Abstract
Background
Ventral incisional hernias (VIH) develop in up to 20% of patients following abdominal surgery. No widely applicable pre-operative risk-assessment tool exists. We aim to develop and validate a risk-assessment tool to predict VIH following abdominal surgery.
Study Design
A prospective study of all patients undergoing abdominal surgery was conducted at a single institution from 2008-2010. Variables were defined in accordance with the National Surgical Quality Improvement Project. VIH was determined through clinical and radiographic evaluation. A multivariate Cox proportional hazard model was built from a development cohort (2008-2009) to identify predictors of VIH. The HERNIAscore was created by converting the hazards ratios (HR) to points. The predictive accuracy was assessed on the validation cohort (2010) using a receiver operator characteristic curve and calculating the area under the curve (AUC).
Results
Of 625 patients followed for a median of 41(0.3-64 months), 93(13.9%) developed a VIH. The training cohort (n=428, VIH=70,16.4%) identified four independent predictors: laparotomy (HR 4.77, 95%CI 2.61-8.70) or hand-assisted laparoscopy (HR=4.00, 95% CI 2.08-7.70), COPD (HR=2.35; 95%CI 1.44-3.83), and BMI≥25 (HR=1.74; 95% CI 1.04-2.91). Factors that were not predictive included age, gender, ASA score, albumin, immunosuppression, prior surgery, and suture material/technique. The predictive score had an AUC=0.77(95%CI0.68-0.86) using the validation cohort (n=197, VIH=23,11.6%). Using the HERNIAscore--HERNIAscore=4*Laparotomy+3*HAL+1*COPD+1* BMI≥25--three classes stratified the risk of VIH: Class I (0-3 points):5.2%, Class II (4-5 points):19.6%, and Class III (6 points):55.0%.
Conclusions
The HERNIAscore accurately identifies patients at increased risk for VIH. While external validation is needed, this provides a starting point to counsel patients and guide clinical decisions. Increasing the use of laparoscopy, weight-loss programs, community smoking prevention programs, and incisional reinforcement may help reduce rates of VIH.
Introduction
In developed countries, one-third of all individuals will undergo abdominal surgery in their lifetime.1 Twenty percent of these patients will develop a ventral incisional hernia (VIH). VIHs cause significant morbidity for patients: they can increase in size, worsen an individual's ability to function, cause skin breakdown, or incarcerate and strangulate requiring emergency surgery.2,3 While outcomes of emergency VIH repair are poor, elective repair can be complex and associated with high rates of complications including surgical site infections (SSI: 20-30%) and hernia recurrences (20-30%).4,5 It is estimated that the United States spends 3.2 billion dollars annually on the surgical management of ventral hernias. Each major complication following the surgical management of a ventral hernia repair costs the healthcare system $30,000 to $210,000 to address.6
There is currently no widely accepted scoring system for predicting VIH formation after abdominal surgery. Several studies have evaluated VIH risk after specific procedures, such as transplant or cardiac surgery, but the applicability to other procedures is unknown.7,8,9 Two recent prospective studies of patients undergoing elective and urgent abdominal surgery have identified risk factors for VIH following open abdominal surgery.10,11 Itatsu et al followed over 4000 consecutive patients while Veljkovik et al used a cohort of 603 patients to develop and validate a hernia risk scoring system equation.10,11 Both models included post-operative complications such as SSIs, which may limit their use preoperatively to counsel patients or to change perioperative management to modify risk. In addition, the generalizability of their findings is unknown as both studies were performed outside of the United States. For example, in the Itatsu study, the mean body mass index (BMI) was 21.9 kg/m2, which is less than the average BMI in the United States of 28.5 kg/m2.10,12 In the Veljkovik study, there was significant variation in use of prophylactic antibiotics due to lack of standardized protocols.11 Lastly, the two studies varied in follow-up time; the Itatsu study had a significantly longer follow-up than the Veljkovik study (median 17 months vs. mean 6.9 months).10,11 The optimal duration of follow-up for diagnosing VIH is controversial but recommended follow-up is for at least 3 years.13 Furthermore, inclusion of patients with less than 6 months of follow-up may bias the results if differential loss to follow-up occurred (i.e., patients were lost because they developed a VIH and sought care elsewhere or patients were lost because they had no complications or ongoing issues). On the other hand, patients who develop a VIH within 6 months of surgery may have different risk factors.
The primary aim of this project was to develop and validate a predictive score for VIH following abdominal surgery. The secondary aims were: (1) to compare whether a score that includes postoperative complications such as SSI more accurately predicts VIH than one that does not, (2) to assess whether the risk factors for VIH differ depending upon the length of follow-up, and (3) to determine whether the risk factors for fascial dehiscence (and early VIH formation) are the same as those for the overall cohort.
Methods
Following institutional review board approval, a prospective cohort study was performed of all consecutive patients undergoing abdominal surgeries, including elective and urgent or emergent cases, performed at a single institution between January 2008 and December 2010. Patients who underwent ventral hernia repair as the primary procedure, any closure reinforced by mesh, or any case where the fascia was not closed were excluded. Because of a lack of consensus regarding the diagnosis of VIH, the primary outcome of VIH was considered to have occurred based on either clinical examination or radiographic imaging.14 Secondary outcomes included fascial dehiscence, SSI, length of stay, and reoperation which was defined as any procedure performed in the operating room suite violating the abdominal fascia or peritoneal cavity.
Patient demographics, comorbidities, and intra-operative data were collected. All comorbidities and complications were defined in accordance with the National Surgical Quality Improvement Project (NSQIP). Variables not utilized by NSQIP were defined as follows. Patients with a temporary abdominal closure were listed as “open abdomen” and the index surgery was when their fascia was closed. Operative approach included purely laparoscopic surgery (all incisions < 15 mm in size), hand-assisted laparoscopy including any laparoscopic surgery with an incision extended beyond 15 mm in size for hand placement or organ extraction, and laparotomy. Fascial incision type was classified as midline incision along the linea alba, any transverse fascial incision, and other which included longitudinal paramedian incisions. Sutures were classified as fast-absorbable if the reported rate of complete absorption was < 6 month (e.g. polyglactin--Vicryl® Ethicon or Polysorb® Covidien), slow-absorbable if the reported rate of complete absorption was > 6 months (e.g polydioxanone--PDS® Ethicon or Dexon® Covidien), and permanent suture (e.g. polypropylene, Prolene® Ethicon or Surgilene® Coviden). Closure technique was reported as running, interrupted, and other suturing techniques including mixed techniques.
VIH was analyzed for the overall cohort using the Kaplan-Meier method. The overall cohort was then divided into development (2008-2009) and validation (2010) cohorts. The predictive model was based on the development cohort. Univariate analyses included unpaired t-test or Wilcoxon rank-sum test for continuous data and Fishers exact test for categorical data. All preoperative and intra-operative variables were initially included for multivariable model development. A backwards stepwise Cox regression, to adjust for follow-up duration and time to event, was performed to identify variables independently predictive of VIH. Hazard ratios (HRs) with 95% confidence intervals (95% CI) were generated. The proportionality of hazards was estimated graphically using log minus log survival curves. Variables with confidence intervals not crossing 1 and a p-value of <0.05 were considered statistically significant. Models were evaluated for interactions and colinearity.
Based upon the final model, the HERNIAscore was developed by rounding the hazard ratios to the nearest integer minus one.15 The HERNIAscore was assessed on the validation cohort using a receiver operator characteristic curve (ROC). An area under the curve (AUC) of >0.70 was considered a viable model. All patients in the development and validation cohorts were assigned a HERNIAscore and actual rates of ventral incisional hernia were reported. Patients were classified into three groups based upon similar rates of VIH formation. All statistical calculations were accomplished using SPSS (IBM Corp, Armonk, NY).
Sensitivity analyses were performed. A Cox regression model for VIH formation was created (1) for the development cohort using preoperative, intra-operative, and postoperative variables (SSI); (2) for all patients with 6 months or more of follow-up; (3) for all patients with less than 6 months of follow-up; and (4) for patients who developed a fascial dehiscence.
Results
Overall Cohort
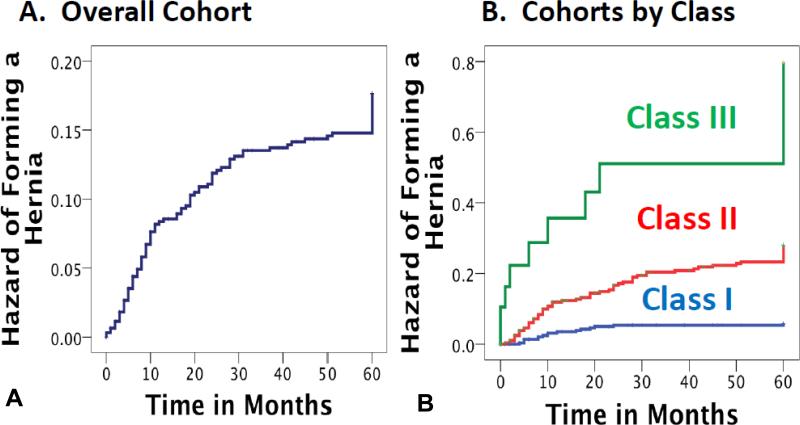
The overall cohort included 625 patients with a median (range) follow-up of 41 (0.3-64) months; 93 (13.9%) developed a VIH (Figure 1A; Table 1, 2, and 3). A CT scan was performed in 439 (70.2%) of patients: among these patients, 346 (78.8%) had no hernia by exam or CT, 59 (13.4%) had hernia by exam and CT, 14 (3.2%) had hernia by CT but not exam, while 20 (4.6%) had a hernia by exam but not CT (p=0.390).
Figure 1.
Kaplan-Meier curve of the hazard of ventral incisional hernia over time (A) of overall cohort and (B) of cohorts by HERNIAscore class.
Table 1.
Demographics and Comorbidities
| Variable | Overall cohort (n=625) | Development cohort (n=428) | ||
|---|---|---|---|---|
| Hernia (n=70) | No hernia (n=358) | p Value | ||
| Age, y | 60.8 ± 11.4 | 62.6 ± 9.11 | 60.3 ± 12.0 | <0.001 |
| Sex, male, n (%) | 588 (94.1%) | 69 (98.6%) | 333 (93.0%) | 0.098 |
| Ethnicity, white, n (%) | 343 (54.9%) | 47 (67.1%) | 192 (53.6%) | 0.048 |
| Functional status, n (%) | 0.846 | |||
| Independent | 551 (88.2%) | 59 (84.3%) | 314 (87.7%) | |
| Partially dependent | 39 (6.2%) | 5 (7.1%) | 24 (6.7%) | |
| Dependent | 35 (5.6%) | 6 (8.6%) | 20 (56%) | |
| ASA score, n (%) | 0.040 | |||
| 1 | 12 (1.9%) | 0 (0%) | 11 (3.1%) | |
| 2 | 94 (15.0%) | 4 (5.7%) | 58 (16.2%) | |
| 3 | 395 (63.2%) | 47 (67.1%) | 221 (61.7%) | |
| 4 | 123 (19.7%) | 19 (27.1%) | 67 (18.7%) | |
| 5 | 1 (0.2%) | 0 (0%) | 1 (0.3%) | |
| BMI, kg/m2 | 28.1 ± 5.7 | 28.7 ± 6.5 | 28.1 ± 5.6 | 0.418 |
| Collagen vascular disorder, n (%) | 30 (4.8%) | 4 (5.7%) | 20 (5.6%) | 1.000 |
| Diabetes Mellitus, n (%) | 160 (25.6%) | 16 (22.9%) | 92 (25.7%) | 0.655 |
| COPD, n (%) | 83 (13.3%) | 19 (27.1%) | 35 (9.8%) | <0.001 |
| Hypertension, n (%) | 430 (68.8%) | 51 (72.9%) | 246 (68.7%) | 0.571 |
| Cardiovascular disease, n (%) | 169 (27.0%) | 21 (30.0%) | 98 (27.4%) | 0.663 |
| Liver disease, n (%) | ||||
| Any | 86 (13.8%) | 7 (10.0%) | 53 (14.8%) | 0.350 |
| Severe | 25 (4.0%) | 2 (2.9%) | 14 (3.9%) | 1.000 |
| Immunosuppression, n (%) | 30 (4.8%) | 4 (5.7%) | 16 (4.5%) | 0.551 |
| Active smoking, n (%) | 215 (34.4%) | 19 (27.1%) | 121 (33.8%) | 0.330 |
| Alcohol use disorder, n (%) | 83 (13.3%) | 9 (12.8%) | 42 (11.7%) | 0.840 |
| Hematocrit, % | 38.1 ± 6.0 | 36.6 ± 6.1 | 38.4 ± 5.9 | 0.022 |
| Albumin, g/dL | 3.4 ± 0.7 | 3.2 ± 0.7 | 3.4 ± 0.7 | 0.120 |
| Creatinine, mg/dL | 1.1 ± 0.7 | 1.1 ± 0.4 | 1.1 ± 0.8 | 0.487 |
| Prior operation | ||||
| Yes, n (%) | 295 (47.2%) | 36 (50.7%) | 160 (44.6%) | 0.359 |
| Number (range) | 0 (0-13) | 1 (0-13) | 0 (0-9) | 0.266 |
| History of SSI, n (%) | 60 (9.6%) | 13 (18.3%) | 33 (9.2%) | 0.033 |
| Open abdomen, n (%) | 16 (2.6%) | 4 (5.7%) | 7 (2.0%) | 0.088 |
ASA, American Society of Anesthesiologist Score; SSI, surgical site infection.
Table 2.
Operative Details
| Variable | Overall cohort (n=625) | Development cohort (n=428) | ||
|---|---|---|---|---|
| Hernia (n=70) | No hernia (n=358) | p Value | ||
| Acute, n (%) | 187 (29.9%) | 26 (37.1%) | 103 (28.8%) | 0.199 |
| Operative service, n (%) | 0.002 | |||
| General | 432 (69.1%) | 53 (75.7%) | 246 (68.7%) | |
| Gynecology | 10 (1.6%) | 0 (0%) | 8 (2.2%) | |
| Plastic | 1 (0.2%) | 1 (1.4%) | 0 (0%) | |
| Thoracic | 2 (0.3%) | 0 (0%) | 1 (0.3%) | |
| Vascular | 16 (2.6%) | 2 (2.9%) | 8 (2.2%) | |
| Urology | 164 (26.2%) | 4 (5.7%) | 95 (26.5%) | |
| Operative duration, h | 3.6 ± 2.1 | 4.1 ± 2.5 | 3.5 ± 2.0 | 0.020 |
| Tranfusion | ||||
| Yes/No, n (%) | 128 (20.5%) | 23 (32.9%) | 63 (17.6%) | |
| Number, mean (range) | 0 (0-17) | 0 (0-7) | 0 (0-17) | |
| EBL, mL (range) | 159 (5-7000) | 200 (10-3000) | 150 (5-7000) | 0.747 |
| Operative approach, n (%) | <0.001 | |||
| Open | 220 (35.2%) | 37 (52.9%) | 112 (31.2%) | |
| HAL | 136 (21.8%) | 21 (30.0%) | 80 (22.3%) | |
| Laparoscopic | 269 (43.0%) | 12 (17.1%) | 166 (46.4%) | |
| Fascial incision type, n (%) | <0.001 | |||
| Midline | 292 (46.7%) | 54 (77.1%) | 152 (42.5%) | |
| Transverse | 59 (9.4%) | 5 (7.1%) | 38 (10.6%) | |
| Other | 274 (43.8%) | 11 (15.7%) | 168 (46.9%) | |
| Wound Class, n (%) | 0.373 | |||
| 1 | 75 (12.0%) | 9 (12.9%) | 39 (10.9%) | |
| 2 | 455 (72.8%) | 45 (64.3%) | 261 (72.9%) | |
| 3 | 50 (8.0%) | 7 (10.0%) | 32 (8.9%) | |
| 4 | 45 (7.2%) | 9 (12.9%) | 26 (7.3%) | |
| Suture, n (%) | <0.001 | |||
| Fast-absorbable | 247 (39.5%) | 11 (15.7%) | 156 (43.6%) | |
| Slow-absorbable | 342 (54.7%) | 52 (74.3%) | 181 (50.6%) | |
| Permanent | 22 (3.5%) | 7 (10.0%) | 13 (3.6%) | |
| Other | 14 (2.2%) | 0 (0%) | 8 (2.2%) | |
| Closure technique, n (%) | 0.194 | |||
| Running | 385 (61.6%) | 46 (65.7%) | 212 (59.2%) | |
| Interrupted | 98 (15.7%) | 7 (10.0%) | 68 (19.0%) | |
| Other | 142 (22.7%) | 17 (24.3%) | 78 (21.8%) | |
EBL, estimated blood loss; HAL, hand assisted laparoscopy.
Table 3.
Outcomes and Follow-up Duration
| Variable | Overall cohort (n=625) | Development cohort (n=428) | ||
|---|---|---|---|---|
| Hernia (n=70) | No hernia (n=358) | p Value | ||
| Ventral incisional hernia, n (%) | 93 (13.9%) | - | - | - |
| Fascial dehiscence, n (%) | 19 (3.0%) | 13 (18.3%) | - | - |
| SSI, n (%) | 51 (11.6%) | 15 (31.9%) | 19 (8.4%) | <0.001 |
| Length of stay, d (range) | 6 (0-384) | 9 (2-64) | 5 (0-147) | <0.001 |
| Reoperation abdominal, n (%) | 120 (19.2%) | 36 (51.4%) | 43 (12.0%) | <0.001 |
| Follow-up duration, mo (range) | 41 (0.25-64) | 48 (1-58) | 46 (0.25-64) | 0.412 |
SSI, surgical site infection.
Development Cohort
In the development cohort (n=428), 70 (16.4%) patients were diagnosed with a VIH. Patients who developed VIH were more likely to be older in age, have an elevated ASA score, be diagnosed with COPD, undergo longer surgery, have an open laparotomy, have a midline incision, and have had permanent sutures used for fascial closure (Table 1 and 2). No interactions were noted; in particular, there was no interaction between COPD and immunosuppression. Patients who developed a VIH were more likely to have had a SSI at the time of the index abdominal surgery, have a longer length of hospital stay, and undergo another abdominal surgery (Table 3).
Model Development
The results of the Cox hazard model are reported in table 4. The model was assessed for predictive accuracy on the validation cohort with an AUC of 0.77 (95% confidence interval 0.68-0.86). The HERNIAscore and rates of VIH formation are reported in table 5. Kaplan-meier curve demonstrates the rate of VIH formation over time for the three risk classes (Figure 1B).
Table 4.
Multivariable Cox Regression Analyses: Main Analysis of Development Cohort and Sensitivity Analysis
| Main analysis | Sensitivity analyses | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Development cohort | Including SSI | >6 mo follow-up | <6 mo follow-up | Fascial dehiscence | ||||||
| (n=428) | (n=428) | (n=583) | (n=42) | (n=625) | ||||||
| Variable | HR | 95% CI | HR | 95% CI | HR | 95%CI | HR | 95% CI | HR | 95% CI |
| COPD | 2.35 | 1.44-3.83 | 2.53 | 1.55-4.13 | 2.8 | 1.54-5.09 | 3.72 | 1.27-10.87 | - | - |
| Laparoscopic | - | |||||||||
| HAL | 4.00 | 2.08-7.70 | 3.42 | 1.78-6.61 | 4.34 | 2.15-8.75 | - | - | 7.35 | 0.82-66.14 |
| Laparotomy | 4.77 | 2.61-8.70 | 3.69 | 2.00-6.83 | 5.48 | 2.89-10.39 | - | - | 12.83 | 1.64-100.50 |
| BMI ≥25 kg/m2 | 1.74 | 1.04-2.91 | - | - | 1.84 | 1.04-3.27 | - | - | - | - |
| SSI | - | - | 2.44 | 1.51-3.94 | - | - | - | - | - | - |
| Open Abdomen | - | - | - | - | - | - | - | - | 4.01 | 1.22-13.13 |
| AUC | 0.77 | 0.68-0.86 | 0.80 | 0.71-0.90 | - | - | - | - | - | - |
Table 5.
Predictive Score for and Actual Rates of Ventral Incisional Hernia after Abdominal Surgery
| Risk of hernia formation based on HERNIAscore points* | |||
|---|---|---|---|
| Class I (0-3 points) | Class II (4-5 points) | Class III (6 points) | |
| Development cohort (n=428) | 14 (6.8%) | 48 (23.2%) | 8 (50.0%) |
| Validation cohort (n=197) | 2 (2.0%) | 18 (19.6%) | 3 (75.0%) |
| Overall cohort (n=625) | 16 (5.2%) | 66 (22.1%) | 11 (55.0%) |
HERNIAscore = 4*Laparotomy + 3* hand-assisted aparoscopy + 1*COPD + 1*BMI>25 kg/m2. Points based on hazard ratios rounded to the nearest integer minus 1.
Sensitivity Analyses
When post-operative variables were included in the Cox regression, SSI was strongly associated with VIH (Table 4). Compared to patients followed 6 months or more, patients followed for less than 6 months were older, more likely to be functionally dependent, have more comorbidities, prior abdominal surgeries and SSIs, undergo an emergency operation, have an open abdomen, a contaminated surgical field, and develop a SSI. Evaluation of only patients who had at least of 6 months of follow-up (n=583) did not change the model. In patients who had less than 6 months of follow-up (n=42), only COPD was associated with VIH. Factors predictive of fascial dehiscence included incision type and closure of an open abdomen.
Discussion
In this prospective study, a model to predict VIH following abdominal surgery was developed and validated. Three pre-operative and intra-operative findings independently associated with VIH formation were identified: surgical approach (open laparotomy versus hand-assisted laparoscopy versus laparoscopy), COPD, and BMI≥25 kg/m2. In the lowest risk patients, the risk of developing a VIH was 5.5% while in the highest risk group over half of all individuals developed a VIH. This risk score is straight-forward to use, practical, and can be estimated preoperatively to determine a patient's risk of forming a VIH.
While prior studies have evaluated risk factors for VIH following abdominal surgery, most would not be able to be used for preoperative counseling because of the inclusion of postoperative complications (such as SSI) or interventions (such as time to suture removal) in the predictive model.7-12,16, 17 In addition, many studies only evaluated a single surgery type. The applicability of these studies to other procedure types or for use in pre-operative planning is limited. The HERNIAscore was derived from abdominal surgeries performed by multiple subspecialties and can potentially be applied in the preoperative period to a wide variety of abdominal surgeries Furthermore, although surgical approach is an intra-operative factor, the final operative approach did not differ from the planned preoperative approach in 99% of cases.
Laparoscopy was associated with a decreased incidence of VIH compared to laparotomy or hand-port assisted surgery. On univariate analysis, fascial incision type (transverse 11.6%, midline 26.2%, and paramedian 6.1%) and suture type used (fast-absorbable 6.6%, slow-absorbable 22.3%, permanent 35.0%) were strongly associated with VIH formation. Midline incisions, compared to transverse or paramedian incisions, have been demonstrated to increase risk of VIH.11,18 However, for open surgery, midline incisions have gained widespread adoption due to relative ease of use as well as flexibility in accessing all areas of the peritoneal cavity. In addition, paramedian and transverse incisions may be associated with defunctionalizing the abdomen by dividing the perforating nerves. While fast-absorbable sutures have been demonstrated to increase the risk of VIH in other studies, in this study, closure with this suture type had the lowest rate of VIH on univariate analysis.19,20 This is likely related to selection bias with laparoscopic port sites being closed with fast absorbable sutures while high-risk incisions may be closed with permanent sutures. The technique that had the greatest impact on outcomes was the use of smaller incisions, in particular laparoscopic incisions < 15 mm in size.16,17 The impact of fascial incision type and suture type may have been mitigated through the increasing use of laparoscopy. One final factor of importance that was not included in this analysis is the use of small bites (0.5×0.5) cm) versus large bites (1.0×1.0cm). Small bites has been shown to decrease SSI rates and VIH rates.21,22 However, in both studies, the patient populations had mean BMI < 25 kg/m2 and benefit of small bites to an obese patient population remains to be evaluated.
Other studies have found age, gender, immunosuppression, diabetes, and wound class to be risk factors for VIH formation. Surprisingly, COPD was either not reported or not associated with VIH in these studies. The association in this study between COPD and VIH and fascial dehiscence may be related to a higher prevalence of COPD in Veterans. Nonetheless, COPD is a biologically plausible risk factor and is known to be associated with VIH and VIH recurrence.23 COPD may play a role through two mechanisms: increasing intra-abdominal pressure through cough and weakening of the tissues through an underlying collagen disorder. Immunosuppression due to steroid treatment could be an additional contributing factor; however, in this study, there was no difference in steroid use between patients with and without a VIH and there was no interaction between steroids and COPD.
Overweight, defined as a BMI≥25 kg/m2, has been correlated to VIH in this study as well as numerous other studies. BMI has been correlated to the presence of a VIH as well as the risk of VIH incarceration.24 Increased intra-abdominal pressure and changes of the abdominal wall configuration (from a vertical linear structure to a circular structure) may explain the strong association of BMI and VIH. Unlike other prospective studies predicting VIH following abdominal surgery, this study assessed a patient population with a higher mean BMI seen in communities consuming a largely Westernized diet.
A number of other factors with potential biologic plausibility and evidence from other studies were found to be significant on univariate analysis but not on the final Cox regression multivariate analysis: ASA score, DM, anemia, hypoalbuminemia, history of SSI, current open abdomen, and operative duration. Reasons for the lack of significance may include: careful patient selection, optimization of patient comorbidities preoperatively, or VIH risk reduction with operative strategies such as use of laparoscopy. In addition, given the number of hernias in the development cohort, the large number of variables, and the relative rarity of certain comorbidities, factors identified in other studies to be associated with VIH (i.e., preoperative chemotherapy or blood transfusions) may not have been statistically significant in this model.
In this study, a sensitivity analyses identified SSI to be associated with VIH (Table 4). Although the predictive accuracy of the model including SSI was no better than the HERNIAscore, the model is consistent with previous literature identifying SSI as a risk factor for VIH formation after abdominal surgery. These studies differ from this one in terms of lack of routine perioperative protocols to prevent SSIs such as antibiotic prophylaxis11 or low incidence of risk factors such as being overweight.10 In addition, SSI and VIH share common risk factors such as incision size and BMI. Further study is necessary to determine what the optimal strategies are for prevention of VIHs: optimization of preoperative comorbidities, prevention of SSIs, or both.
Preoperative risk reduction, patient selection, and intra-operative choices may help to decrease VIH rates. Weight loss programs, nutritional optimization, and decreasing the prevalence of smoking in communities may help decrease rates of VIH. Increasing use of laparoscopic surgery, particularly avoiding increasing incisions to over 15 mm may have an impact on lowering VIH rates. In cases when open surgery or larger incisions are warranted, reinforcement of the incision line with mesh remains an intriguing option. Studies assessing the role of incision reinforcement with stoma formation, the morbidly obese, and with laparoscopic cholecystectomy have been demonstrated to improve outcomes in randomized controlled trials.25 However, concern remains for prosthetic infection, particularly in the face of contamination.26 Further study is needed of the effectiveness of prosthetic incisional reinforcement.
There are a number of limitations to this study. First, this model was based on a Veteran population treated at a single tertiary-referral, academic teaching institution. Thus, the higher incidence of COPD, the low rate of conversion from laparoscopic to open surgery, and the use of standardized perioperative SSI prevention protocols may reduce the generalizability of these results. Second, there may have been misclassification of the primary outcome and intraoperative factors such as wound classification27,28 or under-diagnosis of comorbidities. There is no gold standard for the diagnosis of ventral hernias. Clinical examination is potentially subject to under-diagnosis, can vary widely between clinicians, and is affected by patient factors such as obesity.14 Radiographic imaging has been shown to have significant inter-observer variability. 4 Even patient reported results are inaccurate with up to 1/3 of patients unaware that they have a VIH.3 Consensus is needed to identify a clinically relevant and standardized method of reporting the presence and absence of a ventral hernia. Third, follow-up duration was variable and some patients had limited follow-up. Although less than 10% of patients had less than 6 months of follow up, differential loss to follow up cannot be excluded. In addition, the Cox regression adjusted for follow-up and sensitivity analysis demonstrated no substantial difference in including or excluding those patients with less than 6 months of follow-up. Lastly, a number of factors that have been shown to be clinically important in post-operative VIH risk were not included, such as suture bite size.
Conclusions
Ventral incisional hernia following abdominal surgery is common and the HERNIAscore allows simple, accurate preoperative risk assessment. The HERNIAscore is based upon surgical approach, chronic obstructive pulmonary disease, and obesity.
While further external validation is required, the HERNIAscore offers clinicians and patients a starting point for discussion of risks of surgery, risk-assessment, and potential interventions to decrease the risk for ventral incisional hernia formation. Prospective trials are needed to further validate this score and to assess if changes in peri-operative management can prevent hernias.
Precis.
The HERNIAscore accurately identifies patients at increased risk for ventral incisional hernias. While external validation is needed, this provides a starting point to counsel patients and guide clinical decisions.
Acknowledgments
Support: Center for Clinical and Translational Sciences, which is funded by National Institutes of Health Clinical and Translational Award UL1 TR000371 and KL2 TR000370 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Presented at the Southern Surgical Association 126th Annual Meeting, Palm Beach, FL, November 30-December 3, 2014.
References
- 1.Nunoo-Mensah JW, Rosen M, Chan LS, et al. Prevalence of intra-abdominal surgery: what is an individual's lifetime risk? South Med J. 2009;102:25–29. doi: 10.1097/SMJ.0b013e318182575b. [DOI] [PubMed] [Google Scholar]
- 2.van Ramshorst GH, Eker HH, Hop WC, et al. Impact of incisional hernia on health-related quality of life and body image: a prospective cohort study. Am J Surg. 2012;204(2):144–50. doi: 10.1016/j.amjsurg.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Ah-Kee EY, Kallachil T, O'Dwyer PJ. Patient awareness and symptoms from an incisional hernia. Int Surg. 2014;99(3):241–6. doi: 10.9738/INTSURG-D-14-00039.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li LT, Jafrani RJ, Becker NS, et al. Outcomes of acute versus elective primary ventral hernia repair. J Trauma Acute Care Surg. 2014;76(2):523–8. doi: 10.1097/TA.0b013e3182ab0743. [DOI] [PubMed] [Google Scholar]
- 5.Berger RL, Li LT, Hicks SC, et al. Development and validation of a risk-stratification score for surgical site occurrence and surgical site infection after open ventral hernia repair. J Am Coll Surg. 2013;217(6):974–82. doi: 10.1016/j.jamcollsurg.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Martindale RG, Deveney CW. Preoperative risk reduction: strategies to optimize outcomes. Surg Clin North Am. 2013;93(5):1041–55. doi: 10.1016/j.suc.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Smith CT, Katz MG, Foley D, et al. Incidence and risk factors of incisional hernia formation following abdominal organ transplantation. Surg Endosc. 2014 doi: 10.1007/s00464-014-3682-8. [Epub ahead of print] PMID: 25125093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Goede B, Eker HH, Klitsie PJ, et al. Incisional hernia after liver transplantation: risk factors and health-related quality of life. Clin Transplant. 2014;28(7):829–36. doi: 10.1111/ctr.12386. [DOI] [PubMed] [Google Scholar]
- 9.Kim HS, Kim KB, Hwang HY, et al. Subxiphoid incisional hernia development after coronary artery bypass grafting. Korean J Thorac Cardiovasc Surg. 2012;45(3):161–5. doi: 10.5090/kjtcs.2012.45.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itatsu K, Yokoyama Y, Sugawara G, et al. Incidence of and risk factors for incisional hernia after abdominal surgery. Br J Surg. 2014;101(11):1439–47. doi: 10.1002/bjs.9600. [DOI] [PubMed] [Google Scholar]
- 11.Veljkovic R, Protic M, Gluhovic A, et al. Prospective clinical trial of factors predicting the early development of incisional hernia after midline laparotomy. J Am Coll Surg. 2010;210(2):210–9. doi: 10.1016/j.jamcollsurg.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 12. [11/25/2014]; http://www.dailymail.co.uk/health/article-2301172/Fattest-countries-world-revealed-Extraordinary-graphic-charts-average-body-mass-index-men-women-country-surprising-results.html.
- 13.Fink C, Baumann P, Wente MN, et al. Incisional hernia rate 3 years after midline laparotomy. Br J Surg. 2014;101(2):51–4. doi: 10.1002/bjs.9364. [DOI] [PubMed] [Google Scholar]
- 14.Holihan JL, Karanjawala B, Ko A, et al. Computed Tomography is not Reliable in Diagnosing Ventral Hernia Recurrence: A Blinded Multi-Specialty Evaluation. S Texas ACS. 2015 [Google Scholar]
- 15.Seymour CW, Kahn JM, Cooke CR, et al. Prediction of critical illness during out-of-hospital emergency care. JAMA. 2010;304(7):747–54. doi: 10.1001/jama.2010.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Comajuncosas J, Hermoso J, Gris P, et al. Risk factors for umbilical trocar site incisional hernia in laparoscopic cholecystectomy: a prospective 3-year follow-up study. Am J Surg. 2014;207(1):1–6. doi: 10.1016/j.amjsurg.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 17.DeSouza A, Domajnko B, Park J, et al. Incisional hernia, midline versus low transverse incision: what is the ideal incision for specimen extraction and hand-assisted laparoscopy? Surg Endosc. 2011;25(4):1031–6. doi: 10.1007/s00464-010-1309-2. [DOI] [PubMed] [Google Scholar]
- 18.Bickenbach KA, Karanicolas PJ, Ammori JB, et al. Up and down or side to side? A systematic review and meta-analysis examining the impact of incision on outcomes after abdominal surgery. Am J Surg. 2013;206(3):400–9. doi: 10.1016/j.amjsurg.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Sajid MS, Parampalli U, Baig MK, et al. A systematic review on the effectiveness of slowly-absorbable versus non-absorbable sutures for abdominal fascial closure following laparotomy. Int J Surg. 2011;9(8):615–25. doi: 10.1016/j.ijsu.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Seiler CM, Bruckner T, Diener MK, et al. Interrupted or continuous slowly absorbable sutures for closure of primary elective midline abdominal incisions: a multicenter randomized trial (INSECT: ISRCTN24023541). Ann Surg. 2009;249(4):576–82. doi: 10.1097/SLA.0b013e31819ec6c8. [DOI] [PubMed] [Google Scholar]
- 21.Millbourn D, Cengiz Y, Israelsson LA. Effect of stitch length on wound complications after closure of midline incisions: a randomized controlled trial. Arch Surg. 2009;144(11):1056–9. doi: 10.1001/archsurg.2009.189. [DOI] [PubMed] [Google Scholar]
- 22.Stitch trial
- 23.Ventral Hernia Working Group. Breuing K, Butler CE, Ferzoco S, et al. Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery. 2010;148(3):544–58. doi: 10.1016/j.surg.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Lau B, Kim H, Haigh PI, et al. Obesity increases the odds of acquiring and incarcerating noninguinal abdominal wall hernias. Am Surg. 2012;78(10):1118–21. [PubMed] [Google Scholar]
- 25.Timmermans L, de Goede B, Eker HH, et al. Meta-analysis of primary mesh augmentation as prophylactic measure to prevent incisional hernia. Dig Surg. 2013;30(4-6):401–9. doi: 10.1159/000355956. [DOI] [PubMed] [Google Scholar]
- 26.Choi JJ, Palaniappa NC, Dallas KB, et al. Use of mesh during ventral hernia repair in clean-contaminated and contaminated cases: outcomes of 33,832 cases. Ann Surg. 2012;255(1):176–80. doi: 10.1097/SLA.0b013e31822518e6. [DOI] [PubMed] [Google Scholar]
- 27.Levy SM, Lally KP, Blakely ML, et al. Surgica Wound Misclassification: A multicenter Evaluation. J Am Coll Surg. doi: 10.1016/j.jamcollsurg.2014.11.007. Epub 2014. [DOI] [PubMed] [Google Scholar]
- 28.Snyder RA, Johnson L, Tice J, et al. Wound classification in pediatric general surgery: significant variation exists among providers. J Am Coll Surg. 2013;217(5):819–26. doi: 10.1016/j.jamcollsurg.2013.05.027. [DOI] [PubMed] [Google Scholar]