Abstract
Purpose
To describe the prevalence and interrelationships of epiretinal membranes, vitreomacular traction, macular cysts, paravascular cysts, lamellar macular holes, full-thickness macular holes, and visual impairment in a population-based study of older adults.
Design
Cross-sectional study.
Participants
There were 1913 participants aged 63–102 years at the 20-year Beaver Dam Eye Study follow-up examination in 2008–2010, of whom 1540 (2980 eyes) had gradable spectral-domain optical coherence tomography (SD-OCT) scans of the macula in at least one eye.
Methods
The presence of epiretinal membranes and other retinal lesions was determined by standardized grading of macular SD-OCT scans and photographs of three standard fields.
Main Outcome Measures
Epiretinal membranes, vitreomacular traction, macular cysts, paravascular cysts, lamellar macular holes, full-thickness macular holes, and visual impairment.
Results
Using SD-OCT the prevalences of epiretinal membranes (34.1%), vitreomacular traction (1.6%), macular cysts (5.7%), paravascular cysts (20.0%), lamellar macular holes (3.6%), and full-thickness macular holes (0.4%) were estimated. The prevalences of macular cysts (P<0.001), epiretinal membranes (P<0.001), and vitreomacular traction (P=0.005) increased with age, the prevalence of paravascular cysts (P=0.05) decreased with age, and the prevalence of lamellar macular holes was not associated with age (P=0.70). The prevalences of macular cysts, lamellar macular holes, and epiretinal membranes were higher in eyes with a history of cataract surgery. Macular cysts and epiretinal membranes were more common in eyes with retinal diseases such as proliferative diabetic retinopathy, retinal vein occlusion, and retinal detachment than in eyes without these conditions. Macular cysts, epiretinal membranes, and full-thickness macular holes were associated with visual impairment. While adjusting for age and sex, macular cysts (odds ratio [OR] 3.96; P<0.0001), paravascular cysts (OR 1.45, P=0.007), lamellar macular holes (OR 10.62; P<0.001), vitreomacular traction (OR 2.72, P=0.01) and visual impairment (OR 3.23; p<0.001) were more frequent in eyes with epiretinal membranes compared to eyes without.
Conclusions
Epiretinal membranes are associated with macular cysts, paravascular cysts, lamellar macular holes, vitreomacular traction, and visual impairment. Further follow-up will allow better understanding of the natural history of epiretinal membranes and vitreomacular traction and their relationships to the development of macular cysts and lamellar macular holes in the aging population.
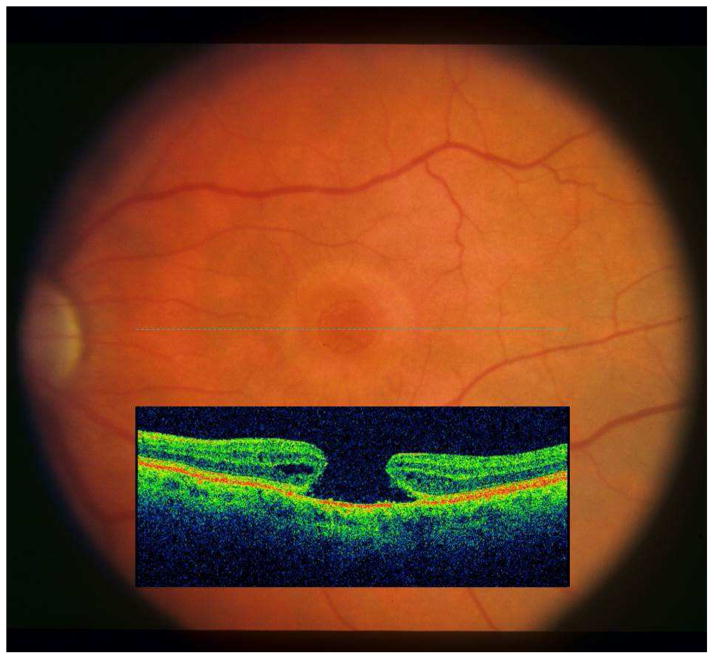
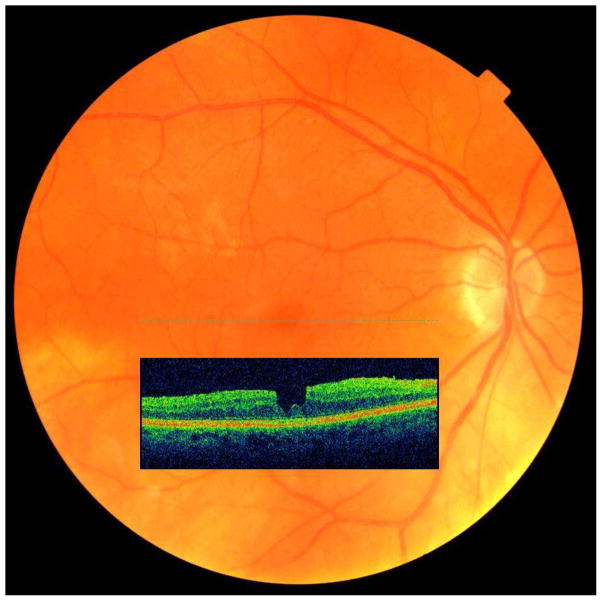
Epiretinal membranes (ERMs) and vitreomacular traction (VMT) increase in frequency with age.1,2 They may be associated with the presence of macular cysts (Figure 1), lamellar macular holes (LMHs), and full-thickness macular holes (FTMHs).3 Prior to the clinical use of spectral-domain optical coherence tomography (SD-OCT), the presence of VMTs, macular cysts, and LMHs often went undetected by ophthalmoscopy or stereoscopic fundus photography. Another lesion, paravascular cysts (PVCs, Figure 2), described as intraretinal hypo-reflective spaces adjacent to the retinal blood vessels, remained undetected clinically. They were first detected in histopathologic studies.4–6 Little is known about their natural history and their relationship to ERMs. Most epidemiological observations were limited to grading of fundus photographs and described the prevalence and incidence of only ERMs and FTMHs.1,2 The purpose of this report is to describe the epidemiology and inter-relationships of vitreomacular interface conditions, ERMs and VMT, with LMHs and FTMHs as well as with non-specific vitreomacular interface findings (e.g., macular cysts and PVCs) as detected from gradings of SD-OCT scans in older adults participating in the population-based Beaver Dam Eye Study (BDES).
Figure 1.
Example of a B-scan with an epiretinal membrane consisting of corrugation of the retinal surface (A) with bridging of the membrane (B) across the top of the corrugation. A cluster of cysts (C) is visible in the outer retinal layers.
Figure 2.
Example of paravascular cysts adjacent to a retinal blood vessel in the inner layers of the retina.
Methods
Participants
Methods used to identify and describe the population have appeared in previous reports.7–12 In brief, a private census of the population of Beaver Dam, Wisconsin was performed from September 15, 1987 to May 4, 1988 to identify all residents in the city or township of Beaver Dam aged 43 to 84 years. Of the 5924 eligible individuals, 4926 participated in the baseline examination between March 1, 1988 and September 14, 1990. Participants were seen approximately every 5 years over the next 20 years. Ninety-nine percent of the population was white. There were 3722 participants in the 5-year examination between 1993 and 1995, 2962 in the 10-year examination from 1998 to 2000, 2375 in the 15-year examination from 2003 to 2005, and 1913 in the 20-year examination from 2008 to 2010. Participation rates among survivors were approximately 80% at every examination.7–12 Institutional Review Board approval was obtained for the study. The work was compliant with the Health Insurance Portability and Accountability Act. The study adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from every participant at each examination.
Procedures
Two SD-OCT cube scans (128 × 512 A-scans) that covered 6 × 6 mm retinal area and centered on the disc and the macula, respectively, were first acquired at the 20-year BDES follow-up examination in 2008–2010 using the Topcon 3D OCT (Topcon Corporation, Tokyo, Japan). They were graded using the Universal OCT Viewer (version 1.01.04), a universal SD-OCT viewing program created by one of the authors of this report (YH). A trained grader determined the center of the fovea by locating the foveal depression and the small peak or separation between the inner segment/outer segment layer (ellipsoid zone) and the top of the RPE, then centered and saved the location of the Early Treatment Diabetic Retinopathy Study (ETDRS) grid.13 Each macular scan was assessed for image quality including layer discrimination and segmentation quality, motion and mirror artifacts, presence of a Z plane offset, and the manufacturer’s layer segmentation lines. The presence of ERMs and VMT, the number of macular cysts present, their location within the grid, and their maximum vertical diameter, as well as the presence of PVCs, LMHs and FTMHs were evaluated. An eye often had more than one of these features graded as present.
Film stereoscopic color photographs of the macula were also acquired at the time of the examination.1 These photographs were graded for macular holes, macular cysts, and ERMs independent of the SD-OCT scan by two different graders.
At all follow-up examinations, before refraction, participants were asked to read the ETDRS chart R modified for a 2 m distance with their current prescription without covering either eye. The number of letters read correctly was recorded. At all examinations, the refraction from a Humphrey 530 refractor (Carl Zeiss, Inc., Oberkochen, Germany) was placed in a trial lens frame and the best-corrected VA was remeasured for each eye by means of the ETDRS protocol with charts R1 and R2 modified for a 2-m distance.7 If the best-corrected visual acuity was 20/40 or worse in either eye, an ETDRS refraction was performed for that eye and the visual acuity was measured.
For each eye, the visual acuity was recorded as the number of letters correctly identified from either the 2 m chart (from 20/10 to 20/200 vision or 70 to 5 letters) or the 1 m chart (20/250 to 20/800 or 0 to −25 letters). The 1 m chart has 25 letters; if all were read correctly, the number of letters assigned was 0; if none were read correctly, the number of letters assigned was −25. For eyes with vision poorer than 20/800, 1 of 3 levels of vision were recorded: hand motions, light perception, and no light perception. These levels were assigned arbitrary values on the visual acuity scale of −40, −55, and −70, respectively.
Definitions
Epiretinal membranes
ERMs are thin membranes of fibrous-like tissue on the surface of the macula. In color photographs, ERMs appear as patches of irregular or increased reflection from the inner surface of the retina associated with fine traction lines and vascular tortuosity. On SD-OCT scans, they are characterized by hyper-reflectivity of the membrane with corrugation (ridges and grooves) along the surface of the internal limiting membrane (Figure 1). Bridging tissue may connect the corrugated sections of the internal limiting membrane. The presence of corrugation or corrugation plus bridging tissue was evaluated in each of three areas defined by the macular grid: the central circle and the inner and/or outer ring of the grid. If ERMs were present, the grader also judged the presence of traction or distortion of the retinal surface in the same grid locations.
Vitreomacular traction
VMT was considered present when the posterior hyaloid had detached from the surface of the retina, but remained adhered in at least one location and pulled the retina into the vitreal cavity. If an adhesion was present but there was no discernible change in the retinal anatomy, the eye was considered to have vitreomacular adhesion only. Any amount of traction, whether straight up into the vitreous or pulled tangentially up and away from its original location, was considered to be VMT. VMT was further classified as broad (>1500 μm area of adhesion and associated distortion) or focal (≤1500 μm area of adhesion and associated distortion) as defined by the International Vitreomacular Traction Study Group,14,15 as well as whether the VMT was located within the central circle of the grid.
Macular cysts
Macular cysts were defined as minimally-reflective spaces with well-defined margins within the retina that were often as dark as the vitreous. A cyst was considered definitely present if it was visible in at least two consecutive B-scans and was larger than 15 μm in vertical diameter. A single cyst was usually circular in shape when present; a cluster of cysts usually appeared oblong and irregular (Figure 3) depending on the surrounding tissue and associated pathology. The perimeter of the cyst was carefully reviewed to rule out other pathology, such as a macular hole with contiguous shifting cavities, which can be mistaken for cysts in adjacent B-scans.
Figure 3.
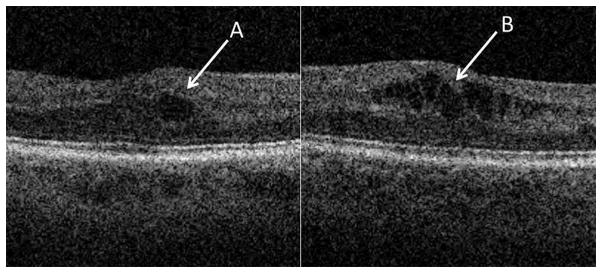
Examples of different cyst shapes. Singular or isolated cysts tend to be circular (A) and clustered cysts are irregular and often angular in shape (B).
Paravascular cysts
PVCs were defined as irregularly shaped hypo-reflective spaces found adjacent to retinal blood vessels in the inner layers of the retina. They are more easily seen abutting the larger retinal blood vessels and therefore rarely seen in the macula.
Full thickness macular holes
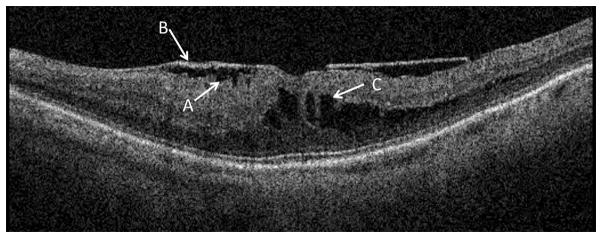
FTMHs are readily seen in both SD-OCT scans and stereoscopic color photographs. In SD-OCT scans, FTMHs were defined as having steep, wide, foveal contours, often with a rim of retinal elevation around the perimeter of the hole (Figure 4). Cysts (fully enclosed) or pockets (small contiguous cavities) around the walls of the hole may be present. An FTMH has no remaining retinal tissue above the retinal pigment epithelium (RPE). In stereoscopic color photographic images, FTMHs appear as a darker red “hole” centered on the fovea with a well-defined margin, with small white precipitates found within. A paler ring of low level detachment often surrounds the central hole.
Figure 4.
Example of a full thickness macular hole.
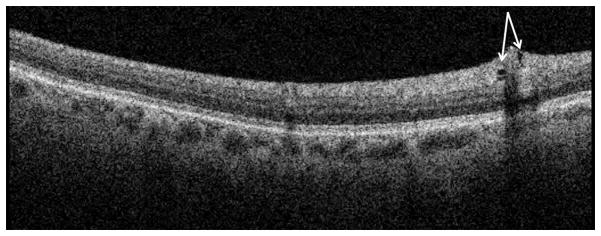
Lamellar macular holes
LMHs (also called partial-thickness holes or pseudoholes) are also easily seen in SD-OCT scans but may be more difficult to discern in stereoscopic color photographs. LMHs are defined as having a concave foveal contour that dips lower and wider than the normal foveal depression but is rarely as steep as an FTMH. Similar to FTMHs, macular cysts and pockets may also be present along the walls of the LMH. Unlike FTMHs, retinal tissue is still present on top of the RPE (Figure 5). In stereoscopic color photographs a LMH appears shallower, with varying color, and with an irregular, less defined margin.
Figure 5.
Example of a lamellar macular hole.
Confounding retinal diseases and conditions
History of retinal detachment as determined from participant self-report, retinal vessel occlusion, presumed ocular histoplasmosis, and proliferative diabetic retinopathy were considered confounding retinal diseases and a history of cataract surgery as a confounding condition.
Visual impairment
Visual impairment was defined as best-corrected visual acuity of poorer than 20/40 (40 or fewer letters read correctly).
Statistical Methods
Distributions of each lesion of interest in the study population and their associations with age and sex were calculated. Frequencies were computed using chi-square tables and means were computed using proc means in SAS version 9.2 (SAS Institute, Cary, North Carolina, USA). Age was divided into three categories: 63–74 years, 75–84 years, and 85 or more years. Associations of the presence of macular cysts, PVCs, and LMHs to the presence of ERMs and VMT, associations of the presence of specific confounding lesions to the presence of macular cysts, PCVs, LMHs, ERMs and VMT, and associations of macular cysts, PCVs, LMHs, ERMs and VMT with measures of visual acuity were examined. The rate of detection of macular cysts, LMHs, FTMHs, and ERMs when using SD-OCT and color photographs was compared. Associations were analyzed in each eye individually using generalized estimating equation models to account for correlation between eyes.
Results
Characteristics of participants
Of the 1913 individuals who participated in the 2008–2010 BDES examination, macular SD-OCT scans were not obtained for 751 eyes and an additional 95 scans were excluded because at least one lesion of interest (macular cysts, PVCs, LMHs, ERMs and VMTs) was ungradable. An individual was excluded from person-level analyses if SD-OCT scans from both eyes were either not taken or the lesions of interest were ungradable in both eyes (N=373 individuals, 746 eyes). A further 100 eyes were excluded from eye-level analyses because a single eye from an individual did not have an SD-OCT scan or the lesions of interest were ungradable in that scan. In 94% of the scans where at least one lesion was ungradable, the grader determined the scan to be unreliable due to poor layer discrimination or severe motion, Z-offset, or mirror image artifact. Characteristics of the 1540 individuals (2980 eyes) included in the study and 373 people (846 eyes) excluded from the study are presented in Table 1. Individuals with at least one gradable macular scan were younger and, after adjusting for age, were more likely to be male, have higher systolic and diastolic blood pressures and greater body mass index, and were less likely to have diabetes. Eyes that were included were less likely to have had cataract surgery, to have late AMD, or to be visually impaired and were more likely to have nuclear cataract and ERMs graded from a stereoscopic fundus photograph compared to eyes that were excluded.
Table 1.
Characteristics of Individuals Included and Excluded from Analysis, Beaver Dam Eye Study, 2008–2010.
| Covariate | Mean (SD) or % |
P value* | |
|---|---|---|---|
| Included (N=1540 persons, 2980 eyes) | Excluded (N=373 persons, 846 eyes) | ||
| Age, years | 74.1 (7.1) | 80.9 (9.1) | <.0001 |
| Sex, male | 43.6 | 33.2 | 0.04 |
| Body mass index, kg/m2 | 31.1 (6.3) | 29.0 (6.5) | 0.01 |
| Systolic blood pressure, mmHg | 130.4 (17.1) | 127.4 (19.0) | 0.01 |
| Diastolic blood pressure, mmHg | 73.8 (10.3) | 68.3 (10.7) | 0.01 |
| Diabetes present | 16.8 | 34.3 | <.0001 |
| AMD severity level | |||
| Minimal early AMD | 9.9 | 9.6 | 0.32† |
| Moderate early AMD | 6.7 | 8.9 | 0.70† |
| Severe early AMD | 2.2 | 3.4 | 0.58† |
| Late AMD | 2.4 | 9.9 | 0.001† |
| Nuclear cataract present | 15.4 | 12.9 | 0.01‡ |
| Positive history of cataract surgery | 24.5 | 68.5 | <.0001‡ |
| Visual impairment present | 3.2 | 23.5 | <.0001 |
| Epiretinal membranes (from fundus photographs) present | 29.2 | 20.0 | 0.001 |
| Axial length/corneal curvature ratio | 3.1 (0.1) | 3.1 (0.1) | 0.29 |
AMD, age-related macular degeneration; SD, standard deviation.
Adjusted for age.
Comparing likelihood of being included vs. excluded for that AMD severity level compared to having no AMD.
Comparing likelihood of being included vs. excluded compared to having no cataract.
An individual was excluded from person-level analyses if SD-OCT scans from both eyes were either not taken or the lesions of interest were ungradable in both eyes (N=373 individuals, 746 eyes). A further 100 eyes were excluded from eye-level analyses because a single eye from an individual did not have an SD-OCT scan or if the lesions of interest were ungradable in that scan.
Epiretinal membranes
ERMs were present in 672 eyes from 525 individuals (34.1% of the 1540 participants whose eyes were gradable for ERM) and were bilateral in 30.3% of individuals who had an ERM present in at least one eye. When an ERM was present in the eye, 17.4% had corrugation only and 82.6% had bridging and corrugation. The frequency of ERMs in right eyes (23.6%, 350/1486) was similar to that in left eyes (21.6%, 322/1494, P=0.14). The prevalence of ERMs increased with age from 28.1% in those aged 63 to 74 years to 53.2% in those aged 85 or more years. The mean age of individuals with ERM with corrugation only was 74.8 years and was 76.2 years in individuals with ERM with corrugation and bridging (P=0.17). After adjusting for age, the frequency of ERM was similar between men and women (33.5% vs. 34.6%; P=0.97).
Vitreomacular adhesions with and without traction
Vitreomacular adhesions without traction were relatively common, found in 551 eyes of 400 individuals (26% of the 1540 participants whose SD-OCT scans were gradable for vitreomacular adhesions with or without traction). VMT was present in 28 eyes in 24 individuals (1.6%) and was bilateral in 16.7% of individuals who had a VMT in at least one eye. Broad VMT was present in 4 eyes (2 with central circle involvement) and focal VMT was present in 24 eyes (11 with central circle involvement). All 4 individuals with bilateral VMT had focal lesions in both eyes. In 3 individuals, the center circle was involved in both eyes. In one, the center circle was not involved in either eye. The frequency of VMT in right eyes (1.2%, 18/1486) was higher than in left eyes (0.67%, 10/1494, P=0.08) but the difference was not statistically significant. The prevalence of VMT increased with age from 1% in individuals aged 63–74 years to 5.6% in individuals aged 85 or more years. After adjusting for age, the frequency of VMT was not statistically significantly different in men and women (1.3% vs. 1.7%; P=0.73).
Macular cysts
Ninety-three eyes from 87 people (5.7%) had at least one macular cyst, and when a macular cyst was present, it was present in both eyes in 11.5% of individuals. The frequency of macular cysts was similar in right eyes (3.0%, 44/1486) and left eyes (3.3%, 49/1494, P=0.56). Of eyes with macular cysts, 15.1% had one cyst present while 63.4% had four or more cysts present. Cysts ranged in size from 28–498 μm in vertical diameter with mean diameter (standard deviation) of 129.1 (95.3) μm and were found most often (81.7%) within the central circle subfield 500 μm from the center of the fovea. The frequency of macular cysts increased with age from 4.2% in those aged 63–74 years to 13.3% in those aged 85 or more years (P=0.0002). After adjusting for age, the presence of macular cysts was similar between men and women (P=0.71).
Paravascular cysts
PVCs were present in 358 eyes from 308 individuals (20%), and, when present, were bilateral in 16.9% of individuals. PVCs were more common in the right eye (13.2%, 196/1486) than in the left (10.84%, 162/1494, P=0.02). The frequency of PVCs decreased with age from 21.4% in those aged 63–74 years to 15.4% in those aged 85 or more years (P=0.05) and, after adjusting for age, were unrelated to sex (P=0.57).
Lamellar macular holes
LMHs were present in 64 eyes from 56 individuals (3.6%) and, when present, were bilateral in 17.9% of individuals. The frequency of LMHs in right eyes (2.4%, 36/1486) was similar to that in left eyes (1.9%, 28/1494, P=0.27). The frequency of LMHs varied with age from 2.1% in those aged 63–74 years to 2.6% in those aged 85 or more years (P=0.95). After adjusting for age, there was no difference in the frequency of LMHs between men and women (P=0.70).
Full thickness macular holes
An FTMH was present in 7 eyes (5 right eyes and 2 left eyes) in 7 participants (0.5%). Two individuals aged 63–74 years, four individuals aged 75–84 years, and one individual aged 85 years or older had a macular hole. Six participants with an FTMH were women and one was a man.
Interrelationships of ERMs, VMTs, and other retinal lesions and ocular conditions
Eyes with ERMs and/or VMT were more likely to have macular cysts and LMHs present than eyes without (Table 2). Eyes with ERM with bridging and corrugation were more likely to have macular cysts but not PVCs, LMHs, FTMHs, or VMT compared to eyes with ERM with corrugation only.
Table 2.
Prevalence of Epiretinal Membranes and Vitreomacular Traction in the Presence of Selected Ocular Conditions in the Beaver Dam Eye Study, 2008–2010.
| Covariate | ERM absent (N=2308 eyes) | ERM present (N=672 eyes) | HR (95% CI) | P value* | ||
|---|---|---|---|---|---|---|
|
| ||||||
| % with | % with | |||||
|
|
|
|||||
| Macular cysts | 1.8 | 7.6 | 4.0 (2.5, 6.3) | <.0001 | ||
| Paravascular cysts | 11.2 | 14.9 | 1.5 (1.1, 1.9) | 0.007 | ||
| Lamellar macular hole | 0.7 | 7.3 | 10.6 (5.8, 19.6) | <.0001 | ||
| Full thickness macular hole | 0.1 | 0.6 | 7.2 (1.5, 35.5)† | 0.06† | ||
| Vitreomacular traction | 0.6 | 2.2 | 2.7 (1.3, 5.5) | 0.01 | ||
|
| ||||||
| Covariate | ERM corrugation only (N=87 eyes) | ERM bridging and corrugation (N=414 eyes) | HR (95% CI) | P value* | ||
|
| ||||||
| % with | % with | |||||
|
|
|
|||||
| Macular cysts | 3.5 | 10.4 | 2.6 (0.9, 7.2) | 0.03 | ||
| Paravascular cysts | 17.2 | 12.6 | 0.7 (0.4, 1.3) | 0.29 | ||
| Lamellar macular hole | 5.8 | 9.2 | 1.5 (0.6, 3.6) | 0.31 | ||
| Full thickness macular hole | 1.2 | 1.0 | 0.7 (0.1, 6.5) | 0.77 | ||
| Vitreomacular traction | 2.3 | 1.9 | 0.7 (0.2, 3.5) | 0.71 | ||
|
| ||||||
| Covariate | VMT absent (N=2952 eyes) | VMT present (N=28 eyes) | HR (95% CI) | P value* | ||
|
| ||||||
| % with | % with | |||||
|
|
|
|||||
| Macular cysts | 3.0 | 21.4 | 7.0 (2.5, 20.1) | 0.06 | ||
| Paravascular cysts | 12.0 | 17.9 | 1.7 (0.6, 4.6) | 0.39 | ||
| Lamellar macular hole | 2.1 | 7.1 | 2.5 (0.4, 16.8) | 0.43 | ||
| Full thickness macular hole | 0.2 | 3.6 | † | |||
|
| ||||||
| Covariate | ERM absent, VMT absent (N=2295 eyes) | ERM present, VMT absent (N=657 eyes) | ERM absent, VMT present (N=13 eyes) | ERM present, VMT present (N=15 eyes) | P value* | |
|
| ||||||
| % with | % with | % with | % with | |||
|
|
|
|||||
| Macular cysts | 1.7 | 7.5 | 30.8 | 13.3 | <.0001 | |
| Paravascular cysts | 11.2 | 14.6 | 7.7 | 26.7 | 0.04 | |
| Lamellar macular hole | 0.7 | 7.2 | 0.0 | 13.3 | † | |
| Full thickness macular hole | 0.1 | 0.5 | 0.0 | 6.7 | † | |
CI, confidence interval; ERM; epiretinal membrane; HR, hazard ratio; VMT, vitreomacular traction
For any difference among groups. Adjusted for age and sex.
Estimate is unreliable due to low number of subjects.
There were 750 eyes from 442 individuals that had at least one confounding ocular condition. ERMs, LMHs, and macular cysts were more likely to be present in eyes with cataract surgery, retinal diseases (e.g., retinal detachment, retinal vein occlusion, proliferative retinopathy) than in eyes without these conditions (Table 3). In diabetic individuals, SD-OCT scans showed that macular cysts were least frequent in those with no retinopathy (0.3%) and increased in frequency with retinopathy severity (5% in eyes with mild non-proliferative retinopathy, 12% in eyes with moderate non-proliferative retinopathy, 33% in eyes with severe non-proliferative retinopathy, and 29% in eyes with proliferative retinopathy).
Table 3.
Relationship of Other Ocular Diseases and Conditions to Prevalence of Macular and Paravascular Cysts, Lamellar and Full Thickness Macular Holes, Epiretinal Membranes, and Vitreomacular Traction.
| Condition | CA (N) | CP (N) | Macular cysts (%) | Paravascular cysts (%) | Lamellar macular holes (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| CA | CP | P value† | CA | CP | P value† | CA | CP | P value† | |||
| Retinal detachment* | 2945 | 29 | 3.0 | 13.8 | 0.002 | 12.1 | 6.9 | 0.40 | 13.8 | 2.0 | 0.0002 |
| Branch RVO | 2952 | 23 | 3.0 | 21.7 | <.0001 | 12.0 | 17.4 | 0.41 | 8.7 | 2.1 | 0.05 |
| Central RVO | 2971 | 4 | 3.1 | 50.0 | 0.0001 | 12.0 | 25.0 | 0.46 | 0.0 | 2.2 | § |
| POHS | 2967 | 6 | 3.1 | 16.7 | 0.01 | 12.0 | 0.0 | § | 0.0 | 2.2 | § |
| PDR‡ | 469 | 14 | 2.6 | 28.6 | 0.43 | 13.4 | 0.0 | § | 0.0 | 1.9 | § |
| Cataract surgery | 2264 | 716 | 2.2 | 6.2 | 0.002 | 12.2 | 11.5 | 0.43 | 3.5 | 1.7 | 0.009 |
|
|
|||||||||||
| Condition | CA (N) | CP (N) | Full-thickness macular holes (%) | Epiretinal membranes (%) | Vitreomacular traction (%) | ||||||
|
| |||||||||||
| CA | CP | P value† | CA | CP | P value† | CA | CP | P value† | |||
|
| |||||||||||
| Retinal detachment* | 2945 | 29 | 3.5 | 0.2 | 0.003 | 22.0 | 72.4 | <.0001 | 1.0 | 0.0 | § |
| Branch RVO | 2952 | 23 | 0.0 | 0.2 | § | 22.4 | 47.8 | 0.009 | 1.0 | 0.0 | § |
| Central RVO | 2971 | 4 | 0.0 | 0.2 | § | 22.5 | 100.0 | 0.001 | 0.9 | 0.0 | § |
| POHS | 2967 | 6 | 0.0 | 0.2 | § | 22.6 | 33.3 | 0.54 | 0.9 | 0.0 | § |
| PDR‡ | 469 | 14 | 0.0 | 0.0 | § | 20.9 | 71.4 | <.0001 | 0.6 | 0.0 | § |
| Cataract surgery | 2264 | 716 | 0.4 | 0.1 | 0.91 | 19.2 | 33.1 | <.0001 | 0.8 | 1.4 | 0.46 |
CA, condition absent; CP, condition present; PDR, proliferative diabetic retinopathy; POHS, presumed ocular histoplasmosis syndrome; RVO, retinal vein occlusion.
Self-reported.
Adjusted for age and sex.
In individuals with diabetes.
Too few individuals to obtain reliable estimate.
Relationships of ERMs, VMT, macular cysts, PVCs, LMHs, and FTMHs to visual acuity
The presence of ERMs, macular cysts, and FTMHs but not PVCs, LMHs, and VMT was associated with poorer vision (Table 4). Those individuals with VMT in the central circle, regardless of whether it was broad or focal adhesion/distortion, had worse visual acuity than those with VMT found elsewhere (mean number of letters read correctly: 42.9 vs. 50.0). After excluding eyes with confounding retinal conditions, the relationships of ERMs, VMT, macular holes, and cysts to visual acuity remained similar (data not shown).
Table 4.
Relationship of Macular and Paravascular Cysts, Lamellar and Full Thickness Macular Holes, and Epiretinal Membranes and Vitreomacular Traction to Visual Acuity.
| Lesion | Lesion present | Lesion absent | P value for VA | VI* | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Mean N letters read | VA | % VI* | Mean N letters read | VA | % VI* | OR (95% CI) | P value | ||
| Macular cysts | 38.5 | 20/40 | 21.6 | 52.5 | 20/25 | 2.6 | <.0001 | 9.5 (5.3, 17.2) | <.0001 |
| Paravascular cysts | 51.7 | 20/25 | 3.4 | 52.1 | 20/25 | 3.2 | 0.66 | 1.1 (0.6, 2.1) | 0.81 |
| Lamellar macular holes | 51.2 | 20/25 | 6.3 | 52.0 | 20/25 | 3.1 | 0.43 | 1.8 (0.6, 5.8) | 0.38 |
| Full thickness macular holes | 3.0 | 20/200 | 83.3 | 52.1 | 20/25 | 3.0 | 0.02 | 164.8 (19.5, 1395.0) | 0.03 |
| Epiretinal membranes | 47.9 | 20/25 | 7.3 | 53.2 | 20/20 | 2.0 | <.0001 | 3.2 (2.1, 5.1) | <.0001 |
| Vitreomacular traction | 46.2 | 20/32 | 14.3 | 52.1 | 20/25 | 3.1 | 0.16 | 3.9 (1.1, 13.6) | 0.03 |
OR, odds ratio; VA, visual acuity; VI, visual impairment.
Defined as having visual acuity worse than 20/40.
Detection of macular cysts, LMHs, FTMHs, and ERMs by grading of SD-OCT scans versus color fundus images
Lesions detected using fundus photography were almost always detected by SD-OCT. However, many lesions detected by SD-OCT were not detected by fundus photography (Table 5).
Table 5.
Detection of Epiretinal Membranes, Macular Cysts, Lamellar Macular Holes, and Full-Thickness Macular Holes by Fundus Photography versus Spectral Domain Optical Coherence Tomography.
| Lesion | Spectral domain optical coherence
tomography scan |
Total | |
|---|---|---|---|
| Present | Absent | ||
| Macular cysts | |||
| Present in fundus image | 9 | 0 | 9 |
| Absent from fundus image | 84 | 2887 | 2971 |
| Total | 93 | 2887 | 2980 |
| Lamellar macular holes | |||
| Present in fundus image | 1 | 7 | 8 |
| Absent from fundus image | 63 | 2890 | 2953 |
| Total (Cannot grade, N=19) | 64 | 2897 | 2961 |
| Full thickness macular holes | |||
| Present in fundus image | 1 | 23 | 24 |
| Absent from fundus image | 5 | 2932 | 2937 |
| Total (Cannot grade, N=19) | 6 | 2955 | 2961 |
| Epiretinal membranes | |||
| Present in fundus image | 325 | 528 | 853 |
| Absent from fundus image | 322 | 1742 | 2064 |
| Total (Cannot grade, N=63) | 647 | 2270 | 2917 |
Discussion
Most previous population-based estimates of the prevalence of macular cysts, LMHs, FTMHs, ERMs, and VMT have been based on detection of the lesions from fundus photographic images.1,2,16 With the development of the SD-OCT technology, more sensitive and specific estimates of prevalence, size, and appearance of various retinal lesions such as PVCs are possible.17–19 Using SD-OCT, the prevalences of ERMs (34.1%), VMT (1.6%), macular cysts (5.7%), PVCs (20.0%), LMHs (3.6%) and FTMHs (0.4%) were estimated in the population-based BDES cohort of persons aged 63–102 years. ERMs were related to the presence of macular cysts, PVCs, and LMHs. Compared to grading of fundus photographs, grading of SD-OCT scans allowed for increased detection of macular cysts (detected in 9 fundus photographs vs. 93 SD-OCT images) and LMHs (detected in 8 fundus photographs vs. 64 SD-OCT images).
The overall prevalence of ERMs (34%) and the age-specific prevalence of ERMs as determined by SD-OCT were higher than that reported in earlier population-based studies. This included studies that assessed the presence of ERMs by grading of stereoscopic fundus photographs (e.g., the BDES) as well as those that used both SD-OCT and fundus photographs (e.g., the Handan Eye Study).1,20 The reason for these differences among and within studies is not known. It may be due to differences in definition as well as variation in imaging.
The prevalence of VMT as determined by SD-OCT and as defined by the International Vitreomacular Traction Study Group14 was lower in the BDES (1.6%) than that found in the Beijing Eye Study (2.4%).21,22 In both the BDES and Beijing Eye Study, the prevalence of VMT increased with age and was higher in women than in men, although it was not statistically significant in the BDES after adjusting for age. VMT has also been reported to be more common in females after menopause, leading to the speculation that this may be due to lack of a protective effect of estrogen on the connective tissue in the vitreous gel in the absence of estrogen in postmenopausal women.23 The cross-sectional nature of our study limited our ability to describe the temporal relationships of ERMs and VMT to the development of lamellar holes and FTMHs. Clinical studies using SD-OCT scans have shown the early involvement of ERMs in the development of lamellar holes and VMT in the development of FTMHs.24
In the BDES cohort, macular cysts were present in approximately 7% of the cohort that had undergone cataract surgery. This is consistent with the findings of a case series in which cystoid macular edema was found via SD-OCT in 5% of eyes following uncomplicated phaco-emulsification cataract surgery.25 In eyes estimated to have had cataract surgery one or more years prior to the most recent BDES examination, the cysts that were found were usually associated with the presence of retinal conditions such as ERMs, diabetic retinopathy, and retinal vein occlusion (Meuer SM, unpublished data). Other studies have shown the temporal relationship of cataract surgery to macular cysts.25
Retinopathy with cystoid macular edema is a common cause of visual impairment in people with diabetes.16,26 Using SD-OCT, a prevalence of approximately 50% has been reported in eyes with diabetic macular edema, with the likelihood of cysts in diabetic individuals found to be directly related to macular thickness. 27,28 Cysts have been found to be more frequent as retinopathy severity increases. Among diabetic individuals in the BDES, SD-OCT scans showed that prevalence of macular cysts varied from 0.3% in eyes with no diabetic retinopathy to 29% in eyes with proliferative retinopathy.
In the BDES, the prevalence of macular cysts was higher in eyes with a VMT (12%) than in eyes with ERMs (5%). These findings are consistent with earlier studies that showed an association of ERMs with the presence of intraretinal cystic changes.29–31 Sebag and colleagues found higher frequencies of macular cysts, FTMHs and other lesions in eyes with vitreopapillary adhesions than in eyes with ERMs only.3 The frequency of vitreopapillary adhesions was not estimated in the BDES and data regarding the history of uveitis and other conditions associated with macular cysts were not collected.
The use of SD-OCT has enabled detection of cysts not detected by grading fundus images. In the BDES, only 9.7% (9/93) eyes with macular cysts detected by SD-OCT were also detected by grading of fundus images. Because of the association of these cysts with visual impairment, further follow-up should provide better understanding of the prognostic implications of finding them in asymptomatic individuals with an ERM or when minimal retinopathy is present.
PVCs were present in 20% of eyes in the BDES cohort and had no clinically measurable effect on visual acuity. They were not related to age or to eye diseases commonly associated with macular cysts. In the BDES, PVCs were related to ERMs with traction and high myopia. The association with high myopia is consistent with other studies in which PVCs have been shown to be related to high myopia or elongation of the eye.5 Vascular microfolds and paravascular retinal holes may develop secondary to PVCs. PVCs were not related to decreased visual acuity in the BDES and have little clinical relevance.
This study found very low sensitivity for detection of macular cysts (9.7%), LMHs (1.6%), and other retinal lesions (50% or less) studied by grading stereoscopic fundus photographic images compared to grading of SD-OCT scans. These findings provide evidence to support the use of SD-OCT for the detection of these retinal lesions in epidemiologic studies and clinical trials.
This study has many strengths, including the use of standardized protocols for measurements as well as systematic grading of stereoscopic photographs and SD-OCT scans. However, there are limitations. There was limited power to detect associations in eyes with infrequent conditions. Further follow-up is necessary to examine SD-OCT as a tool for risk assessment.
In conclusion, using SD-OCT, this study shows that the frequencies of two vitreoretinal interface disorders, ERM and VMT, increase with age and that they are related to macular cysts, LMHs, and visual impairment in persons aged 63 years and older. Population-based estimates of the incidence of these conditions, their relation to the development of macular cysts and holes using SD-OCT, and their effect on visual function are needed.
Acknowledgments
Financial Support: This study was supported by National Institutes of Health grant EY06594 (BEK Klein and R Klein) and by an unrestricted grant from Research to Prevent Blindness, New York, NY. The National Eye Institute provided funding for entire study including collection and analyses of data; Research to Prevent Blindness provided additional support for data analyses. The funding organizations had no role in the design or conduct of this research.
The authors wish to acknowledge Mary Rechek, RN and Jennifer Poetter, BS for their assistance with data collection and grading of SD-OCT scans for this study and Nancy Barrett, MS, Barbara Budig, BS, Charles Chandler, BS, Holly Cohn, MFA, Shirley Craanen, BS, Andrew Ewen, BS, and Anne Mosher, BS for their assistance with grading fundus photographs for this study. The authors also thank Topcon Corporation for providing the SD-OCT equipment used in this study.
Footnotes
Meeting Presentation: An abstract of this study was presented at the 2014 Annual Meeting of the Association for Research and Vision in Ophthalmology (ARVO), Orlando, Florida, USA, May 4-8, 2014.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Eye Institute or the National Institutes of Health.
Conflict of Interest: None of the authors has any conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klein R, Klein BE, Wang Q, Moss SE. The epidemiology of epiretinal membranes. Trans Am Ophthalmol Soc. 1994;92:403–25. [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell P, Smith W, Chey T, et al. Prevalence and associations of epiretinal membranes: the Blue Mountains Eye Study, Australia. Ophthalmology. 1997;104:1033–40. doi: 10.1016/s0161-6420(97)30190-0. [DOI] [PubMed] [Google Scholar]
- 3.Sebag J, Wang MY, Nguyen D, Sadun AA. Vitreopapillary adhesion in macular diseases. Trans Am Ophthalmol Soc. 2009;107:35–44. [PMC free article] [PubMed] [Google Scholar]
- 4.Forte R, Cennamo G, Pascotto F, de Crecchio G. En face optical coherence tomography of the posterior pole in high myopia. Am J Ophthalmol. 2008;145:281–8. doi: 10.1016/j.ajo.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 5.Ohno-Matsui K, Hayashi K, Tokoro T, Mochizuki M. Detection of paravascular retinal cysts before using OCT in a highly myopic patient. Graefes Arch Clin Exp Ophthalmol. 2006;244:642–4. doi: 10.1007/s00417-005-0112-6. [DOI] [PubMed] [Google Scholar]
- 6.Spencer LM, Foos RY. Paravascular vitreoretinal attachments. Role in retinal tears. Arch Ophthalmol. 1970;84:557–64. doi: 10.1001/archopht.1970.00990040559001. [DOI] [PubMed] [Google Scholar]
- 7.Klein R, Klein BE, Linton KL, De Mets DL. The Beaver Dam Eye Study: visual acuity. Ophthalmology. 1991;98:1310–5. doi: 10.1016/s0161-6420(91)32137-7. [DOI] [PubMed] [Google Scholar]
- 8.Klein R, Klein BE, Lee KE. Changes in visual acuity in a population: the Beaver Dam Eye Study. Ophthalmology. 1996;103:1169–78. doi: 10.1016/s0161-6420(96)30526-5. [DOI] [PubMed] [Google Scholar]
- 9.Klein R, Klein BE, Lee KE, et al. Changes in visual acuity in a population over a 10-year period: the Beaver Dam Eye Study. Ophthalmology. 2001;108:1757–66. doi: 10.1016/s0161-6420(01)00769-2. [DOI] [PubMed] [Google Scholar]
- 10.Klein R, Klein BE, Lee KE, et al. Changes in visual acuity in a population over a 15-year period: the Beaver Dam Eye Study. Am J Ophthalmol. 2006;142:539–49. doi: 10.1016/j.ajo.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Klein R, Lee KE, Gangnon RE, Klein BE. Incidence of visual impairment over a 20-year period: the Beaver Dam Eye Study. Ophthalmology. 2013;120:1210–9. doi: 10.1016/j.ophtha.2012.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linton KL, Klein BE, Klein R. The validity of self-reported and surrogate-reported cataract and age-related macular degeneration in the Beaver Dam Eye Study. Am J Epidemiol. 1991;134:1438–46. doi: 10.1093/oxfordjournals.aje.a116049. [DOI] [PubMed] [Google Scholar]
- 13.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs: an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 14.Duker JS, Kaiser PK, Binder S, et al. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120:2611–9. doi: 10.1016/j.ophtha.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 15.Stalmans P, Benz MS, Gandorfer A, et al. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N Engl J Med. 2012;367:606–15. doi: 10.1056/NEJMoa1110823. [DOI] [PubMed] [Google Scholar]
- 16.Klein R, Klein BE, Moss SE. Visual impairment in diabetes. Ophthalmology. 1984;91:1–9. [PubMed] [Google Scholar]
- 17.Sigler EJ. Microcysts in the inner nuclear layer, a nonspecific SD-OCT sign of cystoid macular edema. Invest Ophthalmol Vis Sci. 2014;55:3282–4. doi: 10.1167/iovs.14-14056. [DOI] [PubMed] [Google Scholar]
- 18.Salvatore S, Genead MA, Fishman GA. The prevalence of macular cysts in patients with clinical cone-rod dystrophy determined by spectral-domain optical coherence tomography. Ophthalmic Genet. 2014;35:47–50. doi: 10.3109/13816810.2013.804095. [DOI] [PubMed] [Google Scholar]
- 19.Deak GG, Bolz M, Ritter M, et al. A systematic correlation between morphology and functional alterations in diabetic macular edema. Invest Ophthalmol Vis Sci. 2010;51:6710–4. doi: 10.1167/iovs.09-5064. [DOI] [PubMed] [Google Scholar]
- 20.Duan XR, Liang YB, Friedman DS, et al. Prevalence and associations of epiretinal membranes in a rural Chinese adult population: the Handan Eye Study. Invest Ophthalmol Vis Sci. 2009;50:2018–23. doi: 10.1167/iovs.08-2624. [DOI] [PubMed] [Google Scholar]
- 21.Shao L, Wei W. Vitreomacular traction syndrome. Chin Med J (Engl) 2014;127:1566–71. [PubMed] [Google Scholar]
- 22.Shao L, Xu L, You QS, et al. Prevalence and associations of incomplete posterior vitreous detachment in adult Chinese: the Beijing Eye Study. PLoS One. 2013;8:e58498. doi: 10.1371/journal.pone.0058498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chuo JY, Lee TY, Hollands H, et al. Risk factors for posterior vitreous detachment: a case-control study. Am J Ophthalmol. 2006;142:931–7. doi: 10.1016/j.ajo.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Witkin AJ, Ko TH, Fujimoto JG, et al. Redefining lamellar holes and the vitreomacular interface: an ultrahigh-resolution optical coherence tomography study. Ophthalmology. 2006;113:388–97. doi: 10.1016/j.ophtha.2005.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vukicevic M, Gin T, Al-Qureshi S. Prevalence of optical coherence tomography-diagnosed postoperative cystoid macular oedema in patients following uncomplicated phaco-emulsification cataract surgery. Clin Experiment Ophthalmol. 2012;40:282–7. doi: 10.1111/j.1442-9071.2011.02638.x. [DOI] [PubMed] [Google Scholar]
- 26.Trichonas G, Kaiser PK. Optical coherence tomography imaging of macular oedema. Br J Ophthalmol. 2014;98(Suppl 2):ii24–9. doi: 10.1136/bjophthalmol-2014-305305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto S, Yamamoto T, Hayashi M, Takeuchi S. Morphological and functional analyses of diabetic macular edema by optical coherence tomography and multifocal electroretinograms. Graefes Arch Clin Exp Ophthalmol. 2001;239:96–101. doi: 10.1007/s004170000238. [DOI] [PubMed] [Google Scholar]
- 28.Otani T, Kishi S, Maruyama Y. Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol. 1999;127:688–93. doi: 10.1016/s0002-9394(99)00033-1. [DOI] [PubMed] [Google Scholar]
- 29.Sato H, Kawasaki R, Yamashita H. Observation of idiopathic full-thickness macular hole closure in early postoperative period as evaluated by optical coherence tomography. Am J Ophthalmol. 2003;136:185–7. doi: 10.1016/s0002-9394(03)00094-1. [DOI] [PubMed] [Google Scholar]
- 30.Chen JC, Lee LR. Clinical spectrum of lamellar macular defects including pseudoholes and pseudocysts defined by optical coherence tomography. Br J Ophthalmol. 2008;92:1342–6. doi: 10.1136/bjo.2007.133041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michalewski J, Michalewska Z, Cisiecki S, Nawrocki J. Morphologically functional correlations of macular pathology connected with epiretinal membrane formation in spectral optical coherence tomography (SOCT) Graefes Arch Clin Exp Ophthalmol. 2007;245:1623–31. doi: 10.1007/s00417-007-0579-4. [DOI] [PubMed] [Google Scholar]