Abstract
The chemokine receptor CXCR4 is required for the entry of human immunodeficiency virus type 1 (HIV-1) into target cells and for the development and dissemination of various types of cancers, including gastrointestinal, cutaneous, head and neck, pulmonary, gynecological, genitourinary, neurological, and hematological malignancies. The T-cell (T)-tropic HIV-1 strains use CXCR4 as the entry coreceptor; consequently, multiple CXCR4 antagonistic inhibitors have been developed for the treatment of acquired immune deficiency syndrome (AIDS). However, other potential applications of CXCR4 antagonists have become apparent since its discovery in 1996. In fact, increasing evidence demonstrates that epithelial and hematopoietic tumor cells exploit the interaction between CXCR4 and its natural ligand, stromal cell-derived factor (SDF)-1α, which normally regulates leukocyte migration. The CXCR4 and/or SDF-1α expression patterns in tumor cells also determine the sites of metastatic spread. In addition, the activation of CXCR4 by SDF-1α promotes invasion and proliferation of tumor cells, enhances tumor-associated neoangiogenesis, and assists in the degradation of the extracellular matrix and basement membrane. As such, the evaluation of CXCR4 and/or SDF-1α expression levels has a significant prognostic value in various types of malignancies. Several therapeutic challenges remain to be overcome before the use of CXCR4 inhibitors can be translated into clinical practice, but promising preclinical data demonstrate that CXCR4 antagonists can mobilize tumor cells from their protective microenvironments, interfere with their metastatic and tumorigenic potentials, and/or make tumor cells more susceptible to chemotherapy.
Keywords: AIDS, CXCR4, HIV-1, SDF-1α, Tumor, vMIP-II
INTRODUCTION: PHYSIOLOGICAL AND PATHOLOGIC FUNCTIONS OF CXCR4 AND SDF-1α
CXCR4 is one of the best studied chemokine receptors, as it serves as an important drug target for multiple human diseases, including HIV-1 infection and various types of malignancies (Fig. 1) [1, 2]. CXCR4 belongs to the superfamily of G-protein-coupled receptors (GPCRs) that possess seven transmembrane (TM) domains. The natural chemokine ligand of CXCR4 is SDF-1α (also known as CXCL12), which activates CXCR4 via G-proteins (Table 1) [3-6]. SDF-1α can also interact with another chemokine receptor, CXCR7, but this interaction does not activate signaling pathways typical of G-proteins [6]. The CXCR4–SDF-1α pathway plays many essential roles in the development of immune, nervous, vascular, and hematopoietic systems, and an important role has recently been identified in tissue regeneration [6-9]. The fact that knockout mice lacking either CXCR4 or SDF-1α die prenatally with multiple neurological, cardiac/vascular, and hematopoietic defects further demonstrates the physiological importance of CXCR4/SDF-1α [7, 8, 10]. In fact, CXCR4 is constitutively and widely expressed by numerous stem cell types, including liver oval, neural, hematopoietic, retinal pigment epithelial, endothelial, and embryonic stem cells, as well as skeletal muscle satellite cells and primordial germ cells [6]. SDF-1α is also widely expressed in multiple organs, including colon, liver, brain, lungs, heart, kidneys, and spleen [6].
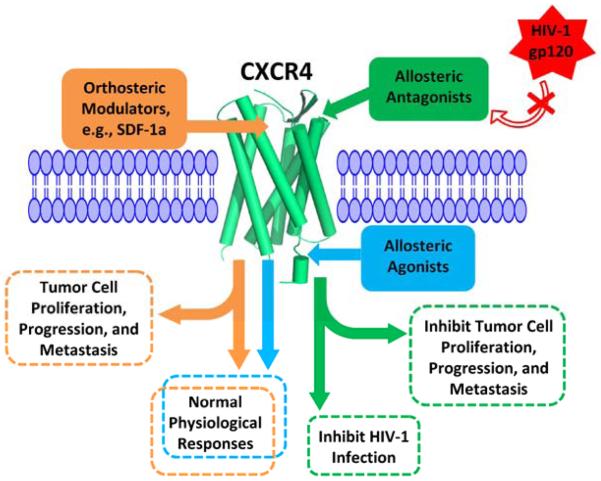
Fig. (1).
Schematic representation of CXCR4 with updated orthosteric/allosteric modulators and their biological effects.
Table 1.
Representative CXCR4 Antagonists and Agonists.
| Name | Chemical Structure | Binding Activity (IC50, nM)b |
Migration Activity |
Calcium Mobilization |
Anti-HIV Activity (IC50, nM)c |
Type of Modulator and References |
|
|---|---|---|---|---|---|---|---|
| SDF-1α | 12G5 | ||||||
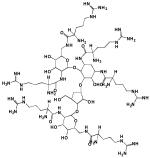
| SDF-1α | KPVSLSYRCPCRFFESHVARANVK HLKILNTPNCALQIVARLKNNNRQ VCIDPKLKWIQEYLEKALNK |
4 | 20 | +f | + | 730 | Linear Peptide Modulator [171-175] |
| ALX40-4C | Ac-NH-RRRRRRRRR-CONH2 | NDe | 3,200 | ND | − g | 3 | Linear Peptide Modulator [37, 43, 173] |
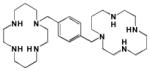
| AMD3100 |
|
33 | 37.5 | − | − | 12.4 | Orthosteric Modulator [46, 176, 177] |
| AMD3465 |
|
18 | 0.75 | − | − | 12.3 | Orthosteric Modulator [46, 177] |
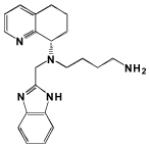
| AMD070 |
|
13 | ND | ND | ND | 2 | Orthosteric Modulator [47, 178] |
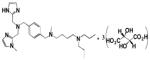
| KRH-3955 |
|
0.61 | 2.8 | ND | − | 0.99 | Orthosteric Modulator [48] |
| GSK812397 |
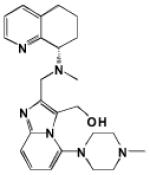
|
0.87 | ND | − | − | 4.60 | Orthosteric Modulator [50] |
| T22 |

|
ND | 48 | ND | − | 5.1 | Cyclic/Orthosteric Modulator [51, 173] |
| T140 | H-Arg-Arg-Nal-Cys-Tyr-Arg-Lys-D- Lys-Pro-Tyr-Arg-Cit-Cys-Arg-NH2a |
2.4 | 2.5 | − | − | 0.43 | Cyclic/Orthosteric Modulator [121, 130, 173, 179] |
| TC14012 | H-Arg-Arg-Nal-Cys-Tyr-Cit-Lys-D- Cit-Pro-Tyr-Arg-Cit-Cys-Arg-NH2a |
2.9 | ND | − | ND | 37 | Cyclic/Orthosteric Modulator [130, 179] |
| FC131 |
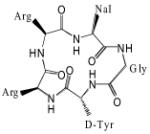
|
4 | ND | ND | ND | 14 | Cyclic/Orthosteric Modulator [54, 55, 180-182] |
| CGP64222 |
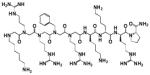
|
ND | ND | ND | − | 1,386 1.8 (μg/ml) |
Orthosteric Modulator [57, 58] |
| R3G |
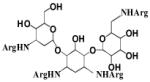
|
ND | 7,700 | ND | − | 15,000 | Orthosteric Modulator [183] |
| NeoR |
|
ND | ND | ND | ND | 800 | Orthosteric Modulator [184, 185] |
| vMIP-II | LGASWHRPDKCCLGYQKRPLPQVL LSSWYPTSQLCSKPGVIFLTKRGR QVCADKSKDWVKKLMQQLPVTAR |
14.8 | 3.0 | ND | − | ND | Linear Peptide Modu- lator [38, 66, 67] |
| V1 | LGASWHRPDKCCLGTQKRPLP | 190 | 640 | − | − | ND | Linear Peptide Modu- lator [38, 39, 62] |
| DV1 | LGASWHRPDKCCLGYQKRPLP d | 13 | 32 | − | − | 12,100 | Linear Peptide Modu- lator [39, 78] |
| RCP168 |
LGASWHRPDKCCLGYQKRPLPQVL LSSWYPTSQLCSKPGVIFLTKRGRQ VCADKSKDWVKKLMQQLPVTAR |
5 | 5 | − | − | 50 | Dimerized/Bivalent/ Orthosteric Modulator [68-70] |
| RSVM | RSVMLSYRCPCRFFESH | ND | ND | + | + | ND | Allosteric Modulator [42, 186] |
| ASLW | ASLWLSYRCPCRFFESH | ND | ND | + | + | ND | Allosteric Modulator [42, 186] |
| DV1 Dimer |

|
ND | 3 | ND | − | 4,400 | Dimerized/Bivalent/ Orthosteric Modulator [78] |
| FC131 Analog |
|
9.9 | ND | ND | ND | ND | Dimerized/Bivalent/ Orthosteric Modulator [187] |
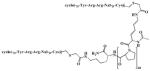
| CTCE-9908 |
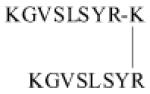
|
ND | ND | − | ND | ND | Dimerized/Bivalent/ Orthosteric Modulator [188-190] |
| CXCL122 |
|
166 | ND | − | + | ND | Dimerized/Bivalent/ Orthosteric Modulator [104, 174, 191] |
| TN14003 | H-Arg-Arg-Nal-Cys-Tyr-Cit-Lys-D-Lys -Pro-Tyr-Arg-Cit-Cys-Arg-NH2a |
ND | ND | − | ND | 140 | Cyclic/Orthosteric Modulator [179, 192] |
| BKT140 (4F- benzoyl -TN14003) |
4-fluorobenzoyl-Arg-Arg-Nal-Cys-Tyr-Cit- Lys-D-Lys-Pro-Tyr-Arg-Cit-Cys-Arg-NH2a |
0.99 | ND | − | − | ND | Cyclic/Orthosteric Modulator [130, 193] |
| ATI-2341 | Palmitic acid-MGYQKKLRSMTDKYRL | ND | ND | + | + | ND | Linear Peptide Modu- lator [168, 194] |
Each peptide has a disulfide linkage between Cys4 and Cys13.
Competition binding assays utilized a single concentration of radioiodinated SDF-1α or monoclonal antibody 12G5 in the presence of various concentrations of unlabeled chemokines.
Infectious virus and/or single-cycle focal infective assays were performed to test the antiviral activities of CXCR4 inhibitors.
The D-amino acids are italicized.
ND: Not determined
+: Promotion
−: Inhibition
Since its discovery in 1996, CXCR4 has become well known as an important coreceptor for HIV-1 infection. A fusion process between HIV-1 and target cells requires HIV-1 envelope glycoprotein, gp120, to first interact with the main receptor of the target cell, CD4, followed by its interaction with either CXCR4 or CCR5 [11-14]. HIV-1 initially uses CCR5 as the entry coreceptor to enter the target cells, and this strain is known as the macrophage (M)-tropic virus [12-14]. However, as the disease progresses, HIV-1 switches its coreceptor usage from CCR5 to CXCR4, and this T-cell (T)-tropic virus causes greater CD4-postive T-cell depletion and faster disease progression [15-17]. Natural chemokines of CXCR4 or CCR5 can directly inhibit HIV-1 infection at the entry level [18, 19] and/or internalize the coreceptors required for HIV-1 infection [20, 21].
In addition to its main role as a HIV-1 coreceptor, CXCR4 is now recognized as the chemokine receptor most widely expressed by various types of malignant tumors (Fig. 1). Indeed, it plays important roles in the development and dissemination of more than 75% of all cancers, including gastrointestinal (esophageal, gastric, pancreatic, hepatocellular, and colorectal), cutaneous, head and neck, pulmonary, gynecological (breast and ovarian), genitourinary (renal and prostate), neurological, and hematological malignancies [6, 22, 23]. SDF-1α expression level is also the highest in common metastatic sites, including lungs, lymph nodes, and liver. This indicates that CXCR4-expressing tumor cells metastasize to SDF-1α-secreting distant organs via the CXCR4–SDF-1α pathway [22]. Therefore, the evaluation of CXCR4/SDF-1α expression level may have a significant prognostic value in various types of malignancies, because the high expression of CXCR4 or SDF-1α has been shown to predict poor survival outcomes in colon [24], pancreatic [25], prostate [26], breast [27], ovarian [28], and lung cancer patients [29]. Furthermore, blocking the interaction between CXCR4 and SDF-1α is supported by the available data as an effective therapeutic strategy that would interfere with the metastatic and tumorigenic potentials of diverse types of malignancies.
One important mechanism that drives the metastatic behavior of tumor cells is hypoxia [1]. Decreased oxygen in the tumor microenvironment increases the concentration of hypoxia-inducible factor-1 (HIF-1), which subsequently upregulates the expression of CXCR4 in many tumor cells [22, 30]. SDF-1α activates CXCR4, and this in turn mediates invasion and proliferation of tumor cells, enhances tumor-associated neoangiogenesis, and assists in the degradation of the extracellular matrix and basement membrane [6, 22]. The important implication of this observation is that although a particular anti-cancer therapy might remove the primary tumor, it can potentially increase the metastatic potential of the surviving tumor cells by promoting a hypoxic environment and increasing CXCR4 expression. Hence, disrupting a particular disease function of CXCR4 or preventing the upregulation of CXCR4 in cancer cells may be impetrative for effective cancer treatment.
Several studies indicate that tumors are composed of different cell subtypes that contribute disproportionately to proliferation and invasion. The identification of so called “cancer stem cells” (CSCs) has led to the viewpoint that cancer therapies should be targeted towards these progenitor cells rather than the entire tumor burden. These CSCs are similar to normal stem cells in that they can self-renew, thereby recapitulating tumors in ectopic settings [31] and initiating tumor growth, therapy resistance, and tumor recurrence [32, 33]. In this regard, CXCR4 plays crucial roles in the maintenance, dissemination, and metastatic colonization of CSCs in various types of malignancies, including renal, prostate, colon, pancreatic, and lung cancers [6, 34].
In this review, we highlight the functions of CXCR4 and SDF-1α, as they pertain to HIV-1 infection, tumor progression, and metastasis, involving gastrointestinal, cutaneous, head and neck, pulmonary, gynecological, genitourinary, neurological, and hematological malignancies. The preclinical and clinical studies supporting the potential efficacy of CXCR4 inhibitors as the treatments of HIV-1 and cancer, and challenges that must be overcome before the translation of these inhibitors into the clinic are also discussed.
INHIBITION OF CXCR4 PREVENTS HIV-1 ENTRY
In 1996, CXCR4 was identified as one of the two essential coreceptors required for HIV-1 infection [11]. The feasibility of a CXCR4-based therapeutic approach for inhibiting HIV-1 infection is supported by the fact that CCR5 mutations confer individuals with resistance to HIV-1 infection [35, 36]. Several earlier CXCR4 antagonistic compounds, including AMD3100 and ALX40-4C (Table 1), were developed by random screening work [37-42]. Both AMD3100 and ALX40-4C inhibit HIV-1 infection by directly blocking the interaction between CXCR4 and HIV-1 [40, 43-45]. Poor oral bioavailability of AMD3100 was overcome by developing derivatives with fewer basic amine groups, such as AMD3465 [46] and AMD070 [47] (Table 1). The KRH-3955 [48, 49] and GSK812397 antagonists [50] were also found to have improved oral bioavailability and more potent antiviral activities than AMD3100 (Table 1). Similarly, T22, T140, TC14012, and FC131 can inhibit HIV-1 entry via CXCR4 (Table 1) [51-53], and smaller, yet more potent, anti-HIV peptide [54, 55] or nonpeptide compounds [56] have been developed more recently, based on the structure of T140. Furthermore, CGP64222, R3G, and NeoR were reported as potent CXCR4 antagonistic inhibitors (Table 1) [57, 58].
Random screening has been supplemented by the use of natural chemokines, such as SDF-1α and viral macrophage inflammatory protein (vMIP)-II (Table 1), as the design templates for synthesis and engineering of a group of short peptides or full-length synthetic chemokines [38, 39, 42, 59-65]. Unlike SDF-1α, vMIP-II binds to multiple chemokine receptors, including CXCR4 [66, 67], and the peptide derived from the first 1-21 residues of vMIP-II, designated as V1 (Table 1), blocks both T- and dual-tropic HIV-1 viruses. Interestingly, the antiviral and binding activities were more potent for an all-D-amino acid analog of V1 peptide (also known as DV1) than for V1 peptide (Table 1) [39]. Modification of only a small sequence of natural chemokines, especially the amino (N)-terminal domain, resulted in the development of a new group of synthetic chemokines called SMM (synthetically and modularly modified)-chemokines [68]. For instance, RCP168 can selectively inhibit CXCR4. It has more potent anti-HIV activity than is observed with SDF-1α but comparable potency to that of T-20 peptide, which is marketed under the trade name Fuzeon (Table 1) [68-70]. Despite its strong binding and antiviral activities, RCP168 does not significantly interfere with SDF-1α signaling, which is important for maintaining the normal physiological functions of CXCR4 [71].
These advances in the development of new CXCR4 antagonistic inhibitors are still limited by the potential adverse outcomes arising from the use of these inhibitors. Blocking the interaction between CXCR4 and SDF-1α for HIV-1 therapy raises concerns, especially with the finding that CXCR4 [8, 10] or SDF-1α [7] knockout mice die prenatally with multiple neurological, cardiac/vascular, and hematopoietic defects [72]. As such, Sachpatzidis et al. reported the development of allosteric agonists, RSVM and ASLW (Table 1), which can activate CXCR4 even in the presence of other CXCR4 antagonistic inhibitors or antibodies [42]. Allosteric modulators can bind to GPCRs at sites that differ from those of endogenous orthosteric agonists [73]. Allosteric agonists may be beneficial in therapeutic applications, as they could potentially allow retention of essential CXCR4 physiological functions.
Recently, the importance of CXCR4 dimerization in CXCR4 functions has been demonstrated by studies on the crystal structure of CXCR4 [74-77]. In this regard, the DV1 dimer (a synthetic bivalent ligand based on the DV1 monomer) showed more potent antiviral and binding activities when compared to the DV1 monomer (Table 1) [78]. Tanaka et al. also synthesized a dimeric form of an FC131 analog (Table 1), and bitopic ligands are currently being developed by combining orthosteric and allosteric pharmacophores in one ligand. Allosteric pharmacophores will target allosteric/therapeutic targets, whereas concurrent interaction with the orthosteric sites will ensure receptor activation and prevent undesired side effects [73]. For instance, pyrazole GPR109 receptor agonists recently provided the proof of concept; analogs of acifran selectively activate the Gi pathway that mediates the beneficial lipolytic effect, but not the β-arrestin pathway involved in the adverse side effect of cutaneous flushing [73, 79, 80]. These findings certainly represent an exciting opportunity for novel drug discovery that specifically targets therapeutically relevant binding sites and/or signaling pathways of CXCR4, which plays an important role in HIV-1 infection, tumor progression, and metastasis. Fig. (1) shows a cartoon representation of orthosteric and allosteric modulators of CXCR4 and their therapeutic potentials for regulating physiological and pathological processes. Table 1 also summarizes representative CXCR4 modulators that are subcategorized into orthosteric, allosteric, cyclic, dimerized, or bivalent groups.
CXCR4 INHIBITION AGAINST GASTROINTESTINAL MALIGNANCIES
The importance of CXCR4 has been described in various types of gastrointestinal tumors, including esophageal, gastric, pancreatic, hepatocellular, and colorectal cancers [22]. A meta-analysis of a total of 1,055 esophageal cancer patients showed that CXCR4 overexpression increases the risk of bone marrow and lymph node metastases and therefore indicates worse survival outcomes [81]. Patients with CXCR4-positive tumors have a median survival of 20 months, whereas the median survival of patients with CXCR4-negative tumors is 76 months [82]. Although medical options are limited for patients with esophageal carcinoma, recent data suggest that CXCR4 antagonists might be attractive therapeutic candidates for treatment of esophageal cancer. For instance, Drenckhan et al. reported that CTCE-9908 (Table 1) targets CXCR4 and prevents both tumor growth and metastases to liver, lungs, and lymph nodes in an orthotopic model of esophageal carcinoma [83]. This finding was further supported by a report that downregulation of CXCR4 expression by small interfering RNA (siRNA) can increase apoptosis and inhibit esophageal tumor growth [84].
Similarly, the prognosis of advanced gastric cancer remains poor, and its therapy relies largely on cytotoxic chemotherapy [85]. Strong CXCR4 expression in gastric cancer is significantly associated with cancer cell migration, lymph node metastases, higher tumor stages, and reduced 5-year survival rate [86]. Eighty-five percent of CXCR4-expressing gastric tumors develop carcinomatosis in the peritoneum, a major cause of gastric carcinoma-related death [87]. A high level of SDF-1α is found in peritoneal mesothelial cells, which promotes the migration of gastric cancer cells that express CXCR4 to the peritoneum. The CXCR4 mRNA level in gastric cancer tissues also correlates with docetaxel sensitivity, and it is significantly higher in resistant specimens [88]. Thus, identifying novel therapeutic approaches to prevent gastric cancer progression and to overcome treatment resistance is an important goal. In this regard, several pre-clinical studies demonstrated that anti-CXCR4 monoclonal antibodies and AMD3100 have anti-tumor activity by significantly suppressing tumor cell migration, proliferation, and survival [85]. Furthermore, AMD3100 can reduce ascitic fluid formation [87] and enhance in vitro docetaxel cytotoxicity [88].
Pancreatic cancer, one of the most lethal human malignancies, is also associated with the high expression level of CXCR4 [89]. Stromal cells and distant organs release SDF-1α, which in turn enhances proliferation, invasion, and metastasis of pancreatic cancer cells, and upregulates matrix-degrading enzymes [89]. Gemcitabine-treated pancreatic cancer cells develop high resistance to chemotherapy in the presence of SDF-1α, indicating that pancreatic cancer cells become more drug resistant upon the activation of CXCR4 by SDF-1α [90]. Interestingly, the pharmacological intervention by AMD3100 can effectively inhibit all these processes. Furthermore, the metastatic potential of CXCR4-expressing pancreatic CSCs can be abrogated by CXCR4 inhibition without affecting their tumorigenic capability [91]. As such, these findings indicate that the modulation of the CXCR4–SDF-1α pathway may have an important role in inhibiting aggressive pancreatic tumors.
In the case of hepatocellular carcinoma (HCC), accumulating evidence indicates that the CXCR4–SDF-1α signaling cascade mediates tumor growth, invasion, and metastasis, which is responsible for the majority of deaths in patients with HCC [92]. In fact, CXCR4 expression is significantly correlated with advanced primary tumor, lymphatic metastasis, distant dissemination, and poor survival rate [93]. The high expression level of CXCR4 also correlates with the activation of the transforming growth factor (TGF)-β pathway, a less differentiated phenotype, and a cirrhotic background [94]. Furthermore, a HCC mouse model demonstrated that SDF-1α-induced CXCR4 activation promotes the secretion of matrix metalloproteinases (MMPs), which is associated with increased metastatic potential [95]. However, antibody neutralization of either CXCR4 or SDF-1α significantly reduces the secretion of MMPs and lymph node metastasis. More recently, an anthraquinone derivative, emodin, was found to downregulate the expression of CXCR4, thus suppressing HCC invasion [92]. Emodin can also significantly suppress lung metastasis in a HCC mouse model.
CXCR4 expression is also associated with recurrence, metastasis, and survival in patients with colorectal cancer [96]. Gao’s analysis of 720 cases of colorectal cancer demonstrated that CXCR4 expression is elevated in colorectal cancer tissues. In addition, lymph node metastasis, higher histological grade and tumor node metastasis (TNM) stage, and liver metastasis are correlated with CXCR4 expression, further indicating that CXCR4 may serve as an important biomarker for both liver metastasis and survival in colorectal cancer patients [96]. Another study showed greater expression of CXCR4 in metastatic foci (such as liver and lymph nodes) than in primary tumors. Colorectal cancer cell lines in which the expression of CXCR4 was reduced via microRNA demonstrated significantly reduced metastases to liver, lymph nodes, and lungs [97]. The pharmacologic inhibition of the CXCR4–SDF-1α interaction by AMD3100 also significantly reduces CXCR4-dependent migration of colorectal cancer cells [98]. Furthermore, a recent demonstration confirmed that CXCR4 can be targeted by microRNA (miR)-126, a tumor suppressor in colorectal cancer that inhibits the Ser/Thr kinase AKT and extracellular signal-related kinase 1/2 (ERK1/2) signaling pathways. Liu et al. reported that miR-126 overexpression negatively regulates CXCR4, inhibits tumor cell proliferation, migration, and invasion, and induces cell cycle arrest in the G0/G1 phase of colorectal cancer cells [99].
CXCR4 INHIBITION AGAINST CUTANEOUS MALIGNANCIES
The CXCR4 expression by malignant melanoma is predictive of metastasis, increased tumor thickness, ulceration, and poor survival rate [100]. Scala et al. demonstrated that CXCR4 expression is associated with a poor prognosis in malignant melanoma patients, with an overall survival time of 35 months [101]. Another study indicated that CXCR4 expression was one of the independent prognostic factors in these patients [101]. Furthermore, CXCR4 plays an important role in the initial implantation of melanoma cells into the lungs, while membrane-bound metalloproteinase MT1-MMP accumulates intracellularly via the Rac-ERK1/2 pathway and mediates invasion and metastasis of the tumor cells [102]. AMD3100 can inhibit the spontaneous and SDF-1α-induced proliferation of melanoma cell lines [103]. More recently, a covalently locked, dimeric variant of SDF-1α, CXCL122 (Table 1), was shown to inhibit implantation of lung metastasis of melanoma cells more effectively than AMD3100, and also to block the growth of established metastatic melanoma in the lungs [104].
CXCR4 overexpression is also significantly associated with tumor size and invasive/infiltrating type in basal cell carcinoma (BCC) [105]. BCC highly expresses transforming growth factor (TGF)-β1, which induces upregulation of CXCR4 through the phosphorylation of the ERK1/2-ETS-1 (v-ets avian erythroblastosis virus E26 oncogene homolog 1) pathway [106]. Chu et al. demonstrated that BCC with higher CXCR4 expression has concomitantly higher microvessel density, and that SDF-1α induces angiogenic activity by upregulating several genes associated with angiogenesis, including interleukin (IL)-6, bone morphogenetic protein (BMP)-6, and cyclooxygenase 2 (COX)-2 [107]. Consistent with the important role of CXCR4 in the pathogenesis of BCC, neutralizing antibodies and the CXCR4-blocking peptide, T22, can both negate the increased proliferation and resistance to apoptosis in BCC following treatment with SDF-1α [108]. Furthermore, transplants of CXCR4-BCC into nude mice showed significant tumor progression, whereas the CXCR4 inhibitor T22 promoted tumor regression.
CXCR4 INHIBITION AGAINST HEAD AND NECK MALIGNANCIES
Approximately 10% of all human cancers are caused by head and neck malignancies [23]. The most common type is head and neck squamous cell carcinoma (HNSCC), representing greater than 90% of all head and neck cancers, and up to a quarter of patients develop metastasis with a poor survival outcome [23, 109]. In the hypoxic environments of the head and neck, the CXCR4–SDF-1α system is significantly upregulated via HIF-1, and is known to promote tumor aggressiveness and metastatic dissemination [23, 110]. In fact, CXCR4-positive patients have a 5-year survival rate of 39.1% versus 71.4% for CXCR4-negative patients [111]. Several groups also have reported a significant association between lymph node metastasis and the expression of CXCR4 in HNSCC [111, 112]. For instance, Uchida et al. demonstrated that lymph node metastasis in HNSCC is controlled by SDF-1α-induced CXCR4 activation via ERK1/2 or AKT/protein kinase B (PKB) pathway, as U0126 (mitogen-activated protein kinase (MEK)/ERK kinase inhibitor) or wortmannin (phosphatidylinositol 3 kinase (PI3K) inhibitor) can inhibit lymph node metastasis [113]. Another study on nude mice injected with B88-SDF-1 (SDF-1α expression into the B88 cell line) reported the development of an increased number of metastatic lymph nodes, more aggressive metastatic foci in the lymph nodes, and dramatic metastasis to the lungs [111]. AMD3100 significantly inhibited the lung metastasis, ameliorated body weight loss, and improved the survival rate of mice. Blocking CXCR4 via an siRNA strategy can also induce anti-tumor effects [114, 115]. Furthermore, TN14003, a small synthetic peptide antagonist of CXCR4, can reduce tumor growth, microvessel density, and lung metastasis in tumor-bearing nude mice (Table 1) [116].
CXCR4 INHIBITION AGAINST PULMONARY MALIGNANCIES
Pulmonary malignancy is the second most frequent human tumor, with a higher mortality rate than the next four common cancers combined (colon, breast, pancreatic, and prostrate cancers) [117]. In fact, the reported overall survival rate within 5 years is a mere 15% [117, 118]. Two types of lung cancers are recognized: non-small cell (NSCLC, 80%) and small cell lung cancers (SCLC, 20%) [117]. All major subtypes of NSCLC express CXCR4, and tumors with higher CXCR4 expression are more prone to metastasis than are low-expression tumors [119]. Similarly, SCLC not only expresses CXCR4 ubiquitously, but also responds to SDF-1α with an increase in cell proliferation, adhesion, and motility [120]. SCLC preferentially metastasizes to the bone marrow, whereas NSCLC tends to metastasize to bones [121].
Stage IV NSCLC patients with high expression of CXCR4 also have an extremely poor median survival time of only 2.7 months (compared to 5.6 months for low CXCR4-expressing patients) [122]. The overexpression of CXCR4 seems to have a greater impact on the clinical outcome of the female population, which has a median overall survival of 1.6 months. Furthermore, recent reports indicate that CXCR4 is expressed by CSCs in NSCLC, and that inhibition and/or siRNA targeting of CXCR4 and of the downstream action of signal transducer and activator of transcription 3 (STAT3) significantly suppress the self-renewal and chemoresistant capacity of various NSCLC cell lines [123]. Taken together, these studies certainly support the notion that CXCR4/SDF-1α plays a pro-malignant role in NSCLC disease progression.
These results also point to an urgent need for novel CXCR4-targeted therapeutics against NSCLC. In fact, the in vitro inhibition of the CXCR4–SDF-1α interaction via CXCR4-specific antibodies was shown to inhibit NSCLC metastasis [119]. Inoculation of nude mice with lung cancer cells with low CXCR4 expression also resulted in a 0- to 2-fold decrease in lung metastatic foci compared to inoculation with cells with high expression of CXCR4. In addition, AMD3100 and BKT140 (Table 1), which specifically block CXCR4, attenuate NSCLC tumor growth, suppress self-renewal capacity, and augment the effects of chemotherapy and radiotherapy [124, 125]. Thus, the CXCR4–SDF-1α axis offers an exciting new target for the development of novel anti-cancer chemokine-based therapeutics.
In SCLC, SDF-1α activates CXCR4 and tumor-associated integrin. This activation increases the adhesion between SCLC cells to collagen and fibronectin within the tumor microenvironment [1, 126]. As such, relapse and residual disease are common in SCLC, as tumor cells adhere to the bone marrow stromal cells and/or extracellular matrix proteins via integrins and are therefore protected from chemotherapy. Indeed, CXCR4 inhibitors, such as T140, can prevent the adhesion of tumor cells to extracellular membranes, thereby increasing the sensitivity of SCLC to chemotherapy [126].
CXCR4 INHIBITION AGAINST FEMALE REPRODUCTIVE MALIGNANCIES
In contrast to normal breast tissue, both primary and metastatic breast tumors highly express CXCR4 [127]. Hung et al. divided cancer patients into low-level and high-level CXCR4 expression groups using immunohistochemical staining, and demonstrated that the high-level CXCR4 expression group had a higher incidence of distant metastasis, especially bone metastasis, during the first year (10.3 % versus 1.1 %) and shorter event-free survival (17.43 months versus 27.5 months) [128]. The CXCR4–SDF-1α pathway mediates the formation of pseudopodia by actin polymerization, which increases the chemotactic and metastatic potentials of breast cancer cells [127]. Yan et al. also observed that abnormal activation of the human epithelial growth factor receptor (HER)-2 in breast cancer cells upregulates CXCR4 expression and mediates tumor invasion and lung metastasis via PI3K/AKT/mammalian targets of the rapamycin (mTOR) pathway [129]. Given the importance of CXCR4 in breast carcinogenesis, the observation that a neutralizing antibody against CXCR4 prevents breast cancer metastasis to lungs and lymph nodes is not surprising [127]. T140 derivatives and CTCE-9908 can also effectively reduce metastasis as well as primary tumor growth [130, 131]. The specific CXCR4 inhibitor AMD3465 was recently shown to trigger a reduction in breast cancer cell invasiveness and metastases to the lungs and liver through the modulation of oncogenic signaling that includes the STAT3 pathway [132]. Furthermore, siRNA duplexes can inhibit invasion and metastasis of breast cancer cells in an animal model by preventing CXCR4 expression [133].
Similarly, the expression of CXCR4 is an independent prognostic marker of ovarian carcinoma, a common gynecological malignancy [134]. The multivariate analysis by Jiang et al. indicated that CXCR4 expression is higher in recurrent and refractory ovarian cancers than in non-recurrent cancers (81% versus 28%) [134]. CXCR4 expression was predictive of significantly reduced overall survival after a median follow-up of 37 months, and the intensity of SDF-1α staining correlated with ascites. CXCR4 expression is also shown to be responsible for SDF-1α-induced ovarian cancer cell invasion and extracellular matrix degradation through αvβ6 integrin-mediated urokinase-type plasminogen activator (uPA) expression via the p38 MAPK and PI3K/AKT pathways [135]. This process can be prevented with the CXCR4 inhibitor AMD3100. Significantly lower cisplatin-based chemosensitivity, a poorer progression-free survival, and a lower overall survival were recently found to be associated with high CXCR4 expression. This suggests that CXCR4 is one of the key molecules responsible for cisplatin-based chemoresistance in epithelial ovarian cancer [136]. In fact, knockdown of CXCR4 by siRNA suppresses in vitro cell proliferation, results in G1/S arrest, and increases apoptosis and chemosensitivity in both cisplatin-sensitive and cisplatin-resistant cells [136].
CXCR4 INHIBITION AGAINST GENITOURINARY MALIGNANCIES
Renal cell carcinoma (RCC) expresses a high level of CXCR4 compared to normal kidney tissues [137]. Strong CXCR4 expression in RCC is significantly associated with advanced T-status, tumor dedifferentiation, and poor overall and recurrence-free survival [138, 139]. Pan et al. used severe combined immunodeficiency (SCID) mouse models to demonstrate that the high expression of CXCR4 in RCC is predictive of increased metastasis, and that enhanced CXCR4 expression is mediated by HIF-1 [30, 140]. The neutralization of SDF-1α in SCID mice abrogates RCC metastasis to SDF-1α-expressing distant organs. Recently, CSCs in RCC were found to express CXCR4 [141]. These CXCR4-positive CSCs exhibit greater tumor sphere formation and in vivo growth potential, and they are more resistant to tyrosine kinase inhibitors, such as sunitinib, sorafenib, and pazopanib. In fact, AMD3100 or siRNA can downregulate CXCR4 expression and increase the sensitivity of CSCs to tyrosine kinase inhibitors, thereby reducing the capability of CSCs to modulate tumor growth [141].
Sun et al. used high density microarrays to demonstrate that CXCR4 expression is also significantly elevated in localized and metastatic prostate cancers [142]. Bone metastatic foci of prostate cancer express higher level of CXCR4 compared to primary tumors and other metastatic lesions [143]. Loss of PTEN (phosphatase and tensin homolog) and subsequent activation of AKT increase the expression of CXCR4 and SDF-1α, as well as tumor cell proliferation and cell cycle progression [144]. In this study, AKT inhibition was shown to reverse CXCR4 expression and cellular invasion in prostate cancers. Furthermore, in the human prostate cancer PC-3 tumor xenograft model, AMD3100 significantly inhibited SDF-1α-induced CXCR4/AKT signal transduction and tumor growth, while AMD3100-treated PC-3 tumors showed lower in vivo levels of microvessel formation, proliferative index (as demonstrated by Ki-67), and anti-apoptotic protein Bcl-2 (B-cell lymphoma 2) compared to control tumors [145]. In addition, AMD3100 can chemosensitize prostate cancer cells to docetaxel [143]. A subcutaneous xenograft mouse model was used to show that combination therapy of AMD3100 and docetaxel has a more potent antitumor effect than does mono-chemotherapy.
CXCR4 INHIBITION AGAINST NEUROLOGICAL MALIGNANCIES
The most common intracranial tumors found in adults are gliomas, and the most aggressive subtype is glioblastoma multiforme (GBM) [34, 146]. The highly infiltrative nature of GBM, with numerous microscopic satellites that often migrate significant distances from the primary tumor, has prevented the currently available surgical techniques and adjuvant therapies from significantly improving the overall survival rate of patients with GBM [34]. However, recent research showed that CXCR4 is overexpressed in primary GBM progenitor cells, and that the administration of SDF-1α stimulates a significant proliferation of CSCs, but not of differentiated tumor cells [147]. This indicates that the CXCR4–SDF-1α pathway is a potent regulator of glioma stem cell proliferation. The evaluation of CXCR4 expression level may in fact have a significant clinical prognostic value in glioma patients. Brian et al. showed that the high expression level of CXCR4 is associated with increased glioma tumor grade and is predictive of poorer prognosis after surgery [148]. The inhibition of CXCR4 further reduces the metastasis of these invasive neoplastic glial cells [149].
A significant blood supply is needed for tumor growth and progression and is maintained by the release of HIF-1 from gliomas in the setting of an increasingly hypoxic environment. This then leads to the activation of a number of genes that function to increase oxygen availability to tumor cells [34]. In this regard, CXCR4 was identified as an activator of HIF-1, and its expression is significantly upregulated by vascular endothelial growth factor (VEGF) in both glioma and endothelial cells, demonstrating the importance of CXCR4 in glioma angiogenesis [150]. Treatment with AMD 3100 can significantly inhibit the mobilization of glioma cells in oxygen-deprived conditions. GBM recurrence can also be prevented by AMD3100 and neutralizing antibodies through inhibition of the development of functional tumor vasculogenesis [151]. Rubin et al. reported that AMD3100 can increase the apoptosis of GBM, thereby decreasing its growth potential [152]. Redjal et al. showed that GBM treated with a combination therapy involving 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) and AMD3100 results in synergistic antitumor efficacy both in vitro and in vivo [153]. Knockdown of CXCR4 via RNA interference or AMD3100 was recently reported to reduce the amount of in vitro VEGF produced by glioma CSCs and to attenuate the in vivo tumor angiogenesis and growth [154].
CXCR4 INHIBITION AGAINST HEMATOLOGICAL MALIGNANCIES
The CXCR4–SDF-1α pathway mediates the interaction between stromal and leukemic cells. Both acute myelogenous leukemia (AML) and chronic lymphocytic leukemia (CLL) can spontaneously infiltrate the bone marrow and avoid chemotherapy-induced apoptosis by activating the CXCR4–SDF-1α pathway [155-157], thereby acquiring necessary mutations required for chemotherapy resistance. In this regard, RCP168 has been shown to inhibit stromal–leukemia cell interaction via CXCR4 and to release CLL and AML cells from the bone marrow microenvironment into peripheral blood, where they can be targeted by chemotherapy [70]. Burger et al. also showed that CXCR4-specific antagonists (T140 or its analogs) can effectively antagonize the migration of CLL cells induced by SDF-1α, and enhance apoptosis of leukemic cells when used in combination with fludarabine [121]. In addition, AMD3100 [158] and AMD3465 [70] achieved similar outcomes, further indicating that the CXCR4–SDF-1α pathway may indeed represent an effective drug target for increasing the number of leukemic cells available in peripheral blood that can be targeted by chemotherapy.
Hematopoietic stem cell (HSC) transplants are most often performed in patients with hematological malignancies, such as multiple myeloma and leukemia. Adult bone marrow contains trilineage hematopoietic elements (i.e., myeloid, erythroid, and megakaryocytic cells), and it is the primary source of SDF-1α [159]. Chemotactic responsiveness of HSCs is restricted to SDF-1α, and this selectivity for SDF-1α is necessary for retention, growth, and differentiation of HSCs in the bone marrow [1]. Both HSCs and hematopoietic progenitor cells (HPCs) can be induced to exit the bone marrow [160, 161] and collected in peripheral blood for hematopoietic stem cell transplantation in patients undergoing high-dose chemotherapy for their hematological malignancies. Currently, granulocyte colony-stimulating factor (GCSF) is used most often in clinical practice to increase the number of HSCs in blood for collection for transplantation, although this treatment is associated with unwanted side effects and requires multiple doses [162]. In this regard, several studies have shown that CXCR4-expressing HSCs and HPCs are retained within the bone marrow that expresses SDF-1α [163-165]. Indeed, AMD3100 [166] or T140 [167] can mobilize HSCs by blocking CXCR4, and for this reason, AMD3100 (Plerixafor) was approved in 2008 for the treatment of patients with lymphoma and multiple myeloma to increase the number of HSCs available for transplantation. Similarly, a lipopeptide pepducin, ATI-2341, can inhibit the function of CXCR4 and increase the mobilization of HSCs and HPCs (Table 1) [168]. HSC mobilization can also be effectively induced by inhibitory CXCR4 nanobodies and AMD3100 [169].
CONCLUSION
Tremendous progress has been made in drug discovery research targeting CXCR4 since the first report in 1996 that CXCR4 is one of the two essential coreceptors for HIV-1 infection [11]. Indeed, the research focus has expanded to include multiple types of cancers, including gastrointestinal, cutaneous, head and neck, pulmonary, gynecological, genitourinary, neurological, and hematological malignancies. Although the collective evidence supports the potential efficacy of CXCR4 inhibitors as therapies for HIV-1 and cancer treatments, important challenges remain before CXCR4 inhibitors will be sanctioned for clinical use, due to the ubiquitous expression of CXCR4 in normal tissues and the functional importance of the CXCR4–SDF-1α interaction, as discussed in this review. One of the major concerns regarding the use of CXCR4 inhibitors as HSC mobilizers is that these may expose not only leukemic cells but also normal hematopoietic cells to cytotoxic chemotherapy. These limitations demonstrate that therapeutic strategies should be aimed at selective disruption of a particular disease function of CXCR4 without compromising its normal functions. In fact, although the use of AMD3100 as a mobilizer of HSCs in patients with non-Hodgkin’s lymphoma and multiple myeloma has shown minimal side effects, its use was discontinued in a clinical study for HIV-1 treatment due to cardiac toxicity [6]. In this regard, several studies have shown that CXCR4 may have two distinct binding sites for HIV-1 gp120 and SDF-1α [170], and that SMM-chemokines, such as RCP168, can selectively disrupt HIV-1 entry without compromising the normal SDF-1α binding and signaling activities [71].
Similarly, the selective targeting of tumor-specific CXCR4–SDF-1α pathways involved in tumor progression and metastasis must not inhibit global CXCR4 signaling. Bitopic ligands that can selectively target therapeutically relevant binding sites and/or signaling pathways of CXCR4, thereby reducing unwanted side effects, represent exciting candidates for novel anti-cancer drug discovery. In summary, most studies on the CXCR4–SDF-1α axis and its inhibition for the treatment of cancers are still in their infancy, but pre-clinical data indicate some encouraging results. This has opened up the possibility that CXCR4 inhibitors may have therapeutic benefits, especially when used in combination with existing treatment strategies.
ACKNOWLEDGEMENTS
All authors listed in the manuscript contributed substantially to drafting and revising the manuscript. We thank all former and current members of our laboratories and collaborators at other institutions who contributed to the work cited in this review. We apologize to our colleagues if we have missed or failed to cite their work and papers in this review due to the specific focus and space constraint of this review and the diverse research topics and rapid development of this field.
Our studies were supported by grants from the National Institutes of Health, the Carol M. Baldwin Breast Cancer Research Fund, the Connolly Endowment/Hendricks Fund, and the LUNGevity Foundation.
LIST OF ABBREVIATIONS
- CXCR4
CXC chemokine receptor 4
- GPCR
G-protein-coupled receptor
- SDF-1α
Stromal cell-derived factor-1α
- vMIP-II
Viral macrophage inflammatory protein-II
- HIV
Human immunodeficiency virus
- AIDS
Acquired immune deficiency syndrome
- HIF-1
Hypoxia-inducible factor-1
- CSC
Cancer stem cell
- HCC
Hepatocellular carcinoma
- BCC
Basal cell carcinoma
- HNSCC
Head and neck squamous cell carcinoma
- NSCLC
Non-small cell lung cancer
- SCLC
Small cell lung cancer
- RCC
Renal cell carcinoma
- GBM
Glioblastoma multiforme
- CLL
Chronic lymphocytic leukemia
- AML
Acute myelogenous leukemia
- HSC
Hematopoietic stem cell
- HPC
Hematopoietic progenitor cell
- G-CSF
Granulocyte colony-stimulating factor
Footnotes
CONFLICT OF INTEREST We declare no conflict of interests.
REFERENCES
- [1].Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107(5):1761–7. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- [2].Zlotnik A. Involvement of chemokine receptors in organ-specific metastasis. Contrib. Microbiol. 2006;13:191–9. doi: 10.1159/000092973. [DOI] [PubMed] [Google Scholar]
- [3].Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu. Rev. Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- [4].Kobilka B. Adrenergic receptors as models for G Protein-coupled receptors. Annu. Rev. Neurosci. 1992;15:87–114. doi: 10.1146/annurev.ne.15.030192.000511. [DOI] [PubMed] [Google Scholar]
- [5].Strader CD, Fong TM, Tota MR, Underwood D. Structure and function of G Protein-coupled Receptors. Annu. Rev. Biochem. 1994;63:101–32. doi: 10.1146/annurev.bi.63.070194.000533. [DOI] [PubMed] [Google Scholar]
- [6].Cojoc M, Peitzsch C, Trautmann F, Polishchuk L, Telegeev GD, Dubrovska A. Emerging targets in cancer management: role of the CXCL12/CXCR4 axis. Onco. Targ. Ther. 2013;6:1347–1361. doi: 10.2147/OTT.S36109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382(6592):635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- [8].Zou Y, Kottmann A, Kuroda M, Taniuchi I, Littman D. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393(6685):595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- [9].Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronsoni RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc. Natl. Acad. Sci. USA. 1998;95(16):9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- [11].Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272(5263):872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- [12].Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Marzio PD, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381(6584):661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- [13].Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381(6584):667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- [14].Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272(5270):1955–8. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- [15].Cheng-Mayer C, Seto D, Tateno M, Levy JA. Biologic features of HIV-1 that correlate with virulence in the host. Science. 1988;240(4848):80–2. doi: 10.1126/science.2832945. [DOI] [PubMed] [Google Scholar]
- [16].Tersmette M, Lange JM, de Goede RE, de Wolf F, Eeftink-Schattenkerk JK, Schellekens PT, Coutinho RA, Huisman JG, Goudsmit J, Miedema F. Association between biological properties of human immunodeficiency virus variants and risk for AIDS and AIDS mortality. Lancet. 1989;1(8645):983–5. doi: 10.1016/s0140-6736(89)92628-7. [DOI] [PubMed] [Google Scholar]
- [17].Schellekens PT, Tersmette M, Roos MT, Keet RP, de Wolf F, Coutinho RA, Miedema F. Biphasic rate of CD4+ cell count decline during progression to AIDS correlates with HIV-1 phenotype. AIDS. 1992;6(7):665–9. doi: 10.1097/00002030-199207000-00008. [DOI] [PubMed] [Google Scholar]
- [18].Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382(6594):829–33. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- [19].Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F, Schwartz O, Heard JM, Clark-Lewis I, Legler DF, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382(6594):833–5. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- [20].Amara A, Gall SL, Schwartz O, Salamero J, Montes M, Loetscher P, Baggiolini M, Virelizier J-L, Arenzana-Seisdedos F. HIV coreceptor downregulation as antiviral principle: SDF-1a-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J. Exp. Med. 1997;186(1):139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Förster R, Kremmer E, Schubel A, Breitfeld D, Kleinschmidt A, Nerl C, Bernhardt G, Lipp M. Intracellular and surface expression of the HIV-1 coreceptor CXCR4/fusin on various leukocyte subsets: rapid internalization and recycling upon activation. J. Immunol. 1998;160:1522–1531. [PubMed] [Google Scholar]
- [22].Lombardi L, Tavano F, Morelli F, Latiano TP, Di Sebastiano P, Maiello E. Chemokine receptor CXCR4: role in gastrointestinal cancer. Crit. Rev. Oncol. Hematol. 2013;88(3):696–705. doi: 10.1016/j.critrevonc.2013.08.005. [DOI] [PubMed] [Google Scholar]
- [23].Albert S, Riveiro ME, Halimi C, Hourseau M, Couvelard A, Serova M, Barry B, Raymond E, Faivre S. Focus on the role of the CXCL12/CXCR4 chemokine axis in head and neck squamous cell carcinoma. Head Neck. 2013;35(12):1819–28. doi: 10.1002/hed.23217. [DOI] [PubMed] [Google Scholar]
- [24].Wu Y, Jin M, Xu H, Shimin Z, He S, Wang L, Zhang Y. Clinicopathologic significance of HIF-1α, CXCR4, and VEGF expression in colon cancer. Clin. Dev. Immunol. 2010;2010(2010):1–10. doi: 10.1155/2010/537531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Maréchal R, Demetter P, Nagy N, Berton A, Decaestecker C, Polus M, Closset J, Devière J, Salmon I, Van Laethem JL. High expression of CXCR4 may predict poor survival in resected pancreatic adenocarcinoma. Br. J. Cancer. 2009;100(9):1444–51. doi: 10.1038/sj.bjc.6605020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jung SJ, Kim CI, Park CH, Chang HS, Kim BH, Choi MS, Jung HR. Correlation between Chemokine Receptor CXCR4 Expression and Prognostic Factors in Patients with Prostate Cancer. Korean J. Urol. 2011;52(9):607–11. doi: 10.4111/kju.2011.52.9.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ramos EA, Grochoski M, Braun-Prado K, Seniski GG, Cavalli IJ, Ribeiro EM, Camargo AA, Costa FF, Klassen G. Epigenetic changes of CXCR4 and its ligand CXCL12 as prognostic factors for sporadic breast cancer. PLoS One. 2011;6(12):e29461. doi: 10.1371/journal.pone.0029461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Popple A, Durrant LG, Spendlove I, Rolland P, Scott IV, Deen S, Ramage JM. The chemokine, CXCL12, is an independent predictor of poor survival in ovarian cancer. Br. J. Cancer. 2012;106(7):1306–13. doi: 10.1038/bjc.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wang M, Chen GY, Song HT, Hong X, Yang ZY, Sui GJ. Significance of CXCR4, phosphorylated STAT3 and VEGF-A expression in resected non-small cell lung cancer. Exp. Ther. Med. 2011;2(3):517–522. doi: 10.3892/etm.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni M, Vago L, Mantovani A, Melillo G, Sica A. Regulation of the chemokine receptor CXCR4 by hypoxia. J. Exp. Med. 2003;198(9):1391–402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138(5):822–9. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- [32].Scheel C, Weinberg RA. Cancer stem cells and epithelialmesenchymal transition: concepts and molecular links. Semin. Cancer. Biol. 2012;22(5-6):396–403. doi: 10.1016/j.semcancer.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance. Nat. Rev. Cancer. 2008;8(7):545–54. doi: 10.1038/nrc2419. [DOI] [PubMed] [Google Scholar]
- [34].Ehtesham M, Min E, Issar NM, Kasl RA, Khan IS, Thompson RC. The role of the CXCR4 cell surface chemokine receptor in glioma biology. J. Neurooncol. 2013;113(2):153–62. doi: 10.1007/s11060-013-1108-4. [DOI] [PubMed] [Google Scholar]
- [35].Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- [36].Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, Farber CM, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, Muyldermans G, Verhofstede C, Burtonboy G, Georges M, Imai T, Rana S, Yi Y, Smyth RJ, Collman RG, Doms RW, Vassart G, Parmentier M. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382(6593):722–5. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- [37].Doranz BJ, Grovit-Ferbas K, Sharron MP, Mao SH, Goetz MB, Daar ES, Doms RW, O’Brien WA. A small-molecule inhibitor directed against the chemokine receptor CXCR4 prevents its use as an HIV-1 coreceptor. J. Exp. Med. 1997;186(8):1395–1400. doi: 10.1084/jem.186.8.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhou N, Luo Z, Luo J, Hall JW, Huang Z. A novel peptide antagonist of CXCR4 derived from the the N-terminus of the viral chemokine vMIP-II. Biochemistry. 2000;39(13):3782–3787. doi: 10.1021/bi992750v. [DOI] [PubMed] [Google Scholar]
- [39].Zhou N, Luo Z, Luo J, Fan X, Cayabyab M, Hiraoka M, Liu D, Han X, Pesavento J, Dong CZ, Wang Y, An J, Kaji H, Sodroski JG, Huang Z. Exploring the stereochemistry of CXCR4-peptide recognition and inhibiting HIV-1 entry with D-peptides derived from chemokines. J. Biol. Chem. 2002;277:17476–17485. doi: 10.1074/jbc.M202063200. [DOI] [PubMed] [Google Scholar]
- [40].Donzella GA, Schols D, Lin SW, Este JA, Nagashima KA, Maddon PJ, Allaway GP, Sakmar TP, Henson G, De Clercq E, Moore JP. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nature Medicine. 1998;4(1):72–77. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- [41].Fenard D, Lambeau G, Maurin T, Lefebvre JC, Doglio A. A peptide derived from bee venom-secreted phospholipase A2 inhibits replication of T-cell tropic HIV-1 strains via interaction with the CXCR4 chemokine receptor. Mol. Pharmacol. 2001;60:341–347. doi: 10.1124/mol.60.2.341. [DOI] [PubMed] [Google Scholar]
- [42].Sachpatzidis A, Benton BK, Manfredi JP, Wang H, Hamilton A, Dohlman HG, Lolis E. Identification of allosteric peptide agonists of CXCR4. J. Biol. Chem. 2003;278:896–907. doi: 10.1074/jbc.M204667200. [DOI] [PubMed] [Google Scholar]
- [43].O’Brien WA, Sumner-Smith M, Mao SH, Sadeghi S, Zhao JQ, Chen IS. Anti-human immunodeficiency virus type 1 activity of an oligocationic compound mediated via gp120 V3 interactions. J. Virol. 1996;70(5):2825–31. doi: 10.1128/jvi.70.5.2825-2831.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sumner-Smith M, Zheng Y, Zhang YP, Twist EM, Climie SC. Antiherpetic activities of N-alpha-acetyl-nona-D-arginine amide acetate. Drugs Exp. Clin. Res. 1995;21(1):1–6. [PubMed] [Google Scholar]
- [45].De Clercq EY,N, Pauwels R, Baba M, Schols D, Nakashima H, Balzarini J, Debyser Z, Murrer BA, Schwartz D, Thornton D, Bridger G, Fricker S, Henson G, Abrams M, Picker D. Potent and selective inhibition of human immunodeficiency virus (HIV)-1 and HIV-2 replication by a class of bicyclams interacting with a viral uncoating event. Proc. Natl. Acad. Sci. U S A. 1992;89:5286–5290. doi: 10.1073/pnas.89.12.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hatse S, Princen K, De Clercq E, Rosenkilde MM, Schwartz TW, Hernandez-Abad PE, Skerlj RT, Bridger GJ, Schols D. AMD3465, a monomacrocyclic CXCR4 antagonist and potent HIV entry inhibitor. Biochem. Pharmacol. 2005;70(5):752–61. doi: 10.1016/j.bcp.2005.05.035. [DOI] [PubMed] [Google Scholar]
- [47].Stone ND, Dunaway SB, Flexner C, Tierney C, Calandra GB, Becker S, Cao YJ, Wiggins IP, Conley J, MacFarland RT, Park JG, Lalama C, Snyder S, Kallungal B, Klingman KL, Hendrix CW. Multiple-dose escalation study of the safety, pharmacokinetics, and biologic activity of oral AMD070, a selective CXCR4 receptor inhibitor, in human subjects. Antimicrob. Agents Chemother. 2007;51(7):2351–8. doi: 10.1128/AAC.00013-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Murakami T, Kumakura S, Yamazaki T, Tanaka R, Hamatake M, Okuma K, Huang W, Toma J, Komano J, Yanaka M, Tanaka Y, Yamamoto N. The novel CXCR4 antagonist KRH-3955 is an orally bioavailable and extremely potent inhibitor of human immunodeficiency virus type 1 infection: comparative studies with AMD3100. Antimicrob. Agents Chemother. 2009;53(7):2940–8. doi: 10.1128/AAC.01727-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Iwasaki Y, Akari H, Murakami T, Kumakura S, Dewan MZ, Yanaka M, Yamamoto N. Efficient inhibition of SDF-1alpha-mediated chemotaxis and HIV-1 infection by novel CXCR4 antagonists. Cancer Sci. 2009;100(4):778–81. doi: 10.1111/j.1349-7006.2009.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jenkinson S, Thomson M, McCoy D, Edelstein M, Danehower S, Lawrence W, Wheelan P, Spaltenstein A, Gudmundsson K. Blockade of X4-tropic HIV-1 cellular entry by GSK812397, a potent noncompetitive CXCR4 receptor antagonist. Antimicrob. Agents Chemother. 2010;54(2):817–24. doi: 10.1128/AAC.01293-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Murakami T, Nakajima T, Koyanagi Y, Tachibana K, Fujii N, Tamamura H, Yoshida N, Waki M, Matsumoto A, Yoshie O, Kishimoto T, Yamamoto N, Nagasawa T. A small molecule CXCR4 inhibitor that blocks T cell line-tropic HIV-1 infection. J. Exp. Med. 1997;186(8):1389–93. doi: 10.1084/jem.186.8.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tamamura H, Omagari A, Hiramatsu K, Gotoh K, Kanamoto T, Xu Y, Kodama E, Matsuoka M, Hattori T, Yamamoto N, Nakashima H, Otaka A, Fujii N. Development of specific CXCR4 inhibitors possessing high selectivity indexes as well as complete stability in serum based on an anti-HIV peptide T140. Bioorg. Med. Chem. Lett. 2001;11(14):1897–902. doi: 10.1016/s0960-894x(01)00323-7. [DOI] [PubMed] [Google Scholar]
- [53].Fujii NO,S, Hiramatsu K, Araki T, Ueda S, Tamamura H, Otaka A, Kusano S, Terakubo S, Nakashima H, Broach JA, Trent JO, Wang ZX, Peiper SC. Molecular-size reduction of a potent CXCR4-chemokine antagonist using orthogonal combination of conformation- and sequence-based libraries. Angew. Chem. Int. Ed. Engl. 2003;42:3251–3253. doi: 10.1002/anie.200351024. [DOI] [PubMed] [Google Scholar]
- [54].Narumi T, Hayashi R, Tomita K, Kobayashi K, Tanahara N, Ohno H, Naito T, Kodama E, Matsuoka M, Oishi S, Fujii N. Synthesis and biological evaluation of selective CXCR4 antagonists containing alkene dipeptide isosteres. Org. Biomol. Chem. 2010;8(3):616–21. doi: 10.1039/b917236j. [DOI] [PubMed] [Google Scholar]
- [55].Ueda S, Oishi S, Wang ZX, Araki T, Tamamura H, Cluzeau J, Ohno H, Kusano S, Nakashima H, Trent JO, Peiper SC, Fujii N. Structure-activity relationships of cyclic peptide-based chemokine receptor CXCR4 antagonists: disclosing the importance of side-chain and backbone functionalities. J. Med. Chem. 2007;50(2):192–8. doi: 10.1021/jm0607350. [DOI] [PubMed] [Google Scholar]
- [56].Ueda S, Kato M, Inuki S, Ohno H, Evans B, Wang ZX, Peiper SC, Izumi K, Kodama E, Matsuoka M, Nagasawa H, Oishi S, Fujii N. Identification of novel non-peptide CXCR4 antagonists by ligand-based design approach. Bioorg. Med. Chem. Lett. 2008;18(14):4124–9. doi: 10.1016/j.bmcl.2008.05.092. [DOI] [PubMed] [Google Scholar]
- [57].Hamy F, Felder ER, Heizmann G, Lazdins J, Aboul-ela F, Varani G, Karn J, Klimkait T. An inhibitor of the Tat/TAR RNA interaction that effectively suppresses HIV-1 replication. Proc. Nat. Acad. Sci. USA. 1997;94(8):3548–53. doi: 10.1073/pnas.94.8.3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Daelemans D, Schols D, Witvrouw M, Pannecouque C, Hatse S, van Dooren S, Hamy F, Klimkait T, de Clercq E, VanDamme AM. A second target for the peptoid Tat/transactivation response element inhibitor CGP64222: inhibition of human immunodeficiency virus replication by blocking CXC-chemokine receptor 4-mediated virus entry. Mol. Pharmacol. 2000;57(1):116–24. [PubMed] [Google Scholar]
- [59].LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111(4):589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- [60].Luo Z, Zhou N, Luo J, Hall JW, Huang Z. The role of positively charged residues in CXCR4 recognition probed with synthetic peptides. Biochem. Biophy. Res. Comm. 1999;263(3):691–695. doi: 10.1006/bbrc.1999.1441. [DOI] [PubMed] [Google Scholar]
- [61].Luo J, Luo Z, Zhou N, Hall JW, Huang Z. Attachment of C-terminus of SDF-1 enhances the biological activity of its N-terminal peptide. Biochem. Biophys. Res. Commun. 1999;264(1):42–47. doi: 10.1006/bbrc.1999.1476. [DOI] [PubMed] [Google Scholar]
- [62].Luo Z, Fan X, Zhou N, Hiraoka M, Luo J, Kaji H, Huang Z. Structure-function study and anti-HIV activity of synthetic peptide analogues derived from viral chemokine vMIP-II. Biochemistry. 2000;39(44):13545–13550. doi: 10.1021/bi000633q. [DOI] [PubMed] [Google Scholar]
- [63].Huang Z. Structural chemistry and therapeutic intervention of protein-protein interaction in immune response, HIV entry and apoptosis. Pharmac. Therap. 2000;86(3):201–215. doi: 10.1016/s0163-7258(00)00052-8. [DOI] [PubMed] [Google Scholar]
- [64].Heveker N, Montes M, Germeroth L, Amara A, Trautmann A, Alizon M, Schneider-Mergener J. Dissociation of the signalling and antiviral properties of SDF-1-derived small peptides. Curr. Biol. 1998;8(7):369–76. doi: 10.1016/s0960-9822(98)70155-1. [DOI] [PubMed] [Google Scholar]
- [65].Loetscher P, Gong JH, Dewald B, Baggiolini M, Clark-Lewis I. N-terminal peptides of stromal cell-derived factor-1 with CXC chemokine receptor 4 agonist and antagonist activities. J. Biol. Chem. 1998;273(35):22279–83. doi: 10.1074/jbc.273.35.22279. [DOI] [PubMed] [Google Scholar]
- [66].Kledal TN, Rosenkilde MM, Coulin F, Simmons G, Johnsen AH, Alouani S, Power CA, Luttichau HR, Gerstoft J, Clapham PR, Clark-Lewis I, Wells TNC, Schwartz TW. A broad-spectrum chemokine antagonist encoded by Kaposi’s sarcoma-associated herpesvirus. Science. 1997;277(5332):1656–9. doi: 10.1126/science.277.5332.1656. [DOI] [PubMed] [Google Scholar]
- [67].Boshoff C, Endo Y, Collins PD, Takeuchi Y, Reeves JD, Schweickart VL, Siani MA, Sasaki T, Williams TJ, Gray PW, Moore PS, Chang Y, Weiss RA. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science. 1997;278(5336):290–4. doi: 10.1126/science.278.5336.290. [DOI] [PubMed] [Google Scholar]
- [68].Kumar S, Choi WT, Dong CZ, Madani N, Tian S, Liu D, Wang Y, Pesavento J, Wang J, Fan X, Yuan J, Fritzsche WR, An J, Sodroski JG, Richman DD, Huang Z. SMMChemokines: a class of unnatural synthetic molecules as chemical probes of chemical receptor biology and leads for therapeutic development. Chemistry & Biology. 2006;13(1):69–79. doi: 10.1016/j.chembiol.2005.10.012. [DOI] [PubMed] [Google Scholar]
- [69].Liu D, Madani N, Li Y, Cao R, Choi WT, Kumar S, Dong C, Russell JD, Lefebure CR, An J, Wilson S, Gao YG, Pallansch LA, Sodroski JG, Huang Z. Crystal structure and structural mechanism of a novel anti-human immunodeficiency virus and D-amino acid-containing chemokine. J. Virol. 2007;81(20):11489–98. doi: 10.1128/JVI.02845-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zeng Z, Samudio IJ, Munsell M, An J, Huang Z, Estey E, Andreeff M, Konopleva M. Inhibition of CXCR4 with the novel RCP168 peptide overcomes stroma-mediated chemoresistance in chronic and acute leukemias. Mol. Cancer Ther. 2006;5(12):3113–21. doi: 10.1158/1535-7163.MCT-06-0228. [DOI] [PubMed] [Google Scholar]
- [71].Choi WT, Tian S, Dong CZ, Kumar S, Liu D, Madani N, An J, Sodroski JG, Huang Z. Unique ligand binding sites on CXCR4 probed by a chemical biology approach: implications for the design of selective human immunodeficiency virus type 1 inhibitors. J. Virol. 2005;79(24):15398–15404. doi: 10.1128/JVI.79.24.15398-15404.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Doranz BJ, Filion LG, Diaz-Mitoma F, Sitar DS, Sahai J, Baribaud F, Orsinis MJ, Benovic JL, Cameron W, Doms RW. Safe use of the CXCR4 inhibitor ALX40-4C in humans. AIDS Res. Hum. Retroviruses. 2001;17:475–486. doi: 10.1089/08892220151126508. [DOI] [PubMed] [Google Scholar]
- [73].Valant C, Robert Lane J, Sexton PM, Christopoulos A. The best of both worlds? Bitopic orthosteric/allosteric ligands of g protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 2012;52:153–78. doi: 10.1146/annurev-pharmtox-010611-134514. [DOI] [PubMed] [Google Scholar]
- [74].Wu B, Chien EY, Mol CD, Fenalti G, Liu W, Katritch V, Abagyan R, Brooun A, Wells P, Bi FC, Hamel DJ, Kuhn P, Handel TM, Cherezov V, Stevens RC. Structures of the CXCR4 Chemokine GPCR with Small-Molecule and Cyclic Peptide Antagonists. Science. 2010;330(330):1066–71. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Babcock GJ, Farzan M, Sodroski J. Ligand-independent dimerization of CXCR4, a principal HIV-1 coreceptor. J. Biol. Chem. 2003;278(5):3378–85. doi: 10.1074/jbc.M210140200. [DOI] [PubMed] [Google Scholar]
- [76].Percherancier Y, Berchiche YA, Slight I, Volkmer-Engert R, Tamamura H, Fujii N, Bouvier M, Heveker N. Bioluminescence resonance energy transfer reveals ligand-induced conformational changes in CXCR4 homo- and heterodimers. J. Biol. Chem. 2005;280(11):9895–903. doi: 10.1074/jbc.M411151200. [DOI] [PubMed] [Google Scholar]
- [77].Hernandez PA, Gorlin RJ, Lukens JN, Taniuchi S, Bohinjec J, Francois F, Klotman ME, Diaz GA. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat. Genet. 2003;34(1):70–4. doi: 10.1038/ng1149. [DOI] [PubMed] [Google Scholar]
- [78].Choi WT, Kumar S, Madani N, Han X, Tian S, Dong CZ, Liu D, Duggineni S, Yuan J, Sodroski JG, Huang Z, An J. A novel synthetic bivalent ligand to probe chemokine receptor CXCR4 dimerization and inhibit HIV-1 entry. Biochemistry. 2012;51(36):7078–86. doi: 10.1021/bi2016712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Walters RW, Shukla AK, Kovacs JJ, Violin JD, DeWire SM, Lam CM, Chen JR, Muehlbauer MJ, Whalen EJ, Lefkowitz RJ. beta-Arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice. J. Clin. Invest. 2009;119(5):1312–21. doi: 10.1172/JCI36806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Jung JK, Johnson BR, Duong T, Decaire M, Uy J, Gharbaoui T, Boatman PD, Sage CR, Chen R, Richman JG, Connolly DT, Semple G. Analogues of acifran: agonists of the high and low affinity niacin receptors, GPR109a and GPR109b. J. Med. Chem. 2007;50(7):1445–8. doi: 10.1021/jm070022x. [DOI] [PubMed] [Google Scholar]
- [81].Wu J, Wu X, Liang W, Chen C, Zheng L, An H. Clinicopathological and prognostic significance of chemokine receptor CXCR4 overexpression in patients with esophageal cancer: a meta-analysis. Tumour Biol. 2013;35(4):3709–15. doi: 10.1007/s13277-013-1490-8. [DOI] [PubMed] [Google Scholar]
- [82].Kaifi JT, Yekebas EF, Schurr P, Obonyo D, Wachowiak R, Busch P, Heinecke A, Pantel K, Izbicki JR. Tumor-cell homing to lymph nodes and bone marrow and CXCR4 expression in esophageal cancer. J. Natl. Cancer Inst. 2005;97(24):1840–7. doi: 10.1093/jnci/dji431. [DOI] [PubMed] [Google Scholar]
- [83].Drenckhan A, Kurschat N, Dohrmann T, Raabe N, Koenig AM, Reichelt U, Kaifi JT, Izbicki JR, Gros SJ. Effective inhibition of metastases and primary tumor growth with CTCE-9908 in esophageal cancer. J. Surg. Res. 2013;182(2):250–6. doi: 10.1016/j.jss.2012.09.035. [DOI] [PubMed] [Google Scholar]
- [84].Wang DF, Lou N, Qiu MZ, Lin YB, Liang Y. Effects of CXCR4 gene silencing by lentivirus shRNA on proliferation of the EC9706 human esophageal carcinoma cell line. Tumour Biol. 2013;34(5):2951–9. doi: 10.1007/s13277-013-0858-0. [DOI] [PubMed] [Google Scholar]
- [85].Lee HJ, Jo DY. The role of the CXCR4/CXCL12 axis and its clinical implications in gastric cancer. Histol. Histopathol. 2012;27(9):1155–61. doi: 10.14670/HH-27.1155. [DOI] [PubMed] [Google Scholar]
- [86].Lee HJ, Kim SW, Kim HY, Li S, Yun HJ, Song KS, Kim S, Jo DY. Chemokine receptor CXCR4 expression, function, and clinical implications in gastric cancer. Int. J. Oncol. 2009;34(2):473–80. [PubMed] [Google Scholar]
- [87].Yasumoto K, Koizumi K, Kawashima A, Saitoh Y, Arita Y, Shinohara K, Minami T, Nakayama T, Sakurai H, Takahashi Y, Yoshie O, Saiki I. Role of the CXCL12/CXCR4 axis in peritoneal carcinomatosis of gastric cancer. Cancer Res. 2006;66(4):2181–7. doi: 10.1158/0008-5472.CAN-05-3393. [DOI] [PubMed] [Google Scholar]
- [88].Xie L, Wei J, Qian X, Chen G, Yu L, Ding Y, Liu B. CXCR4, a potential predictive marker for docetaxel sensitivity in gastric cancer. Anticancer Res. 2010;30(6):2209–16. [PubMed] [Google Scholar]
- [89].Shen B, Zheng MQ, Lu JW, Jiang Q, Wang TH, Huang XE. CXCL12-CXCR4 promotes proliferation and invasion of pancreatic cancer cells. Asian Pac. J. Cancer Prev. 2013;14(9):5403–8. doi: 10.7314/apjcp.2013.14.9.5403. [DOI] [PubMed] [Google Scholar]
- [90].Singh S, Srivastava SK, Bhardwaj A, Owen LB, Singh AP. CXCL12-CXCR4 signalling axis confers gemcitabine resistance to pancreatic cancer cells: a novel target for therapy. Br. J. Cancer. 2010;103(11):1671–9. doi: 10.1038/sj.bjc.6605968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1(3):313–23. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- [92].Manu KA, Shanmugam MK, Ong TH, Subramaniam A, Siveen KS, Perumal E, Samy RP, Bist P, Lim LH, Kumar AP, Hui KM, Sethi G. Emodin suppresses migration and invasion through the modulation of CXCR4 expression in an orthotopic model of human hepatocellular carcinoma. PLoS One. 2013;8(3):e57015. doi: 10.1371/journal.pone.0057015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Schimanski CC, Bahre R, Gockel I, Müller A, Frerichs K, Hörner V, Teufel A, Simiantonaki N, Biesterfeld S, Wehler T, Schuler M, Achenbach T, Junginger T, Galle PR, Moehler M. Dissemination of hepatocellular carcinoma is mediated via chemokine receptor CXCR4. Br. J. Cancer. 2006;95(2):210–7. doi: 10.1038/sj.bjc.6603251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Bertran E, Crosas-Molist E, Sancho P, Caja L, Lopez-Luque J, Navarro E, Egea G, Lastra R, Serrano T, Ramos E, Fabregat I. Overactivation of the TGF-β pathway confers a mesenchymal-like phenotype and CXCR4-dependent migratory properties to liver tumor cells. Hepatology. 2013;58(6):2032–44. doi: 10.1002/hep.26597. [DOI] [PubMed] [Google Scholar]
- [95].Chu H, Zhou H, Liu Y, Liu X, Hu Y, Zhang J. Functional expression of CXC chemokine recepter-4 mediates the secretion of matrix metalloproteinases from mouse hepatocarcinoma cell lines with different lymphatic metastasis ability. Int. J. Biochem. Cell Biol. 2007;39(1):197–205. doi: 10.1016/j.biocel.2006.07.008. [DOI] [PubMed] [Google Scholar]
- [96].Gao Y, Li C, Nie M, Lu Y, Lin S, Yuan P, Sun X. CXCR4 as a novel predictive biomarker for metastasis and poor prognosis in colorectal cancer. Tumour Biol. 2014;35(5):4171–5. doi: 10.1007/s13277-013-1545-x. [DOI] [PubMed] [Google Scholar]
- [97].Murakami T, Kawada K, Iwamoto M, Akagami M, Hida K, Nakanishi Y, Kanda K, Kawada M, Seno H, Taketo MM, Sakai Y. The role of CXCR3 and CXCR4 in colorectal cancer metastasis. Int. J. Cancer. 2013;132(2):276–87. doi: 10.1002/ijc.27670. [DOI] [PubMed] [Google Scholar]
- [98].Heckmann D, Laufs S, Maier P, Zucknick M, Giordano FA, Veldwijk MR, Eckstein V, Wenz F, Zeller WJ, Fruehauf S, Allgayer H. A Lentiviral CXCR4 overexpression and knockdown model in colorectal cancer cell lines reveals plerixafor-dependent suppression of SDF-1α-induced migration and invasion. Onkologie. 2011;34(10):502–8. doi: 10.1159/000332390. [DOI] [PubMed] [Google Scholar]
- [99].Liu Y, Zhou Y, Feng X, An P, Quan X, Wang H, Ye S, Yu C, He Y, Luo H. MicroRNA-126 functions as a tumor suppressor in colorectal cancer cells by targeting CXCR4 via the AKT and ERK1/2 signaling pathways. Int. J. Oncol. 2014;44(1):203–10. doi: 10.3892/ijo.2013.2168. [DOI] [PubMed] [Google Scholar]
- [100].Longo-Imedio MI, Longo N, Treviño I, Lázaro P, Sánchez-Mateos P. Clinical significance of CXCR3 and CXCR4 expression in primary melanoma. Int. J. Cancer. 2005;117(5):861–5. doi: 10.1002/ijc.21269. [DOI] [PubMed] [Google Scholar]
- [101].Scala S, Ottaiano A, Ascierto PA, Cavalli M, Simeone E, Giuliano P, Napolitano M, Franco R, Botti G, Castello G. Expression of CXCR4 predicts poor prognosis in patients with malignant melanoma. Clin. Cancer. Res. 2005;11(5):1835–41. doi: 10.1158/1078-0432.CCR-04-1887. [DOI] [PubMed] [Google Scholar]
- [102].Bartolomé RA, Ferreiro S, Miquilena-Colina ME, Martínez-Prats L, Soto-Montenegro ML, García-Bernal D, Vaquero JJ, Agami R, Delgado R, Desco M, Sánchez-Mateos P, Teixidó J. The chemokine receptor CXCR4 and the metalloproteinase MT1-MMP are mutually required during melanoma metastasis to lungs. Am. J. Pathol. 2009;174(2):602–12. doi: 10.2353/ajpath.2009.080636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Scala S, Giuliano P, Ascierto PA, Ieranò C, Franco R, Napolitano M, Ottaiano A, Lombardi ML, Luongo M, Simeone E, Castiglia D, Mauro F, De Michele I, Calemma R, Botti G, Caracò C, Nicoletti G, Satriano RA, Castello G. Human melanoma metastases express functional CXCR4. Clin. Cancer Res. 2006;12(8):2427–33. doi: 10.1158/1078-0432.CCR-05-1940. [DOI] [PubMed] [Google Scholar]
- [104].Takekoshi T, Ziarek JJ, Volkman BF, Hwang ST. A locked, dimeric CXCL12 variant effectively inhibits pulmonary metastasis of CXCR4-expressing melanoma cells due to enhanced serum stability. Mol. Cancer Ther. 2012;11(11):2516–25. doi: 10.1158/1535-7163.MCT-12-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Xu CZ, Wang PH, Yan XJ, Wang T, Chen D, Zhang ZJ, Shi RJ. Expression of CXCR4 Is Associated with Progression and Invasion in Patients with Nasal-Surface Basal Cell Carcinoma. ORL J. Otorhinolaryngol. Relat. Spec. 2014;75(6):332–341. doi: 10.1159/000357027. [DOI] [PubMed] [Google Scholar]
- [106].Chu CY, Sheen YS, Cha ST, Hu YF, Tan CT, Chiu HC, Chang CC, Chen MW, Kuo ML, Jee SH. Induction of chemokine receptor CXCR4 expression by transforming growth factor-β1 in human basal cell carcinoma cells. J. Dermatol. Sci. 2013;72(2):123–33. doi: 10.1016/j.jdermsci.2013.06.011. [DOI] [PubMed] [Google Scholar]
- [107].Chu CY, Cha ST, Lin WC, Lu PH, Tan CT, Chang CC, B.R. L, Jee SH, Kuo ML. Stromal cell-derived factor-1alpha (SDF-1alpha/CXCL12)-enhanced angiogenesis of human basal cell carcinoma cells involves ERK1/2-NF-kappaB/interleukin-6 pathway. Carcinogenesis. 2009;30(2):205–13. doi: 10.1093/carcin/bgn228. [DOI] [PubMed] [Google Scholar]
- [108].Chen GS, Yu HS, Lan CC, Chow KC, Lin TY, Kok LF, Lu MP, Liu CH, Wu MT. CXC chemokine receptor CXCR4 expression enhances tumorigenesis and angiogenesis of basal cell carcinoma. Br. J. Dermatol. 2006;154(5):910–8. doi: 10.1111/j.1365-2133.2006.07150.x. [DOI] [PubMed] [Google Scholar]
- [109].Kotwall C, Sako K, Razack MS, Rao U, Bakamjian V, Shedd DP. Metastatic patterns in squamous cell cancer of the head and neck. Am. J. Surg. 1987;154(4):439–42. doi: 10.1016/0002-9610(89)90020-2. [DOI] [PubMed] [Google Scholar]
- [110].Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int. J. Radiat. Oncol. Biol. Phys. 1997;38(2):285–9. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- [111].Uchida D, Onoue T, Tomizuka Y, Begum NM, Miwa Y, Yoshida H, Sato M. involvement of an autocrine stromal cell derived factor-1/CXCR4 system on the distant metastasis of human oral squamous cell carcinoma. Mol. Cancer Res. 2007;5(7):685–94. doi: 10.1158/1541-7786.MCR-06-0368. [DOI] [PubMed] [Google Scholar]
- [112].Ishikawa T, Nakashiro K, Hara S, Klosek SK, Li C, Shintani S, Hamakawa H. CXCR4 expression is associated with lymph-node metastasis of oral squamous cell carcinoma. Int. J. Oncol. 2006;28(1):61–6. [PubMed] [Google Scholar]
- [113].Uchida D, Begum NM, Tomizuka Y, Bando T, Almofti A, Yoshida H, Sato M. Acquisition of lymph node, but not distant metastatic potentials, by the overexpression of CXCR4 in human oral squamous cell carcinoma. Lab Invest. 2004;84(12):1538–46. doi: 10.1038/labinvest.3700190. [DOI] [PubMed] [Google Scholar]
- [114].Hong JS, Pai HK, Hong KO, Kim MA, Kim JH, Lee JI, Hong SP, Hong SD. CXCR-4 knockdown by small interfering RNA inhibits cell proliferation and invasion of oral squamous cell carcinoma cells. J. Oral Pathol. Med. 2009;38(2):214–9. doi: 10.1111/j.1600-0714.2008.00671.x. [DOI] [PubMed] [Google Scholar]
- [115].Yu T, Wu Y, Huang Y, Yan C, Liu Y, Wang Z, Wang X, Wen Y, Wang C, Li L. RNAi targeting CXCR4 inhibits tumor growth through inducing cell cycle arrest and apoptosis. Mol. Ther. 2012;20(2):398–407. doi: 10.1038/mt.2011.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Yoon Y, Liang Z, Zhang X, Choe M, Zhu A, Cho HT, Shin DM, Goodman MM, Chen ZG, Shim H. CXC chemokine receptor-4 antagonist blocks both growth of primary tumor and metastasis of head and neck cancer in xenograft mouse models. Cancer Res. 2007;67(15):7518–24. doi: 10.1158/0008-5472.CAN-06-2263. [DOI] [PubMed] [Google Scholar]
- [117].Wald O, Shapira OM, Izhar U. CXCR4/CXCL12 axis in non small cell lung cancer (NSCLC) pathologic roles and therapeutic potential. Theranostics. 2013;3(1):26–33. doi: 10.7150/thno.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E. Cancer treatment and survivorship statistics, 2012. CA Cancer J. Clin. 2012;62(4):220–41. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- [119].Su L, Zhang J, Xu H, Wang Y, Chu Y, Liu R, Xiong S. Differential expression of CXCR4 is associated with the metastatic potential of human non-small cell lung cancer cells. Clin. Cancer Res. 2005;11(23):8273–80. doi: 10.1158/1078-0432.CCR-05-0537. [DOI] [PubMed] [Google Scholar]
- [120].Kijima T, Maulik G, Ma PC, Tibaldi EV, Turner RE, Rollins B, Sattler M, Johnson BE, Salgia R. Regulation of cellular proliferation, cytoskeletal function, and signal transduction through CXCR4 and c-Kit in small cell lung cancer cells. Cancer Res. 2002;62(21):6304–11. [PubMed] [Google Scholar]
- [121].Burger M, Hartmann T, Krome M, Rawluk J, Tamamura H, Fujii N, Kipps TJ, Burger JA. Small peptide inhibitors of the CXCR4 chemokine receptor (CD184) antagonize the activation, migration, and antiapoptotic responses of CXCL12 in chronic lymphocytic leukemia B cells. Blood. 2005;106(5):1824–30. doi: 10.1182/blood-2004-12-4918. [DOI] [PubMed] [Google Scholar]
- [122].Otsuka S, Klimowicz AC, Kopciuk K, Petrillo SK, Konno M, Hao D, Muzik H, Stolte E, Boland W, Morris D, Magliocco AM, Bebb DG. CXCR4 overexpression is associated with poor outcome in females diagnosed with stage IV non-small cell lung cancer. J. Thorac. Oncol. 2011;6(7):1169–78. doi: 10.1097/JTO.0b013e3182199a99. [DOI] [PubMed] [Google Scholar]
- [123].Jung MJ, Rho JK, Kim YM, Jung JE, Jin YB, Ko YG, Lee JS, Lee SJ, Lee JC, Park MJ. Upregulation of CXCR4 is functionally crucial for maintenance of stemness in drug-resistant non-small cell lung cancer cells. Oncogene. 2013;32(2):209–21. doi: 10.1038/onc.2012.37. [DOI] [PubMed] [Google Scholar]
- [124].Fahham D, Weiss ID, Abraham M, Beider K, Hanna W, Shlomai Z, Eizenberg O, Zamir G, Izhar U, Shapira OM, Peled A, Wald O. In vitro and in vivo therapeutic efficacy of CXCR4 antagonist BKT140 against human non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2012;144(5):1167–1175. doi: 10.1016/j.jtcvs.2012.07.031. [DOI] [PubMed] [Google Scholar]
- [125].Wald O, Izhar U, Amir G, Kirshberg S, Shlomai Z, Zamir G, Peled A, Shapira OM. Interaction between neoplastic cells and cancer-associated fibroblasts through the CXCL12/CXCR4 axis: role in non-small cell lung cancer tumor proliferation. J. Thorac. Cardiovasc. Surg. 2011;141(6):1503–12. doi: 10.1016/j.jtcvs.2010.11.056. [DOI] [PubMed] [Google Scholar]
- [126].Hartmann TN, Burger JA, Glodek A, Fujii N, Burger M. CXCR4 chemokine receptor and integrin signaling co-operate in mediating adhesion and chemoresistance in small cell lung cancer (SCLC) cells. Oncogene. 2005;24(27):4462–71. doi: 10.1038/sj.onc.1208621. [DOI] [PubMed] [Google Scholar]
- [127].Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verástegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- [128].Hung CS, Su HY, Liang HH, Lai CW, Chang YC, Ho YS, Wu CH, Ho JD, Wei PL, Chang YJ. High-level expression of CXCR4 in breast cancer is associated with early distant and bone metastases. Tumour Biol. 2013;35(2):1581–8. doi: 10.1007/s13277-013-1218-9. [DOI] [PubMed] [Google Scholar]
- [129].Li YM, Pan Y, Wei Y, Cheng X, Zhou BP, Tan M, Zhou X, Xia W, Hortobagyi GN, Yu D, Hung MC. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6(5):459–69. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- [130].Tamamura H, Hori A, Kanzaki N, Hiramatsu K, Mizumoto M, Nakashima H, Yamamoto N, Otaka A, Fujii N. T140 analogs as CXCR4 antagonists identified as anti-metastatic agents in the treatment of breast cancer. FEBS Lett. 2003;550(1-3):79–83. doi: 10.1016/s0014-5793(03)00824-x. [DOI] [PubMed] [Google Scholar]
- [131].Huang EH, Singh B, Cristofanilli M, Gelovani J, Wei C, Vincent L, Cook KR, Lucci A. A CXCR4 antagonist CTCE-9908 inhibits primary tumor growth and metastasis of breast cancer. J. Surg. Res. 2009;155(2):231–6. doi: 10.1016/j.jss.2008.06.044. [DOI] [PubMed] [Google Scholar]
- [132].Ling X, Spaeth E, Chen Y, Shi Y, Zhang W, Schober W, Hail NJ, Konopleva M, Andreeff M. The CXCR4 antagonist AMD3465 regulates oncogenic signaling and invasiveness in vitro and prevents breast cancer growth and metastasis in vivo. PLoS One. 2013;8(3):e58426. doi: 10.1371/journal.pone.0058426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Liang Z, Yoon Y, Votaw J, Goodman MM, Williams L, Shim H. Silencing of CXCR4 blocks breast cancer metastasis. Cancer Res. 2005;65(3):967–71. [PMC free article] [PubMed] [Google Scholar]
- [134].Jiang YP, Wu XH, Shi B, Wu WX, Yin GR. Expression of chemokine CXCL12 and its receptor CXCR4 in human epithelial ovarian cancer: an independent prognostic factor for tumor progression. Gynecol. Oncol. 2006;103(1):226–33. doi: 10.1016/j.ygyno.2006.02.036. [DOI] [PubMed] [Google Scholar]
- [135].Xue B, Wu W, Huang K, Xie T, Xu X, Zhang H, Qi C, Ge J, Yu Y. Stromal cell-derived factor-1 (SDF-1) enhances cells invasion by αvβ6 integrin-mediated signaling in ovarian cancer. Mol. Cell. Biochem. 2013;380(1-2):177–84. doi: 10.1007/s11010-013-1671-1. [DOI] [PubMed] [Google Scholar]
- [136].Li J, Jiang K, Qiu X, Li M, Hao Q, Wei L, Zhang W, Chen B, Xin X. Overexpression of CXCR4 is significantly associated with cisplatin-based chemotherapy resistance and can be a prognostic factor in epithelial ovarian cancer. BMB Rep. 2013;47(1):33–8. doi: 10.5483/BMBRep.2014.47.1.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Schrader AJ, Lechner O, Templin M, Dittmar KE, Machtens S, Mengel M, Probst-Kepper M, Franzke A, Wollensak T, Gatzlaff P, Atzpodien J, Buer J, Lauber J. CXCR4/CXCL12 expression and signalling in kidney cancer. Br. J. Cancer. 2002;86(8):1250–6. doi: 10.1038/sj.bjc.6600221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Wang L, Chen W, Gao L, Yang Q, Liu B, Wu Z, Wang Y, Sun Y. High expression of CXCR4, CXCR7 and SDF-1 predicts poor survival in renal cell carcinoma. World J. Surg. Oncol. 2012;7(10):212. doi: 10.1186/1477-7819-10-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Wehler TC, Graf C, Biesterfeld S, Brenner W, Schadt J, Gockel I, Berger MR, Thüroff JW, Galle PR, Moehler M, Schimanski CC. Strong expression of chemokine receptor CXCR4 by renal cell carcinoma correlates with advanced disease. J. Oncol. 2008;2008:626340. doi: 10.1155/2008/626340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Pan J, Mestas J, Burdick MD, Phillips RJ, Thomas GV, Reckamp K, Belperio JA, Strieter RM. Stromal derived factor-1 (SDF-1/CXCL12) and CXCR4 in renal cell carcinoma metastasis. Mol. Cancer Ther. 2006;3(5):56. doi: 10.1186/1476-4598-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Gassenmaier M, Chen D, Buchner A, Henkel L, Schiemann M, Mack B, Schendel DJ, Zimmermann W, Pohla H. CXC chemokine receptor 4 is essential for maintenance of renal cell carcinoma-initiating cells and predicts metastasis. Stem Cells. 2013;31(8):1467–76. doi: 10.1002/stem.1407. [DOI] [PubMed] [Google Scholar]
- [142].Sun YX, Wang J, Shelburne CE, Lopatin DE, Chinnaiyan AM, Rubin MA, Pienta KJ, Taichman RS. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J. Cell Biochem. 2003;89(3):462–73. doi: 10.1002/jcb.10522. [DOI] [PubMed] [Google Scholar]
- [143].Domanska UM, Timmer-Bosscha H, Nagengast WB, Oude Munnink TH, Kruizinga RC, Ananias HJ, Kliphuis NM, Huls G, De Vries EG, de Jong IJ, Walenkamp AM. CXCR4 inhibition with AMD3100 sensitizes prostate cancer to docetaxel chemotherapy. Neoplasia. 2012;14(8):709–18. doi: 10.1593/neo.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Conley-LaComb MK, Saliganan A, Kandagatla P, Chen YQ, Cher ML, Chinni SR. PTEN loss mediated Akt activation promotes prostate tumor growth and metastasis via CXCL12/CXCR4 signaling. Mol. Cancer. 2013;12(1):85. doi: 10.1186/1476-4598-12-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Cho KS, Yoon SJ, Lee JY, Cho NH, Choi YD, Song YS, Hong SJ. Inhibition of tumor growth and histopathological changes following treatment with a chemokine receptor CXCR4 antagonist in a prostate cancer xenograft model. Oncol. Lett. 2013;6(4):933–938. doi: 10.3892/ol.2013.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, Groups., E. O. f. R. a. T. o. C. B. T. a. R.; Group., N. C. I. o. C. C. T Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- [147].Ehtesham M, Mapara KY, Stevenson CB, Thompson RC. CXCR4 mediates the proliferation of glioblastoma progenitor cells. Cancer Lett. 2009;274(2):305–12. doi: 10.1016/j.canlet.2008.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Bian XW, Yang SX, Chen JH, Ping YF, Zhou XD, Wang QL, Jiang XF, Gong W, Xiao HL, Du LL, Chen ZQ, Zhao W, Shi JQ, Wang JM. Preferential expression of chemokine receptor CXCR4 by highly malignant human gliomas and its association with poor patient survival. Neurosurgery. 2007;61(3):570–8. doi: 10.1227/01.NEU.0000290905.53685.A2. [DOI] [PubMed] [Google Scholar]
- [149].Ehtesham M, Winston JA, Kabos P, Thompson RC. CXCR4 expression mediates glioma cell invasiveness. Oncogene. 2006;25(19):2801–6. doi: 10.1038/sj.onc.1209302. [DOI] [PubMed] [Google Scholar]
- [150].Zagzag D, Lukyanov Y, Lan L, Ali MA, Esencay M, Mendez O, Yee H, Voura EB, Newcomb EW. Hypoxiainducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: implications for angiogenesis and glioma cell invasion. Lab Invest. 2006;86(12):1221–32. doi: 10.1038/labinvest.3700482. [DOI] [PubMed] [Google Scholar]
- [151].Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J. Clin. Invest. 2010;120(3):694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Rubin JB, Kung AL, Klein RS, Chan JA, Sun Y, Schmidt K, Kieran MW, Luster AD, Segal RA. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc. Natl. Acad. Sci. U S A. 2003;100(23):13513–8. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Redjal N, Chan JA, Segal RA, Kung AL. CXCR4 inhibition synergizes with cytotoxic chemotherapy in gliomas. Clin. Cancer Res. 2006;12(22):6765–71. doi: 10.1158/1078-0432.CCR-06-1372. [DOI] [PubMed] [Google Scholar]
- [154].Ping YF, Yao XH, Jiang JY, Zhao LT, Yu SC, Jiang T, Lin MC, Chen JH, Wang B, Zhang R, Cui YH, Qian C, Wang JM, Bian XW. The chemokine CXCL12 and its receptor CXCR4 promote glioma stem cell-mediated VEGF production and tumour angiogenesis via PI3K/AKT signalling. J. Pathol. 2011;224(3):344–54. doi: 10.1002/path.2908. [DOI] [PubMed] [Google Scholar]
- [155].Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell’Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96(8):2655–63. [PubMed] [Google Scholar]
- [156].Burger JA, Burger M, Kipps TJ. Chronic lymphocytic leukemia B cells express functional CXCR4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cells. Blood. 1999;94(11):3658–67. [PubMed] [Google Scholar]
- [157].Konopleva M, Konoplev S, Hu W, Zaritskey AY, Afanasiev BV, Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16(9):1713–24. doi: 10.1038/sj.leu.2402608. [DOI] [PubMed] [Google Scholar]
- [158].Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, Ritchey JK, Prior JL, Piwnica-Worms D, Bridger G, Ley TJ, DiPersio JF. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009;113(24):6206–14. doi: 10.1182/blood-2008-06-162123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Pelus LM. Peripheral blood stem cell mobilization: new regimens, new cells, where do we stand. Curr. Opin. Hematol. 2008;15(4):285–92. doi: 10.1097/MOH.0b013e328302f43a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Robinson WA, Kurnick JE, Pike BL. Colony growth of human leukemic peripheral blood cells in vitro. Blood. 1971;38(4):500–8. [PubMed] [Google Scholar]
- [161].McCredie KB, Hersh EM, Freireich EJ. Cells capable of colony formation in the peripheral blood of man. Science. 1971;171(968):293–4. doi: 10.1126/science.171.3968.293. [DOI] [PubMed] [Google Scholar]
- [162].Roberts AW, DeLuca E, Begley CG, Basser R, Grigg AP, Metcalf D. Broad inter-individual variations in circulating progenitor cell numbers induced by granulocyte colony-stimulating factor therapy. Stem Cells. 1995;13(5):512–6. doi: 10.1002/stem.5530130508. [DOI] [PubMed] [Google Scholar]
- [163].Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia. 2002;16(10):1992–2003. doi: 10.1038/sj.leu.2402684. [DOI] [PubMed] [Google Scholar]
- [164].Kollet O, Petit I, Kahn J, Samira S, Dar A, Peled A, Deutsch V, Gunetti M, Piacibello W, Nagler A, Lapidot T. Human CD34(+)CXCR4(−) sorted cells harbor intracellular CXCR4, which can be functionally expressed and provide NOD/SCID repopulation. Blood. 2002;100(8):2778–86. doi: 10.1182/blood-2002-02-0564. [DOI] [PubMed] [Google Scholar]
- [165].Kim CH, Broxmeyer HE. In vitro behavior of hematopoietic progenitor cells under the influence of chemoattractants: stromal cell-derived factor-1, steel factor, and the bone marrow environment. Blood. 1998;91(1):100–10. [PubMed] [Google Scholar]
- [166].Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB, Calandra G, Bridger G, Dale DC, Srour EF. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J. Exp. Med. 2005;201(8):1307–18. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Abraham M, Biyder K, Begin M, Wald H, Weiss ID, Galun E, Nagler A, Peled A. Enhanced unique pattern of hematopoietic cell mobilization induced by the CXCR4 antagonist 4F-benzoyl-TN14003. Stem Cells. 2007;25(9):2158–66. doi: 10.1634/stemcells.2007-0161. [DOI] [PubMed] [Google Scholar]
- [168].Tchernychev B, Ren Y, Sachdev P, Janz JM, Haggis L, O’Shea A, McBride E, Looby R, Deng Q, McMurry T, Kazmi MA, Sakmar TP, Hunt S. r., Carlson KE. Discovery of a CXCR4 agonist pepducin that mobilizes bone marrow hematopoietic cells. Proc. Nat. Acad. Sci. USA. 2010;107(51):22255–9. doi: 10.1073/pnas.1009633108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Jähnichen S, Blanchetot C, Maussang D, Gonzalez-Pajuelo M, Chow KY, Bosch L, De Vrieze S, Serruys B, Ulrichts H, Vandevelde W, Saunders M, De Haard HJ, Schols D, Leurs R, Vanlandschoot P, Verrips T, Smit MJ. CXCR4 nanobodies (VHH-based single variable domains) potently inhibit chemotaxis and HIV-1 replication and mobilize stem cells. Proc. Nat. Acad. Sci. USA. 2010;107(47):20565–70. doi: 10.1073/pnas.1012865107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Tian S, Choi WT, Liu D, Pesavento J, Wang Y, An J, Sodroski JG, Huang Z. Distinct functional sites for human immunodeficiency virus type 1 and stromal cell-derived factor 1a on CXCR4 transmembrane helical domains. J. Virol. 2005;79(20):12667–12673. doi: 10.1128/JVI.79.20.12667-12673.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Dong CZ, Tian S, Choi WT, Kumar S, Liu D, Xu Y, Han X, Huang Z, An J. Critical role in CXCR4 signaling and internalization of the polypeptide main chain in the amino terminus of SDF-1 probed by novel N-methylated synthetically and modularly modified chemokine analogues. Biochemistry. 2012;51(30):5951–7. doi: 10.1021/bi3003742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Dong CZ, Tian S, Madani N, Choi WT, Kumar S, Liu D, Sodroski JG, Huang Z, An J. Role of CXCR4 internalization in the anti-HIV activity of stromal cell-derived factor-1α probed by a novel synthetically and modularly modified-chemokine analog. Exp. Biol. Med. 2011;236(12):1413–9. doi: 10.1258/ebm.2011.011260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Tamamura H, Xu Y, Hattori T, Zhang X, Arakaki R, Kanbara K, Omagari A, Otaka A, Ibuka T, Yamamoto N, Nakashima H, Fujii N. A low-molecular-weight inhibitor against the chemokine receptor CXCR4: a strong anti-HIV peptide T140. Biochem. Biophys. Res. Comm. 1998;253(3):877–82. doi: 10.1006/bbrc.1998.9871. [DOI] [PubMed] [Google Scholar]
- [174].Veldkamp CT, Seibert C, Peterson FC, De la Cruz NB, Haugner J. C. r., Basnet H, Sakmar TP, Volkman BF. Structural basis of CXCR4 sulfotyrosine recognition by the chemokine SDF-1/CXCL12. Sci. Signal. 2008;16(1):37. doi: 10.1126/scisignal.1160755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Zhou NL,Z, Luo J, Fan X, Cayabyab M, Hiraoka M, Liu D, Han X, Pesavento J, Dong CZ, Wang Y, An J, Kaji H, Sodroski JG, Huang Z. Exploring the stereochemistry of CXCR4-peptide recognition and inhibiting HIV-1 entry with D-peptides derived from chemokines. J. Biol. Chem. 2002;277:17476–17485. doi: 10.1074/jbc.M202063200. [DOI] [PubMed] [Google Scholar]
- [176].Schols D, Struyf S, Van Damme J, Esté JA, Henson G, De Clercq E. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J. Exp. Med. 1997;186(8):1383–8. doi: 10.1084/jem.186.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Bodart V, Anastassov V, Darkes MC, Idzan SR, Labrecque J, Lau G, Mosi RM, Neff KS, Nelson KL, Ruzek MC, Patel K, Santucci Z, Scarborough R, Wong RS, Bridger GJ, Macfarland RT, Fricker SP. Pharmacology of AMD3465: a small molecule antagonist of the chemokine receptor CXCR4. Biochem. Pharmacol. 2009;78(8):993–1000. doi: 10.1016/j.bcp.2009.06.010. [DOI] [PubMed] [Google Scholar]
- [178].Skerlj RT, Bridger GJ, Kaller A, McEachern EJ, Crawford JB, Zhou Y, Atsma B, Langille J, Nan S, Veale D, Wilson T, Harwig C, Hatse S, Princen K, De Clercq E, Schols D. Discovery of novel small molecule orally bioavailable C-X-C chemokine receptor 4 antagonists that are potent inhibitors of T-tropic (X4) HIV-1 replication. J. Med. Chem. 2010;53(8):3376–88. doi: 10.1021/jm100073m. [DOI] [PubMed] [Google Scholar]
- [179].Tamamura H, Hiramatsu K, Kusano AS, Terakubo S, Yamamoto N, Trent JO, Wang Z, Peiper SC, Nakashima H, Otaka A, Fujii N. Synthesis of potent CXCR4 inhibitors possessing low cytotoxicity and improved biostability based on T140 derivatives. Org. Biomol. Chem. 2003;1(21):3656–62. doi: 10.1039/b306473p. [DOI] [PubMed] [Google Scholar]
- [180].Kobayashi K, Oishi S, Hayashi R, Tomita K, Kubo T, Tanahara N, Ohno H, Yoshikawa Y, Furuya T, Hoshino M, Fujii N. Structure-activity relationship study of a CXC chemokine receptor type 4 antagonist, FC131, using a series of alkene dipeptide isosteres. J. Med. Chem. 2012;55(6):2746–57. doi: 10.1021/jm2016914. [DOI] [PubMed] [Google Scholar]
- [181].Tamamura H, Araki T, Ueda S, Wang Z, Oishi S, Esaka A, Trent JO, Nakashima H, Yamamoto N, Peiper SC, Otaka A, Fujii N. Identification of novel low molecular weight CXCR4 antagonists by structural tuning of cyclic tetrapeptide scaffolds. J. Med. Chem. 2005;48(9):3280–9. doi: 10.1021/jm050009h. [DOI] [PubMed] [Google Scholar]
- [182].Tamamura H, Esaka A, Ogawa T, Araki T, Ueda S, Wang Z, Trent JO, Tsutsumi H, Masuno H, Nakashima H, Yamamoto N, Peiper SC, Otaka A, Fujii N. Structure-activity relationship studies on CXCR4 antagonists having cyclic pentapeptide scaffolds. Org. Biomol. Chem. 2005;3(24):4392–4. doi: 10.1039/b513145f. [DOI] [PubMed] [Google Scholar]
- [183].Cabrera C, Gutiérrez A, Blanco J, Barretina J, Litovchick A, Lapidot A, Evdokimov AG, Clotet B, Esté JA. Anti-human immunodeficiency virus activity of novel aminoglycoside-arginine conjugates at early stages of infection. AIDS Res. Hum. Retroviruses. 2000;16(7):627–34. doi: 10.1089/088922200308855. [DOI] [PubMed] [Google Scholar]
- [184].Catani MV, Corasaniti MT, Ranalli M, Amantea D, Litovchick A, Lapidot A, Melino G. The Tat antagonist neomycin B hexa-arginine conjugate inhibits gp-120-induced death of human neuroblastoma cells. J. Neurochem. 2003;84(6):1237–45. doi: 10.1046/j.1471-4159.2003.01620.x. [DOI] [PubMed] [Google Scholar]
- [185].Litovchick A, Lapidot A, Eisenstein M, Kalinkovich A, Borkow G. Neomycin B-arginine conjugate, a novel HIV-1 Tat antagonist: synthesis and anti-HIV activities. Biochemistry. 2001;40(51):15612–23. doi: 10.1021/bi0108655. [DOI] [PubMed] [Google Scholar]
- [186].Baryshnikova OK, Rainey JK, Sykes BD. Nuclear magnetic resonance studies of CXC chemokine receptor 4 allosteric peptide agonists in solution. J. Pept. Res. 2005;66(Suppl 1):12–21. [Google Scholar]
- [187].Tanaka T, Nomura W, Narumi T, Masuda A, Tamamura H. Bivalent ligands of CXCR4 with rigid linkers for elucidation of the dimerization state in cells. J. Am. Chem. Soc. 2010;132(45):15899–901. doi: 10.1021/ja107447w. [DOI] [PubMed] [Google Scholar]
- [188].Faber A, Roderburg C, Wein F, Saffrich R, Seckinger A, Horsch K, Diehlmann A, Wong D, Bridger G, Eckstein V, Ho AD, Wagner W. The many facets of SDF-1alpha, CXCR4 agonists and antagonists on hematopoietic progenitor cells. J. Biomed. Biotechnol. 2007;2007(3):26065. doi: 10.1155/2007/26065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Kim SY, Lee CH, Midura BV, Yeung C, Mendoza A, Hong SH, Ren L, Wong D, Korz W, Merzouk A, Salari H, Zhang H, Hwang ST, Khanna C, Helman LJ. Inhibition of the CXCR4/CXCL12 chemokine pathway reduces the development of murine pulmonary metastases. Clin. Exp. Metastasis. 2008;25(3):201–11. doi: 10.1007/s10585-007-9133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Kwong J, Kulbe H, Wong D, Chakravarty P, Balkwill F. An antagonist of the chemokine receptor CXCR4 induces mitotic catastrophe in ovarian cancer cells. Mol. Cancer Ther. 2009;8(7):1893–905. doi: 10.1158/1535-7163.MCT-08-0966. [DOI] [PubMed] [Google Scholar]
- [191].Drury LJ, Ziarek JJ, Gravel S, Veldkamp CT, Takekoshi T, Hwang ST, Heveker N, Volkman BF, Dwinell MB. Monomeric and dimeric CXCL12 inhibit metastasis through distinct CXCR4 interactions and signaling pathways. Proc. Natl. Acad. Sci. U S A. 2011;108(43):17655–60. doi: 10.1073/pnas.1101133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Liang Z, Wu T, Lou H, Yu X, Taichman RS, Lau SK, Nie S, Umbreit J, Shim H. Inhibition of breast cancer metastasis by selective synthetic polypeptide against CXCR4. Cancer Res. 2004;64(12):4302–8. doi: 10.1158/0008-5472.CAN-03-3958. [DOI] [PubMed] [Google Scholar]
- [193].Tamamura H, Fujisawa M, Hiramatsu K, Mizumoto M, Nakashima H, Yamamoto N, Otaka A, Fujii N. Identification of a CXCR4 antagonist, a T140 analog, as an anti-rheumatoid arthritis agent. FEBS Lett. 2004;569(1-3):99–104. doi: 10.1016/j.febslet.2004.05.056. [DOI] [PubMed] [Google Scholar]
- [194].Janz JM, Ren Y, Looby R, Kazmi MA, Sachdev P, Grunbeck A, Haggis L, Chinnapen D, Lin AY, Seibert C, McMurry T, Carlson KE, Muir TW, Hunt S. r., Sakmar TP. Direct interaction between an allosteric agonist pepducin and the chemokine receptor CXCR4. J. Am. Chem. Soc. 2011;133(40):15878–81. doi: 10.1021/ja206661w. [DOI] [PubMed] [Google Scholar]