Abstract
Endogenous opioid and non-opioid mechanisms [e.g. dopamine (DA), endocannabinoids (eCB)] have been implicated in the formation of placebo analgesic effects, with initial reports dating back three-decades. Besides the perspective that placebo effects confound randomized clinical trials (RCTs), the information so far acquired points to neurobiological systems that when activated by positive expectations and maintained through conditioning and reward learning are capable of inducing physiological changes that lead to the experience of analgesia and improvements in emotional state. Molecular neuroimaging techniques with positron emission tomography (PET) and the selective μ-opioid and D2/3 radiotracers [11C]carfentanil and [11C]raclopride have significantly contributed to our understanding of the neurobiological systems involved in the formation of placebo effects. This line of research has described neural and neurotransmitter networks implicated in placebo responses and provided the technical tools to examine inter-individual differences in the function of placebo responsive mechanisms, and potential surrogates (biomarkers). As a consequence, the formation of biological placebo effects is now being linked to the concept of resiliency mechanisms, partially determined by genetic factors, and uncovered by the cognitive emotional integration of the expectations created by the therapeutic environment and its maintenance through learning mechanisms. Further work needs to extend this research into clinical conditions where the rates of placebo responses are high and its neurobiological mechanisms have been largely unexplored (e.g. mood and anxiety disorders, persistent pain syndromes, or even Parkinson Disease and multiple sclerosis). The delineation of these processes within and across diseases would point to biological targets that have not been contemplated in traditional drug development.
Keywords: Placebo effects, analgesia, endogenous opioids, dopamine, endocannabinoids, Positron Emission Tomography (PET)
1. Introduction
In science, profound changes in paradigm have emerged when previously unexplained phenomena, typically disregarded as noise or measurement error, are explained by a new theoretical structure. That was the case for physics, when observations unaccounted by classical mechanics led to the development of relativism, while the “noise” in relativity theory became the source of quantum physics. If a comparable phenomenon could be found in medicine, an unexplained source of variance, it would undoubtedly be the so-called “placebo effect”, a psychological experience occurring in the patient’s brain after the administration of an inert substance, or of a sham physical treatment such as sham surgery, along with verbal suggestions (or any other cue) of clinical benefit1.
Accumulating data, stemming primarily from the area of placebo analgesia, show that placebo effects appear in response to the individual expectations and subsequent conditioning1–4. Work in this area has demonstrated that placebo administration and analgesic responses are associated with the activation of specific brain regions and neurotransmitter systems5–8. Furthermore, the neural systems activated by placebo-induced expectations have shown to overlap with those affected by the pathology and treatments under study8–13, adding to the variability in responses associated with the pathologies and treatments themselves.
At the neurotransmitter level, decades of research have supported the role of the opioid system in the neurobiology of placebo analgesic effects. This review article describes the contribution of molecular imaging to our understanding of the role of opioid mechanisms in the formation of placebo effects in healthy humans using pain as the primary paradigm of investigation. It also describes the interaction between opioid and non-opioid mechanisms [e.g. DA and cannabinoid systems] and how individual differences in the function of these systems contribute to the variability observed in placebo responses. These systems could be seen as biomarkers that allow the prediction of placebo responses in clinical trials, helping decisions regarding patient stratification and the separation of the effects of inactive and active treatments. In clinical practice, placebo-responsiveness would inform the likelihood of “non-specific” responses and customized care by indicating lower pharmacological dosages and procedural interventions, or alternatively a preferential response to psychosocial or cognitive approaches, an area that remains to be explored. In this regard, the predictability of the individual’s capacity to recruit “internally mediated” changes in physiological effects (the placebo effect) and its underlying neurobiology represents a shift in paradigm, where internal resources are mobilized, as opposed to traditional therapies that are given or applied to the patient with little individual control and minimal personalization in the selection of therapeutic approaches.
2. Opioid Mechanisms of Placebo Analgesia
The opioid systems consist of a large number of opioid peptides (β-endorphin, the endomorphins, enkephalins and dynorphins) and their opioid receptor sites (μ, β-endorphin, the endomorphins and enkephalins; δ, enkephalins; κ, dynorphins). In particular, the μ-opioid receptors (MORs) are critically involved in the induction of endogenous and exogenous analgesia, reward and stress responsiveness14, 15, as well as the regulation of emotion16 and hedonic responses to natural stimuli, including food17 and social interactions18, 19. They attain their highest concentrations in the thalamus (THA) and periaqueductal grey (PAG), where they regulate pain and stress responses, as well as in the amygdala (AMY), nucleus accumbens (NAC), and the cingulate cortex (ACC), where these receptors modulate reward, emotion, and in the case of the AMY and ACC, also sensory processing20, 21, 22.
Pharmacological23–25 and neuroimaging studies7, 8, 26 have extensively demonstrated the role of the opioid system in placebo analgesia. The first study that described this relationship showed that placebo analgesic effects could be blocked after the administration of the opioid receptor antagonist naloxone25. In a subsequent study23, Amanzio and Benedetti extended these findings investigating the role of non-opioid mechanisms to the formation of placebo analgesic effects. They demonstrated that using an ischemic arm pain experimental model, expectations of analgesia during placebo or morphine administration with and without pre-conditioning with morphine, induced analgesic effects that were blocked by naloxone. However, conditioning with the non-steroidal anti-inflammatory drug, ketorolac, paired with additional expectation cues, induced a placebo antinociceptive response that was only partially blocked by naloxone, while ketorolac conditioning alone produced analgesia that proved to be naloxone insensitive. Overall, these results demonstrated that both, opioid and non-opioid mechanisms were responsible for the formation of placebo effects; the former being induced by positive expectations or conditioning with an opioid agonist, and the latter being induced by conditioning with a non-opioid agonist and independent of positive expectations.
The role of the opioid system to the formation of placebo analgesic effects was further studied using blood flow measures and a pharmacological challenge26. This investigation compared the effects of the short-acting μ-opioid receptor agonist remifentanil on regional cerebral blood flow (rCBF) as measured with PET, with the effects of a placebo under expectations of analgesia. The results of this study demonstrated overlapping brain activity in the rostral anterior cingulate cortex (rACC) for the placebo and remifentanil conditions. Placebo administration also increased the correlation between the activity of this region and that of the midbrain PAG, a region known to exert modulatory effects on pain transmission. Individuals with high placebo analgesic responses further demonstrated greater rCBF responses to remifentanil, suggesting that individual differences in placebo analgesia may involve differences in the concentration or function of μ-opioid receptors26.
Later studies7, 27–31 have investigated the neurobiology of placebo analgesia using in-vivo molecular imaging with PET and validated models to quantify μ-opioid and other neurotransmitter systems, such as DA D2/3 receptors, following up on work involving the DA system in placebo responses in Parkinson Disease10, 11. In these types of functional molecular assays, reductions in the in vivo receptor availability (binding potential, BP) from a pain to a pain with placebo condition reflect placebo-induced activation of either opioid or DA neurotransmission. An initial investigation studied 14 young healthy males under baseline conditions, pain expectation and actual pain7. The latter two were performed with and without the administration of a placebo with expectations of analgesic properties (isotonic saline infused intravenously, 1 mL every 4 min at the sight of the volunteer). The pain model consisted of a steady-state of deep muscle pain maintained for 20 min by a computer-controlled closed-loop system through individually titrated infusion of medication-grade hypertonic saline (5%) into the masseter muscle, at a target pain intensity of 40 visual analog scale (VAS) units32, 33. Volunteers rated pain intensity every 15 s using an electronic version of a 10-cm VAS, placed in front of the scanner gantry. For the baseline condition, the same procedure was followed, except that non-painful isotonic instead of hypertonic saline was administered. This study determined the regional activation of endogenous opioid neurotransmission on μ-opioid receptors with PET and the selective μ-opioid radiotracer [11C]carfentanil. This dataset was the first direct evidence that the administration of a placebo with expectations of analgesia was associated with the activation of the endogenous opioid system and μ-opioid receptors in vivo in the rostral and subgenual (r/sgACC), the dorsolateral prefrontal cortex (DLPFC), anterior insular cortex (aINS) and the NAC. This activation was also associated with quantifiable reductions in physical and emotional elements of the pain experience. The DLPFC was not found to be related to changes in the psychophysical properties of the pain challenge, but instead was negatively associated with the expected analgesic effect of the placebo, as rated by the volunteers prior to its administration, suggesting that the reduction in the inhibitory effect of MOR’s in this cognitive and antinociceptive region was allowing the top-down engagement of subcortical pain regulatory regions through changes in the activation of MORs.
A subsequent study31 using the same pain paradigm showed that the largest proportion of the variance in regional endogenous opioid activity (40–68%, depending on the region) was accounted for a multiple regression model that included the affective (but not sensory) quality of the pain, the Positive and Negative Affectivity Scale (PANAS)34 positive and negative affect ratings, and a measure of individual pain sensitivity (the volume of algesic substance infused to maintain pain at target intensity level). This indicated that the individual affective experience during pain, whether pain-specific (McGill Pain Questionnaire (MPQ)35 pain affect subscale) or not (PANAS ratings of positive and negative internal affective state) were important predictors of the subsequent development of a placebo response, as was the measure of individual pain sensitivity.
The findings described above were replicated in a different sample using the same radiotracer labeling μ-opioid receptors and a modified version of the pain challenge that in this case was kept fixed between pain and pain and placebo conditions to eliminate possible confounds8. In this study, the administration of the placebo was associated with significant endogenous opioid activation in the pre- and subgenual ACC, orbitofrontal cortex (OFC), anterior and posterior INS, medial THA, NAC, AMY and periaqueductal gray (PAG). There was a notable lack of involvement of the DLPFC, while activation in the OFC was observed instead. Regional magnitudes of activation correlated with the subjects expected analgesia (NAC, PAG), the update of these verbally-induced expectations by the subjectively perceived efficacy of the placebo (NAC, AMY), as well as with placebo-induced changes in pain intensity (rACC, NAC, OFC) and positive affect (NAC).
These series of studies have help delineating a μ-opioid dependent network of regions involved in this complex phenomenon. The regions implicated included some involved in cognitive (OFC, DLPFC) and emotional integration (rACC); the representation and modulation of internal states (INS), and reward and saliency assessments (NAC).
2.1. The Contribution of the Opioid System to Current Theories of Placebo Effects
The contribution of the opioid system to theories about the formation of placebo effects has also been the subject of substantial debate. As briefly described above, initial investigations suggested that opioid mediated mechanisms of placebo analgesia were associated with expectation-induced placebo effects, but not conditioning23. Alternatively, recent evidence suggest that opioid mechanisms might play a significant role, not only in expectation-induced placebo analgesia, but also in the formation of placebo responses that appear in context of learning, in particular under a predictive coding framework. Bayesian learning theories support that learning depends on prediction errors, which signal the discrepancy between what it is expected and what it is received36, 37, 38,39. These newer learning theories provide a framework through which classical theories of placebo analgesia, verbally- and environmental context-induced expectations of clinical improvement and conditioning are reconciled, and placebo responses would emerge as a consequence of expectation and outcomes associations. Under this hypothesis, placebo responses would be greater in those who have a positive prediction error signal or experience an unexpected improvement of their symptoms (“the surprised”); and lower in those who have a negative prediction error signal or do not achieve the expected improvement (“the disappointed”).
A recent study supports the role of opioid mechanisms during the encoding of prediction errors in placebo analgesia8. This study examined the effect of expectations alone, or expectation and outcome comparisons on placebo analgesia using PET and the μ-opioid receptor selective radiotracer [11C]carfentanil29. In order to create a measure of expectations and expectations/outcomes comparisons, subjects were assigned to a Low (≤50) or High (>50) Expectations or Effectiveness groups based on their expected analgesic effects (0–100 VAS) before the experiment and their perceived effectiveness of the placebo (0–100 VAS) after the experiment. This study reported a lack of significant relationships between the subjects expected analgesic effects and placebo-associated reductions in pain ratings. Instead, individuals with high expectations showed greater μ-opioid system activation in the DLPFC that were not associated with placebo analgesic effects. Conversely, a learning mechanism defined by the discrepancy between expected analgesia and subjectively perceived effectiveness (prediction error signal) was associated with placebo analgesic responses, and with the activation of regional μ-opioid neurotransmission in a substantial number of regions implicated in opioid-mediated antinociception40 (ACC, OFC, AMY, THA, INS). The largest placebo responses were observed in those with low expectations and high subjective effectiveness (positive prediction error signal) whereas “nocebo”, hyperalgesic responses, were observed in those reporting high expectations and low reported effectiveness (negative prediction error signal). The magnitude of μ-opioid system activation in regions relevant to error detection was further associated with the subjective perception of analgesia. In particular, opioid neurotransmission in the dACC mediated the effect of prediction error on placebo analgesia. These findings then presented an apparent discrepancy with classical theories where the formation of placebo responses is dependent on the development of positive expectations. Instead, this study provided a mechanism through which classical theories of placebo analgesia (expectation vs. conditioning) are reconciled and are likely to facilitate the formation and sustainability of placebo responses over time.
A different study in a subsample of the study described above examined the possibility that the recall of placebo responses (e.g., the persistence of a memory of placebo-induced analgesia) would be associated with greater endogenous opioid neurotransmission during placebo administration41. In this study participants were asked to recall their pain experience by completing the McGill Pain Questionnaire (MPQ) in a phone interview 24 hours after completion using the same scanning protocol used in previous studies8. Subjects were further assigned to a positive placebo effect recall or a negative placebo effect recall based on their responses to the MPQ 24 hours after the each scanning procedure. This data showed that in addition to its immediate placebo-analgesic effects, the μ-opioid receptor system is involved in the subsequent recall of placebo effects. Specifically, the accurate or enhanced recall of analgesic effects 24 hours after the studies (“the recall of the placebo effect”) was associated with μ-opioid system activation during placebo administration in the VTA and the Papez circuit, implicated in reward-motivated learning and memory processing respectively42.
The studies described above support the role of μ-opioid receptor mediated neurotransmission in the cognitive processes involved in the formation of placebo responses. In particular, these studies suggest that in the context of placebo administration opioid neurotransmission is engaged during the encoding of prediction errors and that its activation contributes to the recall of the analgesic experience. This “decision-making”, Bayesian perspective on placebo analgesia, and the particular role that the opioid system takes in it, needs further development but opens up exciting perspectives for future research as recently suggested by Buchel and colleagues38.
2.2. Personality predictors of Placebo-induced activation of regional endogenous opioid neurotransmission
The role of opioid neurotransmission in the neurobiology of personality traits that might predict placebo effects has also been the focus of recent research. Given the role of μ-opioid receptor mediated neurotransmission in the maintenance of homeostasis during various forms of stress, including sustained pain43, personality traits such stress resiliency are likely to be mediated by opioid neurotransmission and potentially likely to explain inter-individual variability in placebo responses. In fact, new evidence suggest that personality traits related to stress resiliency and interpersonal relationships have a substantial impact on the capacity to develop placebo effects and could be employed to reduce variability in treatment trials where placebo effects can be particularly prominent and obscure the effects of potentially active treatments (for a review44). One study has examined the predictive value of scales assessing emotional, psychological, and social well-being, dispositional optimism, satisfaction with life and ego-resiliency on μ-opioid mediated placebo analgesia45. This study also evaluated overall personality traits (NEO Personality Inventory Revised46) and traits specifically related to the trait anxiety and reward processing. In this study Ego-Resiliency, Altruism, Straightforwardness (positive predictors) and Angry Hostility (negative predictor) accounted for 25% of the variation in placebo analgesic responses and had a predictive ability of 18%. Subjects scoring higher in these trait measures also presented greater placebo-induced activation of μ-opioid neurotransmission in the sg/dACC, OFC, INS, NAC, AMY and PAG (Figure 1). Additionally, they found significant reductions in cortisol plasma levels during placebo administration, which were correlated with reductions in subjective pain report and μ-opioid system activation in the dorsal ACC and PAG.
Figure 1. Personality traits effect on placebo-induced activation of regional μ-opioid receptor mediated neurotransmission.
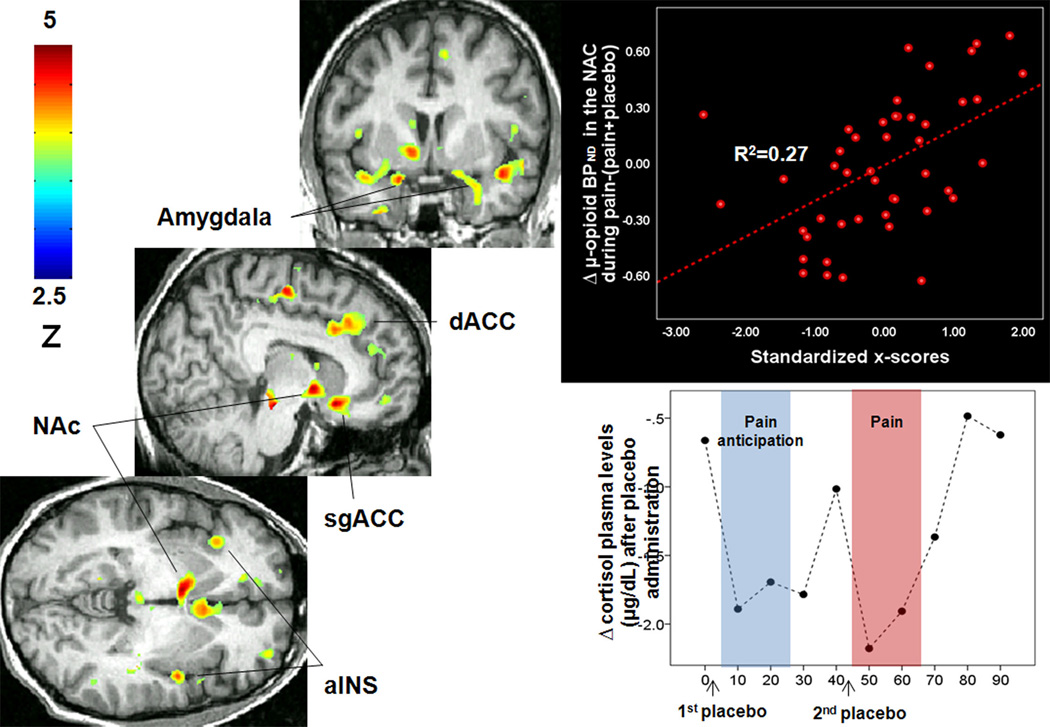
Left: Regions of greater μ-opioid system activation during placebo administration in subjects with high levels of Ego Resilience, Straightforwardness and Altruism and low levels of Angry Hostility. Upper right: Upper right: x-scores correlations with μ-opioid system activation (change in μ-opioid BPND) in the NAc after placebo administration. Lower right: reductions in cortisol plasma levels (mg/dl) after placebo administration. The sustained pain challenge was administered during 20 min, starting at 45 min scan time. Abbreviations: aINS: Anterior Insula; NAc: nucleus accumbens; d/sgACC: dorsal and subgenual anterior cingulate cortex.
Other studies have aimed to investigate personality predictors of placebo analgesia with inconsistent results47–51. This is not surprising considering the variability of this phenomenon, not only between subjects and across diseases but also within subjects. However, a neurobiological approach such as the one proposed above, to linking personality traits and placebo analgesia, promises, in our opinion, to advance our ability to predict placebo effects in the clinical practice by utilizing objective, endophenotypic measures that are not biased by subjective reporting, further elaborated for non-opioid mechanisms in the subsequent section.
3. Non-Opioid Mechanism of Placebo Analgesia
3.1. Dopaminergic Mechanisms of Placebo Analgesia
Apart from the opioidergic system, several studies have linked the mesolimbic system, in particular during reward anticipation, to placebo analgesia. This hypothesis36, 37, 11 was first confirmed in patients with Parkinson’s disease (PD), where DA activation in the NAC was detected with PET during receipt of a placebo in a manner proportional to the anticipated improvement in motor control11. However, no relationship was found between NAC DA activity and the actual placebo effects on motor function. These were, in fact, ascribed to DA activity in nigrostriatal projection regions (caudate and putamen)10. In a different study using intracerebral recordings during surgery for severe PD, placebo treatment was associated with reduced activity in single neurons in the subthalamic nucleus of placebo-responsive patients52. These reports confirmed an involvement of ventral basal ganglia synaptic activity and NAC DA in response to placebo-associated cues, potentially triggering downstream motor regulatory responses in PD. The first study to investigate the role of DA neurotransmission in placebo analgesia8 showed that placebo administration was associated with the activation of DA D2/D3 neurotransmission localized in mesolimbic DA terminal fields, ventral caudate, ventral putamen and NAC. Furthermore, they demonstrated that the magnitude of DA activation in the NAC was positively correlated with the individual expectations of analgesia, the update of those expectations during the study period (the ratio of subjectively rated analgesic efficacy over the initial expectations), and the magnitude of analgesia (the change in pain intensity ratings over the 20 min study period). DA activation in the NAC was also positively correlated with increases in PANAS positive affect ratings during placebo. Consistent with the hypothesis that NAC DA responses to placebo constitute a “trigger” that, responding to the saliency and potential reward value of the placebo would allow for the activation of down-stream adaptive (e.g., opioid) responses, placebo-induced NAC DA release was positively correlated with the magnitude of endogenous opioid release in the NAC, ventral putamen, AMY, aINS, pINS and rACC. Similarly to the opioid system, NAC DA release also differentiated volunteers that were above and below the mean in their analgesic responses (high and low placebo responders) in these trials.
Partly overlapping with the above sample, a different study tested the hypothesis that individual variations in placebo responses may be related to differences in the processing of reward expectation53. In this study participants were studied with a combination of molecular PET with [11C]raclopride and functional magnetic resonance imaging (fMRI) using a variation of the Monetary Incentive Delay (MID) task. This task is known to activate NAC synaptic activity during anticipation of a monetary reward54. This study described that individuals that activated NAC synaptic function to a greater extent during monetary reward anticipation also showed more profound placebo responses. The NAC BOLD signal during monetary reward anticipation was further correlated with placebo-induced DA activity as measured with PET.
The relationship between DA related personality traits and placebo analgesia has also been reported49. Schweinhardt and colleagues49 showed that the magnitude of placebo analgesia was related to gray matter density (GMD) in several brain regions, including the ventral striatum, INS, and PFC. Additionally, GMD in the ventral striatum and PFC was related to DA-related personality traits, such as novelty seeking, harm avoidance, behavioral drive, fun seeking and reward responsiveness. A broader role of DA neurotransmission in placebo responses across disease processes has also been investigated. In patients with irritable bowel syndrome (IBS), Hall et al.55 recently described the first genetic association between a functional polymorphism in the DA pathway, the Catechol-O-methyltransferasa (COMT) val158met, and placebo response. Their findings showed that IBS patients homozygous for the COMT met allele (met/met), which show reduced cortical COMT enzymatic activity and increased DA levels in the prefrontal cortex, were the most responsive to placebo treatment, whereas heterozygous (val/met) patients showed an intermediate response, and homozygous valine (val/val) patients showed essentially no placebo mediated symptom improvement. Similar results have been replicated using a conditioning paradigm during placebo analgesia47. A different study examined the role of genetic variation within the brain-derived neurotrophic factor (BDNF) gene on DA responses to reward anticipation and pain and placebo-induced changes in DA neurotransmission. BDNF has an important role in synaptic plasticity and the survival and function of DA neurons within the VTA-NAc pathway56, and therefore represents a candidate mechanism to examine inter-individual variability in DA related function. This study examined the effects of the BDNF functional single nucleotide polymorphism, Val66Met, on basal ganglia DA-associated mechanisms, which included responses to the anticipation of monetary gains and losses, as well as psychophysical and DA responses to the pain challenge, in the absence and presence of a placebo with putative analgesic properties. This study showed that BDNF met66 carriers, compared to val/val homozygotes, had increased BOLD responses during anticipation of monetary losses (but not gains) in the VTA-NAc-mPFC circuit and greater DA release in the NAc during a pain challenge. Conversely, during placebo administration, val/val homozygotes demonstrated increases in DA neurotransmission in the NAC and greater responses of the NAC during reward anticipation.
The evidence described above strongly supports the role of DA neurotransmission in placebo analgesia. These results directly link D2/3 receptors in the NAC and responses in the same regions during reward processing to the likelihood of developing placebo analgesic responses. Furthermore, DA related personality traits and genetic variation in candidate genes impacting DA neurotransmission seem to explain inter-individual variability in DA mediated pain and placebo responses, and more broadly in stress and reward responses in the human striatum.
3.2. Endocannabinoid Mechanisms in Placebo Analgesia
Another neurotransmitter system recently implicated in placebo analgesic responses is the endocannabinoid (eCB) system. This system, comprised of cannabinoid CB1 and CB2 receptors and their endogenous ligands, including N-arachidonoyl ethanolamine (anandamide, AEA) and 2-arachidonoyl glycerol (2-AG)57, is thought to be involved in analgesia58 and reward/reinforcement59 mechanisms, both of which are thought to be engaged during the development of placebo effects60. Recent work has shown that in the context of a conditioning paradigm, the cannabinoid receptor 1 (CBR1) antagonist SR 141716A (Rimonabant) blocked non-opioid, ketorolac-conditioned placebo analgesia, but not opioid placebo responses after morphine conditioning61. However, CBR1 and μ-opioid receptors are co-localized in brain structures involved in nociceptive control62, and they functionally interact63. This interaction was demonstrated in a study investigating the role of the common, functional missense variant Pro129Thr of the gene coding fatty acid amide hydrolase (FAAH), the major degrading enzyme of endocannabinoids, in pain and placebo-induced opioid and DA neurotransmission64. In this study the FAAH Pro129Thr polymorphism showed a selective effect on placebo responses, independent of other aspects of pain report. Surprisingly, Pro129/Pro129 homozygotes, which have increased activity of the FAAH and therefore lower synaptic endocannabinoid levels65, showed significantly greater psychophysical placebo responses, a more positive internal affective state during the placebo condition and a more positive recall of the placebo experience 24 hours after the pain challenge compared to Thr129 carriers. The neuroimaging data showed that during placebo administration FAAH Pro129/Pro129 homozygotes had greater endogenous opioid system activation, but not DA, in widespread regions, including, cortically, the DLFPC, the dorsal and ventromedial PFC, the lateral and medial OFC, the inferior frontal gyrus (IFG), the dorsal, rostral and subgenual ACC, the anterior and posterior INS and the hippocampus and parahippocampal gyrus. Subcortically, the same effects were detected in the NAC and mammillary bodies, the dorsal and ventral PUT and the anterior and posterior THA (Figure 2). The effects of FAAH on placebo-induced regional activation of μ-opioid neurotransmission were significantly correlated with psychophysical responses to placebo and with an enhancement of the recall of positive placebo effects 24 hours after the pain challenge.
Figure 2. FAAH Pro129Thr effect on opioid release during placebo administration.
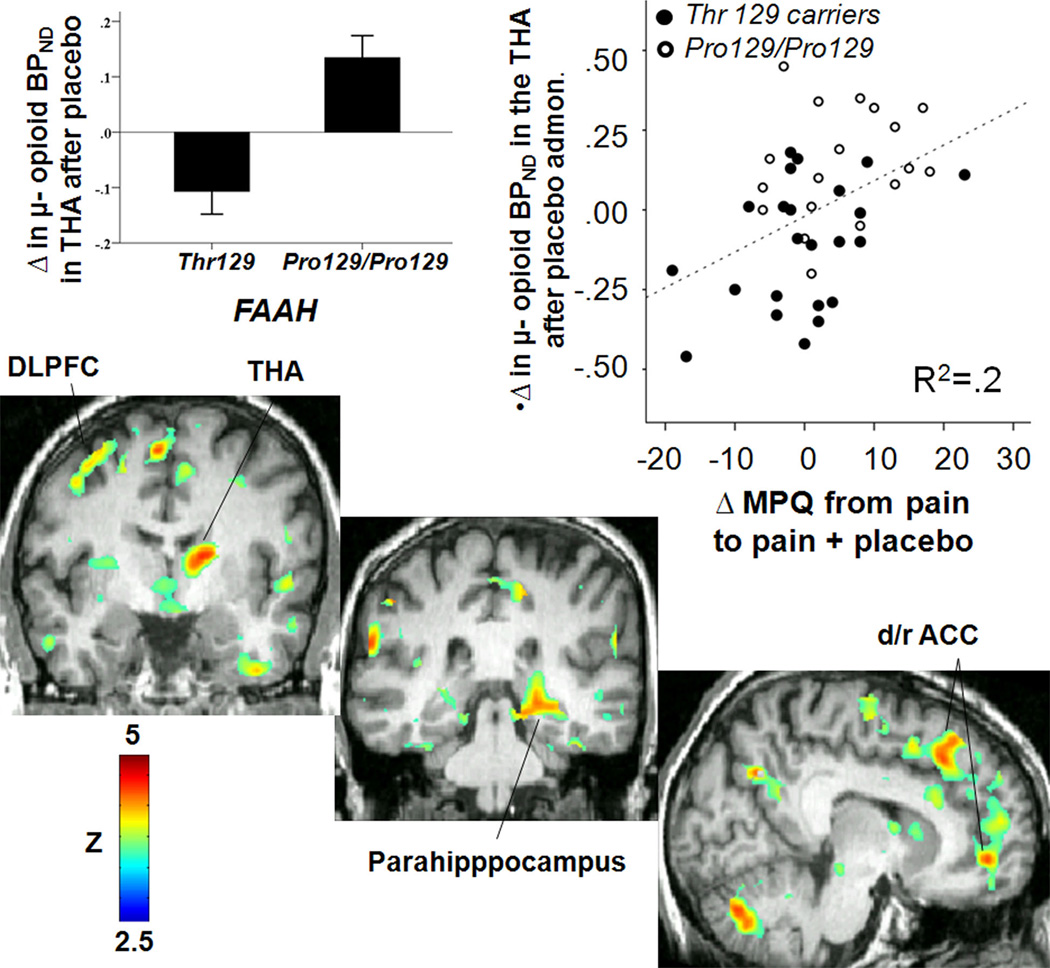
Upper left: regional effects of FAAH Pro129Thr (Pro129/Pro129 > Thr 129 carriers) on Δ μ-opioid BPND in the thalamus (THA) after placebo administration during pain. Upper right: Pearson correlation between ∆ μ-opioid BPND after placebo administration in the thalamus and ∆ in pain ratings after placebo administration. Botton: Voxel-by-voxel brain effects of FAAH Pro129Thr (Pro129/Pro129 > Thr 129 carriers) on ∆ μ-opioid BPND after placebo administration during pain. Abbreviations: DLPFC: dorsolateral prefrontal cortex; THA: thalamus; d/r ACC: dorsal/rostral anterior cingulate cortex; MPQ: McGill Pain Questionnaire; Admon.: Administration.
These results then strongly suggested that functional FAAH genotype variation selectively influenced psychophysical placebo responses and placebo-induced activation of μ-opioid receptor mediated neurotransmission in a network of regions previously involved in placebo-induced analgesia, but not other aspects of the pain experience not associated with placebo administration7, 8. FAAH Thr129 carriers, despite their chronic greater tonic eCB concentrations, showed lower psychophysical placebo responses and regional μ-opioid activation during placebo administration, compared with Pro129/Pro129 homozygotes, suggesting a down-regulation of CBR1 sites or their transduction mechanisms as potentially mediating these reduced placebo responses. These results also demonstrated an interaction between eCB and μ-opioid neurotransmission in the formation of placebo responses in the absence of previous conditioning, and provide new insights into the neurobiology of placebo effects in conditions where these interactions play a critical role, such as substance use disorders66–68. From the perspective of clinical trials, the examination of FAAH Pro129Thr could be used as a marker for patient stratification, in particular for pathological states that are potentially influenced by eCB and endogenous opioid systems.
4. Conclusions
The evidence described above demonstrates the critical role of opioid and non-opioid mechanism in the cognitive and emotional processes that are engaged during the administration of a placebo to modify physiology. These processes are of importance to understand the inter-individual variability that leads to recovery from any illness and appear to be associated with neurobiological resiliency mechanisms that are engaged in response to stressors and pathological states. A network of regions, including the rostral ACC, DLPFC and OFC, INS, NAC, AMY, medial THA and PAG appear involved. Opioid, DA and eCB neurotransmission in these areas modulate various elements of the placebo effect, which include the representation of its subjective value, updates of expectations over time, the recall of pain and placebo experiences and changes in affective state and in pain ratings. The circuitry involved in placebo analgesic effects also have the potential to modulate a number of functions beyond pain, as the regions involved have been implicated in the regulation of stress responses, neuroendocrine and autonomic functions, mood, reward and integrative cognitive processes.
Besides the perspective that placebo effects confound RCTs, the information so far acquired points to neurobiological systems that when activated by positive expectations and maintained through reward learning are capable of inducing physiological changes that lead to the experience of analgesia and changes in emotional state.
Further evidence needs to extend this research to the many clinical conditions with high placebo response rates (e.g. mood and anxiety disorders, persistent pain conditions, Parkinson Disease, multiple sclerosis), where its neurobiological mechanisms have been largely unexplored. It would be of importance to investigate placebo effects in clinical trials of disease states where a dysregulation of endogenous opioid, dopaminergic or endocannabinoid systems have been reported (e.g. substance use disorders, persistent pain conditions); and where a potential interaction between the active treatment and the placebo might occur. This work, by examining the noise in clinical trials and previously unexamined “confounds” is likely to point to new targets for therapeutic development by the enhancement of disease-modifying, resilience mechanisms present in a substantial number of patients diagnosed with these chronic conditions.
Table 1.
Summary of studies investigating the neurobiology of placebo analgesic effects using PET and the selective radiotracers carfentanil (CFN, μ-opioid receptor agonist) and raclopride (RCL, D2/3 receptor antagonist).
| Study* | Year | Tracer, pain model**& subjects*** |
Findings**** | |
|---|---|---|---|---|
| 1 | Zubieta et al. | J Neuro, 2005 | CFN: 14M | ↑ placebo-induced opioid neurotransmission was observed in the pregenual and subgenual rostral ACC, DLPFC, INS, and NAC. |
| 2 | Zubieta et al. | Brain Behav Immun, 2006 | CFN: 19M | A regression model that included the affective qualities of pain and the volume of algesic stimulus required to maintain moderate levels of pain contributed to 40–68% of the variance in placebo-induced opioid release. |
| 3 | Wager et al. | PNAS, 2007 | CFN: 15M | ↑ placebo-induced opioid neurotransmission was observed in the PAG, AMY, OFC, INS, rostral ACC, and LPFC. Placebo treatment increased functional connectivity between the PAG and rostral ACC. |
| 4 | Scott et al. | Neuron, 2007 | MID: 7F, 23M RCL: 7F, 7M |
↑ placebo-induced activation of DA neurotransmission observed in the NAC was associated with the anticipated placebo effects, the perception-anticipation mismatches, and the formation of placebo responses as well as the ↑ BOLD responses during the anticipation of monetary gains in NAC. |
| 5 | Scott et al. | JAMA Psych, 2008 | CFN/RCL: 9F, 11M | ↑ placebo-induced activation of opioid neurotransmission was observed in the ACC, OFC and INS, NAC, AMY, and PAG. ↑ placebo-induced activation of DA neurotransmission was observed in the ventral basal ganglia. Nocebo responses were associated with a deactivation of DA and opioid release. |
| 6 | Peciña et al. | Mol Psych, 2013 | CFN: 18F, 19M | ↑ recall of the placebo experience 24 hours after a pain challenge was associated with greater placebo-induced opioid release in the VTA and the Papez circuit. |
| 7 | Peciña et al. | NPP, 2013 | CFN: 28F, 19M | Personality traits including Ego-Resiliency, NEO-Altruism, NEO-Straightforwardness (positive predictors) and NEO-Angry Hostility (negative predictor) scales predicted 25% of the variance in placebo analgesic responses and were associated with ↑ placebo-induced opioid release in the ACC, OFC, INS, NAC, AMY and PAG. |
| 8 | Peciña et al. | Soc Cogn Affect Neurosci, 2013 | CFN: 27F, 21M | Expectations of improvement were associated with greater opioid release in the DLPFC but not higher placebo respones. ↑ placebo-induced pioid release in the dACC mediated the predictive effect of PE signal on placebo analgesia. |
| 9 | Peciña et al. | Mol Psych, 2014 | CFN/RCL: 23F, 19M | FAAH Pro129/Pro129, compared to Thr129 carriers, reported higher placebo analgesia, more positive affective states inmediatly and 24h after the pain challenge, and increased opiod, but not DA, neurotransmission in DLPFC, d/v MPFC, l/m OFC, ACC, INS, HIPP, paraHIPP, NAC, PUT and THA. |
| 10 | Peciña et al. | J Neuro, 2014 | MID: 34F, 48M RCL: 28F, 21M |
BDNF Met66 carriers, compared to Val/Val homozygotes, showed ↑ BOLD responses during anticipation of monetary losses in the VTA-NAc-mPFC circuit, ↑ DA release in the NAc during a pain challenge and overall reduction in DA neurotransmission in the same region after placebo. |
Studies 4–10 have overlapping sample sizes.
Sustained pain challege (hipertonic saline solution into the masseter muscle) in all cases except Wager et al. (thermal heat induction) CFN: (11C) Carfentanil; RCL: (11C)Raclopride; MID: Monetary Incentive Delayed fMRI Task.
F: Females; M: Males.
ACC: Anterior Cingulate Cortex; DLPFC: Dorsolateral Prefrontal Cortex; INS: Insula; NAC: Nucleus Accumbens; PAG: Periaqueductal Gray, AMY: Amygdala; OFC: Orbitofrontal Cortex; HIPP: Hippocampus VTA: Ventral Tegmental Area; FAAH: Fatty acid amine hydrolase; BDNF: Brain-derived neurotrophic factor; DA: Dopamine; PE: Prediction Error; BOLD: Blood-oxygen-level dependent.
ACKNOWLEDGEMENTS
Work was supported by R01 AT 001415, R01 DA 022520, R01 DA 27494 (JKZ) and the Phil F. Jenkins Foundation. We would also like to acknowledge the contribution of the technologists of the PET Center at the University of Michigan.
Footnotes
CONFLICTS OF INTEREST
The authors have no interests to disclose that are or might be perceived to be in conflict with the work reported in this study.
References
- 1.Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annu Rev Psychol. 2008;59:565–590. doi: 10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed] [Google Scholar]
- 2.Colloca L, Benedetti F. Placebos and painkillers: is mind as real as matter? Nature reviews Neuroscience. 2005;6(7):545–552. doi: 10.1038/nrn1705. [DOI] [PubMed] [Google Scholar]
- 3.Kirsch I. Conditioning, expectancy, and the placebo effect: comment on Stewart-Williams and Podd (2004) Psychological bulletin. 2004;130(2):341–343. doi: 10.1037/0033-2909.130.2.341. discussion 344–345. [DOI] [PubMed] [Google Scholar]
- 4.Stewart-Williams S, Podd J. The placebo effect: dissolving the expectancy versus conditioning debate. Psychological bulletin. 2004;130(2):324–340. doi: 10.1037/0033-2909.130.2.324. [DOI] [PubMed] [Google Scholar]
- 5.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 6.Wager TD, Scott DJ, Zubieta JK. Placebo effects on human μ-opioid activity during pain. Proc Natl Acad Sci U S A. 2007;104(26):11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, et al. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25(34):7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry. 2008;65(2):220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- 9.Mayberg HS, Silva JA, Brannan SK, Tekell JL, Mahurin RK, McGinnis S, et al. The functional neuroanatomy of the placebo effect. Am J Psychiatry. 2002;159(5):728–737. doi: 10.1176/appi.ajp.159.5.728. [DOI] [PubMed] [Google Scholar]
- 10.de la Fuente-Fernandez R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ. Expectation and dopamine release: mechanism of the placebo effect in Parkinson's disease. Science. 2001;293(5532):1164–1166. doi: 10.1126/science.1060937. [DOI] [PubMed] [Google Scholar]
- 11.de la Fuente-Fernandez R, Phillips AG, Zamburlini M, Sossi V, Calne DB, Ruth TJ, et al. Dopamine release in human ventral striatum and expectation of reward. Behav Brain Res. 2002;136(2):359–363. doi: 10.1016/s0166-4328(02)00130-4. [DOI] [PubMed] [Google Scholar]
- 12.Volkow ND, Wang GJ, Ma Y, Fowler JS, Zhu W, Maynard L, et al. Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. J Neurosci. 2003;23(36):11461–11468. doi: 10.1523/JNEUROSCI.23-36-11461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennedy SE, Koeppe RA, Young EA, Zubieta JK. Dysregulation of endogenous opioid emotion regulation circuitry in major depression in women. Arch Gen Psychiatry. 2006;63(11):1199–1208. doi: 10.1001/archpsyc.63.11.1199. [DOI] [PubMed] [Google Scholar]
- 14.Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug and alcohol dependence. 1998;51(1–2):23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- 15.Vaccarino AL, Kastin AJ. Endogenous opiates: 1999. Peptides. 2000;21(12):1975–2034. doi: 10.1016/s0196-9781(00)00345-4. [DOI] [PubMed] [Google Scholar]
- 16.Zubieta JK, Ketter TA, Bueller JA, Xu Y, Kilbourn MR, Young EA, et al. Regulation of human affective responses by anterior cingulate and limbic mu-opioid neurotransmission. Archives of general psychiatry. 2003;60(11):1145–1153. doi: 10.1001/archpsyc.60.11.1145. [DOI] [PubMed] [Google Scholar]
- 17.Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25(50):11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herman BH, Panksepp J. Effects of morphine and naloxone on separation distress and approach attachment: evidence for opiate mediation of social affect. Pharmacology, biochemistry, and behavior. 1978;9(2):213–220. doi: 10.1016/0091-3057(78)90167-3. [DOI] [PubMed] [Google Scholar]
- 19.Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Wang H, et al. Response of the mu-opioid system to social rejection and acceptance. Mol Psychiatry. 2013;18(11):1211–1217. doi: 10.1038/mp.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellingsen DM, Wessberg J, Eikemo M, Liljencrantz J, Endestad T, Olausson H, et al. Placebo improves pleasure and pain through opposite modulation of sensory processing. Proc Natl Acad Sci U S A. 2013;110(44):17993–17998. doi: 10.1073/pnas.1305050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manning BH. A lateralized deficit in morphine antinociception after unilateral inactivation of the central amygdala. J Neurosci. 1998;18(22):9453–9470. doi: 10.1523/JNEUROSCI.18-22-09453.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oroszi G, Goldman D. Alcoholism: genes and mechanisms. Pharmacogenomics. 2004;5(8):1037–1048. doi: 10.1517/14622416.5.8.1037. [DOI] [PubMed] [Google Scholar]
- 23.Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci. 1999;19(1):484–494. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gracely RH, Dubner R, Wolskee PJ, Deeter WR. Placebo and naloxone can alter post-surgical pain by separate mechanisms. Nature. 1983;306(5940):264–265. doi: 10.1038/306264a0. [DOI] [PubMed] [Google Scholar]
- 25.Levine J, Gordon N, Fields H. The mechanism of placebo analgesia. Lancet. 1978;2(8091):654–657. doi: 10.1016/s0140-6736(78)92762-9. [DOI] [PubMed] [Google Scholar]
- 26.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia-- imaging a shared neuronal network. Science. 2002;295(5560):1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 27.Pecina M, Azhar H, Love TM, Lu T, Fredrickson BL, Stohler CS, et al. Personality trait predictors of placebo analgesia and neurobiological correlates. Neuropsychopharmacology. 2013;38(4):639–646. doi: 10.1038/npp.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pecina M, Martinez-Jauand M, Hodgkinson C, Stohler CS, Goldman D, Zubieta JK. FAAH selectively influences placebo effects. Mol Psychiatry. 2014;19(3):385–391. doi: 10.1038/mp.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pecina M, Stohler CS, Zubieta JK. Neurobiology of placebo effects: expectations or learning? Social cognitive and affective neuroscience. 2013 doi: 10.1093/scan/nst079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci U S A. 2007;104(26):11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zubieta JK, Yau WY, Scott DJ, Stohler CS. Belief or Need? Accounting for individual variations in the neurochemistry of the placebo effect. Brain Behav Immun. 2006;20(1):15–26. doi: 10.1016/j.bbi.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Ashton-Miller JA, Stohler CS. A closed-loop system for maintaining constant experimental muscle pain in man. IEEE Trans Biomed Eng. 1993;40(4):344–352. doi: 10.1109/10.222327. [DOI] [PubMed] [Google Scholar]
- 33.Stohler C, Kowalski C. Spatial and temporal summation of sensory and affective dimensions of deep somatic pain. Pain. 1999;79(2–3):165–173. doi: 10.1016/s0304-3959(98)00171-7. [DOI] [PubMed] [Google Scholar]
- 34.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Personal Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 35.Melzack R, Katz J. The McGill Pain Questionnaire: Appraisal and Current Status. In: Turk D, Melzack R, editors. Handbook of Pain Assessment. New York: Guilford Press; 2000. pp. 152–168. [Google Scholar]
- 36.Fields H. State-dependent opioid control of pain. Nature reviews Neuroscience. 2004;5(7):565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- 37.Irizarry KJ, Licinio J. An explanation for the placebo effect? Science. 2005;307(5714):1411–1412. doi: 10.1126/science.307.5714.1411. [DOI] [PubMed] [Google Scholar]
- 38.Buchel C, Geuter S, Sprenger C, Eippert F. Placebo analgesia: a predictive coding perspective. Neuron. 2014;81(6):1223–1239. doi: 10.1016/j.neuron.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 39.Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinformecement. Classical Conditioning, II, Current Research and Theory, edited by Black AH adn Prokasy WF New York: Appleton Century Crofts. 1972:64–69. [Google Scholar]
- 40.Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293(5528):311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- 41.Pecina M, Stohler CS, Zubieta JK. Role of mu-opioid system in the formation of memory of placebo responses. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50(3):507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 43.Ribeiro SC, Kennedy SE, Smith YR, Stohler CS, Zubieta JK. Interface of physical and emotional stress regulation through the endogenous opioid system and mu-opioid receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(8):1264–1280. doi: 10.1016/j.pnpbp.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 44.Jaksic N, Aukst-Margetic B, Jakovljevic M. Does personality play a relevant role in the placebo effect? Psychiatria Danubina. 2013;25(1):17–23. [PubMed] [Google Scholar]
- 45.Pecina M, Azhar H, Love TM, Lu T, Fredrickson BL, Stohler CS, et al. Personality Trait Predictors of Placebo Analgesia and Neurobiological Correlates. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Costa P, McRae R. Normal personality assessment in clinical plactice: the NEO Personality Inventory. Psychological Assessment. 1992;4(1):5–13. [Google Scholar]
- 47.Yu R, Gollub RL, Vangel M, Kaptchuk T, Smoller JW, Kong J. Placebo analgesia and reward processing: Integrating genetics, personality, and intrinsic brain activity. Human brain mapping. 2014;35(9):4583–4593. doi: 10.1002/hbm.22496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vase L, Robinson ME, Verne GN, Price DD. Increased placebo analgesia over time in irritable bowel syndrome (IBS) patients is associated with desire and expectation but not endogenous opioid mechanisms. Pain. 2005;115(3):338–347. doi: 10.1016/j.pain.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 49.Schweinhardt P, Seminowicz DA, Jaeger E, Duncan GH, Bushnell MC. The anatomy of the mesolimbic reward system: a link between personality and the placebo analgesic response. J Neurosci. 2009;29(15):4882–4887. doi: 10.1523/JNEUROSCI.5634-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morton DL, Watson A, El-Deredy W, Jones AK. Reproducibility of placebo analgesia: Effect of dispositional optimism. Pain. 2009;146(1–2):194–198. doi: 10.1016/j.pain.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 51.Geers AL, Wellman JA, Fowler SL, Helfer SG, France CR. Dispositional optimism predicts placebo analgesia. The journal of pain : official journal of the American Pain Society. 2010;11(11):1165–1171. doi: 10.1016/j.jpain.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benedetti F, Colloca L, Torre E, Lanotte M, Melcarne A, Pesare M, et al. Placebo-responsive Parkinson patients show decreased activity in single neurons of subthalamic nucleus. Nature neuroscience. 2004;7(6):587–588. doi: 10.1038/nn1250. [DOI] [PubMed] [Google Scholar]
- 53.Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007;55(2):325–336. doi: 10.1016/j.neuron.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 54.Knutson B, Bjork JM, Fong GW, Hommer D, Mattay VS, Weinberger DR. Amphetamine modulates human incentive processing. Neuron. 2004;43(2):261–269. doi: 10.1016/j.neuron.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 55.Hall KT, Lembo AJ, Kirsch I, Ziogas DC, Douaiher J, Jensen KB, et al. Catechol-O-methyltransferase val158met polymorphism predicts placebo effect in irritable bowel syndrome. PloS one. 2012;7(10):e48135. doi: 10.1371/journal.pone.0048135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pecina M, Martinez-Jauand M, Love T, Heffernan J, Montoya P, Hodgkinson C, et al. Valence-Specific Effects of BDNF Val66Met Polymorphism on Dopaminergic Stress and Reward Processing in Humans. J Neurosci. 2014;34(17):5874–5881. doi: 10.1523/JNEUROSCI.2152-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kogan NM, Mechoulam R. The chemistry of endocannabinoids. Journal of endocrinological investigation. 2006;29(3 Suppl):3–14. [PubMed] [Google Scholar]
- 58.Hohmann AG. Spinal and peripheral mechanisms of cannabinoid antinociception: behavioral, neurophysiological and neuroanatomical perspectives. Chemistry and physics of lipids. 2002;121(1–2):173–190. doi: 10.1016/s0009-3084(02)00154-8. [DOI] [PubMed] [Google Scholar]
- 59.Gardner EL, Vorel SR. Cannabinoid transmission and reward-related events. Neurobiology of disease. 1998;5(6 Pt B):502–533. doi: 10.1006/nbdi.1998.0219. [DOI] [PubMed] [Google Scholar]
- 60.Colloca L, Sigaudo M, Benedetti F. The role of learning in nocebo and placebo effects. Pain. 2008;136(1–2):211–218. doi: 10.1016/j.pain.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 61.Benedetti F, Amanzio M, Rosato R, Blanchard C. Nonopioid placebo analgesia is mediated by CB1 cannabinoid receptors. Nat Med. 2011;17(10):1228–1230. doi: 10.1038/nm.2435. [DOI] [PubMed] [Google Scholar]
- 62.Salio C, Fischer J, Franzoni MF, Mackie K, Kaneko T, Conrath M. CB1-cannabinoid and mu-opioid receptor co-localization on postsynaptic target in the rat dorsal horn. Neuroreport. 2001;12(17):3689–3692. doi: 10.1097/00001756-200112040-00017. [DOI] [PubMed] [Google Scholar]
- 63.Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283(5400):401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- 64.Pecina M, Martinez-Jauand M, Hodgkinson C, Stohler CS, Goldman D, Zubieta JK. FAAH selectively influences placebo effects. Molecular psychiatry. 2013 doi: 10.1038/mp.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chiang KP, Gerber AL, Sipe JC, Cravatt BF. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Human molecular genetics. 2004;13(18):2113–2119. doi: 10.1093/hmg/ddh216. [DOI] [PubMed] [Google Scholar]
- 66.Fattore L, Vigano D, Fadda P, Rubino T, Fratta W, Parolaro D. Bidirectional regulation of mu-opioid and CB1-cannabinoid receptor in rats self-administering heroin or WIN 55,212-2. The European journal of neuroscience. 2007;25(7):2191–2200. doi: 10.1111/j.1460-9568.2007.05470.x. [DOI] [PubMed] [Google Scholar]
- 67.Fattore L, Deiana S, Spano SM, Cossu G, Fadda P, Scherma M, et al. Endocannabinoid system and opioid addiction: behavioural aspects. Pharmacology, biochemistry, and behavior. 2005;81(2):343–359. doi: 10.1016/j.pbb.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 68.Navarro M, Carrera MR, Fratta W, Valverde O, Cossu G, Fattore L, et al. Functional interaction between opioid and cannabinoid receptors in drug self-administration. J Neurosci. 2001;21(14):5344–5350. doi: 10.1523/JNEUROSCI.21-14-05344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


