Abstract
A devastating aspect of cancer cachexia is severe loss of muscle and fat mass. Though cachexia occurs in both sexes, it is not well-defined in the female. The Apc Min/+ mouse is genetically predisposed to develop intestinal tumors; circulating IL-6 is a critical regulator of cancer cachexia in the male Apc Min/+ mouse. The purpose of this study was to examine the relationship between IL-6 signaling and cachexia progression in the female Apc Min/+ mouse. Male and female Apc Min/+ mice were examined during the initiation and progression of cachexia. Another group of females had IL-6 overexpressed between 12-14 weeks or 15-18 weeks of age to determine whether IL-6 could induce cachexia. Cachectic female Apc Min/+ mice lost body weight, muscle mass, and fat mass; increased muscle IL-6 mRNA expression was associated with these changes, but circulating IL-6 levels were not. Circulating IL-6 levels did not correlate with downstream signaling in muscle in the female. Muscle IL-6r mRNA expression and SOCS3 mRNA expression as well as muscle IL-6r protein and STAT3 phosphorylation increased with severe cachexia in both sexes. Muscle SOCS3 protein increased in cachectic females but decreased in cachectic males. IL-6 overexpression did not affect cachexia progression in female Apc Min/+ mice. Our results indicate that female Apc Min/+ mice undergo cachexia progression that is at least initially IL-6-independent. Future studies in the female will need to determine mechanisms underlying regulation of IL-6 response and cachexia induction.
Keywords: IL-6r, STAT3, SOCS3, skeletal muscle, Apc Min/+
1. Introduction
Cachexia is a devastating condition that occurs secondary to several chronic diseases, including AIDS, COPD, chronic renal failure, and many forms of cancer (1). There is no FDA-approved treatment for cachexia, though it occurs in 30-50% of cancers (1-3) and has an annual mortality rate of 80% (1); the investigation into its etiology and progression is therefore essential. The most recent definition of cachexia includes an unintentional 5% weight loss over twelve months, comprising the loss of muscle and fat (1). Other abnormalities associated with cachexia include anemia, fatigue, muscle weakness, increased plasma triglycerides and inflammatory markers, and insulin resistance (1). There has been considerable improvement in our understanding of the regulation of cancer cachexia progression due to research employing male mouse models (4-8). However, there is evidence that sex differences exist in the development of cachexia in rodents (9). Additionally, sex differences have been observed in the loss of muscle strength associated with cachexia severity in humans (10). Although there is clear evidence that cachexia occurs in both males and females, the fundamental differences in the pathophysiology due to sex and underlying mechanisms are unknown.
Systemic inflammation related to cancer is thought to be a mediator of cachexia, as several pro-inflammatory pathways are enhanced during the progression of cachexia (11, 12). Sex hormones can modulate the inflammatory response to a variety of stimuli (13-15). Specifically, estrogen is known to inhibit NfκB and tumor necrosis factor α (TNFα) signaling (13, 16), C-reactive protein (CRP)-induced interleukin-6 (IL-6) production (17), and signal transducer and activator of transcription-3 (STAT3) signaling downstream of IL-6 (18). In several rodent models of cachexia and some human cancers, IL-6 is associated with the development of muscle wasting and body weight loss (6, 8, 19-21). Classical IL-6 signaling involves binding of the cytokine to the membrane-bound IL-6 receptor (mIL-6r) on target tissues, which include hepatocytes, immune cells, and skeletal muscle (22-24). mIL-6r is a heterodimer comprising the ligand-specific gp80 unit and the signal-transducing gp130 unit (25). Activation of mIL-6r induces downstream activation of many signaling pathways, including JAK/STAT, p38, and ERK (23, 26-28). Several of these pathways have been implicated in the regulation of muscle mass loss during cancer cachexia (26, 29).
The Apc Min/+ mouse is genetically predisposed to develop intestinal tumors and becomes cachectic secondary to the tumor burden (4, 30). Our lab initially characterized cachexia in retired female Apc Min/+ breeders (31); however, the progression and etiology of cachexia in the female Apc Min/+ mouse has not been described. Development of cachexia in the male Apc Min/+mouse has an established IL-6 dependence (6-8, 30, 32-34). Administration of an IL-6 receptor antibody to cachectic male Apc Min/+ mice can attenuate further cachexia progression (7). When IL-6 is systemically overexpressed in the male, more body weight is lost and cachexia is more severe, indicating a causative role (6, 8, 26). However, the response of female Apc Min/+ mice to IL-6 overexpression has not been examined. Sex differences have been noted in the inflammatory milieu of humans (35, 36) and mice (14, 37) under multiple pathological circumstances. Additionally, estrogen has been shown to inhibit IL-6 transcription and signaling in several tissues (17, 18, 37-39), which may also lead to sex differences in IL-6 response during cancer cachexia progression. Therefore, the purpose of the present study was to examine the relationship of circulating IL-6 to cancer cachexia progression in the female Apc Min/+ mouse. Our hypothesis is that cachexia progression in the female Apc Min/+ mouse would not be associated with increased circulating IL-6 levels as has been reported in the male. This hypothesis was tested through three experiments. The first experiment followed a cohort of female and male Apc Min/+ mice to 18 weeks of age, at which point the association between cachexia severity and circulating IL-6 level was determined. In the second experiment, 12-week old female Apc Min/+ mice had IL-6 systemically overexpressed for two weeks (until 14 weeks of age) to determine if supraphysiological IL-6 levels could induce cachexia as we have previously shown in the male (8, 33, 40). In the third experiment, 15-week old female Apc Min/+ mice had IL-6 systemically overexpressed for three weeks (until 18 weeks of age) to determine if supraphysiological IL-6 levels could accelerate cachexia progression as we have previously shown in the male (30).
2. Methods
2.1 Animals
Female Apc Min/+mice (N=32), male Apc Min/+ mice (N=12), female C57BL/6 mice (N=6), and male C57BL/6 mice (N=6) were bred and maintained at the University of South Carolina Animal Resource Facility. ApcMin/+ mice used were offspring from breeders originally purchased from Jackson Labs (Bar Harbor, ME, USA). Male and female mice were taken during the three-month period from a standing inbred ApcMin/+ breeding colony. ApcMin/+ mice used were on a C57BL/6 background. Mice were kept on a 12:12h light/dark cycle beginning at 7:00 AM and were given unrestricted access to standard rodent chow (Harlan Teklad Rodent Diet, #8604). Mice were weighed weekly. Blood was collected by retro-orbital eye bleed at 12, 14, 16, 18, and 20 weeks for IL-6 analysis. All experiments were approved by the University of South Carolina’s Institutional Animal Care and Use Committee.
2.2 Procedures
Three experiments were performed. Experiment 1 examined the progression of cachexia. Female Apc Min/+mice (n=18) were sacrificed at 18 weeks of age. Male Apc Min/+ mice were sacrificed at 18-20 weeks of age. Female (n=6) and male (n=6) C57BL/6 (B6) were sacrificed at 18 weeks of age as non-cancer controls. Prior to sacrifice, blood was taken for analysis of IL-6 levels. Experiment 2 examined 2 weeks of IL-6 over-expression in 12 week-old, weight stable female Apc Min/+ mice as we have previously completed in the male (40). Female Apc Min/+ mice were electroporated with a control plasmid or with an IL-6 overexpression plasmid (n=4 per group) at 12 weeks and were sacrificed at 14 weeks. Blood was taken at sacrifice (post-treatment) to determine plasma IL-6 levels. Mice from this experiment were not used for any other analysis. Experiment 3 examined IL-6 over-expression in 15-week-old female Apc Min/+ mice corresponding with the initiation of cachexia, a time course we have previously examined in the male (30). At 15 weeks of age mice were randomly separated into IL-6 over-expression (n=6), control plasmid (N=3), or non-electroporated control (n=3) treatment groups. All female Apc Min/+ mice were sacrificed at 18 weeks of age. Blood was taken at sacrifice (post-treatment) for determination of plasma IL-6 levels. No differences were found in body weight (p=0.78) or muscle mass (p=0.36) between control plasmid and non-electroporated mice. Therefore, the control plasmid and non-electroporated female Apc Min/+ mice were then pooled for analysis. Additionally, these 6 mice are in the cohort of 18 female Apc Min/+ mice related to Experiment 1. In all experiments, blood, inguinal fat, hindlimb muscles, and spleen were removed at the time of sacrifice. Hindlimb muscle mass measurements comprise the sum of left soleus, plantaris, gastrocnemius, tibialis anterior, extensor digitalis longus, and quadriceps masses.
2.3 IL-6 overexpression by electroporation
In vivo intramuscular electroporation of an IL-6 plasmid was used to increase circulating IL-6 levels in mice as previously described (41). The right quadriceps muscle was used to synthesize and secrete exogenous IL-6 into circulation from the injected expression plasmid, and was not used for any analyses in the study. The gastrocnemius muscle used in the study was not subjected to electroporation. Briefly, mice were injected with 50 μg of IL-6 plasmid driven by the CMV promoter, or control plasmid (pV1J), into the right quadriceps muscle. Mice were anesthetized with a 2% mixture of isoflurane (IsoSol, VEDCO, St. Joseph, MO, USA) and oxygen (1 L/min). The leg was shaved, and a small incision was made over the quadriceps muscle. Fat was dissected away from the muscle, and the plasmid was injected in a 50 μl volume of sterile phosphate-buffered saline (PBS). A series of eight 50 ms, 100 V pulses was used to promote uptake of the plasmid into myonuclei, and the incision was closed with a wound clip. In Experiment 2, electroporation was performed at 12 weeks and mice were sacrificed at 14 weeks of age. In Experiment 3, electroporation was performed at 15 weeks and mice were sacrificed at 18 weeks of age.
2.4 RNA isolation and RT-PCR
RNA isolation, cDNA synthesis, and real-time PCR were performed as previously described (42). Reagents from Applied Biosystems (Foster City, CA, USA) were used. Briefly, proximal gastrocnemius muscle was homogenized in TRIzol (Life Technologies, Grand Island, NY, USA) and mixed with chloroform, then centrifuged. The RNA phase was removed and washed several times with ethanol in DEPC-treated water. cDNA was synthesized using 1 μg of RNA. RT-PCR was performed on an Applied Biosystems 7300 thermocycler. A Taqman gene expression assay was used to determine IL-6 mRNA expression (Life Technologies). IL-6 receptor (forward, 5’-CTGCCAGTATTCTCAGCAGCTG-3’, and reverse, 5’-CCTGTGTGGGGTTCCAGAGGAT-3’); SOCS3 (forward, 5’-TGCAGGAGAGCTGATTCTAC-3’, and reverse, 5’-TGACGCTCAACGTGAAGAAG-3’); gp130 and GAPDH primer sequences have been published elsewhere (43).
2.5 Western blotting
Western blots were performed as described previously (7, 44). Briefly, gastrocnemius muscle samples were run on 6-8% acrylamide gels and transferred overnight to PVDF membrane (Thermo Scientific, Waltham, MA, USA). After transfer, Ponceau stains were imaged to verify equal loading. The membrane was blocked for 1 hour in 5% milk-TBS-Tween 20, and incubated with primary antibodies at 1:2000 dilution overnight at 4°C. After several washes, membranes were incubated with secondary antibodies at 1:2000 dilution for 1 hour. Blots were visualized with WesternBright ECL (BioExpress, Kaysville, UT, USA). SOCS3 antibody was obtained from Abcam (Cambridge, MA, USA). STAT3, phopho-STAT3 (Y705), anti-mouse IgG, and anti-rabbit IgG antibodies were obtained from Cell Signaling (Danvers, MA, USA).
2.6 Grip strength measurement
Grip strength measurements were determined as previously described (8). Mice underwent 2 sets of 5 grip strength trials. The first mouse was removed from its cage, held firmly by the base of the tail, and allowed to grasp the top of the grate attached to the force gauge (Chatillon, Largo, FL, USA) with its paws. The mouse was firmly pulled down the grate, and grip strength was recorded in Newtons. The mouse completed the 5 trials of the first set and was returned to its cage. Each mouse tested went through the same procedure for its first trial, and mice were cycled through in the same order for the second trial.
2.7 Fasting glucose
Mice were fasted for five hours prior to blood glucose measurements. Blood glucose measurements were performed using a handheld glucometer (Bayer CONTOUR®, Whippany, NJ, USA ) according to the manufacture’s instructions.
2.8 IL-6 Enzyme-linked immunosorbent assay
Plasma IL-6 concentrations were determined as previously described (8). A commercial IL-6 ELISA kit was obtained from Invitrogen (Fredrick, MD, USA) and the manufacturer’s protocol was followed. Briefly, blood was centrifuged after sacrifice; plasma was removed and stored at −80°C until analysis. A Costar clear 96-well plate (Corning, NY, USA) was coated with IL-6 capture antibody and allowed to incubate overnight. The next morning, the plate was blocked with assay diluent buffer. The plate was washed; plasma samples and IL-6 standards were diluted with assay diluent buffer and added in duplicate to the plate. The plate was again washed and sAV-HRP reagent was added to wells. After several washes, TMB substrate was added to wells and color was allowed to develop. The reaction was stopped with sulfuric acid and absorbance was read in a BioRad iMark plate reader (Hercules, CA, USA) at 450 nm.
2.9 IL-6R Enzyme-linked immunosorbent assay
Muscle IL-6R protein levels were determined using a DuoSet ELISA kit (R&D Systems, Minneapolis, MN, USA) as previously described (45). Briefly, gastrocnemius muscle tissue was homogenized in buffer containing 50mM HEPES, 4mM EGTA, 10mM EDTA, 15mM Na4P2O7, 100mM β-glycerophosphate, 25mM NaF, 5mM NaVO4, 0.1% Triton-X, and 0.1% protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). A Costar 96-well plate (Corning) was coated with IL-6r capture antibody and allowed to incubate overnight. The next morning, the plate was blocked with assay diluent. After washing, IL-6r standards and 50-500 μg protein of samples were added to wells in duplicate. The plate was again washed and detection antibody was added. Another wash was performed and streptavidin-HRP was added to the plate. After a final wash, substrate solution was added to the plate and color was allowed to develop before the addition of stop solution. Absorbance was read at 450 and 570 nm in a BioRad iMark plate reader (BioRad). Standard and sample concentration was determined using a third-order polynomial curve.
2.10 Intestinal polyp quantification
Quantification of intestinal polyps was determined as previously described (46). Briefly, intestinal sections were fixed in formalin at time of sacrifice. At time of analysis, sections were rinsed in deionized water and dipped briefly in 0.1% methylene blue. Polyps from segment 4 were counted under a dissecting microscope; it has been determined that tumor number in segment 4 is representative of total tumor number (47).
2.11 Statistical analysis
All results are reported as means ± SEM. Differences between degrees of cachexia severity were analyzed by one-way ANOVA using Tukey post hoc test where appropriate. Differences between sexes and genotypes were determined by two-way ANOVA with Student-Newman-Keuls post hoc test where appropriate. Correlations were determined by Pearson’s test for correlation. Differences between 12-14 week and 15-18 week IL-6 treatment groups were determined by Student’s t-test. Level of significance was set at 0.05.
3. Results
3.1 Differential cachexia progression in male and female Apc Min/+ mice (Experiment 1)
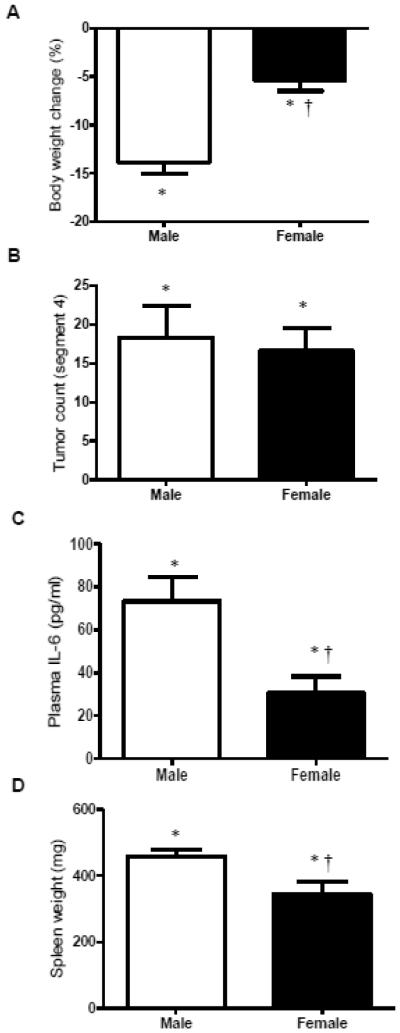
Both male and female Apc Min/+ mice undergo varying degrees of cachexia; however, efforts to characterize progression have focused overwhelmingly on the male. Our lab has previously described the male (7, 8, 19), but limited data has been presented related to the female response (48). Both male (n=12) and female (n=18) Apc Min/+ mice lost a significant amount of body weight from their peak weight (Figure 1A); however, males lost a greater percentage of body weight than females (p<0.0001). Apc Min/+ mice generally have the greatest density of tumors in segment 4 of the small intestine, and the number of tumors in this segment correlates with total tumor number (47). The number of tumors in male and female Apc Min/+ mice, though significantly higher than male and female B6 mice (p<0.0001), did not differ (Figure 1B), indicating that the differences in body weight loss were not due to tumor burden. Regardless of sex, Apc Min/+ mice had significantly higher circulating IL-6 levels than B6 mice (p<0.0001). Despite the consistency in tumor burden, male Apc Min/+ mice had higher circulating IL-6 levels than females (p<0.0001) (Figure 1C). Both male and female Apc Min/+ mice had significantly larger spleens than B6 mice of the same sex (p<0.0001), indicating a higher level of systemic inflammation (Figure 1D); however, male Apc Min/+ had larger spleens than females (p=0.03).
Figure 1. Characteristics of male and female Apc Min/+ mice (Experiment 1).
A) Average body weight change from peak weight in male and female ApcMin/+ mice (n=12 males, n=18 females). Male Apc Min/+ mice had more body weight loss than female Apc Min/+ mice. B) There is no difference in the average number of tumors in segment 4 of the small intestine between male and female Apc Min/+ mice. C) Average level of plasma IL-6 (pg/ml). D) Average spleen weight in male and female Apc Min/+ mice. Male Apc Min/+ mice had larger spleens than female Apc Min/+ mice. * indicates significantly different from B6 mice of the same sex (p<0.05); † indicates significant difference from male Apc Min/+ (p<0.05).
3.2 Progression of cachexia in female Apc Min/+ mice (Experiment 1)
To determine the changes seen with progression of cachexia in the female Apc Min/+ mouse, a cohort of 18 week old female Apc Min/+ mice (n=18) were stratified based on body weight change from peak (Table 1). The cohort was divided based on body weight loss; “Weight Stable” comprises mice with less than 2% body weight loss from peak weight (n=6), “Initial Cachexia” comprises mice with 2-9% body weight loss (n=6), and “Cachexia” comprises mice with greater than 10% body weight loss (n=6). Body weight loss ranged from 0% to −15.8%; ~30% of the mice were classified as “cachectic,” with severe body weight loss, fat and muscle mass loss, and decreased muscle strength (Table 1). As expected, body weight change from peak weight was significantly different between all groups (Table 1). Spleen weight did not differ between weight stable and the initiation of cachexia. Cachectic female Apc Min/+ mice had significantly larger spleens than either of the other groups (p=0.01) (Table 1). Segment 4 tumor count did not differ between weight stable and the initiation of cachexia. Cachectic Apc Min/+ mice had significantly more tumors than either of the other groups (p=0.002) (Table 1). This indicates a relationship between tumor burden and cachexia development in the female that has also been reported in the male (4). Hindlimb muscle mass and inguinal fat mass did not differ between weight stable and initiation of cachexia. Cachectic Apc Min/+ females had significantly less hindlimb muscle mass and inguinal fat mass than weight stable or the initial cachexia groups (Table 1). Importantly, there were no differences in circulating IL-6 levels between any of the groups (Table 1). Cachectic Apc Min/+ females had significantly lower voluntary forelimb grip strength than either of the other groups, though this difference was eliminated when normalized to body weight (Table 1).
Table 1.
Characteristics of female Apc Min/+ mice (Experiment 1).
| Female Apc Min/+ mice Weight stable |
Initial Cachexia | Cachexia | |
|---|---|---|---|
| n | 6 | 6 | 6 |
| Weight change (%) | −1.1 ± 0.5% | −4.2 ± 0.4%* | −11.0 ± 1.3%*† |
| Spleen weight (mg) | 250.5 ± 50.2 | 310.5 ± 64.4 | 485.7 ± 37.7*† |
| Tumor count (seg 4) | 7.8 ± 2.2 | 6.8 ± 3.0 | 26.3 ± 1.0*† |
| Hindlimb muscle mass (mg) | 300.2 ± 9.9 | 299.7 ± 18.9 | 226.8 ± 13.4*† |
| Inguinal fat mass (mg) | 224.0 ± 34.7 | 153.8 ± 35.2 | 14.8 ± 6.6*† |
| Plasma IL-6 (pg/ml) | 17.6 ± 6.7 | 34.4 ± 18.9 | 39.3 ± 13.1 |
| Grip strength (N) | 1.9 ± 0.2 | 1.9 ± 0.1 | 1.6 ± 0.1*† |
| Grip strength/Body weight (N/g) |
0.092 ± 0.008 | 0.098 ± 0.006 | 0.084 ± 0.003 |
Weight change (%): Percent weight change from peak body weight to weight at sacrifice [(weight at sacrifice-peak weight)/peak weight]. Hindlimb muscle mass comprises left soleus, plantaris, gastrocnemius, tibialis anterior, extensor digitalis longus, and quadriceps mass. n, number. seg 4, segment 4 of the small intestine. mg, milligrams. pg, picograms. ml, milliliters. N, Newtons.
indicates significantly different from weight stable (p<0.05).
indicates significantly different from initial cachexia (p<0.05).
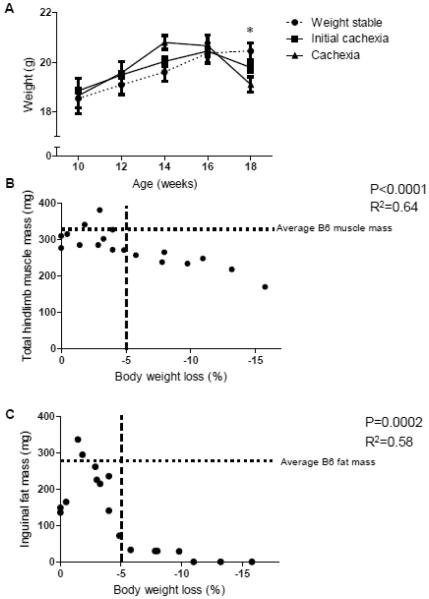
All female ApcMin/+ mice continued to gain weight until 16 weeks of age; differences in body weight between groups are only seen after this point (Figure 2A; p=0.016). Across all female Apc Min/+ mice, there was a significant relationship between hindlimb muscle mass and body weight loss (Figure 2B; p<0.0001, R2=0.64). Inguinal fat mass demonstrated a curvilinear relationship (p=0.0002, R2=0.58) with body weight loss, with fat mass having the most direct relationship with body loss during the initiation of cachexia (Figure 2C). Taken together, this indicates that during the progression of cachexia in the female Apc Min/+ mouse the loss of inguinal fat mass occurs early, while muscle mass loss demonstrates a consistent decline over time.
Figure 2. Cachexia progression in female Apc Min/+ mice (Experiment 1).
A) Average body weight from 10-18 weeks for weight stable, initial cachexia, and cachexia groups (n=6 per group). Groups are significantly different at 18 weeks of age (p=0.016, repeated measures ANOVA). B) Hindlimb muscle mass has a negative correlation with body weight loss during cachexia in the female Apc Min/+ mouse (Pearson’s correlation, p<0.0001). C) Inguinal fat mass has a negative correlation with body weight loss during cachexia in the female Apc Min/+ mouse (Pearson’s correlation, p=0.0002). Horizontal dotted lines in B and C show average B6 muscle and fat masses. Dotted line at -5% body weight loss intended to show the contrast between fat and muscle loss before and after 5% body weight loss. * indicates significant differences between groups (p<0.05).
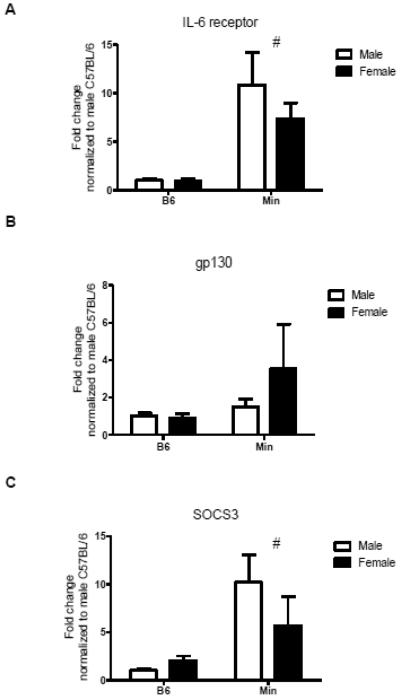
3.3 Muscle IL-6 signaling-associated mRNA expression in male and female Apc Min/+ mice (Experiment 1)
The levels of circulating IL-6 in female Apc Min/+ mice were not associated with body weight loss, hindlimb muscle mass, or inguinal fat mass (Table 2). However, muscle mRNA expression of IL-6 was significantly correlated with both body weight loss and hindlimb muscle mass (Table 2). There was a main effect of cachexia independent of sex to increase muscle IL-6 receptor mRNA expression (p=0.043; Figure 3A). In female Apc Min/+ mice, muscle IL-6 receptor mRNA expression was significantly correlated with muscle gp130 mRNA and SOCS3 mRNA expression (Table 2). Neither sex nor cachexia had a significant effect on muscle gp130 mRNA expression (Figure 3B). Although gp130 mRNA expression was not associated with body weight loss in female Apc Min/+ mice, it was significantly associated with muscle SOCS3 mRNA expression (Table 2). Cachexia increased muscle SOCS3 mRNA expression independent of sex (p=0.01; Figure 3C). However, muscle SOCS3 mRNA expression was not correlated with body weight loss in female Apc Min/+ mice (Table 2).
Table 2.
Relationships between circulating IL-6 and skeletal muscle signaling during cachexia development (Experiment 1).
| Muscle mRNA expression correlations |
| Factor 1 | Factor 2 | R2 | p |
|---|---|---|---|
| Body weight loss | 0.004 | 0.802 | |
| Hindlimb muscle mass | 0.070 | 0.289 | |
| Circulating plasma IL-6 (pg/ml) | Inguinal fat mass | 0.004 | 0.797 |
| IL-6 mRNA | 0.041 | 0.436 | |
| IL-6 receptor mRNA | 0.038 | 0.468 | |
| gp130 mRNA | 0.014 | 0.701 | |
| SOCS3 mRNA | 0.045 | 0.428 | |
|
| |||
| Body weight loss | 0.380 | 0.008* | |
| Muscle | Hindlimb muscle mass | 0.315 | 0.019* |
| IL-6 mRNA expression | Inguinal fat mass | 0.151 | 0.124 |
| IL-6 receptor mRNA | 0.183 | 0.112 | |
| gp130 mRNA | 0.000 | 0.981 | |
| SOCS3 mRNA | 0.224 | 0.075 | |
|
| |||
| Body weight loss | 0.189 | 0.092 | |
| Muscle | Hindlimb muscle mass | 0.142 | 0.150 |
| IL-6 receptor mRNA expression | Inguinal fat mass | 0.218 | 0.068 |
| IL-6 receptor protein | 0.246 | 0.060 | |
| gp130 mRNA | 0.769 | 0.000* | |
| SOCS3 mRNA | 0.913 | 0.000* | |
|
| |||
| Body weight loss | 0.200 | 0.125 | |
| Muscle | Hindlimb muscle mass | 0.130 | 0.226 |
| gp130 mRNA expression | Inguinal fat mass | 0.151 | 0.190 |
| SOCS3 mRNA | 0.757 | 0.000* | |
|
| |||
| Body weight loss | 0.212 | 0.110 | |
| Muscle | Hindlimb muscle mass | 0.124 | 0.181 |
| SOCS3 mRNA expression | Inguinal fat mass | 0.045 | 0.428 |
| Muscle protein expression correlations |
| Factor 1 | Factor 2 | R2 | p |
|---|---|---|---|
| IL-6 receptor protein | 0.098 | 0.221 | |
| STAT3 | |||
| Circulating plasma IL-6 (pg/ml) | phosphorylation/STAT3 | 0.008 | 0.728 |
| Body weight loss | 0.005 | 0.795 | |
|
| |||
| Muscle | Hindlimb muscle mass | 0.007 | 0.745 |
| IL-6 receptor protein (pg/ug) | Inguinal fat mass | 0.001 | 0.913 |
| STAT3 | |||
| phosphorylation/STAT3 | 0.001 | 0.912 | |
|
| |||
| Muscle | Body weight loss | 0.205 | 0.059 |
| pSTAT3/STAT3 | Hindlimb muscle mass | 0.385 | 0.006* |
| Inguinal fat mass | 0.034 | 0.465 | |
Correlations calculated in 18-week female Apc Min/+ mice of varying degrees of cachexia (n=18). R2, coefficient of determination; p, p-value. Correlations determined by Pearson’s correlation. mRNA levels are expressed as fold change from female C57BL/6 mice.
indicates significant correlation between factors (p<0.05).
Figure 3. Muscle IL-6 signaling-associated mRNA levels (Experiment 1).
A) IL-6 receptor mRNA expression is significantly higher in male and female Apc Min/+ mice than B6 mice. B) There is no difference in gp130 mRNA expression between male or female Apc Min/+ mice or B6 mice. C) SOCS3 mRNA expression is higher in male and female Apc Min/+ mice than B6 mice. All differences were analyzed by two-way ANOVA. # indicates main effect of Apc Min/+ (p<0.05).
3.4 Muscle IL-6 signaling-associated protein expression in male and female Apc Min/+ mice (Experiment 1)
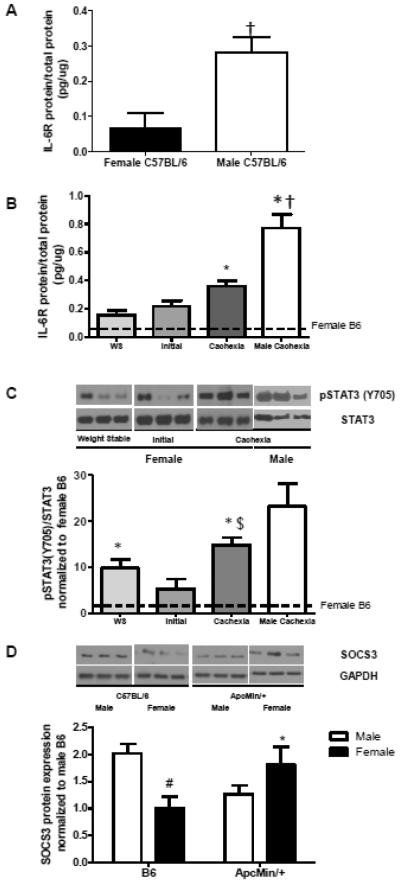
Muscle IL-6 receptor protein expression was examined in gastrocnemius muscle of male and female b6 and Apc Min/+ mice. Interestingly, male B6 mice have significantly higher levels of muscle IL-6r than female B6 mice (Figure 4A; p=0.006). In female Apc Min/+ mice, muscle IL-6r increases with cachexia severity (Figure 4B; p=0.002); however, cachectic male Apc Min/+ mice have significantly higher expression than cachectic females (Figure 4B; p=0.02).
Figure 4. Muscle IL-6 signaling-associated protein levels (Experiment 1).
A) Muscle IL-6 receptor levels (pg/ug total protein) are significantly higher in male B6 than in female B6 mice (0.28 ± 0.04 vs 0.07 ± 0.04, p=0.01, Student’s t-test) B) Muscle IL-6 receptor levels (pg/ug total protein) increases with cachexia severity in female Apc Min/+ mice (one-way ANOVA, p<0.0001). Cachectic levels were different from all other groups (p<0.05, Tukey post hoc). IL-6 receptor levels are significantly higher in male cachexia than female cachexia ± 0.11 vs. 0.42 ± 0.09, p=0.02, Student’s t-test). C) Upper: Representative blots shown. All samples per group were run on a gel with all weight stable samples, and then normalized during analysis. Lower: Quantification of pSTAT3/STAT3 blots. The ratio of phosphorylated STAT3 (Y705)/STAT3 increases with cachexia severity in the female Apc Min/+ mouse (one-way ANOVA, p=0.01). There is no difference in phosphorylation of STAT3 (Y705) between cachectic females and cachectic males (p=0.154, Student’s t-test). No differences were seen between groups in total STAT3. D) Upper: Representative blots shown. All ApcMin/+ samples were run on a gel with male B6 samples for normalization. Lower: Quantification of SOCS3 blots. There is a significant interaction of sex by genotype in muscle SOCS3 protein expression (p=0.006, two-way ANOVA). Female B6 mice had significantly lower SOCS3 expression than male B6 mice (p=0.015, Student-Newman-Keuls post hoc). Female ApcMin/+ mice had significantly higher SOCS3 expression than female B6 (p=0.029, Student-Newman-Keuls post hoc), while male ApcMin/+ mice showed a strong trend towards lower SOCS3 expression than male B6 (p=0.053, Student-Newman-Keuls post hoc). $ indicates significant difference from initial cachexia (p=0.005, Tukey post hoc); * indicates significantly different from same-sex B6; # indicates significant difference from male B6; † indicates significantly different from female. Dotted lines on graphs indicate B6 levels.
Muscle STAT3 phosphorylation was examined during the progression of cachexia in female Apc Min/+ mice (Figure 4C). Muscle STAT3 phosphorylation (Y705) was normalized to female B6 levels of phosphorylation. Total STAT3 muscle expression did not change with the progression of cachexia (B6: 1 ± 0.05; Weight stable: 1.03 ± 0.03; Cachexia: 1.02 ± 0.07). Muscle STAT3 phosphorylation was significantly different across groups (p=0.010). Muscle STAT3 phosphorylation significantly increased in cachectic muscle when compared to the initially cachectic female Apc Min/+ mice (p=0.049). The induction of STAT3 phosphorylation had a strong trend towards correlation with body weight loss in female Apc Min/+ mice, but there was no association with circulating IL-6 levels (Table 2). There was no difference in muscle STAT3 phosphorylation between female and male cachectic muscle (Figure 4C). These results demonstrate that muscle STAT3 phosphorylation is not related to circulating IL-6 level during the progression of cachexia in female Apc Min/+ mice.
SOCS3 protein expression was examined in muscle of male and female B6 and cachectic Apc Min/+ mice. As with the IL-6 receptor, male B6 mice had significantly higher levels of SOCS3 protein than female B6 mice (Figure 4D; p=0.015). However, cachexia had differential effects on SOCS3 protein expression between the sexes. SOCS3 protein expression was significantly higher in female Apc Min/+ mice than female B6 (Figure 4D; p=0.03); however, there was a strong trend towards a decrease in SOCS3 protein with cachexia in the male versus the B6 male (Figure 4D; p=0.053).
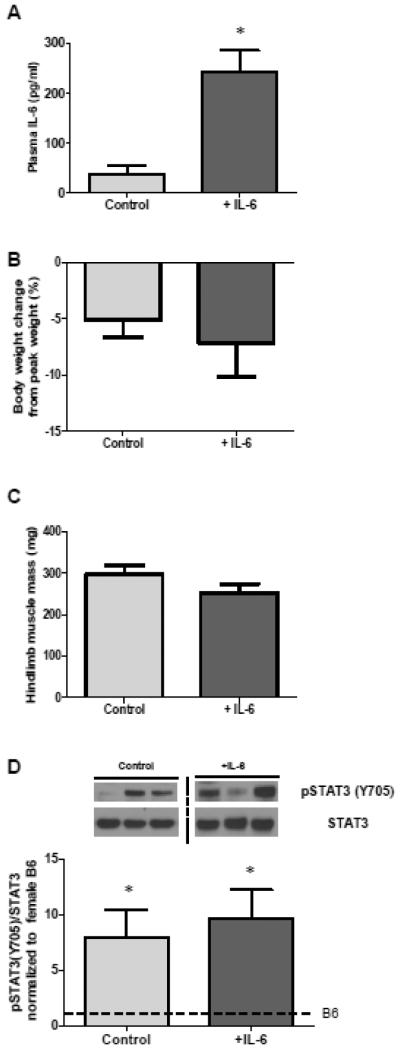
3.4 Effect of IL-6 overexpression on cachexia initiation in Apc Min/+ females (Experiment 2)
We have previously shown that IL-6 overexpression in male Apc Min/+ mice is sufficient to initiate or accelerate cachexia progression (8, 30). To determine the effect of IL-6 on the initiation of cachexia in the female Apc Min/+ mouse, we overexpressed IL-6 from 12-14 weeks of age, when tumor burden is fully present but cachexia has not been initiated. Plasma IL-6 levels were significantly higher in mice treated with the IL-6 overexpression vector (Table 3). There were no differences in body weight between treatments groups at the initiation of IL-6 overexpression, and IL-6 treatment did not alter body weights after two weeks (Table 3). In spite of IL-6 overexpression, 12-14 week-old female Apc Min/+ mice continued to grow (Table 3). Inguinal fat mass and hindlimb muscle mass did not significantly change in response to IL-6 overexpression (Table 3). Tumor number was quantified at the end of the study. Although tumor numbers were variable, there were no significant differences between treatment groups (not shown).
Table 3.
Characteristics of 14-week and 18-week female Apc Min/+ mice with IL-6 overexpression (Experiments 2 and 3).
| Female Apc Min/+ | mice | ||
|---|---|---|---|
|
| |||
| IL-6 overexpression | − | + | |
| 12-14 week treatment | p-value | ||
| n | 4 | 4 | |
| 12-week body weight (g) | 20.0 ± 0.7 | 19.0 ± 0.7 | 0.38 |
| 14-week body weight (g) | 20.3 ± 0.6 | 19.5 ± 0.7 | 0.49 |
| Spleen weight (mg) | 312.3 ± 35.0 | 336.5 ± 22.3 | 0.59 |
| Fasting Glucose (mg/dl) | 140.7 ± 1.3 | 171.0 ± 28.0 | 0.34 |
| Plasma IL-6 (pg/ml) | 1.5 ± 1.5 | 65.0 ± 18.1* | 0.02 |
| Grip strength (N) | 2.1 ± 0.1 | 2.0 ± 0.1 | 0.67 |
|
| |||
| 15-18 week treatment | |||
| n | 6 | 6 | |
| Peak body weight (g) | 20.4 ± 0.3 | 19.4 ± 0.6 | 0.21 |
| 15-week body weight (g) | 19.6 ± 0.3 | 18.8 ± 0.7 | 0.41 |
| 18-week body weight (g) | 19.3 ± 0.3 | 17.9 ± 0.6 | 0.06 |
| Inguinal fat mass (mg) | 130.3 ± 39.9 | 65.5 ± 36.3 | 0.27 |
| Spleen weight (mg) | 318.7 ± 77.6 | 367.3 ± 75.7 | 0.68 |
| Fasting Glucose (mg/dl) | 119.3 ± 6.8 | 111.3 ± 4.7 | 0.37 |
| Grip strength (N) | 1.9 ± 0.1 | 1.9 ± 0.1 | 0.89 |
Female Apc Min/+ mice had the right quadriceps muscle electroporated with control plasmid or IL-6 overexpression plasmid. G, grams; mg, milligrams; dl, deciliter; pg, picograms; ml, milliliters; seg 4, segment 4 of the small intestine; N, Newtons.
denotes significant difference from control group (p<0.05).
3.5 Effect of IL-6 overexpression on cachexia progression in Apc Min/+ females (Experiment 3)
To determine the effect of IL-6 on cachexia progression in the female Apc Min/+ mouse, we overexpressed IL-6 between 15 and 18 weeks of age, which generally corresponds with the development of cachexia. Plasma IL-6 levels were significantly higher in mice overexpressing IL-6 (p=0.002, Figure 5A), though it is important to note that the control mice had significantly higher levels of IL-6 than B6 females (p=0.01, data not shown). IL-6 overexpression above this cancer-induced increase did not induce an acceleration of body weight loss in female Apc Min/+ mice that had initiated cachexia (Table 3, Figure 5B). It is unlikely that tumor counts would have been affected by IL-6 overexpression, as polyp number stabilizes at approximately 12 weeks of age (32). Tumor number was highly variable, but there were no differences between treatment groups in the number of intestine segment 4 tumors (not shown). Spleen weight was not further increased with IL-6 overexpression (Table 3). Hindlimb muscle mass and inguinal fat mass loss were not accelerated by IL-6 overexpression in 18 week female Apc Min/+ mice (Figure 5B, Table 3). Importantly, muscle STAT3 phosphorylation was not increased by systemic IL-6 overexpression (Figure 5D). These results are in direct contrast to previous work by our lab with the male Apc Min/+ mouse, which showed decreases in body weight and muscle mass, and an increase in spleen weight as a result of IL-6 overexpression in addition to the cancer-induced levels between 16 and 18 weeks (30).
Figure 5. Effect of IL-6 overexpression in 18 week-old female Apc Min/+ mice (Experiment 3).
A) Female Apc Min/+ mice that had IL-6 systemically overexpressed from 15-18 weeks of age had higher circulating plasma IL-6 than control mice (p=0.002). B) There is no difference in body weight change from peak body weight (%) between female Apc Min/+ mice that have had IL-6 systemically overexpressed from 15-18 weeks of age and control female Apc Min/+ mice at 18 weeks of age. C) There is no difference in hindlimb muscle mass between female Apc Min/+ mice that have had IL-6 systemically overexpressed from 15-18 weeks of age and control female Apc Min/+ mice at 18 weeks of age. D) There is no difference in the ratio of phosphorylation of STAT3/STAT3 between female Apc Min/+ mice that have had IL-6 systemically overexpressed from 15-18 weeks of age and control female Apc Min/+ mice at 18 weeks of age (p=0.662, Student’s t-test). Upper: Representative blots showing pSTAT3 and STAT3 expression. Dashed line indicates that samples were run on same gel, but separated by other samples. Lower: Quantification of pSTAT3/STAT3 expression. Dotted line on graph indicates female B6 level of phosphorylation/total STAT3. * indicates pSTAT3/STAT3 levels were significantly higher than B6 (p=0.008).
4. Discussion
Circulating IL-6 has been demonstrated to be a regulator of cancer cachexia progression in male Apc Min/+ mice. Circulating IL-6 is a known cachectic factor in human cancer populations as well (21, 49-51). Treatments for cancer cachexia that target IL-6 signaling are currently being examined (52, 53). However, it is known that sex differences exist in inflammatory responses (13, 14, 54, 55), and sex differences have been reported in cancer cachexia progression in both rodents and humans (9, 10, 27). We therefore sought to determine the relationship between circulating IL-6 and cachexia progression the female Apc Min/+ mouse. We have demonstrated for the first time that circulating IL-6 level is not associated with the degree of cachexia in the female Apc Min/+ mouse, as IL-6 levels were similar between weight stable and cachectic mice. We also report that, unlike the male (8, 30), IL-6 overexpression above cancer-induced levels does not induce or accelerate cachexia progression in female Apc Min/+ mice. Additionally, we report that sex alters muscle IL-6 transcription during cachexia. Female muscle IL-6 mRNA expression increased with cachexia severity, which contrasts with male cachexia progression (7, 30). We found that the female muscle may become more sensitive to circulating IL-6 levels during the progression of cachexia, since muscle IL-6r protein and phosphorylated STAT3 increased with severe cachexia. Though the female Apc Min/+ mouse loses both muscle and fat with the progression of cachexia, the development of this loss occurs differently from that reported in the male (7). The male Apc Min/+ mouse initiates cachexia with the rapid loss of both muscle and fat (7). We provide evidence that the initiation of cachexia in the female Apc Min/+ mouse involves a rapid loss of fat mass, while the loss of muscle mass occurs later in the development of cachexia. These data clearly show that sex influences the regulation of cachexia progression in the Apc Min/+ mouse.
As therapies targeting IL-6 for the treatment of cancer cachexia are gaining traction (52, 53), it is of utmost importance that basic research support this modality. The majority of investigations into human cancer cachexia do not separately analyze men and women (49-51, 56), which does not allow for the determination of sex differences in IL-6 levels. Recent investigation into single nucleotide polymorphisms (SNPs) in the IL-6 and IL-6 receptor promoter sequences has shown that certain SNPs affect levels of circulating IL-6 and soluble IL-6r; however, there are no differences between the sexes in allele frequency, indicating that sex differences do not likely occur at the transcriptional level (57). While we have consistently found that cachexia development in the male Apc Min/+ mouse is directly related to tumor burden and the level of circulating IL-6 (6, 8, 19, 30, 33, 40, 58), this relationship has not been firmly established in the female. Though we found that male and female Apc Min/+ mice had similar tumor burdens, females had significantly lower levels of circulating IL-6 than males, even when weight loss was comparable. However, the female also appears to have a differential cachectic response to IL-6 that is independent of circulating level. Specifically, plasma IL-6 did not increase as cachexia progressed, while muscle IL-6 mRNA expression and STAT3 phosphorylation were increased. Additionally, systemic overexpression of circulating IL-6 did not accelerate the progression of cachexia, indicating that higher circulating IL-6 levels are not sufficient to induce cachexia in the female Apc Min/+ mouse. We also found that circulating IL-6 levels were not correlated with increased muscle IL-6r mRNA expression. Though the C26 and Apc Min/+ models of cancer cachexia are IL-6 dependent in the male (26, 29, 58), sex differences have been found in the C26 model of cachexia, providing evidence that this is not a singular phenomenon related to the Apc Min/+ mouse (9). Importantly, estrogen inhibits IL-6 transcription and signaling in many tissues (17, 18, 37-39); however, IL-6 is a known mediator of diseases in females including polycystic ovarian syndrome, ovarian cancer, and osteoporosis (38, 59, 60), indicating that females are susceptible to the pathophysiological effects of IL-6 under multiple circumstances regardless of estrogen status. The overall inflammatory environment related to the underlying disease may also alter IL-6 action. The circulating levels of IL-10, IL-4, and interferon-α (IFNα) have been shown to influence IL-6 action (50, 61). Interestingly, sex differences in inflammation have been reported in IL-10 knockout mice (12). Further investigation is required to determine if the systemic inflammatory environment, particularly the presence of estrogen, is differentially regulating IL-6 function in female Apc Min/+ mice.
A key factor that determines tissue response to the inflammatory environment is receptor expression. IL-6 initiates intracellular signaling through binding with mIL-6r, which interacts with the signal-transducing gp130 (62). The importance of muscle IL-6 signaling through mIL-6r and gp130 in the development of cachexia has been clearly demonstrated in many tumor models (27, 33, 60, 63). Signaling through gp130 activates many signaling pathways, including JAK/STAT, p38, and ERK (23, 26-28). We have previously demonstrated that LLC-induced cachexia in males suppresses muscle gp130 expression (11). However, alterations in the expression level of gp130 or IL-6r during cachexia progression in Apc Min/+ mice have not been previously reported. Interestingly, cachectic muscle from both male and female Apc Min/+ mice demonstrated an induction of muscle IL-6r mRNA expression and protein expression, without a significant change in muscle gp130 mRNA expression. The novel observation that male C57BL/6 mice have significantly higher muscle IL-6r protein level than females may explain why males have a more robust response to IL-6 than females. As IL-6 signaling has been linked to the induction of muscle protein degradation (7, 26), it remains to be determined why this signal is being amplified in cachectic muscle of both sexes at both the mRNA and protein levels. It also appears unlikely that differential muscle gp130 and IL-6r expression can account for differential sex responses to circulating IL-6 in Apc Min/+ mice.
In addition to IL-6 interaction with its receptor complex, downstream regulators have the potential to alter IL-6 signaling in muscle (64-66). STAT3 is activated by phosphorylation at Y705 by Janus kinases downstream of the IL-6r/ gp130 complex (26). Activation of JAK/STAT signaling in cachectic muscle is associated with ubiquitin/proteasome and autophagy-mediated protein degradation (7, 26). SOCS3 has the capacity to directly bind to gp130, inhibiting IL-6 signaling (66). Cachexia increased muscle SOCS3 mRNA expression in Apc Min/+ mice of both sexes. However, the observation that SOCS3 protein has a differential sex response both in B6 and cachectic Apc Min/+ mice may have implications for differential control of STAT3 expression and downstream signaling between the sexes. The pattern of increased SOCS mRNA and decreased SOCS3 protein that we report in the male ApcMin/+ has been previously noted with cachexia in the C26 tumor-bearing model; it has been shown that phosphorylation of SOCS3 by Jak can destabilize SOCS3 and lead to its proteasome-mediated degradation (29). Further, there is evidence that a second chronological activation of STAT3 signaling may occur even in the presence of SOCS3 binding; this activation is induced by binding of the epidermal growth factor receptor (EGFR) to IL-6r in the continued presence of IL-6, though it is unknown if sex differences exist (67). We demonstrate a strong trend towards increased STAT3 phosphorylation during cachexia progression in female Apc Min/+ mice, as previously seen in the male (7). This also corresponds with female muscle becoming more sensitive to inflammatory signaling with the progression of cachexia, and may represent the loss of a protective mechanism present in female muscle that suppresses inflammatory signaling. However, IL-6 signaling is only one of many inflammatory pathways involved in the progression of cachexia, and other inflammatory factors may regulate cachexia development in the female Apc Min/+ mouse. Further work is needed to determine the regulation of JAK/STAT signaling during the progression of cachexia in female muscle and its relationship to wasting.
We have shown that female Apc Min/+ mice undergo cachexia that is, at least initially, independent of IL-6 regulation. This finding has important ramifications, particularly as IL-6 has garnered interest as a potential therapeutic target for cancer cachexia. We report for the first time levels of IL-6r, gp130, and SOCS3 mRNA and protein expression in the muscle of male and female Apc Min/+ mice. We also report the novel finding that there is differential expression of muscle IL-6r protein in male and female mice. Further, we have reported a difference in the timing of muscle and fat loss between the sexes that may have important ramifications for metabolism and cytokine signaling during the progression of cachexia. It appears that factors involved in the regulation of cancer cachexia progression are subject to differential sex regulation, and more work is needed to mechanistically understand these differences. In addition, future work will need to determine the mechanisms in skeletal muscle during the progression of cachexia related to protein turnover, oxidative metabolism, and apoptosis that undergo differential regulation in the female.
Highlights.
Cachexia in female Apc Min/+ mice is unrelated to plasma IL-6 level, unlike the male.
Muscle IL-6r and downstream targets increase with cachexia in the female.
IL-6 overexpression has no effect on cachexia in the female Apc Min/+, unlike the male.
IL-6 expression does not correlate with STAT3 phosphorylation in the female ApcMin/+.
Muscle IL-6r and SOCS3 protein levels are higher in males than females.
5. Acknowledgements
This work was supported by National Institutes of Health grant # NCI-R01CA121249A501 (JAC), National Institutes of Health Grant P20 RR-017698 from the National Center for Research, and by the USC Behavioral-Biomedical Interface Program, which is a NIGMS/NIH-T32 supported program. Contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIGMS or NIH. The authors would like to acknowledge Tia Davis and Dr. M. Marjorie Peña for assistance with mouse breeding and IL-6 overexpression experiments. The authors declare no competing interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Farkas J, von Haehling S, Kalantar-Zadeh K, Morley JE, Anker SD, Lainscak M. Cachexia as a major public health problem: Frequent, costly, and deadly. J Cachexia Saropenia Muscle. 2013;4:173–178. doi: 10.1007/s13539-013-0105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pajak B, Orzechowska S, Pijet B, Pijet M, Pogorzelska A, Gajkowska B, Orzechowski A. Crossroads of cytokine signaling- the chase to stop muscle cachexia. J Physiol Pharmacol. 2008;59:251–264. [PubMed] [Google Scholar]
- 3.Suzuki H, Asakawa A, Amitani H, Nakamura N, Inui A. Cancer cachexia-pathophysiology and management. J Gastroenterol. 2013;48:574–594. doi: 10.1007/s00535-013-0787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baltgalvis KA, Berger FG, Pena MM, Davis JM, White JP, Carson JA. Activity level, apoptosis, and development of cachexia in Apc min/+ mice. Journal of Applied Physiology. 2010;109:1155–1161. doi: 10.1152/japplphysiol.00442.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White JP, Baynes JW, Welle SL, Kostek MC, Matesic LE, Sato S, Carson JA. The regulation of skeletal muscle protein turnover during the progression of cancer cachexia in the Apc(Min/+) mouse. PloS one. 2011;6:e24650. doi: 10.1371/journal.pone.0024650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White JP, Baltgalvis KA, Puppa MJ, Sato S, Baynes JW, Carson JA. Muscle oxidative capacity during IL-6 dependent cancer cachexia. American Journal of Physiology Regul Integr Comp Physiol. 2011;300:R201–211. doi: 10.1152/ajpregu.00300.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White JP, Baynes JW, Welle SL, Kostek MC, Matesic LE, Sato S, Carson JA. The regulation of skeletal muscle protein turnover during the progression of cancer cachexia in the Apc min/+ mouse. PLoS One. 2011;6:e24650. doi: 10.1371/journal.pone.0024650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puppa MJ, White JP, Velazquez KT, Baltgalvis KA, Sato S, Baynes JW, Carson JA. The effect of exercise on IL-6 induced cachexia in the Apc min/+ mouse. J Cachexia Saropenia Muscle. 2012;3:117–137. doi: 10.1007/s13539-011-0047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosper PF, Leinwand LA. Cancer causes cardiac atrophy and autophagy in a sexually dimorphic manner. Cancer Research. 2011;71:1710–1720. doi: 10.1158/0008-5472.CAN-10-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norman K, Stobaus N, Reiss J, Schulzke J, Valentini L, Pirlich M. Effect of sexual dimorphism on muscle strength in cachexia. J Cachexia Saropenia Muscle. 2012;3:111–116. doi: 10.1007/s13539-012-0060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Somasundaram R, Herlyn D. Chemokines and the microenvironment in neuroectodermal tumor-host interaction. Seminars in Cancer Biology. 2009;19:92–96. doi: 10.1016/j.semcancer.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tso VK, Sydora BC, Foshaug RR, Churchill TA, Doyle J, Slupsky CM, Fedorak RN. Metabolomic Profiles are gender, disease, and timespecific in the interleukin-10 gene-deficient mouse model of inflammatory bowel disease. PLoS One. 2013;8:e67654. doi: 10.1371/journal.pone.0067654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xing D, Nozell S, Chen Y-F, Hage F, Oparil S. Estrogen and mechanisms of vascular protection. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29:289–295. doi: 10.1161/ATVBAHA.108.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang M, Tsai BM, Reiger KM, Brown JW, Meldrum DR. 17-beta-estradiol decreases p38 MAPK-mediated myocardial inflammation and dysfunction following acute ischemia. Journal of Molecular and Cellular Cardiology. 2006;40:205–212. doi: 10.1016/j.yjmcc.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Xiao E, Xia-Zheng L, Ferin M, Wardlaw SL. Differential effects of estradiol on the adrenocorticotropin responses to interleukin-6 and interleukin-1 in the monkey. Endocrinology. 2001;142:2736–2741. doi: 10.1210/endo.142.7.8243. [DOI] [PubMed] [Google Scholar]
- 16.Liu CJ, Lo JF, Kuo CH, Chu CH, Chen LM, Tsai FJ, Tsai CH, Tzang BS, Kuo WW, Huang CY. Akt mediates 17beta-estradiol and/or estrogen receptor-alpha inhibition of LPS-induced tumor necrosis factor-alpha expression and myocardial cell apoptosis by suppressing the JNK1/2-NFkB pathway. Journal of Cellular and Molecular Medicine. 2009;13:3655–3667. doi: 10.1111/j.1582-4934.2009.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cossette E, Cloutier I, Tardif K, DonPierre G, Tanguay J-F. Estradiol inhibits vascular endothelial cells pro-inflammatory activation induced by C-reactive protein. Molecular and Cellular Biochemistry. 2013;373:137–147. doi: 10.1007/s11010-012-1482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang LH, Yang XY, Mihalic K, Xiao W, Li D, Farrar WL. Activation of estrogen receptor blocks interleukin-6-inducible cell growth of human multiple myeloma involving molecular cross-talk between estrogen receptor and STAT3 mediated by co-regulator PIAS3. J Biol Chem. 2001;276:31839–31844. doi: 10.1074/jbc.M105185200. [DOI] [PubMed] [Google Scholar]
- 19.Baltgalvis KA, Berger FG, Pena MM, Davis JM, Muga SJ, Carson JA. Interleukin-6 and cachexia in Apc min/+ mice. American Journal of Physiology Regul Integr Comp Physiol. 2008;294:R393–401. doi: 10.1152/ajpregu.00716.2007. [DOI] [PubMed] [Google Scholar]
- 20.Scheede-Bergdahl C, Watt HL, Trutschnigg B, Kilgour RD, Haggarty A, Lucar E, Vigano A. Is IL-6 the best pro-inflammatory biomarker of clinical outcomes of cancer cachexia? Clinical Nutrition. 2012;31:85–88. doi: 10.1016/j.clnu.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Kim HJ, Kim HJ, Yun J, Kim KH, Kim SH, Lee S-C, Bae SB, Kim C-K, Lee NS, Lee KT, Park SK, Won JH, Park HS, Hong DS. Pathophysiological role of hormones and cytokines in cancer cachexia. J Korean Med Sci. 2012;27:128–134. doi: 10.3346/jkms.2012.27.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikolajuk A, Kowlska I, Karczewska-Kupczewska M, Adamska A, Otziomek E, Wolczynski S, Kinalska I, Gorska M, Straczkowski M. Serum soluble glycoprotein 130 concentration is inversely related to insulin sensitivity in women with polcystic ovary syndrome. Diabetes. 2010;59:1026–1029. doi: 10.2337/db09-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SY, Buhimschi IA, Dulay AT, Ali UA, Zhao G, Abdel-Razeq SS, Bahtiyar MO, Thung SF, Funai EF, Buhimschi CS. IL-6 trans-signaling system in intra-amniotic inflammation, preterm birth, and preterm premature rupture of the membranes. Journal of Immunology. 2011;186:3226–3236. doi: 10.4049/jimmunol.1003587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onesti JK, Guttridge DC. Inflammation based regulation of cancer cachexia. BioMed Research International. 2014 doi: 10.1155/2014/168407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao R, Nutu M, Karlsson-Lindahl L, Benrick A, Weijdegard B, Lager S, Egecioglu E, Fernandez-Rodriguez J, Gemzell-Danielsson K, Ohlsson C, Jansson J-O, Billig H. Downregulation of cilia-localized IL-6R alpha by 17beta-estradiol in mouse and human fallopian tubes. American Journal of Physiology Cell Physiology. 2009;297:C140–151. doi: 10.1152/ajpcell.00047.2009. [DOI] [PubMed] [Google Scholar]
- 26.Bonetto A, Aydogdu T, Jin X, Zhang Z, Zhan R, Puzis L, Koniaris LG, Zimmers TA. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. American Journal of Physiology Endocrinology and Metabolism. 2012;303:E410–421. doi: 10.1152/ajpendo.00039.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clinical Science. 2012;122:143–159. doi: 10.1042/CS20110340. [DOI] [PubMed] [Google Scholar]
- 28.Tzeng HE, Tsai CH, Chang ZL, Su CM, Wang SW, Hwang WL, Tang CH. Interleukin-6 induces vascular endothelial growth factor expression and promotes angiogenesis through apoptosis signal-regulating kinase 1 in human osteosarcoma. Biochemical Pharmacology. 2013;85:531–540. doi: 10.1016/j.bcp.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 29.Bonetto A, Aydogdu T, Kunzevitzky N, Guttridge DC, Khuri S, Koniaris LG, Zimmers TA. STAT3 activation in skeletal muscle links muscle wasting and the acute phase response in cancer cachexia. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baltgalvis KA, Berger FG, Pena MMO, Davis JM, White JP, Carson JA. Muscle wasting and interleukin-6-induced atrogin1 expression in the cachectic Apc min/+ mouse. Pflugers Archive. 2009;457:989–1001. doi: 10.1007/s00424-008-0574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehl KA, Davis JM, Berger FG, Carson JA. Myofiber degeneration/regeneration is induced in the cachectis Apc min/+ mouse. Journal of Applied Physiology. 2005;99:2379–2387. doi: 10.1152/japplphysiol.00778.2005. [DOI] [PubMed] [Google Scholar]
- 32.Puppa MJ, White JP, Sato S, Cairns M, Baynes JW, Carson JA. Gut barrier dysfunction in the Apc min/+ mouse model of colon cancer cachexia. Biochim Biophys Acta. 2012;1812:1601–1606. doi: 10.1016/j.bbadis.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White JP, Puppa MJ, Sato S, Gao S, Price RL, Baynes JW, Kostek MC, Matesic LE, Carson JA. IL-6 regulation on skeletal muscle mitochondrial remodeling during cancer cachexia in the Apc min/+ mouse. Skeletal Muscle. 2012;2 doi: 10.1186/2044-5040-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White JP, Puppa MJ, Narsale A, Carson JA. Characterization of the male Apc min/+ mouse as a hypogonadism model related to cancer cachexia. Open Biology. 2013 doi: 10.1242/bio.20136544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdullah SM, Khera A, Leonard D, Das SR, Canham RM, Kamath SA, Vega GL, Grundy SM, McGuire DK, de Lemos JA. Sex differences in the association between leptin and CRP: Results from the Dallas Heart Study. Atherosclerosis. 2006;195:404–410. doi: 10.1016/j.atherosclerosis.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 36.Ghazeeri G, Abdullah L, Abbas O. Immunological differences in women compared with men: Overview and contributing factors. American Journal of Reproductive Immunology. 2011;66:163–169. doi: 10.1111/j.1600-0897.2011.01052.x. [DOI] [PubMed] [Google Scholar]
- 37.Naugler WE, Sakurai T, Kim S, Maeda S, Kim KH, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MYD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 38.Pottratz ST, Bellido T, Mocharia H, Crabb D, Manolagas SC. 17 beta estradiol inhibits expression of human interleukin 6 promoter reporter constructs by a receptor-dependent mechanism. Journal of Clinical Investigation. 1994;93:944–950. doi: 10.1172/JCI117100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edwards KM, Mills PJ. Effects of estrogen versus estrogen and progesterone on cortisol and interleukin-6. Maturitas. 2008;61:330–333. doi: 10.1016/j.maturitas.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White JP, Puppa MJ, Gao S, Sato S, Welle SL, Carson JA. Muscle mTORC1 suppression by IL-6 during cancer cachexia: a role for AMPK. American Journal of Physiology Endocrinology and Metabolism. 2012;304:E1042–1052. doi: 10.1152/ajpendo.00410.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puppa MJ, White JP, Velazquez KT, Baltgalvis KA, Sato S, Baynes JW, Carson JA. The effect of exercise on IL-6-induced cachexia in the Apc ( Min/+) mouse. J Cachexia Sarcopenia Muscle. 2011 doi: 10.1007/s13539-011-0047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hetzler KL, Collins BC, Shanely RA, Sue H, Kostek MC. The homeobox gene Six1 alters myosin heavy chain isoform expression in mouse skeletal muscle. Acta Physiologica. 2013 doi: 10.1111/apha.12168. [DOI] [PubMed] [Google Scholar]
- 43.Puppa MJ, Gao S, Narsale AA, Carson JA. Skeleatl muscle glycoprotein 130's role in Lewis lung carcinoma-induced cachexia. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014;28:998–1009. doi: 10.1096/fj.13-240580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hardee JP, Puppa MJ, Fix DK, Gao S, Hetzler KL, Bateman TA, Carson JA. The effect of radiation dose on mouse skeletal muscle remodeling. Radiology and Oncology. 2014;48:247–256. doi: 10.2478/raon-2014-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steiner JL, Davis JM, McClellan JL, Guglielmotti A, Murphy EA. Effects of the MCP-1 synthesis inhibitor bindarit on tumorigenesis and inflammatory markers in the C3(1)/SV40Tag mouse model of breast cancer. Cytokine. 2014;66:60–68. doi: 10.1016/j.cyto.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehl KA, Davis JM, Clements JM, Berger FG, Pena MM, Carson JA. Decreased intestinal polyp multiplicity is related to exercise mode and gender in Apc min/+ mice. Journal of Applied Physiology. 2005;98:2219–2225. doi: 10.1152/japplphysiol.00975.2004. [DOI] [PubMed] [Google Scholar]
- 47.Murphy EA, Davis JM, McClellan JL, Carmichael MD. Quercetin's effects on intestinal polyp multiplicity and macrophage number in the Apc min/+ mouse. Nutrition and Cancer. 2011;63:421–426. doi: 10.1080/01635581.2011.535954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.You S, Ohmori M, Pena MMO, Nassri B, Quiton J, Al-Assad ZA, Liu L, Wood PA, Berger SH, Liu ZJ, Wyatt MD, Price RL, Berger FG, Hrushesky WJM. Developmental abonormalities in multiple proliferative tissues of Apc min/+ mice. International J Experimental Pathology. 2006;87:227–236. doi: 10.1111/j.1365-2613.2006.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iwase S, Murakami T, Saito Y, Nakagawa K. Steep elevation of blood interleukin-6 (IL-6) associated only with late stage of cachexia in cancer patients. European cytokine network. 2004;15:312–316. [PubMed] [Google Scholar]
- 50.Argiles JM, Lopez-Soriano FJ. The role of cytokines in cancer cachexia. Medicinal Research Reviews. 1999;19:223–248. doi: 10.1002/(sici)1098-1128(199905)19:3<223::aid-med3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 51.Suh S-Y, Choi YS, Yeom CH, Kwak SM, Yoon HM, Kim DG, Koh S-J, Park J, Lee MA, Lee YJ, Seo A-R, Ahn H-Y, Yim E. Interleukin-6 but not tumor necrosis factor-alpha predicts survival in patients with advanced cancer. Support Care Cancer. 2013;21:3071–3077. doi: 10.1007/s00520-013-1878-4. [DOI] [PubMed] [Google Scholar]
- 52.Gandolfi S, Granelli B, Terzoni D, Civardi G, Cavanna L. Favorable responses to Tocilizumab in two patients with cancer-related cachexia. Journal of Pain and Symptom Management. 2013;46:E9–13. doi: 10.1016/j.jpainsymman.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 53.Ando K, Takahashi F, Motojima S, Nakashima K, Kaneko N, Hoshi K, Takahashi K. Possible role for Tocilizumab, and anti-interleukin-6 receptor antibody, in treating cancer cachexia. Journal of Clinical Oncology. 2013;31:e69–72. doi: 10.1200/JCO.2012.44.2020. [DOI] [PubMed] [Google Scholar]
- 54.Senthil Kumar SPD, Shen M, Spicer EG, Goudjo-Ako AJ, Stumph JD, Zhang J, Shi H. Distinct metabolic effects following short-term exposure of different high-fat diets in male and female mice. Endocrine Journal. 2014 doi: 10.1507/endocrj.ej13-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bloor ID, Symonds ME. Sexual dimorphism in white and brown adipose tissue with obesity and inflammation. Hormones and Behavior. 2014 doi: 10.1016/j.yhbeh.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 56.Mantovani G, Maccio A, Madeddu C, Serpe R, Massa E, Dessi M, Panzone F, Contu P. Randomized Phase III Clinical Trial of Five Different Arms of Treatment in 332 patients with cancer cachexia. The Oncologist. 2010;15:200–211. doi: 10.1634/theoncologist.2009-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruzzo A, Catalano V, Canestrari E, Giacomini E, Santini D, Tonini G, Vincenzi B, Fiorentini G, Magnani M, Graziano F. Genetic modulation of the interleukin 6 (IL-6) system in patients with advanced gastric cancer: a background for an alternative target therapy. BMC Cancer. 2014;14 doi: 10.1186/1471-2407-14-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carson JA, Baltgalvis KA. Interleukin-6 as a key regulator of muscle mass during cachexia. Exerc Sport Sci Rev. 2010;38:168–176. doi: 10.1097/JES.0b013e3181f44f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Syed V, Ulinski G, Mok SC, Ho S-M. Reproductive hormone-induced STAT3-mediated interleukin-6 action in normal and malignant human ovarian surface epithelial cells. Journal of the National Cancer Institute. 2002;94:617–629. doi: 10.1093/jnci/94.8.617. [DOI] [PubMed] [Google Scholar]
- 60.Lo C-W, Chen M-W, Hsiao M, Wang S, Chen C-A, Hsiao S-M, Chang J-S, Lai T-C, Rose-John S, Kuo M-L, Wei L-H. IL-6 trans-signaling in formation and progression of malignant ascites in ovarian cancer. Cancer Research. 2011;71:424–434. doi: 10.1158/0008-5472.CAN-10-1496. [DOI] [PubMed] [Google Scholar]
- 61.Batista ML, Jr., Olivan M, Alcantara PSM, Sandoval R, Peres SB, Neves RX, Silverio R, Maximiano LF, Otoch JP, Seelaender M. Adipose tissue-derived factors as potential biomarkers in cachectic cancer patients. Cytokine. 2013;61:532–539. doi: 10.1016/j.cyto.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 62.Klein C, Wusefeld T, Assmus U, Roskams T, Rose-John S, Muller M, Manns MP, Ernst M, Trautwein C. The IL-6-gp130-STAT3 pathway in hepatocytes triggers liver protection in T cell-mediated liver injury. Journal of Clinical Investigation. 2005;115:860–869. doi: 10.1172/JCI200523640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weidle UH, Klostermann S, Eggle D, Kruger A. Interleukin 6/Interleukin 6 receptor interaction and its role as a therapeutic target for treatment of cachexia and cancer. Cancer Genomics and Proteomics. 2010;7:287–302. [PubMed] [Google Scholar]
- 64.Li Y, Han M-F, Li W-N, Shi A-C, Zhang Y-Y, Wang H-Y, Wang F-X, Li L, Wu T, Ding L, Chen T, Yan W-M, Luo X-P, Ning Q. SOCS3 expression correlates with severity of inflammation in mouse hepatitis virus strain 3-induced acute liver failure and HBV-ACLF. Journal of Huazhong University Science and Tehcnology. 2014;34:348–353. doi: 10.1007/s11596-014-1281-5. [DOI] [PubMed] [Google Scholar]
- 65.Liu X, Croker BA, Campbell IK, Gauci SJ, Alexander WS, Tonkin BA, Walsh NC, Linossi EM, Nicholson SE, Lawlor KE, Wicks IP. Supressor of cytokine signaling-3 regulates gp130 cytokine-induced signaling to limit chondrocyte responses during inflammatory arthritis. Arthritis and Rheumatism. 2014 doi: 10.1002/art.38701. [DOI] [PubMed] [Google Scholar]
- 66.White GE, Cotterill A, Addley MR, Soilleux EJ, Greaves DR. Suppressor of cytokine signaling protein SOCS3 expression is increased at sites of acute and chronic inflammation. Journal of Molecular Histology. 2011;42:137–151. doi: 10.1007/s10735-011-9317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Y, van Boxel-Dezaire AH, Cheon HJ, Yang J, Stark GR. STAT3 activation in response to IL-6 is prolonged by the binding of the IL-6 receptor to EGF receptor. Proc Natl Acad Sci. 2013;110:16975–16980. doi: 10.1073/pnas.1315862110. [DOI] [PMC free article] [PubMed] [Google Scholar]