Introduction
Progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD) are neurodegenerative diseases first described almost half a century ago [1, 2] that are both characterized by the deposition of tau, a microtubule associated protein, in an altered aberrant form. Significant advancements have been made over the past half century for both PSP and CBD. In fact, clinical trials have already been completed in PSP. This review covers the most important advancements over the past 3 years in the areas of clinical diagnosis, pathological features, neuroimaging and cerebrospinal fluid (CSF) biomarkers, genetic associations and clinical trials.
Clinical diagnosis
Clinical criteria for PSP have been previously established by the National Institute of Neurological Disease and Stroke and the Society for Progressive Supranuclear Palsy (NINDS-SPSP) [3]. The NINDS-SPSP criteria separate PSP into two diagnostic categories: possible PSP which has high sensitivity and average specificity, and probable PSP which has high specificity and average sensitivity for PSP pathology. More recently, another criterion, the Neuroprotection and Natural History in Parkinson Plus Syndromes (NNIPPS) criteria was published [4]. A recent study compared the NINDS-SPSP criteria to the NNIPPS criteria [5]. The authors concluded that NINDS-SPSP probable criteria should be reserved for clinical trials where specificity is of the utmost importance, whereas a combination of the NINDS possible and probable criteria is best for making diagnosis in routine clinical practice.
Another area worth mentioning is that of clinical syndromes associated with PSP (Figure 1). It has been known for over a decade now that the pathological findings of PSP, with the characteristic tau deposition, can be associated with many different clinical syndromes. In a recent study of 100 pathologically confirmed cases of PSP, the authors found that less than a quarter had the expected typical presentation of PSP [6]. Surprising many had presenting clinical features that did not fit the proposed clinical criteria for PSP [3]. One such clinical presentation that has garnished a lot of attention is that of apraxia of speech. It has been shown that patients who present with an isolated motor speech disorder and classified as having a primary progressive apraxia of speech (PPAOS) [7] later develop other motor features suggestive of PSP [8]. In fact, PSP pathology has been identified in such patients, and hence PPAOS could be an important clinical biomarker of abnormal tau. This is even more important since patients with PPAOS have relatively little functional impairment at the time of presentation, while a recent study has demonstrated that functional disability is high early on in patients with the typical presentation associated with PSP pathology [9].
Figure 1.
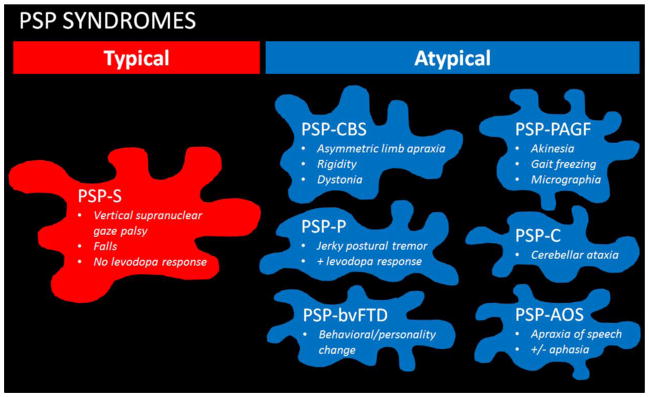
Early presenting syndromes associated with PSP pathology. PSP clinical syndromes are first divided into the typical PSP syndrome and the atypical PSP syndromes. The typical PSP syndrome, or PSP-S, is also sometimes referred to as Richardson’s syndrome (PSP-RS) and is the most common presenting syndrome. The atypical PSP syndromes include PSP presenting as the corticobasal syndrome (PSP-CBS), as Parkinson’s disease-like (PSP-P), with progressive akinesia/gait freezing (PSP-PAGF), with cerebellar ataxia (PSP-C), as the behavioural variant of frontotemporal dementia (PSP-bvFTD) and with apraxia of speech with or without aphasia (PSP-AOS). Of note, PSP-AOS is sometimes incorrectly referred to as PSP-PNFA for non-fluent aphasia.
Like PSP, CBD is characterised by abnormal tau deposition. Corticobasal degeneration tau is morphologically and biochemically distinct from PSP tau. Unlike PSP where a diagnosis of PSP is highly predictive of the underlying pathology, no one clinical syndrome is highly predictive of CBD. Recently, an international consortium was assembled to develop criteria for CBD. The results were a publication with two sets of criteria that mirrored the criteria for PSP. That is, a probable CBD criteria for research purposes and a possible criteria with the intent to be more inclusive to capture other tau-based pathologies such as PSP [10]. Unfortunately, one study assessing the validity of the CBD criteria for research concluded that the new probable criteria was not specific as many of patients in their study met criteria for possible or probable CBD, yet did not have CBD pathology [11]. A second study also demonstrated that the clinical criteria for possible CBD were not very sensitive within the first two years of disease onset [12].
Pathological features
Pathological features of PSP and CBD have been well defined for over a decade. Both diseases are characterised by abnormal tau deposition that affects neurons and glial cells, including astrocytes and oligodendroglia cells. Little is understood, however, regarding astrocytic phenotype which differs between PSP (tufted astrocyte) and CBD (astrocytic plaques). Recently, however, it has been demonstrated that astrocytic differences mirror neuronal differences and depend on many factors such as the primary amino acid sequence of the tau, which specific sites are abnormally phosphorylated, and other post translational modifications, as well as modifications to the cytoskeleton of the astrocytes [13].
Another recent study has demonstrated that there are tau oligomers in PSP that are an important component of the pathology that defines PSP. In fact, the authors demonstrated that oligomers were able to seed other oligomers and concluded that it is the oligomers, and not the neuronal and glial pathology, that are responsible for the progressive nature of PSP [14]. In-keeping with the phenomenon of cell to cell transmission, another group of investigators injected brain extracts from humans who had died with PSP into different brain regions of mice and were able to produce the characteristic lesions of PSP [15]. These findings suggest that once tau aggregates are formed in specific brain regions in PSP they then become self-propagating and can spread in a prionoid manner.
Neuroimaging and CSF biomarkers
One of the most important developments in the neurodegenerative field is the search for biomarkers that can predict underlying pathology; specifically the protein that defines the pathology. For PSP and CBD, that protein is tau. As discussed above, no one clinical syndrome is predictive of PSP or CBD pathology and hence tau deposition. Two areas of significance related to biomarker development are neuroimaging techniques and CSF biomarkers.
Neuroimaging techniques, particularly the assessment of structural MRI, have been applied to the study of PSP and CBD for almost a decade. However, with the advent of newer techniques, the field has seen a blossoming of neuroimaging studies on PSP and CBD. Two such techniques that have received recent attention are the use of tract-based spatial statistics to analyze diffusion tensor imaging data, and resting-state or task-free functional MRI. The former allows the assessment of the integrity of white matter tracts, while the later allows the assessment of functional connectivity across the brain. Studies comparing patients with typical PSP to controls observed white matter tract degeneration in the superior cerebellar peduncles that connect the cerebellum and thalamus, as well as in the superior longitudinal fasciculus [16]. Two others studies have assessed white matter tract degeneration in PSP compared to other neurodegenerative diseases, including Parkinson’s disease and multiple system atrophy [17], and corticobasal syndrome [18]. Both studies found differences in white matter tract degeneration between these different neurodegenerative syndromes. Unfortunately, all studies are lacking pathological confirmation and hence, while important, will need to be replicated in pathologically confirmed cohorts. Similar to diffusion tensor imaging studies, two studies have assessed network connectivity in clinically diagnosed patients with typical PSP [16, 19]. These studies utilized somewhat different analysis methods, yet the findings were basically identical showing disruption of network connectivity between the cerebellum, midbrain, thalamus and premotor cortex.
In addition to studies utilizing these new techniques, two recent studies have also used structural MRI to investigate midbrain atrophy as a potential biomarker of pathologically-confirmed PSP. In one study, the authors concluded that the midbrain to pons ratio was a reliable measurement with high sensitivity and specificity to PSP [20]. Unfortunately, only subjects with the typical PSP syndrome were included in that study. No subjects with any of the other atypical clinical variants discussed above were included. In another study, the authors did include cases of PSP that had presented with one of the atypical clinical diagnoses [21]. They also found that midbrain atrophy was sensitive and specific to PSP when the clinical presentation was typical. However, midbrain atrophy was not a useful biomarker of pathology in patients presenting with one of the atypical clinical syndromes, hence midbrain atrophy appears to correlate to the typical PSP syndrome, and not PSP pathology.
Three studies have assessed longitudinal changes in PSP on MRI over time. The first study demonstrated that changes over time in grey and white matter can be detected in PSP and showed that changes over time correlated with the PSP rating scale; hence validating the PSP rating scale as a useful measure of disease progression over time [22]. A second study showed that changes over time can be useful to differentiate patients with typical PSP from multiple system atrophy [23]; although again autopsy was lacking. The third study modelled changes over time in multiple grey matter regions in typical PSP and demonstrated that rates of regional atrophy in PSP exceeded those of controls and correlated with the observed clinical changes that occur over time in PSP [24]. In most brain regions rates of change were linear, although in some regions they were non-linear.
In addition to MRI techniques, there have been four recent studies that have assessed metabolic changes on 18F-flurodeoxyglocose positron emission tomography (FDG-PET) in PSP and CBD. Of these four studies, one included autopsy confirmed PSP [25] and another autopsy confirmed CBD [25, 26]. One study identified a focal area of midbrain hypometabolism in patients with typical PSP, referred to as the pimple sign of PSP that corresponds to midbrain atrophy on MRI [27]. This sign could be a useful diagnostic marker for typical PSP, although it may not be a useful biomarker of PSP for atypical PSP syndromes. This midbrain area was found to also be affected, in addition to the caudate, thalamus and supplementary motor area in typical PSP in another study with autopsy confirmation [25] (Figure 2). In patients with autopsy confirmed CBD from two studies, the parietal lobe was affected [25, 26] (Figure 2); an area that was not seen to be affected in PSP [25]. Hence, parietal hypometabolism may be a biomarker of CBD pathology. The fourth study demonstrated that hypometabolism of the prefrontal cortex, subthalamic nucleus and pedunculopontine/cuneiform nuclear complex correlated with gait dysfunction in PSP [28], suggesting that these areas may be responsible for the gait and balance problems that haunts patients with PSP.
Figure 2.
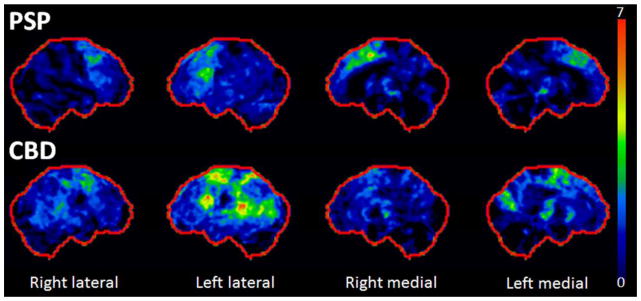
18F-Flurodeoxyglucose PET patterns of hypometabolism in autopsy confirmed PSP and CBD. Results are shown as surface stereotactic projection maps representing Z-scores compared to controls. In PSP, hypometabolism is observed in bilateral premotor extending into prefrontal cortex, supplementary motor area and midbrain. In CBD, hypometabolism is observed predominantly in left frontal and parietal cortices.
Another area that is ripe for biomarker development is CSF analysis. One recent study compared CSF beta-amyloid, total tau, phosphorylated tau and C and N terminal tau fragments in PSP, Alzheimer’s disease and normal controls [29]. The authors found that the most useful CSF marker that differentiated PSP from Alzheimer’s diseases was the C and N terminal fragments. Hence, C and N terminal fragments of tau have the potential to be good biomarkers of PSP pathology and now needs to be replicated in a pathologically confirmed cohort.
Genetic associations
Progressive supranuclear palsy and CBD have never been strongly considered as diseases that are due to genetic abnormalities. There has been, however, some evidence that there may be some genetic influences. In a large genome wide association study of autopsy confirmed PSP, three risk loci that implicate three genes, STX6, EIF2AK3 and MOBP, were identified as being associated with PSP [30]. Additionally, two independent variants in the MAPT gene were identified [30]. The genes identified are of interest as these genes are felt to be involved with proteins that function at the fusion sites between the endosome and the Golgi complexes. In a follow-up study from a different group of investigators that assessed for coding changes, given that genetic associations are often in linkage disequilibrium with the causative polymorphism, none were identified [31].
There have also been two reports of genetic abnormalities identified in patients with pathologically confirmed CBD. In one, the authors identified mutations in the MRS2 and ZHX2 genes in two cousins with CBD [32], while in the other study a novel mutation in the MAPT gene on exon 13, p.N410H was identified [33]. In addition, there was one report of a mutation in the MAPT gene, the A152T mutation, that was associated with a variant of PSP, known as the pallido-nigro-luysial degeneration variant of PSP [34]. All these cases may, however, represent rare associations, as no other genetic abnormalities have been identified in PSP or CBD. Hence, at present, especially given the rarity of a significant family history in PSP and CBD, both diseases should be considered sporadic until proven otherwise.
Clinical trials
One of the most important advances has been the completion of clinical trials in PSP. In 2013, the first clinical trial of a therapy targeting tau was reported in PSP [35]. Tideglusib, a glycogen synthase kinase 3 inhibitor, was assessed in a double blind placebo controlled randomized trial that assessed efficacy, safety and tolerability. One hundred and forty six PSP patients received one of two doses, or a placebo, over 52 weeks. The primary endpoint was change in the PSP rating scale. Tideglusib was found to be safe and generally well tolerated but did not show any clinical efficacy. A second trial targeting tau was reported in 2014 [36]. Davunetide (AL-108, NAP), a drug that affects microtubule stability, was assessed in a double blind placebo controlled randomized trial that assessed safety and efficacy. Three hundred and thirteen PSP patients received either davunetide or placebo. The primary endpoints were change from baseline in the PSP rating scale and the England Activities of Daily Living scale. Davunetide was found to be safe and generally well tolerated but did not show any clinical efficacy.
Two recent studies have assessed rates of decline in PSP as a means to determine sample size estimates for future clinical trials. The first study determined sample size estimates over 6 and 12 months using both clinical and imaging metrics [37]. The smallest sample size estimates for treatment trials over 6 months was observed using rate of midbrain atrophy, followed by whole brain atrophy and ventricular expansion. Estimates were lower over a 12 month interval and estimates were better than those obtained with clinical metrics, including the PSP rating scale. The second study focused on assessing decline in clinical measures over 12 months [38]. The smallest sample size estimates were observed using the PSP rating scale oculomotor score, followed by the Mini-Mental State Examination, and then the Unified Parkinson’s disease rating scale total activities of daily living score. Hence, overall, sample size estimates are feasible for clinical trials using both clinical and imaging metrics, although the later appears to be superior.
Final reflections
There have been great advancements in our understanding of PSP and CBD. We have come a far way in 50 years from the original description of two diseases, to the undertaking of clinical trials focusing on hyperphosphorylated tau which is felt to be at the core of the neurodegenerative process in both diseases. With that said however, there is still a lot to be done. Imaging PET ligands directed at tau are now available [39, 40]. However, there are no published reports of these tau biomarkers in PSP or CBD patients. If these ligands in fact are specific to tau, they would likely become one of the main outcome measures in clinical trials, although showing a reduction in tau may not be essential to prove efficacy of a drug. Regardless, a tau ligand would be a preferred method over CSF analysis given the non-invasive nature. Future genetic studies are also needed to better understand whether genetics plays any role in these two diseases. Additional treatment trials are also needed but should be based on our understanding of the pathogenesis of these diseases and not just a trial of medicines that exist for other neurodegenerative diseases without any mechanistic basis. While targeting hyperphosporylated tau is reasonable, it is still unclear why tau becomes hyperphosphorylated and whether the problem is upstream to tau hyperphosphorylation. Recent evidence suggests cell-to-cell transmission of tau in a prionoid like fashion and hence novel approaches taking this into account could also be fruitful. Additionally, more research is needed on the atypical presentations of PSP, since they are no less important than the typical presentations of PSP. In fact, some might argue that some of the atypical presentations result in less functional impairment at onset, for example PSP-AOS, and hence it might be easier to detect drug related change and hence efficacy in some of the atypical syndromes.
References
- 1.Rebeiz JJ, Kolodny EH, Richardson EP., Jr Corticodentatonigral degeneration with neuronal achromasia. Arch Neurol. 1968;18:20–33. doi: 10.1001/archneur.1968.00470310034003. [DOI] [PubMed] [Google Scholar]
- 2.Steele JC, Richardson JC, Olszewski J. Progressive Supranuclear Palsy. A Heterogeneous Degeneration Involving the Brain Stem, Basal Ganglia and Cerebellum with Vertical Gaze and Pseudobulbar Palsy, Nuchal Dystonia and Dementia. Arch Neurol. 1964;10:333–359. doi: 10.1001/archneur.1964.00460160003001. [DOI] [PubMed] [Google Scholar]
- 3.Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, Hallett M, Jankovic J, Quinn NP, Tolosa E, Zee DS. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Bensimon G, Ludolph A, Agid Y, Vidailhet M, Payan C, Leigh PN. Riluzole treatment, survival and diagnostic criteria in Parkinson plus disorders: the NNIPPS study. Brain. 2009;132:156–171. doi: 10.1093/brain/awn291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Respondek G, Roeber S, Kretzschmar H, Troakes C, Al-Sarraj S, Gelpi E, Gaig C, Chiu WZ, van Swieten JC, Oertel WH, Hoglinger GU. Accuracy of the National Institute for Neurological Disorders and Stroke/Society for Progressive Supranuclear Palsy and neuroprotection and natural history in Parkinson plus syndromes criteria for the diagnosis of progressive supranuclear palsy. Mov Disord. 2013;28:504–509. doi: 10.1002/mds.25327. [DOI] [PubMed] [Google Scholar]
- 6.Respondek G, Stamelou M, Kurz C, Ferguson LW, Rajput A, Chiu WZ, van Swieten JC, Troakes C, Al Sarraj S, Gelpi E, Gaig C, Tolosa E, Oertel WH, Giese A, Roeber S, Arzberger T, Wagenpfeil S, Hoglinger GU. The phenotypic spectrum of progressive supranuclear palsy: A retrospective multicenter study of 100 definite cases. Mov Disord. 2014 doi: 10.1002/mds.26054. [DOI] [PubMed] [Google Scholar]
- 7.Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV, Lowe VJ, Jack CR, Jr, Whitwell JL. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain. 2012;135:1522–1536. doi: 10.1093/brain/aws032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Gunter JL, Schwarz CG, Reid RI, Spychalla AJ, Lowe VJ, Jack CR, Jr, Whitwell JL. The evolution of primary progressive apraxia of speech. Brain. 2014;137:2783–2795. doi: 10.1093/brain/awu223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duff K, Gerstenecker A, Litvan I. Functional impairment in progressive supranuclear palsy. Neurology. 2013;80:380–384. doi: 10.1212/WNL.0b013e31827f0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, Boxer AL, Dickson DW, Grossman M, Hallett M, Josephs KA, Kertesz A, Lee SE, Miller BL, Reich SG, Riley DE, Tolosa E, Troster AI, Vidailhet M, Weiner WJ. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80:496–503. doi: 10.1212/WNL.0b013e31827f0fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander SK, Rittman T, Xuereb JH, Bak TH, Hodges JR, Rowe JB. Validation of the new consensus criteria for the diagnosis of corticobasal degeneration. J Neurol Neurosurg Psychiatry. 2014;85:925–929. doi: 10.1136/jnnp-2013-307035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouchi H, Toyoshima Y, Tada M, Oyake M, Aida I, Tomita I, Satoh A, Tsujihata M, Takahashi H, Nishizawa M, Shimohata T. Pathology and sensitivity of current clinical criteria in corticobasal syndrome. Mov Disord. 2014;29:238–244. doi: 10.1002/mds.25746. [DOI] [PubMed] [Google Scholar]
- 13.Ferrer I, Lopez-Gonzalez I, Carmona M, Arregui L, Dalfo E, Torrejon-Escribano B, Diehl R, Kovacs GG. Glial and neuronal tau pathology in tauopathies: characterization of disease-specific phenotypes and tau pathology progression. J Neuropathol Exp Neurol. 2014;73:81–97. doi: 10.1097/NEN.0000000000000030. [DOI] [PubMed] [Google Scholar]
- 14.Gerson JE, Sengupta U, Lasagna-Reeves CA, Guerrero-Munoz MJ, Troncoso J, Kayed R. Characterization of tau oligomeric seeds in progressive supranuclear palsy. Acta Neuropathol Commun. 2014;2:73. doi: 10.1186/2051-5960-2-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clavaguera F, Akatsu H, Fraser G, Crowther RA, Frank S, Hench J, Probst A, Winkler DT, Reichwald J, Staufenbiel M, Ghetti B, Goedert M, Tolnay M. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc Natl Acad Sci U S A. 2013;110:9535–9540. doi: 10.1073/pnas.1301175110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitwell JL, Avula R, Master A, Vemuri P, Senjem ML, Jones DT, Jack CR, Jr, Josephs KA. Disrupted thalamocortical connectivity in PSP: a resting-state fMRI, DTI, and VBM study. Parkinsonism Relat Disord. 2011;17:599–605. doi: 10.1016/j.parkreldis.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Worker A, Blain C, Jarosz J, Chaudhuri KR, Barker GJ, Williams SC, Brown RG, Leigh PN, Dell’Acqua F, Simmons A. Diffusion tensor imaging of Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy: a tract-based spatial statistics study. PLoS One. 2014;9:e112638. doi: 10.1371/journal.pone.0112638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitwell JL, Schwarz CG, Reid RI, Kantarci K, Jack CR, Jr, Josephs KA. Diffusion tensor imaging comparison of progressive supranuclear palsy and corticobasal syndromes. Parkinsonism Relat Disord. 2014;20:493–498. doi: 10.1016/j.parkreldis.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 19.Gardner RC, Boxer AL, Trujillo A, Mirsky JB, Guo CC, Gennatas ED, Heuer HW, Fine E, Zhou J, Kramer JH, Miller BL, Seeley WW. Intrinsic connectivity network disruption in progressive supranuclear palsy. Ann Neurol. 2013;73:603–616. doi: 10.1002/ana.23844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massey LA, Jager HR, Paviour DC, O’Sullivan SS, Ling H, Williams DR, Kallis C, Holton J, Revesz T, Burn DJ, Yousry T, Lees AJ, Fox NC, Micallef C. The midbrain to pons ratio: a simple and specific MRI sign of progressive supranuclear palsy. Neurology. 2013;80:1856–1861. doi: 10.1212/WNL.0b013e318292a2d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitwell JL, Jack CR, Jr, Parisi JE, Gunter JL, Weigand SD, Boeve BF, Ahlskog JE, Petersen RC, Dickson DW, Josephs KA. Midbrain atrophy is not a biomarker of progressive supranuclear palsy pathology. Eur J Neurol. 2013;20:1417–1422. doi: 10.1111/ene.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitwell JL, Xu J, Mandrekar J, Gunter JL, Jack CR, Jr, Josephs KA. Imaging measures predict progression in progressive supranuclear palsy. Mov Disord. 2012;27:1801–1804. doi: 10.1002/mds.24970. [DOI] [PubMed] [Google Scholar]
- 23.Reginold W, Lang AE, Marras C, Heyn C, Alharbi M, Mikulis DJ. Longitudinal quantitative MRI in multiple system atrophy and progressive supranuclear palsy. Parkinsonism Relat Disord. 2014;20:222–225. doi: 10.1016/j.parkreldis.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Josephs KA, Xia R, Mandrekar J, Gunter JL, Senjem ML, Jack CR, Jr, Whitwell JL. Modeling trajectories of regional volume loss in progressive supranuclear palsy. Mov Disord. 2013;28:1117–1124. doi: 10.1002/mds.25437. [DOI] [PubMed] [Google Scholar]
- 25.Zalewski N, Botha H, Whitwell JL, Lowe V, Dickson DW, Josephs KA. FDG-PET in pathologically confirmed spontaneous 4R-tauopathy variants. J Neurol. 2014;261:710–716. doi: 10.1007/s00415-014-7256-4. [DOI] [PubMed] [Google Scholar]
- 26.Niethammer M, Tang CC, Feigin A, Allen PJ, Heinen L, Hellwig S, Amtage F, Hanspal E, Vonsattel JP, Poston KL, Meyer PT, Leenders KL, Eidelberg D. A disease-specific metabolic brain network associated with corticobasal degeneration. Brain. 2014;137:3036–3046. doi: 10.1093/brain/awu256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Botha H, Whitwell JL, Madhaven A, Senjem ML, Lowe V, Josephs KA. The pimple sign of progressive supranuclear palsy syndrome. Parkinsonism Relat Disord. 2014;20:180–185. doi: 10.1016/j.parkreldis.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 28.Zwergal A, la Fougere C, Lorenzl S, Rominger A, Xiong G, Deutschenbaur L, Schoberl F, Linn J, Dieterich M, Brandt T, Strupp M, Bartenstein P, Jahn K. Functional disturbance of the locomotor network in progressive supranuclear palsy. Neurology. 2013;80:634–641. doi: 10.1212/WNL.0b013e318281cc43. [DOI] [PubMed] [Google Scholar]
- 29.Wagshal D, Sankaranarayanan S, Guss V, Hall T, Berisha F, Lobach I, Karydas A, Voltarelli L, Scherling C, Heuer H, Tartaglia MC, Miller Z, Coppola G, Ahlijanian M, Soares H, Kramer JH, Rabinovici GD, Rosen HJ, Miller BL, Meredith J, Boxer AL. Divergent CSF tau alterations in two common tauopathies: Alzheimer’s disease and progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2014 doi: 10.1136/jnnp-2014-308004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoglinger GU, Melhem NM, Dickson DW, Sleiman PM, Wang LS, Klei L, Rademakers R, de Silva R, Litvan I, Riley DE, van Swieten JC, Heutink P, Wszolek ZK, Uitti RJ, Vandrovcova J, Hurtig HI, Gross RG, Maetzler W, Goldwurm S, Tolosa E, Borroni B, Pastor P, Cantwell LB, Han MR, Dillman A, van der Brug MP, Gibbs JR, Cookson MR, Hernandez DG, Singleton AB, Farrer MJ, Yu CE, Golbe LI, Revesz T, Hardy J, Lees AJ, Devlin B, Hakonarson H, Muller U, Schellenberg GD. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat Genet. 2011;43:699–705. doi: 10.1038/ng.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrari R, Ryten M, Simone R, Trabzuni D, Nicolaou N, Hondhamuni G, Ramasamy A, Vandrovcova J, Weale ME, Lees AJ, Momeni P, Hardy J, de Silva R. Assessment of common variability and expression quantitative trait loci for genome-wide associations for progressive supranuclear palsy. Neurobiol Aging. 2014;35:1514 e1511–1512. doi: 10.1016/j.neurobiolaging.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fekete R, Bainbridge M, Baizabal-Carvallo JF, Rivera A, Miller B, Du P, Kholodovych V, Powell S, Ondo W. Exome sequencing in familial corticobasal degeneration. Parkinsonism Relat Disord. 2013;19:1049–1052. doi: 10.1016/j.parkreldis.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kouri N, Carlomagno Y, Baker M, Liesinger AM, Caselli RJ, Wszolek ZK, Petrucelli L, Boeve BF, Parisi JE, Josephs KA, Uitti RJ, Ross OA, Graff-Radford NR, DeTure MA, Dickson DW, Rademakers R. Novel mutation in MAPT exon 13 (p.N410H) causes corticobasal degeneration. Acta Neuropathol. 2014;127:271–282. doi: 10.1007/s00401-013-1193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graff-Radford J, Whitwell JL, Dickson DW, Josephs KA. Pallidonigroluysian atrophy associated with p.A152T variant in MAPT. Parkinsonism Relat Disord. 2013;19:838–841. doi: 10.1016/j.parkreldis.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 35.Tolosa E, Litvan I, Hoglinger GU, Burn D, Lees A, Andres MV, Gomez-Carrillo B, Leon T, Del Ser T. A phase 2 trial of the GSK-3 inhibitor tideglusib in progressive supranuclear palsy. Mov Disord. 2014;29:470–478. doi: 10.1002/mds.25824. [DOI] [PubMed] [Google Scholar]
- 36.Boxer AL, Lang AE, Grossman M, Knopman DS, Miller BL, Schneider LS, Doody RS, Lees A, Golbe LI, Williams DR, Corvol JC, Ludolph A, Burn D, Lorenzl S, Litvan I, Roberson ED, Hoglinger GU, Koestler M, Jack CR, Jr, Van Deerlin V, Randolph C, Lobach IV, Heuer HW, Gozes I, Parker L, Whitaker S, Hirman J, Stewart AJ, Gold M, Morimoto BH. Davunetide in patients with progressive supranuclear palsy: a randomised, double-blind, placebo-controlled phase 2/3 trial. Lancet Neurol. 2014;13:676–685. doi: 10.1016/S1474-4422(14)70088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitwell JL, Xu J, Mandrekar JN, Gunter JL, Jack CR, Jr, Josephs KA. Rates of brain atrophy and clinical decline over 6 and 12-month intervals in PSP: determining sample size for treatment trials. Parkinsonism Relat Disord. 2012;18:252–256. doi: 10.1016/j.parkreldis.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Litvan I, Kong M. Rate of decline in progressive supranuclear palsy. Mov Disord. 2014;29:463–468. doi: 10.1002/mds.25843. [DOI] [PubMed] [Google Scholar]
- 39.Chien DT, Bahri S, Szardenings AK, Walsh JC, Mu F, Su MY, Shankle WR, Elizarov A, Kolb HC. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J Alzheimers Dis. 2013;34:457–468. doi: 10.3233/JAD-122059. [DOI] [PubMed] [Google Scholar]
- 40.Okamura N, Furumoto S, Harada R, Tago T, Yoshikawa T, Fodero-Tavoletti M, Mulligan RS, Villemagne VL, Akatsu H, Yamamoto T, Arai H, Iwata R, Yanai K, Kudo Y. Novel 18F-labeled arylquinoline derivatives for noninvasive imaging of tau pathology in Alzheimer disease. J Nucl Med. 2013;54:1420–1427. doi: 10.2967/jnumed.112.117341. [DOI] [PubMed] [Google Scholar]


