Abstract
The rising prevalence of type-2 diabetes (T2DM) is becoming a pressing issue based on emerging reports that T2DM can also adversely impact mental health. We have utilized the UCD-T2DM rat model in which the onset of T2DM develops spontaneously across time and can serve to understand the pathophysiology of diabetes in humans. An increased insulin resistance index and plasma glucose levels manifested the onset of T2DM. There was a decrease in hippocampal insulin receptor (InR) signaling in the hippocampus, which correlated with peripheral insulin resistance index along the course of diabetes onset (r=−0.56, p< 0.01). T2DM increased the hippocampal levels of 4-hydroxynonenal (4-HNE; a marker of lipid peroxidation) in inverse proportion to the changes in the mitochondrial regulator PGC-1α. Disrupted energy homeostasis was further manifested by a concurrent reduction in energy metabolic markers, including TFAM, SIRT1, and AMPK phosphorylation. In addition, T2DM influenced brain plasticity as evidenced by a significant reduction of BDNF-TrkB signaling. These results suggest that the pathology of T2DM in the brain involves a progressive and coordinated disruption of insulin signaling, and energy homeostasis, with profound consequences for brain function and plasticity. All the described consequences of T2DM were attenuated by treatment with the glucagon-like peptide-1 receptor agonist, liraglutide. Similar results to those of liraglutide were obtained by exposing T2DM rats to a food energy restricted diet, which suggest that normalization of brain energy metabolism is a crucial factor to counteract central insulin sensitivity and synaptic plasticity associated with T2DM.
Keywords: Energy homeostasis, Dietary energy restriction, Insulin signaling, Liraglutide, Plasticity, Type-2 diabetes
1. Introduction
The prevalence of metabolic disorders such as diabetes and metabolic syndrome (MetS) is rapidly increasing and posing a serious concern for morbidity and mortality around the world. While the effects of metabolic disorders have been characterized in peripheral systems, recent evidence from clinical studies suggest that the complexity of these disorders can extend to the brain, and can compromise cognitive function [1, 2]. We have recently reported the negative effects of metabolic syndrome in brain plasticity and cognitive function induced by consumption of high fructose [3]. In the current study, we wanted to determine whether the same sequelae induced by fructose could occur spontaneously in a novel rat model of type-2 diabetes (T2DM) that closely reproduces the pathophysiology of T2DM in human. The model was produced by crossing obese insulin-resistant Sprague-Dawley (OSD) rats resulting from polygenic adult-onset obesity with Zucker diabetic fatty (ZDF) lean rats that have a defect in pancreatic β-cell function, but normal leptin signaling [4]. We performed these studies in the hippocampus based on the importance of this structure in cognitive functions such as learning and memory.
The brain is a highly metabolic organ, and disruptions of metabolic homeostasis can have dramatic consequences for information processing. Mitochondria are the major bioenergetic machinery of the cell and are highly susceptible to metabolic damage and thus, likely influence the pathology of T2DM in the brain. T2DM has been associated with lower expression of mitochondrial genes, abnormal mitochondrial morphology and impaired oxidative phosphorylation [5]. Insulin receptor (InR) dysfunction, a hallmark of T2DM, is associated with mitochondrial dysfunction in metabolic diseases [6–8]. Therefore, effective control of mitochondrial biogenesis is critical for energy management and subsequent neuronal function. ATP and NAD are small molecules involved in all energy transactions in cells, and they are under regulatory control of proteins such as sirtuins and AMP-activated protein kinase (AMPK). Sirtuins regulate important metabolic functions in the cell working in close association with NAD and AMPK. Multiple endogenous and exogenous factors regulate mitochondrial biogenesis through peroxisome proliferator-activated receptor gamma (PPARγ) coactivator-1alpha (PGC-1α). PGC-1α is a regulator of various transcription factors involved in mitochondrial biogenesis [9], including nuclear respiratory factors (NRFs). In turn, the NRFs activate the mitochondrial transcription factor A (TFAM) that regulates mitochondrial DNA transcription and replication [10]. PGC-1α can also influence neuronal plasticity by acting on brain-derived neurotrophic factor (BDNF) [11]. BDNF is classically recognized for its role on plasticity and behavior [12], and has been lately shown to participate in a range of metabolic events, including glucose utilization and energy management in diabetes [13].
We studied the effects of two interventions, i.e., administration of a GLP-1 receptor agonist, liraglutide and dietary energy restriction, with proven ability to restore systemic metabolic homeostasis [14, 15], and they have great potential to counteract the central effects of T2DM. Liraglutide (Victoza) is a glucagon-like peptide-1 (GLP-1) receptor agonist which has been shown to cross the blood brain barrier [16], and is used clinically as an effective therapeutic agent for T2DM [17]. Liraglutide has the ability to regulate glucose by restoring insulin secretion [18] and inhibiting glucagon secretion [19]. GLP-1 also acts as a growth factor in the brain [20] and has been shown to protect neurons against various insults including Alzheimer’s and Parkinson’s disease [21–23]. In turn, dietary energy restriction stimulates mitochondrial biogenesis [24] and has been shown to restore neuronal function [25] under several pathological conditions. We examine the possibility that interventions that restore metabolic homeostasis and insulin signaling have the efficacy to slow or prevent the progression of the pathophysiology of T2DM in the brain.
2. Materials and methods
2.1. Animals
The University of California, Davis, type 2 diabetes mellitus (UCD-T2DM) rat model was produced by crossing obese Sprague–Dawley (OSD) rats prone to adult-onset obesity and insulin resistance with Zucker diabetic fatty (ZDF) lean rats that have intact leptin signaling, but a defect in pancreatic β-cell insulin gene transcription [26]. This cross resulted in a new rat model that develops polygenic adult-onset obesity and diabetes in both sexes with rats exhibiting insulin resistance, impaired glucose tolerance and eventual β-cell decompensation [4]. These rats had the genetic propensity to develop diabetes at later age when fed a standard rodent chow diet than other rodent models of T2DM, such as the ZDF rat, making them highly suitable for diabetes prevention studies [27–30]. OSD and ZDF lean rats were originally purchased from Charles River Laboratories (Wilmington, MA). Both the founder OSD rats and UCD-T2DM Rats gain weight more rapidly than Sprague-Dawley rats purchased from Harlan Laboratories (Indianapolis, IN), which were used as control in the present study. All animals received a ground rodent chow diet (no. 5012; Ralston Purina, Belmont, CA) in spill-resistant jars and were housed in hanging wire cages in the Department of Nutrition animal facility at the University of California, Davis, and maintained on a 12 h light–dark cycle.
2.2. Experimental design
Male UCD-T2DM rats were followed until the age of ~5.5 months and divided into pre-diabetic (no diabetes onset occurred until sacrifice) and diabetic (had diabetes for a duration of either 2 weeks or 3 months) groups (n=6/group), based on the period after diabetes onset. A group of UCD-T2DM animals were continued for 6 months after diabetes onset to assess the long-term effects of T2DM and animals were sacrificed at the age of 8.5 months. Diabetes onset was defined as a non-fasted blood glucose value above 11·1 mmol/l (200 mg/dl) on two consecutive weeks. Blood glucose was measured with a glucose meter (One-Touch Ultra, LifeScan, Milpitas, CA, USA) using a lancet to collect a drop of blood from the tail.
A separate cohort of UCD-T2DM male rats was divided into vehicle, energy restricted diet and liraglutide groups (n=6/group) at 2 months of age. Only the animals with body weight >375 g were selected for these groups. Data from previous study have shown that animals weighing >375 g at 2 months of age have an average age of diabetes onset of 113 ± 5 days and a diabetes incidence rate of 98%, whereas animals weighing <375 g at 2 months of age have an average age of onset of 219 ± 10 days and a diabetes incidence rate of 89% [4]. Body weights at the time of entry into the study were 400.2 ± 5.25, 400.4 ± 9.76, and 396.5 ± 7.86 g in vehicle, energy restricted diet, and liraglutide groups, respectively. Restricted animals were diet restricted to 9% less energy per kg of body weight compared with liraglutide-treated animals to equalize body weights between these two groups [27]. Liraglutide animals received subcutaneous liraglutide injections (0.2 mg/kg body weight) twice daily, and vehicle and energy restricted diet animals received subcutaneous Dulbecco’s PBS injections (1 ml/kg body weight) twice daily. The dose for Liraglutide (0.2 mg/kg) was selected based on efficacy in diabetes patients (i.e. 0.6 mg-1.8 mg) [31], and designed according to the guidelines provide by the Centre for Drug Evaluation and Research (CDER) at the Food and Drug Administration (http://www.fda.gov/downloads/Drugs/Guidance/UCM078932.pdf July 2005) [32].
The dietary energy restriction and liraglutide treatments were started at 2 months of age and continued until the age of sacrifice at 6.5 months. The average age of diabetes onset in the vehicle treated animals was 136 ± 17 days. Dietary energy restriction and liraglutide treatment delayed the onset of diabetes as none of the energy restricted diet or liraglutide-treated animals had developed diabetes at the time of sacrifice. Data from previous study have shown that dietary energy restriction and liraglutide treatment delays the onset of diabetes by approximately 4 months [27]. The experimental protocols were approved by the University of California, Davis Institutional Animal Care and Use Committee.
2.3. Plasma analyses
Blood samples were collected from tail vein after an overnight (13-h) fast and placed into EDTA-treated tubes. The plasma was separated by centrifugation and assayed for glucose, insulin and triglycerides (TGs). Plasma glucose concentrations were measured with an enzymatic colorimetric assay for glucose (Thermo DMA Louisville, CO) and plasma insulin concentrations were measured with a rat-specific radioimmunoassay (Millipore, St. Charles, MO). Plasma TG was measured with an enzymatic colorimetric assay (L-type TG H kit, Wako Chemicals, Richmond, VA). The homeostasis model assessment ratio (HOMA-R), which is an index of insulin resistance [33], was calculated using the formula: .
2.4. Tissue collection
The animals were killed by decapitation and the whole brain was removed and quickly frozen in powdered dry ice and stored at −70 °C until use. The hippocampus (HP) was dissected out later for the analyses reported here.
2.5. Immunobloting
The hippocampal tissues were homogenized in a lysis buffer containing 137 mM NaCl, 20 mM Tris–HCl pH 8.0, 1% NP40, 10% glycerol, 1 mM phenylmethylsulfonylfluoride (PMSF), 10 μg/ml aprotinin, 0.1 mM benzethonium chloride, 0.5 mM sodium vanadate. The homogenates were then centrifuged, the supernatants were collected and total protein concentration was determined according to MicroBCA procedure (Pierce, IL, USA), using bovine serum albumin (BSA) as standard. Briefly, protein samples were separated by electrophoresis on a 10% polyacrylamide gel and electrotransferred to a PVDF membrane (Millipore, MA, USA). Non-specific binding sites were blocked in Tris-buffered saline (TBS), pH 7.6, containing 5% non-fat dry milk. Membranes were rinsed in buffer (0.05% Tween-20 in TBS) and then incubated with anti-actin or anti-BDNF, anti-pTrk, anti-Trk, anti-AMPK, anti-mtTFA, anti-4HNE anti-pIR-β, anti-IR-β (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-PGC-1α, anti-Sir2, anti-pIRS-1, anti-IRS-1 (1:1000, Millipore, MA, USA), anti-pAMPK (1:1000; Cell signaling technology, MA, USA) followed by anti-rabbit or anti goat IgG horseradish peroxidase-conjugate (1:10,000; Santa Cruz Biotechnology). After rinsing with buffer, the immunocomplexes were visualized by chemiluminescence using the ECL kit (Amersham Pharmacia Biotech Inc., NJ, USA) according to the manufacturer’s instructions. The film signals were digitally scanned and then quantified using ImageJ software. Actin was used as an internal control for western blot such that data were standardized according to actin values.
2.6. Statistical Analysis
The results of metabolic markers are expressed as mean ± standard error of the mean (SEM). Protein expression results are expressed as mean ± SEM of percentage of either control or vehicle group. All statistical analyses were performed by one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison post-hoc test. A level of 5% probability was considered as statistically significant. Pearson correlation analysis was performed on individual samples to evaluate the association between variables.
3. Results
3.1. Alteration in metabolic markers with time course of diabetes onset
We have measured the body weight (F4,25 = 84.94, p<0.01), fasting plasma triglycerides (F4,25 = 5.445, p<0.01), fasting plasma glucose (F4,25 = 24.82, p<0.01) and homeostasis model assessment ratio (HOMA-R; F4,25 = 12.94, p<0.01), an index of insulin resistance in control (Sprague-Dawley rats from Harlan Laboratories), pre-diabetic (UCD-T2DM rats with no diabetes onset until sacrifice) and diabetic rats (UCD-T2DM rats; 2 weeks, 3 months & 6 months after diabetes onset). As shown in Table 1, the pre-diabetic rats gain significantly higher body weight (Pre-db Vs CON, p<0.01) and HOMA-R (Pre-db Vs CON, p<0.01) compared with control, demonstrate the genetic predisposition of UCD-T2DM rats to develop diabetes at later age. Animals that had diabetes since 3 & 6 months, have elevated levels of triglycerides (3 mos-db or 6 mos-db Vs Pre-db, p<0.05) and glucose (3 mos-db or 6 mos-db Vs Pre-db, p<0.01) in comparison to the pre-diabetic group, thus demonstrates the peripheral occurrence of diabetes in UCD-T2DM rats. Animals with diabetes of 3 and 6 months duration exhibited significantly lower body weights (3 mos-db or 6 mos-db Vs Pre-db, p<0.01) compared with pre-diabetic animals, likely due to loss of energy in the form of glucosuria and polyuria.
Table 1.
Body weight (g), triglycerides (mg/dl), glucose (mg/dl), and HOMA-R levels in control, pre-diabetic and diabetic groups:
| Groups | Body weight (g) | Plasma triglycerides (mg/dl) | Plasma glucose (mg/dl) | HOMA-R |
|---|---|---|---|---|
| Control | 399.8 ± 7.22 | 94 ± 10 | 82 ± 1 | 3.12 ± 0.32 |
| Pre-diabetic | 628.8 ± 4.65## | 112 ± 19 | 97 ± 2 | 10.97 ± 1.37## |
| Diabetic (2 weeks) | 677.5 ± 5.87## | 91 ± 13 | 91 ± 5 | 15.22 ± 1.17# |
| Diabetic (3 months) | 478.3 ± 22.54##** | 188 ± 34#* | 220 ± 33##** | 13.44 ± 1.71## |
| Diabetic (6 months) | 495.8 ± 12.28##** | 203 ± 29#* | 331 ± 36##** | 11.47 ± 1.41## |
Values are expressed as mean ± SEM.
P<0.05,
P<0.01 Vs control;
P<0.05,
P<0.01 Vs pre-diabetic; ANOVA (one-way) followed by Bonferroni’s multiple comparison post-hoc test.
3.2. Influence of T2DM on insulin receptor signaling in brain
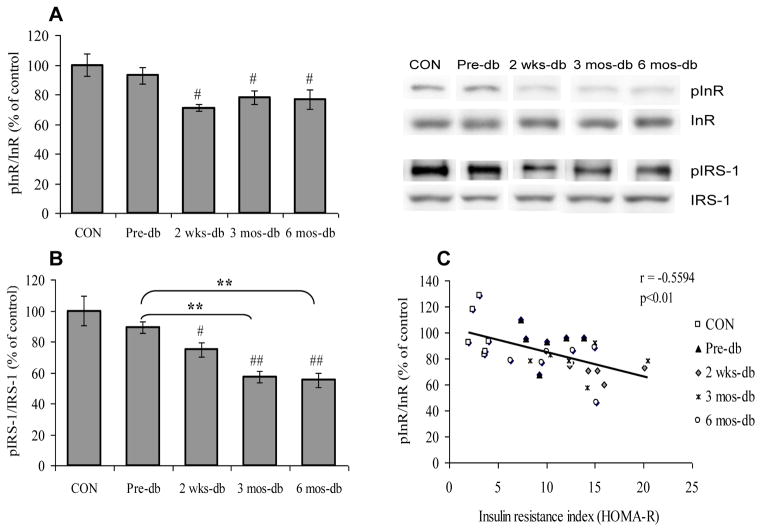
We assessed the time course of T2DM for the development of brain insulin resistance by analyzing molecules involved in the insulin signaling pathway in hippocampus to explore whether the insulin resistance associated with T2DM may extend to the brain. Insulin resistance is the consequence of impaired insulin signaling at the level of the insulin receptor and its downstream effectors as a result of post-translational modifications such as altered phosphorylation. To measure the changes in insulin signaling, we assessed the phosphorylation levels of insulin receptor (InR), and IRS-1 in control, pre-diabetic and diabetic rats. Animals that had diabetes for 2 weeks (2 wks-db Vs CON, p<0.05), 3 months (3 mos-db Vs CON, p<0.05–0.01) and 6 months (6 mos-db Vs CON, p<0.05–0.01) exhibited deficits in insulin signaling in the hippocampus as evidenced by decreased phosphorylation of InR (F4,25 = 4.628, p<0.01; Fig. 1A) and IRS-1 (F4,25 = 11.99, p<0.01; Fig. 1B) in comparison to the control group. Pre-diabetic (UCD-T2DM rats with no diabetes onset) animals did not alter the phosphorylation of InR and IRS-1 (Pre-db Vs CON, p>0.05), however, the occurrence of diabetes onset, decreases the phosphorylation of IRS-1 as observed in animals with 3 (3 mos-db Vs Pre-db, p<0.01) & 6 months (6 mos-db Vs Pre-db, p<0.01) of diabetes. Thus it appears that transition from the pre-diabetic to diabetic state is associated with a marked decrease of insulin signaling, thereby indicating insulin resistance in brain. The negative correlation found between HOMA-R and InR phosphorylation (r = −0.5594, P<0.01; Fig. 1C), suggests that the insulin resistance in the periphery and the brain are closely related phenomena.
Fig. 1.
(A) Phosphorylation of insulin receptor (InR) and (B) IRS-1 in control (CON), pre-diabetic (Pre-db), 2 weeks-diabetic (2 wks-db), 3 months-diabetic (3 mos-db) and 6 months-diabetic (6 mos-db) groups. (C) Negative correlation between insulin resistance index (HOMA-R) and InR phosphorylation. #P<0.05, ##P<0.01 Vs CON; **P<0.01 Vs Pre-db; ANOVA (one-way) followed by Bonferroni’s multiple comparison post-hoc test.
3.3. T2DM and markers related to the brain energy homeostasis
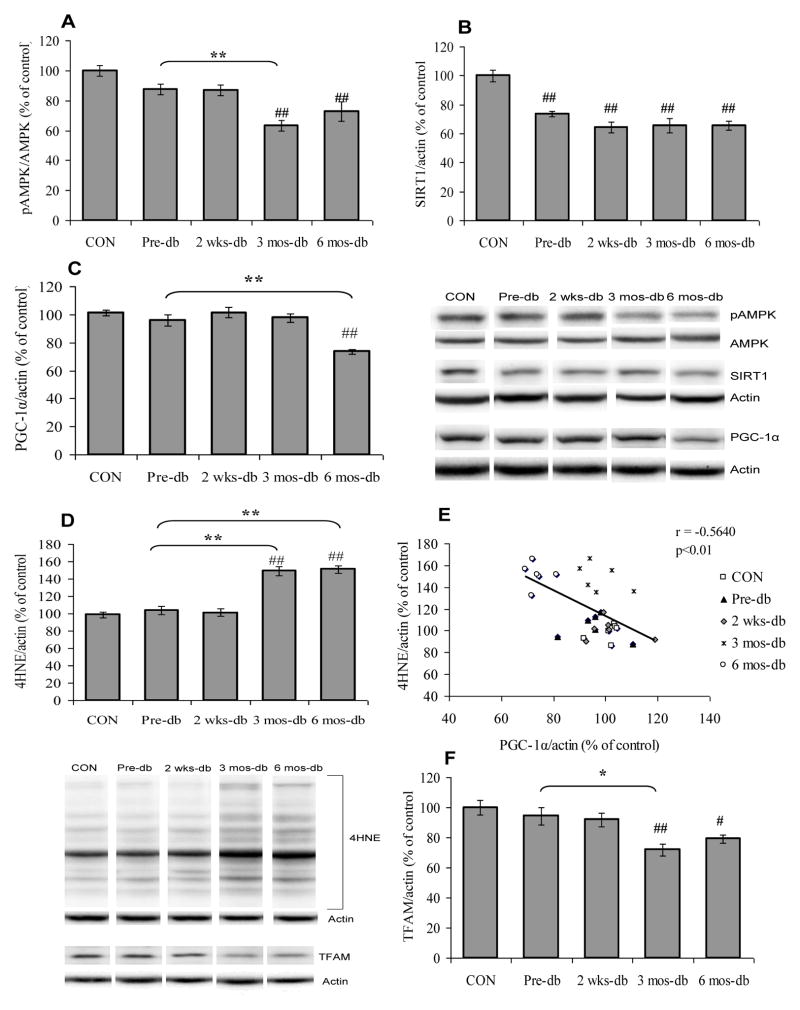
We evaluated the effects of T2DM on brain energy homeostasis by measuring energy sensing molecules including AMPK and SIRT1 and proteins involved in mitochondrial biogenesis that control the balance and transduction of cellular energy metabolism. AMPK, a serine-threonine kinase, has the ability to sense low energy levels and activate or inhibit the appropriate molecules to re-establish cellular energy homeostasis. Phosphorylation of AMPK (F4,25 = 10.78, p<0.01; Fig. 2A) found to be decreased in animals with diabetes of 3 (3 mos-db Vs CON, p<0.01) or 6 months (6 mos-db Vs CON, p<0.01) duration in comparison with control animals, however, no significant change was observed in AMPK phosphorylation in pre-diabetic animals (Pre-db Vs CON, p>0.05). The animals with diabetes of 3 months duration exhibited decreased phosphorylation of AMPK (3 mos-db Vs Pre-db, p<0.01) compared with pre-diabetic animals. In addition, hippocampal SIRT1 (F4,25 = 16.82, p<0.01; Fig. 2B) was down-regulated in both prediabetic and diabetic UCD-T2DM rats.
Fig. 2.
(A) Phosphorylation of AMPK, (B) protein expression of SIRT1, (C) PGC-1α, (D) levels of 4HNE, (E) Negative correlation between PGC-1α expression and 4HNE and (F) TFAM in control (CON), pre-diabetic (Pre-db), 2 weeks-diabetic (2 wks-db), 3 months-diabetic (3 mos-db) and 6 months-diabetic (6 mos-db) groups. #P<0.05, ##P<0.01 Vs CON; *P<0.05, **P<0.01 Vs Pre-db; ANOVA (one-way) followed by Bonferroni’s multiple comparison post-hoc test.
We also assessed molecules associated with mitochondrial biogenesis, including PGC-1α and TFAM to assess the influence of T2DM on energy homeostasis. PGC-1α is a member of a family of transcription co-activators considered to have a central role in the regulation of cellular energy metabolism. The decrease in the levels of PGC-1α (F4,25 = 15.15 p<0.01; Fig. 2C) was observed only at 6 months (6 mos-db Vs CON, p<0.01) following the onset of diabetes. Pre-diabetic UCD-T2DM rats did not exhibit a reduction of PGC-1α (Pre-db Vs CON, p>0.05) in the hippocampus. In contrast, PGC-1α was down-regulated in animals with diabetes of 6 months, but not 2 weeks or 3 months duration (6 mos-db Vs Pre-db, p<0.01). Previous studies have indicated that the actions of PGC-1α are also related to the reactive oxygen species (ROS)-detoxifying capacity of cells [34, 35]. Overproduction of reactive oxygen species (ROS), a byproduct of mitochondrial electron transport chain, leads to oxidative damage. Therefore, we assessed the levels of 4-hydroxynonenal (4HNE), a marker of lipid peroxidation to determine the effects of T2DM on oxidative stress. 4HNE was elevated in animals with diabetes of 3 (3 mos-db Vs CON, p<0.01) and 6 months (6 mos-db Vs CON, p<0.01) duration (F4,25 = 38.30, p<0.01; Fig. 2D) compared with control and pre-diabetic UCD-T2DM animals. 4HNE was not increased in pre-diabetic animals (Pre-db Vs CON, p>0.05). Diabetes for 3 and 6 months resulted in an increase in 4HNE levels with respect to the pre-diabetic group (3 mos-db or 6 mos-db Vs Pre-db, p<0.01). PGC-1α expression was inversely correlated with 4HNE (r = −0.5640, p<0.01; Fig. 2E) suggesting that a decrease in the levels of PGC-1α may contribute to increased lipid peroxidation.
Further, we assessed the mitochondrial transcription factor TFAM, which is a key activator of mitochondrial transcription and mitochondrial genome replication. The level of TFAM (F4,25 = 6.804, p<0.01; Fig. 2F) was reduced in animals that had diabetes since 3 (3 mos-db Vs CON, p<0.01) and 6 months (6 mos-db Vs CON, p<0.05) as compared to control.
3.4. Effect of T2DM on markers of neuronal plasticity
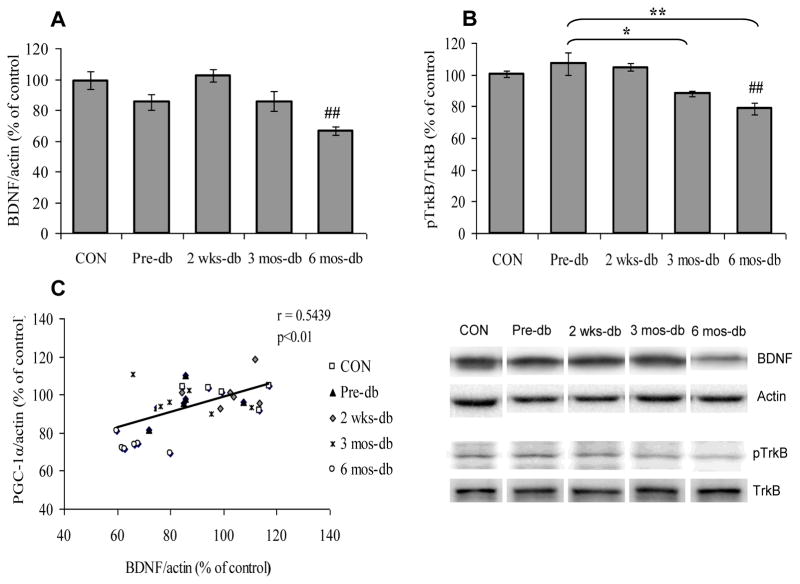
We have measured the BDNF signaling in hippocampus of control, UCD-T2DM pre-diabetic and diabetic rats. Brain-derived neurotrophic factor (BDNF) and its tropomyosin related kinase receptor type B (TrkB) have a demonstrated role on neuronal plasticity and function. BDNF expression was significantly decreased (F4,25 = 8.144, p<0.01; Fig. 3A) as was phosphorylation of TrkB (F4,25 = 9.447, p<0.01; Fig. 3B) in animals diabetes of 6 months duration (6 mos-db Vs CON, p<0.01), but not in animals with diabetes of 2 weeks or 3 months duration. BDNF expression was not reduced in pre-diabetic UCD-T2DM rats compared with control animals (Pre-db Vs CON, p>0.05). The animals with diabetes of 3 or 6 months duration displayed significant reductions of TrkB phosphorylation (3 mos-db or 6 mos-db Vs Pre-db, p<0.01) compared with the pre-diabetic animals. We assessed a possible association between BDNF action and regulation of the PGC-1α, and found that the expression of BDNF was significantly correlated with of PGC-1α expression (r = 0.54, p<0.01; Fig. 3C).
Fig. 3.
(A) BDNF levels, (B) phosphorylation levels of TrkB and (C) Positive correlation between BDNF and PGC-1α expression in control (CON), Pre-diabetic (Pre-db), 2 weeks-diabetic (2 wks-db), 3 months-diabetic (3 mos-db) and 6 months-diabetic (6 mos-db) groups. ##P<0.01 Vs CON; *P<0.05, **P<0.01 Vs Pre-db; ANOVA (one-way) followed by Bonferroni’s multiple comparison post-hoc test.
3.5. Dietary energy restriction or liraglutide treatment delay the onset of T2DM
We investigated the effects of energy restriction and the administration of liraglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist on brain insulin signaling, and markers of brain energy metabolism, and oxidative stress in the hippocampus of UCD-T2DM Rats. We have previously reported that both dietary energy restriction and liraglutide treatment delay the onset of diabetes in UCD-T2DM Rats [27]. While both dietary energy restriction or liraglutide treatment delay the onset of diabetes, which is manifested by decreases of fasting plasma triglycerides and fasting plasma glucose (Table 2). The age of diabetes onset in vehicle-treated animals was 136 ± 17 day, while diabetes had not developed in any of the energy restricted diet or liraglutide treated animals at the time of sacrifice (4.5 months of intervention).
Table 2.
Body weight (g), triglycerides (mg/dl), glucose (mg/dl) and HOMA-R levels in vehicle, energy restricted diet and liraglutide groups:
| Groups | Body weight (g) | Plasma triglycerides (mg/dl) | Plasma glucose (mg/dl) | HOMA-R |
|---|---|---|---|---|
| Vehicle | 565.2 ± 38.8 | 164 ± 26 | 224 ± 59 | 14.69 ± 1.96 |
| Energy restricted | 572.2 ± 9.2 | 97 ± 9# | 127 ± 1 | 19 ± 2.76 |
| Liraglutide | 588.7 ± 16.1 | 107 ± 16# | 111 ± 4 | 11.86 ± 1.57 |
Values are expressed as mean ± SEM.
P<0.05 Vs pre-diabetic; ANOVA (one-way) followed by Bonferroni’s multiple comparison post-hoc test.
3.6. Dietary energy restriction and liraglutide treatment counteract the effects of T2DM in the hippocampus
We examined the effect of dietary energy restriction and liraglutide on insulin signaling, energy metabolism and neuronal plasticity in hippocampal samples from UCD-T2DM rats. There are several reports suggest that dietary energy restriction increases mitochondrial biogenesis [24]. We sought to assess the effects of dietary restriction on insulin signaling and mitochondrial parameters in the pathophysiology of T2DM. The effects of prolonged liraglutide administration were also studied to determine if activation of GLP-1 signaling might also lead to restoration of insulin resistance/signaling and energy homeostasis and neuronal plasticity in the hippocampus of UCD-T2DM Rats.
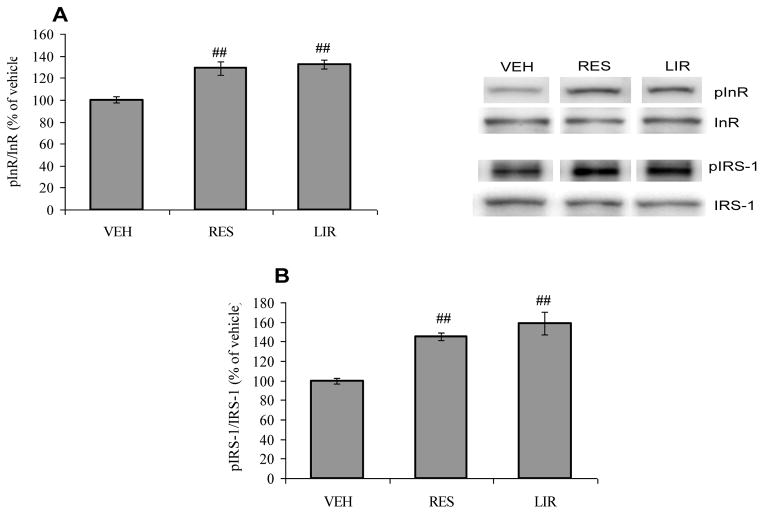
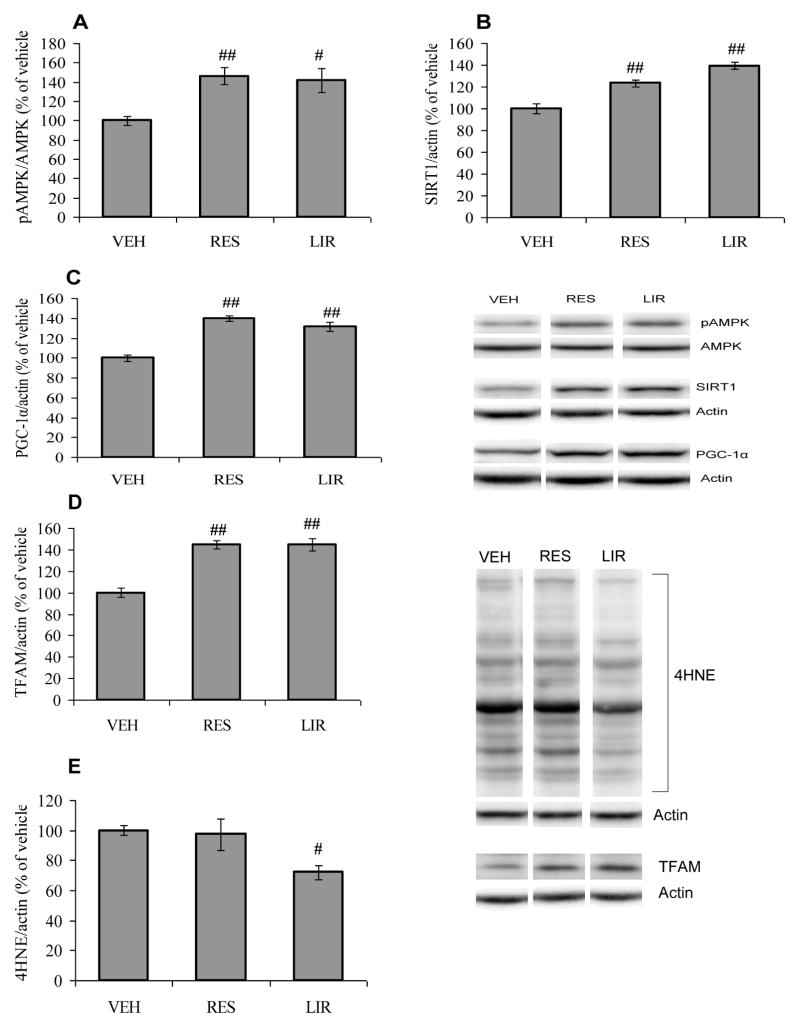
According to our results, the dietary energy restriction and liraglutide treatment offset the effect of T2DM on hippocampal insulin signaling as evidenced by increased phosphorylation of InR (F2,15 = 16.19, p<0.01; Fig. 4A) and IRS-1 (F2,15 = 17.61, p<0.01; Fig. 4B) in comparison with vehicle-injected animals. These results indicate that both energy restriction diet and liraglutide treatment prevent the impairment of insulin signaling in the brain as assessed by improved insulin receptor and IRS-1 phosphorylation in the hippocampus in UCD-T2DM Rats.
Fig. 4.
(A) Phosphorylation of insulin receptor (InR) and (B) IRS-1 in vehicle (VEH), energy restricted (RES) diet and liraglutide (LIR) treated groups. ##P<0.01 Vs VEH; ANOVA (one-way) followed by Bonferroni’s multiple comparison post-hoc test.
Dietary restriction has been suggested to preserve neuronal plasticity resulting from disruption of energy homeostasis [36, 37]. Accordingly, we have assessed AMPK phosphorylation and SIRT1 expression based on the role of these molecules in cellular homeostasis and energy metabolism [38, 39]. We found that either prolonged dietary restriction exposure or liraglutide treatment increased the expression of these metabolic sensors, AMPK phosphorylation (F2,15 = 8.037, p<0.01; Fig. 5A) and SIRT1 (F2,15 = 31.62, p<0.01; Fig. 5B) compared with vehicle-injected animals. We also found that both dietary restriction and liraglutide increased the protein expression of PGC-1α (F2,15 = 33.27, p<0.01; Fig. 5C) and TFAM (F2,15 = 32.07, p<0.01; Fig. 5D) compared with vehicle-injected animals. These results support the previously reported finding that dietary energy restriction improves mitochondrial functions to maintain energy homeostasis under adverse conditions [24]. Treatment with liraglutide, but not energy restricted diet, also appears to counteract the effect of T2DM on 4HNE (F2,15 = 4.727, p<0.05; Fig. 5E).
Fig. 5.
(A) Phosphorylation of AMPK, (B) protein expression of SIRT1, (C) PGC-1α, (D) TFAM and (E) levels of 4HNE in vehicle (VEH), energy restricted (RES) diet and liraglutide (LIR) treated groups. #P<0.05, ##P<0.01 Vs VEH; ANOVA (one-way) followed by Bonferroni’s multiple comparison post-hoc test.
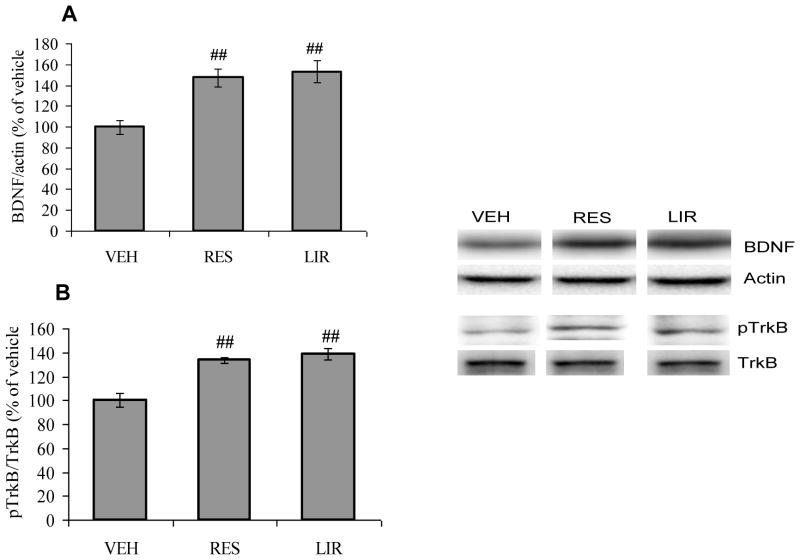
There are evidences that BDNF signaling works at the interface of metabolism and synaptic plasticity [12]. To explore a possible association between energy balance and synaptic plasticity, we assessed the effects of the interventions on BDNF signaling. We found that the either dietary restriction or liraglutide treatments elevated the expression of BDNF protein (F2,15 = 11.00, p<0.01; Fig. 6A), and phosphorylation of its receptor TrkB (F2,15 = 20.26, p<0.01; Fig. 6B) when compared with vehicle-injected animals. Overall, these results suggest a possible crosstalk between insulin signaling and mitochondrial functions in the regulation of neuronal plasticity in the hippocampus.
Fig. 6.
(A) BDNF levels and (B) Phosphorylation levels of TrkB in vehicle (VEH), energy restricted (RES) diet and liraglutide (LIR) treated groups. ##P<0.01 Vs VEH; ANOVA (one-way) followed by Bonferroni’s multiple comparison post-hoc test.
4. Discussion
An increasing body of evidence indicates that T2DM poses a threat for brain function and can increase the risk for neurological and psychiatric disorders. We have embarked in studies to determine how progression of T2DM pathology can disrupt brain plasticity. We have utilized a rat model of T2DM that resembles the pathophysiology of T2DM in humans [4]. We found that the peripheral manifestations of T2DM (insulin resistance and hyperglycemia) coincided with disruptions in insulin signaling in the brain. Moreover, these effects were associated with reductions in markers of energy metabolism (PGC-1α, TFAM and AMPK phosphorylation) and markers of neuronal plasticity (BDNF-TrkB signaling) in hippocampus. The fact that these alterations were more pronounced during the late stages of T2DM suggests that T2DM is associated with a progressive deterioration in the machinery that maintains energy homeostasis and plasticity in the brain. Previous reports indicate that diabetes leads to morphological alterations in cortical and hypothalamic brain regions that could be related to the various pathologic processes observed in neuropsychological disorders [40]. Our animal model of T2DM was centered on the hippocampal formation based on its involvement in neurological disorders associated with cognitive functions. T2DM has been associated with cortical and subcortical atrophy and with diminished regional cerebral perfusion and vasoreactivity [41]. A recent study in T2DM using microarray technology show alterations in genes related to neurotransmission, lipid metabolism, neuronal development, insulin secretion, oxidative damage and DNA repair in hippocampus, striatum and prefrontal cortex [42].
The treatment with a dietary energy restriction paradigm known to promote energy homeostasis [24], counteracted many of the effects of T2DM on hippocampal insulin signaling, energy metabolism and plasticity markers. Similar effects were observed after treatment with the GLP-1 receptor agonist liraglutide, which is known to improve insulin sensitivity and pancreatic islet function [27]. Together, these results show that the effects of T2DM on the brain involve an integrated disruption of insulin signaling, energy homeostasis, and plasticity. The results showing that interventions known for their actions on stabilizing insulin resistance and energy homeostasis, can restore BDNF-TrkB signaling, suggest potential therapeutic targets to reduce the T2DM pathology in the brain.
4.1. T2DM and brain insulin resistance
Results showing that hippocampal insulin receptor (InR) signaling changed in proportion to peripheral insulin resistance, and suggest a possible association between these two parameters. Insulin resistance is the consequence of impaired signaling at the level of the InR and its downstream effectors as a result of post-translational modifications such as altered phosphorylation. The observed reduction in InR phosphorylation and its downstream signaling molecules such as IRS-1 in diabetic rats (observed at 2 weeks, 3 months and 6 months), suggest a comprehensive disruption in the insulin signaling pathways. The counteractive effects of liraglutide on insulin signaling suggest that activation of the GLP-1 receptor may restore insulin sensitivity in brain. GLP-1 receptors are present in the hippocampus and GLP-1 has been suggested to have beneficial effects on synaptic function and learning in rats [43]. The results of our study are consistent with previous reports showing the beneficial effects of liraglutide in brain insulin resistance in mouse model of Alzheimer’s disease [44]. As discussed below, treatment with dietary energy restriction normalized similar parameters affected by liraglutide, and suggests that disrupted metabolic homeostasis and insulin signaling are key aspects of the brain pathology surrounding T2DM.
4.2. T2DM Vs Energy homeostasis
Brain energy metabolism is central to all cellular processes that maintain neuronal functionality. We found that T2DM affected all of the parameters of energy homeostasis, especially at the later stages of diabetes (3 and 6 months). Our results also show that T2DM altered an important regulator of mitochondrial transcription, notably PGC-1α, which is a major orchestrator of several pathways involved in the regulation of energy homeostasis and neuronal plasticity. PGC-1α is a transcriptional coactivator of peroxisome proliferator-activated receptor-gamma (PPARγ) involved in regulation of insulin action and other key metabolic pathways, such as adipogenesis and adiponectin production [45]. PGC-1α is a potent coactivator of a plethora of transcription factors, including nuclear respiratory factors (NRFs), which in turn, activate the mitochondrial transcription factor A (TFAM) that acts on mitochondrial DNA. PGC-1α also regulates several factors involved in mitochondrial homeostasis such as AMPK, a serine-threonine kinase, which has the ability to sense low energy levels and to re-establish the proper energy balance of the cell. Once activated, AMPK switches on catabolic pathways to produce ATP while simultaneously shutting down energy-consuming anabolic processes, in a process involving the actions of PGC-1α [46] and sirtuin-regulated deacetylation [47]. Therefore, our results showing a decrease in AMPK activation in the hippocampus of UCD-T2DM rats indicates a disturbance of energy homeostasis with the progression of diabetes pathology. In turn, the increase in AMPK phosphorylation in energy restricted and liraglutide treated animals, advocates that these interventions activate mechanisms to restore energy homeostasis levels in the hippocampus. In further support of this possibility, the treatment with liraglutide and energy restricted diet normalized PGC-1α and TFAM levels, which are important elements for the maintenance of energy homeostasis.
4.3. Metabolic pathways supporting neuronal signaling systems
Emerging evidence suggests a strong association between cell energy regulation, synaptic plasticity, and behavior [48];[49]. In particular, systems deeply involved with energy metabolism such as SIRT1, AMPK, PGC-1α, and BDNF can also support brain plasticity and function. Therefore, it is not surprising that T2DM reduced the levels of BDNF and its receptor TrkB in our results. BDNF stimulates energy metabolism in developing cortical neurons [50], and appears to function at the interface of metabolism and synaptic plasticity as a metabotrophin [12]. Therefore, our results showing that dysfunction in energy metabolism in T2DM may influence synaptic modulators such as BDNF signaling are particularly significant.
Our results showed that BDNF changed in proportion to levels of PGC-1α in the T2DM rats. We have previously shown that activation of the BDNF receptor TrkB elevates PGC-1α levels [48] which is in line with other studies suggesting that the actions of BDNF and PGC-1α are interrelated [11]. Through tyrosine kinase B (TrkB) receptor, BDNF leads to the activation of cyclic-AMP response element binding protein (CREB), which is a potent activator of PGC-1α [51]. Therefore, the marked reduction in the levels of phosphorylated CREB in T2DM rats may reflex the double function of CREB as a synaptic activator and energy modulator. In addition, consistent with these findings, we observed that dietary restriction and liraglutide treatment preserved the BDNF signaling and energy metabolism following the onset of T2DM.
T2DM also decreased hippocampal SIRT1 levels in harmony with the double action of SIRT1 in cell metabolism [38, 39] and brain plasticity [52, 53]. SIRT1 has the ability to function as protein deacetylases during energy regulation, such that PGC-1α is a deacetylation target of SIRT1 [54, 55]. Previous studies have suggested a mechanistic association between SIRT1 and AMPK, as they showed that NAD, a critical substrate for SIRT1 function, is activated by AMPK in a dose-dependent manner [56]. The capacity of the energy restricted diet to counteract the development of T2DM may be related to the ability of SIRT1 to support metabolic transcriptional adaptations under situations of nutrient scarcity, which are generally coupled to increased NAD+ levels [57]. This condition may be reflected in our results showing that T2DM reduced SIRT1 levels. In turn, the results showing that energy restriction and liraglutide normalized the levels of SIRT1 emphasize the importance of restoring energy homeostasis to reduce the effects of T2DM in the brain. During the last decade, an overwhelming body of evidence indicates that the activity of mammalian sirtuins, most notably SIRT1 and SIRT3, have the ability to enhance fat oxidation and prevent metabolic disease [58–60]. Therefore, strategies aimed to increase intracellular NAD+ levels are gaining interest in order to activate sirtuins and treat metabolic disorders.
5. Conclusions
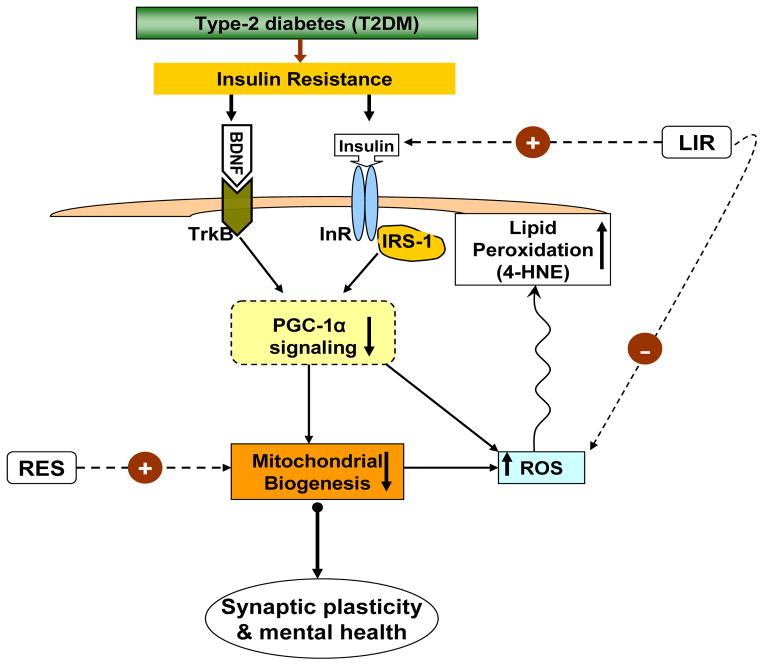
T2DM has been mainly associated with disruptions of peripheral metabolism, and the current results help extend the concept of metabolic impairments to the brain. We previously found that diet-induced metabolic syndrome disrupts hippocampal insulin resistance in proportion to brain plasticity, energy homeostasis and learning performance [3]. The current results show that similar aspects of the metabolic syndrome pathology can be observed under spontaneous development of the T2DM pathology. Results reveal the impact of T2DM on brain function and plasticity centered on the involvement of insulin signaling and mitochondrial homeostasis. These effects were counteracted by either energy restriction or improving central insulin sensitivity. Thus, these results observed in the UCD-T2DM rat model of type-2 diabetes, suggest that brain energy homeostasis and insulin signaling may serve as important targets for neurological interventions directed to improve T2DM patient quality of life and to reduce health care expenses (Fig. 7). The current results are additionally significant since the hippocampus is the locus of learning and memory, which are disrupted in various neurological disorders such as Alzheimer’s disease. Further studies are required to evaluate how T2DM affects other brain regions and selected neurological diseases.
Fig. 7.
Proposed mechanism by which type-2 diabetes (T2DM) can affect the brain and leads to insulin resistance with subsequent effects on synaptic plasticity and mental health. It also suggests a mechanistic pathway by which insulin signaling and BDNF signaling can act on metabolic pathways that regulate brain plasticity and function. T2DM leads to the disruption of insulin and BDNF receptor signaling, thereby affecting the co-transcriptional regulator PGC-1α signaling. Impairment in PGC-1α function decreases mitochondrial biogenesis by suppressing mitochondrial DNA transcription, and mitochondrial proliferation through mitochondrial transcription factor A (TFAM). Reactive oxygen species (ROS), a byproduct of mitochondrial electron transport chain, leads to generation of 4-hydroxynonenal (4HNE) via lipid peroxidation. In turn, 4HNE disrupts mitochondrial function and overall neuronal functions. The treatment with liraglutide (LIR), a glucagon-like peptide-1 (GLP-1) agonist, prevents the detrimental influence of T2DM on synaptic plasticity by enhancing insulin receptor signaling in brain, and possibly by reducing oxidative stress (ROS). Dietary energy restriction (RES) mainly increases mitochondrial biogenesis, thereby helping to preserve brain plasticity and mental health under diabetic condition. Overall, these events are important to understand how T2DM influences brain functions and synaptic plasticity. The insulin and mitochondrial interventions appear crucial for the maintenance of neuronal function and promote mental health.
Highlights.
Progression of T2DM disrupts energy homeostasis and neuronal plasticity in brain.
Liraglutide and energy restriction counteract the pathology of T2DM in brain.
Brain pathology associated with T2DM weakens the substrates for brain plasticity.
Acknowledgments
This work was supported by the National Institute of Health Grants NS050465 and NS056413 (FGP), AT-002993, DK-097307, DK-095980, and Novo Nordisk A/S (PJH). We acknowledge Dr. Kirsten Raun (Novo Nordisk) for her contributions to the design and implementation of the liraglutide/energy restriction study.
Footnotes
There is no conflict of interest for any of the contributing authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moran C, Phan TG, Chen J, Blizzard L, Beare R, Venn A, Münch G, Wood AG, Forbes J, Greenaway TM, Pearson S, Srikanth V. Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care. 2013;36:4036–4042. doi: 10.2337/dc13-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reijmer YD, Brundel M, de Bresser J, Kappelle LJ, Leemans A, Biessels GJ U.V.C.I.S. Group. Microstructural white matter abnormalities and cognitive functioning in type 2 diabetes: a diffusion tensor imaging study. Diabetes Care. 2013;36:137–144. doi: 10.2337/dc12-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal R, Gomez-Pinilla F. ‘Metabolic syndrome’ in the brain: deficiency in omega-3 fatty acid exacerbates dysfunctions in insulin receptor signalling and cognition. J Physiol. 2012;590:2485–2499. doi: 10.1113/jphysiol.2012.230078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings BP, Digitale EK, Stanhope KL, Graham JL, Baskin DG, Reed BJ, Sweet IR, Griffen SC, Havel PJ. Development and characterization of a novel rat model of type 2 diabetes mellitus: the UC Davis type 2 diabetes mellitus UCD-T2DM rat. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1782–1793. doi: 10.1152/ajpregu.90635.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu H, Koshkin V, Allister EM, Gyulkhandanyan AV, Wheeler MB. Molecular and metabolic evidence for mitochondrial defects associated with beta-cell dysfunction in a mouse model of type 2 diabetes. Diabetes. 2010;59:448–459. doi: 10.2337/db09-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szendroedi J, Phielix E, Roden M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:92–103. doi: 10.1038/nrendo.2011.138. [DOI] [PubMed] [Google Scholar]
- 7.Cheng Z, Tseng Y, White MF. Insulin signaling meets mitochondria in metabolism. Trends Endocrinol Metab. 2010;21:589–598. doi: 10.1016/j.tem.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102:401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res. 2008;79:208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 10.Campbell CT, Kolesar JE, Kaufman BA. Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number. Biochim Biophys Acta. 2012;1819:921–929. doi: 10.1016/j.bbagrm.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Cheng A, Wan R, Yang JL, Kamimura N, Son TG, Ouyang X, Luo Y, Okun E, Mattson MP. Involvement of PGC-1α in the formation and maintenance of neuronal dendritic spines. Nat Commun. 2012;3:1250. doi: 10.1038/ncomms2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci. 2008;28:2278–2287. doi: 10.1111/j.1460-9568.2008.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamanaka M, Tsuchida A, Nakagawa T, Nonomura T, Ono-Kishino M, Sugaru E, Noguchi H, Taiji M. Brain-derived neurotrophic factor enhances glucose utilization in peripheral tissues of diabetic mice. Diabetes Obes Metab. 2007;9:59–64. doi: 10.1111/j.1463-1326.2006.00572.x. [DOI] [PubMed] [Google Scholar]
- 14.Horowitz M, Flint A, Jones KL, Hindsberger C, Rasmussen MF, Kapitza C, Doran S, Jax T, Zdravkovic M, Chapman IM. Effect of the once-daily human GLP-1 analogue liraglutide on appetite, energy intake, energy expenditure and gastric emptying in type 2 diabetes. Diabetes Res Clin Pract. 2012;97:258–266. doi: 10.1016/j.diabres.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 15.Duivenvoorde LP, van Schothorst EM, Bunschoten A, Keijer J. Dietary restriction of mice on a high-fat diet induces substrate efficiency and improves metabolic health. J Mol Endocrinol. 2011;47:81–97. doi: 10.1530/JME-11-0001. [DOI] [PubMed] [Google Scholar]
- 16.Hunter K, Hölscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012;13:33. doi: 10.1186/1471-2202-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:262–269. doi: 10.1038/nrendo.2009.48. [DOI] [PubMed] [Google Scholar]
- 18.Flint A, Kapitza C, Hindsberger C, Zdravkovic M. The once-daily human glucagon-like peptide-1 (GLP-1) analog liraglutide improves postprandial glucose levels in type 2 diabetes patients. Adv Ther. 2011;28:213–226. doi: 10.1007/s12325-010-0110-x. [DOI] [PubMed] [Google Scholar]
- 19.Degn KB, Juhl CB, Sturis J, Jakobsen G, Brock B, Chandramouli V, Rungby J, Landau BR, Schmitz O. One week’s treatment with the long-acting glucagon-like peptide 1 derivative liraglutide (NN2211) markedly improves 24-h glycemia and alpha- and beta-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes. 2004;53:1187–1194. doi: 10.2337/diabetes.53.5.1187. [DOI] [PubMed] [Google Scholar]
- 20.Perry T, Lahiri DK, Chen D, Zhou J, Shaw KT, Egan JM, Greig NH. A novel neurotrophic property of glucagon-like peptide 1: a promoter of nerve growth factor-mediated differentiation in PC12 cells. J Pharmacol Exp Ther. 2002;300:958–966. doi: 10.1124/jpet.300.3.958. [DOI] [PubMed] [Google Scholar]
- 21.Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, Silverman MA, Kazi H, Melo HM, McClean PL, Holscher C, Arnold SE, Talbot K, Klein WL, Munoz DP, Ferreira ST, De Felice FG. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease- associated Aβ oligomers. J Clin Invest. 2012;122:1339–1353. doi: 10.1172/JCI57256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McClean PL, Parthsarathy V, Faivre E, Hölscher C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J Neurosci. 2011;31:6587–6594. doi: 10.1523/JNEUROSCI.0529-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K, Brossi A, Lahiri DK, Mattson MP, Hoffer BJ, Wang Y, Greig NH. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A. 2009;106:1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.López-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, de Cabo R. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci U S A. 2006;103:1768–1773. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. 2009;59:293–315. doi: 10.1016/j.brainresrev.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffen SC, Wang J, German MS. A genetic defect in beta-cell gene expression segregates independently from the fa locus in the ZDF rat. Diabetes. 2001;50:63–68. doi: 10.2337/diabetes.50.1.63. [DOI] [PubMed] [Google Scholar]
- 27.Cummings BP, Stanhope KL, Graham JL, Baskin DG, Griffen SC, Nilsson C, Sams A, Knudsen LB, Raun K, Havel PJ. Chronic administration of the glucagon-like peptide-1 analog, liraglutide, delays the onset of diabetes and lowers triglycerides in UCD-T2DM rats. Diabetes. 2010;59:2653–2661. doi: 10.2337/db09-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cummings BP, Strader AD, Stanhope KL, Graham JL, Lee J, Raybould HE, Baskin DG, Havel PJ. Ileal interposition surgery improves glucose and lipid metabolism and delays diabetes onset in the UCD-T2DM rat. Gastroenterology. 2010;138:2437–2446. 2446.e2431. doi: 10.1053/j.gastro.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cummings BP, Bettaieb A, Graham JL, Stanhope KL, Kowala M, Haj FG, Chouinard ML, Havel PJ. Vertical sleeve gastrectomy improves glucose and lipid metabolism and delays diabetes onset in UCD-T2DM rats. Endocrinology. 2012;153:3620–3632. doi: 10.1210/en.2012-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bergeron R, Yao J, Woods JW, Zycband EI, Liu C, Li Z, Adams A, Berger JP, Zhang BB, Moller DE, Doebber TW. Peroxisome proliferator-activated receptor (PPAR)-alpha agonism prevents the onset of type 2 diabetes in Zucker diabetic fatty rats: A comparison with PPAR gamma agonism. Endocrinology. 2006;147:4252–4262. doi: 10.1210/en.2005-1535. [DOI] [PubMed] [Google Scholar]
- 31.Peterson GE, Pollom RD. Liraglutide in clinical practice: dosing, safety and efficacy. Int J Clin Pract Suppl. 2010:35–43. doi: 10.1111/j.1742-1241.2010.02498.x. [DOI] [PubMed] [Google Scholar]
- 32.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 33.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 34.Chen SD, Yang DI, Lin TK, Shaw FZ, Liou CW, Chuang YC. Roles of Oxidative Stress, Apoptosis, PGC-1α and Mitochondrial Biogenesis in Cerebral Ischemia. Int J Mol Sci. 2011;12:7199–7215. doi: 10.3390/ijms12107199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valle I, Alvarez-Barrientos A, Arza E, Lamas S, Monsalve M. PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc Res. 2005;66:562–573. doi: 10.1016/j.cardiores.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 36.Hiona A, Leeuwenburgh C. Effects of age and caloric restriction on brain neuronal cell death/survival. Ann N Y Acad Sci. 2004;1019:96–105. doi: 10.1196/annals.1297.018. [DOI] [PubMed] [Google Scholar]
- 37.Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, Mattson MP. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2007;26:212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 38.Starai VJ, Celic I, Cole RN, Boeke JD, Escalante-Semerena JC. Sir2-dependent activation of acetyl-CoA synthetase by deacetylation of active lysine. Science. 2002;298:2390–2392. doi: 10.1126/science.1077650. [DOI] [PubMed] [Google Scholar]
- 39.Hallows WC, Lee S, Denu JM. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc Natl Acad Sci U S A. 2006;103:10230–10235. doi: 10.1073/pnas.0604392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernández-Fonseca JP, Rincón J, Pedreañez A, Viera N, Arcaya JL, Carrizo E, Mosquera J. Structural and ultrastructural analysis of cerebral cortex, cerebellum, and hypothalamus from diabetic rats. Exp Diabetes Res. 2009;2009:329632. doi: 10.1155/2009/329632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Last D, Alsop DC, Abduljalil AM, Marquis RP, de Bazelaire C, Hu K, Cavallerano J, Novak V. Global and regional effects of type 2 diabetes on brain tissue volumes and cerebral vasoreactivity. Diabetes Care. 2007;30:1193–1199. doi: 10.2337/dc06-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdul-Rahman O, Sasvari-Szekely M, Ver A, Rosta K, Szasz BK, Kereszturi E, Keszler G. Altered gene expression profiles in the hippocampus and prefrontal cortex of type 2 diabetic rats. BMC Genomics. 2012;13:81. doi: 10.1186/1471-2164-13-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tweedie D, Rachmany L, Rubovitch V, Lehrmann E, Zhang Y, Becker KG, Perez E, Miller J, Hoffer BJ, Greig NH, Pick CG. Exendin-4, a glucagon-like peptide-1 receptor agonist prevents mTBI-induced changes in hippocampus gene expression and memory deficits in mice. Exp Neurol. 2013;239:170–182. doi: 10.1016/j.expneurol.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Long-Smith CM, Manning S, McClean PL, Coakley MF, O’Halloran DJ, Holscher C, O’Neill C. The diabetes drug liraglutide ameliorates aberrant insulin receptor localisation and signalling in parallel with decreasing both amyloid-β plaque and glial pathology in a mouse model of Alzheimer’s disease. Neuromolecular Med. 2013;15:102–114. doi: 10.1007/s12017-012-8199-5. [DOI] [PubMed] [Google Scholar]
- 45.Swarbrick MM, Havel PJ. Physiological, pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Metab Syndr Relat Disord. 2008;6:87–102. doi: 10.1089/met.2007.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roy Chowdhury SK, Smith DR, Saleh A, Schapansky J, Marquez A, Gomes S, Akude E, Morrow D, Calcutt NA, Fernyhough P. Impaired adenosine monophosphate-activated protein kinase signalling in dorsal root ganglia neurons is linked to mitochondrial dysfunction and peripheral neuropathy in diabetes. Brain. 2012;135:1751–1766. doi: 10.1093/brain/aws097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett. 2008;582:46–53. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agrawal R, Tyagi E, Vergnes L, Reue K, Gomez-Pinilla F. Coupling energy homeostasis with a mechanism to support plasticity in brain trauma. Biochim Biophys Acta. 2014;1842:535–546. doi: 10.1016/j.bbadis.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 49.Liu J, Head E, Gharib AM, Yuan W, Ingersoll RT, Hagen TM, Cotman CW, Ames BN. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha -lipoic acid. Proc Natl Acad Sci U S A. 2002;99:2356–2361. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burkhalter J, Fiumelli H, Allaman I, Chatton JY, Martin JL. Brain-derived neurotrophic factor stimulates energy metabolism in developing cortical neurons. J Neurosci. 2003;23:8212–8220. doi: 10.1523/JNEUROSCI.23-23-08212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 52.Gao J, Wang WY, Mao YW, Gräff J, Guan JS, Pan L, Mak G, Kim D, Su SC, Tsai LH. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michán S, Li Y, Chou MM, Parrella E, Ge H, Long JM, Allard JS, Lewis K, Miller M, Xu W, Mervis RF, Chen J, Guerin KI, Smith LE, McBurney MW, Sinclair DA, Baudry M, de Cabo R, Longo VD. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30:9695–9707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha} J Biol Chem. 2005;280:16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- 55.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 56.Rafaeloff-Phail R, Ding L, Conner L, Yeh WK, McClure D, Guo H, Emerson K, Brooks H. Biochemical regulation of mammalian AMP-activated protein kinase activity by NAD and NADH. J Biol Chem. 2004;279:52934–52939. doi: 10.1074/jbc.M409574200. [DOI] [PubMed] [Google Scholar]
- 57.Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13:225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hirschey MD, Shimazu T, Capra JA, Pollard KS, Verdin E. SIRT1 and SIRT3 deacetylate homologous substrates: AceCS1,2 and HMGCS1,2. Aging (Albany NY) 2011;3:635–642. doi: 10.18632/aging.100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
Web Reference
- Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. The guidance for industry by the office of new drugs in the center for drug evaluation and research (CDER) at the food and drug administration, Rockville, Maryland, USA. Pharmacology and Toxicology. 2005 Jul; URL: http://www.fda.gov/downloads/Drugs/Guidance/UCM078932.pdf.