Abstract
Objectives
To assess aggressive medical care, hospice utilization, and advance care documentation among ovarian cancer patients in the final thirty days of life.
Methods
Ovarian, fallopian tube, or primary peritoneal cancer patients registered at our institution during 2007–2011 were identified. Statistical analyses included Wilcoxon Mann-Whitney, Chi-square analysis, and multivariate analysis.
Results
183 patients met inclusion criteria. Median age at diagnosis was 58. Most were white and had advanced ovarian cancer.
Fifty percent had experienced at least one form of aggressive care during the last 30 days of life. Patients with provider recommendations to enroll in hospice were more likely to do so (OR 27.7, p=<0.001), with a median hospice stay of 18 days before death.
Seventy-five percent had an in-hospital DNR and 33% had an out-of-hospital DNR order. These orders were created a median of 15 and 12 days prior to death, respectively. Twenty-eight percent had a Medical Power of Attorney and 20% had a Living Will. These documents were created a median of 381 and 378 days prior to death, respectively.
Conclusions
Many ovarian cancer patients underwent some form of aggressive medical care in the last 30 days of life. The time between hospice enrollment and death was short. Patients created Medical Power of Attorney and Living Will documents far in advance of death. DNR orders were initiated close to death.
INTRODUCTION
Early discussions regarding the patient’s treatment goals and need for palliative supportive care may be perceived as premature by some. However, early palliative care interventions provide advanced cancer patients with improved quality of life (QOL), regardless of whether or not they are receiving anti-cancer treatment (1, 2). As a result, both the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) recommend that providers engage in discussions regarding advanced cancer patient’s treatment goals, expectations, and need for palliative care interventions early in the disease process (3, 4). These advance care planning discussions serve two main purposes. First, these discussions give patients time to think about the issues they may have to face in the future. Second they give patients the opportunity to discuss their wishes with their family members and medical care team (5, 6). Often these discussions result in less aggressive care at the end-of-life and increased hospice utilization (7, 8).
Unfortunately, many patients do not have early advance care planning discussions with their providers (9, 10). As a result, many patients experience aggressive medical care ranging from chemotherapeutic administration to multiple hospital admissions in the final days of life (7, 8). While there is no universal definition of what constitutes aggressive medical care at the end of life, several researchers use the following metrics identified by the National Quality Forum: chemotherapy administration within the last 14 days of life, more than one emergency room visit in the last 30 days of life, more than one hospital admission in the last 30 days of life, more than 14 days spent admitted to the hospital in the last 30 days of life, intensive care unit (ICU) admission in the last 30 days of life, death in the hospital, and hospice admission during the last three days of life (7, 8, 11, 12).
For those patients who will eventually succumb to their disease, hospice care provides an alternative to aggressive medical care at the end of life by allowing the patient to transition from the active treatment of disease to the management of symptoms and identification of expectations surrounding death. In addition to the benefits provided to the individual patient, hospice provides benefits on a global healthcare level. Recent studies demonstrate decreased utilization of hospital resources (i.e. procedures, admissions) and increased medical costs savings among patients enrolled in hospice (13–15). In order to efficiently utilize the limited health care dollars available, we must evaluate the benefits of aggressive measures taken at the end of life.
Ovarian cancer is often diagnosed at an advanced stage and has the highest mortality rate among gynecologic cancers (16). Overall survival is poor, with a 5-year survival rate of 40–50% following initial diagnosis (17, 18). Limited studies have evaluated the medical care received by ovarian cancer patients at the end of life (8, 19). The primary objective of this study was to assess patterns of medical care, hospice utilization, and aggressive medical care among ovarian cancer patients at our institution in the last 30 days of life. A secondary objective was to assess the utilization of advance care documentation, such as medical power of attorney documents, living wills, or Do Not Resuscitate (DNR) orders, among deceased ovarian cancer patients at our institution.
MATERIALS AND METHODS
This retrospective study was approved by The University of Texas MD Anderson Cancer Center’s Institutional Review Board (IRB). Deceased patients, ages 18 years or older with a diagnosis of ovarian, fallopian tube, or primary peritoneal cancer who were registered at The University of Texas MD Anderson Cancer Center in Houston, Texas during 2007–2011 were identified through our institution’s Tumor Registry. Patients who did not receive their primary cancer care at The University of Texas MD Anderson Cancer Center, received the majority of their cancer care in the final month of life at an outside institution, or did not have a documented location of death were excluded from the study.
Demographic data and end-of-life care outcomes were collected from the electronic medical record of patients satisfying these inclusion criteria. Data collected included: age, race, cancer type, education level, cancer stage, date of diagnosis, date of death, location of death, provider recommendations regarding hospice care (a documented conversation in the medical record where a medical care provider suggested that the patient transition to hospice care), presence of advance directive document (DNR, Medical Power of Attorney, and Living Will), and date of advance directive document signing (when applicable). Of note, our institution has a Palliative Care unit where patients may be transferred to when they decide to pursue hospice care during a hospital admission. Patients often go to our Palliative Care unit prior to moving to either home hospice or another inpatient hospice unit outside our institution. Deaths occurring in the Palliative Care unit were counted as hospital deaths in the results because the patients were listed as dying at our institution.
Information regarding aggressive medical care during the last 30 days of life was collected. For the purposes of our study, we adopted the metrics outlined by the National Quality Forum (chemotherapy administration within the last 14 days of life, more than one emergency room visit in the last 30 days of life, more than one hospital admission in the last 30 days of life, more than 14 days spent admitted to the hospital in the last 30 days of life, intensive care unit (ICU) admission in the last 30 days of life, death in a hospital, and hospice admission during the last three days of life) as indicators of aggressive medical care (7, 8, 11, 12).
Additionally, we collected information regarding palliative care consultation, including whether or not the first palliative care consult occurred in the in-patient or out-patient setting and the time between palliative care consult and death. For the purposes of our study, late palliative care consultation was defined as a consultation taking place in the final 30 days of life.
Descriptive statistics were used to characterize the study population. Differences between groups were evaluated using Wilcoxon Mann-Whitney, Chi-square, and multivariate analysis. IBM SPSS Statistics (v. 21) was used for the statistical analyses.
RESULTS
One-thousand sixty-eight records were identified in the initial query of The University of Texas MD Anderson Cancer Center’s Tumor Registry. Of these, 480 patients received only treatment recommendations at our institution and then received the remainder of their cancer care at an outside hospital. Eight-hundred and eighty-five patients received cancer care primarily at an outside institution during the last six months of life or were lost to follow-up prior to death. One-hundred and eighty-three patients met all of the inclusion criteria for this study and had information regarding both their cancer care during the last month of life and place of death. The median age of eligible patients at diagnosis was 58 years. Most patients were white with advanced stage ovarian cancer. Table 1 lists pertinent demographics.
Table 1.
Demographics
| Characteristic | N (%) |
|---|---|
| Race/Ethnicity | |
| White | 124 (67.8) |
| Black | 24 (13.1) |
| Hispanic | 24 (13.1) |
| Asian | 10 (5.5) |
| Other | 1 (0.5) |
| Cancer Type | |
| Ovarian | 177 (96.7) |
| Fallopian tube | 5 (2.7) |
| Primary Peritoneal | 1 (0.5) |
| Stage of Cancer | |
| I | 4 (2.2) |
| II | 8 (4.4) |
| III | 79 (43.2) |
| IV | 36 (19.7) |
| Unstaged/Neoadjuvant | 42 (23.0) |
| Not documented | 14 (7.7) |
| Marital Status | |
| Single | 29 (15.8) |
| Married | 114 (62.3) |
| Divorced | 21 (11.5) |
| Widowed | 19 (10.4) |
| Education | |
| Less than College | 99 (54.1) |
| College and Beyond | 61 (33.3) |
| Unknown | 23 (12.6) |
| Insurance | |
| Private | 110 (60.1) |
| Medicare | 58 (31.7) |
| Medicaid/Self-Pay/Indigent | 15 (8.2) |
Median age at diagnosis: 58 years (SD: 11.4, Range: 22–87 years old)
Median age at death: 60 years (SD 11.3, Range 25–90 years old)
Aggressive Care Received in the Last 30 Days of Life
Twelve patients (7%) received chemotherapy or a clinical trial drug in the last 14 days of life. Thirty-four (19%) had more than one ER visit in the last 30 days of life. Of these 34 patients, the mean number of ER visits was two visits (SD 0.55 visits, Range 2–4 visits). Thirty-one (17%) were admitted to the hospital more than one time in the last 30 days of life. Of these 31 patients, the mean number of admissions in the last 30 days of life was two admissions (SD 0.45 admissions, Range 2–3). Twenty-six patients (14%) were admitted for longer than 14 days in the last 30 days of life. Of these 26 patients, the median number of days spent in the hospital was 18 days (SD 4.91 days, Range 15–31 days). Eighteen (10%) were admitted to the ICU in the last 30 days of life. Forty-one (22%) of the patients died in the hospital. Fifteen (8%) went to hospice in the last three days of life. Of the entire study population, ninety-one (50%) had experienced at least one form of aggressive care at the end of life (Figure 1).
Figure 1.
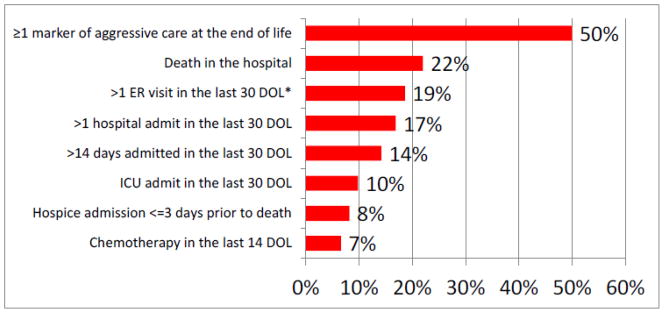
Percentage of patients receiving markers of aggressive medical care at the end of life
*DOL = days of life
After adjusting for the effects of race, age at diagnosis, marital status, education, and years between initial diagnosis and death, only years between diagnosis and death predicted whether patients received aggressive care. The odds of receiving aggressive care decreased by 22% with each passing year after initial diagnosis (OR=0.78; 95% CI [0.66, 0.93]; p=0.006).
Palliative Care Consultation and Location of Death
Seventy-nine percent (n=144) of our patients received a palliative care consult prior to death. The median time between first seeing a palliative care provider and death was 35.5 days (SD 172 days, Range 0–1,064 days prior to death). Among those 144 patients receiving palliative care consults, most received in-patient as opposed to out-patient consults (76% vs. 24%, respectively) and most (47%) received a late palliative care consult (less than thirty days prior to death) (Figure 2). Seventy-four percent (n=135) of patients enrolled in hospice. Among these 135 patients, the median length of hospice stay was 18 days prior to death. Twenty-three percent (n=30) of those who died under hospice care received hospice care for less than or equal to seven days prior to death. Eleven percent (n=15) of those who died under hospice care received hospice care for less than or equal to three days prior to death. Twenty-two percent (n=41) of the patients died during an admission to our hospital. Among those 41 patients dying in the hospital, 43.9% (n=18) died in the Palliative Care unit, 39% (n=16) died in the inpatient gynecologic oncology unit, and 17.1% (n=7) died in the ICU. Figure 3 lists location of death.
Figure 2.
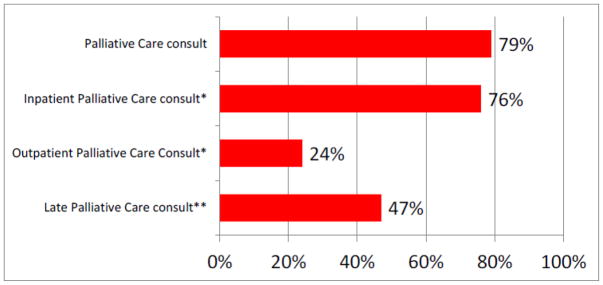
Percentage of patients receiving palliative care consults prior to death
*Among those patients receiving a Palliative Care consult
** Defined as Palliative Care consult within the last 30 days of life
Figure 3.
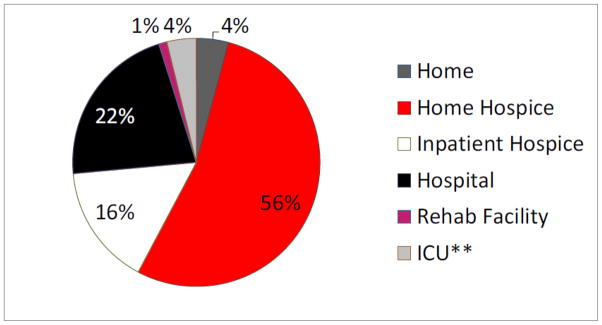
Location of death
*3 patients originally enrolled in hospice care were ultimately admitted and died in the hospital
**Percentile calculated based on all patients in the study
Factors Impacting Hospice Enrollment and Death in the Hospital
Patients who received provider recommendations to enroll in hospice were more likely to do so than those who did not (OR=27.7, 95% CI [8.8, 87.1]; p=<0.001). White patients and those with at least a college education had shorter hospice stays versus non-white patients and patients with less than a college education (p=0.009 and 0.017, respectively). Patient age was not associated with likelihood of dying in the hospital. A longer time period between initial diagnosis and death was associated with reduced odds of death in the hospital (OR=0.72 per year since initial diagnosis, 95% CI [0.57, 0.91]; p=0.006). Compared to married patients, unmarried patients were 2.95 times more likely to die in the hospital (OR=2.95, 95% CI [1.23, 7.07]; p=0.015).
Advance Care Planning Documentation
Seventy-five percent of patients (n=137) had an in-hospital Do Not Resuscitate order (IH-DNR) and 33% (n=61) had an out-of-hospital Do Not Resuscitate order (OH-DNR) listed in the electronic medical record (Figure 4). These documents were created a median of 15 and 12 days prior to death, respectively. The majority (64%) of IH-DNR orders were created by a Gynecologic Oncology attending. The remaining IH-DNR orders were created by the Palliative Care physician (15%), the Clinical Trials physician (17%), the Intensive Care Unit physician (4%), or the Emergency Department physician (<1%). In a similar fashion, the majority (57%) of OH-DNR orders were created by a Gynecologic Oncology attending. The remainder were completed by the Palliative Care physician (29%), the Clinical Trials physician (10%), and the Intensive Care Unit physician (2%).
Figure 4.
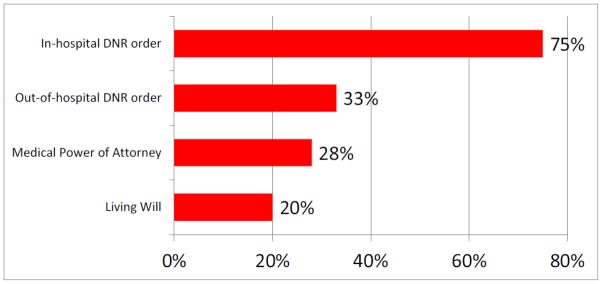
Percentage of patients with advance care planning documents
Patients with DNR orders who went to hospice had an IH-DNR created a median of six days prior to hospice enrollment, and an OH-DNR created a median of one day prior to hospice enrollment. White patients were less likely to have both an IH-DNR (OR=0.44, 95% CI [0.20, 0.99]; p=0.046) and an OH-DNR (OR=0.51, 95% CI [0.26, 0.98]; p=0.04) when compared to non-white patients. Twenty-eight percent of patients (n=51) had a Medical Power of Attorney in their electronic medical record and 20% (n=36) had a Living Will. These documents were created a median of 381 and 378 days prior to death, respectively. Among the 55 patients with either a Living Will or Medical Power of Attorney in the electronic medical record, 76.5% (n=42) had an IH-DNR and 29.4% (n=15) had an OH-DNR (Figure 4).
DISCUSSION
The results of our study demonstrate half of our population experienced at least one form of aggressive medical care in the last 30 days of life. While the majority of our patients ultimately enrolled in hospice care prior to death, enrollment occurred in the time immediately surrounding death. The majority of our patients saw a palliative care provider in the period preceding death, but these visits often occurred in the last month and a half of life and were in the inpatient setting. A very small percentage of patients had advance care planning documents such as a Medical Power of Attorney or a Living Will within their medical record. These findings suggest that our patients are receiving aggressive care at the end of life and are not having early discussions with their providers regarding advance care planning topics. In an era of multiple resource challenges that require us to evaluate and justify our rationale behind pursuing various treatment options, it is important that we discuss with our patients what treatments they desire and encourage them to create the corresponding documentation within their medical record.
Aggressive medical care at the end of life has recently become an important topic as many researchers have demonstrated increased medical costs and decreased patient QOL associated with such care (7, 20). The results of our study demonstrate that 50% of our patients experienced at least one component of aggressive medical care during their last month of life. Those closer to the time of their initial cancer diagnosis were more likely to receive at least one form of aggressive care. Unmarried patients and those closer to the time of their initial cancer diagnosis were more likely to experience aggressive medical care in the form of dying in the hospital. White patients and those with higher education had shorter hospice stays. This suggests that certain demographic factors may impact the level of aggressive care received at the end of life and may impact a patient’s decision to enroll in hospice. It is important that providers take these demographic factors into consideration when discussing end-of-life care topics with patients in order to facilitate productive conversations. Further exploration of the role that demographic factors play in end-of-life care decisions must occur in order to clarify the way these factors alter a patient’s decisions regarding care received at the end of life.
As oncologists, it is our responsibility to provide our patients with cutting edge cancer therapeutics and to provide patients interested in exploring all treatment options prior to death the tools to do so. We must, however, be careful to engage in honest and open discussions with our patients regarding their likely treatment outcomes. It is imperative that we communicate with our patients regarding their desired management at the end of life in order to minimize hospitalizations and time spent away from loved ones during the last days of life.
One way to communicate with patients regarding their end-of-life care desires is to initiate conversations relating to advance care planning documentation (21). These documents, such as a Medical Power of Attorney, Living Will, and DNR order allow patients to think about and communicate their desires to their medical care team (21). Creation of these documents also allows patients to communicate their desires to their loved ones (21). Our study reveals that the majority of our patients did not have these documents present within their medical records. As providers, it is important that we encourage patients to complete these documents early in their disease course. Discussion and completion of these documents will allow providers to learn more about their patient’s end of life care preferences and will encourage an open dialogue regarding these topics.
The majority of our patients died in the hospice setting. This finding is reassuring, in that the majority of our patients were able to transition out of the hospital and away from the active treatment of their disease in the time before death. Despite the positive findings relating to overall hospice utilization in our patient population, many of our patients transitioned to hospice during the last several weeks of their life. Our findings are similar to other studies suggesting that advanced gynecologic oncology patients are not being referred to hospice in a timely manner before death (22).
The delayed hospice referrals noted in our study reiterate the importance of early palliative care consultation and provider discussion regarding advance care planning. Simply discussing these sensitive topics with our patients can alter the treatment received by our patients, and ultimately has the potential to positively impact their QOL (7). Our study demonstrates that providers play an important role in encouraging patients to enroll in hospice care. This further supports prior studies that demonstrate a clear link between hospice enrollment and provider discussions regarding end-of-life care plans (7, 8). Unfortunately, many of the conversations regarding hospice care in our study population first took place in the time immediately surrounding death. We must be mindful not to delay these conversations until the final days of life. Deferred advance care planning represents a missed opportunity to improve our patients’ QOL and often leads to increased anxiety and depression among our patients’ loved ones (23).
Our study has several limitations. First, it is a retrospective review. Accordingly, our findings were limited by the information documented in the electronic medical system. Certainly providers may have had advance care planning discussions with their patients at an earlier time than is indicated in the medical record. Accordingly, the information relating to physician-patient advance care planning discussions reported in our study may not completely represent our physicians’ current practices. However, the lack of advance care planning documentation and delay in hospice enrollment does, to a certain extent, suggest that the data documenting the timing of discussions collected from the medical record for our study was not completely inaccurate.
Second, our data were collected from a single, tertiary-care institution and may not be generalizable to other gynecologic oncology practices. Additional evaluation of medical care, palliative care, and hospice referral patterns must be undertaken at multiple institutions in order to elucidate the current practice patterns of gynecologic oncologists. Finally, the majority of our patients were white, which limits generalizability of our data to other races and ethnicities. This is particularly true since race/ethnicity is a personal determinate known to impact end-of-life care and advance care planning documentation patterns (24, 25).
We should encourage discussions regarding advance care planning with our patients. This is particularly true for patients with cancers with poor overall survival rates, such as women with ovarian cancer. Our medical care system continues to evolve and over the last several years has placed increased emphasis on patient-reported outcomes (i.e. QOL) and the financial burden associated with medical treatments that do not result in improved survival outcomes. Because prior research has demonstrated that early advance care planning has been associated with improved patient QOL and decreased medical costs, advance care planning discussions and documentation have the potential to become a quality indicator for cancer care in the future (7). Advance care planning must no longer be an afterthought for patients and their providers. It must instead become a primary component of comprehensive cancer care.
Highlights.
Most received at least one marker of aggressive medical care at the end of life.
Those going to hospice did so in the time immediately surrounding their death.
Provider recommendations may impact a patient’s decision to enroll in hospice.
Footnotes
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–42. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 2.Bakitas M, Lyons KD, Hegel MT, Balan S, Brokaw FC, Seville J, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741–9. doi: 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. Practice Guidelines in Oncology. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive.
- 4.Smith TJ, Temin S, Alesi ER, Abernethy AP, Balboni TA, Basch EM, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol. 2012;30(8):880–7. doi: 10.1200/JCO.2011.38.5161. [DOI] [PubMed] [Google Scholar]
- 5.Mack JW, Weeks JC, Wright AA, Block SD, Prigerson HG. End-of-life discussions, goal attainment, and distress at the end of life: predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol. 2010;28(7):1203–8. doi: 10.1200/JCO.2009.25.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silveira MJ, Kim SY, Langa KM. Advance directives and outcomes of surrogate decision making before death. N Engl J Med. 2010;362(13):1211–8. doi: 10.1056/NEJMsa0907901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mack JW, Cronin A, Keating NL, Taback N, Huskamp HA, Malin JL, et al. Associations between end-of-life discussion characteristics and care received near death: a prospective cohort study. J Clin Oncol. 2012;30(35):4387–95. doi: 10.1200/JCO.2012.43.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez-Acevedo M, Havrilesky LJ, Broadwater G, Kamal AH, Abernethy AP, Berchuck A, et al. Timing of end-of-life care discussion with performance on end-of-life quality indicators in ovarian cancer. Gynecol Oncol. 2013 doi: 10.1016/j.ygyno.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Cherlin E, Fried T, Prigerson HG, Schulman-Green D, Johnson-Hurzeler R, Bradley EH. Communication between physicians and family caregivers about care at the end of life: when do discussions occur and what is said? J Palliat Med. 2005;8(6):1176–85. doi: 10.1089/jpm.2005.8.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaros MC, Curtis JR, Silveira MJ, Elmore JG. Opportunity lost: End-of-life discussions in cancer patients who die in the hospital. J Hosp Med. 2012 doi: 10.1002/jhm.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Gruenigen V, Daly B, Gibbons H, Hutchins J, Green A. Indicators of survival duration in ovarian cancer and implications for aggressiveness of care. Cancer. 2008;112(10):2221–7. doi: 10.1002/cncr.23391. [DOI] [PubMed] [Google Scholar]
- 12.National Quality Forum. National Voluntary Consensus Standards for Quality of Cancer Care. 2009 May; Available from: http://www.qualityforum.org/Publications/2009/05/National_Voluntary_Consensus_Standards_for_Quality_of_Cancer_Care.aspx.
- 13.Bergman J, Saigal CS, Lorenz KA, Hanley J, Miller DC, Gore JL, et al. Hospice use and high-intensity care in men dying of prostate cancer. Arch Intern Med. 2011;171(3):204–10. doi: 10.1001/archinternmed.2010.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keyser EA, Reed BG, Lowery WJ, Sundborg MJ, Winter WE, 3rd, Ward JA, et al. Hospice enrollment for terminally ill patients with gynecologic malignancies: impact on outcomes and interventions. Gynecol Oncol. 2010;118(3):274–7. doi: 10.1016/j.ygyno.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 15.Greer JA, Jackson VA, Meier DE, Temel JS. Early integration of palliative care services with standard oncology care for patients with advanced cancer. CA Cancer J Clin. 2013;63(5):349–63. doi: 10.3322/caac.21192. [DOI] [PubMed] [Google Scholar]
- 16.American Cancer Society. Cancer Facts and Figures. 2014 Available from: http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-041770.pdf.
- 17.Chi DS, Franklin CC, Levine DA, Akselrod F, Sabbatini P, Jarnagin WR, et al. Improved optimal cytoreduction rates for stages IIIC and IV epithelial ovarian, fallopian tube, and primary peritoneal cancer: a change in surgical approach. Gynecol Oncol. 2004;94(3):650–4. doi: 10.1016/j.ygyno.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 18.Aletti GD, Dowdy SC, Gostout BS, Jones MB, Stanhope CR, Wilson TO, et al. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet Gynecol. 2006;107(1):77–85. doi: 10.1097/01.AOG.0000192407.04428.bb. [DOI] [PubMed] [Google Scholar]
- 19.Fairfield KM, Murray KM, Wierman HR, Han PK, Hallen S, Miesfeldt S, et al. Disparities in hospice care among older women dying with ovarian cancer. Gynecol Oncol. 2012;125(1):14–8. doi: 10.1016/j.ygyno.2011.11.041. [DOI] [PubMed] [Google Scholar]
- 20.Wright AA, Zhang B, Ray A, Mack JW, Trice E, Balboni T, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–73. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Billings JA, Bernacki R. Strategic Targeting of Advance Care Planning Interventions: The Goldilocks Phenomenon. JAMA Intern Med. 2014 doi: 10.1001/jamainternmed.2013.14384. [DOI] [PubMed] [Google Scholar]
- 22.Fauci J, Schneider K, Walters C, Boone J, Whitworth J, Killian E, et al. The utilization of palliative care in gynecologic oncology patients near the end of life. Gynecol Oncol. 2012 doi: 10.1016/j.ygyno.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 23.Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345. doi: 10.1136/bmj.c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mack JW, Paulk ME, Viswanath K, Prigerson HG. Racial disparities in the outcomes of communication on medical care received near death. Arch Intern Med. 2010;170(17):1533–40. doi: 10.1001/archinternmed.2010.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carr D. Racial and ethnic differences in advance care planning: identifying subgroup patterns and obstacles. J Aging Health. 2012;24(6):923–47. doi: 10.1177/0898264312449185. [DOI] [PubMed] [Google Scholar]


