To the Editor:
Anxiety disorders are the most common psychiatric conditions, affecting as many as 1 in 10 individuals. The diagnosis of anxiety disorders peak during adolescence, a period characterized by pronounced changes in the ability to regulate emotional thoughts and behavior. These changes occur in parallel with development of frontolimbic circuitry captured by the “imbalance model” of adolescence (Figure 1A). Clarifying the nature of neurobehavioral changes during this developmental phase may provide important insight into both the etiology and treatment of anxiety disorders.
Figure 1.
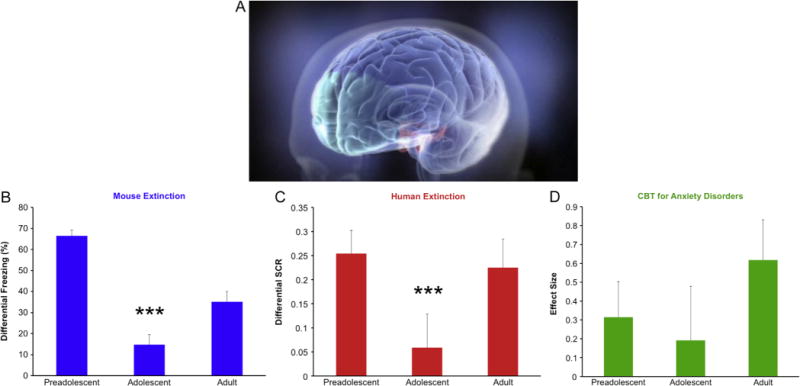
Fear extinction learning and improvement of anxiety by age. (A) Representative illustration of frontolimbic circuitry, highlighting the earlier developing limbic areas (red) and later developing prefrontal cortex (green) suggested by the imbalance model of adolescence (Reproduced with permission from McPhee) (9). (B) Adolescent (P29) mice show diminished extinction learning relative to adults (P70) and preadolescents (P23). (C) Human adolescents (mean age: 13.9 ± 1.47 years, n = 25) show diminished fear extinction learning as indexed by skin conductance responses (SCR) compared with adults (mean age: 22.8 ± 2.57 years, n = 28) and children (mean age: 8.8 ± 1.78 years, n = 30) (4). (D) A similar pattern emerges when the effect size of cognitive behavioral therapy (CBT) relative to placebo is calculated separately for children (CBT: children, mean age: 9.46 ± 1.36 years, n = 90; adolescents, mean age: 14.34 ± 1.74 years, n = 49; placebo: children, mean age: 9.20 ± 1.31 years, n = 50; adolescents, mean age: 14.55 ± 1.64 years, n = 26) (1) and adults (7). All results presented as mean ± SEM. ***p < .001 compared with other groups.
Current Practice
Cognitive behavioral therapy (CBT) is the only evidence-based behavioral treatment for anxiety disorders. Yet 40% to 50% of patients do not improve with this treatment (1), underscoring the need to predict who will respond to this treatment. A primary therapeutic component of CBT involves exposure exercises in which a patient is systemically desensitized to anxiety triggers through repeated exposures. Desensitization is based on principles of classical fear learning and extinction. Recent advances in our understanding of the development of fear extinction provide reason to investigate whether exposure therapy is best suited for all age groups.
A Matter of Timing
The imbalance model of adolescence links structural and functional brain changes in brain circuitry mediating emotional reactivity and regulation to their behavioral consequences. The amygdala, a subcortical structure that mediates fear learning and reactivity, appears to be functionally mature early in life. In contrast, the prefrontal cortex, involved in emotion regulation, continues to develop well into young adulthood. These distinct region-specific developmental trajectories may lead to an imbalance in how these structures drive behavior during adolescence. As a result, teenagers show elevated emotional reactivity that is more difficult to regulate (2). With age and experience, connectivity between these regions strengthens, allowing for top-down modulation of emotional responses. Failure to regulate emotional responses correlates with symptoms of anxiety in healthy and anxious youth (2,3), highlighting a common reduction in amygdala regulation in both typical adolescence and anxiety disorders.
Translational Mouse and Human Studies
In our laboratories, human and rodent studies shed light on the development of mammalian fear circuitry. In mice, fear is measured by quantifying the characteristic “freezing” in reaction to a potential threat. Mice of various ages were fear conditioned by pairing a neutral tone with a mild electrical shock. On subsequent days, the mice were exposed to the conditioned tone without the shock and freezing gradually decreased in a process known as extinction. These studies demonstrated a striking attenuation in extinction learning during adolescence. Both preadolescent (23-day-old) and adult (70-day-old) mice showed a greater decrease in freezing than adolescent (29-day-old) mice (Figure 1B) (4).
Fear neurocircuitry is highly conserved across species, enabling the translation of basic animal work to humans. In our human fear-induction paradigm, participants were conditioned by pairing the presentation of a colored square with an unpleasant sound. During extinction the following day, the conditioned colored square was presented repeatedly without the aversive sound. We quantified fear extinction by comparing physiologic conditioned responses during early and late extinction trials. As in rodents, healthy human adolescents showed significantly less extinction learning than children or adults (Figure 1C).
Reduced abilities to acquire and maintain extinction learning are thought to confer vulnerability to anxiety (5). Taken together, our cross-species results indicate that adolescents show diminished fear extinction learning. Other studies have shown that adolescent retention of this learning is also compromised (6).
Clinical Patterns
Our data suggest the hypothesis that the efficacy of extinction-based exposure therapies might be reduced in adolescence. To date, no study has been designed to specifically assess age differences in the efficacy of exposure therapy in anxious patients. As a proof of concept, we examined a subset of data from a large-scale placebo-controlled trial of anxious children and adolescents assigned to CBT or pill placebo (1). We quantified an effect size of the benefit of CBT-only treatment versus placebo for each age group. Anxiety symptoms were measured in children and adolescents after 12 weeks of CBT or placebo. A second published study provided an estimate of CBT’s effect size versus placebo in adults (7). Comparing response rates for CBT across age groups reveals a nonsignificant pattern in which adolescents show reduced treatment efficacy (Figure 1D). However, it is important to note that the Child/Adolescent Anxiety Multimodal Study was not designed to test our hypothesis. The CBT component of this study included many elements in addition to exposure, and the patient population had far fewer adolescents than children. Although it is impossible to segregate the benefits of anxiety-management training from the effects of exposure-therapy in this study (1), these data provide an effect size for estimating the sample size that would be needed to test our hypothesis with exposure-therapy alone.
Our findings in rodent and human laboratory studies demonstrate the power of basic research to uncover patterns of potential clinical importance in psychiatry. Developmental changes in fear extinction circuitry and function have potential relevance for any treatment that incorporates exposure therapy. Although it has been suggested that anxiety syndromes in children may be more likely than adolescents to remit spontaneously (8), we highlight that they may also be more responsive to therapy. We hope that our findings lead to more precise psychiatric treatments by defining the optimal period for treating individuals with anxiety disorders. Such an approach is necessary to translate laboratory findings into better treatments.
Acknowledgments
A very special thank you to the Child/Adolescent Anxiety Multimodal Study (CAMS) team, a collaborative workgroup responsible for the clinical research presented in this report. The CAMS team research was supported by Johns Hopkins Medical Institutions (Weill Cornell Medical College; J Walkup, M.D.; Grant No. U01 MH064089), the New York State Psychiatric Institute (AM Albano, Ph.D.; Grant No. U01 MH64092), the Western Psychiatric Institute and Clinic/University of Pittsburgh Medical Center (B. Birmaher, M.D.; Grant No. U01 MH64003), Temple University (P. Kendall, Ph.D.; Grant No. U01 MH63747), Duke University Medical Center (J. March, M.D.; Grant No. U01 MH64107), the University of California at Los Angeles (J. Piacentini, Ph.D.; Grant No. U01 MH64088), Data Center (S. Compton, Ph.D.; Grant No. U01 MH064003), and the National Institute of Mental Health (J. Sherrill, Ph.D.). Mouse and human laboratory research presented in this study was supported by Weill Cornell Medical College (B.J. Casey, Ph.D. and Francis Lee, Ph.D.; Grant No. P50 MH079513). Andrew T. Drysdale was supported by a Medical Scientist Training Program grant from the NIGMS to the Weill Cornell/Rockefeller/Sloan-Kettering Tri-Institutional MD-PhD Program (Grant No. T32GM07739). The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Dr. Compton has received research funding and consulting fees from Shire Pharmaceuticals. He currently serves as Associate Editor to Journal of Consulting and Clinical Psychology and Journal of Child and Adolescent Psychopharmacology. Dr. Walkup has received research funding and honoraria from the Tourette Syndrome Association. He has received free medication and pill placebos from Lilly, Pfizer, and Abbott Pharmaceuticals for National Institutes of Health–funded studies. He currently receives royalties from Oxford Press and Guilford Press. He currently consults for Shire Pharmaceuticals. Mr. Drysdale, Ms. Ruberry, and Drs. Hartley, Pattwell, Lee, and Casey report no biomedical financial interests or potential conflicts of interest.
References
- 1.Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359:2753–2766. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, et al. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- 4.Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, et al. Altered fear leaning across development in both mouse and human. Proc Natl Acad Sci. 2012;109:16319–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: Ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCallum J, Kim JH, Richardson R. Impaired extinction retention in adolescent rats: Effects of D-cycloserine. Neuropsychopharmacology. 2010;35:2134–2142. doi: 10.1038/npp.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson JRT, Foa EB, Huppert JD, Keefe FJ, Franklin ME, Compton JS, et al. Fluoxetine, comprehensive cognitive behavioral therapy, and placebo in generalized social phobia. Arch Gen Psychiatry. 2004;61:1005–1013. doi: 10.1001/archpsyc.61.10.1005. [DOI] [PubMed] [Google Scholar]
- 8.Beesdo-Baum K, Knappe S. Developmental epidemiology of anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012;21:457–478. doi: 10.1016/j.chc.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 9.McPhree L. Depression: Out of the Shadows [video] Saint Paul, MN/Boston, MA: Twin Cities Public Television, Inc. and WGBH Boston; 2008. Available at: http://www.pbs.org/wgbh/takeonestep/depression/video-ch_03.html. Accessed April 29, 2013. [Google Scholar]


