Abstract
Objectives
Flavonoids are a common group of plant polyphenols that give color and flavor to fruits and vegetables. In recent years, flavonoids have gained importance in the pharmaceutical field through their beneficial effects on human health and are widely available as nutritional supplements. Several pharmacological activities of the bioflavonoids may be useful in the prevention or treatment of ocular diseases responsible for vision loss such as diabetic retinopathy, macular degeneration, and cataract. This review summarizes potential therapeutic applications of various bioflavonoids in different ocular diseases and also discusses delivery of these agents to the ocular tissues.
Key findings
It is apparent that the flavonoids are capable of acting on various mecha- nisms or aetiological factors responsible for the development of different sight threatening ocular diseases. From a drug delivery perspective, ocular bioavailability depends on the physicochemical and biopharmaceutical characteristics of the selected flavonoids and very importantly the route of administration.
Summary
The potential therapeutic applications of various bioflavonoids in ocular dis- eases is reviewed and the delivery of these agents to the ocular tissues is discussed. Whereas oral administration of bioflavonoids may demonstrate some pharmacological activity in the outer sections of the posterior ocular segment, protection of the retinal ganglionic cells in vivo may be limited by this delivery route. Systemic or local administration of these agents may yield much higher and effective concentrations of the parent bioflavonoids in the ocular tissues and at much lower doses.
Keywords: anti-angiogenic, anti-inflammatory, antioxidant, flavonoids, ocular drug delivery
1. Introduction
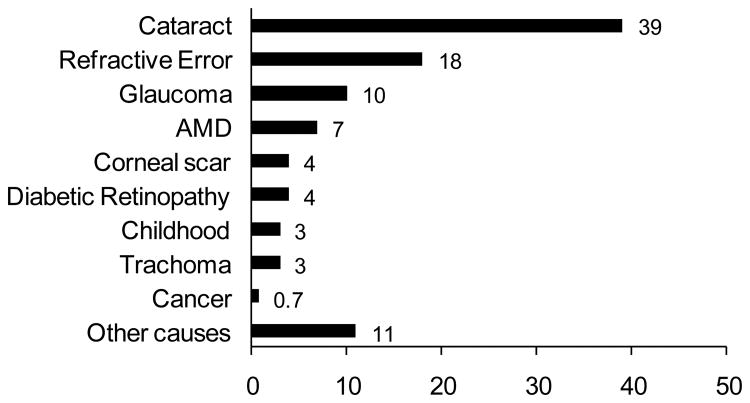
The eye, the organ of sight, has a very unique structural and biochemical organization. Age and certain disease conditions, however, can affect the function of this vital organ. A report from the World Health Organization (WHO) in 2002 estimated that approximately 161 million people were suffering from visual impairment worldwide out of which 37 million face blindness while 124 million suffer from low vision. Age seems to be a causative factor in blindness as 82% of the population with blindness are aged above 50 years[1]. In the United States, in 2002, among civilian non-institutionalized adults, 19.1 million people were suffering from visual impairment including 0.3% with blindness[2]. Although, blindness is necessarily associated with ageing, cataract is still the leading cause of blindness followed by refractive error, glaucoma, age-related macular degeneration, trachoma, childhood blindness and diabetic retinopathy[1, 3] (Fig. 1).
Figure 1. Proportion (percentage) of cases of blindness by major ocular diseases (adapted from Ref [1]).
Over the century, flavonoids or bioflavonoids have been identified as the most common group of plant polyphenols that give color and flavor to fruits and vegetables. They are secondary metabolites in plants and their biosynthesis takes place via the shikimate and arogenate pathways[4]. In plants, flavonoids occur as the glycosides (with attached sugar) or occasionally as the aglycones. Initially they were considered as vitamins and the term ‘Vitamin P’ was coined to describe them; however, subsequently this term was discontinued. Flavonoids play an important role in plant physiology including pigmentation, flavor, growth, and reproduction. Furthermore, these molecules also provide resistance against pathogens[5].
The common structural feature of the flavonoids is the flavone nucleus (2-phenyl chromone or 2-phenyl benzopyrone), characterized by a C6-C3-C6 carbon skeleton with the C6 component being aromatic in nature (Fig. 2). This basic skeleton may contain numerous substituent groups like i) hydroxyl groups: these are generally present at the 4′, 5 and 7 positions ii) sugars: these are generally linked with the hydroxyl group positioned at 7 and iii) methyl and isopentyl units. Hydroxyl groups and sugars impart hydrophilicity, while methyl groups and isopentyl units impart lipophilicity to the flavonoids. Till now more than 8000 polyphenolic compounds have been identified and these flavonoids can be classified into different subclasses which include flavones, flavonols, flavanones, flavanols, anthocyanins and isoflavones[6] (Fig. 2 and Table 1).
Figure 2. Basic chemical structures of different flavonoid classes.
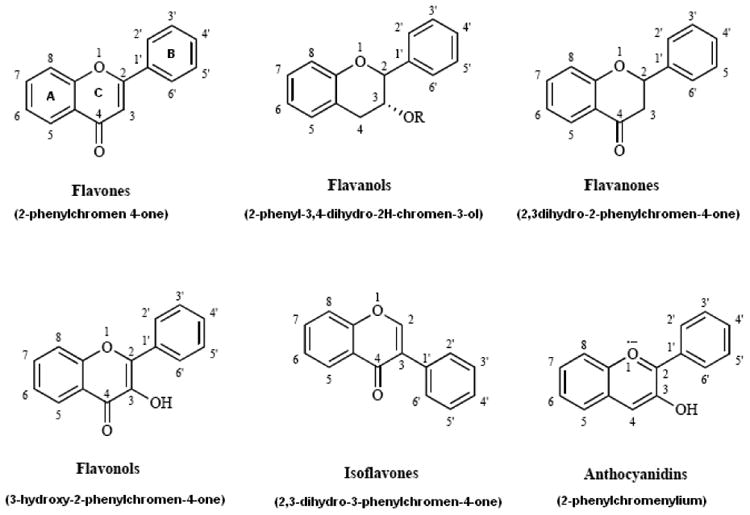
Table 1. Major flavonoid classes: examples under each category with their substituent groups.
| Flavonoid class | Examples | Substituent groups | ||||||
|---|---|---|---|---|---|---|---|---|
| 3 | 5 | 7 | 3′ | 4′ | 5′ | Others | ||
| Flavones | Apigenin | H | OH | OH | H | OH | H | |
| Luteolin | H | OH | OH | OH | OH | H | ||
| Chrysin | H | OH | OH | H | H | H | ||
| Baicalein | H | OH | OH | H | H | H | 6=OH | |
| Nobiletin | H | OCH3 | OCH3 | OCH3 | OCH3 | H | 6, 8=OCH3 | |
| Wogonin | H | OH | OH | H | H | H | 8=OCH3 | |
| Flavanols | Epicatechin | H | OH | OH | OH | OH | H | R=H |
| ECG1 | H | OH | OH | OH | OH | H | R=Gallate | |
| EGCG2 | H | OH | OH | OH | OH | OH | R=Gallate | |
| Flavanones | Naringenin | H | OH | OH | H | OH | H | |
| Naringin | H | OH | O-Rha3 | H | OH | H | ||
| Taxifolin | OH | OH | OH | OH | OH | H | ||
| Eriodictyol | H | OH | OH | OH | OH | H | ||
| Diosmin | - | OH | O-Rha3 | OH | OCH3 | H | ||
| Hesperetin | H | OH | OH | OH | OCH3 | H | ||
| Hesperidin | H | OH | O-Rha3 | OH | OCH3 | H | ||
| Linarin | - | OH | O-Rha3 | H | OCH3 | H | ||
| Isorhoifolin | - | OH | O- Rha3 | H | OH | H | ||
| Flavonols | Kaempferol | OH | OH | OH | H | OH | H | |
| Galangin | OH | OH | OH | H | H | H | ||
| Morin | OH | OH | OH | H | OH | H | ||
| Myricetin | OH | OH | OH | OH | OH | OH | 2′=OH | |
| Quercetin | OH | OH | OH | OH | OH | H | ||
| Fisetin | OH | H | OH | OH | OH | H | ||
| Quercetrin | O-Rha3 | OH | OH | OH | OH | H | ||
| Isoflavones | Daidzein | H | H | OH | H | OH | H | |
| Genistein | H | OH | OH | H | OH | H | ||
| Puerarin | H | H | OH | H | OH | H | 8=O-Glucosyl | |
| Anthocyanidins | Cyanidin | OH | OH | OH | OH | OH | H | |
| Delphinidin | OH | OH | OH | OH | OH | OH | ||
| Malvidin | OH | OH | OH | OCH3 | OH | OCH3 | ||
| Petunidin | OH | OH | OH | OH | OH | OCH3 | ||
ECG: Epicatechin-3-gallate
ECGC: Epigallocatechin-3-gallate
Rha: Rhamnoglucose
Flavonoids have gained prominence in the pharmaceutical arena by virtue of their therapeutically beneficial properties. Bioflavonoids possess antioxidant, anti-angiogenic, and/or anti-inflammatory activities and are also capable of reducing fluid retention and strengthening capillary walls. Interestingly, the etiology of most ocular diseases involve free radical mediated oxidative damage, hypoxia, decreased blood supply to ocular tissues and, in certain conditions, angiogenesis, increased vascular permeability and leakage of vascular contents[7, 8]. Thus, select bioflavonoids may be effective in the prevention or treatment of ocular diseases (e.g. diabetic retinopathy, macular degeneration, and cataract) that lead to vision loss if left untreated.
This review summarizes different factors associated with the initiation and progression of some of these conditions, potential therapeutic role of the flavonoids and ocular drug delivery aspects.
2. Ocular Diseases/Disorders
The following section briefly describes the pathophysiology of various ocular diseases and pathways that can be targeted by the flavonoids.
2.1 Cataract
Multiple mechanisms have been proposed with respect to the development of cataractus lens such as nonenzymatic glycation, oxidative stress and polyol pathway[7, 9, 10]. There is a body of evidence which suggests that H2O2 and hydroxyl radicals, the most reactive and damaging free radicals, contribute to cataract formation. When compared to normal eyes, significantly higher amounts of these free radicals were found in the cataractous lenses and in the aqueous humor. Additionally, glutathione reductase activity was found to be inversely proportional to the severity of cataract formation[7]. In the case of the polyol pathway, when hyperglycemia occurs, glucose is converted to sorbitol by aldose reductase. The sorbitol thus produced does not cross cell membranes easily and thus accumulates in the cells causing a disturbance in homeostasis. Intralenticular accumulation of polyols has long been suggested to be a major factor in acute models of sugar cataract[10].
2.2 Diabetic Retinopathy
Diabetes is considered to be the primary causative factor in the development of diabetic retinopathy (DR). The disease can be broadly categorized into three stages; background DR, pre-proliferative DR and proliferative DR (PDR). In the first stage of DR, hyperglycemia initiates thickening of capillary basement membrane and causes death of pericytes that support the vessel wall. Following this, microaneurysms and vascular leakage takes place leading to blockage of retinal capillaries and inducing local hypoxia. Subsequently, endothelial cells die resulting in closure of capillaries and increased areas of non-perfusion. Pre-proliferative DR is identifiable by the areas of increased retinal hypoxia and multiple hemorrhages because of loss of vascular patency. Increased areas of non-perfusion stimulates the generation of angiogenic factors leading to the formation of new blood vessels, a characteristic feature of PDR. Subsequently retinal detachment may take place causing vision loss or blindness. Hyperglycemia and hypoxia are the two principal factors in the initiation and progression of DR. Production of a variety of local agents in the ocular tissues such as vascular endothelial growth factor (VEGF), prostaglandins, cyclooxygenase-2 (COX-2) and nitric oxide (NO) is indicated in the process, all of which contribute to vascular permeability and angiogenesis[11, 12].
2.3 Age-Related Macular Degeneration
Age-related macular degeneration (AMD) is another leading cause of vision impairment and blindness, especially in the western countries[13]. The well known risk factor for AMD is age. Retinal tissues most affected in this disease are the photoreceptors and the retinal pigmented epithelium (RPE). There are two types of AMD: an atrophic form, which is associated with pigmentary changes in the macula without hemorrhage or scar formation, and disciform macular degeneration, which is characterized by exudative mound formation and sub and intraretinal hemorrhage. However, leakage of plasma from small blood vessels in the macula following breakdown of the blood-retinal barrier can lead to macular edema and can endanger vision. Besides age, macular pigmentary change, hypertension, smoking and obesity are other risk factors. Importantly, in AMD, like in diabetic macular edema, free radicals and reactive oxygen intermediates (ROI) are implicated in the initiation and progression of the disease[7, 8, 14].
2.4 Glaucoma
Glaucoma is a complex disease that can damage the optic nerve of the eye leading to vision loss and blindness. It is often times called as ‘silent blinder’, since many people are incognizant about the presence of the disease. It is prevalent in almost all age groups; however, it most affects the elderly. There are different types of glaucoma; the most common ones are, primary open-angle glaucoma (POAG) and primary angle-closure glaucoma (PACG), normal tension glaucoma and the less common types are congenital glaucoma, and secondary open-angle glaucoma. Conventionally, POAG has been characterized as a disease of elevated IOP. However, recent scientific evidences suggest that both vascular and biochemical factors are also involved. It is now defined as group of ocular diseases that may cause changes in the optic nerve head, visual field or both[15, 16].
Recent evidence also suggests that reactive oxygen species (ROS) plays a significant role in the pathogenesis of POAG. Oxidative damage to the epithelial tissue regulating aqueous humor outflow, i.e. the trabecular meshwork, is significantly higher in glaucoma patients compared to the normotensive subjects. Moreover, oxidative damage to the retinal cells and neuronal cells of the optic nerve can also lead to POAG[17, 18].
In glaucoma patients, average blood flow to various ocular tissues like iris, choroid, retina, and optic nerve is reduced. This reduction in blood flow is more noticeable in normal tension glaucoma compared to high tension glaucoma. Decreased ocular blood flow can lead to glaucomatous optic neuropathy (GON)[17, 19].
2.5 Dry Eye Syndrome
The international dry eye workshop in 2007 described dry eye as a “multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface. This syndrome is most prevalent among the elderly and is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface” [20]. It has been classified into aqueous tear deficient dry eye and evaporative dry eye and there are additional subclasses under each category.
Dry eye is generally associated with inflammation of the surface of the eye, the lacrimal gland or the conjunctiva and any disease that changes the composition of tears. In dry eye syndrome whatever may be the initial cause, chronic dryness of the surface of the eye leads to neurogenic inflammation, subsequent to activation of T-cells and release of inflammatory cytokines into the lacrimal glands, tear fluid and conjunctiva. In later stages, these inflammatory mediators may even cause gradual dysfunction and destruction of the lacrimal glands and impairment of conjunctival epithelium. Thus, lacrimal glands are deprived of normal trophic stimulation required for regular maintenance. Once the disease is initiated, inflammation becomes the key mechanism of ocular surface injury. Recent evidence also suggests a role of oxidative stress in the primary initiating events that lead to the corneal, conjunctival and lacrimal gland injury. Thus, oxidative stress with associated inflammatory process can trigger this disease state [21].
3. The Pharmacological Actions of the Flavonoids
The following section briefly describes various pharmacological activities of the bioflavonoids, which can be useful in the prevention or treatment of different ocular diseases.
3.1 Antioxidant Activity
A number of flavonoids have been documented as potential antioxidants and include quercetin, apigenin, hesperidin, hesperetin, luteolin, epigallocatechin gallate, epicatechin gallate, rutin, cyanidin, naringenin, myricetin, chrysin, eriodictyol, kaempferol [22-24]. These molecules exhibit their antioxidant activity through different mechanisms.
A) By scavenging the free radicals directly
Because of their low redox potential (i.e. high reactivity of the hydroxyl groups) flavonoids are able to reduce the highly oxidizing free radicals (e.g. superoxide, peroxyl, alkoxyl, and hydroxyl), resulting in more stable, less-reactive radicals[22, 23].
B) By inhibiting the nitric oxide production
Nitric oxide (NO) is produced by several types of cells including endothelial cells and macrophages. The constitutive production of NO is necessary to maintain the dilation of blood vessels. However, inducible nitric oxide synthases (iNOS) are responsible for the production of higher concentrations of NO during oxidative damage. NO reacts with free radicals and generates the highly reactive and damaging peroxynitrite. Flavonoids through their free radical scavenging properties can prevent the generation of peroxynitrite[23]. Moreover, the flavonoids appear to be capable of inhibiting iNOS directly and thus decrease production of NO [25-28].
C) By inhibiting certain enzymes
Flavonoids have been reported to inhibit the enzymes responsible for the production of superoxide anions such as xanthine oxidase, protein kinase C. Some flavonoids are also capable of inhibiting cyclooxygenase, lipoxygenase, microsomal monooxygenase, glutathione S-transferase, mitochondrial succinoxidase, and NADH oxidase, all involved in ROS generation[22].
D) By chelating trace elements
Trace elements, e.g. free iron and copper, are potential enhancers of ROS generation and also play an important role in oxygen metabolism. Certain flavonoids are capable of chelating these trace elements and thereby prevent the generation of ROS[22].
3.2 Effect on Ocular Blood Flow
A decrease in ocular blood flow can lead to a number of diseases like glaucoma, diabetic retinopathy and macular degeneration[29]. Literature suggests a significant effect of bioflavonoids on ocular blood flow[30-32]. In 1996, Liu et al. reported that hesperetin was able to increase the blood flow in iris, ciliary body and choroid[32]. Further investigations using different flavonoids revealed that this activity is dependent on the number of hydroxyl groups present in the flavones and flavanones and on dihydrogenation of the flavone molecules [30, 31, 33] (Fig. 2). Topical administration of the flavonoids in rabbits suggested that flavonoids with two hydroxyl groups, e.g. hesperidin and naringin, had very little effect or no effect on blood flow, while those compounds with three hydroxyl groups, e.g. hesperetin and naringenin, exhibited highest activity with respect to increasing ocular blood flow. With flavanones containing four hydroxyl groups, e.g. rutin, a mixed effect was observed i.e. blood flow increased at some time points and decreased at other time points. Interestingly, compounds with five hydroxyl groups, e.g. quercetin, had a negative effect on ocular blood flow. A significant improvement in ocular blood flow was also attained when the flavones were dehydrogenated to the flavanones. Additionally, compounds which increased blood flow also showed a marked increase in retinal function recovery following ischemic insult[31, 34].
Ginkgo biloba extract (GBE), which contains approximately 25 % flavone glycosides, was also found to increase blood flow in the ophthalmic artery, in healthy human volunteers, when administered orally at a dose of 40 mg three times a day for 2 days [35]. No effect was observed on arterial BP, heart rate, and IOP. However, Wimpissinger et al. recently reported that a single administration of 240 mg GBE did not produce significant changes in the ocular and systemic hemodynamic parameters compared to the placebo treated group [36].
In another study, administration of the food supplement Mirtogenol® (Mirtoselect® and Picnogenol®), which contains anthocyanosides, to 38 asymptomatic subjects with intraocular hypertension resulted in a significant improvement in ocular blood flow (central retinal, ophthalmic, and posterior ciliary arteries) and reduced the IOP[37].
3.3 Effect on Oxidative Damage to the Retinal Cells
The ability of the flavonoids to protect the retinal ganglion cells (RGC) from oxidative stress induced death, in vitro, was investigated by Maher et al [38]. Oxidative stress was induced by three different methods; glutathione depletion, t-butyl peroxide (t-BOOH) treatment, and H2O2 treatment. Some of the flavones (baicalein and luteolin), flavonols (3, 6-dihydroxyflavone, 3, 7-dihydroxyflavone, galangin, fisetin and quercetin) and flavanones (eriodictyol) were found to be effective in preventing retinal cell death induced by the above three methods of oxidative stress. Few others were effective against either two or one of the oxidative stress induction methods. However, some flavonoids (myricetin and epigallocatechin gallate (EGCG)) were completely ineffective. The differences in activity were attributed to the capability of the flavonoids to induce the biosynthesis of glutathione and to prevent the accumulation of ROS.
In another in vitro study by the same authors, ischemia was induced in the rat retinal ganglion cell line, RGC-5, using iodoacetic acid (IAA), and the protective effect of different flavonoids was investigated[39]. Several of the neuroprotective flavonoids (3, 6 dihydroxyflavone, 3, 7 dihydroxyflavone, galangin, baicalein, luteolin, fisetin, quercetin, and eriodictyol) were effective in preventing ischemia induced cell death. Interestingly, the classical antioxidants (vitamin E, vitamin C, trolox and resveratrol) demonstrated weak protection against IAA induced toxicity. Another clinically significant observation made by the authors of this study was that the flavonoids were effective even when they were administered subsequent to the ischemic insult to the RGC. The neuroprotective function of the flavonoids, in this study, was attributed to several mechanisms such as prevention of ROS accumulation, inhibition of calcium influx and induction of the expression and activity of phase-II detoxification proteins.
Hanneken et al. evaluated the ability of specific dietary and synthetic flavonoids to protect ARPE-19 and human RPE cells from oxidative stress induced death in vitro. Oxidative stress was induced by treatment with t-BOOH or H2O2. It was found that some of the flavonoids exhibited good efficacy, high potency and low toxicity in RPE cells. Dietary flavonoids with good efficacy include fisetin, luteolin, quercetin, eriodictyol, baicelein, galangin and EGCG and synthetic flavonoids include 3, 6- dihydroxy flavonol, and 3, 7- dihydroxy falvonol. Structure activity studies revealed that minor differences in the structures made a dramatic difference to the efficacy. For example, luteolin is very effective while apigenin is not and the only difference in their structure is a single hydroxyl group in the B ring (Fig. 2). Additionally, it was observed that the effective flavonoids were hydrophobic in nature and mostly belonged to the flavone and flavonol class. Importantly, some of the flavonoids tested (quercetin, fisetin, luteolin, and eriodictyol) were observed to be effective even after the RPE cells were exposed to oxidative stress, but before cell death occurred. The authors suggested that the flavonoids were probably acting through the inhibition of ROS accumulation and through induction of transcription factor, Nrf2 (Nuclear erythroid 2 p45-related factor 2), and its downstream phase-2 gene, heme-oxygenase 1, in human RPE cells[40]. Similar mechanisms in the protection of the ARPE-19 cells by eriodictyol were suggested by Johnson et al.[41].
Protection of retinal cells against oxidative stress and ischemia/reperfusion in vivo were reported for baicalein[42] and EGCG[43, 44]. Also pretreatment with GBE in drinking water was able to protect the retinal ganglion cells in a rat model of chronic glaucoma[45]. High levels of NO mediate glutamate-induced neurotoxicity following interaction with oxygen radicals. Flavonoid content of GBE was reported to strongly inhibit NO free radicals. Thus GBE's flavonoid content can be effectively used for the treatment of glaucoma.
The retinal neuroprotective effect of various flavonoids studied to date, along with their effective and lethal concentrations, is summarized in Table 2.
Table 2. The protective effect of various flavonoids on retinal cell lines against oxidative stress induced cell death in terms of effective (EC50) and lethal (LD50) concentrations.
| ARPE-19 [40] | RPE159 [40] | RGC-5 [39] | RGC-5[38] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Flavonoid class | Common name | EC50 (μM) | LD50 (μM) | EC50 (μM) | LD50 (μM) | EC50 (μM) | EC50 (μM) | ||||
| t-BOOH | H2O2 | t-BOOH | H2O2 | IAA | Post-IAA | Glutamate plus BSO | t-BOOH | ||||
| Flavone | Apigenin | No | No | <50 | - | - | 15 | No | |||
| Baicalein | 14 | No | 84 | 8 | 21 | ≫100 | 3.5 | 8 | 3 | 10 | |
| Luteolin | 14 | 9 | 104 | 2 | 3 | >50 | 3.5 | 7.5 | 2 | 7 | |
| Flavonol | Galangin | 32 | 31 | 112 | 26 | 61 | 70 | 7.5 | 30 | 10 | 50 |
| Fisetin | 15 | 11 | 101 | 3 | 5 | >50 | 8 | 17 | 15 | 10 | |
| Kaempferol | No | No | ∼50 | ∼50 | No | ∼50 | 1 | No | |||
| Quercetin | 18 | 19 | 230 | 6 | 11 | >50 | 14 | 24 | 17 | 18 | |
| Myricetin | No | No | >50 | >50 | No | ≫50 | No | - | |||
| Isoflavone | Genistein | No | No | ≫50 | No | No | >50 | 100 | No | ||
| Flavanone | Naringenin | No | No | ≫50 | No | No | ≫50 | No | - | ||
| Eiodictyol | 6 | 17 | 153 | 7 | 11 | >100 | 20 | 34 | 5 | 25 | |
| Hesperetin | 50 | - | |||||||||
| Flavanol | Catechin | No | No | >50 | No | No | ≫50 | No | - | ||
| Epicatechin, | No | No | >50 | No | No | ≫50 | 50 | No | |||
| epicatechin-3-gallate | No | No | >50 | 22 | 30 | >100 | No | - | |||
| Anthocyanidin | Cyanidin | No | No | >100 | No | No | >50 | No | - | ||
No: Little or no efficacy upto 50 μM
t-BOOH: Tert-Butyl hydroperoxide
H2O2: Hydrogen peroxide
IAA: Idoacetic acid
BSO: Buthionine sulphoximine
3.4 Effect on Angiogenesis and Vascular Leakage
Angiogenesis is the process of formation of new blood vessels and is characterized by early degradation of the extracellular matrix followed by migration and proliferation of the endothelial cells and, finally, maturation of the new blood vessels. Several factors are associated with the pathophysiology of angiogenesis, e.g. matrix metalloproteases (MMP-2 and MMP-9), and pro-angiogenic factors expressed in response to local injury, ischemia or inflammation, such as hypoxia-inducible factor-1α (HIF-1α), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), tumor necrosis factor-α (TNF-α), and interleukin-1, 6 and 8 and others[46, 47]. The newly formed blood vessels are leaky in nature and this hyperpermeability causes interstitial edema, which leads to physical compression of the capillaries resulting in a no-reflow phenomenon. Retinal vascular hyperpermeability is observed in the later stages of diabetic retinopathy and age related macular degeneration[8]. Thus, treatment with agents capable of decreasing capillary hyperpermeability and inhibiting angiogenesis is highly desired. Some flavonoids have been reported to possess these properties.
The micronized purified flavonoid fraction (Daflon® 500mg) (MPFF) and its individual flavonoid components (diosmin, hesperidin, linarin, and isorhoifolin) were evaluated for their anti-leakage effect in a hamster cheek pouch animal model, where hyperpermeability was induced by ischemia-reperfusion. The activity displayed by hesperidin, linarin, and isorhoifolin was similar to or greater than that of diosmin, the major component (90%) of MPFF. MPFF activity was greater than that of any single flavonoid, indicating synergetic activity[48]. Furthermore, recently, it has been reported that hesperidin (at concentrations of 10 and 100 μM) is capable of inhibiting the expression of hypoxia-inducible factor-1α (HIF-1α) and inflammatory cytokine production in the human mast cell line (HMC-1) in addition to inhibition of tumor necrosis factor (TNF-α). HIF-1α is an important mediator of inflammatory response, and one of the major transcriptional activators of vascular endothelial growth factor (VEGF) gene expression, plays a critical role in the process of angiogenesis[49].
Zou et al. demonstrated the ability of apigenin to inhibit the process of angiogenesis in vitro and in vivo[50]. Apigenin inhibited the proliferation of human umblical vein endothelial cells (HUVEC) and also choroidal endothelial cells (CEC), in vitro, through the degradation of HIF-1α protein and inhibition of VEGF expression. Moreover, at a dose of 15 and 30 mg/kg (i.p. administration), apigenin exhibited a similar anti-angiogenic activity in a laser induced rat model of chorodial neovascularization (CNV). In an in vitro study, homoisoflavanone was also found to inhibit CNV. It was demonstrated that this flavonoid inhibits expression of fibroblast growth factor (FGF-2), responsible for the blood vessel growth in CNV, induced tube formation and cell invasion of HUVECs[51].
Quercetin, abundantly found in red wine, grapes and other fruits, also inhibited retinal and choroidal angiogenesis, in the rhesus choroid-retina endothelial cell line, RF/6A. Quercetin prevented endothelial cell proliferation, migration, and tube formation in a dose dependent manner[52]. Quercetin's anti-angiogenic activity was thought to be mediated through the inhibition of MMP-2 activation[53]. Other reports also substantiate the anti-angiogenic activity of quercetin[54, 55]. However, it has also been reported that one of the metabolites of quercetin has an opposite effect. Quercetin and quercetin-3′-glucuronide were found to inhibit the VEGF receptor-2 but quercetin-3′-sulphate stimulated the VEGF receptor-2[56].
EGCG, a catechin component of green tea, is another potent inhibitor of angiogenesis. EGCG was reported to inhibit angiogenesis by inhibiting HIF-1α protein expression [57] and in turn VEGF expression[57, 58]. Jung et al. observed that treatment with EGCG (intraperitonial administration) in nude mice decreased tumor growth, microvessel density and tumor cell proliferation. However, the authors reported that other tea catechins such as (-)-epigallocatechin (EGC), (-)-epicatechin gallate (ECG), and (-)-epicatechin (EC) were ineffective in vitro against Erk1/2 (Extracellularly-Regulated Kinase-1 and -2; important mediators in the up-regulation of VEGF expression) activation, whereas EGCG inhibited Erk1/2 activation in a dose dependent manner[59]. Several other flavonoids such as delphinidin[60], silibinin[60], keampferol[60], fisetin[61, 62], luteolin[62, 63] and chrysin[64, 65] are also reported to be anti-angiogenic.
Recently, effect of the number of hydroxyl groups on the B-ring (Fig. 2) of the flavonoid nucleus on anti-angogenic properties has been investigated. Quercetin, kaempferol, galagin, and myrecitin were studied with regard to angiogenesis and cell adhesion inhibition in HUVECs. However, a correlation between the number of hydroxyl groups and VEGF inhibitory potential could not be established [60].
3.5 Anti-inflammatory Activity
Some flavonoids have been reported to inhibit several mediators, that are activated in certain inflammatory conditions, such as nitric oxide (NO), prostanoids and leukotrienes, cytokines, adhesion molecules[66]. NO is produced from L-arginine by three nitric oxide synthase (NOS) enzymes; endothelial NOS (eNOS), neuronal NOS (nNOS) and inducible NOS (iNOS). iNOS is responsible for the production of larger amounts of NO for longer durations. Prostanoids and luekotrienes are also involved in inflammation. Prostaglandins and thromboxan A2 are produced by the cyclooxygenases (COX-1, COX-2 and COX-3). Generally COX-1 is expressed in most tissues (acts in response to hormones and other stimuli) whereas COX-2 is highly expressed in inflammatory cells. Leukotrienes are generated by lipooxygenases (LOX) and 5- and 12-LOXs are associated with the inflammatory processes. Moreover, different cytokines are involved in inflammation and can be pro-inflammatory (interleukins; IL-1β, IL-2, IL-6, IFN-γ or TNF-α) or anti-inflammatory (IL-10, TGF-β). Adhesion molecules also play a role in inflammation; blood vessel endothelial cells characteristically respond to pro-inflammatory stimuli and recruit leukocytes by selectively expressing adhesion molecules on the surface, such as vascular cell adhesion molecules (VCAM-1), intercellular adhesion molecules (ICAM-1) and endothelial cell selectin (E-selectin)[66]. Flavonoids can act on multiple pathways in the inflammation process as summarized in Table 3.
Table 3. General mechanisms by which various flavonoids exert their anti-inflammatory activity.
| S. No | Target pathway | Flavonoid |
|---|---|---|
| 1 | Inhibition of iNOS expression | Quercetin[27]; Quercetin gallate[26]; Baicalin, Baicalein[25]; Hesperidin [28] |
| 2 | Inhibition of COX-2 expression | Hesperidin[28, 85]; Quercetin, kaempferol, genistein, resveratrol[86] |
| 3 | Inhibition of COX-2 and iNOS expression | Hesperidin [28]; Quercetin, galangin, apigenin, and naringenin[87]; Quercetin[88] |
| 4 | Inhibition of vascular cell adhesion molecule-1 (VCAM-1) | Hesperidin[89]; |
| 5 | Inhibition of VCAM-1, Intercellular Adhesion Molecule-1 (ICAM-1), Endothelial Cell Selectin (E-selectin) | Quercetin and kaempferol[88] |
| 6 | Inhibition of Tumor necrosis factor-alpha (TNF-alpha), Interleukin (IL)-1beta, IL-6, and IL-8 | Fisetin, quercetin, and rutin[90] |
| 7 | TNF-α and IL-6 | Myricetin[90] |
Biacalein has been reported to exert anti-inflammatory effects[67]. In a study by Yang et al., treatment with biacalein reduced the inflammatory process in a diabetic retinopathy rat model. This was evident from a decreased secretion of inflammatory (IL-18 and IL-1β) and/or cytotoxic factors (TNF- α)[68]. In another study Nakamura et al., studied the anti-inflammatory effects of baicalin, baicalein, and wogonin on ARPE-19 cell line. It was found that baicalin did not suppress IL-1β induced IL-6 and IL-8 production, but baicalein, and wogonin, significantly suppressed IL-6 and IL-8 production. In addition, nuclear factor-kappaB binding activities were not suppressed by baicalin and baicalein, but were suppressed by wogonin[69].
Naringin and naringenin were able to suppress uveitis in rats induced by foot pad injection of lipopolysaccharide. To see the effect of these two compounds, they were administered intravenously (0.4, 4, and 40 μg/Kg dose) at three different time points; simultaneously, 30 min before and 30 min after injection of the lipopolysaccharide. At the end of 24 h, aqueous humor was collected and prostaglandin E2 and nitric oxide was estimated. It was found that both compounds decreased the levels of prostaglandin E2 and nitric oxide, compared to a control group, in a dose dependent manner[70].
3.6 Aldose Reductase (AR) Inhibitory Activity
In diabetic patients, hyperglycemia is also observed in the aqueous humor. These sugars can passively diffuse into the lens, where aldose reductase converts glucose to sorbitol or galactose to galactilol. High levels of these polyols, thus generated, cannot diffuse out of the lens passively and either accumulates, or is converted to fructose. Therefore an osmotic gradient is generated, inducing diffusion of water into the lens. The resultant swelling and electrolyte imbalance leads to cataract formation[10].
Several flavonoids are reported to inhibit the enzyme AR. Quercitrin is the most promising AR inhibitor and is used as a positive control in many studies. Additionally, a number of the flavonoids including luteolin, Luteolin-7-β-glucoside, nepetin and its 7-glucoside, nepitrin, kaempferol, kaempferol 3-O-glucuronic acid, eriodictyol 7-O-glucuronide and hesperidin were also found to be effective[10, 71].
A comprehensive tabulation of various published reports investigating the potential benefits of different bioflavonoids, particularly those dealing with ocular manifestations, is presented in Table 4.
Table 4. A comprehensive review of various studies carried out till date investigating the potential use of different bioflavonoids in the prevention or treatment of ocular diseases or disorders.
| Flavonoid | Type of study | Type of model used/Route of administration/Dose | Inference |
|---|---|---|---|
| Anthocyanins | Ocular bioavailability[80] | Rabbits and rats; oral, intravenous (i.v.) and intraperitoneal (i.p.) administration | Blackcurrant anthocyanins were absorbed and distributed in ocular tissues in intact forms following i.v. and i.p. administration |
| Anthocyanosides | Effect on night vision | Young normal volunteers; Single oral administrations of 12, 24 and 36 mg[71] and Multiple oral administrations of 12 and 24 mg[72] | Single (12-36mg) and Multiple oral administrations of (12 and 24 mg) anthocyanosides twice a day had insignificant effect on night vision |
| Anthocyanins (Delphinidin, Cyanidin, Petunidin and Malvidin) | Antioxidant effect[91] | Human retinal pigment epithelia (ARPE-19) cell line | Anthocyanins can serve as antioxidants by suppressing photooxidative processes initiated in RPE cells by the lipofuscin fluorophore, A2E |
| Baicalein | A pharmacokinetic and bioavailability study[79] | Topical administration (1% w/v) in rabbits | Baicalein:HP-β-CD solution exhibited superior BA in the aqueous humor compared to plain baicalein suspension |
| Effect on inflammatory process[68] | Oral administration (150 mg/kg/d) in a diabetes induced rat model | Baicalein treatment was able to inhibit inflammatory processes, characterized by microglial activation and Müller cell dysfunction, and inhibited vascular abnormality and neuron loss in diabetic retinas. | |
| Baicalin | Preventive effect against ischemic and oxidative insult to the retinal cells[42] | Intraperitonial (12.5 mg/kg) administration just before and after ischemic insult to the retina in rats Retinal ganglion cell-5 (RGC-5) line (0.1–10 μM) |
Baicalin statistically inhibited most of the effects induced by ischemia/reperfusion (IR); however, the increase in caspase-3 and caspase-8 mRNAs caused by ischemia/reperfusion was unaffected. Baicalin significantly attenuated the negative insult of light, hydrogen peroxide and serum withdrawal on RGC-5 cells in a dose dependent manner. In lipid peroxidation studies, baicalin was found to be equivalent to EGCG in terms of antioxidant activity. |
| Baicalin, Baicalein and Wogonin | Anti-inflammatory activity[69] | ARPE-19 cell line | Baicalin did not suppress interleukin-1beta (IL-1beta) induced IL-6 and IL-8 production, but baicalein, and wogonin, significantly suppressed IL-6 and IL-8 production.Baicalin and baicalein did not suppress Nuclear Factor-kappaB (NF-kappaB) binding activities, which was suppressed by wogonin. |
| Catechin | Protective effect against glutamate induced retinal toxicity[92] | Porcine retinal homogenates (8 nM Concentration) | Catechin suppressed the damage of retinal lipoproteins. |
| (-)-epigallocatechin gallate | Protective effect on UVA-induced damage [74]. | ARPE-19 cell line | EGCG inhibited UVA-induced H2O2 production, mitogen-activating protein kinase activation, and expression of COX-2 |
| Epigallocatechin gallate (EGCG) | Protective effect on UV irradiated human lens epithelial cells[93] | Cultured human lens epithelial cells | EGCG increased the cell count and cell viability after UV irradiation of cultured human lens epithelial cells |
| Attenuating effect on light induced photoreceptor damage[94] | Oral administration in rats, 400 mg/kg body weight/day | EGCG suppressed negative effects of light induced insult to the retina | |
| Protective effect against oxidative stress induced apoptosis [77] | Human lens epithelial cells (HLEB-3) | EGCG protected against cell death caused by H2O2 in HLEB-3 cells | |
| Protective effect on retinal ganglion cells following IR [95] | Intraperitonial injection in female Wistar rats before the IR | EGCG pretreatment decreased retinal ganglion cell death from IR by approximately 10% probably by attenuating neuronal nitric oxide synthase expression and activity. | |
| Protective effect on retinal pigment epithelial cells[96] | ARPE-19 cell line | The administration of EGCG increased the cell count and the cell activity after UV irradiation, suggesting a protective role | |
| Protective effect on retina[34] | Rats and RGC-5 cell line | EGCG provided protection to retinal neurons from oxidative stress and ischemia/reperfusion. | |
| Protection against oxidation-induced retinal degeneration[97] | Wistar rats5 μL of a combination of sodium nitropruside (SNP) and and EGCG (100 and 15 μM in vitreous chamber, respectively) was injected intravitreally | A single bolus injection attenuated the SNP-induced oxidative photoreceptor apoptosis | |
| IOP lowering effect[98] | Intravenous administration of flavonoids in normotensive rabbits (1 mg dose showed effect) | Phenolic antioxidants containing a pyrogallol B-ring system and nonaromatic C-ring (epigallocatechin, epigallocatechin gallate and myricetin) were found to be effective | |
| Daflon® 500mg (Diosmin and hesperidin) | Protective action in ischemia[80] | Gerbil with ischemia-reperfusion injury. 200, 100 and 50 mg/kg, for 6 days before left carotid occlusion | Daflon 500 mg significantly reversed the increase of stroke index only at the dose of 100 mg/kg and significantly decreased levels of hydroxyl free radicals at all 3 doses with a maximum effectiveness for the dose of 100 mg/kg |
| Deguelin | Protective effect in retinopathy[99, 100] | Mouse model of retinopathy, (0.1 μm/1μL i.v.) | Deguelin was found to be a potent inhibitor of choroidal neovascularization (CNV) and may be useful in the treatment of other vasoproliferative retinopathies |
| Eriodictyol | Long term protective effect on RPE cells[41] | ARPE-19 cell line | Eriodictyol was able to induce Nrf2 and phase-2 proteins, heme-oxygenase (HO)-1 and quinone oxidoreductase (NQO)-1. These proteins play a significant role in protecting RPE against oxidative stress. Eriodictyol induced long-term protection was significantly greater than its short-term protection. |
| Eriodictyol, Luteolin, Quercetin, and Taxifolin | Antioxidant activity[101] | Cultured retinal cells | Antioxidant activity was found to be in the order of eriodictyol > quercetin > luteolin > taxifolin |
| Ginkgo Biloba Extract (EGB 761) | Protective effect on retinal injury[102] | Ischemic injury induced in cat retina, 100 mg/kg i.v. | Free radical scavenger EGb 761 efficiently protected the retina from ischemic injury |
| Fisetin | Antioxidant effect on lens epithelial cells[85] | SRA01/04 cell line | Fisetin exhibited anti-catarogenic activity by activating NF-kappaB and mitogen activated protein kinase (MAPK) in UV-induced oxidative stress |
| Fisetin, genistein, luteolin | Effect on corneal neovascularization[62] | Topical application of microemulsion containing flavonoid, 0.5 and 1 ng/mL | Fisetin exhibited the strongest effect followed by Genistein and Luteolin |
| Genistein | Aldose reductase inhibitory activity[103] | Human epithelial cell line (HLE-B3) | Genistein inhibited AR activity in a dose dependent manner. Genistein also exhibited antioxidant activity in HEL-B3 cells. |
| Effect on cataract [104] | The animal model of dietary galactose-induced cataracts in adult male rats 15 mg/kg body weight |
Genistein was not able to completely prevent cataract formation, but did delay progression. | |
| Protective effect on retinal neovascularization [105] | Retinal pigment epithelia-19 cell line 50, 100, 200 μM |
Pretreatment with genistein reduced the expression of interleukin-8, indicated in the development of retinal neovascularization, in a dose dependent manner. | |
| ARPE-19 cell line (10, 20, 50, 100, and 200 μM) [106] | Pretreatment with genistein reduced the expression of basic fibroblast growth factor, indicated in the development of retinal neovascularization, in a dose dependent manner. | ||
| ARPE-19 cell line (50, 100, and 200 μM) [107] [108] | After pretreatment with genistein, hypoxia-evoked HIF-1alpha and VEGF expression was inhibited. Activity was concentration dependent. | ||
| Effect on retinal vascular permeability[109] | Diabetic rats, 150 and 300mg/kg with food, ad libitum | Chronic oral administration of genistein significantly reduced retinal vascular leakage in an animal model of diabetic retinopathy | |
| Effect on proliferative vitreoretinopathy[110] | Rat retinal pigment epithelial cell line, RPE-J | Genistein inhibited RPE cell growth and induced apoptosis. 10 μM inhibited cell proliferation, 50 μM caused growth inhibition and subsequent apoptotic death. | |
| Model of ischemia-reperfusion injury in the rat retina. Intraperitonial administration[111] | Genistein (3.4mg) inhibited the increase in tyrosine phosphorylation and protected the eyes from the induced ischemic retinal degeneration. 0.034 mg and 0.34 mg did not show a significant effect. | ||
| Hesperidin | Transocular permeation[84] | Isolated rabbit ocular tissues | Hesperidin is capable of permeating across ocular tissues like cornea, sclera and choroid-RPE |
| Ocular blood flow[32] | Rabbits, topical application (50 μL of 1 % W/V solution) | Hesperetin increased ocular blood flow in all eye tissues except retina. | |
| Homoisoflavone | Inhibitory effect on CNV[51, 112] | Mouse model of laser-photocoagulation-induced CNV, intravenous administration. Human umbilical vein endothelial cells (HUVECs) |
Homoisoflavanone significantly reduced CNV and capillary leakage. Homoisoflavanone effectively inhibited tube formation and cell migration of HUVECs, in vitro. |
| Mirtogenol® | Effect on ocular blood flow and intraocular hypertension[28] | Asymptomatic human subjects with intraocular hypertension, oral administration for six months | Treatment with Mirtogenol is useful for lowering the risk of developing symptomatic glaucoma by controlling IOP and improving ocular blood flow. |
| Myricetin, quercetin, kaempferol | Protective effect against retinal cells[113] | Bovine retinal cell line | Myricetin, quercetin and kaempferol exhibited approximately 100% protection against A2E (a major fluorophore of lipofuscin) induced toxicity but quercetin was ineffective and kaempferol was poorly active against blue light induced toxicity |
| Naringin and naringenin | Uveitis[70] | Rats, i.v. administration | Both exhibited anti-inflammatory activity by suppressing PGE2 and NO expression. Particularly 40 μg/kg dose (i. v.) demonstrated the activity. |
| Puerarin | Protective effect against diabetic retinopathy[114] | Rats | Puerarin exerts significant protective effects against DR in rats,by regulating the expression of angiogenesis factors (VEGF and HIF-1alpha). |
| Retinal blood flow[115] | Rats, topical application | Puerarin and all its derivatives, except ET (puerarin disubstituted with -CH2CH2OH), showed marked increase of choroidal blood flow at various time periods. | |
| Quercetin | Protective action against oxidative stress [116] | Cultured human RPE cells | Quercetin was able to protect RPE cells from oxidative damage and cellular senescence in vitro in a dose-dependent manner |
| Therapeutic benefit in cataract[117] | Cultured human lens epithelial (HLE) cell line | Quercetin inhibited both a UV- and H2O2-induced decrease of collagen type-I, which has a significant role in cataract formation, via the inhibition of JNK/c-Jun activity | |
| Therapeutic benefit in cataract[118] | HLE cell line | Quercetin, at a low concentration (0.1 μM), protected HLECs and reversed the toxic effects of DMSO (1% v/v). However, at higher concentrations, quercetin was toxic to HLECs with an LD50 of 90.85 μM | |
| Therapeutic benefit in cataract[119] | Rat lens | Quercetin and 3′-O-methyl quercetin both (10 μM)inhibited H2O2 -induced (500 μM) sodium and calcium influx and lens opacification. | |
| Rat lens organ cultured model[120] | Quercetin was active when incubated in the culture medium together with hydrogen peroxide, or when the lens was pre-treated with quercetin prior to oxidative insult, whereas (+)epicatechin and chlorogenic acid, were much less effective | ||
| Flavonoids | Ocular blood flow[30, 31, 33] | Rabbit topical administration | Structure activity relationship with respect to activity of various flavonoids to increase the ocular blood flow was elucidated (refer the text for details). |
| Retinal function recovery[33, 34] | Rat | Structure activity relationship of various flavonoids on retinal function following ischemic insult was elucidated (refer the text for details). | |
| Aldose reductase inhibitory activity[121] | Relationship between the structure and AR inhibitory activity was studied (refer the text for details). |
4. Ocular Drug Delivery
From the above discussion it is apparent that flavonoids hold immense potential in the prevention or treatment of several sight threatening eye diseases or disorders. However, drug delivery to the ocular tissues is a challenging task[72]. Drug delivery to the target site can be attempted through topical, periocular, intravitreal, systemic or oral routes of administration depending on the physicochemical properties of the molecule.
Topical application is the most favored for ocular conditions and involves application of solution, suspension or ointment formulations into the cul-de-sac of the eye. The topical route is mainly used to deliver drugs to the anterior segment of the eye. However, several factors e.g. formulation (aqueous solubility and stability) and permeability / delivery (precorneal drainage, corneal ultrastructure and drainage through the conjunctival vasculature or nasolacrimal duct) issues, limit bioavailability of the administered drug by this route [72, 73]. Periocular administration is the more effective, minimally invasive route of drug administration for the posterior segment of the eye and includes subconjunctival, subtenon, retrobulbar, peribulbar, posterior juxtascleral routes. The physical barriers associated with this route are the sclera, choroid-Bruch's membrane, and retinal pigmented epithelium (RPE). Systemic or oral administration is another option for delivering therapeutic agents to the ocular tissues. However, this route is challenged by several physiological barriers (blood-ocular-barrier (BOB), blood-retinal-barrier (BRB)), and involves unnecessary systemic exposure of the drug. Intravitreal administration, which delivers the drug directly into the vitreous humor is very effective but is invasive in nature.
Physicochemical properties of drug molecules play a very important role in determining ocular bioavailability following topical application. In general there exists a parabolic relationship between oil/water partition-coefficient and corneal bioavailability[72]. Maximum corneal permeability was observed for compounds with log octanol-water partition coefficient (logP) in the range of 2 - 3, for a series of steroids tested[74]. Thus, for efficient ocular tissue permeation, compounds should neither be too lipophilic nor too hydrophilic[72]. Among the different flavonoids, aglycones are more lipophilic compared to their corresponding conjugates. LogP was found to be highly variable within the flavonoid subclass[75] and mostly range between X to X. While considering drug delivery aspects, solubility issues are additional parameters encountered. Solubility of the flavonoids depends to a large extent on the form in which they are available; compounds with one or more sugar moieties are more polar while the alycones will be less polar, with the highly alkylated flavonoids being lipid soluble[76]. Besides solubility and logP, the three dimensional configuration, isomeric structure, number of ring substituents, interaction with membrane influx and efflux transporters, molecular weight and hydrogen bond donors/acceptors of the molecule are other important determinants of ocular tissue diffusion of the flavonoids.
Till date, only a few studies have investigated delivery of flavonoids to the eye. The following section briefly discusses some of these reports.
A pH responsive in situ gelling system for puerarin was developed by Wu et al. to improve the precorneal residence time and thus bioavailability of the drug[77]. Two polymers, carbopol® 980NF and HPMC E4M, were used to develop the formulation. Based on gelling capability, pH, transparency, viscosity and in vitro release profiles, a formulation containing 0.1 % (w/v) carbopol and 0.4 % (w/v) HPMC E4M was identified as an optimized formulation. Bioavailability from this formulation, following topical application, was evaluated in vivo in rabbits. Puerarin eye drops containing 4 % PVP was used as a control. The pH-triggered in situ gelling formulation yielded a 2.17-fold greater AUC0-24h compared to the aqueous solution. The authors also evaluated the in vitro permeability of puerarin across the rabbit cornea and it was found that an aqueous peurarin solution containing 5 % hydroxyl propyl beta cyclodextrin (HP-β-CD) exhibited a 2.5 fold higher permeability than 4 % PVP solution.
Based on the same concept, of increasing precorneal residence time of the drug, another thermosensitive and mucoadhesive in situ gelling system for puerarin was developed by Qi et al.[78]. Using a two-factor, five level central composite design, a formulation containing 21 % (w/v) poloxamer P407 and 5 % (w/v) polaxamer P188 (F1) was considered to be ideal. The formulation exhibited a gelation temperature of 34.8 °C on dilution with artificial tear fluid. In order to improve the mucoadhesive properties of the above formulation, 0.1 % (w/v) (F2) and 0.2 % (w/v) (F3) carbopol 1342P NF was incorporated. These formulations were evaluated in vivo in rabbits, in addition to other control formulations like aqueous solution of the drug in HP-β-CD (F4) and in 0.2% carbopol 1342 NF (F5). Concentration of the puerarin in tear fluids and IOP lowering effect of the drug were evaluated. The AUC of the drug in tears was 4.43 and 5.26 times higher for F2 and F3, respectively, compared to F4. All the formulations were observed to decrease the IOP (a maximum drop of about 4 to 5 mmHg); however effect of F2 and F3 lasted for 24 h compared to 8 h with F4 and between 8 – 24 h for F1 and F5.
Zhang et al studied pharmacokinetics of topically applied baicalein in rabbits[79]. Two formulations of 1% (w/v) baicalein were tested; drug suspension and solution in 10 % HP-β-CD. Drug concentration in aqueous humor and cornea were determined after 5, 10, 20, 30, 45, 60, 90 and 120 min, post dosing. Baicalein-HP-β-CD demonstrated a 2.1-fold higher bioavailability than the suspension formulation.
The distribution of blackcurrant anthocyanins (BAC) in the ocular tissues following oral (100 mg/Kg body weight, rats), intraperitonial (108 mg/Kg body weight, rats) and intravenous (20 mg/Kg body weight, rabbits) administration was investigated by Matsumoto et al. to evaluate the barrier characteristics of the BOB and BRB[80]. Concentration of all four anthocyanins (delphidin-3-rutinoside, delphidin-3-glucoside, cyaniding-3-rutinoside, and cyaniding-3-glucoside) were determined in different ocular tissues and summed together. Following oral administration in rats, intact BACs were detected in the plasma and in the eye; however the concentrations in the eye were very low. Interestingly, when these BAC were administered orally in rabbits (authors did not mention the dose), detectable levels were not observed even in plasma suggesting poor absorption of anthocyanins in rabbits. Comparing the plasma AUCs of BACs in rats following oral (2.56 μg.h/mL) and i.p. (12.3 μg.h/mL) administration, it can be speculated that oral bioavailability of BACs is low even in rats. Following i.p. administration, detectable BAC levels were observed in all the ocular tissues tested and levels are in the following order 1 h after drug administration; sclera with choroid > cornea > ciliary body with iris > retina > aqueous humor > vitreous > lens. However, at the 24 h time point, BAC levels were not detectable in any of the tissues, indicating that anthocyanins were rapidly eliminated from the eye. Following i.v. administration in rabbits, the AUC in various ocular tissues were in the following order; choroid > sclera > ciliary body > cornea > aqueous humor > iris > retina > vitreous > lens. The elimination half-life of the drug ranged between 1.4 to 1.8 h, explaining the absence of drug levels in the ocular tissues at 24 h after i.p. administration. Overall the results, taken together, suggest that BACs exhibit low oral bioavailability but are capable of permeating across the BOB and BRB to reach the inner ocular tissues following i.v or i.p administration. Other reports also support the observation that following oral administration, the bioavailability is limited and mainly the respective Phase-II metabolites (e.g. sulphate, glucuronide conjugates) of the flavonoids are principally observed in the systemic circulation [81-83].
In a recent study, we investigated in vitro permeability of hesperidin across various isolated rabbit ocular tissues [84]. Hesperidin appeared to be fairly permeable across the cornea, sclera, and sclera with choroid-RPE. Expectedly, its permeability across the sclera was much higher, almost 10-fold, compared to the cornea and sclera with choroid-RPE.
5. Conclusion
In summary, it is apparent that the flavonoids are capable of acting on various mechanisms or etiological factors responsible for the development of different sight threatening ocular diseases. Oral bioavailability of the flavonoids, however, is limited by poor intrinsic transmembrane diffusion characteristics, poor solubility and intestinal and hepatic metabolism. The activity of the flavonoid metabolites have not been properly evaluated as yet. From a drug delivery perspective, ocular bioavailability depends on the physicochemical and biopharmaceutical characteristics of the selected flavonoids and very importantly the route of administration. When administered by the oral route, diffusion of the hydrophilic metabolites (the parent compounds undergo rapid hepatic metabolism) from the plasma into the neural retina will be severely restricted by the inner and outer blood retinal barriers. Thus whereas oral administration may demonstrate some pharmacological activity in the outer sections of the posterior ocular segment, protection of the retinal ganglionic cells in vivo may be limited by this delivery route. Systemic or local administration of these agents may yield much higher and effective concentrations of the parent bioflavonoids in the ocular tissues and at much lower doses.
Acknowledgments
This project was supported in part by Grant Numbers, P20RR021929 from the National Center for Research Resources (NIH/NCRR) and EY018426-02 from the National Eye Institute (NIH/NEI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Eye Institute, National Institutes of Health.
List Of Abbreviations
- AMD
Age Related Macular Degeneration
- AR
Aldose Reductase
- ARPE
Cell line arising from Retinal Pigmented Epithelium
- AUC
Area Under Curve
- BAC
Blackcurrant Anthocyanins
- BOB
Blood-Ocular-Barrier
- BRB
Blood-Retinal-Barrier
- CEC
Choroidal Endothelial Cells
- CNV
Choroidal Neovascularization
- COX
Cyclooxygenase
- DR
Diabetic Retinopathy
- ECG
Epicatechin Gallate
- EGC
Epigallocatechin
- EGCG
Epigallocatechin gallate
- EGF
Epidermal Growth Factor
- eNOS
Endothelial Nitricoxide Synthase
- Erk1/2
Extracellularly Regualted Kinase-1 and -2
- FGF-2
Fibroblast Growth Factor-2
- GBE
Ginkgo Biloba Extract
- GON
Glaucomatous Optic Neuropathy
- H2O2
Hydrogen Peroxide
- HIF
Hypoxia Inducible Factor
- HMC-1
Human mast cell line
- HPMC
Hydroxy Propyl Methyl Cellulose
- HPβCD
Hydroxy Propyl beta Cyclodextrin
- HUVEC
Human Umblical Vein Endothelial cells
- i.p
intraperitonial
- i.v
intravenous
- IAA
Iodoacetic Acid
- ICAM
Intercellular Adhesion Molecules
- IL
Interleukins
- iNOS
Inducible Nitricoxide Synthase
- IOP
Intra Ocular Pressure
- LOX
Lipooxygenases
- MPFF
Micronized Purified Flavonoid Fraction
- NADH
Nicotinamide Adenine Dinucleotide
- nNOS
Neuronal Nitricoxide Synthase
- NO
Nitric oxide
- Nrf2
Nuclear erythoid-2 p45-related factor-2
- PACG
Primary Angle-Closure Glaucoma
- PDR
Proliferative Diabetic Retinopathy
- POAG
Primary Open-Angle Glaucoma
- PVP
Poly Vinyl Pyrrolidone
- ROI
Reactive Oxygen Intermediates
- ROS
Reactive Oxygen Species
- t-BOOH
tertiary-butyl peroxide
- TNF-α
Tumor Necrosis Factor-α
- VCAM
Vascular Adhesion Molecules
- VEGF
Vascular Endothelial Growth Factor
- WHO
World Health Organization
References
- 1.Resnikoff S, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 2.Ryskulova A, et al. Self-reported age-related eye diseases and visual impairment in the United States: results of the 2002 national health interview survey. Am J Public Health. 2008;98:454–461. doi: 10.2105/AJPH.2006.098202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foster A, et al. Changing patterns in global blindness: 1988-2008. Community Eye Health. 2008;21:37–39. [PMC free article] [PubMed] [Google Scholar]
- 4.Davies KM, Schwinn KE. Flavonoids: chemistry, biochemistry and applications. CRC Press; 2006. [Google Scholar]
- 5.Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 6.Crozier A, et al. Dietary phenolics: chemistry, bioavailability and effects on health. Nat Prod Rep. 2009;26:1001–1043. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- 7.Ohia SE, et al. Pharmacological consequences of oxidative stress in ocular tissues. Mutat Res. 2005;579:22–36. doi: 10.1016/j.mrfmmm.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 8.Erickson KK, et al. Vascular permeability in ocular disease and the role of tight junctions. Angiogenesis. 2007;10:103–117. doi: 10.1007/s10456-007-9067-z. [DOI] [PubMed] [Google Scholar]
- 9.Kyselova Z, et al. Pharmacological prevention of diabetic cataract. J Diabetes Complications. 2004;18:129–140. doi: 10.1016/S1056-8727(03)00009-6. [DOI] [PubMed] [Google Scholar]
- 10.Head KA. Natural therapies for ocular disorders, part two: cataracts and glaucoma. Altern Med Rev. 2001;6:141–166. [PubMed] [Google Scholar]
- 11.Wilkinson-Berka JL. Vasoactive factors and diabetic retinopathy: vascular endothelial growth factor, cycoloxygenase-2 and nitric oxide. Curr Pharm Des. 2004;10:3331–3348. doi: 10.2174/1381612043383142. [DOI] [PubMed] [Google Scholar]
- 12.Singh R, et al. Diabetic retinopathy: an update. Indian J Ophthalmol. 2008;56:178–188. [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman DS, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 14.Beatty S, et al. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 15.Infeld DA, O'Shea JG. Glaucoma: diagnosis and management. Postgrad Med J. 1998;74:709–715. doi: 10.1136/pgmj.74.878.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coleman AL. Glaucoma. Lancet. 1999;354:1803–1810. doi: 10.1016/S0140-6736(99)04240-3. [DOI] [PubMed] [Google Scholar]
- 17.Mozaffarieh M, et al. Oxygen and blood flow: players in the pathogenesis of glaucoma. Mol Vis. 2008;14:224–233. [PMC free article] [PubMed] [Google Scholar]
- 18.Izzotti A, et al. The role of oxidative stress in glaucoma. Mutat Res. 2006;612:105–114. doi: 10.1016/j.mrrev.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Flammer J, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–393. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 20.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 21.Wakamatsu T, et al. Tearful relations: oxidative stress, inflammation and eye diseases. Arquivos Brasileiros de Oftalmologia. 2008;71:72–79. doi: 10.1590/s0004-27492008000700015. [DOI] [PubMed] [Google Scholar]
- 22.Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000;63:1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 23.Nijveldt RJ, et al. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–425. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 24.Rice-Evans CA, et al. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 25.Chen YC, et al. Wogonin, baicalin, and baicalein inhibition of inducible nitric oxide synthase and cyclooxygenase-2 gene expressions induced by nitric oxide synthase inhibitors and lipopolysaccharide. Biochem Pharmacol. 2001;61:1417–1427. doi: 10.1016/s0006-2952(01)00594-9. [DOI] [PubMed] [Google Scholar]
- 26.Kim BH, et al. Down-regulatory effect of quercitrin gallate on nuclear factor-kappa B-dependent inducible nitric oxide synthase expression in lipopolysaccharide-stimulated macrophages RAW 264.7. Biochem Pharmacol. 2005;69:1577–1583. doi: 10.1016/j.bcp.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Florez S, et al. Quercetin attenuates nuclear factor-kappaB activation and nitric oxide production in interleukin-1beta-activated rat hepatocytes. J Nutr. 2005;135:1359–1365. doi: 10.1093/jn/135.6.1359. [DOI] [PubMed] [Google Scholar]
- 28.Sakata K, et al. Inhibition of inducible isoforms of cyclooxygenase and nitric oxide synthase by flavonoid hesperidin in mouse macrophage cell line. Cancer Lett. 2003;199:139–145. doi: 10.1016/s0304-3835(03)00386-0. [DOI] [PubMed] [Google Scholar]
- 29.Pemp B, Schmetterer L. Ocular blood flow in diabetes and age-related macular degeneration. Can J Ophthalmol. 2008;43:295–301. doi: 10.3129/i08-049. [DOI] [PubMed] [Google Scholar]
- 30.Park YH, Chiou GC. Structure-activity relationship (SAR) between some natural flavonoids and ocular blood flow in the rabbit. J Ocul Pharmacol Ther. 2004;20:35–42. doi: 10.1089/108076804772745446. [DOI] [PubMed] [Google Scholar]
- 31.Xu XR, et al. Effects of dihydrogenation of flavones and number of hydroxy groups in the molecules on ocular blood flow in rabbits and retinal function recovery in rats. J Ocul Pharmacol Ther. 2004;20:311–320. doi: 10.1089/1080768041725290. [DOI] [PubMed] [Google Scholar]
- 32.Liu SX, et al. Increase of ocular blood flow by some phytogenic compounds. J Ocul Pharmacol Ther. 1996;12:95–101. doi: 10.1089/jop.1996.12.95. [DOI] [PubMed] [Google Scholar]
- 33.Park YH, et al. Structural requirements of flavonoids for increment of ocular blood flow in the rabbit and retinal function recovery in rat eyes. J Ocul Pharmacol Ther. 2004;20:189–200. doi: 10.1089/1080768041223666. [DOI] [PubMed] [Google Scholar]
- 34.Chiou GC, Xu XR. Effects of some natural flavonoids on retinal function recovery after ischemic insult in the rat. J Ocul Pharmacol Ther. 2004;20:107–113. doi: 10.1089/108076804773710777. [DOI] [PubMed] [Google Scholar]
- 35.Chung HS, et al. Ginkgo biloba extract increases ocular blood flow velocity. J Ocul Pharmacol Ther. 1999;15:233–240. doi: 10.1089/jop.1999.15.233. [DOI] [PubMed] [Google Scholar]
- 36.Wimpissinger B, et al. Influence of Ginkgo biloba on ocular blood flow. Acta Ophthalmol Scand. 2007;85:445–449. doi: 10.1111/j.1600-0420.2007.00887.x. [DOI] [PubMed] [Google Scholar]
- 37.Steigerwalt RD, et al. Effects of Mirtogenol on ocular blood flow and intraocular hypertension in asymptomatic subjects. Mol Vis. 2008;14:1288–1292. [PMC free article] [PubMed] [Google Scholar]
- 38.Maher P, Hanneken A. Flavonoids protect retinal ganglion cells from oxidative stress-induced death. Invest Ophthalmol Vis Sci. 2005;46:4796–4803. doi: 10.1167/iovs.05-0397. [DOI] [PubMed] [Google Scholar]
- 39.Maher P, Hanneken A. Flavonoids protect retinal ganglion cells from ischemia in vitro. Exp Eye Res. 2008;86:366–374. doi: 10.1016/j.exer.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Hanneken A, et al. Flavonoids protect human retinal pigment epithelial cells from oxidative-stress-induced death. Invest Ophthalmol Vis Sci. 2006;47:3164–3177. doi: 10.1167/iovs.04-1369. [DOI] [PubMed] [Google Scholar]
- 41.Johnson J, et al. The flavonoid, eriodictyol, induces long-term protection in ARPE-19 cells through its effects on Nrf2 activation and phase 2 gene expression. Invest Ophthalmol Vis Sci. 2009;50:2398–2406. doi: 10.1167/iovs.08-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jung SH, et al. The flavonoid baicalin counteracts ischemic and oxidative insults to retinal cells and lipid peroxidation to brain membranes. Neurochem Int. 2008;53:325–337. doi: 10.1016/j.neuint.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 43.Zhang B, et al. Orally administered epigallocatechin gallate attenuates retinal neuronal death in vivo and light-induced apoptosis in vitro. Brain Res. 2008;1198:141–152. doi: 10.1016/j.brainres.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 44.Zhang B, et al. Epigallocatechin gallate, an active ingredient from green tea, attenuates damaging influences to the retina caused by ischemia/reperfusion. Brain Res. 2007;1159:40–53. doi: 10.1016/j.brainres.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 45.Hirooka K, et al. The Ginkgo biloba extract (EGb 761) provides a neuroprotective effect on retinal ganglion cells in a rat model of chronic glaucoma. Curr Eye Res. 2004;28:153–157. doi: 10.1076/ceyr.28.3.153.26246. [DOI] [PubMed] [Google Scholar]
- 46.Mojzis J, et al. Antiangiogenic effects of flavonoids and chalcones. Pharmacol Res. 2008;57:259–265. doi: 10.1016/j.phrs.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Stoclet JC, et al. Vascular protection by dietary polyphenols. Eur J Pharmacol. 2004;500:299–313. doi: 10.1016/j.ejphar.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 48.Paysant J, et al. Different flavonoids present in the micronized purified flavonoid fraction (Daflon 500 mg) contribute to its anti-hyperpermeability effect in the hamster cheek pouch microcirculation. Int Angiol. 2008;27:81–85. [PubMed] [Google Scholar]
- 49.Choi IY, et al. Hesperidin inhibits expression of hypoxia inducible factor-1 alpha and inflammatory cytokine production from mast cells. Mol Cell Biochem. 2007;305:153–161. doi: 10.1007/s11010-007-9539-x. [DOI] [PubMed] [Google Scholar]
- 50.Zou Y, Chiou GC. Apigenin inhibits laser-induced choroidal neovascularization and regulates endothelial cell function. J Ocul Pharmacol Ther. 2006;22:425–430. doi: 10.1089/jop.2006.22.425. [DOI] [PubMed] [Google Scholar]
- 51.Kim JH, et al. Inhibition of choroidal neovascularization by homoisoflavanone, a new angiogenesis inhibitor. Mol Vis. 2008;14:556–561. [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y, et al. Quercetin inhibits choroidal and retinal angiogenesis in vitro. Graefes Arch Clin Exp Ophthalmol. 2008;246:373–378. doi: 10.1007/s00417-007-0728-9. [DOI] [PubMed] [Google Scholar]
- 53.Tan WF, et al. Quercetin, a dietary-derived flavonoid, possesses antiangiogenic potential. Eur J Pharmacol. 2003;459:255–262. doi: 10.1016/s0014-2999(02)02848-0. [DOI] [PubMed] [Google Scholar]
- 54.Jackson SJ, Venema RC. Quercetin inhibits eNOS, microtubule polymerization, and mitotic progression in bovine aortic endothelial cells. J Nutr. 2006;136:1178–1184. doi: 10.1093/jn/136.5.1178. [DOI] [PubMed] [Google Scholar]
- 55.Igura K, et al. Resveratrol and quercetin inhibit angiogenesis in vitro. Cancer Lett. 2001;171:11–16. doi: 10.1016/s0304-3835(01)00443-8. [DOI] [PubMed] [Google Scholar]
- 56.Donnini S, et al. Divergent effects of quercetin conjugates on angiogenesis. Br J Nutr. 2006;95:1016–1023. doi: 10.1079/bjn20061753. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Q, et al. Green tea extract and (-)-epigallocatechin-3-gallate inhibit hypoxia- and serum-induced HIF-1alpha protein accumulation and VEGF expression in human cervical carcinoma and hepatoma cells. Mol Cancer Ther. 2006;5:1227–1238. doi: 10.1158/1535-7163.MCT-05-0490. [DOI] [PubMed] [Google Scholar]
- 58.Shirakami Y, et al. (-)-Epigallocatechin gallate suppresses the growth of human hepatocellular carcinoma cells by inhibiting activation of the vascular endothelial growth factor-vascular endothelial growth factor receptor axis. Cancer Sci. 2009 doi: 10.1111/j.1349-7006.2009.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jung YD, et al. EGCG, a major component of green tea, inhibits tumour growth by inhibiting VEGF induction in human colon carcinoma cells. Br J Cancer. 2001;84:844–850. doi: 10.1054/bjoc.2000.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim JD, et al. Chemical structure of flavonols in relation to modulation of angiogenesis and immune-endothelial cell adhesion. J Nutr Biochem. 2006;17:165–176. doi: 10.1016/j.jnutbio.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 61.Sung B, et al. Fisetin, an inhibitor of cyclin-dependent kinase 6, down-regulates nuclear factor-kappaB-regulated cell proliferation, antiapoptotic and metastatic gene products through the suppression of TAK-1 and receptor-interacting protein-regulated IkappaBalpha kinase activation. Mol Pharmacol. 2007;71:1703–1714. doi: 10.1124/mol.107.034512. [DOI] [PubMed] [Google Scholar]
- 62.Joussen AM, et al. Treatment of corneal neovascularization with dietary isoflavonoids and flavonoids. Exp Eye Res. 2000;71:483–487. doi: 10.1006/exer.2000.0900. [DOI] [PubMed] [Google Scholar]
- 63.Bagli E, et al. Luteolin inhibits vascular endothelial growth factor-induced angiogenesis; inhibition of endothelial cell survival and proliferation by targeting phosphatidylinositol 3′-kinase activity. Cancer Res. 2004;64:7936–7946. doi: 10.1158/0008-5472.CAN-03-3104. [DOI] [PubMed] [Google Scholar]
- 64.Fu B, et al. Chrysin inhibits expression of hypoxia-inducible factor-1alpha through reducing hypoxia-inducible factor-1alpha stability and inhibiting its protein synthesis. Mol Cancer Ther. 2007;6:220–226. doi: 10.1158/1535-7163.MCT-06-0526. [DOI] [PubMed] [Google Scholar]
- 65.Lin CM, et al. Chrysin inhibits lipopolysaccharide-induced angiogenesis via down-regulation of VEGF/VEGFR-2(KDR) and IL-6/IL-6R pathways. Planta Med. 2006;72:708–714. doi: 10.1055/s-2006-931602. [DOI] [PubMed] [Google Scholar]
- 66.Tunon MJ, et al. Potential of flavonoids as anti-inflammatory agents: modulation of pro-inflammatory gene expression and signal transduction pathways. Curr Drug Metab. 2009;10:256–271. doi: 10.2174/138920009787846369. [DOI] [PubMed] [Google Scholar]
- 67.Wakabayashi I. Inhibitory effects of baicalein and wogonin on lipopolysaccharide-induced nitric oxide production in macrophages. Pharmacol Toxicol. 1999;84:288–291. doi: 10.1111/j.1600-0773.1999.tb01496.x. [DOI] [PubMed] [Google Scholar]
- 68.Yang LP, et al. Baicalein reduces inflammatory process in a rodent model of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50:2319–2327. doi: 10.1167/iovs.08-2642. [DOI] [PubMed] [Google Scholar]
- 69.Nakamura N, et al. Effects of baicalin, baicalein, and wogonin on interleukin-6 and interleukin-8 expression, and nuclear factor-kappab binding activities induced by interleukin-1beta in human retinal pigment epithelial cell line. Exp Eye Res. 2003;77:195–202. doi: 10.1016/s0014-4835(03)00116-7. [DOI] [PubMed] [Google Scholar]
- 70.Shiratori K, et al. The effects of naringin and naringenin on endotoxin-induced uveitis in rats. J Ocul Pharmacol Ther. 2005;21:298–304. doi: 10.1089/jop.2005.21.298. [DOI] [PubMed] [Google Scholar]
- 71.Kawanishi K, et al. Aldose reductase inhibitors from the nature. Curr Med Chem. 2003;10:1353–1374. doi: 10.2174/0929867033457304. [DOI] [PubMed] [Google Scholar]
- 72.Sreeraj M, et al. Ophthalmic drug delivery systems. Vol. 2. Marcel Dekker, Inc.; 2003. [Google Scholar]
- 73.Maurice D, Mishima S. Ocular Pharmacokinetics. In: Sears ML, editor. Handbook of experimental pharmacology: pharmacology of the eye. Vol. 69. 1986. pp. 19–116. [Google Scholar]
- 74.Schoenwald RD, Ward RL. Relationship between steroid permeability across excised rabbit cornea and octanol-water partition coefficients. J Pharm Sci. 1978;67:786–788. doi: 10.1002/jps.2600670614. [DOI] [PubMed] [Google Scholar]
- 75.Rothwell JA, et al. Experimental determination of octanol-water partition coefficients of quercetin and related flavonoids. J Agric Food Chem. 2005;53:4355–4360. doi: 10.1021/jf0483669. [DOI] [PubMed] [Google Scholar]
- 76.Bohm B. Introduction to flavonoids. Taylor & Francis; 1998. [Google Scholar]
- 77.Wu C, et al. Preparation and evaluation of a Carbopol/HPMC-based in situ gelling ophthalmic system for puerarin. Yakugaku Zasshi. 2007;127:183–191. doi: 10.1248/yakushi.127.183. [DOI] [PubMed] [Google Scholar]
- 78.Qi H, et al. Development of a poloxamer analogs/carbopol-based in situ gelling and mucoadhesive ophthalmic delivery system for puerarin. Int J Pharm. 2007;337:178–187. doi: 10.1016/j.ijpharm.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 79.Zhang L, et al. Ocular pharmacokinetics and availability of topically applied baicalein in rabbits. Curr Eye Res. 2009;34:257–263. doi: 10.1080/02713680902725962. [DOI] [PubMed] [Google Scholar]
- 80.Matsumoto H, et al. Comparative assessment of distribution of blackcurrant anthocyanins in rabbit and rat ocular tissues. Exp Eye Res. 2006;83:348–356. doi: 10.1016/j.exer.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 81.Manach C, et al. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 82.Manach C, Donovan JL. Pharmacokinetics and metabolism of dietary flavonoids in humans. Free Radic Res. 2004;38:771–785. doi: 10.1080/10715760410001727858. [DOI] [PubMed] [Google Scholar]
- 83.Prasain JK, Barnes S. Metabolism and bioavailability of flavonoids in chemoprevention: current analytical strategies and future prospectus. Mol Pharm. 2007;4:846–864. doi: 10.1021/mp700116u. [DOI] [PubMed] [Google Scholar]
- 84.Majumdar S, Srirangam R. Solubility, stability, physicochemical characteristics and in vitro ocular tissue permeability of hesperidin: a natural bioflavonoid. Pharm Res. 2009;26:1217–1225. doi: 10.1007/s11095-008-9729-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hirata A, et al. Kinetics of radical-scavenging activity of hesperetin and hesperidin and their inhibitory activity on COX-2 expression. Anticancer Res. 2005;25:3367–3374. [PubMed] [Google Scholar]
- 86.Mutoh M, et al. Suppression of cyclooxygenase-2 promoter-dependent transcriptional activity in colon cancer cells by chemopreventive agents with a resorcin-type structure. Carcinogenesis. 2000;21:959–963. doi: 10.1093/carcin/21.5.959. [DOI] [PubMed] [Google Scholar]
- 87.Raso GM, et al. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage J774A.1. Life Sci. 2001;68:921–931. doi: 10.1016/s0024-3205(00)00999-1. [DOI] [PubMed] [Google Scholar]
- 88.Crespo I, et al. A comparison of the effects of kaempferol and quercetin on cytokine-induced pro-inflammatory status of cultured human endothelial cells. Br J Nutr. 2008;100:968–976. doi: 10.1017/S0007114508966083. [DOI] [PubMed] [Google Scholar]
- 89.Nizamutdinova IT, et al. Hesperidin, hesperidin methyl chalone and phellopterin from Poncirus trifoliata (Rutaceae) differentially regulate the expression of adhesion molecules in tumor necrosis factor-alpha-stimulated human umbilical vein endothelial cells. Int Immunopharmacol. 2008;8:670–678. doi: 10.1016/j.intimp.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 90.Park HH, et al. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch Pharm Res. 2008;31:1303–1311. doi: 10.1007/s12272-001-2110-5. [DOI] [PubMed] [Google Scholar]
- 91.Jang YP, et al. Anthocyanins protect against A2E photooxidation and membrane permeabilization in retinal pigment epithelial cells. Photochem Photobiol. 2005;81:529–536. doi: 10.1562/2004-12-14-RA-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Siu AW, et al. Glutamate-induced retinal lipid and protein damage: the protective effects of catechin. Neurosci Lett. 2008;432:193–197. doi: 10.1016/j.neulet.2007.12.046. [DOI] [PubMed] [Google Scholar]
- 93.Heo J, et al. Protective effects of epigallocatechin gallate after UV irradiation of cultured human lens epithelial cells. Korean J Ophthalmol. 2008;22:183–186. doi: 10.3341/kjo.2008.22.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Costa BL, et al. Orally administered epigallocatechin gallate attenuates light-induced photoreceptor damage. Brain Res Bull. 2008;76:412–423. doi: 10.1016/j.brainresbull.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 95.Peng PH, et al. Epigallocatechin-3-gallate reduces retinal ischemia/reperfusion injury by attenuating neuronal nitric oxide synthase expression and activity. Exp Eye Res. 2008;86:637–646. doi: 10.1016/j.exer.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 96.Yang SW, et al. Protective effects of epigallocatechin gallate after UV irradiation in cultured human retinal pigment epithelial cells. Korean J Ophthalmol. 2007;21:232–237. doi: 10.3341/kjo.2007.21.4.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang B, Osborne NN. Oxidative-induced retinal degeneration is attenuated by epigallocatechin gallate. Brain Res. 2006;1124:176–187. doi: 10.1016/j.brainres.2006.09.067. [DOI] [PubMed] [Google Scholar]
- 98.Hodges LC, et al. Intraocular pressure-lowering activity of phenolic antioxidants in normotensive rabbits. Curr Eye Res. 1999;19:234–240. doi: 10.1076/ceyr.19.3.234.5320. [DOI] [PubMed] [Google Scholar]
- 99.Kim JH, et al. Deguelin inhibits retinal neovascularization by down-regulation of HIF-1alpha in oxygen-induced retinopathy. J Cell Mol Med. 2008;12:2407–2415. doi: 10.1111/j.1582-4934.2008.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim JH, et al. Antiangiogenic effect of deguelin on choroidal neovascularization. J Pharmacol Exp Ther. 2008;324:643–647. doi: 10.1124/jpet.107.132720. [DOI] [PubMed] [Google Scholar]
- 101.Areias FM, et al. Antioxidant effect of flavonoids after ascorbate/Fe(2+)-induced oxidative stress in cultured retinal cells. Biochem Pharmacol. 2001;62:111–118. doi: 10.1016/s0006-2952(01)00621-9. [DOI] [PubMed] [Google Scholar]
- 102.Kim SY, et al. The protection of the retina from ischemic injury by the free radical scavenger EGb 761 and zinc in the cat retina. Ophthalmologica. 1998;212:268–274. doi: 10.1159/000027305. [DOI] [PubMed] [Google Scholar]
- 103.Kim YS, et al. Genistein inhibits aldose reductase activity and high glucose-induced TGF-beta2 expression in human lens epithelial cells. Eur J Pharmacol. 2008;594:18–25. doi: 10.1016/j.ejphar.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 104.Huang R, et al. Effect of the isoflavone genistein against galactose-induced cataracts in rats. Exp Biol Med (Maywood) 2007;232:118–125. [PubMed] [Google Scholar]
- 105.Li H, et al. Inhibitive effect of genistein on interleukin-8 expression in cultured human retinal pigment epithelial cells. Methods Find Exp Clin Pharmacol. 2006;28:295–299. doi: 10.1358/mf.2006.28.5.1000337. [DOI] [PubMed] [Google Scholar]
- 106.Pan JS, et al. Inhibitive effect of genistein on hypoxia-induced basic fibroblast growth factor expression in human retinal pigment epithelium cells. J Ocul Pharmacol Ther. 2006;22:103–109. doi: 10.1089/jop.2006.22.103. [DOI] [PubMed] [Google Scholar]
- 107.Wang B, et al. Genistein inhibited hypoxia-inducible factor-1alpha expression induced by hypoxia and cobalt chloride in human retinal pigment epithelium cells. Methods Find Exp Clin Pharmacol. 2005;27:179–184. doi: 10.1358/mf.2005.27.3.890875. [DOI] [PubMed] [Google Scholar]
- 108.Wang B, et al. Genistein suppressed upregulation of vascular endothelial growth factor expression by cobalt chloride and hypoxia in rabbit retinal pigment epithelium cells. J Ocul Pharmacol Ther. 2003;19:457–464. doi: 10.1089/108076803322473015. [DOI] [PubMed] [Google Scholar]
- 109.Nakajima M, et al. Normalization of retinal vascular permeability in experimental diabetes with genistein. Invest Ophthalmol Vis Sci. 2001;42:2110–2114. [PubMed] [Google Scholar]
- 110.Yoon HS, et al. Genistein produces reduction in growth and induces apoptosis of rat RPE-J cells. Curr Eye Res. 2000;20:215–224. [PubMed] [Google Scholar]
- 111.Hayashi A, et al. Genistein, a protein tyrosine kinase inhibitor, ameliorates retinal degeneration after ischemia-reperfusion injury in rat. Invest Ophthalmol Vis Sci. 1997;38:1193–1202. [PubMed] [Google Scholar]
- 112.Kim JH, et al. Homoisoflavanone inhibits retinal neovascularization through cell cycle arrest with decrease of cdc2 expression. Biochem Biophys Res Commun. 2007;362:848–852. doi: 10.1016/j.bbrc.2007.08.100. [DOI] [PubMed] [Google Scholar]
- 113.Laabich A, et al. Protective effects of myricetin and related flavonols against A2E and light mediated-cell death in bovine retinal primary cell culture. Exp Eye Res. 2007;85:154–165. doi: 10.1016/j.exer.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 114.Teng Y, et al. Protective effect of puerarin on diabetic retinopathy in rats. Mol Biol Rep. 2009;36:1129–1133. doi: 10.1007/s11033-008-9288-2. [DOI] [PubMed] [Google Scholar]
- 115.Xuan B, et al. Improvement of ocular blood flow and retinal functions with puerarin analogs. J Ocul Pharmacol Ther. 1999;15:207–216. doi: 10.1089/jop.1999.15.207. [DOI] [PubMed] [Google Scholar]
- 116.Kook D, et al. The protective effect of quercetin against oxidative stress in the human RPE in vitro. Invest Ophthalmol Vis Sci. 2008;49:1712–1720. doi: 10.1167/iovs.07-0477. [DOI] [PubMed] [Google Scholar]
- 117.Jiang Q, et al. Quercetin attenuates UV- and H(2)O(2)-induced decrease of collagen type I in cultured human lens epithelial cells. J Ocul Pharmacol Ther. 2008;24:164–174. doi: 10.1089/jop.2007.0073. [DOI] [PubMed] [Google Scholar]
- 118.Cao XG, et al. Responses of human lens epithelial cells to quercetin and DMSO. Invest Ophthalmol Vis Sci. 2007;48:3714–3718. doi: 10.1167/iovs.06-1304. [DOI] [PubMed] [Google Scholar]
- 119.Cornish KM, et al. Quercetin metabolism in the lens: role in inhibition of hydrogen peroxide induced cataract. Free Radic Biol Med. 2002;33:63–70. doi: 10.1016/s0891-5849(02)00843-2. [DOI] [PubMed] [Google Scholar]
- 120.Sanderson J, et al. Quercetin inhibits hydrogen peroxide-induced oxidation of the rat lens. Free Radic Biol Med. 1999;26:639–645. doi: 10.1016/s0891-5849(98)00262-7. [DOI] [PubMed] [Google Scholar]
- 121.Matsuda H, et al. Structural requirements of flavonoids and related compounds for aldose reductase inhibitory activity. Chem Pharm Bull (Tokyo) 2002;50:788–795. doi: 10.1248/cpb.50.788. [DOI] [PubMed] [Google Scholar]