Abstract
The nematode Angiostrongylus cantonensis is the causative agent of human angiostrongyliasis, the main clinical manifestation of which is eosinophilic meningitis. Although this parasite has been found recently in its definitive rat host in Tenerife (Canary Islands, Spain), showing a widespread distribution over the north-east part of the island, there are no available data regarding which snail and/or slug species are acting as intermediate hosts on this island. Consequently, the objective of this work was to determine the possible role of three mollusc species, Plutonia lamarckii, Cornu aspersum and Theba pisana, as intermediate hosts of A. cantonensis in Tenerife. Between 2011 and 2014, 233 molluscs were collected from five biotopes where rats had been found previously to harbor either adult worms or antibodies against A. cantonensis, and the identification was carried out on the basis of morphological features and a LAMP technique. The prevalence of A. cantonensis larvae in the mollusc samples, based on morphological identification, was 19.3%, whereas 59 out of the 98 individuals (60.2%) analyzed by LAMP were positive. Positive results were obtained for the three mollusc species analyzed and two of the positive samples, both obtained from P. lamarckii, were confirmed as positive by 18S rRNA and ITS1 PCR. Sequence analysis of 18S rRNA PCR products showed 100% similarity with previously published A. cantonensis sequences. These results may be relevant from a public health point of view, since all the biotopes from which the samples were obtained were in inhabited areas or areas with human activity, but it is also important from the perspective of a possible transmission to other accidental hosts, such as dogs and horses, animals that are present in some of the areas analyzed.
Introduction
The nematode Angiostrongylus cantonensis is the causative agent of human angiostrongyliasis, which in its severe form is characterized by eosinophilic meningitis (or meningoencephalitis), with marked cerebrospinal fluid (CSF) eosinophilia [1]. The life cycle of this nematode involves rats and molluscs as definitive and intermediate hosts, respectively, whereas humans are accidental hosts infected through the consumption of raw or undercooked molluscs that contain the infective third stage larvae (L3) [2]. Once the nematode has been ingested by a person, it can reach the central nervous system or, less frequently, the eye, causing eosinophilic meningitis or ocular angiostrongyliasis, respectively [3]. Human angiostrongyliasis presents a broad clinical spectrum, from a mild disease to a form of eosinophilic meningitis or, uncommonly, encephalitis [4]. As a result, neurological damage and even death may occur, especially if prompt and proper treatment is not administered [5–7].
From its original range in southeastern China, A. cantonensis spread throughout many tropical and sub-tropical regions of the world during the 20th century [8,9]. This rapid geographical spread coincided with globalization and has probably been facilitated by the unintentional transport of infected hosts in ships and planes [10]. Once introduced into a new area, the nematode may easily establish itself in the local fauna since a large number of mollusc species can act as intermediate hosts and rats are ubiquitous [8].
Detection of A. cantonensis L3s in snails by digestion of snail tissues, followed by microscopic examination, is time-consuming and requires expertise in identifying A. cantonensis larvae [11]. Molecular detection using the polymerase chain reaction (PCR) has been used to circumvent these problems associated with morphological identification of Angiostrongylus worms. Genomic DNA suitable for PCR detection can be extracted from various types of material, including intact worms in all developmental stages, tissues from intermediate, definitive and paratenic hosts, and rat droppings [11]. A loop-mediated isothermal DNA amplification (LAMP) technique has also been used to detect A. cantonensis in invasive snail species [12,13] and presents several advantages when compared to PCR. For instance, the LAMP technique does not require special equipment; amplification of the target DNA can be performed in a water bath or heat block and the end-point analysis can be achieved by visual inspection directly in the reaction tube. The LAMP assay has already been applied to the detection and identification of other parasites of humans and animals [13].
A. cantonensis has been detected recently in black rats (Rattus rattus) in the Canary Islands [14], an archipelago very rich in gastropod species, among which many are endemic [15]. To our knowledge, this is the only location in Europe where the parasite has been detected. Nevertheless, there are no data regarding which snail and/or slug species are acting as intermediate hosts in the Canary Islands. Hence, the possible sources of human infection have not been identified until now. The aim of this study was to determine the possible role of three mollusc species, Plutonia lamarckii, Cornu aspersum and Theba pisana, as intermediate hosts of A. cantonensis in Tenerife.
Materials and Methods
Sampling areas
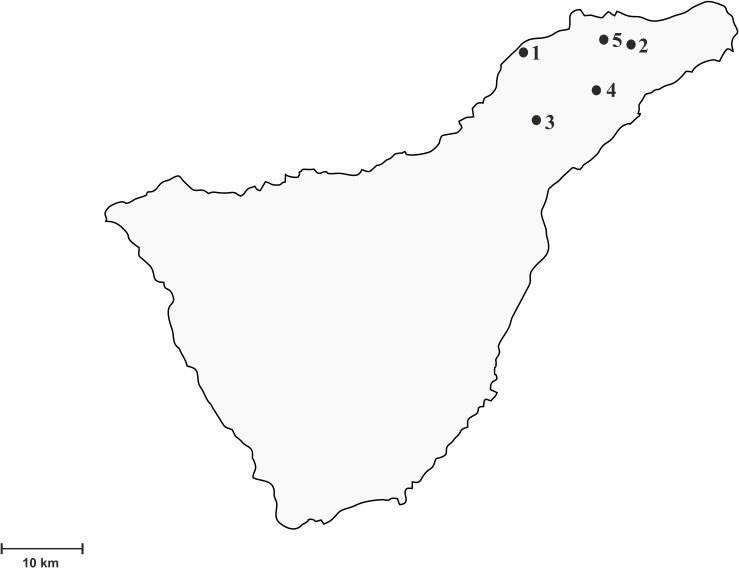
Between 2011 and 2014, 233 molluscs were collected at several locations in Tenerife. This island, with an area of 2068 km2, is the largest of the Canary Islands and of the Macaronesian region as a whole. The biotopes sampled in this study included three laurel forest areas (Pedro Álvarez, La Esperanza and Pico del Inglés) and two inhabited areas close to urban centers (La Laguna and El Pris) (Fig. 1). The mollusc species analyzed were the endemic semi-slug P. lamarckii and the snails T. pisana and C. aspersum (Fig. 2). Either adult worms of A. cantonensis or black rats carrying antibodies against A. cantonensis had been previously found in all the five locations [2].
Fig 1. Geographical distribution of the sampling areas in Tenerife.
1, El Pris; 2, Pico del Inglés; 3, La Esperanza; 4, La Laguna; 5, Pedro Álvarez.
Fig 2. Mollusc species included in the study.

Theba pisana (a), Cornu aspersum (b), Plutonia lamarckii (c).
Ethical statement
This field study did not involve endangered or protected species. Animal trapping in protected areas (Pedro Álvarez and Pico del Inglés) was approved by the Área de Medio Ambiente del “Excmo. Cabildo Insular” de Tenerife.
Morphological identification of Angiostrongylus cantonensis larvae
Tissue samples were cut from the posterior end of the mollusc's foot and placed in 1 ml of 0.01% pepsin-0.7% HCl in individual wells of a 24-well culture dish for digestion of the tissue. This method was combined with direct observation of mollusc tissues after mincing. Taxonomic identification of nematodes was based on morphological characters and morphometric parameters [16].
DNA extraction
Other individuals of the same species were used for molecular detection of A. cantonensis larvae. Tissue samples were cut from the posterior end of the mollusc’s foot and DNA was extracted by digestion with 4 mg proteinase K/ml in a buffer consisting of 30 mM Tris-HCl (pH 8), 0.4% SDS, and 10 mM EDTA overnight at 56°C, followed by incubation at 95°C for 10 min to inactivate the proteinase K. After having inactivated the proteinase K, DNA extraction continued by following the method used by López et al. [17].
LAMP method for the detection of A. cantonensis in mollusc tissue
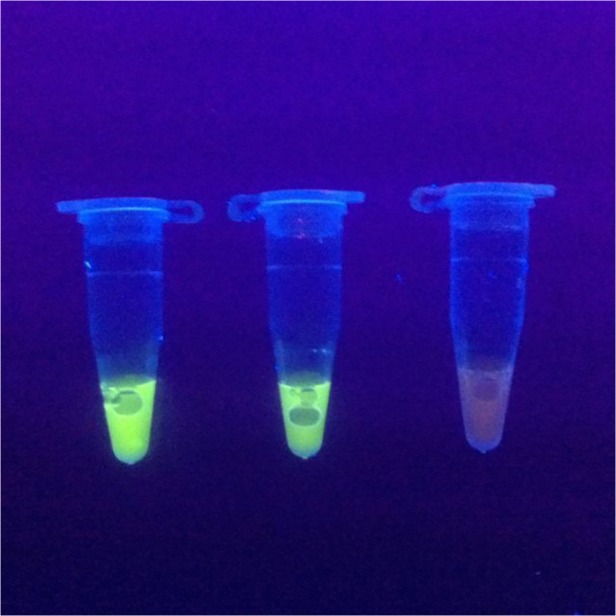
The LAMP assay was performed according to [13]. Briefly, the assay was carried out in a 25 μl reaction system containing 10x Bst-DNA polymerase buffer (3 μl), deoxynucleotide triphosphates (0.5 mM for each), MgSO4 (2 mM), a forward inner primer (FIP) (5′- CTCATCATCAACCACCCACCCCTAGCATCATCTACGTCGTC-3′) and a backward inner primer (BIP) (5′-AGAAACCACCAACACATATACACGTATACCACCAACTTTAGCGA-3′) (1.6 μM for each), loop-F (5′- GGGTGGTGATGTAGTAGCTA-3′) and loop-B (5′- TCACCTAGTGTATGATGGT-3′) (0.8 μM for each), 0.4 μM of outer primers F3(5′- CCACCACAAAACACAAACA-3′) and B3 (5′-GTGTTGAGCTCTAACGGT-3′), Bst DNA polymerase (8 U) (New England BioLabs) and DNA template (1 μl, approximately 30 ng). A reaction system with no DNA template was used as the negative control. The mixtures were incubated at 65°C for 45 min, and then heated at 80°C for 10 min to terminate the reaction. LAMP amplification results were visually detected under UV light after adding 2 μl of 20x EvaGreen I (Biotium) to the reaction tubes. Solutions turned green in the case of positive LAMP amplification; otherwise, they remained orange (Fig. 3).
Fig 3. Detection of Angiostrongylus cantonensis by LAMP reaction.
tube 1, DNA isolated from Plutonia lamarckii tissue; tube 2, DNA obtained from A. cantonensis adult worms; tube 3, negative control.
PCR amplification
DNA extracted from LAMP-positive tissue samples was amplified by conventional PCR targeting the 18S rRNA [18]. Briefly, the 50 μl PCR mixtures contained 0.4 μM of each primer (Angio-F and Angio-R) and 1.25 U of BioTaq DNA pol (Bioline). Amplification was carried out using the following cycling parameters: 95°C for 5 min, 45 cycles of 95°C for 15 s, 65°C for 15 s, and 72°C for 1 min, and 72°C for 10 min.
18S rRNA positive samples were confirmed to be infected with A. cantonensis by PCR targeting the first internal transcribed spacer (ITS1), which is comparatively more variable than the rRNA coding regions and therefore allows the identification of closely related species [19]. The ITS1 PCR was adapted from a real-time PCR assay [19], and it was conducted in a 50 μL reaction mixture containing 200 μM of each dNTP (Bioline), 1.5 mM MgCl2 (Bioline) 0.4 μM of each primer (AcanITS1F1 and AcanITS1R1) and 1 U of BioTaq DNA pol (Bioline). Amplification was performed using the following cycling parameters: 94°C for 2 min, 35 cycles of 94°C for 30 s, 62°C for 30 s, and 72°C for 30 s, and 72°C for 7 min.
Statistical analysis
Binary logistic regression was carried out to analyze the influence of the covariables (mollusc species and location) on the presence or absence of A. cantonensis (outcome variable), determined by LAMP. Odds ratios (ORs) and 95% confidence intervals (CI) were calculated for these associations. Interactions between covariables within the logistic model were also tested by adding an interaction variable for mollusc species and location. A probability value less than < 0.05 was considered as statistically significant. 95% CI for prevalence obtained with the LAMP technique were calculated.
Results
A. cantonensis larvae were present in 19.3% of the molluscs analyzed morphologically but in 60.2% (59/98) of those analyzed by LAMP (Table 1). Morphological features of third-stage larvae are illustrated in Fig. 4. All three species showed positive LAMP results, whereas A. cantonensis was detected by the morphological method only in T. pisana and P. lamarckii, although the sample size of C. aspersum specimens was limited. With regard to the results obtained by LAMP technique, the binary logistic regression showed that only the covariable mollusc species was significantly associated with the presence of A. cantonensis (P<0.01). More concretely, P. lamarckii specimens showed a more than 6-fold increased odd of being infected with A. cantonensis, when compared with T. pisana specimens (OR = 6.9, 95% CI 1.99–23.87, P<0.01). On the other hand, no significant interaction was found between the two covariables, mollusc species and location.
Table 1. Percentage prevalence of Angiostrongylus cantonensis larvae in molluscs and numbers of molluscs testing positive in samples from Tenerife, based on morphological (P) and molecular identification (P*).
| Area of study | Mollusc species | P (%) (+/n) | P* (%) (+/n) [95% CI] |
|---|---|---|---|
| El Pris | |||
| Theba pisana | 53.1 (17/32) | - | |
| Plutonia lamarckii | - | - | |
| Cornu aspersum | 0 (0/1) | - | |
| Total | 51.5 (17/33) | - | |
| Pico del Inglés | |||
| Theba pisana | 33.3 (1/3) | - | |
| Plutonia lamarckii | 0 (0/32) | 77.8 (7/9) [45.3–93.7] | |
| Cornu aspersum | 0 (0/5) | - | |
| Total | 2.5 (1/40) | 77.8 (7/9) [45.3–93.7] | |
| La Laguna | |||
| Theba pisana | 21.9 (25/114) | 35 (7/20) [18.1–56.7] | |
| Plutonia lamarckii | - | - | |
| Cornu aspersum | 0 (0/3) | 50 (9/18) [29–71] | |
| Total | 21.3 (25/117) | 42.1 (16/38) [27.9–57.8] | |
| La Esperanza | |||
| Theba pisana | 0 (0/18) | - | |
| Plutonia lamarckii | 0 (0/5) | 55.6 (10/18) [33.7–75.4] | |
| Cornu aspersum | - | - | |
| Total | 0 (0/23) | 55.6 (10/18) [33.7–75.4] | |
| Pedro Álvarez | |||
| Theba pisana | - | - | |
| Plutonia lamarckii | 10 (2/20) | 78.8 (26/33) [62.2–89.3] | |
| Cornu aspersum | - | - | |
| Total | 10 (2/20) | 78.8 (26/33) [62.2–89.3] | |
| Total | 19.3 (45/233) | 60.2 (59/98) [50.5–69.8] |
*n., number of molluscs studied; +, number of positive samples; P, prevalence; CI, confidence interval.
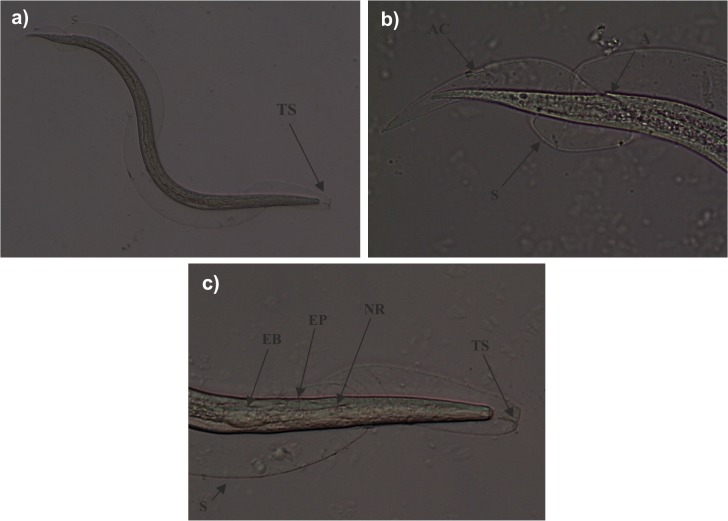
Fig 4. Light microscopy of third stage larvae (L3) morphologically compatible with Angiostrongylus cantonensis, obtained from Theba pisana.
General view of third-stage larvae with one sheath (S). A characteristic “T”-shaped structure (TS) is apparent at the anterior end of the sheath that surrounds the L3 larva (a). Posterior end of L3 larva with anus (A) at subterminal position. Anus cuticle (AC) is completely molted and can be seen on the sheath (b). Anterior end of L3 larva with esophagus bulbus (EB), excretory pore (EP) and nervous ring (NR) (c).
Two of the samples that tested positive with LAMP were confirmed as positive by 18S rRNA PCR (GenBank Accession number KM096415). Both were from P. lamarckii specimens. Sequences exhibited 100% similarity to an 18S rRNA gene partial sequence of A. cantonensis retrieved from GenBank (AY295804.1) [18] and to a sequence obtained from A. cantonensis third stage larvae of Parmarion cf. martensi [20]. Both samples were confirmed to be infected with A. cantonensis by amplification of the ITS1 region.
Discussion
A. cantonensis was detected by morphological and/or molecular methods in all three mollusc species and from all the areas studied. The three species are common and widespread over the north-east part of Tenerife.
This finding is in concordance with the previously demonstrated capacity of A. cantonensis to infect naturally many diverse freshwater and terrestrial mollusc species [8]. The prevalence recorded in this study via LAMP (60.2%) is higher than that found in other studies: 9% via real-time PCR in Pomacea maculata [21]; 14–31% by morphological identification in Pomacea canaliculata [22]; and 25% and 6.5% by morphological identification in Achatina fulica and P. canaliculata, respectively [23]. These differences could be due in part to a higher sensitivity of LAMP compared to the other methods. On the other hand, the prevalence observed in this work was lower than those found in P. martensi by PCR (73.5% and 74.1%) [19,20].
There is a huge variability of mollusc species in the Canary Islands [15], and we only analyzed three mollusc species in this study. Therefore, the rest of mollusc species should be studied to assess the real range of mollusc species that are acting as intermediate hosts of A. cantonensis in this archipelago. To our knowledge, this work constitutes the first finding of A. cantonensis in the mollusc species T. pisana and P. lamarckii. The genus Plutonia belongs to the family Vitrinidae, which contains mainly Palaearctic semislugs [24]. This genus has colonized the Macaronesian Islands (Azores, Madeira, Canary Islands and Cape Verde) [24], with six endemic species, including P. lamarckii, now occurring in the Canary Islands. Whereas the highest diversity of European vitrinids can be found above 1000 m altitude, their highest diversity in the Canary Islands is below 500 m [24], corresponding to the most inhabited zones of these islands. The possible role as intermediate hosts of A. cantonensis of the other vitrinid species in Tenerife should be assessed. Nevertheless, P. lamarckii is mainly distributed in laurel forest and rarely occurs in the inhabited areas sampled in this study. Thus, this study suggests that T. pisana and C. aspersum may be important species implicated in the transmission of the parasite to its definitive and accidental hosts, including humans, in inhabited areas such as La Laguna and El Pris, whereas P. lamarckii might be involved in this transmission in laurel forest areas. Supporting this hypothesis, introduced rats in Tenerife are known to prey on endemic mollusc species, such as Plutonia spp. [25], a phenomenon that could explain the notable percentage of rats (55.6%) carrying antibodies against A. cantonensis in a previous study carried out mainly in rural areas of Tenerife [2], along with the high prevalence of P. lamarckii specimens carrying A. cantonensis larvae in this study (71.67% by LAMP technique). With regard to C. aspersum, this snail species has been reported as an unsuitable host [26], although it has been used recently to transmit infection to rats experimentally [27]. This snail species is currently cooked for food in the Canary Islands, where several snail gardens have been created to produce them. In accordance with the introduced T. pisana, it is native to coastal regions of the Mediterranean and Western Europe, as far north as Ireland and Wales [28], whereas C. aspersum is also prevalent in Western Europe, along the Atlantic coast and at the Balkan. Because of its gastronomic use this species also occurs in Africa, Oceania, America and Australia [29].
Molecular methods to detect A. cantonensis were used in this study because morphological identification based on pepsin digestion can only identify the larvae to the superfamily level [19]. In this study, we found that only 3.4% of the molluscs testing positive via LAMP were confirmed by PCR, possibly because LAMP technique is able to detect DNA quantities as low as 0.001 ρg of A. cantonensis genomic DNA [12,13]. Therefore, LAMP technique can demonstrate the presence of A. cantonensis genomic DNA in lightly-infected molluscs. It should be also taken into account that LAMP is hardly inhibited by impurities such as those found in tissue-derived DNA samples [30]. LAMP shows not only high sensitivity but also exhibits high specificity, since several authors have demonstrated the absence of cross-reactions by using DNA samples obtained from various other parasites [13].
Our findings may be important from a public health point of view, since all the biotopes analyzed were in inhabited areas (La Laguna and el Pris) near to urban centers or other places frequented by people for leisure (Pedro Álvarez, Pico del Inglés and La Esperanza). There is also the possibility of infecting other mammal species in Tenerife as both dogs and horses, since these animals have been reported as accidental hosts [31]. Natural infections of horses may produce verminous encephalomyelitis with tetraparesis as the principal clinical features. Canine neural angiostrongyliasis caused by this nematode has been reported in Australia and elsewhere [32].
Although raw snails and slugs are not part of the Canary Islands diet, there is a risk associated with ingestion of undercooked C. aspersum or accidentally ingesting snails or slugs in vegetable produce, and a slight risk of transmission to humans from contact with the mucus deposited by them [33]. The zoonotic relevance of this study is underlined by the finding of the first case of a human patient carrying antibodies against A. cantonensis in Tenerife (Martin-Alonso pers. comm.), indicating that transmission to humans may be taking place at present. The historical absence of the parasite in the Canary Islands may be an artefact of the lack of familiarity with this nematode and the diseases that it causes among physicians, which is reflected in the fact that human angiostrongyliasis is not currently included in the differential diagnosis of patients with meningitis in the Canary Islands. Consequently, further investigations to ascertain the real incidence of human angiostrongyliasis in the Canary Islands are required, in order to assess the role of this nematode as a cause of eosinophilic meningitis in the archipelago.
Acknowledgments
We thank ‘Excmo. Cabildo Insular’ of Tenerife for allowing us to conduct this field study. We also thank L. Moro for his help in the identification of the mollusc species.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by projects Red de Investigación Cooperativa en Enfermedades Tropicales—RICET (RD12/0018/0013), CGL 2009-07759BOS and 2014SGR 1241 (Generalitat de Catalunya). A. M-A was supported by a PhD grant from Agencia Canaria de Investigación, Innovación y Sociedad de la Información. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Eamsobhana P, Yong HS. Immunological diagnosis of human angiostrongyliasis due to Angiostrongylus cantonensis (Nematoda: Angiostrongylidae). Int J Infect Dis. 2009;13: 425–431. 10.1016/j.ijid.2008.09.021 [DOI] [PubMed] [Google Scholar]
- 2. Martin-Alonso A, Foronda P, Quispe-Ricalde MA, Feliu C, Valladares B. Seroprevalence of Angiostrongylus cantonensis in wild rodents from the Canary Islands. PloS ONE. 2011;6: e27747 10.1371/journal.pone.0027747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang Q, Lai D, Zhu X, Chen X, Lun Z. Human angiostrongyliasis. Lancet Infect Dis. 2008;8: 621–630. 10.1016/S1473-3099(08)70229-9 [DOI] [PubMed] [Google Scholar]
- 4. Slom TJ, Cortese MM, Gerber SI, Jones RC, Holtz TH, Lopez AS, et al. An outbreak of eosinophilic meningitis caused by Angiostrongylus cantonensis in travelers returning from the Caribbean. N Engl J Med. 2002;346: 668–675. [DOI] [PubMed] [Google Scholar]
- 5. Yii CY. Clinical observations on eosinophilic meningitis and meningoencephalitis caused by Angiostrongylus cantonensis on Taiwan. Am J Trop Med Hyg. 1976;25: 233–249. [DOI] [PubMed] [Google Scholar]
- 6. Bowden DK. Eosinophilic meningitis in the New Hebrides: two outbreaks and two deaths. Am J Trop Med Hyg. 1981;30: 1141–1143. [DOI] [PubMed] [Google Scholar]
- 7. Chotmongkol V, Sawanyawisuth K. Clinical manifestations and outcome of patients with severe eosinophilic meningoencephalitis presumably caused by Angiostrongylus cantonensis . Southeast Asian J Trop Med Public Health. 2002;33: 231–234. [PubMed] [Google Scholar]
- 8. Kim JR, Hayes KA, Yeung NW, Cowie RH. Diverse gastropod hosts of Angiostrongylus cantonensis, the rat lungworm, globally and with a focus on the Hawaiian Islands. PloS ONE. 2014;9: e94969 10.1371/journal.pone.0094969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cowie RH. Biology, systematics, life cycle, and distribution of Angiostrongylus cantonensis, the cause of rat lungworm disease. Hawai'i J Med Public Health. 2013;72 (Supplement 2): 6–9. [PMC free article] [PubMed] [Google Scholar]
- 10. Kliks MM, Palumbo NE. Eosinophilic meningitis beyond the Pacific Basin: The global dispersal of a peridomestic zoonosis caused by Angiostrongylus cantonensis, the nematode lungworm of rats. Soc Sci Med. 1992;34: 199–212. [DOI] [PubMed] [Google Scholar]
- 11. Qvarnstrom Y, Bishop HS, da Silva AJ. Detection of rat lungworm in intermediate, definitive, and paratenic hosts obtained from environmental sources. Hawaii J Med Public Health. 2013;72 (Supplement 2): 63. [PMC free article] [PubMed] [Google Scholar]
- 12. Chen R, Tong Q, Zhang Y, Lou D, Kong Q, Lv S, et al. Loop-mediated isothermal amplification: rapid detection of Angiostrongylus cantonensis infection in Pomacea canaliculata . Parasit Vectors. 2011;4: 204 10.1186/1756-3305-4-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu C, Song H, Zhang R, Chen M, Xu M, Ai L, et al. Specific detection of Angiostrongylus cantonensis in the snail Achatina fulica using a loop-mediated isothermal amplification (LAMP) assay. Mol Cell Probes. 2011;25: 164–167. 10.1016/j.mcp.2011.04.002 [DOI] [PubMed] [Google Scholar]
- 14. Foronda P, López-González M, Miquel J, Torres J, Segovia M, Abreu-Acosta N, et al. Finding of Parastrongylus cantonensis (Chen, 1935) in Rattus rattus in Tenerife, Canary Islands (Spain). Acta Trop. 2010;114: 123–127. 10.1016/j.actatropica.2010.02.004 [DOI] [PubMed] [Google Scholar]
- 15. Groh K, Garcıa A. Phylum Mollusca In: Izquierdo I, Martín JL, Zurita N, Arechavaleta M, editors. Lista de especies silvestres de Canarias (hongos, plantas y animales terrestres). Tenerife: Consejería de Medio Ambiente y Ordenación Territorial. Gobierno de Canarias; 2004. pp. 149–154. [Google Scholar]
- 16. Ash LR. Diagnostic morphology of the third-stage larvae of Angiostrongylus cantonensis, Angiostrongylus vasorum, Aelurostrongylus abstrusus, and Anafilaroides rostratus (Nematoda: Metastrongyloidea). J Parasitol. 1970;56: 249–253. [PubMed] [Google Scholar]
- 17.López C, Clemente S, Almeida C, Brito A. Hernández M. A genetic approach to the origin of Millepora sp. in the eastern Atlantic. Coral Reefs. 2014. 10.1007/s00338-015-1260-8 [DOI]
- 18. Carreno RA, Nadler SA. Phylogenetic analysis of the Metastrongyloidea (Nematoda: Strongylida) inferred from ribosomal RNA gene sequences. J Parasitol. 2003;89: 965–973. [DOI] [PubMed] [Google Scholar]
- 19. Qvarnstrom Y, da Silva AC, Teem JL, Hollingsworth R, Bishop H, Graeff-Teixeira C, et al. Improved molecular detection of Angiostrongylus cantonensis in mollusks and other environmental samples with a species-specific internal transcribed spacer 1-based TaqMan assay. Appl Environ Microbiol. 2010;76: 5287–5289. 10.1128/AEM.00546-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qvarnstrom Y, Sullivan JJ, Bishop HS, Hollingsworth R, da Silva AJ. PCR-based detection of Angiostrongylus cantonensis in tissue and mucus secretions from molluscan hosts. Appl Environ Microbiol. 2007;73: 1415–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Teem JL, Qvarnstrom Y, Bishop HS, da Silva AJ, Carter J, White-McLean J, et al. The occurrence of the rat lungworm, Angiostrongylus cantonensis, in nonindigenous snails in the Gulf of Mexico region of the United States. Hawaii J Med Public Health. 2013;72: 11 [PMC free article] [PubMed] [Google Scholar]
- 22. Yen CM, Chen ER, Cheng CW. A survey of Ampullarium canaliculatus for natural infection of Angiostrongylus cantonensis in south Taiwan. J Trop Med Hyg. 1990;93: 347–350. [PubMed] [Google Scholar]
- 23. Chen D, Zhang Y, Shen H, Wei Y, Huang D, Tan Q, et al. Epidemiological survey of Angiostrongylus cantonensis in the west-central region of Guangdong Province, China. Parasitol Res. 2011;109: 305–314. 10.1007/s00436-011-2255-1 [DOI] [PubMed] [Google Scholar]
- 24. Hausdorf B. Phylogeny and biogeography of the Vitrinidae (Gastropoda: Stylommatophora). Zool J Linn Soc. 2002;134: 347–358. [Google Scholar]
- 25. Delgado JD, Arévalo JR, Fernández‐Palacios JM. Road and topography effects on invasion: Edge effects in rat foraging patterns in two oceanic island forests (Tenerife, Canary Islands). Ecography. 2001;24: 539–546. [Google Scholar]
- 26. Alicata JE. Biology and distribution of the rat lungworm, Angiostrongylus cantonensis, and its relationship to eosinophilic meningoencephalitis and other neurological disorders of man and animals. Adv Parasitol. 1965;3: 223–248. [DOI] [PubMed] [Google Scholar]
- 27. Prociv P, Carlisle MS. The spread of Angiostrongylus cantonensis in Australia. Southeast Asian J Trop Med Public Health. 2001;32 (Supplement 2): 126–128. [PubMed] [Google Scholar]
- 28. Johnson MS. Founder effects and geographic variation in the land snail Theba pisana . Heredity. 1988;61: 133–142. [Google Scholar]
- 29.Nicolai A. The impact of diet treatment on reproduction and thermophysiological processes in the land snails Cornu aspersum and Helix pomatia. M. Sc. Thesis, The University of Sheffield. 2010. Available: https://hal.archives-ouvertes.fr/file/index/docid/525296/filename/These_Annegret_Nicolai2010.pdf. Accessed 2015 March 5.
- 30. Thekisoe OM, Kuboki N, Nambota A, Fujisaki K, Sugimoto C, Igarashi I, et al. Species-specific loop-mediated isothermal amplification (LAMP) for diagnosis of trypanosomosis. Acta Trop. 2007;102: 182–189. [DOI] [PubMed] [Google Scholar]
- 31. Lunn JA, Lee R, Smaller J, MacKay BM, King T, Hunt GB, et al. Twenty two cases of canine neural angiostrongylosis in eastern Australia (2002–2005) and a review of the literature. Parasit Vectors. 2012;70: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eamsobhana P. The rat lungworm Parastrongylus (= Angiostrongylus) cantonensis: parasitology, immunology, eosinophilic meningitis, epidemiology and laboratory diagnosis Bangkok: Wankaew (IQ) Book Center; 2006. [Google Scholar]
- 33. Cowie RH. Pathways for transmission of angiostrongyliasis and the risk of disease associated with them. Hawai'i J Med Public Health. 2013;72 (Supplement 2): 70–74. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.