Abstract
Purpose
Translocator protein (TSPO) concentrations are elevated in glioma, suggesting a role for TSPO Positron Emission Tomography (PET) imaging in this setting. In preclinical PET studies, we evaluated a novel, high-affinity TSPO PET ligand, [18F]VUIIS1008, in healthy mice and glioma-bearing rats.
Procedures
Dynamic PET data were acquired simultaneously with [18F]VUIIS1008 injection, with binding reversibility and specificity evaluated in vivo by non-radioactive ligand displacement or blocking. Compartmental analysis of PET data was performed using metabolite-corrected arterial input functions. Imaging was validated with histology and immunohistochemistry.
Results
[18F]VUIIS1008 exhibited rapid uptake in TSPO-rich organs. PET ligand uptake was displaceable with non-radioactive VUIIS1008 or PBR06 in mice. Tumor accumulation of [18F]VUIIS1008 was blocked by pre-treatment with VUIIS1008 in rats. [18F]VUIIS1008 exhibited improved tumor-to-background ratio and higher binding potential in tumors compared to a structurally similar pyrazolopyrimidine TSPO ligand, [18F]DPA-714.
Conclusions
The PET ligand [18F]VUIIS1008 exhibits promising characteristics as a tracer for imaging glioma. Further translational studies appear warranted.
Keywords: PET, DPA-714, VUIIS1008, TSPO, glioma, cancer imaging
Introduction
The most common primary tumors of the brain are malignant gliomas, and they are exemplified by invasive growth and resistance to therapy. Routine methods to diagnose, grade, and classify gliomas include pathology of biopsy or surgically resected specimens, and these methods frequently are limited by sampling errors [1–2]. Consequently, clinical decisions are frequently supported by non-invasive imaging [3]. Though ubiquitously employed clinically, x-ray computed tomography (CT) and magnetic resonance imaging (MRI) tend to poorly discriminate disease margins and provide no molecular information attributable to the pathology of glioma, particularly where tumors are highly infiltrative. Stemming from the sensitivity and the ability to discriminate tissues based upon certain molecular characteristics, positron emission tomography (PET) imaging has the potential to overcome many of the limitations associated with conventional brain tumor imaging methods. However, PET imaging tracers available for routine use in brain tumor studies are limited. For example, PET with 2-deoxy-2-[18F]fluoro-D-glucose ([18F]FDG), which is used widely for grading brain tumors [4], tends to suffer from modest to poor tumor-to-background ratios due to significant accumulation of 18F-FDG in normal brain. Thus, improved molecular imaging biomarkers suitable for detection and characterization of gliomas could positively impact patient care.
Translocator protein (TSPO), formerly referred to as peripheral benzodiazepine receptor (PBR) is an 18-kDa protein primarily localized in mitochondria. TSPO plays numerous roles in key biological processes in healthy tissue and disease, which include the metabolism of cholesterol, biosynthesis of steroids biosynthesis, proliferation, and apoptosis [5–6]. Accordingly, TSPO expression is elevated in several clinically important disease states, including Alzheimer’s disease[7–8], Huntington’s disease[9], Parkinson’s disease[10], and cancer. In cancer, TSPO expression is reportedly associated with advanced disease and diminished survival [11–13]. We have explored TSPO as a molecular imaging target in colon cancer [14], breast cancer [15], and glioma [16–18], as TSPO PET could potentially serve as a useful cancer imaging biomarker [6].
In pursuit of improved TSPO ligands for cancer imaging, we reported the synthesis and structure-activity relationship (SAR) analysis of a library of novel pyrazolopyrimidines [18]. In that study, we identified a novel ligand, (2-(5,7-diethyl-2-(4-(2-fluoroethoxy)phenyl)pyrazolo[1,5-a]pyrimidin-3-yl)-N,N-diethylacetamide, VUIIS1008), that exhibited a 36-fold enhancement in binding affinity (Ki = 0.3nM) compared to the previously reported parent compound, DPA-714 (Ki = 10.9 nM) [16, 18] (Fig. 1). Radiolabeling this compound with fluorine-18 resulted in [18F]VUIIS1008, which exhibited promising preliminary characteristics for cancer imaging in preliminary studies [18]. The purpose of the present study was to explore the in vivo imaging characteristics of the new ligand quantitatively in healthy mice and a rat model of glioma. Quantitative imaging data obtained in a preclinical glioma model using [18F]VUIIS1008 were compared to an analogous study using a structurally similar pyrazolopyrimidine, [18F]DPA-714, which has been advanced as a PET agent for neuroinflammation [19] and oncology [6, 16, 20].
Figure 1.
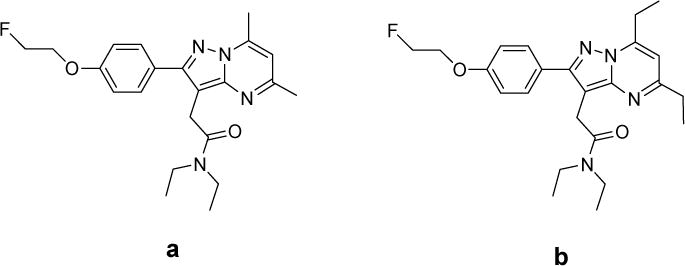
Chemical structure of DPA-714 (a) and VUIIS1008 (b) [18].
Materials and Methods
Chemistry and Radiochemistry
PBR06 was prepared as previously described [17]. [18F]VUIIS1008, the requisite tosylate ester precursor, and non-radioactive VUIIS1008 were prepared as previously described [18]. Specific activities of the [18F]VUIIS1008 produced in this study were 4600 ± 2160 Ci/mmol (170 ± 80 TBq/mmol) (Mean ± SD)(n = 10).
Animals
Studies involving animals were conducted in accordance with institutional and federal guidelines. Prior to imaging, all animals were allowed food and water ad libitum. Healthy, 10 week-old C57BL/6 mice (~25 g; The Jackson Laboratory, Bar Harbor, Maine, USA) were used for tracer biodistribution studies. Healthy, 10 week-old male Wistar rats (~300 g; Harlan Laboratories, Indianapolis, IN, USA) were inoculated with 1.0 × 105 C6 glioma cells (ATTC, Manassas, VA, USA) in the right brain hemisphere two weeks prior to imaging studies as described [16–18, 21]. Rats were surgically affixed with venous and arterial catheters prior to imaging.
MRI
For anatomical registration with PET, rats were secured prone in a radiofrequency coil (38-mm inner diameter) and placed in a 4.7-T horizontal bore MRI system (Varian Inc., Palo Alto, CA, USA). Body temperature (37°C) was maintained using heated airflow. An initial multislice gradient-echo imaging sequence (repetition time, 150 ms; echo time, 3.5 ms; matrix, 128 × 128; field of view, 40 × 40 mm2; slice thickness, 2 mm) was used to acquire 7 slices in each imaging plane (axial, coronal, sagittal) for proper positioning of subsequent scans. A multi-slice T2-weighted fast spin-echo scan with 8 echoes and 8.0-ms echo spacing (effective echo time, 32 ms) was then collected with a repetition time of 2,000 ms; field of view of 32 × 32 mm2; matrix of 128 × 128; 16 acquisitions; and 8 coronal slices of 2-mm thickness. PET/CT tumor studies in rats followed MRI by less than 24 hours.
PET/CT Acquisition and Reconstruction
For imaging studies, the tracer was administered intravenously (bolus) with initiation of a dynamic PET scan, either via retro-orbital injection (mice; 0.25 mCi, 9.25 MBq) or jugular catheter (rats; 1.2 ± 0.2 mCi, 44.9 ± 8.56 MBq), while in a microPET Focus 220 scanner (Siemens, Knoxville, TN, USA). For displacement studies, non-radioactive TSPO ligand challengers (VUIIS1008 or PBR06; 10 mg/kg) were administered via retro-orbital injection 30 minutes after tracer infusion. For blocking studies, VUIIS1008 (10 mg/kg) was administered intravenously five minutes prior to [18F]VUIIS1008. PET data were collected in list-mode format for 60 or 90 minutes, followed by CT (microCAT II; Siemens) for attenuation correction (rats only). The dynamic PET acquisitions were divided into twelve 10-second frames for the first two minutes, three 60-second frames for the following three minutes, and 300-second frames for the remainder of the scans. The raw data within each frame were binned into three-dimensional sinograms, with a span of three and ring difference of 47. The sinograms were reconstructed into tomographic images (128 × 128 × 95) with voxel sizes of 0.095 × 0.095 × 0.08 cm3 after scatter and attenuation corrections were applied using a two-dimensional ordered-subsets expectation-maximization algorithm with 16 subsets and four iterations. Attenuation correction (rats only) was accomplished by generating an attenuation map from the CT images, which were first coregistered with PET, then segmented and projected into sinogram space with a span of 47 and ring difference of 23.
Thin-Layer Chromatography for Radiometabolite Analysis
Plasma radiometabolites of [18F]VUIIS1008 were evaluated by thin-layer chromatography (TLC). Rat arterial blood (200 μL) was collected at 2, 12, 30, 60, and 90 minutes following administration of 1.2 ± 0.2 mCi (44.9 ± 8.56 MBq) of [18F]VUIIS1008 in rats (n = 4). Plasma (145 μL) was denatured with a mixture of acetonitrile/water (340 μL, 7/1 v/v), centrifuged, and the supernatant spotted on silica/glass TLC plates (Waterman, GE Healthcare, Little Chalfont, UK). TLC plates were developed in a solution of 10% methanol in dichloromethane and scanned using an AR-2000 radio-TLC imaging scanner (Bioscan/TriFoil, Washington, DC, USA). TLC plates were evaluated by determining the ratio of the parent radioligand ([18F]VUIIS1008) with respect to the total radioactivity in the plasma.
PET Imaging Analysis
Three-dimensional regions of interest (ROIs) were manually drawn around the heart, lung, kidney, and liver in mouse images using ASIPro (Siemens). Time-activity curves (TACs) were generated over these regions for the duration of the scan. For glioma-bearing rats, TACs were generated by manually drawing three-dimensional ROIs around the tumor and contralateral normal brain using ASIPro. Tumor ROIs included areas of central necrosis, if present. The arterial input function (AIF) was manually computed from plasma sampling (15 μL) that occurred concomitantly with imaging. Using TLC data, a metabolite-corrected plasma TAC was used as the input function. In this study, 1-tissue and 2-tissue models were evaluated using the PMOD software package (version 2.6) as published [16]. Total distribution volume (VT) and binding potential (BPND; k3/k4) were estimated using the kinetic parameters of K1, k2, k3, and k4 for various tissues [22].
Histology
Whole brains were harvested and fixed in 4% buffered formalin for 48 hours, followed by paraffin embedding for histology and immunohistochemistry (IHC). Tissue sections (5.0 μm) were stained with TSPO-specific rabbit polyclonal anti-rat/anti-mouse antibody (Novus Biologicals, LLC, Littleton, CO). Immunoreactivity was assessed using a horseradish peroxidase detection kit (Dako, Glostrup, DK). Hematoxylin and eosin staining was used to evaluate cell density and tumor localization. Sections were visualized and documented using bright field microscopy (Leica Microsystems, Inc., Buffalo Grove, IL, USA).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA). The significance (p-value) of tumor to brain comparisons was determined using a paired t-test. Data was considered significant when p-values were less than 0.05.
Results
In vivo accumulation and reversibility of [18F]VUIIS1008 in healthy tissues
Major organ accumulation and reversibility of [18F]VUIIS1008 uptake was evaluated in healthy mice using dynamic PET scanning (n = 2). In mice, the overall uptake of [18F]VUIIS1008 was consistent with known TSPO densities inherent to healthy organs [18] and hepatic excretion. Thirty minutes after intravenous administration of [18F]VUIIS1008, the greatest organ accumulation was observed in myocardium, lung, renal tissue and liver (Fig. 2a and 2b). Intravenous administration of non-radioactive VUIIS1008 (10 mg/kg) at the scan mid-point resulted in rapid and significant displacement of [18F]VUIIS1008 from myocardium and lung tissue, and partial displacement from renal tissue (Fig. 2b, 2d, and 2e). Following displacement, slightly elevated radioactivity was observed in liver, which appeared to be consistent with excretion (Fig. 2b, 2d, and 2e). Similar displacement of [18F]VUIIS1008 from myocardium, renal and lung tissue was achieved with an equivalent dosage of PBR06 (10 mg/kg), another well-documented TSPO ligand [23] (Fig. 2f). Overall these studies illustrated that [18F]VUIIS1008 exhibits reversible binding in healthy, TSPO-rich tissues and exhibits in vivo accumulation patterns not unlike other structurally similar TSPO ligands, such as [18F]DPA-714 [24].
Figure 2. 18F-VUIIS1008 accumulation and displacement in mice.
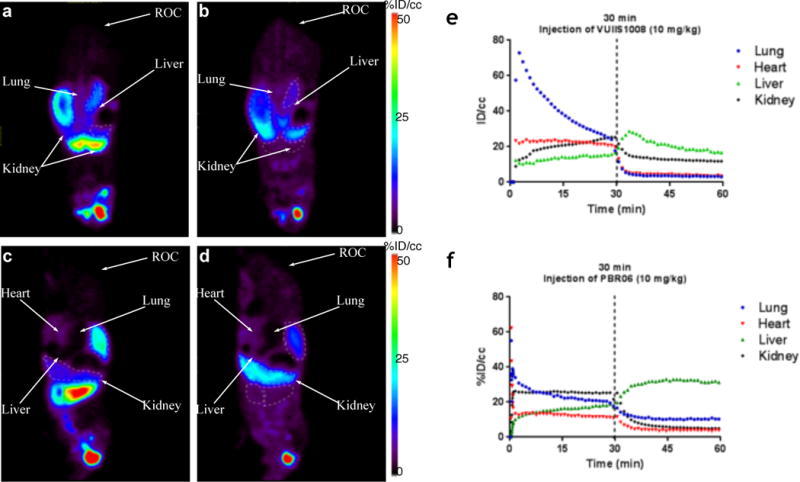
Summation of the first 30 minutes of a 60-minute dynamic PET acquisition following administration of 18F-VUIIS1008, prior to VUIIS1008 challenge (coronal view, a; sagittal view, c). Summation of the last 30 minutes of the dynamic acquisition following administration of 18F-VUIIS1008, following non-radioactive VUIIS1008 challenge (coronal view, b; sagittal view d). TACs for lung, heart, kidney and liver; displacement with VUIIS1008 (10 mg/kg) (e). (f) TACs for lung, heart, kidney and liver; displacement with PBR06 (10 mg/kg). ROC: retro-orbital cavity; %ID/cc = percentage injected dose per cubic centimeter. %ID/cc for ROC at 60 min is 45.7% in the image shown.
[18F]VUIIS1008 uptake and specificity in glioma-bearing rats
Uptake and specificity of [18F]VUIIS1008 were evaluated in C6 glioma-bearing rats. Accumulation (n = 6, 90-minute scans) and blocking (n = 4, 60-minute scans) were evaluated in dynamic PET studies (Fig. 3). Prior to PET, C6 gliomas were localized with T2-weighted MRI (Fig. 3a). Following administration, [18F]VUIIS1008 exhibited accumulation in glioma tissue in preference to normal brain (Fig. 3b/3c). Relative to tumor, [18F]VUIIS1008 exhibited modest accumulation in normal tissue. Mean TACs for the cohort of C6-bearing rats (n = 6) illustrated rapid tracer delivery to both tumor and normal brain, with each tissue exhibiting unique clearance profiles. Additionally, the tracer cleared rapidly from plasma (Fig. 3h). In contrast to normal brain, [18F]VUIIS1008 accumulated in tumors over time. Based upon the percentage of the injected dose per gram of tissue (%ID/g), a tumor-to-brain ratio of 3:1 was achieved by the conclusion of a 90-minute scan (Fig. 3h).
Figure 3. 18F-VUIIS1008 accumulation and target specificity in C6 glioma.
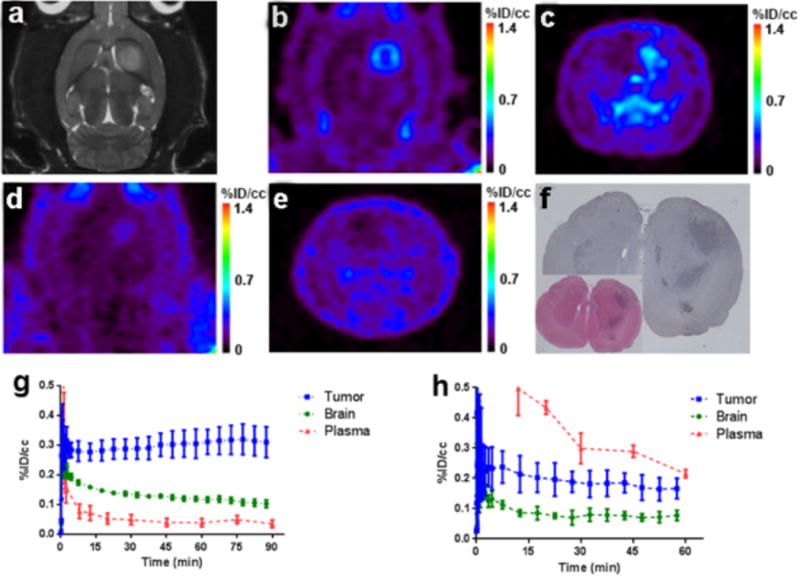
(a) T2-weighted MRI of a representative C6-bearing rat; lesion exhibits hyperintensity in the right hemisphere. Corresponding PET study 18F-VUIIS1008 (transverse view, b; coronal view, c). Uptake characteristics of 18F-VUIIS1008 following a pre-PET block with VUIIS1008 (10 mg/kg) in the same rat (transverse view, d; coronal view, e). (f/g) Imaging-matched brain from the study rat shown; TSPO immunoreactivity (f) and H&E staining (g). (h) Mean TACs for the un-blocked 90-min dynamic scan (n = 6). (i) Mean TACs for the pre-blocked scan with 60-min dynamic PET scans (n = 4). %ID/cc = percentage injected dose per cubic centimeter. In TACs, Data = Mean ± SD. R and L in the image denote the right and left side of the rats imaged individually.
In blocking studies with non-radioactive VUIIS1008, [18F]VUIIS1008 uptake was attenuated by approximately 50% in normal brain and tumor (Fig. 3d/3e; n = 4). A blocking dosage of 10 mg/kg of VUIIS1008 also led to significantly elevated plasma radioactivity compared with the unblocked scenario. In addition to lower tissue uptake, the blocking dose accelerated the clearance of [18F]VUIIS1008 from tumor and normal tissue (Fig. 3i). To validate PET, imaging-matched brains were processed for post-mortem staining and IHC, where close agreement between elevated TSPO levels (Fig. 3f) and tumor tissue by IHC were observed (Fig. 3g).
Compartmental modeling
The pharmacokinetics of [18F]VUIIS1008 uptake and clearance were modeled in tumor and normal brain. As with other TSPO PET ligands evaluated in this setting [16–18], [18F]VUIIS1008 TACs for tumor and normal brain more closely fit a 2-tissue, 4-rate-constant kinetic model compared to a 1-tissue model, as determined by inspection and chi-squared values (Fig. 4). In the 2-tissue model, terms reflecting delivery and efflux from a non-binding compartment (K1, k2) were not significantly different for [18F]VUIIS1008 in normal brain and tumor tissue. Delivery to the specific binding compartment (k3) was also similar for tumor and normal brain. However, a statistically significant difference was observed between k4 values derived from tumor and normal brain (Table 1; Supplementary Table 1). Here, we found measurable k4 values for normal brain, where k4 reflects efflux from the specific binding compartment, that were significantly greater than tumor tissue, which approached zero.
Figure 4. Pharmacokinetic modeling of 18F-VUIIS1008.
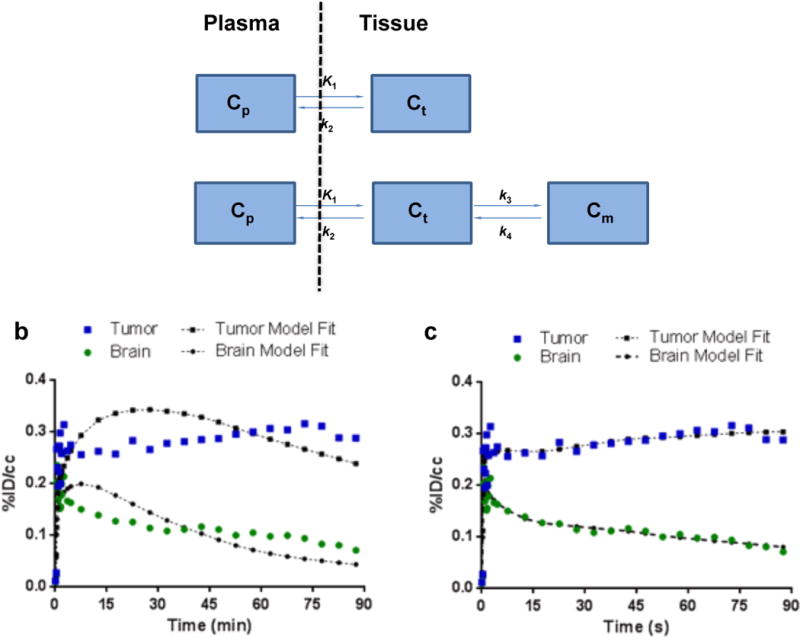
(a) Pharmacokinetic models evaluated to describe 18F-VUIIS1008 kinetics in tumor and brain. In these two models: Cp represents the concentration of 18F-VUIIS1008 in the plasma; Ct represents the concentration of tracer in a nonspecific compartment; Cm represents the concentration of tracer in a specific binding compartment. The parameters of K1, k2, k3, and k4 reflect transport between the compartments. (b) Representative TACs and pharmacokinetic model fits for 18F-VUIIS1008 in a 1-tissue, 2-kinetic-parameter model (calculated χ2 for one-tissue modeling fit = 26.311 ± 5.210; Mean ± SEM, n = 5). (c) Representative TACs and pharmacokinetic model fits for 18F-VUIIS1008 in a 2-tissue, 4-kinetic-parameter model (calculated χ2 for two-tissue modeling fit = 9.458 ± 2.623; Mean ± SEM, n = 5). TACs for tumor (blue) and contralateral brain (green) are shown with associated model fit. %ID/cc = percentage injected dose per cubic centimeter.
Table 1.
Parameter estimates for 18F-VUIIS1008 and 18F-DPA-714 pharmacokinetics in normal brain and tumor tissue using a 2-tissue, 4-parameter. Results = Mean ± SEM.
| Probe | Region | K1 | k2 | k3 | k4 |
|---|---|---|---|---|---|
| 18F-VUIIS1008 | Brain (n = 5) | 0.881 ± 0.392 | 2.078 ± 1.666 | 0.172 ± 0.070 | 0.037 ± 0.004 |
| Tumor (n = 5) | 0.709 ± 0.306 | 0.442 ± 0.101 | 0.113 ± 0.013 | 0.009 ± 0.002 | |
| 18F-DPA-714a | Brain (n = 11) | 5.674 ± 0.852 | 2.141 ± 0.540 | 0.070 ± 0.0132 | 0.023 ± 0.005 |
| Tumor (n = 11) | 5.708 ± 0.916 | 1.060 ± 0.174 | 0.098 ± 0.013 | 0.011 ± 0.001 |
Originally reported in reference [16]
Compared to a similar TSPO PET ligand we previously evaluated in analogous studies, [18F]DPA-714, [18F]VUIIS1008 exhibited lower influx-to-efflux parameter ratios (K1/k2) in the non-binding compartment for both tumor (1.90 mL/g versus 6.87 mL/g) and brain (0.86 mL/g versus 3.62 mL/g) (Table 2) [16]. In normal brain, binding potentials (k3/k4) were similar for both tracers, yet interestingly, tumor binding potentials were higher for 1[18F]VUIIS1008 compared to [18F]DPA-714 (12.63 versus 8.91) [16]. These results likely reflect the relative affinities of the two probes in cancer cells [16, 18] and suggest potentially greater tumor avidity for [18F]VUIIS1008 compared to [18F]DPA-714. Calculated VT levels for tumor and normal brain were higher for [18F]DPA-714, yet VT ratios between tumor and normal brain were higher for [18F]VUIIS1008 (6.0 versus 4.4), which led to an apparent improved tumor-to-normal ratio with this tracer [16]. The %ID/cc ratio between tumor and normal brain at 90 min was slightly higher for [18F]DPA-714 (4:1) than [18F]VUIIS1008 (3:1) (Table 2). Blocking the uptake of [18F]VUIIS1008 with excess non-radioactive VUIIS1008 (10 mg/kg) led to significantly lower VT in both tumor and normal brain and a decreased tumor binding potential (k3/k4) (Table 2).
Table 2.
K1/k2, k3/k4, and VT obtained from 18F-VUIIS1008 (n = 5) and 18F-VUIIS1008 in pre-blocking study (n = 2). Results = Mean ± SEM.
| Probe | Region | K1/k2 | k3/k4 | VT=(K1/k2)(1+k3/k4) | %ID/cc (90 min) |
|---|---|---|---|---|---|
| 18F-VUIIS1008a | Brain | 0.855 ± 0.325 | 4.435 ± 1.387 | 4.210 ± 1.612 | 0.103 ± 0.031 |
| Tumor | 1.902 ± 0.816 | 12.634 ± 1.406 | 25.224 ± 10.035 | 0.310 ± 0.051 | |
| 18F-DPA-714b | Brain | 3.619 ± 0.551 | 4.024 ± 0.842 | 15.963 ± 3.566 | 0.088 ± 0.011 |
| Tumor | 6.867 ± 1.226 | 8.913 ± 1.155 | 70.033 ± 14.729 | 0.331 ± 0.036 | |
| 18F-VUIIS1008c (Pre-blocking study) | Braind | 0.059 – 0.061 | 4.888 – 13.507 | 0.380 – 0.615 | N/A |
| Tumore | 0.124 – 0.143 | 3.972 – 4.368 | 0.618 – 0.767 | N/A |
C6-glioma bearing rats (n = 5). Results = Mean ± SEM
Originally reported in reference [16]. Results = Mean ± SEM
Pre-blocking study with C6-glioma bearing rats (n = 2). Results reflect the range of values from the 2 rats.
Discussion
Our previous research has explored TSPO expression as a target for molecular imaging of cancer [14–17, 25]. Formerly referred to as peripheral benzodiazepine receptor (PBR), TSPO is an 18-kDa protein typically localized to the outer mitochondria membrane. Tissues such as liver and brain exhibit comparatively modest TSPO levels [5]. While frequently exploited as a target in neuroscience, elevated TSPO expression is also observed in cancers of the breast [12, 26], prostate [11, 27], oral cavity [28], colon [14, 29–30], liver [31–32], and brain [33–34]. In oncology, TSPO expression is linked with disease progression and diminished survival [28, 30, 35–36] and is a hallmark of aggressive and potentially metastatic tumors [11–13].
We have reported preclinical utilization of the PET agents N-[18F]fluoroacetyl-N-(2,5-dimethoxybenzyl)-2-phenoxyaniline ([18F]PBR06) [17] and N,N-diethyl-2-(2-(4-(2-[18F]fluoroethoxy)phenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl)acetamide ([18F]DPA-714) [16] for quantitative assessment of TSPO expression in glioma. In these proof-of-principle PET imaging studies, tumors proved detectable among surrounding normal brain and, importantly, quantitative analyses of PET data agreed with ex vivo measures of TSPO levels [16–17]. However, drawbacks were observed with both agents in this context, including tracer accumulation in normal brain that reached levels potentially sufficient to preclude detection of gliomas with modest TSPO expression, such as lower grade disease. Furthermore, both tracers exhibited significant metabolism in vivo, which required correction of plasma input functions for quantitative analysis. While promising, these studies prompted our development of novel TSPO PET ligands with potentially improved properties for cancer imaging.
In this study, we quantitatively evaluated the in vivo performance of a novel TSPO PET ligand, [18F]VUIIS1008. A seemingly minor structural alteration, substitution of the 5,7-methyl groups on the pyrazolopyrimidine ring of [18F]DPA-714 with ethyl groups, yielded 18F-VUIIS1008 (Fig. 1), a probe with approximately 36-fold greater affinity for TSPO [18]. As shown in this study, this structural modification resulted in a number of intriguing and potentially improved properties for cancer imaging. For example, [18F]VUIIS1008 exhibited lower accumulation in healthy rat brain, improved tumor-to-background ratio (VT) and higher binding potential in tumors compared to [18F]DPA-714. Interestingly, despite improved binding potential of [18F]VUIIS1008 in tumor, the tracer exhibited similar binding potential to [18F]DPA-714 in normal brain. This study also evaluated the specificity of [18F]VUIIS1008 in vivo. In tumor studies, reduced tracer absorption was observed following pre-blocking of the binding sites with non-radioactive VUIIS1008. In support of high target specificity, pharmacokinetic modeling of PET data derived from the blocking studies demonstrated significantly lower tumor VT and tumor binding potential (k3/k4), suggesting reduced availability of TSPO binding sites compared with the un-blocked scenario. Overall, these characteristics suggest that [18F]VUIIS1008 might be superior for detecting tumors, particularly for those with modest TSPO expression profiles. Although not directly compared here, substitution of the 5,7-methyl with ethyl groups did not appear to confer a significant in vivo stability advantage, as metabolism profiles for the new probe (Supplemental Table 2), and when compared with [18F]DPA-714 were not remarkably different [16].
Certain attributes of the new tracer and the attendant PET data that stem from its use warrant further exploration. For example, simple quantification of tracer uptake at a fixed time following injection (%ID/cc; 90 minutes), as would be routine in the clinical setting, actually yield a slightly lower tumor-to-normal brain ratio for [18F]VUIIS1008 (3:1) compared to [18F]DPA-714 (4:1) [16]. Only with more sophisticated analysis of the dynamic PET acquisitions, which included pharmacokinetic modeling, arterial sampling, and metabolite correction, did we observe significantly greater tumor-to-normal brain VT and BPND ratios inherent to [18F]VUIIS1008. While these techniques can obviously be incorporated in the research setting, they are not routine in clinical practice, highlighting a potential translational hurdle. Longer dynamic PET scans and compartmental analyses are not routine in clinical practice. Further preclinical studies should evaluate the most appropriate acquisition and data analysis metrics, which could be feasibly translated to human studies.
Conclusion
These studies suggest that [18F]VUIIS1008 exhibits several promising and unique characteristics as a PET tracer for imaging glioma. Further translational studies, including those conducted in more complex glioma models and potentially other solid tumors, appear warranted.
Supplementary Material
Acknowledgments
The authors thank George H. Wilson and Daniel Colvin for assistance with microPET and MR imaging studies, respectively, Allie Fu for preclinical model support, and Matthew R. Hight and Michael L. Schulte for editing the manuscript and helpful discussions. The authors acknowledge funding from the National Institutes of Health (K25 CA127349, P50 CA128323, S10 RR17858, U24 CA126588, 1R01 CA163806) and the Kleberg Foundation.
Footnotes
Conflict of Interest Statement
The authors do not have any conflicts of interest.
References
- 1.Jackson RJ, Fuller GN, Abi-Said D, et al. Limitations of stereotactic biopsy in the initial management of gliomas. Neuro-oncology. 2001;3:193–200. doi: 10.1093/neuonc/3.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Law M, Yang S, Wang H, et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR American journal of neuroradiology. 2003;24:1989–1998. [PMC free article] [PubMed] [Google Scholar]
- 3.Dhermain FG, Hau P, Lanfermann H, et al. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010;9:906–920. doi: 10.1016/S1474-4422(10)70181-2. [DOI] [PubMed] [Google Scholar]
- 4.Dimitrakopoulou-Strauss A, Seiz M, Tuettenberg J, et al. Pharmacokinetic studies of (6)(8)Ga-labeled Bombesin ((6)(8)Ga-BZH(3)) and F-18 FDG PET in patients with recurrent gliomas and comparison to grading: preliminary results. Clin Nucl Med. 2011;36:101–108. doi: 10.1097/RLU.0b013e318203bb24. [DOI] [PubMed] [Google Scholar]
- 5.Papadopoulos V, Baraldi M, Guilarte TR, et al. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Awde AR, Boisgard R, Theze B, et al. The Translocator Protein Radioligand 18F-DPA-714 Monitors Antitumor Effect of Erufosine in a Rat 9L Intracranial Glioma Model. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2013;54:2125–2131. doi: 10.2967/jnumed.112.118794. [DOI] [PubMed] [Google Scholar]
- 7.Edison P, Archer HA, Gerhard A, et al. Microglia, amyloid, and cognition in Alzheimer’s disease: An [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiology of disease. 2008;32:412–419. doi: 10.1016/j.nbd.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Yasuno F, Ota M, Kosaka J, et al. Increased binding of peripheral benzodiazepine receptor in Alzheimer’s disease measured by positron emission tomography with [11C]DAA1106. Biological psychiatry. 2008;64:835–841. doi: 10.1016/j.biopsych.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 9.Pavese N, Gerhard A, Tai YF, et al. Microglial activation correlates with severity in Huntington disease: a clinical and PET study. Neurology. 2006;66:1638–1643. doi: 10.1212/01.wnl.0000222734.56412.17. [DOI] [PubMed] [Google Scholar]
- 10.Ouchi Y, Yoshikawa E, Sekine Y, et al. Microglial activation and dopamine terminal loss in early Parkinson’s disease. Annals of neurology. 2005;57:168–175. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- 11.Han Z, Slack RS, Li W, Papadopoulos V. Expression of peripheral benzodiazepine receptor (PBR) in human tumors: relationship to breast, colorectal, and prostate tumor progression. J Recept Signal Transduct Res. 2003;23:225–238. doi: 10.1081/rrs-120025210. [DOI] [PubMed] [Google Scholar]
- 12.Hardwick M, Fertikh D, Culty M, et al. Peripheral-type benzodiazepine receptor (PBR) in human breast cancer: correlation of breast cancer cell aggressive phenotype with PBR expression, nuclear localization, and PBR-mediated cell proliferation and nuclear transport of cholesterol. Cancer Res. 1999;59:831–842. [PubMed] [Google Scholar]
- 13.Hardwick M, Rone J, Han Z, et al. Peripheral-type benzodiazepine receptor levels correlate with the ability of human breast cancer MDA-MB-231 cell line to grow in SCID mice. Int J Cancer. 2001;94:322–327. doi: 10.1002/ijc.1472. [DOI] [PubMed] [Google Scholar]
- 14.Deane NG, Manning HC, Foutch AC, et al. Targeted imaging of colonic tumors in smad3−/− mice discriminates cancer and inflammation. Mol Cancer Res. 2007;5:341–349. doi: 10.1158/1541-7786.MCR-06-0225. [DOI] [PubMed] [Google Scholar]
- 15.Wyatt SK, Manning HC, Bai M, et al. Molecular imaging of the translocator protein (TSPO) in a pre-clinical model of breast cancer. Mol Imaging Biol. 2010;12:349–358. doi: 10.1007/s11307-009-0270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang D, Hight MR, McKinley ET, et al. Quantitative preclinical imaging of TSPO expression in glioma using N,N-diethyl-2-(2-(4-(2–18F-fluoroethoxy)phenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimi din-3-yl)acetamide. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2012;53:287–294. doi: 10.2967/jnumed.111.095653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buck JR, McKinley ET, Hight MR, et al. Quantitative, preclinical PET of translocator protein expression in glioma using 18F-N-fluoroacetyl-N-(2,5-dimethoxybenzyl)-2-phenoxyaniline. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2011;52:107–114. doi: 10.2967/jnumed.110.081703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang D, McKinley ET, Hight MR, et al. Synthesis and structure-activity relationships of 5,6,7-substituted pyrazolopyrimidines: discovery of a novel TSPO PET ligand for cancer imaging. Journal of medicinal chemistry. 2013;56:3429–3433. doi: 10.1021/jm4001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boutin H, Prenant C, Maroy R, et al. [18F]DPA-714: direct comparison with [11C]PK11195 in a model of cerebral ischemia in rats. PloS one. 2013;8:e56441. doi: 10.1371/journal.pone.0056441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winkeler A, Boisgard R, Awde AR, et al. The translocator protein ligand [(1)(8)F]DPA-714 images glioma and activated microglia in vivo. European journal of nuclear medicine and molecular imaging. 2012;39:811–823. doi: 10.1007/s00259-011-2041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buck JR, Saleh S, Uddin MI, Manning HC. Rapid, Microwave-Assisted Organic Synthesis of Selective (V600E)BRAF Inhibitors for Preclinical Cancer Research. Tetrahedron letters. 2012;53:4161–4165. doi: 10.1016/j.tetlet.2012.05.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 23.Fujimura Y, Kimura Y, Simeon FG, et al. Biodistribution and radiation dosimetry in humans of a new PET ligand, (18)F-PBR06, to image translocator protein (18 kDa) Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2010;51:145–149. doi: 10.2967/jnumed.109.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arlicot N, Vercouillie J, Ribeiro MJ, et al. Initial evaluation in healthy humans of [18F]DPA-714, a potential PET biomarker for neuroinflammation. Nuclear medicine and biology. 2012;39:570–578. doi: 10.1016/j.nucmedbio.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Manning HC, Goebel T, Thompson RC, et al. Targeted molecular imaging agents for cellular-scale bimodal imaging. Bioconjug Chem. 2004;15:1488–1495. doi: 10.1021/bc049904q. [DOI] [PubMed] [Google Scholar]
- 26.Carmel I, Fares FA, Leschiner S, et al. Peripheral-type benzodiazepine receptors in the regulation of proliferation of MCF-7 human breast carcinoma cell line. Biochem Pharmacol. 1999;58:273–278. doi: 10.1016/s0006-2952(99)00093-3. [DOI] [PubMed] [Google Scholar]
- 27.Fafalios A, Akhavan A, Parwani AV, et al. Translocator protein blockade reduces prostate tumor growth. Clin Cancer Res. 2009;15:6177–6184. doi: 10.1158/1078-0432.CCR-09-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagler R, Ben-Izhak O, Savulescu D, et al. Oral cancer, cigarette smoke and mitochondrial 18kDa translocator protein (TSPO) – In vitro, in vivo, salivary analysis. Biochim Biophys Acta. 2010;1802:454–461. doi: 10.1016/j.bbadis.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Maaser K, Hopfner M, Jansen A, et al. Specific ligands of the peripheral benzodiazepine receptor induce apoptosis and cell cycle arrest in human colorectal cancer cells. Br J Cancer. 2001;85:1771–1780. doi: 10.1054/bjoc.2001.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maaser K, Grabowski P, Sutter AP, et al. Overexpression of the peripheral benzodiazepine receptor is a relevant prognostic factor in stage III colorectal cancer. Clin Cancer Res. 2002;8:3205–3209. [PubMed] [Google Scholar]
- 31.Venturini I, Zeneroli ML, Corsi L, et al. Up-regulation of peripheral benzodiazepine receptor system in hepatocellular carcinoma. Life Sci. 1998;63:1269–1280. doi: 10.1016/s0024-3205(98)00388-9. [DOI] [PubMed] [Google Scholar]
- 32.Venturini I, Zeneroli ML, Corsi L, et al. Diazepam binding inhibitor and total cholesterol plasma levels in cirrhosis and hepatocellular carcinoma. Regulatory peptides. 1998;74:31–34. doi: 10.1016/s0167-0115(98)00013-5. [DOI] [PubMed] [Google Scholar]
- 33.Black KL, Ikezaki K, Toga AW. Imaging of brain tumors using peripheral benzodiazepine receptor ligands. Journal of neurosurgery. 1989;71:113–118. doi: 10.3171/jns.1989.71.1.0113. [DOI] [PubMed] [Google Scholar]
- 34.Starosta-Rubinstein S, Ciliax BJ, Penney JB, et al. Imaging of a glioma using peripheral benzodiazepine receptor ligands. Proc Natl Acad Sci U S A. 1987;84:891–895. doi: 10.1073/pnas.84.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galiegue S, Casellas P, Kramar A, et al. Immunohistochemical assessment of the peripheral benzodiazepine receptor in breast cancer and its relationship with survival. Clin Cancer Res. 2004;10:2058–2064. doi: 10.1158/1078-0432.ccr-03-0988. [DOI] [PubMed] [Google Scholar]
- 36.Miettinen H, Kononen J, Haapasalo H, et al. Expression of peripheral-type benzodiazepine receptor and diazepam binding inhibitor in human astrocytomas: relationship to cell proliferation. Cancer Res. 1995;55:2691–2695. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


