Abstract
Background
Radioiodine (RAI) lobe ablation in lieu of completion thyroidectomy is not recommended. This study describes RAI utilization patterns and outcomes in patients with well-differentiated thyroid cancer (DTC) after thyroid lobectomy (TL).
Methods
A total of 170,330 patients diagnosed with DTC between 1998 and 2011 were identified using the National Cancer Database. Demographic, tumor and treatment variables were analyzed using both univariate and multivariate regression.
Results
A total of 32,119 (20%) patients underwent TL as the definitive procedure. Mean age at diagnosis was 48 years; median tumor size was 1cm, 4% had extrathyroidal extension, 4% had positive lymph nodes and <1% distant metastases. RAI was administered to 24% of patients in the TL cohort and represented 10% of the overall RAI use. In multivariate analysis, RAI use was associated with age<45 (OR 1.51), community facilities (OR 1.26), >1cm tumors (OR 5.67), Stage II or III (OR 1.54 and 2.05), positive lymph nodes (OR 1.78) and extrathyroidal extension (OR 1.36). On both univariate and multivariate analysis, RAI after TL was associated with improved survival at both 5 and 10 years follow up (97% vs. 95% and 91% vs. 89% respectively, HR 0.53, CI: 0.38–0.72, p<0.001)
Conclusions
Nearly a quarter of TL patients received RAI. The strongest predictors of RAI utilization were larger cancers and advanced stage. The use of RAI in these patients was associated with improved overall survival. Future studies and guidelines will need to more clearly address this practice and educate providers about the appropriate use of RAI in TL patients.
Introduction
Radioiodine (RAI) has been used in the post surgical treatment of well-differentiated thyroid cancer (DTC) for over 50 years.1 While the indications and recommendations for its utilization have changed over time, current American Thyroid Association (ATA) and National Comprehensive Cancer Network (NCCN) guidelines recommend RAI ablation in select patients after total thyroidectomy (TT).2, 3 The role of RAI ablation after lobectomy (TL), however, is unclear. The 2009 ATA guidelines for the management of DTC state: “Ablation of the remaining lobe has been used as an alternative to completion thyroidectomy. It is unknown whether this approach results in similar long-term outcomes. Consequently, routine RAI ablation in lieu of completion thyroidectomy is not recommended.”2
Several small retrospective studies have described the use of RAI lobe ablation after TL as a safe and effective alternative to completion thyroidectomy in DTC.4–8 Santra and colleagues studied the long-term outcomes of RAI lobe ablation in a cohort of 364 TL patients with DTC and compared them to a group of 372 patients who underwent completion thyroidectomy and RAI remnant ablation. After a median follow-up of 5 years, recurrence-free, disease-free survival and cause-specific mortality were similar between the groups.8 The authors concluded that RAI lobar ablation is a safe, simple, effective and less expensive alternative to completion thyroidectomy in patients with DTC. To our knowledge, there are no randomized studies or large retrospective studies that compare the outcomes of patients who undergo TL with or without postoperative RAI.
Using the National Cancer Database (NCDB), this study describes RAI utilization rates and factors associated with its use after TL in DTC. It also compares the overall survival of patients who underwent TL alone with that of patients who underwent TL followed by RAI.
Methods
Data source and patient acquisition
After obtaining institutional review board approval, patients ≥18 years of age diagnosed with DTC between 1998–2011 were identified in the National Cancer Database (NCDB) utilizing the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) as the reference for histology coding (papillary thyroid cancer [PTC], codes 8050, 8340–8344, and 8350; follicular thyroid cancer [FTC], codes 8330–8332, 8335 and 8337; Hurthle cell thyroid cancer [HCTC], code 8290). The NCDB is a joint program of the American College of Surgeons and the Commission on Cancer (CoC) that captures newly diagnosed malignancies from CoC approved hospitals, which account for >75% of all cancers and close to 85% of all thyroid cancers in the United States.9, 10 Data are abstracted, coded and reported according to nationally established protocols coordinated by the National American Association of Central Cancer Registries by CoC trained and certified cancer registrars.
Demographic, clinical characteristics and definitions
Patient age at diagnosis, sex, race, urban or rural residence, insurance status, facility type and location as well as survival status were obtained. Age at diagnosis was classified into two groups (18–44, ≥45 years). Race was grouped into white, white Hispanic, black and other. A residence was defined as urban if it was located in a metro area or the population was greater than 2,500 and was designated rural if it was not located in a metropolitan area and the population was less than 2,500. Insurance status was divided into private insurance, Medicaid, Medicare, military or uninsured. Facility type included academic/research facilities and community based health centers. Facility location was grouped by region: East, South, Mid-West, and Mountain/Pacific.
Clinical variables of interest included number of primary cancers per patient, Charlson/Deyo comorbidity score11, extent of thyroid surgery, utilization of RAI and overall survival. Only patients with one primary cancer who underwent lobectomy were included. TL was defined as removal of a lobe with or without isthmusectomy and also included removal of a lobe with partial removal of contralateral lobe (Surgery of primary site codes 20–23, 30). RAI was documented if patients received RAI or combination external beam radiation therapy and RAI. Overall survival was analyzed at 5 and 10 years.
Pathologic characteristics included histology, stage, tumor size, regional lymph node status, extrathyroidal extension and distant metastases. Histologic type included PTC, FTC, and HCC based on the ICD-O-3 codes. Tumor stage was coded according to the NCDB analytic stage group, which is assigned the value of the reported pathologic stage group but utilizes clinical stage group if pathologic stage is not available. Tumor size was classified as greater than or less than 1 cm. Lymph nodes status was grouped into negative or positive and was only documented in patients who had lymph nodes examined. Extrathyroidal extension was defined as extension of tumor beyond the pericapsular soft tissue/connective tissue.
Statistical analysis
Summary statistics were used to compare the demographic, clinical and pathologic characteristics of patients who received RAI to those who did not. Chi-square, student’s t-test and analysis of variance (ANOVA) were used to compare categorical and continuous variables. Logistic regression was utilized to identify independent factors associated with utilization of RAI.
Kaplan-Meier analysis was used to determine overall survival, and to perform univariate analysis of the impact of RAI on overall survival; the log rank test was used to calculate statistical significance of the comparison. For multivariate analysis, Cox proportional hazards regression modeling was performed to identify apriori chosen factors that were independently associated with overall survival. Hazard ratios and 95% confidence intervals were calculated to evaluate the strength of association between each variable and survival. A proportional-hazards assumption test based on Schoenfled residuals was used to check the proportional hazards assumption of the variables included in the model. Only patients diagnosed between 1998–2006 were included in the survival analysis to ensure at least five-year follow-up. Statistical significance was defined as a p-value of <0.05. Missing data were excluded from the analysis after a sensitivity analysis limited to patients with no missing variables was shown to be equivalent.
Results
A total of 170,330 patients aged ≥ 18 years with a single primary cancer diagnosis of DTC were identified in the NCDB between 1998 and 2011. Of these patients, 32,119 (20%) underwent TL as the definitive procedure. Demographics, clinical characteristics and pathologic features of TL patients are shown in Table 1. The median age at diagnosis was 48 years, 79% were women, and 66% were treated in community health care facilities. The predominant tumor histology was PTC (83%) and the median tumor size was 1cm. At the time of TL, 4% of patients had extrathyroidal extension, 4% had positive lymph nodes and <1% of patients had distant metastases.
Table 1.
Demographics, Clinical Characteristics and Pathologic Features of Patients who Underwent Thyroid Lobectomy
| Lobectomy n=32,119 |
|
|---|---|
| Median Age, years (IQR) | 48 (38–59) |
| Female | 25,438 (79) |
| Race | |
| White | 23,620 (74) |
| White Hispanic | 3,578 (11) |
| Black | 2,646 (8) |
| Other | 2,275 (7) |
| Urban/Rural | |
| Metro | 25,336 (84) |
| Urban | 4,213 (14) |
| Rural | 510 (2) |
| Facility Type | |
| Community | 20,495 (66) |
| Academic | 10,639 (34) |
| Region | |
| East | 9,045 (28) |
| South | 10,811 (34) |
| Mid-West | 7,536 (23) |
| Mountain/Pacific | 4,727 (15) |
| Insurance status | |
| Private | 23,054 (73) |
| Uninsured | 840 (3) |
| Medicaid | 1,629 (5) |
| Medicare | 5,546 (18) |
| Military | 297 (1) |
| Charlson-Deyo Comorbidity Score | |
| 0 | 17,735 (86) |
| 1 | 2,440 (12) |
| 2 | 486 (2) |
| Histology | |
| Papillary Carcinoma | 26,759 (83) |
| Follicular Carcinoma | 3,550 (11) |
| Hürthle Cell Carcinoma | 1,810 (6) |
| Stage | |
| I | 18,087 (78) |
| II | 3,297 (14) |
| III | 1,575 (7) |
| IV | 328 (1) |
| Median Tumor Size, cm (IQR) | 1 (0.4–2.5) |
| Extrathyroidal Extension | |
| No | 20,584 (96) |
| Yes | 927 (4) |
| Lymph Node Status | |
| Not examined | 23,699 (75) |
| No | 6,628 (21) |
| Yes | 1,123 (4) |
| Distant Metastases | |
| No | 18,019 (99.6) |
| Yes | 74 (0.41) |
| Radioiodine Utilization | |
| No | 23,815 (76) |
| Yes | 7,585 (24) |
Values are presented as n (%) or median with interquartile range (IQR) as indicated
Postoperative RAI was administered to 24% of patients. Demographics, clinical characteristics and pathologic features of patients who received RAI are compared to patients who did not receive RAI and displayed in Table 2. Patients treated with RAI were younger (47 vs. 49 years, p<0.001) more likely male (23% vs. 20%, p <0.001) and more likely to receive care in a community health care facility (67% vs. 65%, p=0.028). There was a greater proportion of FTC (18% vs. 9%, p<0.001) and HCC (8% vs. 5%, p<0.001) and a lower percentage of PTC (74% vs. 86%, p<0.001) in the RAI cohort. Patients treated with RAI after TL presented at later stages (p<0.001), had larger cancers (2.2 vs. 0.7cm, p<0.001), and were more likely to have extrathyroidal extension (9% vs. 3%, p<0.001) and positive lymph nodes (25% vs. 10%, p<0.001).
Table 2.
Demographics, Clinical Characteristics and Pathologic Features of Patients who Underwent Thyroid Lobectomy by Radioiodine Status
| Lobectomy without RAI n=23,815 |
Lobectomy with RAI n=7,585 |
p-value | |
|---|---|---|---|
| Median Age, years (IQR) | 49 (39–60) | 47 (36–57) | <0.001 |
| Female | 19,023 (80) | 5,830 (77) | <0.001 |
| Race | 0.001 | ||
| White | 17,491 (74) | 5,600 (74) | |
| White Hispanic | 2,601 (11) | 897 (12) | |
| Black | 1,973 (8) | 622 (8) | |
| Other | 1,750 (7) | 466 (6) | |
| Urban/Rural | <0.001 | ||
| Metro | 18,916 (85) | 5,865 (83) | |
| Urban | 3,023 (13) | 1,093 (15) | |
| Rural | 350 (2) | 137 (2) | |
| Facility Type | 0.028 | ||
| Community | 15,044 (65) | 4,927 (67) | |
| Academic | 7,992 (35) | 2,460 (33) | |
| Region | <0.001 | ||
| East | 7,099 (30) | 1,704 (22) | |
| South | 7,806 (33) | 2,711 (37) | |
| Mid-West | 5,418 (23) | 1,967 (25) | |
| Mountain/Pacific | 3,492 (14) | 1,203 (16) | |
| Insurance status | <0.001 | ||
| Private | 16,881 (73) | 5,657 (76) | |
| Uninsured | 623 (3) | 201 (3) | |
| Medicaid | 1,211 (5) | 358 (5) | |
| Medicare | 4,306 (18) | 1,108 (15) | |
| Military | 202 (1) | 88 (1) | |
| Charlson-Deyo Comorbidity Score | <0.001 | ||
| 0 | 12,891 (85) | 4,479 (88) | |
| 1 | 1,850 (12) | 531 (10) | |
| 2 | 394 (3) | 85 (2) | |
| Histology | <0.001 | ||
| Papillary Carcinoma | 20,546 (86) | 5,612 (74) | |
| Follicular Carcinoma | 2,128 (9) | 1,349 (18) | |
| Hürthle Cell Carcinoma | 1,141 (5) | 624 (8) | |
| Stage | <0.001 | ||
| I | 17,924 (82) | 4,302 (62) | |
| II | 2,640 (12) | 1,619 (23) | |
| III | 1,022 (5) | 862 (12) | |
| IV | 231 (1) | 161 (2) | |
| Median Tumor Size, cm (IQR) | 0.7 (0.3–2.0) | 2.2 (1.3–3.5) | <0.001 |
| Extrathyroidal Extension | <0.001 | ||
| No | 15,506 (97) | 4,664 (91) | |
| Yes | 469 (3) | 448 (9) | |
| Positive Lymph nodes | <0.001 | ||
| No | 4,856 (90) | 1,636 (75) | |
| Yes | 563 (10) | 536 (25) | |
| Distant Metastases | <0.001 | ||
| No | 13,201 (99.71) | 4,458 (99.22) | |
| Yes | 39 (0.29) | 35 (0.78) | |
Values are presented as n (%) or median with interquartile range (IQR) as indicated
A multivariate analysis of factors associated with greater utilization of RAI after TL is presented in Table 3. RAI use was associated with age <45 (OR 1.51, 95% CI 1.24–1.83), receipt of care in community health care facilities (OR 1.26, 95% CI 1.06–1.48), in a mid-west facility (OR 1.47, 95% CI 1.18–1.83), Stage II or III disease (OR 1.54, 95% CI 1.17–2.03 and OR 2.05, 95% CI 1.51–2.80), >1cm tumors (OR 5.67, 95% CI 4.68–6.88), extrathyroidal extension (OR 1.36, 95% CI 1.01–1.93) and lymph node positive disease (OR 1.78, 95% CI 1.38–2.31). Sex, race, urban or rural residence, insurance status, comorbidity score, histology, or presence of distant metastases did not affect utilization patterns of RAI.
Table 3.
Factors Associated with Utilization of Radioiodineafter Thyroid Lobectomy
| Factor | OR | 95% CI | p-value |
|---|---|---|---|
| Age (ref = 45–64) | |||
| <45 | 1.51 | 1.24–1.83 | <0.001 |
| ≥65 | 0.79 | 0.52–1.19 | 0.273 |
| Sex (ref=female) | |||
| Male | 1.03 | 0.85–1.25 | 0.727 |
| Race (ref=white) | |||
| White/Hispanic | 1.03 | 0.80–1.32 | 0.811 |
| Black | 0.77 | 0.55–1.08 | 0.138 |
| Other | 0.85 | 0.62–1.15 | 0.313 |
| Urban/Rural (ref=urban) | |||
| Rural | 1.12 | 0.57–2.19 | 0.738 |
| Facility Type (ref=academic/research) | |||
| Community | 1.26 | 1.06–1.48 | 0.007 |
| Facility Location (ref = East) | |||
| South | 1.24 | 1.00–1.53 | 0.043 |
| Mid-West | 1.47 | 1.18–1.83 | <0.001 |
| Mountain/Pacific | 1.15 | 0.89–1.49 | 0.278 |
| Insurance status (ref=private) | |||
| Uninsured | 0.89 | 0.87–1.39 | 0.616 |
| Medicaid | 0.84 | 0.59–1.18 | 0.327 |
| Medicare | 0.86 | 0.58–1.28 | 0.474 |
| Military | 1.47 | 0.66–3.25 | 0.338 |
| Charlson-Deyo Comorbidity Score (ref=0) | |||
| 1 | 0.83 | 0.64–1.09 | 0.208 |
| 2 | 0.60 | 0.32–1.11 | 0.107 |
| Histology (ref = Papillary Carcinoma) | |||
| Follicular Carcinoma | 1.15 | 0.88–1.51 | 0.283 |
| Hurthle Cell Carcinoma | 1.12 | 0.80–1.54 | 0.500 |
| Stage (ref=stage I) | |||
| Stage II | 1.54 | 1.17–2.03 | 0.002 |
| Stage III | 2.05 | 1.51–2.80 | <0.001 |
| Stage IV | 1.17 | 0.69–1.97 | 0.556 |
| Tumor Size, cm (ref <1cm) | |||
| ≥1cm | 5.67 | 4.68–6.88 | <0.001 |
| Extrathyroidal extension (ref = no) | |||
| Yes | 1.36 | 1.01–1.93 | 0.042 |
| Lymph node status (ref = negative) | |||
| Positive | 1.78 | 1.38–2.31 | <0.001 |
| Distant Metastases (ref = no) | |||
| Yes | 0.36 | 0.12–1.02 | 0.056 |
OR, odds ratio; CI, confidence interval; ref, reference value;
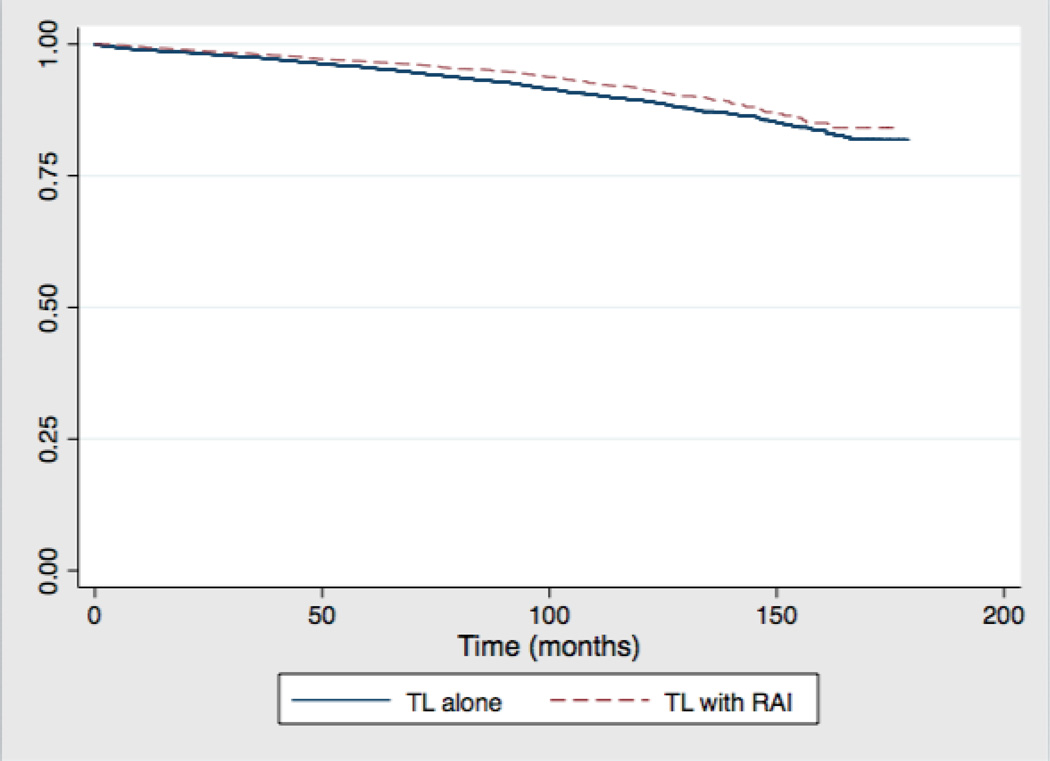
A total of 20,751 patients who underwent TL from 1998–2006 were included in the survival analysis. Median follow up was 81 months in the TL group and 86 months in the TL with RAI group. There were 1136 (4.8%) deaths in the TL group compared to 290 (3.8%) in the TL with RAI group. A Kaplan-Meier estimate of overall survival of patients who underwent lobectomy by RAI status is shown in Figure 1. By log-rank analysis, overall survival was slightly greater in the RAI group at five (97% vs. 95%, p<0.001) and ten years (91% vs. 89%, p<0.001). On multivariate cox proportional hazards regression (Table IV) adjusting for multiple patient, tumor and hospital factors, there was a statistically significant survival benefit in the RAI group (HR 0.53, 95% CI 0.38–0.72). Factors associated with decreased overall survival included age ≥45 (HR 4.53, 95% CI 2.66–7.70), male gender (HR 1.69, 95% CI 1.31–2.17), black race (HR 1.62, 95% CI 1.14–2.32), Medicaid insurance (HR 2.83, 95% CI 1.53–5.23), Medicare insurance (5.98, 95% CI 4.55–7.86), tumor size ≥ 1cm (HR 1.49, 95% CI 1.12–1.99), extrathyroidal extension (HR 2.41, 95% CI 1.71–3.40), distant metastases (HR 10.38, 95% CI 5.23–20.53).
Figure 1.
Overall Survival of Patients who Underwent Thyroid Lobectomy
Table 4.
Cox Proportional Hazards Regression: Factors that Affected Overall Survival in Patients who Underwent Thyroid Lobectomy
| Factor | HR | 95% CI | p-value |
|---|---|---|---|
| Age (ref <45 years) | |||
| ≥ 45 years | 4.53 | 2.66–7.70 | <0.001 |
| Sex (ref=female) | |||
| Male | 1.69 | 1.31–2.17 | <0.001 |
| Race (ref=white) | |||
| White/Hispanic | 1.08 | 0.74–1.57 | 0.685 |
| Black | 1.62 | 1.14–2.32 | 0.007 |
| Other | 0.63 | 0.30–1.29 | 0.211 |
| Insurance status (ref=private) | |||
| Uninsured | 1.35 | 0.48–3.75 | 0.559 |
| Medicaid | 2.83 | 1.53–5.23 | 0.001 |
| Medicare | 5.98 | 4.55–7.86 | <0.001 |
| Military | 1.80 | 0.43–7.41 | 0.416 |
| Tumor Size, cm (ref <1cm) | |||
| ≥1cm | 1.49 | 1.12–1.99 | 0.006 |
| Extrathyroidal extension (ref = no) | |||
| Yes | 2.41 | 1.71–3.40 | <0.001 |
| Distant Metastases (ref = no) | |||
| Yes | 10.38 | 5.23–20.53 | <0.001 |
| Radioiodine Utilization (ref=no) | |||
| Yes | 0.53 | 0.38–0.72 | <0.001 |
HR, hazard ratio; CI, confidence interval; ref, reference value
also included in the model urban/rural, facility type and location, histology
Discussion
The results of this study provide insight into the utilization patterns of RAI for management of DTC after TL. Despite lack of guideline recommendations for the utilization of RAI after TL, this practice is prevalent in approximately one-quarter of patients undergoing TL in the current series and is consistent with the literature.12 In fact, 10% of all RAI administered from 1998 to 2011, recorded in the NCDB, was given to patients who underwent TL. This study found that patients who are younger, those receiving care in community health facilities and those with larger tumors or advanced disease stage at presentation were more likely to receive RAI after TL. Despite the lack of recommendations however, we also found a significant overall survival advantage of postoperative RAI after TL when compared to TL alone.
The current study demonstrates that more advanced disease is associated with increased utilization of RAI after TL. Tumors >1cm, Stage II and III disease, extrathyroidal extension and lymph node positive disease were all independently associated with RAI use. While it is expected that patients with more advanced disease receive additional care after TL, the current standard of care recommends completion thyroidectomy prior to RAI ablation or treatment.2, 3 It is possible that practitioners are utilizing RAI lobe ablation to avoid a second thyroid procedure with its associated anesthetic and technical complications. On the other hand, RAI ablation in the setting of an intact lobe has been associated with delays in treatment and slightly higher elevation of thyroglobulin as well as increased risk of radiation thyroiditis, chronic sialoadenitis, odynophagia, and facial edema when compared to remnant ablation after total thyroidectomy secondary to the use of higher or multiple doses.13–15 Complication data are not reported in the NCDB, however, and therefore cannot be analyzed using this dataset.
Age <45 years was also associated with increased utilization of RAI after TL. This finding is surprising since such patients tend to have low-risk disease and prolonged survival, even with advanced locoregional disease or large tumors.16, 17 Neither the NCCN nor ATA guidelines have age-specific recommendations regarding the use of RAI. Several groups have reported a lack of survival advantage when RAI is utilized in patients <45 years of age in the presence of lymph node positive disease or large tumors.3, 16, 18–20 In our series, subgroup analysis of patients <45 years of age also failed to show a significant difference in the overall survival of patients who underwent TL and RAI vs. those who underwent TL without RAI (data not shown).
Increased utilization of RAI after TL was also higher in community health facilities and in the mid-west region. Previous studies have shown that TL is performed at higher rates when compared to total thyroidectomy in community health facilities and by low volume surgeons.12, 21 TL is also associated with lower complication rates when compared to total thyroidectomy and therefore, RAI is possibly being utilized in lieu of completion thyroidectomy.21 In an analysis of RAI utilization, Haymart and colleagues demonstrated that the wide variation in RAI use in the United States could be explained by hospital type, case volume and other hospital characteristics.9 The lack of strong evidence to support or refute the use of RAI after TL likely further contributes to its continued utilization and decreases guideline concordance.
On unadjusted analysis, RAI after TL conferred a small survival advantage compared to TL alone at 5 and 10 years of follow up. After controlling for a variety of covariates, however RAI was independently associated with a significantly improved survival. Consistent with previous studies, age >45, male gender, black race, larger tumor size, extrathyroidal extension and distant metastases were all associated with decreased overall survival.2, 22, 23 Additionally, insurance status seems to greatly impact survival outcomes. While this is not the focus of this study, similar findings have been shown in other cancer types24, 25 and warrant further investigation as an important disparity in health care delivery and outcomes.
This study is unique because it compares TL to TL and RAI. The finding of improved overall survival in the TL and RAI group may indicate that not all thyroid cancers should be treated with lobectomy alone or without RAI, and proper patient selection for treatment is paramount. Recent data have shown that RAI has little or no impact on disease specific survival in low or intermediate risk thyroid cancer patients (<4cm tumors, without extrathyroidal extension and clinically node negative disease).19, 20, 26, 27 Furthermore, adjusted analysis of the NCDB and SEER databases comparing TL to total thyroidectomy have demonstrated that the extent of surgery fails to provide a survival advantage when based solely on cancer size28 or in patients <45 years of age, with <4cm tumors and without distant metastases.29 Due to these findings experts are advocating for a more selective approach to RAI utilization and the practice of always performing a total thyroidectomy to allow for RAI ablation is being reassessed.26–29 This study was underpowered to perform survival analysis by risk group to determine which patients benefitted the most from RAI after TL. Although the findings in this study do not definitively support the routine use of RAI after TL as an alternative to completion thyroidectomy and remnant ablation, a subset of patients undergoing TL may benefit from RAI. Studies comparing morbidity and oncologic outcomes of TL followed by RAI vs. total thyroidectomy with or without RAI in properly selected patients are needed to ultimately answer this question.
This study has several limitations. First, large administrative database analysis is limited by the number of variables collected. Specific comorbid conditions, previous thyroid surgery, complications, surgeon volume or patient preference may have all impacted utilization patterns of RAI, however, none of these factors are captured in the NCDB. Second, studies have shown that quality of coding for certain data points varies.30 In this study, it is possible that diagnostic I131 scans are coded as RAI treatment therefore over estimating the number of patients truly treated with RAI and that primary site procedures may be coded incorrectly as TL for patients who underwent subsequent completion thyroidectomy; however, many studies have demonstrated that procedure coding in cancer registries and administrative databases is highly accurate.31 Third, there is a paucity of recurrence and disease specific survival data in the NCDB; therefore overall survival is the only outcome measure available. Survival data stops at 2006 to insure 5-year follow up on all patients and comorbidity score was not reported until 2003, both may affect overall survival.
In conclusion, nearly a quarter of patients undergoing thyroid lobectomy for definitive treatment of DTC will receive RAI. There appears to be a survival advantage of RAI after TL when compared to TL alone. Future studies comparing TL alone, RAI after TL, and RAI after total thyroidectomy are needed to further elucidate the role of RAI after TL and to identify the risks and benefits of this treatment approach.
Footnotes
There are no financial disclosures
Presented at the Southern Surgical Association 126th Annual Meeting, Palm Beach, FL November 30-December 3, 2014.
References
- 1.Robbins RJ, Schlumberger MJ. The evolving role of (131)I for the treatment of differentiated thyroid carcinoma. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2005;46(Suppl 1):28s–37s. [PubMed] [Google Scholar]
- 2.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid : official journal of the American Thyroid Association. 2009;19(11):1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 3.Network. NCC. [November 1, 2014];Thyroid Carcinoma (Version 2.2013) Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp-site. [Google Scholar]
- 4.Bal CS, Kumar A, Pant GS. Radioiodine lobar ablation as an alternative to completion thyroidectomy in patients with differentiated thyroid cancer. Nuclear medicine communications. 2003;24(2):203–208. doi: 10.1097/00006231-200302000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Barbesino G, Goldfarb M, Parangi S, Yang J, Ross DD, Daniels GH. Lobe ablation with radioactive iodine as an alternative to completion thyroidectomy after hemithyroidectomy in patients with follicular thyroid cancer: long-term follow-up. Thyroid : official journal of the American Thyroid Association. 2011 doi: 10.1089/thy.2011.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbesino G, Goldfarb M, Parangi S, Yang J, Ross DS, Daniels GH. Thyroid lobe ablation with radioactive iodine as an alternative to completion thyroidectomy after hemithyroidectomy in patients with follicular thyroid carcinoma: long-term follow-up. Thyroid : official journal of the American Thyroid Association. 2012;22(4):369–376. doi: 10.1089/thy.2011.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Randolph GW, Daniels GH. Radioactive iodine lobe ablation as an alternative to completion thyroidectomy for follicular carcinoma of the thyroid. Thyroid : official journal of the American Thyroid Association. 2002;12(11):989–996. doi: 10.1089/105072502320908321. [DOI] [PubMed] [Google Scholar]
- 8.Santra A, Bal S, Mahargan S, Bal C. Long-term outcome of lobar ablation versus completion thyroidectomy in differentiated thyroid cancer. Nuclear medicine communications. 2011;32(1):52–58. doi: 10.1097/MNM.0b013e328340e74c. [DOI] [PubMed] [Google Scholar]
- 9.Haymart MR, Banerjee M, Stewart AK, Koenig RJ, Birkmeyer JD, Griggs JJ. Use of radioactive iodine for thyroid cancer. JAMA : the journal of the American Medical Association. 2011;306(7):721–728. doi: 10.1001/jama.2011.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raval MV, Bilimoria KY, Stewart AK, Bentrem DJ, Ko CY. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. Journal of surgical oncology. 2009;99(8):488–490. doi: 10.1002/jso.21173. [DOI] [PubMed] [Google Scholar]
- 11.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 12.Bilimoria KY, Bentrem DJ, Linn JG, Freel A, Yeh JJ, Stewart AK, et al. Utilization of total thyroidectomy for papillary thyroid cancer in the United States. Surgery. 2007;142(6):906–913. doi: 10.1016/j.surg.2007.09.002. discussion 13.e1-2. [DOI] [PubMed] [Google Scholar]
- 13.Allweiss P, Braunstein GD, Katz A, Waxman A. Sialadenitis following I-131 therapy for thyroid carcinoma: concise communication. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1984;25(7):755–758. [PubMed] [Google Scholar]
- 14.Burmeister LA, du Cret RP, Mariash CN. Local reactions to radioiodine in the treatment of thyroid cancer. The American journal of medicine. 1991;90(2):217–222. [PubMed] [Google Scholar]
- 15.DiRusso G, Kern KA. Comparative analysis of complications from I-131 radioablation for well-differentiated thyroid cancer. Surgery. 1994;116(6):1024–1030. [PubMed] [Google Scholar]
- 16.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer. 1998;83(12):2638–2648. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [see commetns]. [DOI] [PubMed] [Google Scholar]
- 17.Verburg FA, Mader U, Tanase K, Thies ED, Diessl S, Buck AK, et al. Life expectancy is reduced in differentiated thyroid cancer patients >/= 45 years old with extensive local tumor invasion, lateral lymph node, or distant metastases at diagnosis and normal in all other DTC patients. The Journal of clinical endocrinology and metabolism. 2013;98(1):172–180. doi: 10.1210/jc.2012-2458. [DOI] [PubMed] [Google Scholar]
- 18.Hay ID, McConahey WM, Goellner JR. Managing patients with papillary thyroid carcinoma: insights gained from the Mayo Clinic's experience of treating 2,512 consecutive patients during 1940 through 2000. Transactions of the American Clinical and Climatological Association. 2002;113:241–260. [PMC free article] [PubMed] [Google Scholar]
- 19.Jonklaas J, Sarlis NJ, Litofsky D, Ain KB, Bigos ST, Brierley JD, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid : official journal of the American Thyroid Association. 2006;16(12):1229–1242. doi: 10.1089/thy.2006.16.1229. [DOI] [PubMed] [Google Scholar]
- 20.Sawka AM, Brierley JD, Tsang RW, Thabane L, Rotstein L, Gafni A, et al. An updated systematic review and commentary examining the effectiveness of radioactive iodine remnant ablation in well-differentiated thyroid cancer. Endocrinology and metabolism clinics of North America. 2008;37(2):457–480. doi: 10.1016/j.ecl.2008.02.007. x. [DOI] [PubMed] [Google Scholar]
- 21.Hauch A, Al-Qurayshi Z, Randolph G, Kandil E. Total Thyroidectomy is Associated with Increased Risk of Complications for Low- and High-Volume Surgeons. Annals of surgical oncology. 2014;21(12):3844–3852. doi: 10.1245/s10434-014-3846-8. [DOI] [PubMed] [Google Scholar]
- 22.Mazurat A, Torroni A, Hendrickson-Rebizant J, Benning H, Nason RW, Pathak KA. The age factor in survival of a population cohort of well-differentiated thyroid cancer. Endocrine connections. 2013;2(3):154–160. doi: 10.1530/EC-13-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harari A, Li N, Yeh MW. Racial and socioeconomic disparities in presentation and outcomes of well-differentiated thyroid cancer. The Journal of clinical endocrinology and metabolism. 2014;99(1):133–141. doi: 10.1210/jc.2013-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parikh AA, Robinson J, Zaydfudim VM, Penson D, Whiteside MA. The effect of health insurance status on the treatment and outcomes of patients with colorectal cancer. Journal of surgical oncology. 2014 doi: 10.1002/jso.23627. [DOI] [PubMed] [Google Scholar]
- 25.Zaydfudim V, Whiteside MA, Griffin MR, Feurer ID, Wright JK, Pinson CW. Health insurance status affects staging and influences treatment strategies in patients with hepatocellular carcinoma. Annals of surgical oncology. 2010;17(12):3104–3111. doi: 10.1245/s10434-010-1181-2. [DOI] [PubMed] [Google Scholar]
- 26.Jonklaas J, Cooper DS, Ain KB, Bigos T, Brierley JD, Haugen BR, et al. Radioiodine therapy in patients with stage I differentiated thyroid cancer. Thyroid : official journal of the American Thyroid Association. 2010;20(12):1423–1424. doi: 10.1089/thy.2010.0308. [DOI] [PubMed] [Google Scholar]
- 27.Schvartz C, Bonnetain F, Dabakuyo S, Gauthier M, Cueff A, Fieffe S, et al. Impact on overall survival of radioactive iodine in low-risk differentiated thyroid cancer patients. The Journal of clinical endocrinology and metabolism. 2012;97(5):1526–1535. doi: 10.1210/jc.2011-2512. [DOI] [PubMed] [Google Scholar]
- 28.Adam MA, Pura J, Gu L, Dinan MA, Tyler DS, Reed SD, et al. Extent of surgery for papillary thyroid cancer is not associated with survival: an analysis of 61,775 patients. Annals of surgery. 2014;260(4):601–605. doi: 10.1097/SLA.0000000000000925. discussion 5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adam MA, Pura J, Goffredo P, Dinan MA, Hyslop T, Reed SD, et al. Impact of extent of surgery on survival for papillary thyroid cancer patients younger than 45 years. The Journal of clinical endocrinology and metabolism. 2014:jc20143039. doi: 10.1210/jc.2014-3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.In H, Bilimoria KY, Stewart AK, Wroblewski KE, Posner MC, Talamonti MS, et al. Cancer recurrence: an important but missing variable in national cancer registries. Annals of surgical oncology. 2014;21(5):1520–1529. doi: 10.1245/s10434-014-3516-x. [DOI] [PubMed] [Google Scholar]
- 31.Cooper GS, Virnig B, Klabunde CN, Schussler N, Freeman J, Warren JL. Use of SEER-Medicare data for measuring cancer surgery. Medical care. 2002;40(8 Suppl):Iv-43–Iv-8. doi: 10.1097/00005650-200208001-00006. [DOI] [PubMed] [Google Scholar]