Abstract
Background
Inhalation injury, which is among the causes of acute lung injury and acute respiratory distress syndrome (ARDS), continues to represent a significant source of mortality in burned patients. Inhalation injury often requires mechanical ventilation, but the ideal tidal volume strategy is not clearly defined in burned pediatric patients. The aim of the present study is to determine the effects of low and high tidal volume on the number of ventilator days, ventilation pressures, and incidence of atelectasis, pneumonia and ARDS in pediatric burned patients with inhalation injury within one year post burn injury.
Methods
From 1986–2014, inhalation injury was diagnosed by bronchoscopy in pediatric burned patients (n=932). Patients were divided into three groups: (1) unventilated (n=241), (2) high tidal volume (HTV, 15 ± 3 ml/kg, n=190), and (3) low tidal volume (LTV, 9 ± 3 ml/kg, n = 501).
Results
HTV was associated with significantly decreased ventilator days (p<0.005) and maximum positive end expiratory pressure (p<0.0001) and significantly increased maximum peak inspiratory pressure (p<0.02) and plateau pressure (p<0.02) compared to patients with LTV. The incidence of atelectasis (p<0.0001) and ARDS (p<0.02) was significantly decreased with HTV compared to LTV. However, the incidence of pneumothorax was significantly increased in the HTV group compared with LTV (p<0.03).
Conclusions
HTV significantly decreases ventilator days and the incidence of both atelectasis and ARDS compared to low tidal volume in pediatric burned patients with inhalation injury. Thus, the use of HTV may interrupt sequences leading to lung injury in our patient population.
Keywords: Mechanical ventilation, inhalation injury, burns, positive expiratory end pressure, peak inspiratory pressure, plateau pressure
INTRODUCTION
Approximately 1.25 million individuals in the United States suffer from thermal injury, which results in 3,400 deaths each year.1 Approximately 23,000 of these burned patients suffer concomitant injury from smoke inhalation. Despite advances in critical care and wound management, inhalation injury remains a major source to mortality and morbidity in burn patients.2, 3 A recent ten-year review from the National Burn Repository documents that mortality was greater for burned patients with inhalation injury (27.3%) than those without inhalation injury (4.5%). Additionally, the incidence of inhalation injury along with pneumonia increased the probability of death by 60% in burned patients.4
Inhalation injury is associated with the formation of casts in the airway and the reduction of surfactant in the alveoli.5, 6 The pathophysiology of inhalation injury may also cause decreased pulmonary compliance and increased airway resistance, which often necessitates mechanical ventilation. Often, severe inhalation injury results in acute lung injury (ALI), which frequently develops into ARDS in burned patients.7 ARDS is associated with protein-rich pulmonary edema, which reflects injury of the lung endothelium and epithelium and impairs carbon dioxide release.6 The clinical definition of ARDS according to the 1994 American-European Consensus Conference includes acute onset, bilateral lung infiltrates by radiography, and a partial pressure of oxygen to fraction inspired of oxygen ratio (PaO2:FiO2) of less than 200 mmHg.8 In 2012, the ARDS Definition Task Force revamped the definition to the ‘Berlin Definition, which classified ARDS solely based on the PaO2:FiO2. Mild ARDS was classified by a PaO2:FiO2 of between 200–300 mmHg, while moderate ARDS was classified by a PaO2:FiO2 of 100–200 mmHg and severe ARDS was classified by a PaO2:FiO2 of less than 100 mmHg.9
Burned patients with inhalation injury often require mechanical ventilation support. Traditionally, high tidal volume ventilation (HTV) was used in the burned pediatric population at the Shriners Hospitals for Children-Galveston from 1986 to 1996 to improve oxygenation and to achieve normal values for partial pressure of arterial carbon dioxide and for pH. However, the ARDS Network Study then showed that low tidal volume ventilation (LTV) decreased mortality in non-burned patients with acute respiratory distress syndrome (ARDS).10 Additionally, HTV was shown to cause over distended alveoli, alveolar capillary membrane disruption, and increased inflammation in non-burned patients.11 The outcome studies of patients treated with HTV identified residual pulmonary abnormalities,12 and the standard of care at SHC was then modified to a LTV protocol for ventilated patients from 1997 to 2014.
The optimal ventilation strategy for patients with burns and inhalation injury is not well defined.13 The ARDS Network Study has shown a significant decrease in mortality when low tidal volumes are used for the treatment of ARDS. However, these studies excluded pediatric patients, as well as patients with greater than 30% total burned surface area (TBSA), and whether the results hold true for patients of inhalation injury and burned patients has yet to be determined in a large scale study. We hypothesize that LTV compared to HTV in burned pediatric patients with inhalation injury will improve pulmonary outcomes, including decreased ventilator days and decreased incidence of ARDS and atelectasis. Our study compared three groups of burned pediatric patients (Unventilated, HTV, LTV) with inhalation injury that were admitted to the Shriners Hospital for Children-Galveston from 1986–2014.
METHODS
Patient demographics and injury characteristics
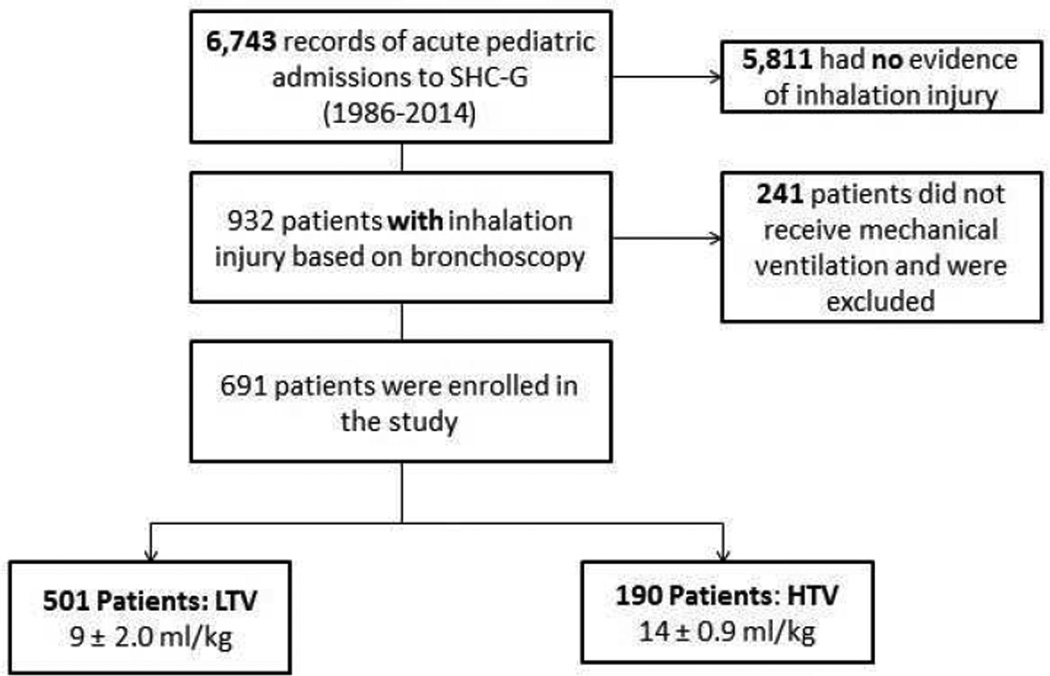
Inclusion criteria for the study were as follows: (1) 0 –18 years of age at the time of the admission, (2) diagnosis with inhalation injury, and (3) the need for ventilation (Fig. 1). Patient age, gender, ethnicity, TBSA, and third-degree TBSA were recorded at the time of admission. Age-appropriate diagrams were used to determine burn size.14 Approval was obtained by the Institutional Review Board from the University of Texas Medical Branch for our retrospective study.
Figure 1.
Out of 6,743 acute pediatric admissions to the Shriners Hospitals for Children-Galveston between 1986 and 2014, 932 patients were included in our study. Inclusion criteria were based on age (0 –18 years at the time of the admission), diagnosis with inhalation injury, and the need for ventilation.
Inhalation Injury Diagnosis
Inhalation injury was confirmed by bronchoscopy in all patients. Findings included soot deposits, erythema, edema, mucosal blisters and erosion, and hemorrhage.
Atelectasis and ARDS Diagnosis
Atelectasis was determined by radiological interpretation of the chest x-ray. ARDS was also diagnosed by the radiological interpretation of the chest x-ray, as well as a PaO2:FiO2 ratio of less than 200 mm Hg.
Pneumonia Diagnosis
Pneumonia was defined using the criteria set by the National Trauma Data Bank: (1) presence of fever, which was defined as <96.8 F or >102.2 F, (2) leukocytosis, which was defined by a white blood cell count of >12, (3) Gram stain of sputum with a predominant organism and moderate to many white blood cells, (4) chest radiograph with a pneumonic infiltrate, and (5) culture of sputum demonstrating a pathogen.
Statistical Analysis
Multiple linear regression was used to model the relation of ventilator days, maximum positive end-expiratory pressures (PEEP) and peak inspiratory pressures (PIP), plateau pressure, and admission nadir PaO2 and PaO2/FiO2 ratio as functions of age, TBSA, treatment group (nonventilated, LTV, or HTV), and death. Age was square root transformed to better approximate a normal distribution. The outcome variables of ventilator days and maximum PEEP exhibited count or count-like distributions, so a negative binomial generalized linear model with a log link was used to model ventilator days and maximum PEEP, accounting for overdispersion in the Poisson model. Maximum PIP, plateau pressure, and admission nadir PaO2 were all log transformed to better approximate a normal distribution and modeled with a standard linear model. The binary outcome variables for pneumonia, atelectasis and ARDS were modeled by multiple logistic regression. Resulting regression parameter estimates and standard errors were reverse transformed and presented as adjusted means, factors, or odds ratios with confidence intervals and p-values. The Berlin ARDS classification was modeled by ordinal logistic regression assuming equidistant intervals.15 The survival analysis compared LTV with HTV using the Peto & Peto modification of the Gehan-Wilcoxon test,16 with Kaplan-Meier survival curves. All statistical testing assumed a 95% level of confidence, and all analyses were performed using R statistical software.17
RESULTS
Table 1 indicates the demographic information from our burned patient population with inhalation injury. Non-ventilated patients did not receive any type of ventilation (‘Non-Ventilated’, n=241), while patients who received a tidal volume of 15 ± 3 ml/kg were classified into the ‘HTV’ group (n=190) and patients who received a tidal volume of 9 ± 3 ml/kg were classified into the ‘LTV’ group (n=501). There were no significant differences among the three groups in our study in gender (p<0.15), and there was a significant different in ethnicities (p=0.0035). The length of hospital stay between the HTV and LTV groups was not significant (p=0.42).
Table 1.
Patient Characteristics
| Non-ventilated (n=241) |
LTV (n=501) |
HTV (n=190) |
|
|---|---|---|---|
| Age, y, median | 9 ± 58 | 8 ± 56 | 7 ± 55 |
| Sex, male, n (%) | 168 (70) | 321 (64) | 126 (66) |
| Hispanic, n (%) | 130 (54) | 339 (68) | 53 (28) |
| TBSA, % [median] | 36 ± 23 [33] | 55 ± 24 [56] | 59 ± 23 [59] |
| 3rd Degree TBSA, % [median] | 24 ± 23 [17] | 45 ± 29 [45] | 50 ± 29 [53] |
| Burn to admission, d [median] | 9 ± 23 [2] | 5 ± 21 [2] | 4 ± 10 [1] |
| Mortality, n (%) | 2 (1) | 77 (15) | 42 (22) |
| Length of stay, d [median] | 28 ± 28 [22] | 41 ± 44 [30] | 44 ± 34 [37] |
| Nadir PaO2:FiO2 | 330 ± 112 | 206 ± 137 | 219 ± 110 |
| Nadir PaO2, mmHg | 92 ± 41 | 79 ± 34 | 89 ± 43 |
| Admit pH | 7.4 ± 0.1 | 7.3 ± 0.1 | 7.4 ± 0.1 |
| Admit base excess, mEq/L | −0.8 ± 3.8 | −2.1 ± 5.4 | −2.3 ± 5.2 |
| Admit hematocrit, % | 36 ± 7 | 35 ± 9 | 35 ± 9 |
Data presented as mean ± standard deviation.
Regression results for each outcome variable are summarized in Tables 2–5. Each table shows the factor, odds ratio, or adjusted mean associated with each predictor variable (treatment group, TBSA, age) on the pulmonary outcome variables. The tables include corresponding 95% confidence intervals and p-values, where appropriate.
Table 2.
Ventilation Parameters and Pulmonary Complications in High Tidal Volume and Low Tidal Volume Patients
| , HTV | LTV | HTV/LTV | 95% CI Minimum |
95% CI Maximum |
p Value* | |
|---|---|---|---|---|---|---|
| Ventilator days [median], mean ± SD | 9 ± 15 [4] | 11 ± 16 [5] | 0.73† | 0.62 | 0.87 | 0.0004 |
| Maximum PEEP, mmHg, mean ± SD | 7 ± 4 | 9 ± 4 | 0.68† | 0.64 | 0.73 | <0.0001 |
| Maximum PIP, mmHg, mean ± SD | 43 ± 18 | 38 ± 16 | 1.07† | 1.02 | 1.13 | 0.0119 |
| Plateau pressure, mmHg, mean ± SD | 37 ± 16 | 33 ± 14 | 1.07† | 1.02 | 1.13 | 0.0106 |
| Pneumonia, n (%) | 44 (25) | 125 (25) | 0.80‡ | 0.52 | 1.24 | 0.3238 |
| Atelectasis, n (%) | 79 (43) | 292 (58) | 0.44‡ | 0.31 | 0.63 | <0.0001 |
| ARDS, n (%) | 20 (11) | 74 (15) | 0.46‡ | 0.25 | 0.84 | 0.0115 |
| Pneumothorax, n (%) | 52 (28) | 75 (19) | 1.59‡ | 1.06 | 2.41 | 0.0268 |
p<0.05 accepted as significant.
Factor.
Odds ratio.
Table 5.
Nadir PaO2 and Pulmonary Complications in Non-Ventilated (NV) and Low Tidal Volume (LTV) Patients
| NV | LTV | NV/LTV | 95% CI Minimum |
95% CI Maximum |
p Value* | |
|---|---|---|---|---|---|---|
| Nadir PaO2, mmHg, mean ± SD | 93 ± 41 | 79 ± 34 | 1.22† | 1.12 | 1.31 | <0.0001 |
| Pneumonia, n (%) | 9 (5) | 125 (26) | 0.24‡ | 0.12 | 0.49 | 0.0001 |
| Atelectasis, n (%) | 17 (10) | 292 (58) | 0.10‡ | 0.06 | 0.17 | <0.0001 |
| ARDS, n (%) | 2 (1) | 74 (15) | 0.12‡ | 0.03 | 0.53 | 0.0046 |
p<0.05 accepted as significant.
Factor.
Odds ratio.
High and low tidal volume
Burned patients with HTV had a significantly decreased number of ventilator days compared to patients with LTV (p=0.004, Table 2). Belonging to the HTV treatment group as opposed to the LTV group was associated with a 27% decrease in ventilator days, with a 95% confidence interval (CI) spanning 13 to 38. Surviving patients with HTV also had a decreased number of ventilator days compared to surviving patients with LTV (p=0.0003), with a CI spanning 58 to 85.
Burned patients with HTV had a significantly decreased maximum PEEP (p<0.0001, Table 2), significantly increased PIP (p=0.0119, Table 2), and significantly increased plateau pressure (p=0.0106, Table 2) compared to patients with LTV. Belonging to the HTV treatment group as opposed to the LTV group was associated with a 32% decrease in max PEEP, with a CI spanning 27 to 36. Belonging to the HTV treatment group as opposed to the LTV group was associated with a 7% increase in maximum PIP, with a CI spanning 2 to 13. Additionally, belonging to the HTV treatment group as opposed to the LTV group was associated with a 7% increase in plateau pressure, with a CI spanning 2 to 13.
Burned patients with HTV had a significantly decreased incidence of atelectasis (p<0.0001, Table 2) compared to patients with LTV. Being a member of the HTV group as opposed to the LTV group reduced the odds of atelectasis by 56% (CI from 37 to 70). Also, burned patients with HTV showed a significantly lower incidence of ARDS (p=0.0115, Table 2) compared to patients with LTV using the American-European Consensus Conference definition8 and the Berlin definition (p<0.0001, CI 12 to 24).18 The incidence of pneumothorax also significantly increased in the HTV group compared to the LTV group (p=0.0268, Table 2).
Survivors and non-survivors
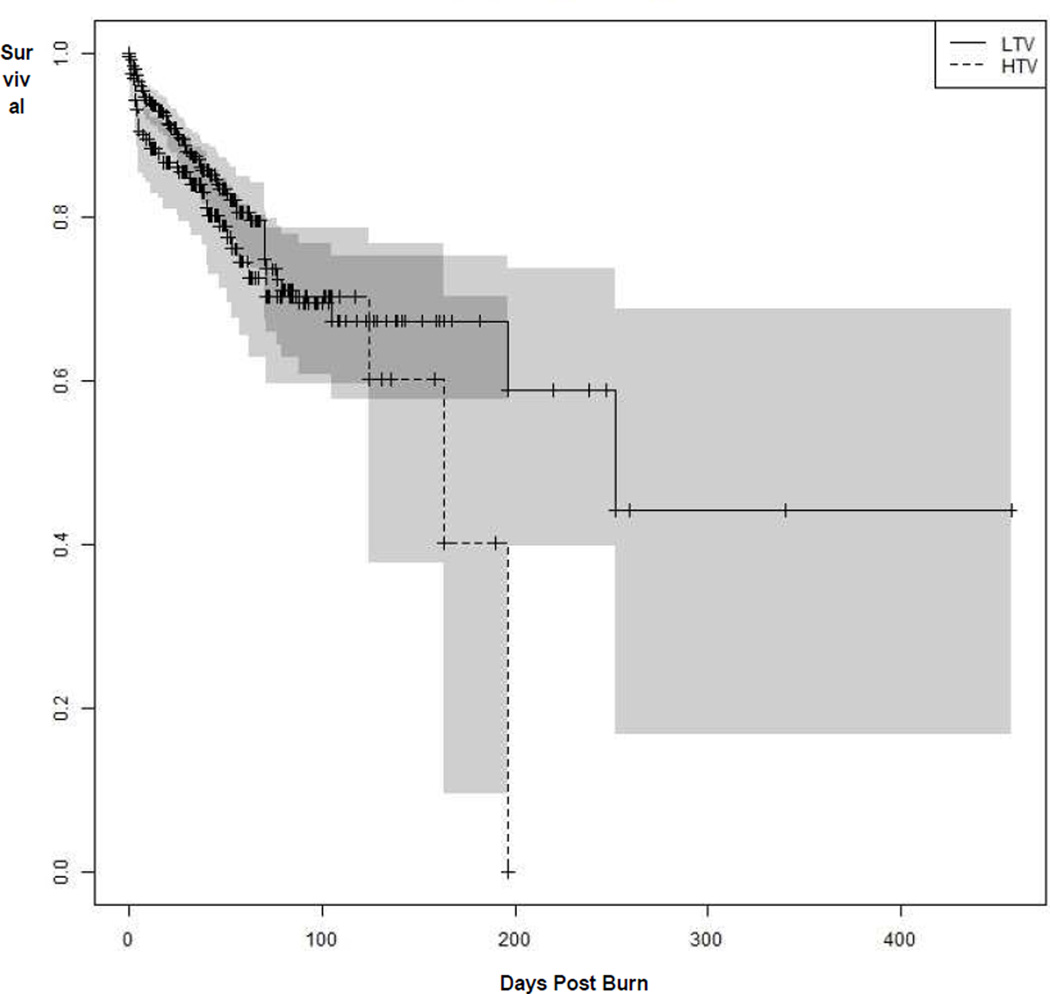
Table 3 indicates the demographic information between survivors and non-survivors with both HTV and LTV. Figure 2 shows a Kaplan-Meier survival curve comparing the LTV and HTV groups; the log-rank test failed to show evidence of a significant difference in mortality between HTV and LTV (p=0.10). The median time to death for the HTV group was 163 days post burn (CI ranged upwards from 124 days), while the median time to death for the LTV was 252 days post burn (CI ranged upwards from 196 days). Among ventilated patients, mortality was approximately 22% for those ventilated with HTV, as compared with 15% for those ventilated with LTV.
Table 3.
Characteristics of HTV and LTV Survivor and Non-Survivors
| Survivors, HTV (n=148) |
Survivors, LTV (n=424) |
Non-survivors, HTV (n=42) |
Non-survivors, LTV (n=77) |
|
|---|---|---|---|---|
| Age, y [median] | 7 ± 5 [6] | 7 ± 5 [6] | 4 ± 4 [3] | 9 ± 6 [8] |
| Sex, male, n (%) | 100 (68) | 272 (64) | 26 (62) | 49 (64) |
| Hispanic, n (%) | 36 (24) | 285 (67) | 17 (40) | 54 (70) |
| TBSA, % [median] | 55 ± 22 [56] | 51 ± 23 [51] | 71 ± 25 [76] | 74 ± 19 [78] |
| 3rd Degree TBSA, % [median] | 46 ± 27 [51] | 41 ± 27 [40] | 65 ± 29 [72] | 65 ± 28 [73] |
| Nadir PaO2:FiO2 | 237 ± 99 | 212 ± 140 | 157 ± 127 | 178 ± 121 |
| Nadir PaO2 (mmHg) | 89 ± 26 | 79 ± 34 | 89 ± 77 | 80 ± 33 |
| Admit pH | 7.4 ± 0.1 | 7.4 ± 0.1 | 7.3 ± 0.2 | 7.3 ± 0.2 |
| Admit base excess, mEq/L | −1.5 ± 4.6 | −1.5 ± 4.8 | −5.1 ± 6.2 | −5.2 ± 7.1 |
| Admit hematocrit, % | 36 ± 9 | 35 ± 9 | 31 ± 10 | 34 ± 10 |
Data presented as mean ± SD.
Figure 2.
Figure 2 shows a Kaplan-Meier survival curve comparing the LTV and HTV groups; the log-rank test failed to show evidence of a significant difference in mortality between HTV and LTV (p=0.10).
There were significant relationship between ventilator days and death (p=0.0137, Table 4). Patients who died had a 30% increase in ventilator days compared to those who survived. Maximum PEEP was also significantly related to death (p<0.0001, Table 4). Patients who died had a 53% increase in maximum PEEP compared to patients who survived. There were significant relationships with maximum PIP due to death (p<0.0001, Table 4). Patients who died had a 51% increase in maximum PIP compared to patients who survived. Additionally, there were significant relations with plateau pressure due to death (p<0.0001, Table 4). Patients who died had a 50% increase in plateau pressure compared to those who survived.
Table 4.
Ventilation Parameters and Pulmonary Complications in Non-Survivors and Survivors
| Survivors | Non-survivors | 95% CI Minimum |
95% CI Maximum |
p Value* | ||
|---|---|---|---|---|---|---|
| Ventilator days, mean ± SD | 9 ± 15 | 16 ± 20 | 1.30† | 1.06 | 1.60 | 0.0137 |
| Maximum PEEP, mmHg, mean ± SD | 8 ± 3 | 12 ± 5 | 1.53† | 1.43 | 1.65 | <0.0001 |
| Maximum PIP, mmHg, mean ± SD | 35 ± 11 | 59 ± 24 | 1.51† | 1.41 | 1.61 | <0.0001 |
| Plateau pressure, mmHg, mean ± SD | 30 ± 9 | 51 ± 21 | 1.50† | 1.41 | 1.60 | <0.0001 |
| Pneumonia, n (%) | 109 (15) | 69 (59) | 6.02‡ | 3.81 | 9.52 | <0.0001 |
| Atelectasis, n (%) | 314 (42) | 74 (62) | 1.17‡ | 0.75 | 1.82 | 0.4904 |
| ARDS, n (%) | 41 (5) | 55 (46) | 10.30‡ | 6.04 | 17.57 | <0.0001 |
| Pneumothorax, n (%) | 1.99‡ | 1.23 | 3.22 | 0.0052 | ||
| Nadir PaO2/FiO2, mean ± SD | 248 ± 135 | 172 ± 124 | −50.118§ | −77.47 | −22.77 | 0.0003 |
p<0.05 accepted as significant.
Factor.
Odds ratio.
Adjusted mean.
Patients who died had nearly 6 times the odds of pneumonia as those who survived (p<0.0001, Table 4). The nadir PaO2/FiO2 ratio was significantly related to death (p<0.0003, Table 4). Patients who died showed a mean 50 unit decrease in this ratio as compared to those who survived. Atelectasis was shown to be not significantly related to death (p=0.4904, Table 4), while ARDS was significantly related to death (p<0.0001, Table 4). Death increased the odds of developing ARDS by a factor of 10. The incidence of pneumothorax also significantly increased in the non-survivor group compared to survivors (p=0.0052, Table 4).
The histopathology of non-surviving patients with barotrauma includes the presence of chest tubes, bubbles in the mediastinum, pulmonary interstitial and soft tissue emphysema, and the presence of crepitants. All autopsies were performed by two independent pathologists. Among the non-surviving patients treated with HTV, 66% exhibited no histopathology associated with barotrauma, while 34% showed chest tubes and less than 1% showed bubbles in the mediastinum and emphysema. On the other hand, among the non-surviving patients treated with LTV, 60% exhibited no histopathology associated with barotrauma, while 30% showed chest tubes, 19% showed bubbles in the mediastinum, and less than 1% showed emphysema.
Non-ventilated patients
Patients in the non-ventilated group as opposed to the LTV group had 1/5 odds of pneumonia, (p<0.0001, Table 5). Additionally, atelectasis was shown to be significantly related to treatment group (p<0.0001, Table 5). Being a member of the non-ventilated group as opposed to the LTV group reduced the odds of atelectasis by a factor of 0.1 (CI from 0.06 to 0.17); alternately, being in the LTV group as opposed to the non-ventilated group increased the odds by a factor of 10.
DISCUSSION
High tidal volumes were used from 1986 to 1996 in our study. The volume is considered more aggressive, but clinical outcomes were significantly improved in our patients (Table 2). HTV may be necessary in the burned pediatric population to improve oxygenation and ventilation. Our results show that burned pediatric patients with inhalation injury that are ventilated with HTV have a significantly decreased number of days on the ventilator and a significantly decreased incidence of both atelectasis and ARDS (Table 2). Also, in non-surviving patients, we demonstrated a significant increase in days alive using HTV compared to LTV (Figure 2). Our findings correlate with the randomized prospective trial of Maslow et al, who showed that HTV (10 mL/kg) caused a significantly lower alveolar dead space ratio and a significantly higher dynamic pulmonary compliance compared to LTV (5 mL/kg) in patients with elective pulmonary resection requiring single-lung ventilation.19 The use of HTV also showed no increase in morbidity and was associated with less hypercarbia and postoperative atelectasis.19 In a 2014 systematic review and meta-analysis of observational studies in mechanically ventilated PICU patients (n=1,756), there was no association between mortality and tidal volumes regardless of the presence of ARDS/ALI.20 Additionally, Zick et al showed that high tidal volumes (10 mL/kg) increased regional respiratory system compliance in an porcine model of ALI.21 Sly et al showed that HTV (21 mL/kg) did not exacerbate lung injury induced by acid in infant rats compared to LTV (7 mL/kg), and tissue elastance and airway resistance were less deteriorated.22 There was no difference in histological lung scores and the concentration of IL-6 in bronchoalveolar lavage fluid between HTV and LTV in the rats.
Traditionally, high tidal volumes of 10 to 15 mL/kg were used to ventilate patients with lung dysfunction. In 1974, Webb and Tierney first explored the possibility of ventilator-associated lung injury after high tidal volume administration by showing an increase in pulmonary edema.23 Currently, lower tidal volumes (6–8 ml/kg) are used because they may not excessively distend or stretch the lung compared to high tidal volumes. The ARDS Network showed that lower tidal volumes (6 mL/kg of predicted body weight) decreased mortality by 22% and decreased the number of ventilator days in patients with ALI and ARDS compared to high tidal volume (12 mL/kg of predicted body weight).10 However, the exclusion criteria listed patients younger than 16 years old and patients with over 30% TBSA. In 2006, Villar et al showed that a higher PEEP and LTV improved mortality and decreased ventilator days in 103 patients with ARDS.24 Their exclusion criteria included patients younger than 15 years, as well as patients with a high risk of mortality within 3 months for reasons other than ARDS such as having more than two extrapulmonary organ failures or having severe neurological damage. In 2006, Wheeler et al showed that lower tidal volume ventilation reduced mortality of patients with ALI from 40% to 25%, but exclusion criteria included the presence of ALI for more than 48 hours as well as having irreversible conditions for which the estimated six-month mortality rate exceeded 50%.25 Additionally, in 1998 Amato et al showed that lower tidal volumes (6 mL/kg) decreased mortality and resulted in a higher rate of weaning from the mechanical ventilator and a lower rate of barotrauma in 53 ARDS patients after 28 days compared to higher tidal volumes (12 mL/kg).26 Their exclusion criteria listed patients less than 14 years and mechanical ventilation for more than one week.
The most effective volume for mechanical ventilation in patients diagnosed with ARDS has been controversial. Phase II and III clinical trials have evaluated pharmacological therapies such as inhaled vasodilators, antioxidants, fluid and hemodynamic management, surfactant therapy, glucocorticoids and other anti-inflammatory agents for the treatment of ALI and ARDS.27 However, none of these treatments have been significantly effective.28 There are numerous inflammatory molecules released into an alveolus during the acute phase of ARDS, including neutrophils, cytokines, interleukins-1, -6, -8, and -10, and macrophages.29 Additionally, alveolar and bronchial epithelial cells are sloughed and protein-rich hyaline membranes are formed along the basement membrane of the alveoli.29 There were no significant differences in net fluid balances between the HTV and LTV groups (p=0.17), and our findings show that the incidence of ARDS significantly decreases with HTV (Table 2). We showed this decrease using both the American-European Consensus Conference Definition and the Berlin Definition.9 The longer inspiration time associated with HTV may open collapsed lung units, which may improve ventilation and gas exchange.
Figure 2 shows a Kaplan-Meier survival curve comparing the LTV and HTV groups. Among ventilated patients, mortality was approximately 22% for those ventilated with HTV, as compared with 15% for those ventilated with LTV. The Kaplan-Meier estimate of the median time to death for the HTV group was 163 days post burn (CI ranged upwards from 124 days), while the median time to death for the LTV was 252 days post burn (CI ranged upwards from 196 days). Mortality between the HTV and LTV groups was assessed by chi-square test, by log-rank test,30 and by Cox proportional hazards regression31 adjusting for age and TBSA. Although the chi-square test suggests that the mortality between HTV and LTV is significantly different, the test is the least appropriate because it fails to account for censoring or adjust for effects of prognostic covariates. The log-rank test failed to show evidence of a significant difference in mortality between HTV and LTV (p=0.10). However, the log-rank test appropriately accounts for censoring, but makes no adjustment for differences prognostic covariates. The Cox model provides the most comprehensive test of differences in mortality between the groups, accounting for censoring while adjusting for age and TBSA. The model also found no evidence for a significant difference in mortality between HTV and LTV (p=0.15, Estimate: 0.286, Standard Error: 0.194, Hazard Ratio: 1.318).
The limitations of our study are primarily based on the large span of time between 1986 and 2014. The major differences over time include (1) changes in anesthesiologists and their extubation techniques, (2) the development of multiple drug resistant organisms (MDRO) and the increasing use of antibiotics, and (3) the admission of a more homogenous population into our burn unit. From 1986 to 1996 (HTV), anesthesiologists prioritized the early extubation of the endotracheal tube from the patient, while from 1997 to 2014 (LTV) anesthesiologists would allow the patients to be intubated longer. However, the clinical care from 1986 to 2014 has been consistently guided by one attending Chief-of-Staff and burn surgeon to ensure the continuity of care. Although antibiotics such as vancomycin have been used consistently, noteworthy changes over the past twenty years include the increased use of colistins in the 2000s, as well as the combination of penicillinase inhibitors with penicillins. The use of broad-spectrum antibiotics has also increased gradually over time. Finally, HTV was used between 1986–1996, a time in which the pediatric burned population was 28% Hispanic, 27% African American, and 43% Caucasian. LTV was used between 1997–2014, a time in which the pediatric burned population was 68% Hispanic, 9% African American, and 23% Caucasian. This may be because of changing referral patterns over time, and it is important to note the differences among the ethnicities. The HTV group (1986 to 1996) was primarily from the southern region of the United States with a racial and ethnic distribution indicative of that time. However, the LTV group (1996 to 2014) included a higher population of burned pediatric patients from Mexico and therefore a larger Hispanic population from an underdeveloped versus developed country.
Increased ventilation pressures may increase the chances of ventilator-induced lung injury (VILI). As illustrated in Table 2, patients with HTV were associated with a 7% increase in maximum PIP (43 ± 18 mm Hg HTV vs 38 ± 16 LTV, p=0.0119) and a 7% increase in plateau pressure (37 ± 16 mm Hg HTV vs 33 ± 14 LTV, p=0.0106) compared to LTV. However, the increases in pressures do not require clinical intervention. The ARDS network determined that plateau pressure should be no higher than 30 mmHg. Additionally, non-survivors were sicker than survivors, and thus required a higher PEEP and PIP (Table 3).
The Pediatric Acute Lung Injury Mechanical Ventilation study, which is a compilation of 47 pediatric intensive care units in 11 countries, found that over 25% of pediatric patients diagnosed with ALI/ARDS were ventilated with tidal volumes above 10 mL/kg.32 Based on our findings, a randomized trial with high and low tidal volume administration in our burned pediatric population is warranted. Future studies should explore the differences in lung compliance and resistance, as well as work of breathing and blood flow, between high and low tidal volume use. Furthermore, future studies should include the different modes of ventilation such as high frequency percussive ventilation and airway pressure release ventilation.
ACKNOWLEDGMENT
The authors would like to thank the staff of Shriners Hospitals for Children-Galveston for their valuable assistance, especially Vicki Walker, Stacey Brewster, and the respiratory therapy team. We would also like to thank Dr Hal Hawkins for his assistance in autopsy findings and Dr Kristofer Jennings for his assistance regarding the statistics of the manuscript.
Support: This work was supported by Grants P50GM060338, R01GM056687, R01HD049471, H133A120091, and T32GM008256 from the National Institutes of Health and Grants 84080, 79135, 71009, 80100, and 71008 from Shriners Hospitals for Children.
ABBREVIATIONS
- HTV
High tidal volume
- LTV
Low tidal volume
- ARDS
Acute respiratory distress syndrome
- ALI
Acute lung injury
- TBSA
Total burned surface area
- PEEP
Positive end-expiratory pressures
- PIP
Peak inspiratory pressures
- CI
Confidence interval
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the Southern Surgical Association 126th Annual Meeting, Palm Beach, FL, November 30–December 3, 2014.
Disclosure Information: Nothing to disclose.
Disclosures outside the scope of this work: Dr Herndon is a consultant for SimQuest and receives royalities from Elsevier.
REFERENCES
- 1.Pruitt BA, Jr, Goodwin CW, Mason AD., Jr . Epidemiology, demographic, and outcome characteristics of burn injury. In: Herndon D, editor. Total burn care. Philadelphia: Saunders/Elsevier; 2007. pp. 16–32. [Google Scholar]
- 2.Mlcak R, Desai MH, Robinson E, et al. Lung function following thermal injury in children--an 8-year follow up. Burns. 1998;24:213–216. doi: 10.1016/s0305-4179(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 3.Palmieri TL, Warner P, Mlcak RP, et al. Inhalation injury in children: A 10 year experience at shriners hospitals for children. J Burn Care Res. 2009;30:206–208. doi: 10.1097/BCR.0b013e3181923ea4. [DOI] [PubMed] [Google Scholar]
- 4.Shirani KZ, Pruitt BA, Jr, Mason AD., Jr The influence of inhalation injury and pneumonia on burn mortality. Ann Surg. 1987;205:82–87. doi: 10.1097/00000658-198701000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Traber DLEP. Fischman's pulmonary diseases and disorders. 4th ed. New York: McGraw-Hill Medical Publishing Company; 2008. [Google Scholar]
- 6.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2:319–323. [Google Scholar]
- 7.Traber DL, Enkhabaatar P. Thermal lung injury and acute smoke inhalation. In: Fischman DA, editor. Fischman's pulmonary diseases and disorders. Vol. 1. New York: McGraw-Hill Medical Publishing Company; 2008. pp. 1053–1064. [Google Scholar]
- 8.Bernard GR, Artigas A, Brigham KL, et al. The american-european consensus conference on ards. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 9.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: The berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 10.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The acute respiratory distress syndrome network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 11.Marcolin R, Mascheroni D, Pesenti A, et al. Ventilatory impact of partial extracorporeal co2 removal (pecor) in arf patients. ASAIO Transactions. 1986;32:508–510. doi: 10.1097/00002480-198609000-00025. [DOI] [PubMed] [Google Scholar]
- 12.McHugh LG, Milberg JA, Whitcomb ME, et al. Recovery of function in survivors of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1994;150:90–94. doi: 10.1164/ajrccm.150.1.8025779. [DOI] [PubMed] [Google Scholar]
- 13.Peck MD, Harrington D, Mlcak RP, Cartotto R. Potential studies of mode of ventilation in inhalation injury. J Burn Care Res. 2009;30:181–183. doi: 10.1097/BCR.0b013e3181923c7a. [DOI] [PubMed] [Google Scholar]
- 14.Mlcak R, Buffalo M. Pre-hospital management, transport, and emergency care. In: Herndon DN, editor. Total burn care. Vol. 3. Philadelphia: Saunders; 2007. pp. 81–92. [Google Scholar]
- 15.Ordinal---regression models for ordinal data R package 2012. 2014 Available at: http://www.cran.r-project.org/package=ordinal/ [Google Scholar]
- 16.PETO RaP, J. Asymptotically efficient rank invariant test procedures (with discussion) J. R. Statist. Soc. A. 1972;135:185–206. [Google Scholar]
- 17.Team RC. R: A language and environment for statistical computing. Vienna: Foundation for Statistical Computing; 2012. [Google Scholar]
- 18.Ferguson ND, Fan E, Camporota L, et al. The berlin definition of ards: An expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 19.Maslow AD, Stafford TS, Davignon KR, Ng T. A randomized comparison of different ventilator strategies during thoracotomy for pulmonary resection. J Thorac Cardiovasc Surg. 2013;146:38–44. doi: 10.1016/j.jtcvs.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 20.de Jager P, Burgerhof JG, van Heerde M, et al. Tidal volume and mortality in mechanically ventilated children: A systematic review and meta-analysis of observational studies*. Crit Care Med. 2014;42:2461–2472. doi: 10.1097/CCM.0000000000000546. [DOI] [PubMed] [Google Scholar]
- 21.Zick G, Elke G, Becher T, et al. Effect of peep and tidal volume on ventilation distribution and end-expiratory lung volume: A prospective experimental animal and pilot clinical study. PLoS One. 2013;8:e72675. doi: 10.1371/journal.pone.0072675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sly PD, Nicholls PK, Berry LJ, et al. High tidal volume ventilation does not exacerbate acid-induced lung injury in infant rats. Respir Physiol Neurobiol. 2013;189:129–135. doi: 10.1016/j.resp.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am Rev Respir Dis. 1974;110:556–565. doi: 10.1164/arrd.1974.110.5.556. [DOI] [PubMed] [Google Scholar]
- 24.Villar J, Kacmarek RM, Perez-Mendez L, Aguirre-Jaime A. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: A randomized, controlled trial. Crit Care Med. 2006;34:1311–1318. doi: 10.1097/01.CCM.0000215598.84885.01. [DOI] [PubMed] [Google Scholar]
- 25.Wheeler AP, Bernard GR, Thompson BT, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213–2224. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 26.Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 27.Matthay MA, Zemans RL. The acute respiratory distress syndrome: Pathogenesis and treatment. Annu Rev Pathol. 2011;6:147–163. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cepkova M, Matthay MA. Pharmacotherapy of acute lung injury and the acute respiratory distress syndrome. J Intensive Care Med. 2006;21:119–143. doi: 10.1177/0885066606287045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 30.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 31.Cox DR. Regression models and life-tables. Journal of the Royal Statistical Society. 1972;34:187–220. [Google Scholar]
- 32.Santschi M, Randolph AG, Rimensberger PC, Jouvet P. Mechanical ventilation strategies in children with acute lung injury: A survey on stated practice pattern. Pediatr Crit Care Med. 2013;14:e332–e337. doi: 10.1097/PCC.0b013e31828a89a2. [DOI] [PubMed] [Google Scholar]