Abstract
A fundamental issue in psychiatric medicine is the lack of empirical evidence indicating when, during development, a treatment will be most effective for a patient. We review behavioral and brain changes that occur across development, focusing on the period of adolescence, when there is a peak in diagnosis of many psychiatric disorders. We use anxiety disorders as an example because of their high prevalence in youth (affecting as many as 1 in 10). Basic forms of fear learning, which are at the core of anxiety disorders and are the targets of behavioral therapeutics, are examined as a function of age. We also discuss how fear learning has been genetically modulated in mice and humans. Based on these findings, we provide future directions for determining the efficacy of innovative therapies and preventive strategies for anxiety disorders as a function of age and potential genetic effects inferred from mice and humans.
Keywords: adolescence, anxiety, development, genetics, psychiatric treatment
INTRODUCTION
The fundamental goal of psychiatric medicine has been to characterize mental illness through diagnostics to direct therapeutics. In the past two decades, the field has moved toward preventive and personalized medicine using biological and epidemiological factors that suggest who is at risk and what treatments may be most effective. A fundamental issue that remains is the lack of empirical evidence for when, during development, an intervention or treatment will be most (or least) effective for preventing or treating mental illness. A mainstream assumption about treatment is that the earlier a disorder is treated, the better the outcome. Although this assumption may hold true in part, it ignores a large literature on significant changes that occur across development. In this article, we review behavioral and brain changes that occur across development and vary across individuals, focusing on the period of adolescence when there is a peak in diagnosis of many psychiatric disorders. Because the anxiety disorders are the most common of the psychiatric illnesses in our youth today, affecting as many as 1 in 10 (1–5), we use anxiety as an example for illustrating the importance of considering the developing brain when considering treatment of mental illnesses.
We examine forms of fear learning, which are at the core of anxiety disorders and are the targets of behavioral therapeutics, as a function of age and how they are genetically modulated in mice and humans. We then build on these empirical findings to suggest for whom and when behavioral treatments for anxiety disorders may be most effective and suggest novel therapies and preventive strategies.
Anxiety and Fear
A core feature of anxiety disorders is difficulty in learning which contexts or cues signal safety and which signal a threat and in learning to suppress these associations when they no longer apply. Fear learning is an adaptive process that involves the formation, or acquisition, of associations between aversive events and co-occurring cues and contexts that may be predictive of danger (6–8). Without the ability to process and respond to fear, we would be unable to identify danger-associated cues necessary for our survival. However, when previously threatening cues become safe, we must be able to reassess them and learn to suppress previous fear associations. In the case of anxiety disorders, fear may persist long after an environmental threat has passed. This unremitting form of fear is a core component of many anxiety disorders (9–12).
Evidenced-Based Behavioral Therapies
The only evidenced-based behavioral therapies for anxiety disorders build upon the basic principles of fear learning. Specifically, cognitive behavioral therapy identifies an individual’s triggers of anxiety and then desensitizes the individual to that fear. This desensitization process of repeated exposure to the fear-eliciting event, in the absence of actual negative consequences, is based on the principles of fear extinction learning. The patient learns to acquire new associations so that a cue previously associated with threat gains a new association of safety. Successful treatment results in activation of the safe association over the threatening one and thus a diminished fear response. Unfortunately, only 50%–60% of individuals with anxiety disorders respond to this form of treatment (13), a rate that invites the questions of who responds and when during development do patients have the best treatment outcome.
This article examines the development of neural circuitry underlying core features of anxiety (e.g., persistence of fear when no threat is present), especially during adolescence, when the diagnosis peaks. We begin by describing the basic neural circuitry implicated in fear learning and regulation. We then provide scientific evidence from three domains that together may inform the timing, duration, and type of treatment for individuals with anxiety disorders. First, we describe findings from human imaging studies that show changes in neural circuitry underlying features of anxiety in healthy individuals during the transition into and out of adolescence. Second, we provide parallel evidence in humans and mice for attenuation of basic fear regulation processes during adolescence and the underlying biological substrates that parallel the human imaging work. Third, we describe a translational genetic approach from mouse to human to identify individual factors that alter fear regulation and the underlying neural circuitry during adolescence. Together, these approaches provide an appreciation of how brain development impacts emotional responses and risk for pathologic fear. Having established ways in which developmental and individual factors influence fear responses and fear-related disorders, we discuss ways in which this information can be used to deliver optimal treatments to the most responsive people at the most appropriate time.
FEAR REGULATION CIRCUITRY
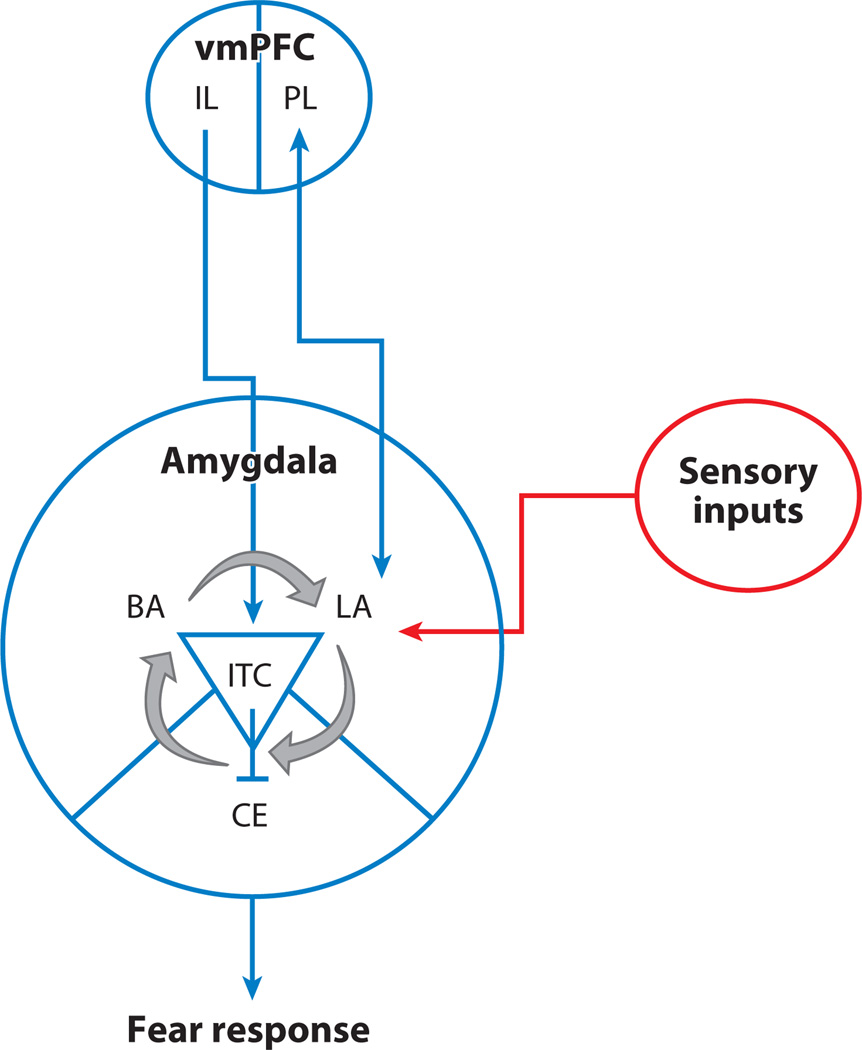
The circuitry involved in fear regulation in adults has been delineated in human imaging and rodent studies (6, 14). Figure 1 illustrates how top-down input from the infralimbic prefrontal cortex to the amygdala, a region important for detecting cues of safety and threat, can reduce fear responses generated there. Sensory input is received primarily by the lateral nuclei of the amygdala. The lateral nuclei project to the basal and central nuclei of the amygdala. The basal nuclei project predominantly to cortical regions (e.g., ventromedial prefrontal cortex), whereas the central nuclei project mainly to subcortical regions involved in various physiologic components of the fear response, including neuromodulatory systems, the hypothalamus, the periaqueductal gray area, and the vagus nerve. The infralimbic cortex can dampen fear responses via projections to a cluster of inhibitory intercalated cells located within the amygdala. Via bidirectional projections to the prefrontal cortex, the amygdala can both signal about the significance of a cue and receive input from the prefrontal cortex that modulates fear responses by suppressing central nucleus output, thereby dampening downstream physiological responses associated with a fear response (6, 15). This suppression of fear responses by prefrontal cortical regions and how it varies across adolescence are the focus of this review.
Figure 1.
Fear circuit. A simplified cartoon of the brain circuitry involved in the fear response to cues of threat and fear regulation. Abbreviations: BA, basal amygdala; CE, central amygdala; IL, infralimbic prefrontal cortex; ITC, intercalated; LA, lateral amygdala; PL, prefrontal cortex; vmPFC, ventromedial prefrontal cortex.
Development of Fear Regulation Circuitry
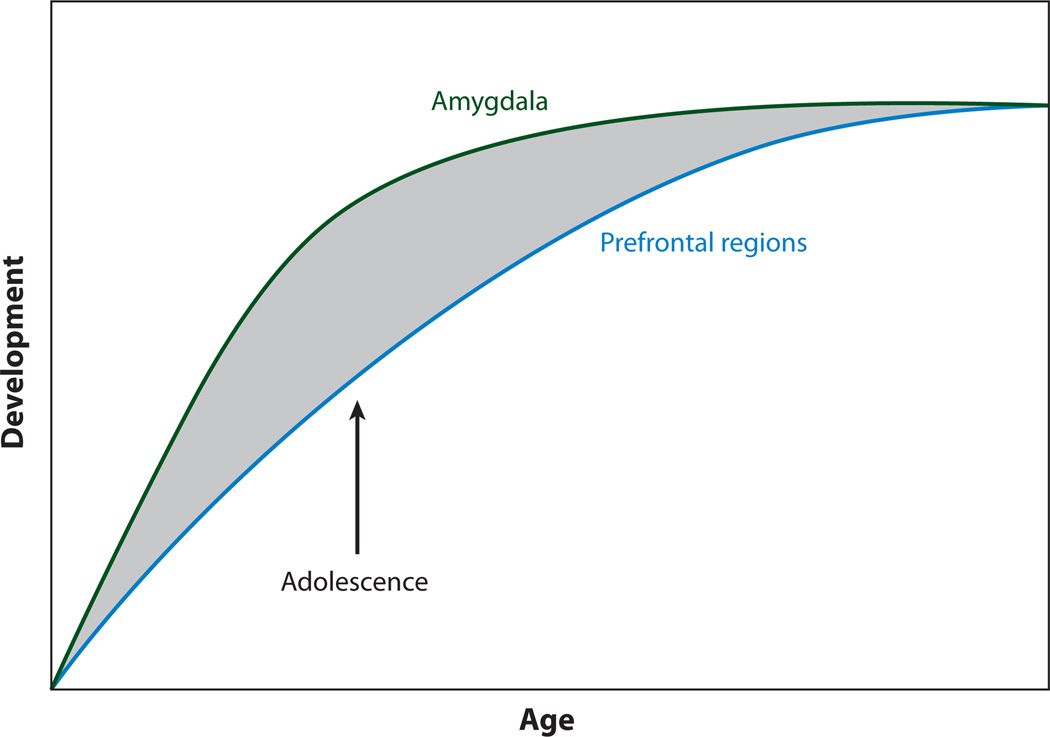
Characterization of adolescent development requires knowing what precedes and follows this period to delineate unique changes during this developmental time frame. This characterization of transitions into and out of adolescence goes beyond simple linear changes by focusing on disjunctions in the development of subcortical limbic regions involved in desire and in fight and flight responses—which are reminiscent of teens’ heightened emotional reactions— and the development of top-down control regions in prefrontal cortex across childhood, adolescence, and adulthood. Different developmental trajectories of these regions, with subcortical limbic structures maturing earlier than prefrontal cortical regions, may result in functional imbalances in this circuitry between these regions during adolescence that may explain some of the unique behavioral characteristics of this life stage as well as the heightened risk for anxiety-related psychopathology in adolescence (16, 17; see Figure 2). The developmental disjunction between limbic and cortical structures and connections between these regions have been described across mammalian species; rodent, nonhuman primate, and human postmortem studies have shown that the prefrontal cortex is one of the last brain regions to mature (18, 19) and that subcortical and sensorimotor regions mature sooner as indexed by peaks in synaptogenesis and subsequent synaptic pruning. The phylogenetic consistency of the early development of structures involved in primal drives and later development of those involved in complex integrative control of these responses suggests that there is important adaptive value in the relative imbalance of these regions in adolescence and its effects on behavior.
Figure 2.
An imbalance results from earlier development of amygdala regions involved in the fear response and later development of the prefrontal cortex, which is involved in regulation of fear. Adapted with permission from Reference 16.
Probing Fear Regulation Circuitry
Studies have commonly used two basic paradigms to probe fear circuitry in humans. The first involves examining behavioral and neural responses to naturalistic cues that over a lifetime come to be associated with the presence of a threat (e.g., a frightened face). The second involves experimental Pavlovian fear conditioning in which a neutral cue (e.g., tone) is paired repeatedly with an aversive event (e.g., shock), so that the neutral cue takes on aversive properties through associative learning processes. Both behavioral paradigms provide a measure of how well an individual can suppress a fear response when danger-associated cues and contexts are no longer a source of threat and can be used in typically and atypically developing humans. We present recent behavioral and neural evidence for developmental variation in fear regulation using both of these approaches below.
DEVELOPMENTAL VARIATION IN FEAR REGULATION
Evidence from Developmental Human Imaging Studies of Emotion Regulation
In a series of human imaging studies, we and others have examined emotional responses during adolescence relative to developmental stages preceding or following it (20–22). Across these studies, naturalistic cues that have been associated with the presence of a threat (e.g., a frightened face) over a lifetime of experiences are repeatedly presented. For example, Hare and colleagues showed that adolescents have an initial exaggerated amygdala response to cues that signal possible threat (fearful faces) relative to children and adults (21). This initial heightened response in amygdala activity is age-dependent and does not correlate with trait anxiety. These results are consistent with other studies that have identified elevated amygdala activity to emotional pictures in adolescents relative to adults (23, 24) and extend those studies to demonstrate that adolescents differ from children as well. Moreover, Hare et al. demonstrated that fearful faces induced behavioral inhibition, as measured by increased latency to respond to the fearful stimulus over other expressions that was significantly correlated with the degree of amygdala activation to the fearful face and inversely correlated with activation of the ventromedial prefrontal cortex (vmPFC) (21). These findings emphasize the balance needed between top-down prefrontal systems and subcortical amygdala regions in regulating emotional behavior.
Examination of the magnetic resonance signal in the amygdala with repeated presentation of the fearful face across experimental trials allowed Hare et al. to assess the ability of individuals to recognize the face as an empty (i.e., not dangerous) threat. Repeated presentation resulted in attenuation in the amygdala response over time (i.e., habituation). Individuals with higher trait anxiety showed less suppression of the amygdala response over time. The failure of the amygdala response to return to baseline over time was associated with coupling of frontoamygdala activity. Specifically, inverse functional coupling of these regions, consistent with greater top-down regulation (higher vmPFC activity) of the amygdala, was correlated with greater diminution of signal in the amygdala (21). The extent to which activation of the amygdala diminished with repeated trials was correlated with self-report ratings of anxiety, demonstrating that the ability of the vmPFC to modulate amygdala responses is associated with emotional states in human subjects.
These findings suggest that heightened initial emotional reactivity as indexed by elevated amygdala activity may be typical of or normal for adolescence, consistent with the likelihood of encountering novel dangers in this developmental stage. However, failure of this response to subside over time with no actual impending threat is atypical and may be indicative of anxiety. Clinical imaging data using similar behavioral paradigms with older children and adolescents diagnosed with anxiety and depression show elevated amygdala activity in response to fearful faces (25). These findings may be due to persistent activation of this region with repeated exposures—as seen in adolescent females, who are at greater risk of these disorders relative to males (26)—rather than elevated activations per se. Future studies of populations at risk for anxiety will need to examine carefully not only what triggers a heightened threat response in the amygdala, but also what brain processes support anxiety responses that are sustained over time (27).
The observation of imbalanced activity in the amygdala–vmPFC network in anxious individuals is consistent with a variety of work in animals (28, 29), in humans (30–34), and in childhood and adolescent mood and anxiety disorders (35, 36), implicating an inverse relationship between these structures that governs affective output. Specifically, increased responses in the vmPFC are inversely correlated with responses in the amygdala and predict behavioral outcomes such as fear extinction, downregulation of autonomic responses (15), and more positive interpretations of emotionally ambiguous information (37). Thus, inverse functional coupling of these structures is key for adaptive downregulation of heightened emotional responses. During adolescence, the relatively immature vmPFC circuitry cannot efficiently regulate responses in the maturing amygdala, leading to the heightened emotional reactivity typically associated with the adolescent years that can, in individual cases, lead to anxiety disorders.
Evidence from Parallel Mouse and Human Developmental Studies of Fear Extinction
The preceding work used paradigms with naturalistic cues that come to be associated with the presence of a threat (e.g., a frightened face) with experience, cues that can be examined across both typical and atypical human populations. Unfortunately, our experiences over a lifetime are not equivalent and are limited by age and opportunity for such experiences. For example, a child may have fewer experiences of dangerous situations or threats than an adult, and an anxious child may have many more experiences of threat than a nonanxious child. These experiences will differentially impact fear-related circuitry. Fear learning paradigms are advantageous in that they can assess fear learning equivalently in typically and atypically developing humans. Second, because fear learning is critical to survival, there is a high degree of neural and behavioral conservation across species; thus, fear learning can be assessed equivalently in humans and mice. These advantages, in addition to the demonstrated relevance of fear learning to human anxiety states, make this behavioral paradigm a useful translational tool for understanding human anxiety disorders.
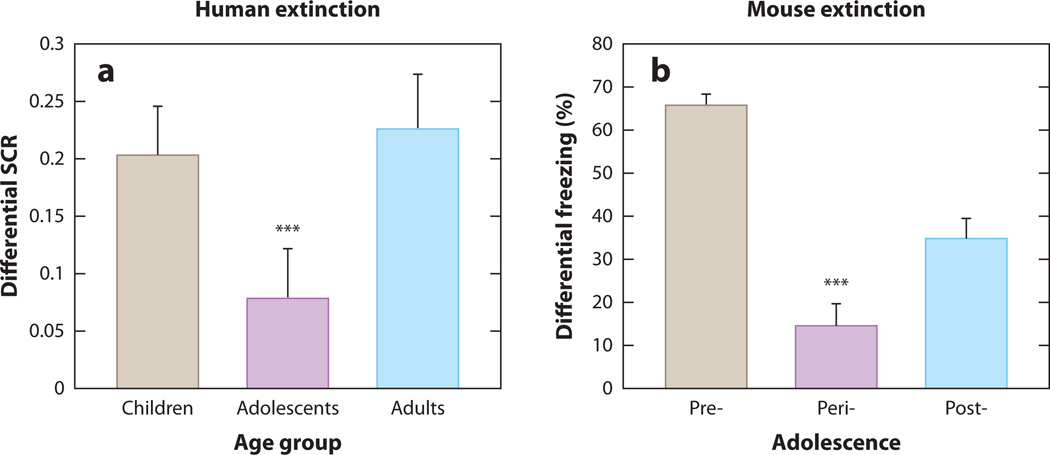
Fear conditioning paradigms have been used to more directly examine how immature functional connectivity between the vmPFC and amygdala in adolescents affects neural, behavioral, and emotional responses to fearful emotional stimuli. In a recent study, we used Pavlovian fear conditioning tasks in mice and humans. We examined fear conditioning and extinction in humans as a function of age and stage on the Tanner Pubertal Scale: children (5–11 years old, Tanner pubertal stage of 1, n = 30), adolescents (12–17 years old, Tanner pubertal stage >1, n = 28), and adults (18–28 years old, Tanner pubertal stage of 5, n = 28). Skin conductance response (SCR) was assessed (58) to measure prototypical physiological fear responses during conditioned fear acquisition and extinction (6, 38–41). During fear acquisition, an aversive noise was the unconditioned stimulus (US) and a colored square served as the conditioned stimulus (CS). Although there were no age differences in fear acquisition, there was a main effect of age on extinction learning (decreased SCR to repeated presentations of the CS only), such that adolescents displayed attenuated fear extinction learning compared to children or adults (Figure 3a). This effect of age group on fear extinction was present when gender and trait anxiety were entered as covariates. Children and adults did not differ in extinction learning. Unlike this study, those using naturalistic cues such as fearful or screaming faces find heightened amygdala activity (21) and fear learning in adolescents (42) compared to younger children, as well as diminished habituation and/or extinction learning during adolescence.
Figure 3.
Developmental variation in fear extinction learning. (a) Extinction learning is attenuated during adolescence in the human as measured by less change in galvanic skin response with repeated presentation of the conditioned stimulus alone during extinction trials. (b) This finding is paralleled in the mouse as measured by less change in freezing behavior. From Reference 58 with permission.
A parallel study to the human one, in mice across comparable postnatal ages [preadolescent (postnatal day 23), adolescent (day 29), and adult (day 70)], used freezing behavior as the prototypical species-specific fear response, an electric shock for the US, and a neutral acoustic tone for the CS. Consistent with previous reports describing permanent erasure of conditioned fear memories in young rats (43), preadolescent mice exhibited minimal spontaneous recovery of fear responses, suggestive of a fragile memory trace that is persistently attenuated, or erased, via extinction trials. In contrast, adolescent mice, like human subjects, displayed attenuated fear extinction learning compared to their preadolescent and adult counterparts (Figure 4b). These findings are consistent with previous rodent studies that show adolescent rats require twice as many extinction trials as adults, a pharmacological manipulation such as d-cycloserine, or CS presentations of prolonged duration to achieve reductions in conditioned fear behavior comparable to those seen in preadolescent or adult rats (43–45).
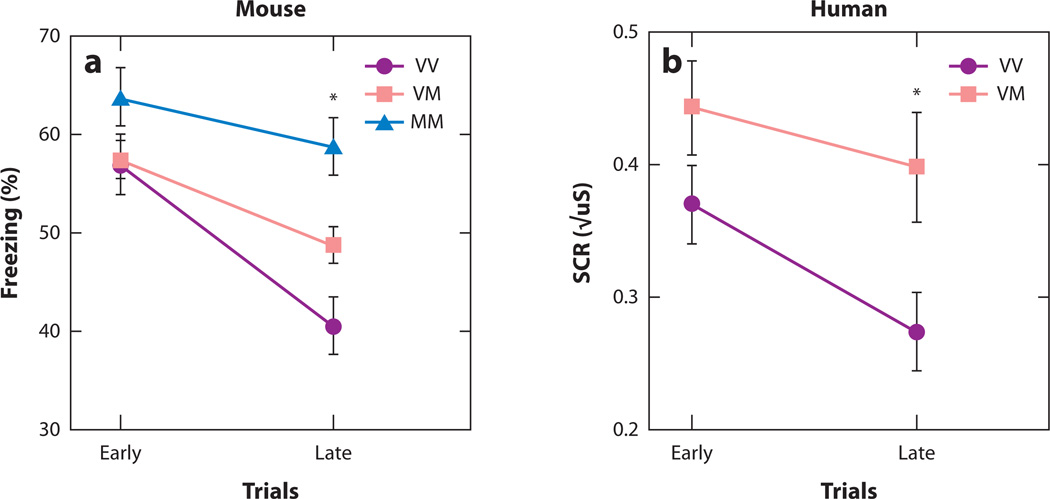
Figure 4.
Individual variation in fear extinction learning. (a) Extinction learning is attenuated in mice with the BNDF Met (M) allele relative to nonMet allele (V) carriers as measured by less change in freezing behavior with repeated presentation of the conditioned stimulus alone during extinction trials. (b) This finding is paralleled in the human as measured by less change in galvanic skin response. Reproduced with permission from Reference 39.
Leveraging the Mouse to Illuminate the Mechanism of Age Effects on Fear Regulation
To assess the mechanism underlying the phylogenetically conserved developmental differences in the fear circuitry and fear extinction learning, we performed immunohistochemical and electrophysiological experiments across development stages in mice. We hypothesized that there would be alterations in vmPFC connectivity and plasticity across development that would account for the differences in amygdala responses and behavior. Because the most prominent developmental effects identified in aspects of fear learning relate to fear extinction learning, we focused on the infralimbic cortex (IL). The IL provides input to the inhibitory intercalated cells, located within the amygdala, necessary for supressing fear responses and amygdala output during extinction learning. To investigate neuronal activity in the IL, we measured expression of the activity-dependent immediate early gene c-Fos because activity-induced expression of c-Fos in the IL of adult rodents has been associated with successful fear extinction learning (46). Consistent with previous studies, the density of c-Fos-labeled cells in the IL of adult mice after fear extinction learning was significantly higher than nonextinguished, fear-conditioned controls (46). In contrast, extinction exposures induced no change in density of c-Fos labeling in adolescent (29-day-old) mice. These data suggest that the neural circuit engaged by fear extinction learning in adults is not active during adolescence, providing a likely neural substrate for the inefficiency of cortical control of fear responses during adolescence.
To probe developmental influences on fear-associated synaptic plasticity within the IL, we performed electrophysiological recordings in vmPFC brain slices of mice after both fear acquisition and fear extinction. A previous study showed that fear conditioning involved a decrease in intrinsic excitability of IL neurons whereas fear extinction reversed this decrease in excitability (47). However, the specific synaptic mechanisms in the vmPFC that are involved in fear conditioning or extinction had not been explored. Therefore, we measured non-N-methyl-D-aspartate (NMDA) receptor transmission mediated primarily by aminomethylphosphonic acid (AMPA) receptors in IL layer V pyramidal neurons after conditioned fear acquisition and fear extinction. Electrophysiological recordings at IL and prelimbic cortex synapses across developmental stages reveal that a fear-conditioning-induced potentiation of PL synapses present in adult mice is absent in adolescent mice. Furthermore, extinction-induced enhancement of IL synaptic plasticity in adult mice is lacking in adolescent mice (58).
Taken together, these studies reveal a nonlinear pattern in fear extinction learning and blunted regulation of amygdala-dependent fear responses during fear extinction in adolescents. These findings may help provide novel insights into the heightened prevalence of anxiety disorders during this developmental period and on treating adolescent populations with anxiety, as the main form of cognitive behavioral therapy relies on principles of extinction learning.
INDIVIDUAL VARIATION IN FEAR REGULATION
We and others have begun to identify ways in which the different developmental trajectories of cortical and subcortical brain regions involved in adaptive fear responses can impact behavior. However, within any developmental stage there is marked individual variability in human fear responses that relates to intrinsic and experiential differences from one person to the next. One important source of individual variability is heritable genetic variation. The main avenues for understanding gene function in anxiety disorders have been human genetic association studies on one end and genetically engineered mouse models on the other. Attempts to bridge these approaches have used brain imaging to link morphological abnormalities seen in knockout/transgenic mouse models and abnormal patterns of brain activity seen in humans. It has been proposed that translating from mouse behavior to human disorder is difficult and perhaps inappropriate because human disorders are defined by complex and subjective emotional experiences that cannot be assessed in nonhuman systems (48). Instead, the focus should be shifted to important survival behaviors and the neural circuits that drive them. Adaptive fear responses are critical to the success of an organism, are highly conserved across mammalian species, and display conserved differences across developmental stages. These attributes make fear learning a good translational model to study the effects of genetic variants on the normal development of fear responses in humans and mice.
Translational Approach from Gene to Molecule to Circuit to Behavior
Recently we completed a study using parallel analysis of behavioral and imaging genetics in humans and a genetic knock in mouse model of a human polymorphism. The respective strengths of different levels of analysis, from molecular to neural to behavioral, all provide external validation for the findings of any single genetic analysis (49). Moreover, this integrative approach provides bridges between the relevant but complex and imprecise phenomenology of human behavior and the solid findings of rodent neurobiology that can be difficult to extrapolate to human behavior and disease.
To implement this translational approach to human genetic variability, we focused on a common polymorphism in the human gene for brain-derived neurotrophic factor (BDNF). BDNF is a growth factor that plays a central role in neuronal survival, growth, and synaptic plasticity—all core aspects of associative learning in the central nervous system and adaptive fear learning in particular. Human populations contain a common single nucleotide polymorphism (SNP) that causes a valine-to-methionine substitution at codon 66 (Val66Met). This polymorphism leads to decreased trafficking of BDNF into the regulated secretory pathway, which in turn leads to reduced activity-dependent release of BDNF. The BDNF gene is highly conserved from mouse to human, and wild-type mice naturally express the ancestral valine-containing form of the BDNF peptide. To study the effects of the human Val66Met polymorphism in mice, we created a knockin mouse with a BDNF protein identical to the wild type except it contains a methionine in codon 66 (BDNFMet). Neurons obtained from these BDNFMet mice have impaired activity-dependent BDNF secretion. In addition, the mice have hippocampal anatomical alterations and impaired hippocampal-dependent learning similar to the findings in humans with the variant human BDNF, validating this mouse as a model of the human Val66Met polymorphism.
Evidence from Parallel Genetic Mouse and Human Imaging Studies
We examined the impact of the variant BDNF on fear regulation using similar fear conditioning and extinction paradigms in mice and humans (39). In both mice and humans, we observed less extinction in Met allele carriers than in Val allele carriers, as shown in Figure 4b and c (50). Moreover, human functional MRI data provided neuroanatomical validation of the cross-species translation. Specifically, we showed alterations in frontoamygdala circuitry, shown to support fear conditioning and extinction in previous rodent (6, 51) and human (15, 52–54) studies, as a function of BDNF genotype. During extinction, Met allele carriers showed less vmPFC activity (Figure 4c) but greater amygdala activity (Figure 4d) than noncarriers. These findings suggest that cortical regions essential for extinction in animals and humans are less responsive in Met allele carriers. Moreover, amygdala activity, which should have diminished progressively during extinction, was elevated in Met allele carriers, further suggesting less dampening by the vmPFC.
These genetic findings are provocative as they provide an example of bridging human behavioral and imaging genetics with a molecular mouse model to suggest a role for BDNF in anxiety in adults. In the context of our neurobiological model of adolescence, individuals with the BDNF Met allele may be more vulnerable to developing symptoms of anxiety as teens, in that they show higher and prolonged patterns of amygdala activity and less vmPFC activity in response to emotional cues. During a period when evaluating social cues from peers is essential in forming and maintaining healthy peer relationships, the failure to suppress heightened emotional responses to empty threat (e.g., failure of a peer to notice or smile at a teenager, without any negative intent) could lead to overinterpretation and ruminations of self-doubt. The genetic data provide an example of how an imbalance in amygdala–vmPFC coupling during typical development could predispose to anxiety and, when exacerbated by an individual factor such as the BDNF Met66 allele, lead to clinical levels of anxiety.
IMPLICATIONS AND NOVEL TREATMENTS FOR THE DEVELOPING BRAIN
Converging evidence from animal and human studies demonstrates that modulatory prefrontal cortical structures develop along a different trajectory than the primary subcortical amygdalar structures that generate and integrate fear responses. These developmental differences explain why fear extinction learning displays different attributes as individuals transition into and out of adolescence and may explain the peak in diagnosis of anxiety during this period of development. An appreciation of the development and individual variation in fear responses can also inform the treatment of anxiety disorders. The most common behavioral treatments for anxiety disorders operate through extinction learning processes in which a stimulus that is experienced as fearful is repeatedly presented in nonthreatening circumstances. Thus, treatment response will correlate with the ability to successfully extinguish fear associations. Yet, it is the combination of individual and developmental inefficiencies in extinction learning that predisposes to anxiety disorders in the first place, so those most in need of desensitization therapies may benefit the least.
These studies of adaptive fear learning may provide a way forward with significant implications for novel evidence-based treatments that go beyond the current standard of care. Studies in rodents have shown that, despite the inefficiency of extinction learning in adolescents, providing adolescent rats with additional extinction learning trials can lead to substantial extinction, suggesting that additional desensitization sessions for nonresponding adolescents may provide some benefit. Furthermore, d-cycloserine is a partial agonist at glutamate receptors and can enhance extinction learning in adolescent rats, suggesting that it may be a useful adjunct to behavioral therapies in adolescent humans (43). Similarly, a growing literature suggests that serotonin selective reuptake inhibitors (SSRIs), a commonly used class of anxiolytic drugs, act by enhancing the retention of extinction learning; thus, combined behavioral and SSRI therapy may improve response in adolescent anxiety disorders, as has been shown in a large-scale clinical study (13, 55). Finally, recent reports in mice and humans have shown that the basic phenomenology of fear learning can be leveraged to enhance extinction learning (56, 57). These studies have shown that a single, isolated presentation of a fear-associated cue opens a “reconsolidation window” during which extinction learning to that cue is enhanced. These studies were conducted in adults and must be tested in adolescents, but they suggest that informed modifications of standard behavioral therapies for anxiety disorders may improve the treatment of these common and debilitating disorders.
SUMMARY POINTS.
Adolescence is a life stage during which the differential developmental trajectories of regions of the brain that generate fear responses and those that regulate them are imbalanced. This imbalance contributes to inefficient fear regulation that is adaptive to the behavioral demands of adolescence but can also contribute to anxiety disorders.
The evolutionary conservation of the neural and behavioral attributes of conditioned fear learning facilitates cross-species translation. Leveraging the strengths of different experimental species should improve understanding and treatment of anxiety disorders.
Behavioral therapies for anxiety disorders are based on the principles of fear extinction. Understanding developmental and individual variation in extinction learning will allow development of novel behavioral therapies and allow them to be targeted to the most responsive people and the most responsive time.
FUTURE ISSUES.
Does age impact the efficacy of cognitive behavioral therapy in youth with anxiety disorders?
How should current behavioral exposure therapies be modified or tailored for patients as a function of age?
How should current psychiatric treatments be modified or tailored for patients as a function of genetics?
As fear memories can undergo erasure in adults, can similar techniques be used to “erase” unwanted fear memories in developing individuals, particularly during adolescence, when fear memories are resistant to classic extinction training?
ACKNOWLEDGMENTS
This work was supported in part by funding from the National Institute of Mental Health (P50 MH079513), the Weill Cornell Medical College Citgroup Biomedical Imaging Center and Imaging Core, and a generous gift from the Mortimer D. Sackler, MD family.
Glossary
- Cognitive behavioral therapy (CBT)
a validated behavioral therapy for anxiety disorders that identifies fearful triggers and desensitizes them with repeated exposure
- Fear extinction
an active learning process in which, after fear conditioning, a fear response is diminished by presenting a conditioned stimulus alone repeatedly
- Infralimbic prefrontal cortex (IL)
a region in the ventromedial prefrontal cortex important in tonic inhibition of subcortical structures and emotional responses such as fear
- Amygdala
an almond-shaped group of nuclei in the medial temporal lobes involved in processing emotional information and responses
- Ventromedial prefrontal cortex (vmPFC)
region in the frontal lobes implicated in processing fear, risk, and choice behavior
- Fear conditioning (acquisition)
a form of associative learning whereby a neutral stimulus is paired with an intrinsically aversive stimulus to trigger a conditioned fear response
- Tanner Pubertal Scale
a scale from 1 to 5 of physical development based on external primary and secondary characteristics
- Skin conductance response (SCR)
a measure of electrical conductance of the skin that varies with sweat and indexes changes in arousal
- Unconditioned stimulus (US)
an intrinsically aversive stimulus that results in a fear response and is used in fear conditioning
- Conditioned stimulus (CS)
a neutral stimulus that triggers a conditioned fear response after pairing with an intrinsically aversive stimulus
- Prelimbic cortex
a region in the ventromedial prefrontal cortex important in the activation of subcortical structures and fear expression
- Brain-derived neurotrophic factor (BDNF)
a secreted protein and member of the neurotrophin family of growth factors involved in plasticity and development
Footnotes
DISCLOSURE STATEMENT
F.S.L. is a consultant for Ono Pharmaceuticals.
LITERATURE CITED
- 1.Kessler RC, Demler O, Frank RG, et al. Prevalence and treatment of mental disorders, 1990 to 2003. N. Engl. J. Med. 2005;352:2515–2523. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication-Adolescent Supplement (NCS-A) J. Am. Acad. Child Adolesc. Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman DL, Moffitt TE, Caspi A, et al. Psychiatric disorder in a birth cohort of young adults: prevalence, comorbidity, clinical significance, and new case incidence from ages 11 to 21. J. Consult. Clin. Psychol. 1996;64:552–562. [PubMed] [Google Scholar]
- 4.Pollack MH, Otto MW, Sabatino S, et al. Relationship of childhood anxiety to adult panic disorder: correlates and influence on course. Am. J. Psychiatry. 1996;153:376–381. doi: 10.1176/ajp.153.3.376. [DOI] [PubMed] [Google Scholar]
- 5.Kim-Cohen J, Caspi A, Moffitt TE, et al. Prior juvenile diagnoses in adults with mental disorder: developmental follow-back of a prospective-longitudinal cohort. Arch. Gen. Psychiatry. 2003;60:709–717. doi: 10.1001/archpsyc.60.7.709. [DOI] [PubMed] [Google Scholar]
- 6.LeDoux JE. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 7.Davis M, Ressler K, Rothbaum BO, et al. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol. Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 8.Petrovich GD, Swanson LW. Projections from the lateral part of the central amygdalar nucleus to the postulated fear conditioning circuit. Brain Res. 1997;763:247–254. doi: 10.1016/s0006-8993(96)01361-3. [DOI] [PubMed] [Google Scholar]
- 9.Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci. STKE. 2004;(225):re5. doi: 10.1126/stke.2252004re5. [DOI] [PubMed] [Google Scholar]
- 10.Pine DS. Research review: a neuroscience framework for pediatric anxiety disorders. J. Child Psychol. Psychiatry. 2007;48:631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- 11.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch. Gen. Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 12.Nestler EJ, Barrot M, DiLeone RJ, et al. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 13.Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N. Engl. J. Med. 2008;359:2753–2766. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol. Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 15.Phelps EA, Delgado MR, Nearing KI, et al. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 16.Casey BJ, Getz S, Galvan A. The adolescent brain. Dev. Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casey BJ, Duhoux S, Malter Cohen M. Adolescence: what do transmission, transition, and translation have to do with it? Neuron. 2010;67:749–760. doi: 10.1016/j.neuron.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb. Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- 19.Huttenlocher PR. Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 20.Galvan A, Hare TA, Parra CE, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J. Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hare TA, Tottenham N, Galvan A, et al. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol. Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Curr. Opin. Neurobiol. 2010;20:1–6. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guyer AE, Monk CS, McClure-Tone EB, et al. A developmental examination of amygdala response to facial expressions. J. Cogn. Neurosci. 2008;20:1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monk CS, Grillon C, Baas JM, et al. A neuroimaging method for the study of threat in adolescents. Dev. Psychobiol. 2003;43:359–366. doi: 10.1002/dev.10146. [DOI] [PubMed] [Google Scholar]
- 25.Thomas KM, Drevets WC, Dahl RE, et al. Amygdala response to fearful faces in anxious and depressed children. Arch. Gen. Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- 26.Thomas KM, Drevets WC, Whalen PJ, et al. Amygdala response to facial expressions in children and adults. Biol. Psychiatry. 2001;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- 27.Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol. Psychiatry. 2010;68(5):416–424. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baxter MG, Parker A, Lindner CC, et al. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J. Neurosci. 2000;20:4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 30.Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol. Psychol. 2006;73:39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Etkin A, Egner T, Peraza DM, et al. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 32.Haas BW, Omura K, Constable RT, et al. Emotional conflict and neuroticism: personality-dependent activation in the amygdala and subgenual anterior cingulate. Behav. Neurosci. 2007;121:249–256. doi: 10.1037/0735-7044.121.2.249. [DOI] [PubMed] [Google Scholar]
- 33.Johnstone T, van Reekum CM, Urry HL, et al. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J. Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urry HL, van Reekum CM, Johnstone T, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J. Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guyer AE, Lau JY, McClure-Tone EB, et al. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch. Gen. Psychiatry. 2008;65:1303–1312. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monk CS, Telzer EH, Mogg K, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch. Gen. Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim H, Somerville LH, Johnstone T, et al. Contextual modulation of amygdala responsivity to surprised faces. J. Cogn. Neurosci. 2004;16:1730–1745. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- 38.Pattwell SS, Bath KG, Casey BJ, et al. Selective early-acquired fear memories undergo temporary suppression during adolescence. Proc. Natl. Acad. Sci. USA. 2011;108:1182–1187. doi: 10.1073/pnas.1012975108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soliman F, Glatt CE, Bath KG, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laviola G, Adriani W, Terranova ML, et al. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci. Biobehav. Rev. 1999;23:993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- 41.Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behav. Neurosci. 1998;112:1152–1166. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- 42.Glenn CR, Klein DN, Lissek S, et al. The development of fear learning and generalization in 8–13 year-olds. Dev. Psychobiol. 2011;54:675–684. doi: 10.1002/dev.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCallum J, Kim JH, Richardson R. Impaired extinction retention in adolescent rats: effects of D-cycloserine. Neuropsychopharmacology. 2010;35:2134–2142. doi: 10.1038/npp.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim JH, Richardson R. Extinction in preweanling rats does not involve NMDA receptors. Neurobiol. Learn. Mem. 2010;94:176–182. doi: 10.1016/j.nlm.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 45.Lai CS, Franke TF, Gan WB. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature. 2012;483(7387):87–91. doi: 10.1038/nature10792. [DOI] [PubMed] [Google Scholar]
- 46.Santini E, Ge H, Ren K, et al. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J. Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santini E, Quirk GJ, Porter JT. Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. J. Neurosci. 2008;28:4028–4036. doi: 10.1523/JNEUROSCI.2623-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Casey BJ, Soliman F, Bath KG, et al. Imaging genetics and development: challenges and promises. Hum. Brain Mapp. 2010;31:838–851. doi: 10.1002/hbm.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hajcak G, Castille C, Olvet DM, et al. Genetic variation in brain-derived neurotrophic factor and human fear conditioning. Genes Brain Behav. 2009;8:80–85. doi: 10.1111/j.1601-183X.2008.00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- 52.Delgado MR, Nearing KI, Ledoux JE, et al. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat. Neurosci. 2004;7:1144–1152. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- 54.LaBar KS, Gatenby JC, Gore JC, et al. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 55.Karpova NN, Pickenhagen A, Lindholm J, et al. Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science. 2011;334:1731–1734. doi: 10.1126/science.1214592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schiller D, Monfils MH, Raio CM, et al. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2009;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Monfils MH, Cowansage KK, Klann E, et al. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pattwell SS, Duhoux S, Hartley CA, et al. Altered fear learning across development in both mouse and human. Proc. Natl. Acad. Sci. 2012;109:16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]