Abstract
Objective
To systematically review and synthesize available epidemiological data on hepatitis C virus (HCV) prevalence and incidence in the Maghreb region and to estimate the country-specific population-level HCV prevalence.
Methods
We conducted a systematic review of HCV antibody prevalence and incidence in the Maghreb countries as outlined by the PRISMA guidelines. Meta-analyses were conducted using DerSimonian-Laird random-effect models with inverse variance weighting to pool HCV prevalence estimates among general population groups.
Results
We identified 133 HCV prevalence measures and two HCV incidence measures. Among high risk groups, HCV prevalence ranged between 22% and 94% among people who inject drugs, 20% and 76% among dialysis patients, and 2% and 51% among hemophiliacs. Among intermediate-risk groups, considerable but widely variable HCV prevalence was found. Most common risk factors cited across studies were the duration of dialysis, number of transfusions, and having a history of surgery or dental work. The national HCV prevalence in Algeria was estimated at 0.3% (95%CI: 0.1–0.5), Libya 1.2% (95%CI: 1.1–1.3), Mauritania 1.1% (95%CI: 0–2.3), Morocco 0.8% (95%CI: 0.5–1.2), and Tunisia 0.6% (95%CI: 0.5–0.8).
Conclusions
HCV prevalence in the Maghreb region of the Middle East and North Africa is comparable to that in developed countries of about 1%. HCV exposures appear often to be linked to medical care and are suggestive of ongoing transmission in such settings. Injecting drug use appears also to be a major, though not dominant, contributor to HCV transmission. Further research is needed to draw a more thorough understanding of HCV epidemiology, especially in the countries with limited number of studies. HCV prevention policy and programming in these countries should focus on the settings of exposure.
Introduction
The Middle East and North Africa (MENA) region appears to have the highest prevalence of hepatitis C virus (HCV) worldwide [1, 2]. The highest national prevalence of HCV globally is found in Egypt (14.7%) [3, 4]. Questions remain as to the prevalence of HCV in the rest of North Africa and to what extent, if any, are this region’s rates influenced by the large HCV reservoir in Egypt.
The Maghreb region of North Africa borders Egypt to the east. Geographically, the region encompasses Algeria, Libya, Mauritania, Morocco and Tunisia. The combined population of this region is approximately 88 million people [5], or over one-fifth of the population of MENA. Our objective in this study was to systematically review and synthesize all epidemiological data on HCV antibody prevalence and incidence among the different population groups in the Maghreb; and to estimate the national population-level HCV prevalence for each of its five countries. The study is conducted under the umbrella of the MENA HCV Synthesis Project; an ongoing effort to characterize HCV epidemiology in the MENA region. The ultimate goal of this project is to provide the empirical evidence necessary for policy makers and public health stakeholders to set the key research, policy, and programming priorities for the MENA region.
Methods
Data Sources and Search Strategy
We conducted a systematic review of the literature following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (S1 Table) [6]. The primary outcome measure of interest was HCV antibody prevalence (sero-prevalence) and incidence (sero-incidence) in the Maghreb region. A secondary outcome measure of interest was HCV RNA prevalence among HCV antibody positive patients.
Main data sources for this review were PubMed (Medline) and Embase databases. No language restrictions were imposed in either database. While our PubMed search had no year limit, we restricted the search in Embase to articles published after 1980. However, since HCV was discovered in 1989, no effect of this restriction on our results is expected. The search criteria utilized MeSH/Emtree terms in PubMed and Embase, respectively, and text terms. Full details of the search criteria are presented in S1 Box. We used broad search criteria to insure completeness. Additional sources of data were primarily obtained through the MENA HIV/AIDS Epidemiology Synthesis Project database [7, 8]. These included international organizations’ reports and country-level reports.
Study Selection
All records found through PubMed and Embase were initially screened to remove duplicates. The title and abstract for the remaining records were screened by two investigators (FAF and YAM) for relevance. Any uncertainty was settled through consultation with members of the study team. All relevant and potentially relevant full-text articles on the sero-prevalence or sero-incidence of HCV in the Maghreb were identified and subsequently screened. Case series, case reports, and reviews were excluded. We only included studies with primary data in our analysis. Publications reporting the same data were considered duplicates and counted as one publication. The bibliographies of relevant reviews were compared to our records, and studies reporting prevalence or incidence, not initially identified or retrieved, were added to our study.
Data Extraction
Data from relevant records were abstracted by two investigators (FAF and YAM) for the following indicators: author, year of publication, year of study, country of study, study site, study design, sampling technique, population, sample size, sero-prevalence, RNA prevalence, incidence, and risk factors for HCV infection. Any disagreements were discussed and settled among the study team. Articles in French were abstracted by one author (GRM). Articles in Arabic were abstracted by two of the authors (FAF) and (LJA). Data was extracted from abstracts when the full-text was not available. Though articles identifying HCV risk factors were not specifically searched for, risk factor data was abstracted from relevant reports when available.
Data Synthesis
Studies were organized by country and by study population. Study population was divided into categories according to the perceived risk of acquiring HCV infection. Four categories were defined: 1) high risk groups consisting of people who inject drugs (PWID), dialysis patients, hemophiliacs, and multi-transfused patients, among others; 2) intermediate risk groups including diabetics, barbers and others potentially exposed to HCV at intermediate risk such as healthcare workers; 3) low risk groups representing the general population including blood donors, pregnant women, healthy adults, army recruits, and healthy controls from case-control studies, among others; and 4) special clinical populations including renal transplant patients, hepatocellular carcinoma (HCC) patients, liver disease patients, and patients with psoriasis, among others. This latter category represents patients with clinical conditions associated with HCV infection or patients with specific diseases that require clinical care, and thus can be exposed to HCV in medical care facilities. These populations were grouped together as special clinical populations since the level of exposure to HCV is uncertain, and accordingly, it is difficult to categorize them among any of the other three population groups.
Quantitative Analysis
Pooled estimates for HCV prevalence among the general population were calculated for Algeria, Morocco and Tunisia since we had a sufficient number of studies to power a meta-analysis in each of these countries. The analysis was conducted using Stata/SEv13 and R2.15.3. Estimates were pooled using a DerSimonian-Laird random effects model which assumes that the true effect size could vary from study to study, and that the true effects are normally distributed [9]. The model therefore accounts for both sampling variation and heterogeneity in effect size. Individual studies’ effect sizes were weighted using their inverse variance. The variance of the raw proportions was stabilized by transforming the proportions using the Freeman-Tukey type double-arcsine square-root transformation [10]. The back-transformed pooled proportions were then calculated using Miller’s inverse transformation with the harmonic mean of the sample sizes [11]. To examine the magnitude of the variation between studies due to heterogeneity rather than chance, we quantified the heterogeneity using the I2 measure and its confidence interval [12]. We considered a two-sided probability value <0.10 as significant.
A meta-analysis was not performed for each of Libya and Mauritania. While Libya presented various studies estimating the prevalence of HCV in the general population; the best estimate was chosen as that reported through the Libyan National Survey [13] due to its nationally representative sampling technique and large sample size (n = 65,711). Only one study was identified in Mauritania and therefore a meta-analysis was not possible.
Results
Search Results
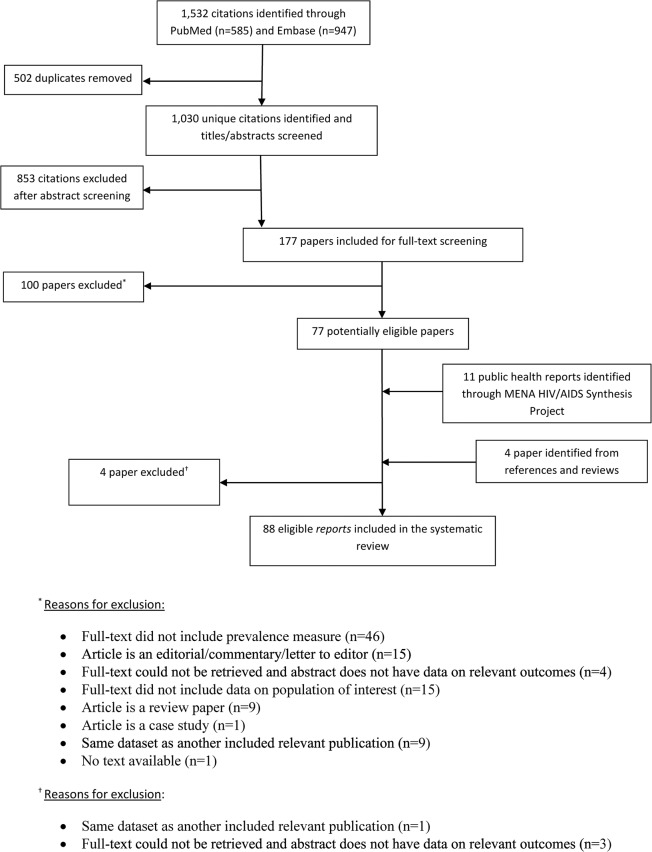
A schematic diagram of the selection process is outlined in Fig. 1, adapted from the PRISMA 2009 flow diagram [6]. We identified a total of 1,532 records (585 through PubMed and 947 through Embase) as of December 11, 2013. Of these records, 502 duplicates were identified and excluded. Screening of the remaining titles and abstracts yielded 177 potentially relevant articles. Full-text records of those were retrieved and screened with the exception of four articles for which the full-text could not be retrieved and the abstracts did not report the relevant outcome. Screening of full-texts identified 77 articles for inclusion in the review. Four additional records were identified through screening of bibliographies of reviews; however, they were subsequently excluded as their full-texts could not be retrieved or they were a duplicate for a study already included. An additional 11 relevant records were included through the MENA HIV/AIDS Synthesis Project database (one international organization report and 10 country-level reports).
Fig 1. Flow chart of article selection adapted from the PRISMA 2009 guidelines [6].
HCV Prevalence Overview
Data on HCV prevalence as well as other indicators were abstracted from the 88 relevant records, and presented in Table 1. In total, 133 HCV prevalence measures were identified by our study. Most reports presenting HCV prevalence in the Maghreb were from Morocco (n = 38) and Tunisia (n = 60). Populations studied to estimate HCV prevalence ranged from general population groups such as blood donors, pregnant women, and healthy adults, to high risk groups such as PWID, dialysis patients, hemophiliacs, and multi-transfused patients. The following is a synthesis of HCV prevalence in each of the Maghreb countries:
Table 1. Studies reporting hepatitis C virus (HCV) prevalence in the Maghreb countries.
| Citation | Year of Data Collection | Study Site | Study Design | Population | Sample Size | Antibody prevalence | RNA Prevalence among Anti-HCV Positive |
|---|---|---|---|---|---|---|---|
| ALGERIA | |||||||
| High Risk | |||||||
| Saidane,11 [14] | 2010–2011 | Hospital | CS | Hemophiliacs | 64 | 30% | |
| General Population | |||||||
| Aidaoui,08 [15] | 2008 | Hospital | CS | Pregnant women | 3044 | 0.63% | |
| Ayed, 95 [16] | 1992–1993 | Hospital | Pregnant women | 715 | 0.19% | ||
| Ayed, 95 [16] | 1992–1993 | Blood transfusion center | CS | Blood donors | 1112 | 0.18% | |
| LIBYA | |||||||
| High Risk | |||||||
| Mirzoyan,13 [17] | 2010 | CS | People who inject drugs | 328 | 94.2% | ||
| Yerly,01 [18] | 1998–1999 | Hospital | CS | HIV patients (children) suspected of acquiring infection paranterally | 111 | 46% | |
| Yerly,01 [18] | 1998–1999 | Hospital | CS | HIV patients (children) suspected of acquiring infection paranterally | 37 | 43% | |
| Elzouki,93 [19] | Hospital | CS | Dialysis patients | 47 | 42.5% | ||
| Alashek,12 [20] | 2009–2010 | Hemodialysis centers | CS | Dialysis patients | 2382 | 32.3% | |
| Elzouki,95 [21] | Hospital | CS | Dialysis patients | 153 | 21% | 72% | |
| Daw,02 [22] | 1999–2001 | CS | Dialysis patients | 200 | 20.5% | ||
| Daw,02 [22] | 1999–2001 | Multi-transfused patients | 250 | 10.8% | |||
| Intermediate Risk | |||||||
| Yerly,01 [18] | 1998–1999 | Hospital | CS | Parents of children with HIV suspected of acquiring infection paranterally | 46 | 4.35% | |
| Kutrani,07 [23] | 2003 | Hospital | CS | Non-Libyan patients referred to the infectious disease department | 281 | 54.09% | |
| Kutrani,07 [23] | 2003 | Hospital | CS | Libyan patients referred to the infectious disease department | 1019 | 44.95% | |
| Alashek,10 [24] | 2009 | Dialysis units | CS | Diabetic patients | 749 | 24.4% | |
| Ziglam,12 [25] | 2006 | Prisons | CS | Prisoners | 6371 | 23.7% | |
| Valadez,13 [26] | 2010–2011 | Community probability based sample | CS | Male sex workers | 227 | 7.3% | |
| Saleh,94 [27] | 1992 | Hospital | CS | Hospital workers | 190 | 6.8% | 69.23% |
| Valadez,13 [26] | 2010–2011 | Community probability based sample | CS | Female sex workers | 69 | 5.2% | |
| Abddulsalam Bagar,10 [28] | 2007–2008 | Hospital | CS (Retrospective) | Patients with unspecified disease | 17419 | 3.67% | |
| Franka,09 [29] | 2004 | Hospital | CS | Medical waste handlers | 300 | 2.7% | |
| Daw,02 [22] | 1999–2001 | CS | Hospital workers | 459 | 2.0% | ||
| Franka,09 [29] | 2004 | Medical management facility | CS | Non-medical waste handlers | 300 | 0% | |
| General Population | |||||||
| Shabash,10 [30] | General population | 878 | 23.2% | ||||
| Saleh,94 [27] | 1992 | Hospital | CS | Blood donors | 76 | 6.6% | 60% |
| Shabash,10 [31] | 2003–2008 | Laboratory | CS (Retrospective) | General population | 1008214 | 1.77% | |
| Daw,02 [22] | 1999–2001 | CS | Healthy adults | 800 | 1.6% | ||
| Daw,02 [22] | 1999–2001 | CS | Blood donors | 1200 | 1.2% | ||
| Daw,14 [13] | 2008 | Libyan national survey | CS | General population | 65711 | 1.19% | |
| Elzouki,95 [32] | Blood Bank | CS | Blood donors | 86 | 0.9% | ||
| Special Clinical Population | |||||||
| Elbouaishi,10 [33] | Hospital | CS (Retrospective) | Nephrotic syndrome patients (children) | 329 | 8.51% | ||
| MAURITANIA | |||||||
| General Population | |||||||
| Lo,99 [34] | 1998 | Hospital | CS | Blood donors | 349 | 1.1% | |
| MOROCCO | |||||||
| High Risk | |||||||
| HIV Integrated Behavioral and Biological Surveillance Survey,12 [35] | 2011–2012 | CS | People who inject drugs | 274 | 79.2% | ||
| Boulaajaj,05 [36] | 1983–2002 | Hospital | CS (Retrospective) | Dialysis patients | 126 | 76% | |
| Sekkat,08 [37] | 2003–2004 | Dialysis units | CS | Dialysis Patients | 303 | 68.3% | |
| Amar,05 [38] | Dialysis units | CS | Dialysis patients | 85 | 54.12% | ||
| HIV Integrated Behavioral and Biological Surveillance Survey,12 [35] | 2010–2011 | CS | People who inject drugs | 261 | 45.6% | ||
| Benjelloun,96 [39] | Hemophilia treatment center | CS | Hemophiliacs | 118 | 42.4% | ||
| Bousfiha,99 [40] | 1999 | Hemophilia treatment center | CS | Hemophiliacs (children) | 39 | 41% | |
| Benjelloun,96 [39] | Dialysis units | CS | Dialysis patients | 114 | 35.1% | ||
| HIV Integrated Behavioral and Biological Surveillance Survey,12 [35] | 2010–2011 | CS | People who inject drugs | 22 | 31.8% | ||
| HIV Integrated Behavioral and Biological Surveillance Survey,12 [35] | 2011–2012 | CS | People who inject drugs | 83 | 22.9% | ||
| El Khorassani,10 [41] | 1981–2006 | Hospital | CS (Retrospective) | Hemophiliacs | 262 | 2.29% | |
| Intermediate Risk | |||||||
| Benjelloun,96[39] | STD center | CS | HIV patients | 116 | 19.8% | ||
| Cacoub,00 [42] | 1995–1996 | Hospital | CS | Inpatients | 280 | 10.36% | 75% |
| Cacoub,00 [42] | 1995–1996 | Hospital | CS | Outpatients | 503 | 6.16% | |
| Rebbani,13 [43] | 2006–2010 | Infectious disease center | CS | HIV patients | 503 | 5.4% | |
| Zahraoui-Mehadji,04 [44] | 2001 | CS | Traditional barbers | 150 | 5% | ||
| Benjelloun,96[39] | STD center | CS | STD patients | 2088 | 3% | ||
| Belbacha,11 [45] | 2007 | CS | Traditional barbers | 267 | 1.10% | ||
| Lahlou Amine,10 [46] | 2005–2006 | Hospital | CS (Retrospective) | Inpatients | 2350 | 0.76% | |
| General Population | |||||||
| Benouda,09 [47] | 2005–2007 | Laboratory | CS | General population | 8326 | 1.93% | |
| Baha,13 [48] | 2005–2011 | Nationwide | CS | General population | 41269 | 1.58% | |
| Belbacha,11 [45] | 2007 | Clients of barbers | 529 | 1.30% | |||
| Benani,11 [49] | CS | General population | 24646 | 1.10% | |||
| Benjelloun,96 [39] | Blood transfusion center | CS | Blood donors | 1000 | 1.1% | ||
| Benjelloun,96 [39] | Hospital | CS | Pregnant women | 676 | 1.0% | ||
| Aqodad,11 [50] | 2006–2007 | Blood bank | CS | Blood donors | 777 | 0.8% | |
| Baha,13 [48] | 2005–2011 | Blood transfusion center | CS | Blood donors | 169605 | 0.62% | |
| Regional Database on HIV/AIDS. WHO Regional Office for the Eastern Mediterranean,11 [51] | CS | ANC and family planning clinic attendees | 0.5% | ||||
| Lahlou Amine,11 [46] | 2005–2006 | Hospital | CS (Retrospective) | Army recruits | 16000 | 0.35% | |
| Zohoun,11 [52] | 2008–2009 | Hospital | CS (Retrospective) | Blood donors | 19801 | 0.2% | |
| Regional Database on HIV/AIDS WHO Regional Office for the Eastern Mediterranean,11 [53] | 2010 | CS | Blood donors | 132197 | 0.16% | ||
| Special Clinical Population | |||||||
| Rioche,91 [54] | 1983–1986 | Dialysis units | CS | Non-A and Non-B chronic hepatitis patients | 38 | 73.7% | |
| Ezzikouri,09 [55] | 2003–2006 | Hospital | CC | Hepatocellular carcinoma patients | 96 | 57.3% | |
| Rioche,91 [54] | 1983–1986 | Hospital | CS | Non-A and Non-B acute hepatitis patients | 90 | 44.4% | |
| Lakhoua Gorgi,10 [56] | 1987–2004 | Hospital | CS | Renal transplant patients | 57 | 19.3% | |
| Radoui,10 [57] | 1998–2008 | Hospital | CS | Renal transplant patients | 69 | 10.1% | |
| Mohammed,12 [58] | 2005–2011 | Hospital | CS (Retrospective) | Hepatic steatosis patients | 79 | 3.8% | |
| Lahlou Amine,10 [46] | 2005–2006 | Hospital | CS (Retrospective) | Patients consulting for HCV infection | 7050 | 2.32% | |
| Bousfiha,99 [59] | 1999 | Hospital | CS | Acute hepatitis patients (children) | 130 | 0% | |
| TUNISIA | |||||||
| High Risk | |||||||
| Ayed,03 [60] | 2001 | Dialysis units/Overall | CS | Dialysis patients | 1394 | 22.24% | 76.7% |
| Belarbi,13 [61] | 2012 | Outpatient hospital | CS (Retrospective) | People who inject drugs | 23 | 21.7% | |
| Ayed,03 [60] | 2001 | Dialysis units/Central | 1314 | 18.47% | 60.5% | ||
| Ayed,03 [60] | 2001 | Dialysis units/Northwest | 358 | 15.36% | 70.9% | ||
| Ayed,03 [60] | 2001 | Dialysis units/Northern | 279 | 15.7% | 90.9% | ||
| Ayed,03 [60] | 2001 | Dialysis units/Southern | 796 | 14.57% | 71.5% | ||
| Bejaoui,13 [62] | Medical institutions | CS (Retrospective) | Thalassemia patients | 391 | 6.1% | ||
| Hannachi,11 [63] | 2008–2009 | Hospital | CC | Multi-transfused patients | 107 | 4.7% | |
| Tunisia Ministry of Health,10 [64] | 2009 | CS | People who inject drugs | 715 | 29.1% | ||
| Djebbi,08 [65] | 2003 | Hospital | CS | Hemophiliacs | 95 | 50.5% | 87.5% |
| Sassi,00 [66] | Dialysis units | CS | Dialysis patients | 58 | 46.5% | 51% | |
| Hmida,95 [67] | Dialysis units | CS | Dialysis patients | 235 | 45.1% | ||
| Ben Othman,04 [68] | 2000–2002 | Dialysis units | CS | Dialysis patients | 276 | 32.6% | 78.89% |
| Ayed,03 [60] | 2001 | Dialysis units/Northeastern | 199 | 30.15% | 93.33% | ||
| Hachicha,95 [69] | Dialysis units | CS | Dialysis patients | 235 | 42% | ||
| Hmaied,06 [70] | 2001–2003 | Dialysis units | CS | Dialysis patients | 395 | 20% | 73% |
| Jemni,94 [71] | Dialysis units | CS | Dialysis patients | 63 | 42% | ||
| Langar,05 [72] | Hospital | CS | Hemophiliacs | 70 | 50% | ||
| Intermediate Risk | |||||||
| Kilani,07 [73] | 1997–2005 | Hospital | CS | HIV patients | 362 | 39.7% | |
| Maaref,11 [74] | 2006 | Hospital | CS | HIV patients | 125 | 26.4% | |
| Larabi,01 [75] | 1997–1999 | Hospital | CS | Inpatients | 542 | 20.3% | |
| Kaabia,09 [76] | 2003 | Hospital | CS | Diabetic patients | 1269 | 1.3% | |
| Znazen,10 [77] | 2007 | Hospital | CS | Female sex workers | 188 | 1.1% | |
| Kaabia,09 [78] | 2005 | Hospital | CS | Hospital workers | 885 | 1% | |
| General Population | |||||||
| Coursaget,90 [79] | 1982–1986 | CS | Blood donors | 99 | 3.0% | ||
| Coursaget,95 [80] | Hospital | CS | Blood donors | 45 | 2.2% | ||
| Krichen,01 [81] | 1995–1997 | CS | Blood donors | 42623 | 1.71% | ||
| Mejri,05 [82] | 1996 | Households | CS | General population | 4157 | 1.7% | 82% |
| Larabi,01 [75] | 1997–1999 | Hospital | CS | Blood donors | 3480 | 1.18% | |
| Slama,91 [83] | Secondary schools | CS | Blood donors | 2006 | 1.09% | ||
| HIV/AIDS Quarterly Report,07 [84] | 2007- 2nd Quarter | CS | 24247 | 0.87% | |||
| Gorgi,98 [85] | 1994–1996 | Community | CS | General population | 3079 | 0.71% | |
| HIV/AIDS Quarterly Report,07 [84] | 2007- 3rd Quarter | CS | 10526 | 0.64% | |||
| Hannachi,11 [63] | 2008–2009 | Hospital | CC | Controls of a case-control study | 160 | 0.6% | |
| Kaabia,09 [76] | 2003 | Hospital | CS | Controls of a case-control study | 1315 | 0.6% | |
| Hatira,00 [86] | 1994–1997 | Blood bank | CS | Blood donors | 34130 | 0.56% | |
| HIV/AIDS Quarterly Report,06 [87] | 2006- 3rd Quarter | CS | 20803 | 0.54% | |||
| Triki,94 [88] | Laboratory | CS | Healthy adults | 735 | 0.40% | ||
| Triki,97 [89] | 1987–1994 | CS | Blood donors | 785 | 0.4% | ||
| HIV/AIDS Quarterly Report,07 [84] | 2007- 4th Quarter | CS | 58368 | 0.36% | |||
| HIV/AIDS Quarterly Report,06 [87] | 2006- 2nd Quarter | CS | Blood donors | 31115 | 0.34% | ||
| Kallel,11 [90] | CC | Controls of a case-control study | 300 | 0.33% | |||
| HIV/AIDS Quarterly Report,06 [87] | 2006- 4th Quarter | CS | 28428 | 0.30% | |||
| HIV/AIDS Quarterly Report,07 [84] | 2007- 1st Quarter | CS | 259890 | 0.26% | |||
| Hannachi,11 [91] | 2006 | Hospital | CS | Pregnant women | 404 | 0.2% | |
| Mejri,05 [82] | 1996 | Households | CS | General population | 7350 | 0.2% | 71% |
| Abid,97 [92] | 1994 | Blood bank | CS | Blood donors | 43000 | 0.18% | |
| Mahjoub,13 [93] | 2000–2010 | CS | Blood donors (military) | 182996 | 0.14% | ||
| Samoud,11 [94] | CC | Healthy adults | 64 | 0% | |||
| Special Clinical Populations | |||||||
| Bouzgarrou,11 [95] | 2005–2008 | Hospital | CS | Chronic hepatitis patients | 77 | 80.52% | |
| Coursaget,90[79] | 1982–1986 | CS | Non-A and Non-B acute hepatitis patients | 25 | 48.0% | ||
| Triki,94 [88] | Laboratory | CS | Cirrhosis patients | 168 | 43% | ||
| Coursaget,92 [96] | CS | Cirrhosis patients | 23 | 31% | |||
| Lakhoua Gorgi,10 [56] | 1987–2004 | Hospital | CS | Renal transplant patients | 115 | 20.9% | 91.7% |
| Triki,94 [88] | Laboratory | CS | Hepatocellular carcinoma patients | 31 | 19% | ||
| Coursaget,95 [80] | Hospital | CS | Acute hepatitis patients | 45 | 18% | ||
| Coursaget,90[79] | 1982–1986 | CS | HBV patients | 28 | 14.3% | ||
| Samoud,11 [94] | CC | Psoriatic patients | 41 | 9.75% | |||
| Coursaget,92 [96] | CS | Acute hepatitis patients | 25 | 8% | |||
| Hannachi,10 [97] | 2010 | CS | HBV patients | 273 | 3.8% | ||
| Kallel,11 [90] | CC | Inflammatory bowel disease patients | 150 | 2% | |||
| ALGERIA, MOROCCO and TUNISIA | |||||||
| General Population | |||||||
| Bahri,11 [98] | 2002–2005 | Multi-center study | CC | Controls of a case-control study | 250 | 4.40% | |
CS: cross-sectional study design, CC: case-control study design, STD: sexually transmitted disease, HBV: hepatitis B virus
Note: Citations are sorted within each risk group in descending order of prevalence.
Algeria
The single study from Algeria reporting on a high risk group examined hemophiliacs, reporting a prevalence of 30% [14] (Table 1). Three studies reported general population HCV prevalence estimates in Algeria. Prevalence of 0.6% [15] and 0.2% [16] was reported among pregnant women, and HCV prevalence of 0.2% [16] was reported among blood donors.
Libya
Among high risk groups in Libya, PWID were reported to have the highest HCV prevalence at 94% [17] (Table 1). Dialysis patients had a prevalence ranging from 21% [22] to 43% [19]. One study examined the prevalence among children infected with HIV and children with HIV who were referred to a specific hospital (both children groups were suspected to have had parenteral exposure to HIV) [18]. HCV prevalence was 46% and 43%, respectively [18]. Among the parents of the children with HIV who were referred to this specific hospital, HCV prevalence was 4% [18].
HCV was measured among several populations at intermediate risk including diabetics (24%) [24], prisoners (24%) [25], male sex workers (7%) [26], female sex workers (5%) [26], hospital care workers (2%) [22], and medical (3%) and non-medical (0%) waste handlers [29] (Table 1). Among patients referred to an infectious disease department, with no further specification, persons of non-Libyan backgrounds had an HCV prevalence of 54% while their Libyan counterparts had a prevalence of 45% [23].
Five reports and one national survey reported HCV prevalence among the general population in Libya [13, 22, 27, 30–32]. Estimates hovered around 1–2% with two outliers of much larger prevalence (Table 1).
Only one study was conducted among special clinical populations in Libya (Table 1). The study reported HCV prevalence of 9% among children with nephrotic syndrome [33].
Mauritania
Only one study measured HCV prevalence in Mauritania. The study tested 349 blood donors at a national hospital, reporting a prevalence of 1% [34]. The majority of donors were males (333 vs. 16).
Morocco
Among high risk groups, HCV prevalence was measured among PWID, but through small samples, at 32% in Nador and 23% in Tanger [35] (Table 1). However, more recently, it was measured among PWID in the same cities, using large probability-based samples (respondent-driven sampling), to be 79% in Nador and 46% in Tanger [35]. Among dialysis patients, HCV prevalence ranged from 35% [39] to as high as 76% [36]. Hemophiliacs had variable rates as reported from three studies with one examining rates in children. At 42%, the earliest of the three studies reported the highest HCV prevalence among hemophiliacs in Morocco [39]. The second study, conducted among children, reported a comparable prevalence of 41% [40]. The third, and most recent study, reported the lowest HCV prevalence among hemophiliacs at 2% [41].
Benjelloun et al. examined HCV prevalence among two intermediate risk populations including those having a history of a sexually transmitted disease (STD) and those infected with HIV (likely through a sexual mode of transmission) [39]. For those with a history of STD, HCV prevalence was 3%, while for those who tested positive for HIV, HCV prevalence was 20% [39]. Another study of HIV infected patients reported an HCV prevalence of 5% [43]. Among hospital attendees, an early study reported HCV prevalence of 10% among inpatients and 6% among outpatients [42]. A more recent study reported an HCV prevalence of 0.8% among inpatients [46]. Traditional barbers were tested for HCV in two studies in Morocco where the prevalence was reported to be 1% [45] and 5% [44].
Estimates of HCV prevalence in the general population in Morocco came from eight reports including 11 prevalence measures on pregnant women, blood donors, and army recruits, among others. HCV prevalence ranged between 0.2% and 2% (Table 1).
Among special clinical populations, two studies examined HCV prevalence among those who had a renal transplant reporting estimates of 19% [56] and 10% [57]. HCV prevalence among HCC patients was 57% [55]. Individuals with chronic or acute hepatitis had prevalence estimates of 74% and 44% [54].
Tunisia
Among high risk groups, HCV prevalence was measured among PWID to be 22% (small sample) [61] and 29% [64] (Table 1). Among dialysis patients, several studies reported different measures in different areas which ranged between 15% [60] and 47% [66]. Djebbi et al. and Langar et al. measured HCV prevalence among hemophiliacs and reported similar results of 51% [65] and 50% [72], respectively. HCV prevalence among multi-tranfused patients was 5% [63], while it was 6% among thalassemia patients [62].
Among intermediate risk populations, HCV prevalence was measured to be 1% among diabetics [76], 20% among inpatients and referred patients (non-specified) [75], and 1% among hospital employees [78]. Among individuals testing positive for HIV, HCV prevalence was 26% [74] and 40% [73]. Among female sex workers, it was 1% [77].
Of the Maghreb countries, Tunisia had the largest number of studies measuring HCV prevalence among general population groups. Many of the studies had large samples (typically over 1,000), and were among populations such as blood donors, pregnant women, and healthy adults, among others. In total, 15 articles and seven country-level reports provided measures for Tunisia’s HCV prevalence in the population at large (24 prevalence measures). HCV prevalence in the general population abstracted from these studies ranged between 0% and 3% (Table 1).
Notably, relatively high HCV prevalence was reported among special clinical populations. HCV prevalence ranged between 31% [96] and 43% [88] among cirrhosis patients, and was as high as 21% [56] among renal transplant recipients, and as high as 19% [88] among HCC patients. Two studies examining chronic and Non-A and Non-B acute hepatitis reported high HCV prevalence at 81% [95] and 48% [79], respectively. Two other studies among individuals with acute viral hepatitis estimated HCV prevalence at 8% [96] and 18% [80].
HCV Incidence Overview
Only two studies, both in dialysis units, reported empirical measures of HCV incidence in the Maghreb. The first in Tunisia, by Ben Othman et al., reported an HCV incidence rate of 2.8 per 100 person-years in 2000–2002 [68]. The second study in Morocco, by Sekkat et al., estimated HCV incidence rate at 9.7 per 100 person-years in 2003–2004 [37].
Risk Factors
Risk factors were most typically identified in studies conducted among high risk or intermediate risk populations. These included dialysis patients, inpatients, and patients referred to an infectious disease department. Common risk factors identified across countries were the duration of dialysis [21, 38, 66], history and number of transfusions [38, 66], and whether individuals had a history of surgery or dental work [23, 42].
HCV Genotypes
Genotype 1 is the most common HCV genotype consistently reported for most countries in the Maghreb [99, 100]. According to a study by Messina et al., HCV genotype 1 accounts for 82% of HCV infections in Algeria, 44% of infections in Libya, 74% of infections in Morocco, and 41% of infections in Tunisia [99]. Another study by Ezzikouri et al. places genotype 4 as the most common genotype in Libya, and genotype 1 as the second most common (genotype 4: 36% vs. genotype 1: 33%) [100]. For Algeria, Morocco, and Tunisia, genotype 2 is consistently reported as the second most common genotype [99, 100]. The genotype distribution diversity, as measured by the Shannon Diversity Index, shows high diversity in Libya and Tunisia and low diversity in Algeria and Morocco [99]. No data seems to be available for HCV genotype distribution in Mauritania.
National Population-Level HCV Prevalence Estimates
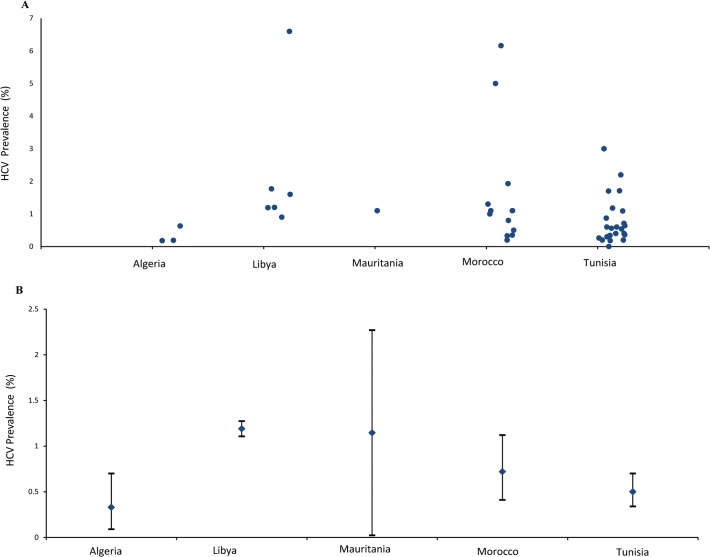
All individual study estimates for HCV prevalence in the general population are presented in Fig. 2A. Using these measures, we estimated the national population-level HCV prevalence for each of the five countries (Fig. 2B). In Libya, since there was a population-based survey, we reported HCV prevalence as measured in this survey, of 1.2% (95% CI: 1.1–1.3) [101]. In Mauritania, since there was only one general population study conducted, we reported HCV prevalence in this study, of 1.1% (95% CI: 0–2.3), as an estimate of the national population-level HCV prevalence [34].
Fig 2. Hepatitis C virus (HCV) prevalence in the general population of the Maghreb countries.
A. Available HCV prevalence measures among the general population as abstracted from studies included in the systematic review. B. Estimated HCV prevalence at the national level in each of the Maghreb countries.
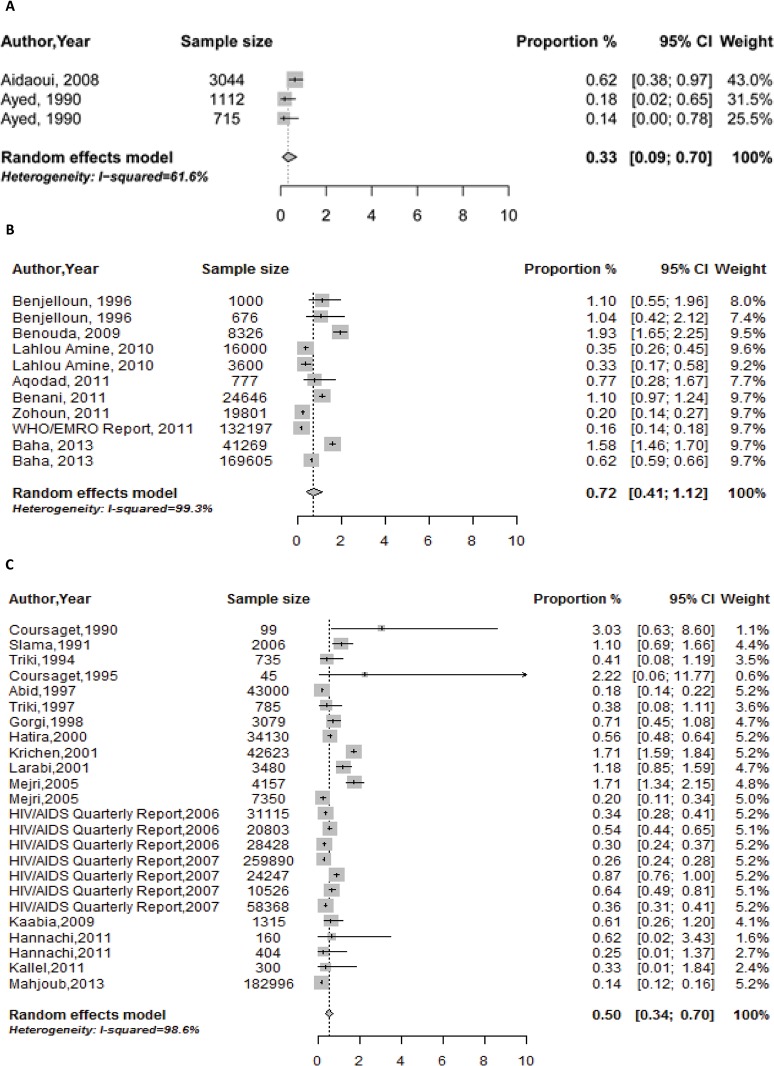
Fig. 3A, 3B, and 3C provide forest plots depicting the specific study estimates as well as the meta-analysis estimates for Algeria, Morocco and Tunisia. The pooled estimate for the national population-level HCV prevalence in Algeria was 0.3% (95% CI 0.1–0.7), I2 = 61% (95% CI 0.0–87.7, p = 0.10). The pooled estimate in Morocco was 0.7% (95% CI 0.4–1.1), I2 = 99% (95% CI 99.0–99.3, p < 0.001). The pooled estimate in Tunisia was 0.5% (95% CI 0.3–0.7), I2 = 99% (95% CI 98.8–99.1, p < 0.001).
Fig 3. Pooled summary estimates of hepatitis C virus (HCV) prevalence among general population groups in A. Algeria; B. Morocco; C. Tunisia.
Discussion
We provided a comprehensive systematic review and synthesis of HCV prevalence and incidence in the Maghreb countries using a well-defined and thorough methodology and following the PRISMA guidelines. We highlighted the key features of HCV epidemiology in this region and, importantly, estimated the national population-level HCV prevalence in each of the Maghreb countries. With the recent remarkable successes in HCV treatment with direct-acting antivirals [102–105], our results take on additional importance as they provide the evidence base necessary for planning of health services, articulation of HCV policy guidelines, and implementation of HCV programming.
Our findings highlight that overall HCV prevalence in the Maghreb countries is at a level of about 1%, comparable to that observed in developed countries [1, 106]. This prevalence level is at odds with that observed in Egypt, the immediate eastern neighbor to the Maghreb, where HCV prevalence is estimated at 15% of the adult population [3, 4]. Additionally, the genotypes most reported in this region vary from that in Egypt (>95% of infections in Egypt belong to genotype 4) [99]. While no genotype information is available for Mauritania, reports from Algeria, Libya, Morocco, and Tunisia suggest genotype 1 as most common [37, 60, 99, 100, 107], in similar pattern to most countries globally [99, 108]. This finding highlights the uniqueness of the Egyptian epidemic and that the major drivers of this epidemic do not appear to be present in North Africa apart from Egypt. It further suggests that the infection transmission networks across the countries of North Africa may not have a sizable overlap.
Nonetheless, there is some evidence that the Egyptian epidemic may have affected HCV patterns in Libya, the neighboring country to the west of Egypt, and possibly Tunisia, the second nearest neighbor to Egypt of the Maghreb countries. HCV genotype 4 appears to account for more than one-third of HCV infections in Libya and one-quarter of infections in Tunisia [22, 99]. This suggests possible cross-over from Egypt into Libya, and then possibly into Tunisia. As many Egyptian migrant workers seek better employment opportunities in Libya, cross border migration may have facilitated avenues for the circulation of HCV from Egypt into Libya. It is possible too that a large fraction of genotype 4 cases in Libya could be reflecting Egyptian migrant workers rather than Libyan nationals. Since genotype 4 is also prevalent in Central Africa [99], it is possible that genotype 4 may have circulated into Libya and Tunisia through links to this part of Africa [109]. Of note that genotype 4 appears to have very limited presence in Algeria and Morocco [99].
Further epidemiological studies are needed to clarify the overlapping chains of transmission across North Africa. Despite the existence of at least some circulation of HCV from Egypt into Libya, the national HCV prevalence in Libya is comparable, though slightly higher, to the rest of the Maghreb countries, and an order of magnitude smaller than that in Egypt [4]. This further highlights the uniqueness of the Egyptian epidemic.
Overall, relatively high HCV prevalence levels were documented among individuals in high risk groups. Dialysis patients and transplant recipients, for example, have rates ranging from 16% [60] to as high as 76% [36]. Studies on HCV incidence among dialysis patients reported high HCV incidence rates of 3 [68] and 10 [37] per 100 person-years. Additionally, duration of dialysis, number of transfusions, and surgical and dental procedures were consistently cited as major risk factors across studies. HCV prevalence levels remained high in these populations, regardless of year of study, indicating possible ongoing transmission in healthcare settings. These findings suggest that a substantial fraction of HCV infections reflect healthcare-related exposures.
Injecting drug use (IDU) is the largest contributor to current HCV incidence in developed countries [110]. However, our findings indicate that this is not necessarily the case in the Maghreb countries. The high HCV prevalence and incidence levels among high risk populations exposed at healthcare facilities (Table 1), such as dialysis patients, suggest that IDU may not be the dominant contributor. Moreover, the prevalence of IDU in the Maghreb countries is lower than global levels, and smaller than that in the eastern part of MENA [111]. The prevalence of IDU was estimated at 0.22% in Algeria, 0.14% in Libya, 0.10% in Morocco, and 0.21% in Tunisia [111]. These rather low levels, in addition to an overall HCV prevalence of about 50% among PWID in MENA [111], suggest that IDU can explain only a minority of prevalent infections. However, given the severity of the HCV and HIV epidemics in Libya among PWID [112], it is possible that IDU may play a proportionally larger role in Libya than the rest of the Maghreb countries.
Our results further suggest that other exposures to HCV, in populations at intermediate risk, appear to be present. Much of these seem to be also related to healthcare, as can be seen by the higher HCV prevalence among diabetics, health care workers, and hospitalized populations (Table 1). Prisons appear to be a setting where a significant level of HCV exposure is found (Table 1), probably because of IDU prior to or after incarceration, but also possibly due to tattooing and sharing of utensils in prisons [7, 111]. Some exposures also seem to be related to certain professions or practices, such as among traditional barbers where somewhat considerable HCV prevalence is found (Table 1). It appears also that there is a rather significant HCV prevalence among sexual high risk populations, such as male and female sex workers and STD patients (Table 1). It is not clear though whether these prevalence levels reflect HCV sexual transmission or probably just higher IDU levels among these populations [111, 113].
There is a hint of geographic variability in HCV prevalence between different regions within the same country in both Libya and Tunisia. According to the Libyan population-based survey, HCV prevalence ranged from as low as 0.6% in Misrata to as high as 2.2% in Fezzan [13]. Similarly in Tunisia, HCV prevalence was estimated at 0.2% in the south and 1.7% in the northwest [82]. Such localized higher HCV prevalence levels have been observed in other settings globally such as in Japan and Taiwan [114–116]. They may suggest specific risk factors for HCV exposure at these localities that need to be investigated and identified. It is not clear for example whether such regional variations may reflect variations in infection control measures across the country, say in central health care facilities versus local facilities, or differences in the prevalence of IDU, or possibly the existence of localized traditional medicine practices that may expose individuals to HCV infection such as cautery and skin scarifications. Such factors that can drive geographically different infection transmission patterns appear to be present in MENA [7, 117]. Mapping of HCV exposure spatially, as has been done recently in Egypt [118], can shed light on such local risk factors and may point to wider issues of infection control that are beyond HCV concerns.
Our study provided country-specific estimates of HCV prevalence in the Maghreb using systematic review and meta-analysis methodology, a distinct approach from that used by the Global Burden of Disease (GBD) study [1]. Our approach is empirically focused whereby data on HCV prevalence are compiled through a systematic methodology and then pooled through formal statistical methods to yield estimates. The GBD approach utilizes mathematical modeling whereby systematically-compiled input data are used to parametrize complex models to generate the estimates. While our country-specific estimates are produced using data only from the respective country, GBD models are parametrized by data from different countries. While the overarching aim of our approach is to delineate the epidemiology of the infection, the GBD’s approach aims to reach estimates for the disease burden resulting from complications of this infection. These two approaches therefore should be seen as complementary approaches, each of which has its strengths and limitations, and each of which is informing the other.
Among the limitations of our study are the variability of the number studies across countries and the low number of studies from Algeria and Mauritania (only one study was identified in Mauritania). The general population studies included in the meta-analyses may not have been representative of the population at large. Nearly all studies were on convenient samples and there was only one study on a nationally representative and probability-based sample (in Libya; Table 1). Many of the studies, particularly the large ones, were among blood donors or pregnant women. HCV prevalence in these populations may underestimate HCV prevalence in the whole population; there could be selection towards lower risk among blood donors, and women tend to have lower HCV prevalence than men.
Our meta-analyses highlighted that there is substantial heterogeneity among the studies conducted in general population groups. This is not surprising considering the differences between studies in terms of the specific general population studied, sampling methodology and participant recruitment, age-group representation in the sample, year of study, location and geographic sub-region of study, and assay used. However, due to the small number of observations for each country, we were unable to conduct a meta-regression analysis to identify potential sources of variation to explain the observed heterogeneity for each country.
We classified the populations into high risk, intermediate risk, and general population (low risk) groups by convention in HCV epidemiology literature. However, there is no established existing classification of risk for some populations, and the information available in some studies was not sufficient to determine the level of risk. In these situations the level of risk was determined based on our best judgment of the risk of exposure to HCV infection in this population. For example, HIV infected patients who have acquired HIV likely through a parenteral mode of transmission, were classified as a high risk population, whereas HIV infected persons who have acquired HIV likely though a sexual mode of transmission, were classified as an intermediate risk population. Furthermore, clinical populations for which the risk of exposure was uncertain were classified into an independent category as special clinical populations.
Another limitation in our study is that there was variability in the diagnostic assays used across studies. Earlier studies typically reported the use of 1st and 2nd generation ELISA tests, which lack the sensitivity and specificity of the 3rd generation ELISA tests. Such variability in assays may impact the representativeness of earlier studies. Lastly, only two studies in this region provided empirical measures of HCV incidence, and these were fairly dated.
Conclusion
HCV prevalence in the Maghreb region of MENA is comparable to that in developed countries of about 1%. Yet, the evidence synthesized here suggests ongoing HCV transmission through specific high-risk healthcare exposures such as dialysis and blood transfusions. IDU is also a major, though probably not dominant, contributor to HCV transmission in these countries. Other exposures, though at much reduced risk, appear to be related to intermediate-risk healthcare settings or procedures and specific professions or community practices. Further research is needed to draw a more thorough and complete understanding of HCV epidemiology in this part of MENA, especially so in the countries where a limited number of studies have been conducted (Algeria and Mauritania). Our findings suggest the need for a targeted approach to control HCV transmission by focusing the response on the settings of exposure. The findings also provide the evidence base necessary to inform planning of health service provision, articulation of HCV policy guidelines, and implementation of HCV programming to reduce HCV transmission and decrease the burden of its associated diseases.
Supporting Information
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was made possible by NPRP grant number 04-924-3-251 from the Qatar National Research Fund (a member of Qatar Foundation). Additional support was provided by the Biostatistics, Epidemiology, and Biomathematics Research Core at the Weill Cornell Medical College in Qatar. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The statements made herein are solely the responsibility of the authors. More information related to Qatar National Research Fund can be found on the (URL: http://www.qnrf.org/).
References
- 1. Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333–42. Epub 2012/11/23. 10.1002/hep.26141 . [DOI] [PubMed] [Google Scholar]
- 2. Lavanchy D. Evolving epidemiology of hepatitis C virus. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2011;17(2):107–15. Epub 2010/11/26. 10.1111/j.1469-0691.2010.03432.x . [DOI] [PubMed] [Google Scholar]
- 3. El-Zanaty F, Way A. Egypt Demographic and Health Survey 2008 Egyptian: Ministry of Health. Cairo: El-Zanaty and Associates, and Macro International; 2009. [Google Scholar]
- 4. Mohamoud YA, Mumtaz GR, Riome S, Miller D, Abu-Raddad LJ. The epidemiology of hepatitis C virus in Egypt: a systematic review and data synthesis. BMC infectious diseases. 2013;13:288 Epub 2013/06/27. 10.1186/1471-2334-13-288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.United Nations. United Nations, Department of Economic and Social Affairs Population Division, Populationa Estimates and Projections Section 2010 [cited 7/11/2012]. Available from: http://esa.un.org/unpd/wpp/unpp/panel_population.htm.
- 6. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. International journal of surgery (London, England). 2010;8(5):336–41. Epub 2010/02/23. 10.1016/j.ijsu.2010.02.007 . [DOI] [PubMed] [Google Scholar]
- 7. Abu-Raddad L, Akala FA, Semini I, Riedner G, Wilson D, Tawil O. Characterizing the HIV/AIDS epidemic in the Middle East and North Africa: Time for Strategic Action Middle East and North Africa HIV/AIDS Epidemiology Synthesis Project. World Bank/UNAIDS/WHO Publication. Washington DC: The World Bank Press; 2010. [Google Scholar]
- 8. Abu-Raddad LJ, Hilmi N, Mumtaz G, Benkirane M, Akala FA, Riedner G, et al. Epidemiology of HIV infection in the Middle East and North Africa. AIDS. 2010;24 Suppl 2:S5–23. Epub 2010/07/17. 10.1097/01.aids.0000386729.56683.33 . [DOI] [PubMed] [Google Scholar]
- 9. Neyeloff JL, Fuchs SC, Moreira LB. Meta-analyses and Forest plots using a microsoft excel spreadsheet: step-by-step guide focusing on descriptive data analysis. BMC research notes. 2012;5:52 Epub 2012/01/24. 10.1186/1756-0500-5-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Freeman MF, Tukey JW. Transformations Related to the Angular and the Square Root. Ann Math Statist. 1950;21(4):607–11. [Google Scholar]
- 11. Miller JJ. The inverse of the Freeman-Tukey double arcsine transformation. American Statistician. 1978;32(4):138. [Google Scholar]
- 12. Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed). 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Daw MA, El-Bouzedi A. Prevalence of hepatitis B and hepatitis C infection in Libya: results from a national population based survey. BMC infectious diseases. 2014;14:17 Epub 2014/01/11. 10.1186/1471-2334-14-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saidane N, Saidi M, Derdous C, Rouabhia S, Soltani F, Ouarhlent Y. Various complications in haemophiliacs managed by Hospital University of Batna, Algeria. Journal of Thrombosis and Haemostasis. 2011;9:923 10.1111/j.1538-7836.2011.04380_4.x . [DOI] [Google Scholar]
- 15. Aidaoui M, Bouzbid S, Laouar M. Seroprevalence of HIV infection in pregnant women in the Annaba region (Algeria). [French]Seroprevalence de l'infection VIH chez les femmes enceintes dans la region de Annaba (Algerie). Revue d'Epidemiologie et de Sante Publique. 2008;56(4):261–6. 10.1016/j.respe.2008.05.023 [DOI] [PubMed] [Google Scholar]
- 16. Ayed Z, Houinato D, Hocine M, Ranger-Rogez S, Denis F. Prevalence of serum markers of hepatitis B and C in blood donors and pregnant women in Algeria. [French]Prevalence des marqueurs seriques des virus des hepatites B et C chez les donneurs de sang et les femmes enceintes en Algerie. Bulletin de la Societe de pathologie exotique (1990). 1995;88(5):225–8. . [PubMed] [Google Scholar]
- 17. Mirzoyan L, Berendes S, Jeffery C, Thomson J, Othman HB, Danon L, et al. New evidence on the HIV epidemic in Libya: Why countries must implement prevention programs among people who inject drugs. Journal of Acquired Immune Deficiency Syndromes. 2013;62(5):577–83. 10.1097/QAI.0b013e318284714a . [DOI] [PubMed] [Google Scholar]
- 18. Yerly S, Quadri R, Negro F, Barbe KP, Cheseaux JJ, Burgisser P, et al. Nosocomial outbreak of multiple bloodborne viral infections. The Journal of infectious diseases. 2001;184(3):369–72. Epub 2001/07/10. 10.1086/322036 . [DOI] [PubMed] [Google Scholar]
- 19. El-Zouki AY, Bendard AB, Sharif MS. HCV in hemodialysis patients in Benghazi, Libya. Annals of Saudi medicine. 1993;13(2):203 Epub 1993/03/01. . [DOI] [PubMed] [Google Scholar]
- 20. Alashek WA, McIntyre CW, Taal MW. Hepatitis B and C infection in haemodialysis patients in Libya: prevalence, incidence and risk factors. BMC Infectious Diseases. 2012;12(265). 10.1186/1471-2334-12-265 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elzouki AN, Bushala M, Tobji RS, Khfaifi M. Prevalence of anti-hepatitis C virus antibodies and hepatitis C virus viraemia in chronic haemodialysis patients in Libya. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 1995;10(4):475–6. Epub 1995/01/01. . [DOI] [PubMed] [Google Scholar]
- 22. Daw MA, Elkaber MA, Drah AM, Werfalli MM, Mihat AA, Siala IM. Prevalence of hepatitis C virus antibodies among different populations of relative and attributable risk. Saudi medical journal. 2002;23(11):1356–60. Epub 2002/12/31. . [PubMed] [Google Scholar]
- 23. Kutrani H, El-Gatit A, Shekhteryea A, El-Gitait Y, Sudani O, Akoub S. Demographic factors influencing hepatitis B and C infection in Benghazi, Libyan Arab Jamahiriya. [Arabic]. Eastern Mediterranean Health Journal. 2007;13(1):85–97. . [PubMed] [Google Scholar]
- 24. Alashek W, McIntyre C, Taal M. Morbidity of diabetic end-stage kidney disease patients treated by dialysis in Libya. Diabetes, Obesity and Metabolism. 2010;12:76–7. 10.1111/j.1463-1326.2010.01284.x . [DOI] [Google Scholar]
- 25. Ziglam H, Zorgani AA, Balouz A, Abudhe AH, Elahmer O. Prevalence of antibodies to human immunodeficiency virus, hepatitis B, and hepatitis C in prisoners in Libya. Libyan Journal of Medicine. 2012;7(1). . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Valadez JJ, Berendes S, Jeffery C, Thomson J, Ben Othman H, Danon L, et al. Filling the Knowledge Gap: Measuring HIV Prevalence and Risk Factors among Men Who Have Sex with Men and Female Sex Workers in Tripoli, Libya. PLoS ONE. 2013;8(6). 10.1371/journal.pone.0066701 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saleh MG, Pereira LM, Tibbs CJ, Ziu M, al-Fituri MO, Williams R, et al. High prevalence of hepatitis C virus in the normal Libyan population. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1994;88(3):292–4. Epub 1994/05/01. . [DOI] [PubMed] [Google Scholar]
- 28. Abddulsalam Bagar S, Abdallah B, Neferro F. Prevalence of hepatitis C virus among patients attending a hospital in Sirte, Libya. Clinical Microbiology and Infection. 2010;16:S311 10.1111/j.1469-0691.2010.03239.x . [DOI] [Google Scholar]
- 29. Franka E, El-Zoka AH, Hussein AH, Elbakosh MM, Arafa AK, Ghenghesh KS. Hepatitis B virus and hepatitis C virus in medical waste handlers in Tripoli, Libya. The Journal of hospital infection. 2009;72(3):258–61. Epub 2009/05/16. 10.1016/j.jhin.2009.03.019 . [DOI] [PubMed] [Google Scholar]
- 30. Shabash A, Habas M, Fara A, Daw M. Epidemiological analysis of potential risk factors contributing to infection of HBV, HCV and HIV among the population in Tripoli Area, Libya. Clinical Microbiology and Infection. 2010;16:S309 10.1111/j.1469-0691.2010.03239.x . [DOI] [Google Scholar]
- 31. Shabash A, Habas M, Alhajrasi A, Furarah A, Bouzedi A, Daw M. Forecast modelling for prediction of hepatitis B and hepatitis C seropositivity among Libyan population. Clinical Microbiology and Infection. 2010;16:S310 10.1111/j.1469-0691.2010.03239.x . [DOI] [Google Scholar]
- 32. Elzouki AN, Beeching NJ, Mutton KJ, Garson JA. Anti-HCV antibodies and HCV RNA in Libyan blood donors. Vox sanguinis. 1995;68(1):65 Epub 1995/01/01. . [DOI] [PubMed] [Google Scholar]
- 33. Elbouaishi A, Rhoma N, Turki M, Fituri O, Elbousaife A, Grera A. Nephrotic syndrome in Libyan children. Jamahiriya Medical Journal. 2010;10(3):199–205. . [Google Scholar]
- 34. Lo BB, Meymouna M, Boulahi MA, Tew M, Sow A, Ba A, et al. Prevalence of serum markers of hepatitis B and C virus in blood donors of Nouakchott, Mauritania. [French] Prevalence des marqueurs seriques des virus des hepatites B et C chez les donneurs de sang a Nouakchott, Mauritanie. Bulletin de la Societe de pathologie exotique (1990). 1999;92(2):83–4. . [PubMed] [Google Scholar]
- 35.HIV Integrated Behavioral and Biological Surveillance Surveys Morocco 2011–2012. Injecting drug users in Tanger and Nador, Morocco. 2012.
- 36. Boulaajaj K, Elomari Y, Elmaliki B, Madkouri B, Zaid D, Benchemsi N. [Prevalence of hepatitis C, hepatitis B and HIV infection among haemodialysis patients in Ibn-Rochd university hospital, Casablanca]. Nephrologie & therapeutique. 2005;1(5):274–84. Epub 2006/08/10. 10.1016/j.nephro.2005.06.012 . [DOI] [PubMed] [Google Scholar]
- 37. Sekkat S, Kamal N, Benali B, Fellah H, Amazian K, Bourquia A, et al. Prevalence of anti-HCV antibodies and seroconversion incidence in five haemodialysis units in Morocco. [French] Prevalence des anticorps anti-VHC et incidence de seroconversion dans cinq centres d'hemodialyse au Maroc. Nephrologie et Therapeutique. 2008;4(2):105–10. 10.1016/j.nephro.2007.11.007 . [DOI] [PubMed] [Google Scholar]
- 38. Amar Y, Benamar L, Laouad I, Ezaitouni F, Ouzeddoun N, Balafrej L. Hepatitis C virus infection in a Moroccan hemodialysis unit: prevalence and risk factors. [French] L'hepatite virale C dans un centre d'hemodialyse marocain: prevalence et fracturs de risque. Gastroenterologie clinique et biologique. 2005;29(6–7):746–7. . [DOI] [PubMed] [Google Scholar]
- 39. Benjelloun S, Bahbouhi B, Sekkat S, Bennani A, Hda N, Benslimane A. Anti-HCV seroprevalence and risk factors of hepatitis C virus infection in Moroccan population groups. Research in virology. 1996;147(4):247–55. Epub 1996/07/01. . [DOI] [PubMed] [Google Scholar]
- 40. Bousfiha AA, Hachim J, Benjelloun S, Benslimane A, Mikou N, Hadj Khalifa H. Prevalence of infection with the hepatitis B and C viruses and the human immunodeficiency virus in 39 Maroccan children with hemophilia. [French] Prevalence des hepatites virales B et C et du VIH chez 39 enfants hemophiles Marocains. Annales de Pediatrie. 1999;46(3):199–204. . [Google Scholar]
- 41. El Khorassani M, Kababri M, Hessissen L, Khattab M, Kili A. Hemophilia in Morocco: Current state and prospects. Haemophilia. 2010;16:61 10.1111/j.1365-2516.2010.02283.x . [DOI] [Google Scholar]
- 42. Cacoub P, Ohayon V, Sekkat S, Dumont B, Sbai A, Lunel F, et al. Epidemiologic and virologic study of hepatitis C virus infections in Morocco. [French] Etude epidemiologique et virologique des infections par le virus de l'hepatite C au Maroc. Gastroenterologie clinique et biologique. 2000;24(2):169–73. . [PubMed] [Google Scholar]
- 43. Rebbani K, Ouladlahsen A, Bensghir A, Akil A, Lamdini H, Issouf H, et al. Co-infections with hepatitis B and C viruses in human immunodeficiency virus-infected patients in Morocco. Clinical Microbiology and Infection. 2013;19(10):E454–E7. 10.1111/1469-0691.12252 . [DOI] [PubMed] [Google Scholar]
- 44. Zahraoui-Mehadji M, Baakrim MZ, Laraqui S, Laraqui O, El Kabouss Y, Verger C, et al. [Infectious risks associated with blood exposure for traditional barbers and their customers in Morocco]. Sante (Montrouge, France). 2004;14(4):211–6. Epub 2005/03/05. . [PubMed] [Google Scholar]
- 45. Belbacha I, Cherkaoui I, Akrim M, Dooley KE, El Aouad R. Seroprevalence of hepatitis B and C among barbers and their clients in the Rabat region of Morocco Seroprevalence de l'hepatite B et C chez les barbiers et leurs clients dans la Region de Rabat (Maroc). Eastern Mediterranean Health Journal. 2011;17(12):911–9. . [DOI] [PubMed] [Google Scholar]
- 46. Lahlou Amine I, Zouhair S, Chegri M, L'Kassmi H. Seroprevalence of anti-HCV in patients of the Military Hospital Moulay Ismail (Meknes, Morocco): Data analysis of the medical biology laboratory (2002–2005). [French] Seroprevalence des anticorps anti-VHC chez les patients de l'hopital militaire Moulay Ismail (Meknes, Maroc): Analyse des donnees du laboratoire de biologie medicale (2002–2005). Bulletin de la Societe de Pathologie Exotique. 2010;103(4):255–8. 10.1007/s13149-010-0064-x . [DOI] [PubMed] [Google Scholar]
- 47. Benouda A, Boujdiya Z, Ahid S, Abouqal R, Adnaoui M. Prevalence of hepatitis C virus infection in Morocco and serological tests assessment of detection for the viremia prediction. [French] Prevalence de l'infection par le virus de l'hepatite-C au Maroc et evaluation des tests serologiques de depistage pour la prediction de la viremie. Pathologie Biologie. 2009;57(5):368–72. 10.1016/j.patbio.2008.07.006 . [DOI] [PubMed] [Google Scholar]
- 48. Baha W, Foullous A, Dersi N, They-they TP, El alaoui K, Nourichafi N, et al. Prevalence and risk factors of hepatitis B and C virus infections among the general population and blood donors in Morocco. BMC public health. 2013;13:50 Epub 2013/01/22. 10.1186/1471-2458-13-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Benani A, Baha W, Dersi N, Ennaji M, Lazaar F, El Malki A, et al. Molecular epidemiology of HCV in Morocco. Clinical Microbiology and Infection. 2011;17:S658 10.1111/j.1469-0691.2011.03558.x . [DOI] [Google Scholar]
- 50. Aqodad N, Lahbabi M, Elyousfi M, Mellouki I, Benajah D, Elabkari M, et al. Prevalence of VHC-Ab and HBsAg among blood donors in Guelmim in the south of Morocco. Hepatology International. 2011;5 (1):96 10.1007/s12072-010-9241-z . [DOI] [Google Scholar]
- 51. WHO/EMRO. Regional database on HIV/AIDS. WHO Regional Office for the Eastern Mediterranean; 2006. [Google Scholar]
- 52. Zohoun A, Hadef R, Zahid H, Benkirane M. [Seroprevalence of HBV and HCV in blood donors at the Blood Transfusion Center of Mohammed V Military Teaching Hospital in Rabat Morocco]. [French] Prevalence des marqueurs seriques des virus de l'hepatite B et C chez les donneurs de sang au Centre de Transfusion Sanguine de l'Hopital Militaire d'Instruction Mohammed V de Rabat (Maroc). Medecine tropicale: revue du Corps de sante colonial. 2011;71(5):513–4. . [PubMed] [Google Scholar]
- 53. WHO/EMRO. Regional database on HIV/AIDS WHO Regional Office for the Eastern Mediterranean; 2011. [Google Scholar]
- 54. Rioche M, Himmich H, Cherkaoui A, Mourid A, Dubreuil P, Zahraoui M, et al. High incidence of sporadic non-A, non-B hepatitis in Morocco: epidemiologic study. [French] Forte incidence des hepatites non-A, non-B sporadiques au Maroc: etude epidemiologique. Bulletin de la Societe de pathologie exotique (1990). 1991;84(2):117–27. . [PubMed] [Google Scholar]
- 55. Ezzikouri S, El Feydi AE, Afifi R, El Kihal L, Benazzouz M, Hassar M, et al. MDM2 SNP309T>G polymorphism and risk of hepatocellular carcinoma: a case-control analysis in a Moroccan population. Cancer detection and prevention. 2009;32(5–6):380–5. Epub 2009/02/24. 10.1016/j.cdp.2009.01.003 . [DOI] [PubMed] [Google Scholar]
- 56. Lakhoua Gorgi Y, Gorgi F, Madkouri G, Abderrahim E, Sfar I, Ramadani B, et al. Hepatitis viral C in kidney transplantation: Comparative study between two Maghrebin centers: Casablanca and Tunis. [French] L'hepatite virale C au cours de la transplantation renale: Etude comparative entre deux centres Maghrebins: Casablanca et Tunis. Tunisie Medicale. 2010;88(12):902–9. . [PubMed] [Google Scholar]
- 57. Radoui A, Skalli Z, Haddiya I, Benamar L, Ezzaitouni F, Ouzeddoun N, et al. Prevalence and predictive factors of anemia after renal transplantation: A moroccan report. Transplantation Proceedings. 2010;42(9):3542–9. 10.1016/j.transproceed.2010.07.092 . [DOI] [PubMed] [Google Scholar]
- 58. Mohammed T, Fatima Zohra L, Ahmed B, Rhimou A. Echographic prevalence of hepatic steatosis in Moroccan hospitalized patients. Hepatology International. 2012;6 (1):283 10.1007/s12072-011-9333-4 . [DOI] [Google Scholar]
- 59. Bousfiha AA, Bouchrit M, Abid A, Benslimane A. Children's acute hepatitis in Casablanca. [French] Hepatites virales icteriques aigues de l'enfant a Casablanca. Medecine et Maladies Infectieuses. 1999;29(12):749–52. 10.1016/S0399-077X(00)88284-0 . [DOI] [Google Scholar]
- 60. Ayed K, Gorgi Y, Ben Abdallah T, Aouadi H, Jendoubi-Ayed S, Sfar I, et al. Hepatitis C virus infection in hemodialysis patients from Tunisia: national survey by serologic and molecular methods. Transplantation proceedings. 2003;35(7):2573–5. Epub 2003/11/13. . [DOI] [PubMed] [Google Scholar]
- 61. Belarbi A, Ben Ammar H, Moula O, Bouasker A, Ghachem R. Addiction to high dose buprenorphine and sexually transmitted diseases in Tunisia. European Psychiatry. 2013;28 . [Google Scholar]
- 62. Bejaoui M, Guirat N. Beta thalassemia major in a developing country: Epidemiological, clinical and evolutionary aspects. Mediterranean Journal of Hematology and Infectious Diseases. 2013;5(1). 10.4084/MJHID.2013.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hannachi N, Boughammoura L, Marzouk M, Tfifha M, Khlif A, Soussi S, et al. Viral infection risk in polytransfused adults: Seroprevalence of seven viruses in central Tunisia. [French] Le risque infectieux viral chez le polytransfuse: Seroprevalence de sept agents viraux dans le centre tunisien. Bulletin de la Societe de Pathologie Exotique. 2011;104(3):220–5. 10.1007/s13149-010-0103-7 . [DOI] [PubMed] [Google Scholar]
- 64.Tunisie MdlSPe, SIDA ATdLClMel. Enquête sérocomportementale auprès des hommes ayant des rapports sexuels avec des hommes en Tunisie (French) [Biobehavioral survey among men who have sex with men in Tunisia]. Tunis, Tunisia: 2010.
- 65. Djebbi A, Bahri O, Langar H, Sadraoui A, Mejri S, Triki H. Genetic variability of genotype 1 hepatitis C virus isolates from Tunisian haemophiliacs. The new microbiologica. 2008;31(4):473–80. Epub 2009/01/07. . [PubMed] [Google Scholar]
- 66. Sassi F, Gorgi Y, Ayed K, Abdallah TB, Lamouchi A, Maiz HB. Hepatitis C virus antibodies in dialysis patients in Tunisia: a single center study. Saudi journal of kidney diseases and transplantation: an official publication of the Saudi Center for Organ Transplantation, Saudi Arabia. 2000;11(2):218–22. Epub 2008/01/23. . [PubMed] [Google Scholar]
- 67. Hmida S, Mojaat N, Chaouchi E, Mahjoub T, Khlass B, Abid S, et al. HCV antibodies in hemodialyzed patients in Tunisia. [French] Anticorps anti-HCV chez les hemodialyses en Tunisie. Pathologie-biologie. 1995;43(7):581–3. . [PubMed] [Google Scholar]
- 68. Ben Othman S, Bouzgarrou N, Achour A, Bourlet T, Pozzetto B, Trabelsi A. [High prevalence and incidence of hepatitis C virus infections among dialysis patients in the East-Centre of Tunisia]. Pathologie-biologie. 2004;52(6):323–7. Epub 2004/07/21. 10.1016/j.patbio.2003.07.001 . [DOI] [PubMed] [Google Scholar]
- 69. Hachicha J, Hammami A, Masmoudi H, Ben Hmida M, Karray H, Kharrat M, et al. Hepatitis C virus in haemodialysis patients in the South of Tunisia. Prevalence and risk factors. [French]Hepatite Virale C Chez Les Hemodialyses Chroniques Dans Le Sud Tunisien. Prevalence Et Facteurs De Risque. Annales de Medecine Interne. 1995;146(5):295–8. . [PubMed] [Google Scholar]
- 70. Hmaied F, Ben Mamou M, Saune-Sandres K, Rostaing L, Slim A, Arrouji Z, et al. Hepatitis C virus infection among dialysis patients in Tunisia: incidence and molecular evidence for nosocomial transmission. Journal of medical virology. 2006;78(2):185–91. Epub 2005/12/24. 10.1002/jmv.20526 . [DOI] [PubMed] [Google Scholar]
- 71. Jemni S, Ikbel K, Kortas M, Mahjoub J, Ghachem L, Bidet JM, et al. Seropositivity to hepatitis C virus in Tunisian haemodialysis patients. Nouvelle revue francaise d'hematologie. 1994;36(5):349–51. Epub 1994/10/01. . [PubMed] [Google Scholar]
- 72. Langar H, Triki H, Gouider E, Bahri O, Djebbi A, Sadraoui A, et al. Blood-transmitted viral infections among haemophiliacs in Tunisia. [French] Infections par des virus transmissibles par le sang chez des hemophiles en Tunisie. Transfusion Clinique et Biologique. 2005;12(4):301–5. 10.1016/j.tracli.2005.07.001 . [DOI] [PubMed] [Google Scholar]
- 73. Kilani B, Ammari L, Marrakchi C, Letaief A, Chakroun M, Ben Jemaa M, et al. Seroepidemiology of HCV-HIV coinfection in Tunisia. La Tunisie medicale. 2007;85(2):121–3. Epub 2007/08/02. . [PubMed] [Google Scholar]
- 74. Maaref F, Kilani B, Ammari L, Ben Othman A, Zribi M, Fendri C, et al. Prevalence of hepatitis G, B and C virus infections among positive HIV population in a Tunisian Hospital, La Rabta, Tunis. [French] Prevalence de l'hepatite G et des hepatites virales B et C dans la population VIH (+) de l'hopital La Rabta, Tunis, Tunisie. Pathologie Biologie. 2011;59(4):213–6. 10.1016/j.patbio.2009.10.004 . [DOI] [PubMed] [Google Scholar]
- 75. Larabi K, Tourjemene K. Epidemiology of hepatitis C virus infection in the Menzel-Bourguiba region. [French] Epidemiologie de l'infection par le virus de l'hepatite C dans la region de Menzel—Bourguiba. La Tunisie medicale. 2001;79(12):672–5. . [PubMed] [Google Scholar]
- 76. Kaabia N, Ben Jazia E, Slim I, Fodha I, Hachfi W, Gaha R, et al. Association of hepatitis C virus infection and diabetes in central Tunisia. World journal of gastroenterology: WJG. 2009;15(22):2778–81. Epub 2009/06/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Znazen A, Frikha-Gargouri O, Berrajah L, Bellalouna S, Hakim H, Gueddana N, et al. Sexually transmitted infections among female sex workers in Tunisia: high prevalence of Chlamydia trachomatis. Sexually transmitted infections. 2010;86(7):500–5. Epub 2010/07/27. 10.1136/sti.2010.042770 . [DOI] [PubMed] [Google Scholar]
- 78. Kaabia N, Ben Jazia E, Hannachi N, Khalifa M, Dhouibi S, Dabbabi F, et al. Prevalence of hepatitis C virus among health care workers in central Tunisia. [French]Prevalence de l'hepatite virale C chez le personnel de sante au Centre tunisien. Medecine et Maladies Infectieuses. 2009;39(1):66–7. 10.1016/j.medmal.2008.10.007 . [DOI] [PubMed] [Google Scholar]
- 79. Coursaget P, Bourdil C, Kastally R, Yvonnet B, Rampanarivo Z, Chiron JP, et al. Prevalence of hepatitis C virus infection in Africa: anti-HCV antibodies in the general population and in patients suffering from cirrhosis or primary liver cancer. Research in virology. 1990;141(4):449–54. Epub 1990/07/01. . [DOI] [PubMed] [Google Scholar]
- 80. Coursaget P, Leboulleux D, Gharbi Y, Enogat N, Ndao MA, Coll-Seck AM, et al. Etiology of acute sporadic hepatitis in adults in Senegal and Tunisia. Scandinavian journal of infectious diseases. 1995;27(1):9–11. Epub 1995/01/01. . [DOI] [PubMed] [Google Scholar]
- 81. Krichen C, Rekik H, Feki H, Dammak J, Gargouri J. Prevalence of viral markers among blood donors in Tunisia. Clinical Laboratory. 2001;47(9–10):509–16. . [PubMed] [Google Scholar]
- 82. Mejri S, Ben Salah A, Triki H, Ben Alaya N, Djebbi A, Dellagi K. Contrasting patterns of hepatitis C virus infection in two regions from Tunisia. Journal of Medical Virology. 2005;76(2):185–93. 10.1002/jmv.20342 . [DOI] [PubMed] [Google Scholar]
- 83. Slama H, Mojaat N, Dahri R, Boukef K. Epidemiologic study of anti-HCV antibodies in Tunisian blood donors. [French] Etude epidemiologique des anticorps anti-HCV chez les donneurs de sang en Tunisie. Revue francaise de transfusion et d'hemobiologie: bulletin de la Societe nationale de transfusion sanguine. 1991;34(6):459–64. . [DOI] [PubMed] [Google Scholar]
- 84.Tunisia Ministry of Health. HIV/AIDS Quarterly Case Notification. 2007.
- 85. Gorgi Y, Yalaoui S, Ben Nejma HL, Azzouz MM, Hsairi M, Ben Khelifa H, et al. Detection of hepatitis C virus in the general population of Tunisia. [French] Depistage de l'hepatite virale C dans la population generale en Tunisie. Bulletin de la Societe de pathologie exotique (1990). 1998;91(2):177 . [PubMed] [Google Scholar]
- 86. Hatira SA, Yacoub-Jemni S, Houissa B, Kaabi H, Zaeir M, Kortas M, et al. Hepatitis C virus antibodies in 34130 blood donors in Tunisian Sahel. [French] Les anti V.H.C chez 34130 donneurs de sang du Sahel Tunisien. La Tunisie medicale. 2000;78(2):101–5. . [PubMed] [Google Scholar]
- 87.Health TMo. HIV/AIDS Quarterly Case Notification. 2006.
- 88. Triki H. Epidemiology of hepatitis B virus, hepatitis C virus and Delta virus in the general population and in liver cirrhosis in Tunisia. [French] Epidemiologie des virus des hepatites B, C et Delta dans la population generale et les cirrhoses hepatitiques en Tunisie. Archives de l'Institut Pasteur de Tunis. 1994;71(3–4):403–6. . [PubMed] [Google Scholar]
- 89. Triki H, Said N, Ben Salah A, Arrouji A, Ben Ahmed F, Bouguerra A, et al. Seroepidemiology of hepatitis B, C and delta viruses in Tunisia. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1997;91(1):11–4. Epub 1997/01/01. . [DOI] [PubMed] [Google Scholar]
- 90. Kallel L, Fekih M, Ouaz A, Ben Mustapha N, Karoui S, Serghini M, et al. Prevalence and risk factors of viral B and C hepatitis among Tunisian inflammatory bowel disease: A case control study. Hepatology International. 2011;5 (1):89–90. 10.1007/s12072-010-9241-z . [DOI] [Google Scholar]
- 91. Hannachi N, Hidar S, Harrabi I, Mhalla S, Marzouk M, Ghzel H, et al. Seroprevalence and risk factors of hepatitis E among pregnant women in central Tunisia. [French] Seroprevalence et facteurs de risque de l'hepatite virale E chez la femme enceinte dans le centre tunisien. Pathologie Biologie. 2011;59(5):e115–e8. 10.1016/j.patbio.2009.06.004 . [DOI] [PubMed] [Google Scholar]
- 92. Abid S, Fkih S, Khlass B, Cherif W, Toumi NH, Jenhani F, et al. Screening and confirmation of anti-HCV antibodies in Tunisian blood donors. [French] Depistage et confirmation des anticorps anti-VHC chez les donneurs de sang tunisiens. Transfusion clinique et biologique: journal de la Societe francaise de transfusion sanguine. 1997;4(2):221–6. . [DOI] [PubMed] [Google Scholar]
- 93. Mahjoub S, Kdous A, Doghri A, Ksontini R, Nsiri B. Epidemiology of hepatitis B and C virus among military blood donors. Vox Sanguinis. 2013;105:182 10.1111/vox.12048 . [DOI] [Google Scholar]
- 94. Samoud S, Hannachi N, Boukadida J. High detection of hepatitis c infection RNA in psoriatic sera. Journal of Investigative Dermatology. 2011;131:S99 10.1038/jid.2011.214 . [DOI] [Google Scholar]
- 95. Bouzgarrou N, Hassen E, Bahri O, Gabbouj S, Mami NB, Triki H, et al. Combined effect of pro- and anti-inflammatory cytokine gene polymorphisms on susceptibility to liver cirrhosis in Tunisian HCV-infected patients. Hepatology International. 2011;5(2):681–7. 10.1007/s12072-010-9232-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Coursaget P, Simpson B, el Goulli N, Ben Khelifa H, Kastally R. Hepatitis C core antibody detection in acute hepatitis and cirrhosis patients from Tunisia. Pathologie-biologie. 1992;40(6):646–8. Epub 1992/06/01. . [PubMed] [Google Scholar]
- 97. Hannachi N, Bahri O, Boukatef B, Triki H, Boukadida J. Isolated anti-HBc profile should be investigated in a country of intermediate endemicity for hepatitis B. Clinical Microbiology and Infection. 2010;16:S306 10.1111/j.1469-0691.2010.03239.x . [DOI] [Google Scholar]
- 98. Bahri O, Ezzikouri S, Alaya-Bouafif NB, Iguer F, Feydi AE, Mestiri H, et al. First multicenter study for risk factors for hepatocellular carcinoma development in North Africa. World journal of hepatology. 2011;3(1):24–30. Epub 2011/02/11. 10.4254/wjh.v3.i1.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61(1):77–87. 10.1002/hep.27259 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ezzikouri S, Pineau P, Benjelloun S. Hepatitis C virus infection in the Maghreb region. Journal of medical virology. 2013;85(9):1542–9. Epub 2013/06/20. 10.1002/jmv.23643 . [DOI] [PubMed] [Google Scholar]
- 101.Libya National Center for the Prevention of and Control of Infectious Diseases. Results of the National Seroprevalence Survey. 2005.
- 102. Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. The New England journal of medicine. 2013;368(20):1867–77. Epub 2013/04/24. 10.1056/NEJMoa1214854 . [DOI] [PubMed] [Google Scholar]
- 103. Kowdley KV, Lawitz E, Crespo I, Hassanein T, Davis MN, DeMicco M, et al. Sofosbuvir with pegylated interferon alfa-2a and ribavirin for treatment-naive patients with hepatitis C genotype-1 infection (ATOMIC): an open-label, randomised, multicentre phase 2 trial. Lancet. 2013;381(9883):2100–7. Epub 2013/03/19. 10.1016/S0140-6736(13)60247-0 . [DOI] [PubMed] [Google Scholar]
- 104. Lawitz E, Lalezari JP, Hassanein T, Kowdley KV, Poordad FF, Sheikh AM, et al. Sofosbuvir in combination with peginterferon alfa-2a and ribavirin for non-cirrhotic, treatment-naive patients with genotypes 1, 2, and 3 hepatitis C infection: a randomised, double-blind, phase 2 trial. The Lancet infectious diseases. 2013;13(5):401–8. Epub 2013/03/19. 10.1016/S1473-3099(13)70033-1 . [DOI] [PubMed] [Google Scholar]
- 105. Younossi ZM, Stepanova M, Henry L, Gane E, Jacobson IM, Lawitz E, et al. Minimal Impact of Sofosbuvir and Ribavirin on Health Related Quality of Life in Chronic Hepatitis C (CH-C). Journal of hepatology. 2013. Epub 2013/12/18. 10.1016/j.jhep.2013.12.006 . [DOI] [PubMed] [Google Scholar]
- 106. Cornberg M, Razavi HA, Alberti A, Bernasconi E, Buti M, Cooper C, et al. A systematic review of hepatitis C virus epidemiology in Europe, Canada and Israel. Liver international: official journal of the International Association for the Study of the Liver. 2011;31 Suppl 2:30–60. Epub 2011/06/18. 10.1111/j.1478-3231.2011.02539.x . [DOI] [PubMed] [Google Scholar]
- 107. Safer L, Ben Chaabene N, Melki W, Saffar H. Epidemiology of viral hepatitis in Tunisia. [French] Epidemiologie des hepatites virales en Tunisie. Revue d'epidemiologie et de sante publique. 2006;54(4):377–80. 10.1016/S0398-7620(06)76732-3 . [DOI] [PubMed] [Google Scholar]
- 108. Te HS, Jensen DM. Epidemiology of hepatitis B and C viruses: a global overview. Clinics in liver disease. 2010;14(1):1–21, vii. Epub 2010/02/04. 10.1016/j.cld.2009.11.009 . [DOI] [PubMed] [Google Scholar]
- 109. Iles JC, Raghwani J, Harrison GL, Pepin J, Djoko CF, Tamoufe U, et al. Phylogeography and epidemic history of hepatitis C virus genotype 4 in Africa. Virology. 2014;464–465:233–43. 10.1016/j.virol.2014.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hellard M, Doyle JS, Sacks-Davis R, Thompson AJ, McBryde E. Eradication of hepatitis C infection: the importance of targeting people who inject drugs. Hepatology. 2014;59(2):366–9. Epub 2013/07/23. 10.1002/hep.26623 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mumtaz GR, Weiss HA, Thomas SL, Riome S, Setayesh H, Riedner G, et al. HIV among people who inject drugs in the Middle East and North Africa: systematic review and data synthesis. PLoS medicine. 2014;11(6):e1001663 Epub 2014/06/18. 10.1371/journal.pmed.1001663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mirzoyan L, Berendes S, Jeffery C, Thomson J, Ben Othman H, Danon L, et al. New evidence on the HIV epidemic in Libya: why countries must implement prevention programs among people who inject drugs. J Acquir Immune Defic Syndr. 2013;62(5):577–83. Epub 2013/01/23. 10.1097/QAI.0b013e318284714a . [DOI] [PubMed] [Google Scholar]
- 113. Mumtaz G, Hilmi N, McFarland W, Kaplan RL, Akala FA, Semini I, et al. Are HIV epidemics among men who have sex with men emerging in the Middle East and North Africa?: a systematic review and data synthesis. PLoS medicine. 2010;8(8):e1000444 Epub 2011/08/11. 10.1371/journal.pmed.1000444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kao JH, Chen DS. Transmission of hepatitis C virus in Asia: past and present perspectives. Journal of gastroenterology and hepatology. 2000;15 Suppl:E91–6. Epub 2000/08/02. . [DOI] [PubMed] [Google Scholar]
- 115. Kiyosawa K, Tanaka E, Sodeyama T, Yoshizawa K, Yabu K, Furuta K, et al. Transmission of hepatitis C in an isolated area in Japan: community-acquired infection. The South Kiso Hepatitis Study Group. Gastroenterology. 1994;106(6):1596–602. Epub 1994/06/01. . [DOI] [PubMed] [Google Scholar]
- 116. Wang CS, Chang TT, Chou P. Differences in risk factors for being either a hepatitis B carrier or anti-hepatitis C+ in a hepatoma-hyperendemic area in rural Taiwan. Journal of clinical epidemiology. 1998;51(9):733–8. Epub 1998/09/10. . [DOI] [PubMed] [Google Scholar]
- 117.Mohamoud YA, Miller FD, Abu-Raddad LJ. Assessing the potential for HIV paranteral transmission in the Middle East and North Africa: an analysis using HCV as a proxy biomarker. World journal of gastroenterology: WJG. 2014:inpress. [DOI] [PMC free article] [PubMed]
- 118.Cuadros DF, Branscum AJ, Miller FD, Abu-Raddad LJ. Spatial epidemiology of hepatitis C virus infection in Egypt: Analyses and implications. Hepatology. 2014. Epub 2014/06/11. 10.1002/hep.27248 . [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.