Our model results suggest that many individuals at increased risk for pancreatic ductal adenocarcinoma may benefit from MR imaging screening.
Abstract
Purpose
To identify when, from the standpoint of relative risk, magnetic resonance (MR) imaging–based screening may be effective in patients with a known or suspected genetic predisposition to pancreatic cancer.
Materials and Methods
The authors developed a Markov model of pancreatic ductal adenocarcinoma (PDAC). The model was calibrated to National Cancer Institute Surveillance, Epidemiology, and End Results registry data and informed by the literature. A hypothetical screening strategy was evaluated in which all population individuals underwent one-time MR imaging screening at age 50 years. Screening outcomes for individuals with an average risk for PDAC (“base case”) were compared with those for individuals at an increased risk to assess for differential benefits in populations with a known or suspected genetic predisposition. Effects of varying key inputs, including MR imaging performance, surgical mortality, and screening age, were evaluated with a sensitivity analysis.
Results
In the base case, screening resulted in a small number of cancer deaths averted (39 of 100 000 men, 38 of 100 000 women) and a net decrease in life expectancy (−3 days for men, −4 days for women), which was driven by unnecessary pancreatic surgeries associated with false-positive results. Life expectancy gains were achieved if an individual’s risk for PDAC exceeded 2.4 (men) or 2.7 (women) times that of the general population. When relative risk increased further, for example to 30 times that of the general population, averted cancer deaths and life expectancy gains increased substantially (1219 of 100 000 men, life expectancy gain: 65 days; 1204 of 100 000 women, life expectancy gain: 71 days). In addition, results were sensitive to MR imaging specificity and the surgical mortality rate.
Conclusion
Although PDAC screening with MR imaging for the entire population is not effective, individuals with even modestly increased risk may benefit.
© RSNA, 2014
Introduction
Pancreatic cancer is the fourth leading cause of cancer mortality in the United States and accounted for 45 220 cancer diagnoses and 38 460 deaths in 2013 (1). On the basis of current epidemiologic trends, it is expected to be the second leading cause of cancer mortality by 2030 (2). Incurable disease is seen in 80% of patients at presentation, and most patients die within a year of diagnosis (1,3). The current 5-year relative survival rate is 6%, an improvement of only three percentage points since 1975 (4). New approaches to the detection and control of pancreatic cancer are critically needed.
Pancreatic ductal adenocarcinoma (PDAC), which accounts for 95% of all pancreatic cancers, can be categorized into two subtypes on the basis of presumed biologic pathways: solid and cystic (4–6). Dominant precursors include pancreatic intraepithelial neoplasia (solid lesions) and intraductal papillary mucinous neoplasms (IPMNs, cystic lesions), but most are indolent and will not progress to cancer (5,7). Although pancreatic intraepithelial neoplasia is not reliably seen at imaging, improvements in cross-sectional imaging have led to increased detection of IPMNs (8–10). Notably, observational data also indicate IPMNs to be a marker of elevated whole-gland risk (11).
To date, evidence on PDAC screening draws largely from single-arm trials in which magnetic resonance (MR) imaging, endoscopic ultrasonography (US), or computed tomography (CT) have been used in individuals with a known or suspected genetic predisposition (12–23). A small number of cancers have been detected relative to a large number of precursor lesions, the majority of which have been IPMNs (12–23). Most patients who underwent surgery in these trials did not have invasive cancer (12–23).
From a population standpoint, these findings can be difficult to interpret (24). In settings of low disease prevalence, even a modest number of false-positive cases can lead to a low positive predictive value. The acceptability of a low positive predictive value is dependent on what happens to individuals who test positive. If a sufficient proportion of test-positive patients undergo relatively high-mortality procedures such as pancreatic surgery, then a low positive predictive value implies that screening may not benefit many patients.
With increased PDAC risk—and therefore disease prevalence—this balance may be tipped to favor screening. Several patient populations are known to be at increased risk for PDAC relative to the general population (Table 1) owing to a family history or a known genetic mutation (p16, BRCA 2, STK11/LKB1, etc); these individuals are expected to account for approximately 10% of all PDAC cases (20,24–28). However, our knowledge of the relationship between PDAC risk and screening benefits remains limited. Patient data from recent trials provide important insights (12–23,29), but restricted eligibility requirements, years of achievable follow-up, and high costs limit the extent to which even the best clinical trials can explore this relationship.
Table 1.
Relative Risk and Lifetime Risk of PDAC in High-Risk Populations
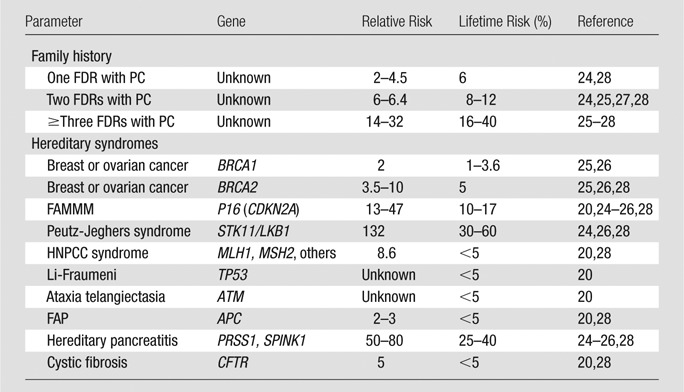
Note.—Relative risks and lifetime risk estimates were obtained from Klein et al (27), Steinberg et al (24), Grover and Syngal (25), Jimenez and Fernandez-del Castillo (26), Schneider et al (20), and Templeton and Brentnall (28). FAMMM = familial atypical multiple mole melanoma syndrome, FAP = familial adenomatous polyposis, FDR = first-degree relative, HNPCC = hereditary nonpolyposis colorectal cancer syndrome, PC = pancreatic cancer.
In other cancer settings, disease modeling methods have been used to complement clinical trials, allowing for broader interrogation of many factors that influence the success of cancer screening (30–37). In this study, we developed a mathematic model of PDAC to evaluate pancreatic cancer screening in hypothetical populations. In the model, patient cohorts were assigned varied levels of risk for PDAC, ranging from that of the general population (average risk) to a risk of 70 times that of the general population. These risk-stratified cohorts were developed to span most populations with known or suspected genetic predispositions (Table 1). In building the model, disparate data sources were merged to fill gaps in evidence, and factors governing test performance, cancer biology, and population characteristics were integrated in ways that are not possible with use of patient studies alone. Our purpose was to identify when, from the standpoint of relative risk, MR imaging–based pancreatic cancer screening may be effective in patients with a known or suspected genetic predisposition to PDAC.
Materials and Methods
Model Overview
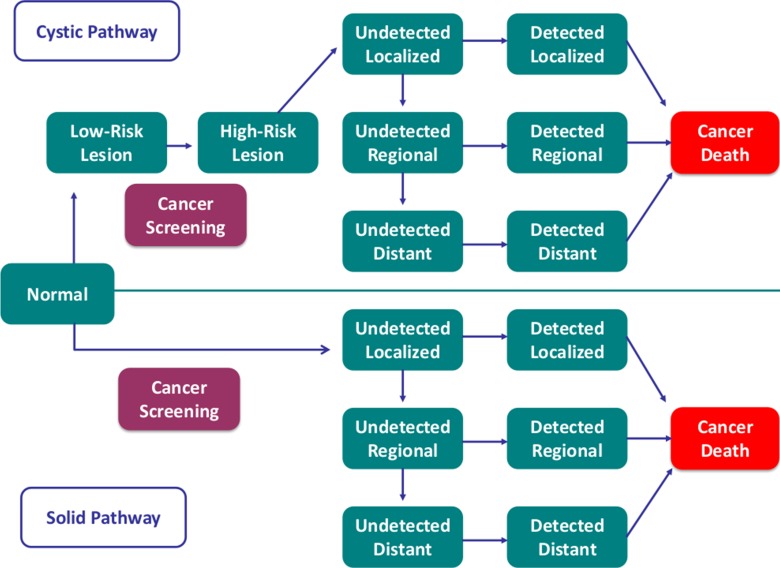
A Markov model was developed to simulate the natural history of PDAC and evaluate effects of MR imaging screening (including MR cholangiopancreatography) on population life expectancy (Fig 1) (38–40). The model had two pathways to PDAC: solid and cystic (4–6). Patients in a hypothetical population were at risk for developing a solid cancer or a low-risk cystic lesion. A low-risk cystic lesion could progress to a high-risk cystic lesion, which, in turn, could progress to cancer.
Figure 1:
Simplified schematic of Markov state transition model. Model replicates solid (90%) and cystic (10%) pathways to PDAC. In solid pathway, screening allowed for detection of localized cancers and prompted surgery. In cystic pathway, affected patients developed a low-risk cystic lesion, which could progress to a high-risk cystic lesion or localized cancer. Patients with high-risk lesions and localized cancers were triaged to surgery and those with low-risk lesions underwent imaging surveillance, with surgery performed in instances of progression.
In the solid pathway, screening allowed for the potential detection of cancers and prompted surgery for those that were localized. In the cystic pathway, screening allowed for the potential detection of low-risk cystic lesions, high-risk cystic lesions, and cancers. Patients with screening-detected high-risk cystic lesions and localized cancers were triaged to surgery; those with low-risk lesions underwent annual surveillance for 10 years and surgery in instances of progression. We defined low-risk lesions as cysts with low malignant potential on the basis of imaging features and high-risk lesions as those that warranted surgery (15). In practice, suspicious cyst features include a size of at least 3 cm, mural nodules, wall thickening, and associated main duct dilatation or abrupt caliber change (41). In the model, the proportion of patients with cysts who underwent subsequent pancreatic surgery (five of 84 patients, 6%) in a single-arm multimodal screening trial was used as a proxy for the proportion of high-risk cysts (Table 2) (15). Patients with screening-detected advanced cancers did not undergo surgery, in keeping with standard clinical practices.
Table 2.
Calibration Targets, Fixed Model Inputs, and Screening Performance: Estimates from the Literature
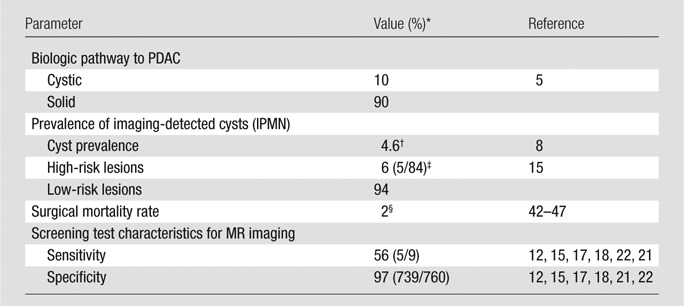
Note.—The proportion of PDAC that develop via cystic versus solid pathways, cyst prevalence, and the proportion of low-risk versus high-risk cystic lesions are calibration targets. The surgical mortality rate is a fixed input parameter.
Numbers in parentheses are raw data.
Reported imaging-detected prevalence was 2.6% (73 of 2832 cysts) (8); because assumed sensitivity for cyst identification was 56%, presumed prevalence was 0.026/0.56 = 0.046 (or 4.6%). Importantly, the 4.6% value was used as a calibration target, but in the calibrated model, cyst prevalence varied with age (see Materials and Methods and Appendix E1 [online] for details).
Number of reported surgical cases in which a cyst was seen in a large multimodal screening study (15).
Cancer states common to both pathways were subdivided according to stage (historic Surveillance, Epidemiology, and End Results stages: local, regional, distant, and unstaged) and as to whether the cancer had been detected (Fig 1). Causes of death included age-specific all-cause mortality (48), pancreatic surgery (42–47), and PDAC (3). Patients in all states were subject to all-cause mortality and, upon entering a cancer state, were also subject to a PDAC-specific mortality rate, which was dependent on stage and detection status.
Each model simulation began with a hypothetical cohort of 20-year-old individuals in the normal health state and continued until an age of 99 years or death. In each model cycle (cycle length, 1 year), an individual could remain in his or her current health state or progress to a new state on the basis of an annual transition probability. Separate simulations were run for each sex.
Parameters of the Natural History Model
Parameters within the model—and those used to develop the model—can be broadly categorized as follows: fixed inputs, calibration targets, and calibrated parameters. Fixed inputs included rates of all-cause mortality (age and sex specific) (48), surgical mortality (42–47), and relative survival according to stage for detected cancers (3) (Tables 2, 3). Most transition probabilities in the model, however, addressed “unobservable” events (eg, progression from undetected regional to undetected distant PDAC) (Fig 1), a typical feature of natural history models (50). These probabilities were estimated with use of a process known as calibration, in which the model was run iteratively to find parameter sets (solutions) that produced model outputs that most closely matched observed clinical or epidemiologic data (calibration targets) (50). Calibration targets included the following: lifetime risk of developing and dying from PDAC (49), PDAC stage distribution (3), proportion of cancers that develop via solid versus cystic pathways (5), cyst prevalence (8), and proportion of low-risk versus high-risk cystic lesions (15) (Tables 2, 3).
Table 3.
Additional Calibration Targets and Fixed Model Inputs: Estimates from National Cancer Institute Surveillance, Epidemiology, and End Results Data
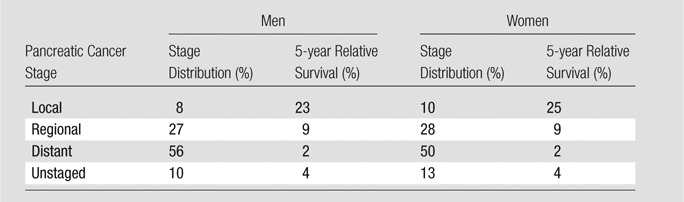
Note.—Data were obtained with the Surveillance, Epidemiology, and End Results registry (3). Stage distribution was a calibration target, and survival rates were fixed model inputs. The lifetime risk of pancreatic cancer development was 1.52% for men and 1.50% for women. The lifetime risk of death from pancreatic cancer was 1.40% for men and 1.38% for women. Both the lifetime risk of pancreatic cancer development and the lifetime risk of death from pancreatic cancer were calculated with software (DevCan: Probability of Developing or Dying of Cancer software, version 6.7.0; Statistical Research and Applications Branch, National Cancer Institute, 2013; http://surveillance.cancer.gov/devcan) (49) and are unadjusted estimates. Estimates were decreased by 0.95 times these values to exclude non–PDAC-related pancreatic cancer deaths (Appendix E1 [online]). Lifetime risk estimates were calibration targets.
It is important to note that the prespecification of a given calibration target—for example, cyst prevalence—did not imply that the final model produced the same cyst prevalence for every age, which would be clinically unrealistic. Instead, cyst prevalence varied with age in such a way that its drivers—namely, the calibrated transition probabilities that bracketed the pancreatic cyst states in the model (Fig 1)—generated, as an output, the calibration target for cyst prevalence. The calibration process is further described in Appendix E1 (online), including application of the χ2 goodness-of-fit metric to compare model outputs to calibration targets.
Addressing Lead Time in the Model
In a calibrated natural history model such as ours, detected cancer states typically have higher cancer-specific mortality rates than corresponding undetected cancer states. This is plausible because it is expected that, in the absence of screening, detected cancers will have progressed further, on average, than asymptomatic cancers. However, care must be taken when imposing screening; it would not be realistic for a positive screening examination to increase a patient’s cancer mortality. To address this problem in our model, patients with screening-detected cancers were subject to cancer-specific mortality rates of undetected cancers until such time as the cancer would have been detected in the absence of screening. This ensured that, in the absence of intervention, screening itself would not affect cancer mortality. This refinement was needed only for localized cancers; screening was not expected to meaningfully affect the time of detection for regional and distant cancers.
MR Imaging Test Performance
A literature search was conducted to identify studies about PDAC screening that used MR imaging as a first-line screening modality. Data were pooled from six patient studies to compute sensitivity and specificity for model inputs (sensitivity = 56% [five of nine patients], specificity = 97% [739 of 760 patients]) (Table 2) (12,15,17,18,21,22). Across studies, patients were classified as having true-positive findings if a screening-detected cancer was resectable and stage N0M0 at surgery, as having false-negative findings if advanced cancer was detected after a normal screening examination, and as having false-positive findings if a detected abnormality led to surgery and no cancer was found or if advised surgery was declined without a known negative consequence. All other cases were designated as true-negative with the following exceptions: Patients found to have neuroendocrine tumors were excluded from analysis (n = 2) because of their rarity and uncertain clinical significance (12,15), and patients in whom advanced PDAC (or other advanced malignancy) was discovered at screening were also excluded (n = 4) (21,22).
Notably, in our analysis, screening MR imaging was considered a strategy rather than an isolated test. In the six screening studies mentioned earlier, among cases that met the specified criteria for being true-positive or false-positive at MR imaging, endoscopic US was also reportedly performed before surgery in most patients (22 of 26 patients, 85%)—concurrently or as a follow-up test—in keeping with standard practices for the evaluation of suspicious pancreatic findings at cross-sectional imaging (12,15,17,18,21,22,51). Conversely, endoscopic US likely spared some patients surgery for MR imaging–detected abnormalities; such cases were considered “test negative” with our criteria because further work-up of MR imaging abnormalities did not prompt a recommendation for surgery. However, on the basis of the data reported in these six studies, it was not possible to accurately reconstruct the proportion of cases in which the latter circumstance occurred (12,15,17,18,21,22).
Implementation of PDAC Screening in the Model
For the solid pathway, MR imaging performance defined the probability of misidentifying a patient with PDAC as healthy or a healthy patient as one with PDAC. For the cystic pathway, the probability of misidentifying a patient with PDAC as healthy was applied identically. However, precursor cystic lesions could also be missed or misclassified with imaging and progress to cancer in later years. Without more granular data available to inform this type of misidentification, we applied the same false-negative rate to the misidentification of a high-risk cystic lesion as a low-risk cystic lesion and to the misidentification of localized cancer as a high-risk cystic lesion. False-positive cases in the cystic pathway were those in which a low-risk cystic lesion was misidentified as a high-risk lesion or cancer.
Patients with true- or false-positive results (indicative of high-risk cystic lesions or PDAC) were treated with surgery and subject to surgical mortality (42–47). Surgery on cysts was assumed to be effective from a cancer control standpoint; patients who survived surgery returned to the normal health state. Correct identification of patients with localized cancer prevented them from progressing to more advanced stages before detection but did not necessarily result in a cure. Patients were subject to stage-specific survival derived from the Surveillance, Epidemiology, and End Results registry (5-year relative survival rates of 23% and 25% for men and women, respectively) (Table 3) (3). Thus, the benefit of screening was a cancer downstaging effect.
Modeling Varied Risk: Designation of Base-Case and High-Risk Populations
For the base-case analysis, one-time MR imaging screening was conducted in all individuals at age 50 years. Two primary outcomes were projected: PDAC deaths averted and life expectancy gained. PDAC deaths averted represented the difference in the proportions of pancreatic cancer deaths with—versus without—screening. Secondary outcomes included numbers of cysts and cancers detected.
Central to our analysis was the comparative evaluation of outcomes according to an individual’s relative risk for PDAC. In the model, an individual’s risk for PDAC was varied from one (average risk) to 70 times that of the general population, a range that incorporates most populations with increased PDAC risk (Table 1) (20,24–28). To vary an individual’s risk for PDAC in the model, lifetime risks of developing and dying from PDAC were scaled to match different relative risk levels (eg, 10 or 20 times that of the general population). Model parameters were then recalibrated to fit these new targets, and screening effects were recomputed. Notably, cyst prevalence was not a calibration target in the high-risk models despite being a target for the average-risk model. This allowed for a higher cyst prevalence in high-risk compared with average-risk populations, as is consistent with the literature (15).
Notably, in our analysis, in patients with low- or high-risk cysts, elevated risk for PDAC in other areas of the pancreas (distinct from cysts) was governed by a patient’s underlying relative risk for PDAC and was not conditional on the presence of a cyst. In practice, it has been observed that patients with cystic precursors are at elevated risk for PDAC in a separate area of the pancreas (11). Our model design accounts for this association because high-risk cohorts are more likely to develop cysts. If such patients were encountered in routine clinical practice—alongside average-risk individuals—the presence of a cystic precursor would indicate a greater likelihood of being part of a high-risk population and thus a greater likelihood of cancer elsewhere in the pancreas. Put another way, the precursor would be a marker of elevated whole-gland risk.
Sensitivity Analysis
Given the Markov modeling methods used, we evaluated additional sources of input variability and uncertainty by using deterministic univariate sensitivity analysis (38–40). Specifically, the following key parameters were varied in one-way deterministic analysis: age at screening (40, 60, and 70 years), MR imaging sensitivity (range, 0.25–1.0) and specificity (range, 0.5–1.0), and surgical mortality (range, 0%–10%).
Results
Results corresponding to one-time MR imaging screening in average-risk cohorts are shown in Table 4. In the base case, we found that PDAC screening at age 50 years for 100 000 average-risk men would identify 2375 low-risk cysts, 159 high-risk cysts, and 56 cancers (53 arising from the solid pathway and three arising from the cystic pathway) and result in 39 cancer deaths averted (three from the solid pathway and 36 from the cystic pathway). We found similar trends for women. For 100 000 average-risk women of the same age, PDAC screening would identify 2323 low-risk cysts, 146 high-risk cysts, and 58 cancers (55 arising from the solid pathway and two from the cystic pathway) and result in 38 cancer deaths averted (three from the solid pathway, 35 from the cystic pathway). For both sexes, screening at age 50 years resulted in a small loss in net life expectancy (−3 days for men, −4 days for women) because screening benefits were outweighed by surgical mortality risks from false-positive diagnoses.
Table 4.
Screening for PDAC in Average-Risk Individuals according to Age
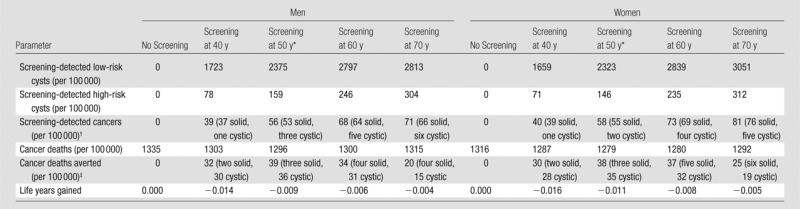
Base-case screening age.
Total number of cancers (PDAC). Whether cancers arose from solid versus cystic pathways is also specified.
Total number of cancer deaths averted. Whether cancers arose from solid versus cystic pathways is also specified.
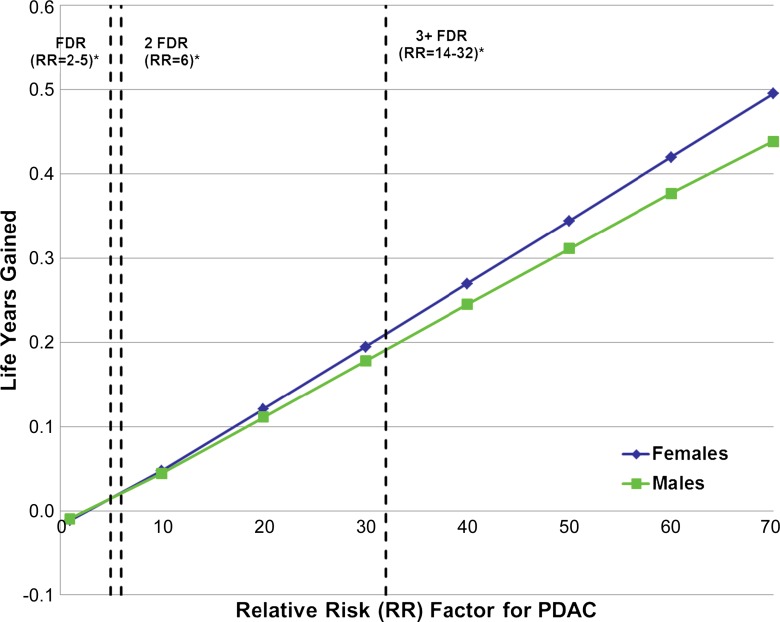
We found that as relative risk for PDAC increases, health benefits from a one-time MR imaging screening examination increase substantially (Fig 2). Net life expectancy gains would be achieved if an individual’s risk for PDAC exceeded 2.4 (men) or 2.7 (women) times that of the general population. For 50-year-old men with a relative risk for PDAC that is 30 times that of the general population, cancer deaths averted would increase from 39 to 1219 per 100 000 men (83 from the solid pathway and 1136 from the cystic pathway) and result in a life expectancy gain of 65 days. In this population, screening would help identify 21 392 low-risk cysts, 1544 high-risk cysts, and 1904 cancers (1800 arising from the solid pathway and 104 from the cystic pathway). Trends were similar in women, with 1204 of 100 000 cancer deaths averted (90 from the solid pathway, 1113 from the cystic pathway), 71 days gained, and 21 038 low-risk cysts, 1435 high-risk cysts, and 1975 cancers identified (1889 arising from the solid pathway and 86 from the cystic pathway). When relative risk further increases to 70 times that of the general population, the benefits of screening increased to 2630 per 100 000 cancer deaths averted (206 from the solid pathway, 2424 from the cystic pathway) and 160 days gained in men. Screening this population would help identify 24 539 low-risk cysts, 2082 high-risk cysts, and 5579 cancers (5310 arising from the solid pathway and 270 from the cystic pathway). In women with the same risk (70 times that of the general population), trends were again similar: 2639 per 100 000 cancer deaths averted (251 from the solid pathway, 2388 from the cystic pathway), 181 days gained, and 24 447 low-risk cysts, 1972 high-risk cysts, and 6222 cancers identified (5997 arising from the solid pathway and 225 from the cystic pathway).
Figure 2:
Relationship of PDAC risk to life expectancy gains from screening. With increased risk for PDAC (relative to that of the general population), screening benefits increase substantially. * = Relative risk (RR) levels among individuals with a positive family history are plotted as vertical dashed lines for reference (one affected first-degree relative [FDR] and two or three or more affected first-degree relatives; the high end of range was used for each category). Relative risks for known hereditary syndromes that predispose to PDAC are shown in Table 1.
With the sensitivity analysis, when varying the age at screening (Table 4), we found that for both men and women, screening individuals at age 50 years would avert the greatest number of PDAC deaths; however, screening at any of these ages for individuals of average risk continued to result in a loss of life expectancy owing to the competing risks of surgical mortality.
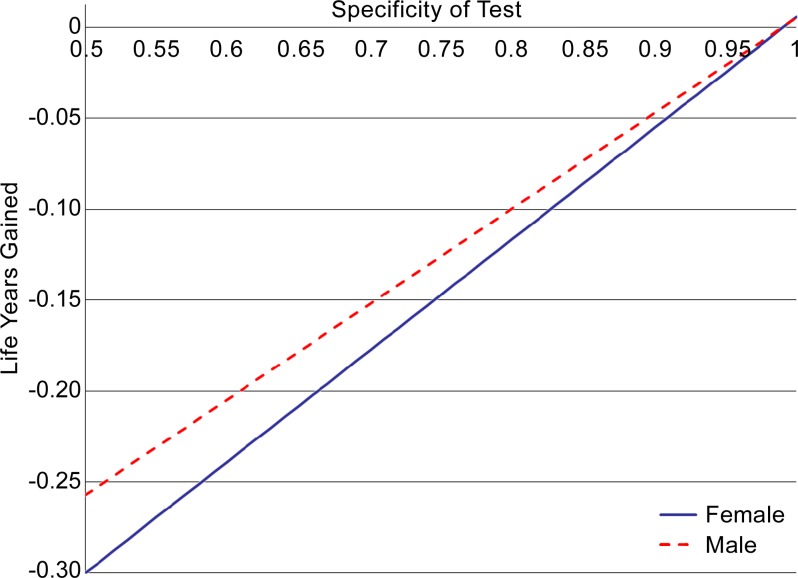
Our results were also sensitive to variability in MR imaging specificity (Fig 3). When screening 50-year-old men and women with an average risk for the tested range of 50%–100%, changes in life expectancy varied from −94 days to +2 days for men and from −110 days to +2 days for women. Results were less sensitive to variability in MR imaging sensitivity. For the tested range of 25%–100%, corresponding changes in life expectancy varied from −4 days to −2 days for men and from −5 days to −2 days for women.
Figure 3:
Graph shows effects of varied MR imaging specificity on life expectancy gains from screening. With decreased specificity, life expectancy losses from screening increase substantially owing to deaths from unnecessary pancreatic surgeries (prompted by false-positive results). Note that, in the base case, MR imaging specificity was 0.97.
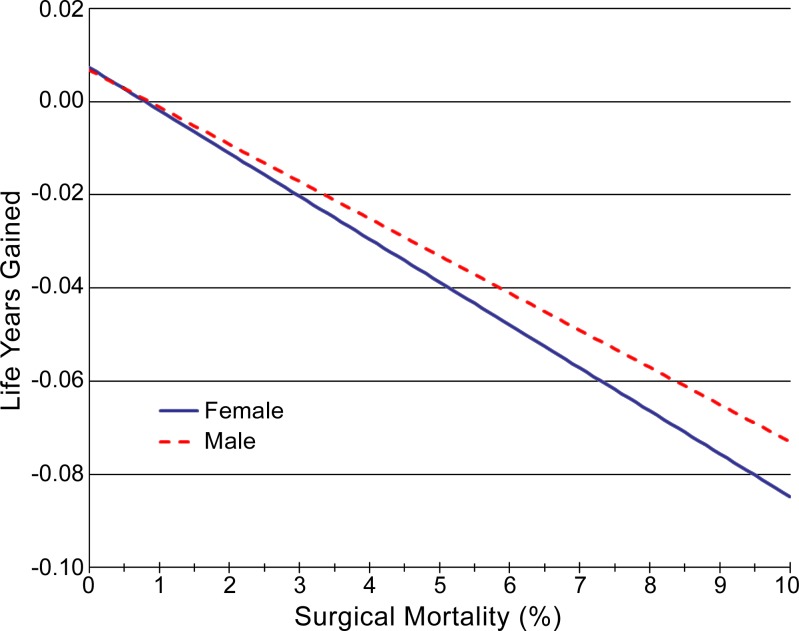
Results were also sensitive to the rate of surgical mortality (Fig 4). For the tested range of 0%–10%, changes in life expectancy varied from +2 days to −27 days for men and from +3 days to −31 days for women.
Figure 4:
Graph shows effects of varied surgical mortality rate on life expectancy gains from screening. Increased rates of surgical mortality resulted in increased life expectancy losses from screening, as would be expected. Note that in the base case, surgical mortality was 2%.
Discussion
We developed a simulation model of PDAC to evaluate when, from the standpoint of individuals’ relative risk for PDAC, MR imaging screening may be effective. We found that one-time MR imaging screening of average-risk 50-year-old individuals led to a net decrease in life expectancy owing to unnecessary pancreatic surgeries incurred as a result of false-positive results, validating current recommendations against screening the general population (14). However, we found that even individuals with modestly elevated risk exceeding thresholds of 2.4 (men) or 2.7 (women) times that of the general population may incur life expectancy gains from screening, an effect largely attributable to the detection of cystic precursors and, to a lesser extent, to the detection of early PDAC. As relative risk for PDAC increases to 30 times that of the general population, as is expected in those with at least three affected first-degree relatives, attributable life expectancy gains are comparable to those achieved from breast (52,53) and colorectal (54) cancer screening. Additional populations with known hereditary disorders who have an even higher relative risk for PDAC, for example those with hereditary pancreatitis (50–80 times that of the general population) (24–26,28) or Peutz-Jeghers syndrome (132 times that of the general population) (24,26,28), could achieve even greater life expectancy gains from screening. Our model results suggest that many individuals at increased risk for PDAC may benefit from MR imaging screening.
This study contributes new information to the field of PDAC screening by introducing contemporary simulation methods that are being used to develop and analyze cancer policy models in other organ systems (33,36,50,55,56). Our model is calibrated to National Cancer Institute Surveillance, Epidemiology, and End Results data, thereby leveraging high-quality data that are representative of the U.S. population to model pancreatic cancer. This approach is necessary for informing cancer control guidelines and health policy. In addition, the calibration process provides a form of model validation because the model results are compared with prespecified targets with revisions to achieve a good model fit (49).
Few studies have been published in which PDAC screening strategies have been modeled (57). In 2003, Rulyak and colleagues (57) performed a cost-effectiveness analysis of endoscopic US–based PDAC screening in high-risk individuals and found screening to be cost-effective beyond an endoscopic US sensitivity of 84% and a 16% prevalence of precursor dysplasia (14). Since that time, substantial developments have occurred on multiple fronts, specifically in the classification and management of precursor lesions (IPMNs and pancreatic intraepithelial neoplasia), implementation and reporting of imaging-based screening trials (including all six used in our analysis), technical capabilities of imaging, and computational methods for disease simulation (12,14,15,17,18,21,22,41,58,59). Our study brings new methods and evidence to this clinically challenging problem, providing insights into the relationship between cancer risk and screening benefits.
More broadly, the evaluation of risk-tailored screening strategies by means of modeling research has the potential to improve screening recommendations across several cancer settings. Such efforts have already led to the identification of individuals most likely to benefit from yearly CT for lung cancer screening, stratified according to age and pack-years of smoking (30). Future cancer screening models will likely be able to integrate more granular—and more accurate—information about individual patient risk (hereditary and acquired) and patient preferences than is readily available today. The generation of such information is a goal that is well aligned with the National Cancer Institute’s Population-based Research Optimizing Screening through Personalized Regimens initiative (60). Data generated from this initiative, when combined with cancer models, will ultimately enable further optimization of imaging-based screening choices.
Several study limitations merit mention. As with all modeling analyses, ours is a simplification of reality. By simplifying granular elements of a cancer’s natural history, model results can be affected. In our model, this limitation pertains most to our treatment of precursor lesions. Data reported on the natural history of precursor lesions are sparse and susceptible to verification bias because only lesions that are sufficiently worrisome with imaging or clinical criteria are removed (10,11,14,15,41). It is difficult to know the true proportion of low- and high-risk cysts within modeled populations. We used referral to surgery as a proxy for a cyst’s high-risk nature; however, unrelated factors such as an individual’s surgical candidacy or risk tolerance may confound such decisions (15). Further data on the natural history of precursor lesions, particularly in high-risk individuals, are needed to inform screening guidelines (14,61). Although the cystic pathway accounts for the minority of PDAC, cysts dominate the reason for positive screening (12,15,17,18,21,22); therefore, how cysts are managed has a substantial influence on the viability of PDAC screening.
There are also limited available data to inform the performance of MR imaging screening (or any imaging-based screening) for PDAC. Although MR imaging and CT are commonly used to diagnose and stage PDAC after symptomatic presentation, corresponding data are not applicable to screening settings. In the former, test performance commonly pertains to lesion characterization, whereas in screening, test performance pertains to lesion detection. To address this obstacle, we categorized findings of six screening studies to allow for estimates of MR imaging sensitivity and specificity (12,15,17,18,21,22). Simplifying assumptions were required in this process, given heterogeneous trial designs. In addition, disease-positive cases were limited in number across studies (12,15,17,18,21,22). Nevertheless, MR imaging sensitivity influenced our results much less than MR imaging specificity, providing reassurance of the stability of our results. Should MR imaging emerge as a first-line tool for PDAC screening, further standardization of screening protocols, image interpretation, and reporting language will be essential, as will measures to decrease interreader variability, commensurate with other cancer screening settings. Furthermore, the role of endoscopic US as an adjunct screening modality will need to be more clearly established. At present, endoscopic US is commonly performed to further evaluate pancreatic abnormalities encountered at MR imaging and CT (51), but this practice did not evolve in the context of cancer screening. The conditional dependence of MR imaging and endoscopic US in a screening setting must be explicitly evaluated to determine how to use the complementary performance attributes of each to optimize patient outcomes.
In addition, we did not specifically model competing effects on life expectancy for each subpopulation of patients at elevated risk for PDAC. Many genetic mutations known to produce elevated risk for PDAC are associated with the development of other malignancies. Ultimately, when considering the management of specific populations, additional competing risks must be considered to prioritize clinical recommendations. In addition, we did not account for pancreatic neuroendocrine tumors or extrapancreatic findings in our analysis owing to limited data about their relevance to the life expectancy of patients undergoing PDAC screening.
Surgical mortality, an additional competing source of death, is expected to vary with multiple factors such as institutional expertise, patient age, and performance status; this type of dependence was not explicitly modeled. Moreover, published mortality rates from pancreatic surgery vary, with most series reporting values within 1%–6% (42–47). We assumed a low mortality rate within this range for our analysis (2%) given that asymptomatic patients with screening-detected cancers are presumed to be healthier, in general, than patients with symptom-detected cancers. We addressed the uncertainty of surgical mortality estimates by evaluating the effects, on our results, of varying surgical mortality rates from 0% to 10% in sensitivity analysis (42–47).
Finally, many questions remain with regard to what age to start and terminate screening, what screening intervals should be used, and which screening strategies are optimal (14). For example, it may be best to alternate MR imaging and endoscopic US to leverage the benefits of each. Furthermore, serum biomarkers may be used alongside imaging tests to enhance diagnostic accuracy. Although known biomarkers such as CA19–9 have not proved to be effective for early detection, new and evolving biomarkers—such as circulating tumor cells—demonstrate promise for early detection efforts (7,62). Our model can be further developed to evaluate a wide spectrum of possible screening strategies and identify optimized strategies that are tailored to individual patient risk. Furthermore, new information about the natural history and genetic risk of PDAC can be rapidly incorporated into the model as it arises, and endpoints of costs, disutility, and harms of overdiagnosis can be further explored in future model iterations (63). Ultimately, a cost-effectiveness analysis in which long-term screening costs are estimated from a lifetime perspective will be necessary for understanding the value of an MR imaging–based screening approach.
In conclusion, we have developed a disease simulation model of PDAC to evaluate the effects of PDAC screening across a spectrum of an individual’s risk for PDAC. We found that MR imaging–based screening of individuals with even modestly elevated PDAC risk could increase life expectancy by averting cancer deaths. Notably, individuals with a known or suspected genetic predisposition account for only a small proportion of PDAC, and potential life expectancy gains remain tempered by the limited effectiveness of currently available screening technologies and therapies. Further modeling initiatives in PDAC screening, however, are warranted. Such initiatives will complement evidence gained from clinical trials, provide insights into requisite test performance characteristics for emerging imaging technologies and serum biomarkers, and ultimately inform risk-stratified guidelines that may reduce the burden of pancreatic cancer.
Advances in Knowledge
■ We projected that MR imaging screening for pancreatic ductal adenocarcinoma (PDAC) in average-risk individuals aged 50 years would lead to a net decrease in life expectancy (−3 days for men, −4 days for women) owing to unnecessary pancreatic surgeries associated with false-positive results.
■ However, even individuals with a modestly elevated risk for PDAC, exceeding thresholds of 2.4 (men) or 2.7 (women) times that of the general population, were projected to incur life expectancy gains from MR imaging screening.
■ Relative risks exceeding these thresholds are common in individuals with a known or suspected genetic predisposition for PDAC; our results suggest that many such individuals may benefit from MR imaging screening, predominantly with the detection of cystic precursors and, to a lesser extent, early PDAC.
Implication for Patient Care
■ MR imaging screening of the entire U.S. population for pancreatic cancer is not effective, but individuals with even modestly increased risk may benefit, including those with a strong family history or known hereditary predisposition.
SUPPLEMENTAL FIGURE
Appendix E1
Model Calibration Process to Estimate Unobservable Parameters
Hierarchical Approach to Calibration Targets
Calibration targets were prioritized according to importance. Primary targets received higher weighting in the calibration process than did secondary targets. Primary targets included the lifetime risk of developing pancreatic cancer (from age 20 years to death, conditional on being cancer-free at age 20), the lifetime risk of dying from pancreatic cancer (from age 20 years to death, without necessarily being cancer-free at age 20 years) (49), and the proportion of cancers that develop through solid versus cystic pathways (5). Lifetime risk estimates extracted from the Surveillance, Epidemiology, and End Results registry were decreased (relatively) by 5% to adjust for pancreatic cancer cases that were not adenocarcinoma (Table 3) (4,6,49). PDAC stage distribution (3), cyst prevalence (8), and the proportion of low-risk versus high-risk cystic lesions (15) were secondary targets (Tables 2, 3).
Age Dependence of Calibration Targets, Calibrated Parameters, and Model Outputs
As addressed briefly in the Materials and Methods, it is important to conceptually distinguish the age dependence of calibration targets, calibrated parameters, and model outputs. Here, we describe how patient age was—or was not—associated with each.
In the calibration process, all calibration targets were used in an age-independent fashion. This meant that, for example, a single age-independent cyst prevalence target was used when evaluating how well model outputs matched calibration targets.
When trying to find optimally calibrated parameter set “solutions” for unobservable transition probabilities, we imposed specific age-related constraints. The probability of developing a cancer via the solid pathway increased linearly with age; however, the slope and intercept of the linear function were determined by means of calibration. Transition probabilities were otherwise constant with age, with the exception of age-specific all-cause mortality (48), as specified in Materials and Methods.
Model outputs that were generated from calibrated parameter sets—for example, cyst prevalence—varied freely with age. These trends were reviewed to ensure the validity of observed age-dependent variability. As an example, Figure E1 depicts the age dependence of cyst prevalence in the calibrated model for an average-risk population.
χ2 Goodness-of-Fit Comparison of Model Outputs to Calibration Targets
The χ2 goodness-of-fit metric was used to compare model outputs to calibration targets. Model calibration consisted of minimizing this score using Solver, an optimization facility developed by Frontline Systems and available as a module within Microsoft Excel. All transition probabilities were determined with calibration, with the exception of relative survival by stage for detected cancers (3), surgical mortality (42–47), and all-cause mortality (48), which were fixed model inputs.
Notably, the algorithm used by Solver for nonlinear optimization problems is susceptible to selection of local (rather than absolute) minima and can generate parameter set solutions that deviate from clinical plausibility. We addressed this in two ways: (a) the algorithm was assisted by means of manual calibration, and (b) final model parameter sets were selected by a combination of goodness-of-fit score and visual inspection to ensure optimally calibrated model inputs.
Received June 2, 2014; revision requested July 24; revision received August 15; final version accepted August 26.
The research content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Funding: This research was supported by the National Institutes of Health (grant K07CA133097).
Abbreviations:
- IPMN
- intraductal papillary mucinous neoplasm
- PDAC
- pancreatic ductal adenocarcinoma
Disclosures of Conflicts of Interest: P.V.P. disclosed no relevant relationships. C.H. disclosed no relevant relationships. E.C.D. disclosed no relevant relationships. C.Y.K. disclosed no relevant relationships. A.T. disclosed no relevant relationships. K.E.P. disclosed no relevant relationships. W.B. disclosed no relevant relationships. C.H. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: received personal fees from Gilead Sciences. Other relationships: disclosed no relevant relationships.
References
- 1.American Cancer Society . Cancer facts and figures 2013. Atlanta, Ga: American Cancer Society, 2013. [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74(11):2913–2921. [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, et al., eds. SEER cancer statistics review, 1975–2010, National Cancer Institute. Bethesda, Md, http://seer.cancer.gov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER web site, April 2013. Accessed September 6, 2013. [Google Scholar]
- 4.Key C. Cancer of the pancreas. In: Ries LAG, Young JL Jr, Keel GE, Eisner MP, Lin YD, Horner MJD, eds. SEER survival monograph: cancer survival among adults: U.S. SEER program, 1988–2001, patient and tumor characteristics. National Cancer Institute, SEER program, NIH pub. no. 07-6215. Bethesda, Md: National Cancer Institute, 2007.
- 5.Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med 2004;351(12):1218–1226. [DOI] [PubMed] [Google Scholar]
- 6.Tempero MA, Arnoletti JP, Behrman S, et al. Pancreatic adenocarcinoma. J Natl Compr Canc Netw 2010;8(9):972–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin EJ, Canto MI. Pancreatic cancer screening. Gastroenterol Clin North Am 2012;41(1): 143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol 2008; 191(3):802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol 2010;105(9):2079–2084. [DOI] [PubMed] [Google Scholar]
- 10.Sahora K, Mino-Kenudson M, Brugge W, et al. Branch duct intraductal papillary mucinous neoplasms: does cyst size change the tip of the scale? A critical analysis of the revised international consensus guidelines in a large single-institutional series. Ann Surg 2013;258(3):466–475. [DOI] [PubMed] [Google Scholar]
- 11.Lafemina J, Katabi N, Klimstra D, et al. Malignant progression in IPMN: a cohort analysis of patients initially selected for resection or observation. Ann Surg Oncol 2013; 20(2):440–447. [DOI] [PubMed] [Google Scholar]
- 12.Al-Sukhni W, Borgida A, Rothenmund H, et al. Screening for pancreatic cancer in a high-risk cohort: an eight-year experience. J Gastrointest Surg 2012;16(4):771–783. [DOI] [PubMed] [Google Scholar]
- 13.Canto MI, Goggins M, Yeo CJ, et al. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol 2004;2(7):606–621. [DOI] [PubMed] [Google Scholar]
- 14.Canto MI, Harinck F, Hruban RH, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 2013;62(3): 339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canto MI, Hruban RH, Fishman EK, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 2012;142(4):796–804; quiz e14–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimmey MB, Bronner MP, Byrd DR, Brentnall TA. Screening and surveillance for hereditary pancreatic cancer. Gastrointest Endosc 2002;56(4,Suppl):S82–S86. [DOI] [PubMed] [Google Scholar]
- 17.Langer P, Kann PH, Fendrich V, et al. Five years of prospective screening of high-risk individuals from families with familial pancreatic cancer. Gut 2009;58(10):1410–1418. [DOI] [PubMed] [Google Scholar]
- 18.Ludwig E, Olson SH, Bayuga S, et al. Feasibility and yield of screening in relatives from familial pancreatic cancer families. Am J Gastroenterol 2011;106(5):946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poley JW, Kluijt I, Gouma DJ, et al. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol 2009;104(9):2175–2181. [DOI] [PubMed] [Google Scholar]
- 20.Schneider R, Slater EP, Sina M, et al. German national case collection for familial pancreatic cancer (FaPaCa): ten years experience. Fam Cancer 2011;10(2):323–330. [DOI] [PubMed] [Google Scholar]
- 21.Vasen HF, Wasser M, van Mil A, et al. Magnetic resonance imaging surveillance detects early-stage pancreatic cancer in carriers of a p16-Leiden mutation. Gastroenterology 2011;140(3):850–856. [DOI] [PubMed] [Google Scholar]
- 22.Verna EC, Hwang C, Stevens PD, et al. Pancreatic cancer screening in a prospective cohort of high-risk patients: a comprehensive strategy of imaging and genetics. Clin Cancer Res 2010;16(20):5028–5037. [DOI] [PubMed] [Google Scholar]
- 23.Brentnall TA, Bronner MP, Byrd DR, Haggitt RC, Kimmey MB. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med 1999;131(4):247–255. [DOI] [PubMed] [Google Scholar]
- 24.Steinberg WM, Barkin JS, Bradley EL, III, et al. Should patients with a strong family history of pancreatic cancer be screened on a periodic basis for cancer of the pancreas? Pancreas 2009;38(5):e137–e150. [DOI] [PubMed] [Google Scholar]
- 25.Grover S, Syngal S. Hereditary pancreatic cancer. Gastroenterology 2010;139(4):1076–1080, 1080.e1–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jimenez RE, Fernandez-del Castillo C. Tumors of the pancreas. In: Feldman M, Friedman LS, Brandt LJ, eds. Sleisenger and Fordtran’s gastrointestinal and liver disease. 9th ed. Philadelphia, Pa: Saunders Elsevier, 2010; 1017–1034. [Google Scholar]
- 27.Klein AP, Brune KA, Petersen GM, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res 2004;64(7):2634–2638. [DOI] [PubMed] [Google Scholar]
- 28.Templeton AW, Brentnall TA. Screening and surgical outcomes of familial pancreatic cancer. Surg Clin North Am 2013;93(3):629–645. [DOI] [PubMed] [Google Scholar]
- 29.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol 2006;4(6):766–781; quiz 665. [DOI] [PubMed] [Google Scholar]
- 30.de Koning HJ, Meza R, Plevritis SK, et al. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med 2014;160(5):311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hur C, Nishioka NS, Gazelle GS. Cost-effectiveness of photodynamic therapy for treatment of Barrett’s esophagus with high grade dysplasia. Dig Dis Sci 2003;48(7):1273–1283. [DOI] [PubMed] [Google Scholar]
- 32.Hur C, Nishioka NS, Gazelle GS. Cost-effectiveness of aspirin chemoprevention for Barrett’s esophagus. J Natl Cancer Inst 2004; 96(4):316–325. [DOI] [PubMed] [Google Scholar]
- 33.Knudsen AB, Hur C, Gazelle GS, Schrag D, McFarland EG, Kuntz KM. Rescreening of persons with a negative colonoscopy result: results from a microsimulation model. Ann Intern Med 2012;157(9):611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMahon PM, Kong CY, Bouzan C, et al. Cost-effectiveness of computed tomography screening for lung cancer in the United States. J Thorac Oncol 2011;6(11):1841–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McMahon PM, Kong CY, Johnson BE, et al. Estimating long-term effectiveness of lung cancer screening in the Mayo CT screening study. Radiology 2008;248(1):278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMahon PM, Kong CY, Johnson BE, et al. Chapter 9: The MGH-HMS lung cancer policy model: tobacco control versus screening. Risk Anal 2012;32(Suppl 1):S117–S124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rutter CM, Knudsen AB, Pandharipande PV. Computer disease simulation models: integrating evidence for health policy. Acad Radiol 2011;18(9):1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beck JR, Kassirer JP, Pauker SG. A convenient approximation of life expectancy (the “DEALE”). I. Validation of the method. Am J Med 1982;73(6):883–888. [DOI] [PubMed] [Google Scholar]
- 39.Beck JR, Pauker SG. The Markov process in medical prognosis. Med Decis Making 1983; 3(4):419–458. [DOI] [PubMed] [Google Scholar]
- 40.Beck JR, Pauker SG, Gottlieb JE, Klein K, Kassirer JP. A convenient approximation of life expectancy (the “DEALE”). II. Use in medical decision-making. Am J Med 1982;73(6): 889–897. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012;12(3):183–197. [DOI] [PubMed] [Google Scholar]
- 42.Pellegrini CA, Heck CF, Raper S, Way LW. An analysis of the reduced morbidity and mortality rates after pancreaticoduodenectomy. Arch Surg 1989;124(7):778–781. [DOI] [PubMed] [Google Scholar]
- 43.Crist DW, Sitzmann JV, Cameron JL. Improved hospital morbidity, mortality, and survival after the Whipple procedure. Ann Surg 1987;206(3):358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernández-del Castillo C, Morales-Oyarvide V, McGrath D, et al. Evolution of the Whipple procedure at the Massachusetts General Hospital. Surgery 2012;152(3,Suppl 1):S56–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med 2011; 364(22):2128–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg 2006;10(9):1199–1210; discussion 1210–1211. [DOI] [PubMed] [Google Scholar]
- 47.Reames BN, Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and operative mortality in the modern era. Ann Surg doi: 10.1097/SLA.0000000000000375. Published online December 13, 2013, [DOI] [PMC free article] [PubMed]
- 48.Arias E. United States life tables, 2008. In: National vital statistics reports. Volume 61, number 3. Hyattsville, Md: National Center for Health Statistics, 2012. [PubMed] [Google Scholar]
- 49.Surveillance, Epidemiology, and End Results Results (SEER) Program DevCan database. SEER 18 incidence and mortality, 2000–2010, with Kaposi sarcoma and mesothelioma. National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released June 2013, based on the November 2012 submission. Underlying mortality data provided by NCHS (www.cdc.gov/nchs). Accessed August 22, 2013.
- 50.Stout NK, Knudsen AB, Kong CY, McMahon PM, Gazelle GS. Calibration methods used in cancer simulation models and suggested reporting guidelines. Pharmacoeconomics 2009;27(7):533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baron TH, Mallery JS, Hirota WK, et al. The role of endoscopy in the evaluation and treatment of patients with pancreaticobiliary malignancy. Gastrointest Endosc 2003;58(5):643–649. [DOI] [PubMed] [Google Scholar]
- 52.Wright JC, Weinstein MC. Gains in life expectancy from medical interventions—standardizing data on outcomes. N Engl J Med 1998;339(6):380–386. [DOI] [PubMed] [Google Scholar]
- 53.van Ravesteyn NT, Miglioretti DL, Stout NK, et al. Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years: a comparative modeling study of risk. Ann Intern Med 2012;156(9):609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med 2008;149(9):659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hur C, Hayeck TJ, Yeh JM, et al. Development, calibration, and validation of a U.S. white male population-based simulation model of esophageal adenocarcinoma. PLoS ONE 2010;5(3):e9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yeh JM, Kuntz KM, Ezzati M, Hur C, Kong CY, Goldie SJ. Development of an empirically calibrated model of gastric cancer in two high-risk countries. Cancer Epidemiol Biomarkers Prev 2008;17(5):1179–1187. [DOI] [PubMed] [Google Scholar]
- 57.Rulyak SJ, Kimmey MB, Veenstra DL, Brentnall TA. Cost-effectiveness of pancreatic cancer screening in familial pancreatic cancer kindreds. Gastrointest Endosc 2003;57(1):23–29. [DOI] [PubMed] [Google Scholar]
- 58.Sipos B, Frank S, Gress T, Hahn S, Klöppel G. Pancreatic intraepithelial neoplasia revisited and updated. Pancreatology 2009;9(1-2):45–54. [DOI] [PubMed] [Google Scholar]
- 59.Fernández-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg 2003;138(4):427–433; discussion 433–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Onega T, Beaber EF, Sprague BL, et al. Breast cancer screening in an era of personalized regimens: a conceptual model and National Cancer Institute initiative for risk-based and preference-based approaches at a population level. Cancer 2014;120(19):2955–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nehra D, Oyarvide VM, Mino-Kenudson M, et al. Intraductal papillary mucinous neoplasms: does a family history of pancreatic cancer matter? Pancreatology 2012;12(4):358–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu M, Ting DT, Stott SL, et al. RNA sequencing of pancreatic circulating tumour cells implicates WNT signalling in metastasis. Nature 2012;487(7408):510–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harinck F, Nagtegaal T, Kluijt I, et al. Feasibility of a pancreatic cancer surveillance program from a psychological point of view. Genet Med 2011;13(12):1015–1024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.