Abstract
Objective
Muscle carnosine and its methylated form anserine are histidine-containing dipeptides. Both dipeptides have the ability to quench reactive carbonyl species and previous studies have shown that endogenous tissue levels are decreased in chronic diseases, such as diabetes.
Design and Methods
Rodent study: Skeletal muscles of rats and mice were collected from 4 different diet-intervention studies, aiming to induce various degrees of glucose intolerance: 45% high-fat feeding (male rats), 60% high-fat feeding (male rats), cafeteria feeding (male rats), 70% high-fat feeding (female mice). Body weight, glucose-tolerance and muscle histidine-containing dipeptides were assessed. Human study: Muscle biopsies were taken from m. vastus lateralis in 35 males (9 lean, 8 obese, 9 prediabetic and 9 newly diagnosed type 2 diabetic patients) and muscle carnosine and gene expression of muscle fiber type markers were measured.
Results
Diet interventions in rodents (cafeteria and 70% high-fat feeding) induced increases in body weight, glucose intolerance and levels of histidine-containing dipeptides in muscle. In humans, obese, prediabetic and diabetic men had increased muscle carnosine content compared to the lean (+21% (p>0.1), +30% (p<0.05) and +39% (p<0.05), respectively). The gene expression of fast-oxidative type 2A myosin heavy chain was increased in the prediabetic (1.8-fold, p<0.05) and tended to increase in the diabetic men (1.6-fold, p = 0.07), compared to healthy lean subjects.
Conclusion
Muscle histidine-containing dipeptides increases with progressive glucose intolerance, in male individuals (cross-sectional). In addition, high-fat diet-induced glucose intolerance was associated with increased muscle histidine-containing dipeptides in female mice (interventional). Increased muscle carnosine content might reflect fiber type composition and/or act as a compensatory mechanism aimed at preventing cell damage in states of impaired glucose tolerance.
Introduction
Histidine-containing dipeptides (HCD) are peptides consisting of a histidine (or a methylated form of histidine) and the atypical amino acid beta-alanine [1]. HCD are predominantly and abundantly present in skeletal muscle, but they are also measurable in other tissues such as brain, kidney and liver, although in concentrations 10- to 1000-fold lower [1–4]. Human skeletal muscles only possess the HCD carnosine (beta-alanyl-L-histidine), whereas rodent muscles contain carnosine along with its methylated variant anserine (beta-alanyl-N-π-methylhistidine) [2].
Carnosine supplementation has recently been associated with a delayed progression of type 1 and 2 diabetes in rodents [5–8]. The mechanism has been attributed to its biochemical property to quench and scavenge damaging species such as reactive oxygen species [9], metal-ions [10], protein carbonyls [11] and reactive carbonyl species (RCS) such as 4-hydroxy-2-nonenal (HNE) [12] and acrolein [13]. Aldini et al. (2011) was the first to report that obese Zucker rats have higher levels of carnosine-HNE adducts in urine compared to their lean counterparts, which points to HNE overproduction in the obese animals and confirms the role of carnosine as an endogenous detoxifying agent of RCS such as HNE [14]. In addition, Baba et al. (2013) confirmed the presence of carnosine-RCS conjugates (HNE and acrolein) in urine from normal, healthy, non-smoking adults and also demonstrated its existence in skeletal muscle of C57BL/6 mice [15]. Interestingly, anserine has similar characteristics as carnosine [2], although less extensively investigated.
Because of these scavenging characteristics, it is suggested that carnosine levels in diabetic tissues are decreased, as it has been shown that diabetic tissues have high production of reactive oxygen and carbonyl species [16]. Indeed, decreased carnosine and anserine levels are demonstrated in retina, kidney and liver of obese and diabetic (type 1 and 2) rodents [4,6,7,17]. However, for skeletal muscle, contradiction exists. In rodents, there is only one paper available which shows decreased carnosine levels in diaphragm muscle of STZ-induced diabetic rats [18]. In humans, Gualano et al. [19] reported reduced carnosine content (-45%) in gastrocnemius muscle in type 2 diabetic patients, but not in soleus muscle nor in type 1 diabetic patients, compared to control subjects matched for age, BMI, gender and diet. However, another study [20] demonstrated an increase in carnosine content in soleus muscle in drug naive type 2 diabetic patients compared to non-diabetic controls.
Although evidence is scarce, a new research topic about supplementing carnosine in humans to combat age related chronic diseases i.e. diabetes and cardiovascular diseases is emerging because of its physiological and scavenging properties. Beta-alanine may be used as an alternative [19,21,22], because it is the rate-limiting precursor for carnosine synthesis in muscle [23]. However, given the conflicting findings in literature concerning muscle carnosine content in a diabetic state, we investigated whether muscle carnosine content is indeed decreased in a diabetic state. We investigated this by feeding rodents various degrees of high-fat diets inducing various degrees of glucose intolerance (interventional study) and by comparing muscle carnosine content in humans with different degrees in glucose tolerance (cross-sectional study). Moreover, as it is known that exercise can reverse metabolic stress in skeletal muscle [24,25], the additional effect of daily endurance training (rodent study) or physical activity (human study), will be examined.
Materials and Methods
Study Design
Rodent study (intervention study). Skeletal muscles of rats and mice were collected from 4 different diet-intervention studies, aiming to induce various degrees of glucose intolerance. The specifications of the animals and the diets are presented in Table 1. Shortly, rodents were fed with a 45% high-fat diet, a 60% high-fat diet, a cafeteria diet or a 70% high-fat diet, and they were compared with their own control group (receiving a control diet). In the HF 70%study, an additional group was added to assess the effect of exercise, namely HF 70% diet in combination with endurance training (12 m/min for 60 min per day, 5 days a week during 6 weeks). All rodents were housed at 22°C in a 12 h light/dark cycle and were given ad libitum access to their water and specific diet.
Table 1. Rodent study: Specifications of the animals and their diets.
| Energy content of the diet (% total kJ) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Species | Strain | Gender | Age at sacrifice | Intervention period | Macro-nutrients | CON | HF/CAF |
| Rat studies | ||||||||
| HF 45% | Rats | Wistar | M | 18 weeks | 14 weeks | Total CHO | 70 | 36 |
| Total fat | 10 | 45 (mainly lard) | ||||||
| Total protein | 20 | 19 | ||||||
| HF 60% | Rats | Sprague-Dawley | M | 12 weeks | 8 weeks | Total CHO | 70 | 20 |
| Total fat | 10 | 60 (mainly lard) | ||||||
| Total protein | 20 | 20 | ||||||
| CAF | Rats | Wistar | M | 18 weeks | 12 weeks | Total CHO | 63 | 69 (mainly sugar) |
| Total fat | 12 | 16 | ||||||
| Total protein | 25 | 15 | ||||||
| Mouse study | ||||||||
| HF 70% | Mice | C57BL/6J | F | 15 weeks | 6 weeks | Total CHO | Standard chow | <1 |
| Total fat | 72 (lard + corn oil) | |||||||
| Total protein | 28 | |||||||
Abbreviations: M: male, F: female, CHO: carbohydrates, CON: control group, HF: high-fat group, CAF: cafeteria group.
In all rodent studies, body weight was recorded during the intervention study and glucose intolerance was measured at the end of the intervention (cfr infra for the description of the glucose tolerance tests). Thereafter, all animals were sacrificed by an intra-peritoneal injection of sodium pentobarbital solution and muscles were dissected for the determination of muscle HCD (carnosine and anserine). In the rat studies, the white gastrocnemius (GW) and the red gastrocnemius (GR) were dissected because of a different muscle fiber type distribution [26]. In the mouse study, the gastrocnemius was taken as a whole (GAS), because it is difficult to make a visual distinction between the GR and GW in mice. The muscle samples were frozen in liquid nitrogen and stored at −80°C until HCD content analysis. All rodent studies were approved by the local animal Ethical Committee (the rat studies by KU Leuven: permit number P037/2010, the mouse study by Université catholique de Louvain: permit number LA 1220548). All surgery was performed under anesthesia (cfr. Infra) and all efforts were made to minimize suffering.
Human study (cross-sectional study). The study population consisted of 35 sedentary non-vegetarian males with different degrees of body weight and glucose intolerance: 9 healthy lean, 8 obese with normal glucose tolerance, 9 prediabetic (preT2D) and 9 newly diagnosed drug naïve type 2 diabetic men (T2D). Prediabetes (impaired glucose tolerance and/or impaired fasting glucose) and type 2 diabetes were diagnosed by fasting glycemia or 2 hours OGTT (oral glucose tolerance test) as defined by the American Diabetes Association 2006 [27]. Individuals were age-matched across the groups, with a mean age of 45 ± 7 yrs. The obese, preT2D and T2D were BMI-matched across the groups. The samples of m. vastus lateralis were obtained by the Bergstrom needle biopsy. Muscle samples were immediately frozen in liquid nitrogen and stored at −80°C until gene expression and carnosine content analyses. Fasting insulinemia was measured by IRMA (Immunotech, France). Daily free living ambulatory activity was monitored by accelerometers (Lifecorder Plus, Kenz, USA) during three consecutive working days. Medium and high intensity ambulatory activity was defined as an activity with the energy requirements exceeding 3-times the resting energy expenditure (>3 REE) [28].
The study was approved by the Ethics Committee of the University Hospital Bratislava, Comenius University Bratislava and the Ethics Committee of the Bratislava Region Office and it conforms to the ethical guidelines of the Helsinki declaration of 1964, as revised in 2000. All participants provided witnessed written informed consent prior entering the study.
Glucose tolerance tests
Rodent study. The intravenous glucose tolerance tests (IVGTT) in the rat studies (the HF 45%, HF 60% and CAF-study) were performed according to Vaisy et al. (2011)[29]. Briefly, rats were anaesthetized by an intraperitoneal injection of a ketamine-xylazine mixture and surgically prepared for the IVGTT, which involved catheter insertion into the left jugular vein. After an overnight fast (16–18 h) a glucose solution (1g glucose.kg-1 body weight using a 30% w.v.-1 glucose solution in w.v-1 saline) was injected into the catheter of the conscious rats and blood glucose and plasma insulin concentrations were measured at regular intervals. Total AUCglucose and AUCinsulin were calculated using the trapezoidal rule.
The oral glucose tolerance tests (OGTT) from the mouse study is described in Deldicque et al. (2013) [30]. Plasma glucose was determined on regular intervals following oral glucose gavage (glucose 1 mg/g body weight), plasma insulin was only determined 30 min before and 15min after glucose gavage. Total AUC for glucose (AUCglucose) was calculated using the trapezoidal rule.
Quantification of muscle carnosine, anserine and total HCD
HCD (carnosine and anserine) were quantified by means of reversed-phase HPLC (high-performance liquid chromatography). Skeletal muscles were dissolved in phosphate buffer (rat studies: 1mg dw muscle/100μL PBS; mouse study: 10mg ww muscle/100 μLPBS; human study: 1mg ww muscle/15 μL PBS) for homogenization. Muscle homogenates were deproteinized using 35% sulfosalicylic acid (SSA) and centrifuged (5min, 14000g). 100μL of deproteinized supernatant was dried under vacuum (40°C). Dried residues were resolved with 40μL of coupling reagent: methanol/triethylamine/H2O/phenylisothiocyanate (PITC) (7/1/1/1) and allowed to react for 20 minutes at room temperature. The samples were dried again and resolved in 100μL of sodium acetate buffer (10mM, pH 6.4). The same method was applied to the combined standard solutions of carnosine (Flamma) and anserine (Sigma). The derivatized samples (20 μL) were chromatographed on a Waters HPLC system with ODS2 guard column (80Å, 5 μm, 4.6 mm X 10 mm), a Spherisorb C18/ODS2 column (4.6 x 150 mm, 5 μm), and UV detector (wavelength: 210 nm). The columns were equilibrated with buffer A (10 mM sodium acetate adjusted to pH 6.4 with 6% acetic acid) and buffer B (60% acetonitrile-40% buffer A) at a flow rate of 0.8 ml/min at 25°C. The limit of quantification of muscle homogenates was 7 μM (~0,7 mmol/kg DW muscle). Total HCD are the sum of muscle carnosine and anserine.
The gene expression analysis
Gene expression analysis of muscle fiber type markers MYH1 (encoding the protein MyHC ‘myosin heavy chain’ 2X, present in fast-glycolytic muscle fibers), MYH2 (encoding the protein MyHC 2A, present in fast-oxidative muscle fibers) and MYH7 (encoding the protein MyHC-β/slow, present in slow-oxidative muscle fibers) was performed in m. vastus lateralis. Total RNA from skeletal muscle was isolated using TriReagent (Molecular Research Center, Inc., USA), purified with RNeasy mini Kit (Qiagen, USA) and DNAse treated (Qiagen, USA). Gene expression was measured by the qRT-PCR with ABI7900HT (Applied Biosystems, USA), using following set of primers designed with the PrimerExpress software (Applied Biosystems, USA): MYH1 (FWD: 5´- TAA GAC CGA GGC AAA AAG GA-3´ REV: 5´- TGC ATC AGC CAA GCT GTC-3´); MYH2 (FWD: 5´-TGT CTC ACT CCC AGG CTA CA-3´ REV: 5´- CCA AAA ACA GCC AAT TCT GAG-3´); MYH7 (FWD: 5´-CTT CGT GCC TGA TGA CAA ACA-3´ REV: 5´- CAC GGT CAC TGT CTT GCC ATA-3´) and Maxima SYBRgreen/ROX gPCR master mix (Fermentas Thermo Scientific, USA). B-2-microglobulin (B2m), Ribosomal protein L13a (Rpl13a) & Hypoxanthine phosphoribosyltransferase 1 (HPRT1) were stably expressed across the experimental groups and therefore used as the set of internal reference genes to calculate dCt expression values. HPRT1 was determined with the aid of Taq-man gene expression assay (Hs03929098_m1, Applied Biosystems, USA) while B2m (fwd: CGCTCCGTGGCCTTAGC; rev: AATCTTTGGAGTACGCTGGATAGC) and Rpl13a (fwd: GGACCGTGCGAGGTATGCT; rev: ATGCCGTCAAACACCTTGAGA) with the above specified set of primers. PCR efficiency for the genes of interest and reference genes was tested and optimized before the real time PCR experiment.
Statistics
Rodent study. To compare body weight, AUCglucose, AUCinsulin, muscle carnosine, anserine and HCD between the HF/CAF groups (HF 45%, HF 60%, CAF or HF 70%) and their own control group, data were analyzed by an independent t-test. To compare muscle carnosine, anserine and HCD between the 3 groups (CON, HF 70% and HF 70%+ex) in the HF 70% study, one-way ANOVA was performed. When a significant group effect was shown, a post hoc LSD test was performed. The pearson correlation coefficient was calculated to explore the relationship between muscle HCD and glucose tolerance (bloodglucose during OGTT/IVGTT).
Human study. To compare muscle carnosine and gene expression levels between the 4 groups (lean, obese, preT2D and T2D), one-way ANOVA was performed. When a significant group effect was shown, a post hoc LSD test was done. All correlations were evaluated by Pearson correlations. All analyses (human and rodent) were done with SPSS statistical software (SPSS 20, Chicago, IL) and statistical significance was set at p < 0.05.
Results
Body weight and glucose tolerance
Rodent study (Table 2). In the HF 45% study, body weight tended (p = 0.08) to be higher in HF compared to CON, but glucose tolerance (AUCglucose and AUCinsulin) was not significantly altered. In the 60% HF study, body weight and AUCinsulin were higher in HF (+ 12% and + 71% respectively, p<0.05) compared to CON, and AUCglucose tended to be higher (+ 22%, p = 0.054). In the CAF study, body weight, AUCglucose and AUCinsulin were all higher in HF compared to CON (+ 12%, 25% and 139% respectively, p<0.05). In the HF 70% study, both body weight and AUCglucose were higher in HF compared to CON (+ 14% and + 81% respectively, p<0.05). In exercised high-fat diet mice (HF 70%+ex), body weight was normalized back to levels of CON mice, however, AUCglucose did not decrease by exercise. Plasma insulin in fasted state and at 15 min after the OGTT was not different between the three groups.
Table 2. Rodent study: Degree of obesity and glucose tolerance for the 4 diet-interventions.
| Study | Obesity | Glucose tolerance test | |||||
|---|---|---|---|---|---|---|---|
| Delta body weight (g) | End body weight (g) | Total AUC for glucose | Total AUC for insulin | ||||
| mmol.l-1.min | % increase | ng.ml-1.min | % increase | ||||
| Rat studies | |||||||
| HF 45% | CON (n = 8) | 323 ± 32 | 430 ± 34 | 999 ± 138 | 463 ± 134 | ||
| HF (n = 7) | 377 ± 61 * | 480 ± 69 $ | 1038 ± 127 | 4 | 533 ± 246 | 15 | |
| HF 60% | CON (n = 9) | 334 ± 48 | 486 ± 57 | 1144 ± 186 | 171 ± 74 | ||
| HF (n = 9) | 390 ± 44 * | 545 ± 47 * | 1397 ± 305 $ | 22 | 293 ± 139 * | 71 | |
| CAF a | CON (n = 11) | 255 ± 14 | 490 ± 47 | 579 ± 29 | 185 ± 28 | ||
| CAF (n = 11) | 305 ± 10* | 548 ± 55 * | 721 ± 44 * | 25 | 443 ± 39 * | 139 | |
| Mouse study | |||||||
| HF 70%a | CON (n = 8) | 3.9 ± 1.0 | 21.6 ± 1.9 | 168 ± 68 | |||
| HF (n = 8) | 6.1 ± 1.1 * | 23.1 ± 1.5 | 304 ± 127 * | 81 | not available | ||
| HF + ex (n = 6) | 4.7 ± 1.3 ** | 20.7 ± 1.4** | 280 ± 71 * | 67 | |||
Values are expressed as mean ± SD.
* p<0.05 versus CON,
** p<0.05 HF+ex versus HF and
$ p<0.1 versus CON.
Underlined % increase (compared to CON) are significant with p<0.05.
aData from the CAF and HF 70% study are obtained from respectively Vaisy et al. (27) and Deldicque et al.(28).
Abbreviations: CON: control group, HF: high-fat group, CAF: cafeteria group, HF+ex: high-fat+exercise group.
Human study. Body weight and BMI of the lean subjects was significantly lower compared to the other three groups (body weight: lean: 78±10, obese: 99±21, preT2D: 103±8, T2D: 97±12 kg; BMI: lean: 25±1, obese: 29±3, preT2D: 32±2, T2D: 31±3 kg/m2).
Muscle HCD and muscle fiber type markers
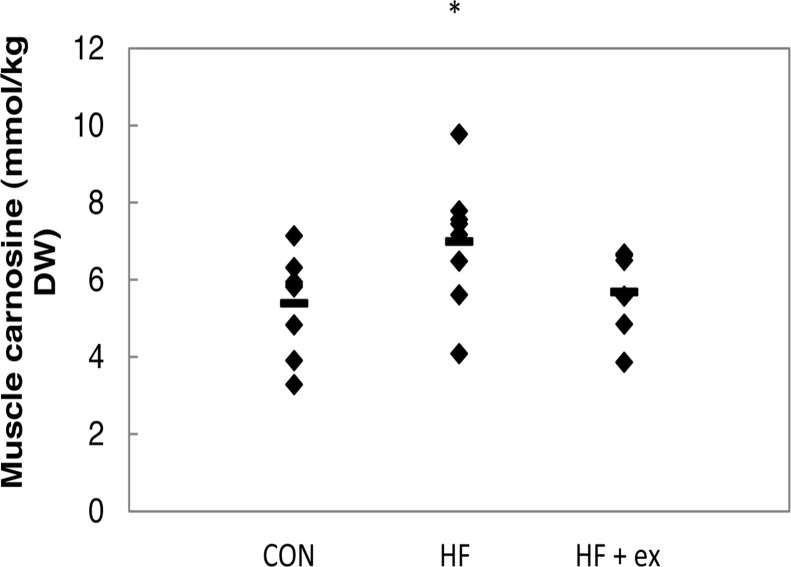
Rodent study (Table 3). In the HF 45% and HF 60% study, neither muscle carnosine, nor anserine, nor the sum (total HCD) was altered by high-fat feeding (for both muscle types GR and GW). Carnosine in GW muscle correlated positively with the AUC of blood glucose during the OGTT (r = 0.542, p<0.05) in the HF 45% study, but not in the HF 60% study or in the GR muscle. In the CAF study, muscle anserine and total HCD had tendency to increase in GW by 14% (p = 0.085) and 12% (p = 0.076), respectively. No changes were reported for muscle carnosine and GR and no correlation was found between muscle carnosine and glucose tolerance. In the HF 70% study, muscle carnosine significantly increased with 30% (p<0.05) and muscle HCD tended to increase (+21%) in HF compared to CON (p = 0.095) (Fig. 1). Muscle anserine followed a similar pattern, but it was not significant. Daily exercise training (HF 70%+ex) seemed to counteract the high-fat diet-induced increase in muscle carnosine (Fig. 1), anserine and total HCD compared to HF. Muscle carnosine (of the CON and HF mice) correlated positively with the AUC for glucose during the OGTT (r = 0.494, p = 0.052). In addition, muscle carnosine tended to correlate with blood glucose at every time point during the OGTT (fasting: r = 0.478, p = 0.061; after 15 min: r = 0.463, p = 0.071; after 30 min: r = 0.485, p = 0.057; after 60 min: r = 0.514, p<0.05, after 90 min: r = 0.553, p<0.05; after 120 min: r = 0.353, p = 0.18). Anserine did not correlate with glucose intolerance in neither of the rodent studies.
Table 3. Rodent study: Muscle carnosine, anserine and total histidine-containing dipeptides after the diet-intervention.
| Carnosine | Anserine | HCD | |||||
|---|---|---|---|---|---|---|---|
| mmol/kg DW | mmol/kg DW | mmol/kg DW | |||||
| Study | Group | GW | GR | GW | GR | GW | GR |
| Rat studies | |||||||
| HF 45% | CON (n = 8) | 19.1 ± 4.1 | 9.2 ± 2.1 | 34.6 ± 2.4 | 29.2 ± 7.6 | 53.7 ± 3.6 | 38.5 ± 7.8 |
| HF (n = 7) | 18.2 ± 4.0 | 8.3 ± 1.4 | 35.7 ± 6.5 | 29.4 ± 5.4 | 53.9 ± 9.3 | 37.7 ± 6.0 | |
| HF 60% | CON (n = 9) | 13.7 ± 3.6 | 5.9 ± 2.0 | 38.3 ± 4.3 | 25.4 ± 7.0 | 52.0 ± 4.9 | 31.3 ± 7.3 |
| HF (n = 9) | 12.8 ± 2.0 | 4.9 ± 2.0 | 37.7 ± 4.5 | 21.8 ± 4.4 | 50.5 ± 4.7 | 26.7 ± 4.1 | |
| CAF | CON (n = 11) | 9.7 ± 1.8 | 7.1 ± 0.9 | 30.4 ± 5.1 | 24.0 ± 4.0 | 40.1 ± 6.4 | 31.1 ± 4.9 |
| CAF (n = 11) | 10.5 ± 2.3 | 7.9 ± 1.3 | 34.6 ± 5.7 $ | 23.3 ± 3.1 | 45.1 ± 6.2 $ | 31.2 ± 3.9 | |
| Mouse study | |||||||
| GAS | GAS | GAS | |||||
| HF 70% | CON (n = 8) | 5.4 ± 1.3 | 11.1 ± 2.4 | 16.4 ± 3.6 | |||
| HF (n = 8) | 7.0 ± 1.7 * | 12.9 ± 2.5 | 19.9 ± 4.1 $ | ||||
| HF+ex (n = 6) | 5.7 ± 1.1 | 10.7 ± 1.7 $$ | 16.4 ± 2.6 $$ | ||||
Values are expressed as mean ± SD.
* p< 0.05 HF versus CON,
$ p< 0.1 HF vs CON and
$$ p<0.1 HF+ex vs HF.
Abbreviations: CON: control group, HF: high-fat group, HF+ex: high-fat+exercise group, DW: dry weight, GW, GR and GAS represent the white, the red and the gastrocnemius as a whole, respectively.
Fig 1. Rodent study: The effect of a 70% high-fat diet on muscle carnosine in female mice.
Diamonds and stripes represent individual and group values, respectively.* p<0.05 versus CON. Abbreviations: CON: control group, HF: high-fat diet group, HF+ex: high-fat diet+exercise group.
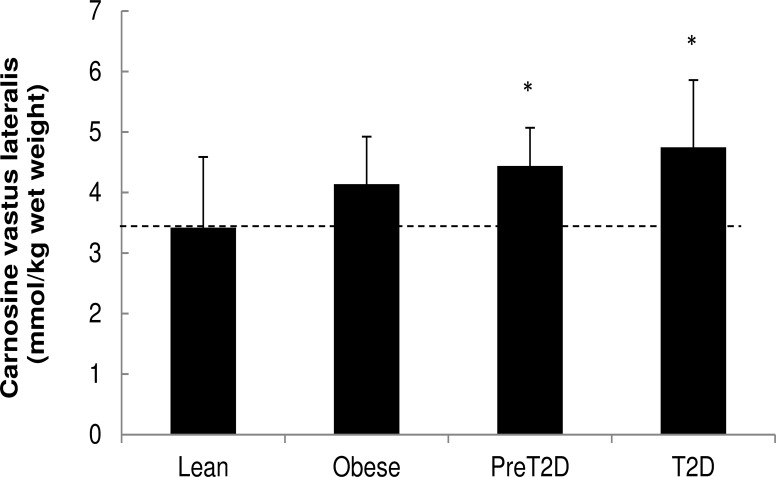
Human study. Muscle carnosine gradually increased with obesity and progressive glucose intolerance. Muscle carnosine of the obese, preT2D and T2D is higher [+21% (p>0.1), +30% (p<0.05) and +39% (p<0.05), respectively], compared to the lean (Fig. 2). Muscle carnosine correlated negatively with the level of medium and high intensity free living ambulatory activity as monitored by accelerometers (r = -0.41, p<0.05). The gene expression analysis of muscle fiber type markers indicated a shift in muscle fiber type composition, with a significant increase in fast-oxidative type 2A fibers (MYH2 mRNA) and a similar trend for fast-glycolytic type 2X fibers (MYH1 mRNA) in muscle of prediabetic and T2D individuals compared to the healthy lean subjects (MYH2 mRNA: obese: 1.1-fold (p>0.1), preT2D: 1.8-fold (p<0.05), T2D: 1.6-fold (p = 0.07) over lean and MYH1 mRNA: obese: 2.2-fold (p>0.1), preT2D: 2.7-fold (p = 0.07), T2D: 2.5-fold (p>0.1) over lean). In addition, expression of MYH1 gene (marker of the fast-glycolytic type 2X muscle fiber content) was positively associated with fasting insulinemia (r = 0.527, p = 0.002). On the other hand, the lack of differences in MYH7 mRNA between the 4 groups indicates that slow-oxidative type I muscle fiber content does not vary with obesity, prediabetes or type 2 diabetes. Levels of muscle carnosine were not associated with the variability in gene expression of muscle fiber type markers (data not shown).
Fig 2. Human study: Muscle carnosine content in lean, obese, prediabetic and diabetic type 2 patients.
Values are expressed as mean ± SD. * p<0.05 versus lean. ANOVA p-value: 0.038. Abbreviations: preT2D: prediabetic patients, T2D: diabetic type 2 patients.
Discussion
It has been suggested that carnosine levels in diabetic tissues are decreased because carnosine can form adducts with reactive carbonyls species such as HNE and acrolein [14,15], which are abundantly present in diabetic tissues [25]. Indeed, a decrease in carnosine content was reported in retina, kidney and liver of diabetic rodents [6,7,17]. However, literature about carnosine levels in diabetic muscle was contradictive [18–20]. Therefore, we have now examined the effect of different degrees of glucose intolerance on muscle HCD (carnosine and anserine) in both rodents (intervention studies) and men (a cross-sectional study).
The current paper shows that muscle HCD content does not decrease, but instead increases with obesity and progressive glucose intolerance in male individuals. In addition, high-fat diet-induced glucose intolerance was associated with increased muscle HCD in female mice. The observation that muscle tissue responds differently in a diabetic state compared to tissues such as retina, kidney and liver, is probably related to the fact that the enzyme carnosine synthase is considerably more expressed in skeletal muscle compared to the other tissues [31,32], whereby restoration of initially decreased muscle carnosine levels is more easily achieved. Yet, it is possible that a compensation mechanism is activated, ultimately leading to net increased carnosine levels.
In search for an alternative explanation for this unexpected finding, we looked at the known determinants of muscle carnosine content and whether or not glucose intolerance could play an interfering role. In addition to age and sex, muscle fiber type is one of the most important determinants of muscle carnosine content, both in rodents and humans [33–37]. The fast type 2 muscle fibers (both the fast-glycolytic as well as the fast-oxidative muscle fibers) possess almost a double amount of carnosine compared to the oxidative type I muscle fibers [38]. As several studies have shown that obesity and type 2 diabetes are associated with an increased proportion of fast glycolytic type 2 fibers and a reduced percentage of slow type 1 fibers compared with lean healthy individuals, it is plausible to speculate that a shift towards a faster fiber type might contribute to the higher carnosine content in type 2 diabetic muscle.
There are some indications in this paper that support our fiber-type explanation. First, both our rodent and human study demonstrated that aerobic exercise (mice) or physical activity (human), which is known to stimulate or preserve oxidative type 1 fibers [39], counteracted the increase in muscle carnosine. However, it can also be related to the fact that exercise prevents the formation of reactive carbonyl species in skeletal muscle [25], whereby the compensation mechanism to increase muscle carnosine will not be activated. Second, a tendency towards increased gene expression of MYH1 (encoding the protein myosin heavy chain 2X, present in fast-glycolytic type 2 fibers) and MYH2 (encoding the protein myosin heavy chain 2A, present in fast-oxidative type 2 fibers) in individuals with prediabetes or type 2 diabetes suggests a higher proportion of more fast muscle fibers (fast-glycolytic and fast-oxidative) in patients with metabolic disease. Third, the fact that the increase in muscle HCD was only clearly present in mice receiving the extreme 70% high-fat diet, and not in the rats receiving a 45–60% high-fat diet or a cafeteria diet, is perhaps due to the fact that fiber type proportions can change throughout the diet intervention. In the beginning of a high-fat diet intervention, muscle fiber type changes in favor of the oxidative metabolism, including increased type I fibers, in an attempt to remove lipid overload. On the other hand, towards the end of the diet, when glucose intolerance increases, the percentage of fast-glycolytic type 2 fibers increases [40].
But why did Gualano et al. (2012) found a decrease in muscle carnosine in Brazilian T2D patients? Compared to the younger, newly diagnosed drug naïve male T2D subjects in this study, the Brazilian diabetic group is represented by both older (± 60 years) males and females, with longer duration of the disease (± 7 years), receiving oral antidiabetic pharmacotherapy (metformin and/or sulfonylurea). In both studies, patients with micro- and macrovascular complications were excluded. It can be speculated that the compensation mechanism, which is responsible for the initial increase in muscle carnosine, is exhausted by the longer duration of the disease and older age. In other words, it can be expected that carnosine would be largely used to scavenge reactive carbonyl species in the older diabetic muscle, resulting in a net decrease in muscle carnosine content as compared to muscles of younger, drug-naïve newly diagnosed diabetic patients.
Finally, in this work, we demonstrated that muscle HCD content increases with obesity and progressive glucose intolerance in male subjects. So, it can be questioned whether this observation would also occur in females. We cannot ignore that the carnosine metabolism is partly gender dependent. Muscle carnosine storage and serum carnosinase (the enzyme that hydrolyses carnosine) is respectively lower and higher in women than in men [41,42]. However, determinants of muscle carnosine storage (such as age and fiber type) and muscle carnosine loading (by beta-alanine supplementation) are gender independent [34,43]. In addition, a 70% high-fat diet increased muscle carnosine in female mice. We suggest we can extrapolate this to female humans. Although rodents do not have the enzyme serum carnosinase and although they possess next to carnosine also the methylated variant anserine, it has previously been shown that muscle HCD storage in rodents and humans is quite similar. Both species share the similar determinants of muscle HCD content (age, sex, fiber type, beta-alanine supplementation)[23,33–37].
Both an increase and decrease in muscle carnosine (or anserine) with prediabetes or type 2 diabetes were reported in existing literature [19,20]. However, our results demonstrate a gradual increase in muscle HCD with progressive glucose intolerance in male individuals. In addition, high-fat diet-induced glucose intolerance is associated with increased muscle HCD in female mice. We underscore the importance of future studies aiming to better understand the metabolic pathways and the (patho)physiological significance of HCD in muscle in particular and in the body in general, in order to optimally design therapeutic strategies interfering with the carnosine system in metabolic diseases.
Acknowledgments
We greatly acknowledge the work of Monique Ramaekers, Bram Stegen and Peter Hespel for their assistance during the glucose tolerance tests (rodent study) as well as Timea Kurdiova, Miroslav Balaz, Marek Vician, Alica Mitkova, Daniela Gasperikova & Iwar Klimes for their versatile and enthusiastic help in human study. We also thank the volunteers for participating in the study.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by: 1) The Research Foundation—Flanders (FWO G035213N and G024311: http://www.fwo.be/en/), Wim Derave; 2) Grants from the Scientific Grant Agency of the Slovak Academy of Sciences (VEGA 2/0192/14 and 2/0174/12: http://erawatch.jrc.ec.europa.eu), Barbara Ukropcova and Jozef Ukropec; and 3) EFSD New Horizons Collaborative Research Initiative 2010, Barbara Ukropcova and Jozef Ukropec. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Boldyrev AA, Severin SE. The histidine-containing dipeptides, carnosine and anserine: distribution, properties and biological significance. Adv Enzyme Regul. 1990;30: 175–194. [DOI] [PubMed] [Google Scholar]
- 2. Boldyrev AA, Aldini G, Derave W. Physiology and pathophysiology of carnosine. Physiol Rev. 2013;93: 1803–1845. 10.1152/physrev.00039.2012 [DOI] [PubMed] [Google Scholar]
- 3. Kamal MA, Jiang H, Hu Y, Keep RF, Smith DE. Influence of genetic knockout of Pept2 on the in vivo disposition of endogenous and exogenous carnosine in wild-type and Pept2 null mice. Am J Physiol Regul Integr Comp Physiol. 2009;296: R986–R991. 10.1152/ajpregu.90744.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peters V, Schmitt CP, Zschocke J, Gross ML, Brismar K, Forsberg E. Carnosine treatment largely prevents alterations of renal carnosine metabolism in diabetic mice. Amino Acids. 2011;42: 2411–2416. 10.1007/s00726-011-1046-4 [DOI] [PubMed] [Google Scholar]
- 5. Sauerhofer S, Yuan G, Braun GS, Deinzer M, Neumaier M, Gretz N et al. L-carnosine, a substrate of carnosinase-1, influences glucose metabolism. Diabetes. 2007;56: 2425–2432. [DOI] [PubMed] [Google Scholar]
- 6. Pfister F, Riedl E, Wang Q, vom HF, Deinzer M, Harmsen MC et al. Oral carnosine supplementation prevents vascular damage in experimental diabetic retinopathy. Cell Physiol Biochem. 2011;28: 125–136. 10.1159/000331721 [DOI] [PubMed] [Google Scholar]
- 7. Riedl E, Pfister F, Braunagel M, Brinkkotter P, Sternik P, Deinzer M et al. Carnosine prevents apoptosis of glomerular cells and podocyte loss in STZ diabetic rats. Cell Physiol Biochem. 2011;28: 279–288. 10.1159/000331740 [DOI] [PubMed] [Google Scholar]
- 8. Soliman K, Mohamed A, Metwally N. Attenuation of some metabolic deteriorations induced by diabetes mellitus using carnosine. Journal of Applied Sciences. 2007;7: 2252–2260. [Google Scholar]
- 9. Decker EA, Livisay SA, Zhou S. A re-evaluation of the antioxidant activity of purified carnosine. Biochemistry (Mosc). 2000;65: 766–770. [PubMed] [Google Scholar]
- 10. Mineo P, Vitalini D, La Mendola D, Rizzarelli E, Scamporrino E, Vecchio G. Electrospray mass spectrometric studies of L-carnosine (beta-alanyl-L-histidine) complexes with copper(II) or zinc ions in aqueous solution. Rapid Commun Mass Spectrom. 2002;16: 722–729. [DOI] [PubMed] [Google Scholar]
- 11. Hipkiss AR, Brownson C, Bertani MF, Ruiz E, Ferro A. Reaction of carnosine with aged proteins: another protective process? Ann N Y Acad Sci. 2002;959: 285–294. [DOI] [PubMed] [Google Scholar]
- 12. Carini M, Aldini G, Beretta G, Arlandini E, Facino RM. Acrolein-sequestering ability of endogenous dipeptides: characterization of carnosine and homocarnosine/acrolein adducts by electrospray ionization tandem mass spectrometry. J Mass Spectrom. 2003;38: 996–1006. [DOI] [PubMed] [Google Scholar]
- 13. Orioli M, Aldini G, Beretta G, Facino RM, Carini M. LC-ESI-MS/MS determination of 4-hydroxy-trans-2-nonenal Michael adducts with cysteine and histidine-containing peptides as early markers of oxidative stress in excitable tissues. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;827: 109–118. [DOI] [PubMed] [Google Scholar]
- 14. Aldini G, Orioli M, Rossoni G, Savi F, Braidotti P, Vistoli G et al. The carbonyl scavenger carnosine ameliorates dyslipidaemia and renal function in Zucker obese rats. J Cell Mol Med. 2011;15: 1339–1354. 10.1111/j.1582-4934.2010.01101.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baba SP, Hoetker JD, Merchant M, Klein JB, Cai J, Barski OA et al. Role of aldose reductase in the metabolism and detoxification of carnosine-acrolein conjugates. J Biol Chem. 2013;288: 28163–28179. 10.1074/jbc.M113.504753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414: 813–820. [DOI] [PubMed] [Google Scholar]
- 17. Mong MC, Chao CY, Yin MC. Histidine and carnosine alleviated hepatic steatosis in mice consumed high saturated fat diet. Eur J Pharmacol. 2011;653: 82–88. 10.1016/j.ejphar.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 18. Buse MG, Weigand DA, Peeler D, Hedden MP. The effect of diabetes and the redox potential on amino acid content and release by isolated rat hemidiaphragms. Metabolism. 1980;29: 605–616. [DOI] [PubMed] [Google Scholar]
- 19. Gualano B, Everaert I, Stegen S, Artioli GG, Taes Y, Roschel H et al. Reduced muscle carnosine content in type 2, but not in type 1 diabetic patients. Amino Acids. 2012;43: 21–24. 10.1007/s00726-011-1165-y [DOI] [PubMed] [Google Scholar]
- 20. Srikanthan P, Singhal A, Lee CC, Nagarajan R, Wilson N, Roberts CK et al. Characterization of Intra-myocellular Lipids using 2D Localized Correlated Spectroscopy and Abdominal Fat using MRI in Type 2 Diabetes. Magn Reson Insights. 2012;5: 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Del Favero S, Roschel H, Solis MY, Hayashi AP, Artioli GG, Otaduy MC et al. Beta-alanine (Carnosyn) supplementation in elderly subjects (60–80 years): effects on muscle carnosine content and physical capacity. Amino Acids. 2012;43: 49–56. 10.1007/s00726-011-1190-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sale C, Artioli GG, Gualano B, Saunders B, Hobson RM, Harris RC. Carnosine: from exercise performance to health. Amino Acids. 2013;44: 1477–1491. 10.1007/s00726-013-1476-2 [DOI] [PubMed] [Google Scholar]
- 23. Harris RC, Tallon MJ, Dunnett M, Boobis L, Coakley J, Kim HJ et al. The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids. 2006;30: 279–289. [DOI] [PubMed] [Google Scholar]
- 24. Kiens B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol Rev. 2006;86: 205–243. [DOI] [PubMed] [Google Scholar]
- 25. Samjoo IA, Safdar A, Hamadeh MJ, Raha S, Tarnopolsky MA. The effect of endurance exercise on both skeletal muscle and systemic oxidative stress in previously sedentary obese men. Nutr Diabetes. 2013;3: e88 10.1038/nutd.2013.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol. 1996;80: 261–270. [DOI] [PubMed] [Google Scholar]
- 27. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29 Suppl 1: S43–S48. [PubMed] [Google Scholar]
- 28. Kurdiova T, Balaz M, Vician M, Maderova D, Vlcek M, Valkovic L et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: in vivo and in vitro studies. J Physiol. 2014;592: 1091–1107. 10.1113/jphysiol.2013.264655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vaisy M, Szlufcik K, De Bock K, Eijnde BO, Van Proeyen K, Verbeke K et al. Exercise-induced, but not creatine-induced, decrease in intramyocellular lipid content improves insulin sensitivity in rats. J Nutr Biochem. 2011;22: 1178–1185. 10.1016/j.jnutbio.2010.10.004 [DOI] [PubMed] [Google Scholar]
- 30. Deldicque L, Cani PD, Delzenne NM, Baar K, Francaux M. Endurance training in mice increases the unfolded protein response induced by a high-fat diet. J Physiol Biochem. 2013;69: 215–225. 10.1007/s13105-012-0204-9 [DOI] [PubMed] [Google Scholar]
- 31. Miyaji T, Sato M, Maemura H, Takahata Y, Morimatsu F. Expression profiles of carnosine synthesis-related genes in mice after ingestion of carnosine or ss-alanine. J Int Soc Sports Nutr. 2012;9: 15 10.1186/1550-2783-9-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Everaert I, De Naeyer H, Taes Y, Derave W. Gene expression of carnosine-related enzymes and transporters in skeletal muscle. Eur J Appl Physiol. 2013;113: 1169–1179. 10.1007/s00421-012-2540-4 [DOI] [PubMed] [Google Scholar]
- 33. Harris R, Dunnett M, Greenhaff PL. Carnosine and taurine contents in individual fibres of human vastus lateralis muscle. J Sports Sci. 1998;16: 639–643. [Google Scholar]
- 34. Baguet A, Everaert I, Achten E, Thomis M, Derave W. The influence of sex, age and heritability on human skeletal muscle carnosine content. Amino Acids. 2012;43: 13–20. 10.1007/s00726-011-1197-3 [DOI] [PubMed] [Google Scholar]
- 35. Penafiel R, Ruzafa C, Monserrat F, Cremades A. Gender-related differences in carnosine, anserine and lysine content of murine skeletal muscle. Amino Acids. 2004;26: 53–58. [DOI] [PubMed] [Google Scholar]
- 36. Derave W, Jones G, Hespel P, Harris RC. Creatine supplementation augments skeletal muscle carnosine content in senescence-accelerated mice (SAMP8). Rejuvenation Res. 2008;11: 641–647. 10.1089/rej.2008.0699 [DOI] [PubMed] [Google Scholar]
- 37. Everaert I, Stegen S, Vanheel B, Taes Y, Derave W. Effect of Beta-Alanine and Carnosine Supplementation on Muscle Contractility in Mice. Med Sci Sports Exerc. 2013;45: 43–51. 10.1249/MSS.0b013e31826cdb68 [DOI] [PubMed] [Google Scholar]
- 38. Kendrick IP, Kim HJ, Harris RC, Kim CK, Dang VH, Lam TQ et al. The effect of 4 weeks beta-alanine supplementation and isokinetic training on carnosine concentrations in type I and II human skeletal muscle fibres. Eur J Appl Physiol. 2009;106: 131–138. 10.1007/s00421-009-0998-5 [DOI] [PubMed] [Google Scholar]
- 39. Thompson LV. Skeletal muscle adaptations with age, inactivity, and therapeutic exercise. J Orthop Sports Phys Ther. 2002;32: 44–57. [DOI] [PubMed] [Google Scholar]
- 40. Yasuda K, Nishikawa W, Iwanaka N, Nakamura E, Seino Y, Tsuda K et al. Abnormality in fibre type distribution of soleus and plantaris muscles in non-obese diabetic Goto-Kakizaki rats. Clin Exp Pharmacol Physiol. 2002;29: 1001–1008. [DOI] [PubMed] [Google Scholar]
- 41. Peters V, Kebbewar M, Jansen EW, Jakobs C, Riedl E, Koeppel H et al. Relevance of allosteric conformations and homocarnosine concentration on carnosinase activity. Amino Acids. 2010;38: 1607–1615. 10.1007/s00726-009-0367-z [DOI] [PubMed] [Google Scholar]
- 42. Everaert I, Mooyaart A, Baguet A, Zutinic A, Baelde H, Achten E et al. Vegetarianism, female gender and increasing age, but not CNDP1 genotype, are associated with reduced muscle carnosine levels in humans. Amino Acids. 2011;40: 1221–1229. 10.1007/s00726-010-0749-2 [DOI] [PubMed] [Google Scholar]
- 43.Stegen S, Bex T, Vervaet C, Vanhee L, Achten E, Derave W. The Beta-Alanine Dose for Maintaining Moderately Elevated Muscle Carnosine Levels. Med Sci Sports Exerc. 2014. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.