Abstract
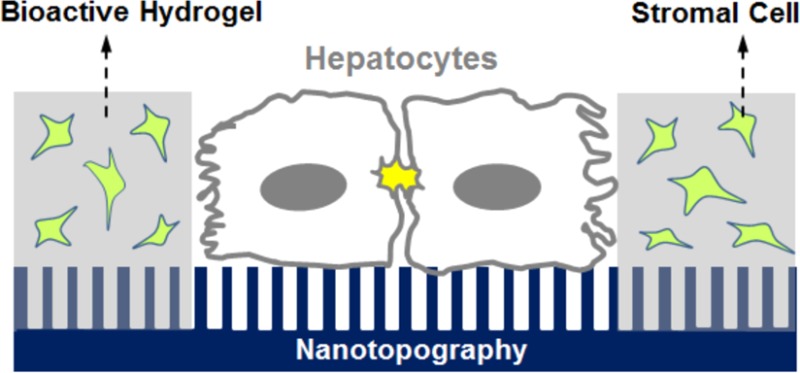
Hepatocytes, the main epithelial cell type in the liver, perform most of the biochemical functions of the liver. Thus, maintenance of a primary hepatocyte phenotype is crucial for investigations of in vitro drug metabolism, toxicity, and development of bioartificial liver constructs. Here, we report the impact of topographic cues alone and in combination with soluble signals provided by encapsulated feeder cells on maintenance of the primary hepatocyte phenotype. Topographic features were 300 nm deep with pitches of either 400, 1400, or 4000 nm. Hepatocyte cell attachment, morphology and function were markedly better on 400 nm pitch patterns compared with larger scale topographies or planar substrates. Interestingly, topographic features having biomimetic size scale dramatically increased cell adhesion whether or not substrates had been precoated with collagen I. Albumin production in primary hepatocytes cultured on 400 nm pitch substrates without collagen I was maintained over 10 days and was considerably higher compared to albumin synthesis on collagen-coated flat substrates. In order to investigate the potential interaction of soluble cytoactive factors supplied by feeder cells with topographic cues in determining cell phenotype, bioactive heparin-containing hydrogel microstructures were molded (100 μm spacing, 100 μm width) over the surface of the topographically patterned substrates. These hydrogel microstructures either carried encapsulated fibroblasts or were free of cells. Hepatocytes cultured on nanopatterned substrates next to fibroblast carrying hydrogel microstructures were significantly more functional than hepatocytes cultured on nanopatterned surfaces without hydrogels or stromal cells significantly elevated albumin expression and cell junction formation compared to cells provided with topographic cues only. The simultaneous presentation of topographic biomechanical cues along with soluble signaling molecules provided by encapsulated fibroblasts cells resulted in optimal functionality of cultured hepatocytes. The provision of both topographic and soluble signaling cues could enhance our ability to create liver surrogates and inform the development of engineered liver constructs.
Keywords: tissue engineering, hepatocyte cultivation, nanotopography, heparin, hydrogel, coculture
1. Introduction
Cell fate and functions in vivo are known to be regulated by the extracellular matrix (ECM) microenvironment comprised of various biophysical and biochemical cues.1−3 It is therefore important to develop cell culture scaffolds that provide essential biophysical and biochemical cues for the optimal control of cell fate and functions. Although delivery of morphogens and designing physiochemical properties of scaffolds for improved cell differentiation and phenotype maintenance in vitro have been investigated extensively,4−10 a knowledge gap exists regarding the impact of topographic cues and their interaction with soluble cytoactive factors on hepatic cell function.11−13
Primary hepatocytes are employed as liver surrogates for in vitro toxicology as well as for the development of bioartificial liver assist devices.14 These cells perform complex metabolic and detoxification functions in vivo and tend to rapidly lose their ability to perform these functions in vitro. In vivo, hepatocytes are embedded within an ECM containing collagen fibrils, structures with width on the scale of hundreds of nanometers and length on the scale of micrometers.15,16 This led us to hypothesize that topographic cues may contribute to development and maintenance of the hepatocyte phenotype. Considering cellular responses to topography are highly dependent on cell type, feature geometry, and feature size, it is important to determine the optimal features for maintenance of hepatocyte phenotype.
In addition, the importance of heterotypic cell–cell interactions has been noted for many tissues including the liver.17−19 In an attempt to mimic interactions between epithelium and stromal elements present in liver, cocultivation of hepatocytes with 3T3 fibroblasts has been demonstrated to significantly increase hepatocyte phenotype expression and maintenance.10,20−22 Bhatia et al. conducted a 3D coculture by encapsulating both hepatocytes and fibroblasts inside PEG based hydrogels. This study demonstrated that fibroblasts are capable of promoting hepatocyte function within hydrogels.23 However, the encapsulation of target cells, such as hepatocytes, within PEG hydrogels creates difficulty in the characterization of cell function as well as the recovery of target cells for regenerative medicine applications.24 It may therefore be beneficial to combine 2D surfaces that allow easy access to hepatocytes with 3D microstructures that confine stromal cells and contain their growth in the culture system.
In this paper, we report the utility of nanopatterned substrates for cultivation of primary hepatocytes. In addition, we explored the impact of integrating hydrogel microstructures with nanopatterned surfaces to create a complex yet biologically relevant cellular microenvironment. Heparin hydrogel was chosen as the biomaterial for constructing hydrogel structures because of its utility for growth factor incorporation and fibroblast cell encapsulation.7−10 Inherent differences in the extent of extracellular matrix protein adsorption on the topographies were determined in addition to the differential cellular response such precoating elicits. Our investigations revealed that substrates patterned with alternating grooves and ridges possessing 400 nm pitch (pitch = ridge width + groove width) most strongly promoted attachment and function of hepatocytes and did not require precoating with hepato-adhesive proteins such as collagen I. Cell function was enhanced further by integrating heparin hydrogel microstructures (stripes) across the surface of underlying topographically patterned substrates. The hydrogel microstructures containing stromal cells further boosted albumin expression of primary hepatocytes. Overall, a novel approach is described here for the provision of both topographic and soluble signaling cues could enhance our ability to create liver surrogates in vitro and inform the development of engineered liver constructs.
2. Experimental Section
2.1. Chemical and Materials
Heparin (sodium salt, from porcine intestinal mucosa) was purchased from Tocris Bioscience (Bristol, UK) and Sigma-Aldrich (St. Louis, MO, USA). Thiolated heparin (Hep-SH) was synthesized with the modification of carboxylic groups of heparin using carbodiimide chemistry, as previously reported.25 Poly(ethylene glycol) diacrylate (PEG-DA, MW 6 kDa, 98% degree of substitution) was purchased from SunBio Inc. (Anyang, Korea). 4-(2-Hydroxyethoxy) phenyl-(2-hydroxy-2-propyl) ketone (Irgacure 2959) was purchased from Ciba Specialty Chemicals Inc. (Basel, Switzerland). Sulfuric acid, ethanol, bovine serum albumin (BSA), and toluidine blue O were purchased from Sigma-Aldrich (St. Louis, MO, USA). Glucagon and recombinant human insulin were obtained from Eli Lilly (Indianapolis, IN, USA), and hydrocortisone sodium succinate was obtained from Pfizer Inc. (Ann Arbor, MI, USA). Phosphate-buffered saline (PBS) was purchased from Gibco (Grand Island, NY, USA). Dulbecco’s modified Eagle’s medium (DMEM), sodium pyruvate, fetal bovine serum (FBS), penicillin/streptomycin were purchased from Life Technologies (Carlsbad, CA, USA). Rat albumin ELISA kit was obtained from Bethyl Laboratories (Montgomery, TX, USA) and urea analysis kit was purchased from Bioassay Systems (Hayward, CA, USA). Paraformaldehyde was purchased from Election Microscopy Sciences (Hatfield, PA, USA). Sheep antialbumin and FITC-antisheep IgG were obtained from Bethyl Laboratories and Santa Cruz Biotechnologies, Inc. (Santa Cruz, CA, USA). Mouse anti-E-cadherin and Alexafluor 546 antimouse were purchased from BD Science and Life Technologies. Mounting medium with DAPI was purchased from Vector Laboratories, Inc. (Burlingame, CA, USA).
2.2. Fabricating Nanotopographically Patterned and Planar Substrates
Silicon masters serving as master stamps were fabricated using X-ray lithography as previously described.26 The silicon masters with large area (6.5 cm2) were 300 nm in depth and 400, 1400, or 4000 nm pitch. The ridge to groove width ratio was 1:1. Chemically identical planar surfaces were used as controls. To conserve silicon master stamps, the topographic patterns were transferred into polydimethylsiloxane (PDMS) stamps that were fabricated using standard soft lithography approaches (Figure 1A).27 To create topographically patterned substrates for cell cultivation, 60 mm diameter tissue culture polystyrene (TCPS) dishes were spin-coated (WS-400–6NPP, Laurell technologies, North Wales, PA) with a thin layer of NOA81 polyurethane (Norland Products, Cranbury, NJ) at 4000 rpm for 40 s and subsequently cured in an XL-1500 UV cross-linker under 365 nm light for 100 min. The detailed protocol for creating these topographically patterned substrates is described elsewhere.28 NOA81has previously been documented to support cell cultivation.28−30 NOA81 substrates were used either uncoated or coated with collagen I prior to seeding with primary hepatocytes.
Figure 1.
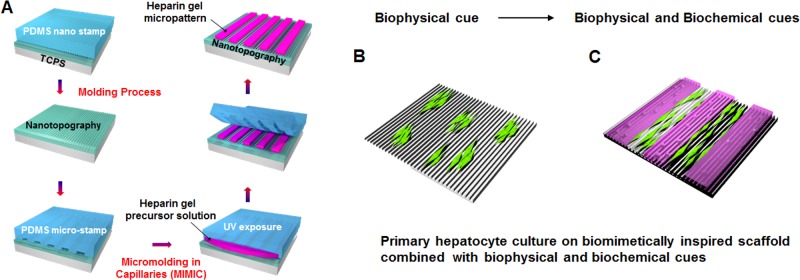
Fabrication of biomimetically inspired scaffolds enable the simultaneous delivery of biophysical and biochemical cues. (A) Schematic illustration of the fabrication of microscale hydrogel patterns across the surface of the topographically patterned substrates used in our study. A combination of soft lithographic methods and micromolding in capillaries was used to create multiscale patterned substrate where microscale hydrogel patterns were constructed on top of nanotopographically patterned substrates having features of differing size scale. Schematic illustration of culturing primary hepatocytes either (B) on topographically patterned substrates alone or (C) in combination with 3T3 cell encapsulated heparin gel microstructures.
2.3. Rhodamine Labeled Fibronectin Adsorption on Planar and 400 nm Pitch Patterned Substrates
A solution of rhodamine-labeled fibronectin in PBS (10 ug/mL) was incubated on substrates for 4 h at 37 °C. The substrates were washed out with PBS three times to remove unattached proteins and then imaged using a laser scanning confocal microscope (LSM700, Carl Zeiss, Jena, Germany)
2.4. Fabricating Hydrogel Microstructures Across the Surface of Topographically Patterned Surfaces
Heparin-based hydrogel microstructures were patterned using molding and UV-initiated thiol–ene polymerization of Hep-SH and PEG-DA. PDMS templates were fabricated using standard soft lithography approaches to contain channels 100 um wide and 20 um deep. These channels could be filled with prepolymer solution by capillary action. The prepolymer solution was comprised of 6 kDa PEG-DA with or without thiolated Hep-SH (1:1 molar ratio of thiol to acrylate group) for fabrication of hydrogel microstructures. The components were dissolved in PBS containing 0.5% w/v photoinitiator (Irgacure 2959) to achieve 10% w/v of gel precursor solutions. The final concentration of photoinitiator was 0.2% w/v. A 20 μL of precursor solution was injected into PDMS channels and exposed to UV light (365 nm, 18 W/cm2, OmniCure series 1000 light source, EXFO, Vanier, Quebec, Canada) for 10 s. Once the gel pattern formed, the stamp was carefully removed. Toluidine blue O staining was conducted to identify the presence of heparin in gel microstructures, as reported previously.31 Staining of negatively charged heparin molecules by this dye (purple color) was visualized using optical microscopy.
2.5. Measurement of Substrate Stiffness Based on Contact Mechanics
A 10 × 10 μm of the substrate was first obtained using an MFP3D-Bio atomic force microscope (Asylum Research, Santa Barbara, CA). From the image scanned, at least 5 locations on various ridges were marked and force vs indentation curved were obtained in contact mode using a silicon nitride probe with a square pyramid tip (κ = 0.32 N/m; α = 36°, PNP-TR-50, Nano and More, Lady’s Island, SC). Five force curves were obtained per position. Applying Hertz model for a square pyramid, elastic modulus was determined as described previously.7 No statistically significant differences in substrate stiffness were observed between planar and topographically patterned surfaces.
2.6. AFM Imaging of Nanotopographically Patterned Substrates
To ensure the preservation of topographic features after collagen adsorption, collagen preadsorbed topographically patterned substrates were imaged using an MFP-3D Bio atomic force microscope (AFM; Asylum Research) with an AC40TS bio lever (Nano and More) in tapping mode in fluid.
2.7. Cell Culture on Nanotopographically Patterned Substrates
Topographically patterned substrates possessing alternating ridges and grooves of 400, 1400, 4000 nm pitch as well as planar controls, were used to culture hepatocytes with and without collagen I preadsorption. To adsorb collagen I, substrates were incubated with rat tail collagen solution (BD Biosciences 0.1 mg/mL) for 1 h at room temperature after which they were washed with PBS solution. Primary hepatocytes were isolated from adult female Lewis rats (Charles River Laboratories, Boston, MA, USA) weighing 125–200 g, using a two-step collagenase perfusion procedure as described previously.32 Primary hepatocytes were maintained in DMEM supplemented with 10% FBS, 200 U/mL penicillin, 200 mg/mL streptomycin, 7.5 mg/mL hydrocortisone sodium succinate, 20 ng/mL EGF, 14 ng/mL glucagon, and 0.5 U/mL recombinant human insulin at 37 °C in a humidified 5% CO2 atmosphere. Topographically patterned substrates and planar control were seeded with 5 × 105 rat primary hepatocytes suspended in 2.5 mL of cell culture medium. After 2 h of incubation at 37 °C, the samples were washed twice in PBS to remove unattached hepatocytes and fresh medium added to the samples.
For coculture experiments based on hepatocyte cultivation on nanotopography and 3T3 cell encapsulation inside heparin gels next to hepatocytes, murine 3T3 fibroblasts were maintained in the DMEM supplemented with 10% FBS, 200 U/ml penicillin and 200 mg/mL streptomycin. To encapsulate 3T3 cells in heparin hydrogels, 3T3 cells were detached and suspended in the precursor gel solution to a final seeding density of 8 × 105 cells/mL and injected into PDMS channels and exposed to UV light source as described before. The viability and proliferation rate of 3T3 cells encapsulated inside heparin gels were examined by LIVE/DEAD staining (Molecular Probes, Eugene, OR) and MTT assay. The 3T3 cells encapsulated heparin hydrogels were washed with PBS solution two times and immediately hepatocytes (1 × 106 cells) were added to 3T3 cells encapsulated heparin gel molded as alternating microscale ridges across the surface of the underlying topographically patterned substrates. After 2 h of incubation at 37 °C, the substrates were washed twice with PBS solution to remove unattached hepatocytes and then hepatocytes and fibroblasts were cultured in hepatocyte culture medium at 37 °C in a humidified 5% CO2 atmosphere.
2.8. Analysis of Hepatocyte Phenotype
The number of hepatocytes attached on each sample was counted by acquiring six microscopic images at 10× magnification per each condition, 1 day after cell seeding. For analysis of hepatic function, culture medium was collected and analyzed for urea and albumin using a urea kit and rat albumin ELISA kit. For intracellular albumin and E-cadherin, the samples were fixed in 4% paraformaldehyde and were washed in PBS solution and then incubated with primary antibodies for albumin (1:100 dilution in PBS) and for E-cadherin (1:50 dilution in PBS). After overnight incubation in 4 °C, samples were washed in PBS solution and then stained with Alexa-Fluor conjugated secondary antibodies for albumin (1:200 dilution in PBS) and for E-cadherin (1:750 dilution in PBS). After 1 h of incubation at room temperature, the samples were washed in PBS and were mounted using a mounting medium containing DAPI to determine the location of nuclei. Stained cells were visualized and imaged using a laser scanning confocal microscope (LSM700, Carl Zeiss, Jena, Germany).
2.9. Statistical Analysis
The results were expressed as the mean ± standard deviation. Student t -test analysis was used for statistical analysis. Differences were considered to be statistically significant at p < 0.05.
3. Results and Discussion
The goal of this study was to explore how substratum/basal topographic cues, in the presence and/or absence of heparin hydrogel microstructures with/without encapsulated fibroblasts, affect the phenotype of hepatocytes (Figure 1). First, we demonstrate that polyurethane-based basal topographic cues of specific dimensions induce elevated expression of hepatocyte markers. Next, we discuss the development of a complex culture environment wherein heparin containing micropatterned hydrogels (with/without encapsulated fibroblasts) are overlaid onto the basal nanotopographic substrates. This allows for the provision of a constrained environment for cell seeding while allowing for biophysical and biochemical stimuli to be simultaneously presented. Using this approach, we demonstrate that culturing primary hepatocytes within the grooves of the heparin hydrogel, while simultaneously being stimulated by the underlying anisotropic topography, promotes the maintenance of the primary hepatocyte phenotype. This work highlights how simultaneous presentation of topographic biomechanical cues along with soluble signaling cues could be introduced onto cell culture surfaces to induce desired cellular phenotype.
3.1. Fabricating Substrates with Micro- and Nanostructures
The micro/nanopatterned substrates were fabricated in two steps as shown in Figure 1A. First, photocurable urethane layer was coated onto TCPS dishes and was used to create topographic cues of differing size scales via soft lithographic methods as previously described.27 Alternating ridges and grooves with pitches of 400, 1400, and 4000 nm and the depth of 300 nm were fabricated onto polystyrene substrates with patterned PDMS molds. Chemically identical planar urethane layers on TCPS served as controls in our experiments.
In a second step, bioactive heparin hydrogel microstructures aligned with the direction of underlying nanotopography were integrated into the substrate using a combination of micromolding and UV induced photopolymerization with micropatterned PDMS mold (Figure 1A). This step was specifically developed to enable delivery of soluble cytoactive factors by encapsulating fibroblasts inside hydrogel microstructures (Figure 1C).
3.2. Characterizing Topographically Patterned Substrates and Cultivating Hepatocytes
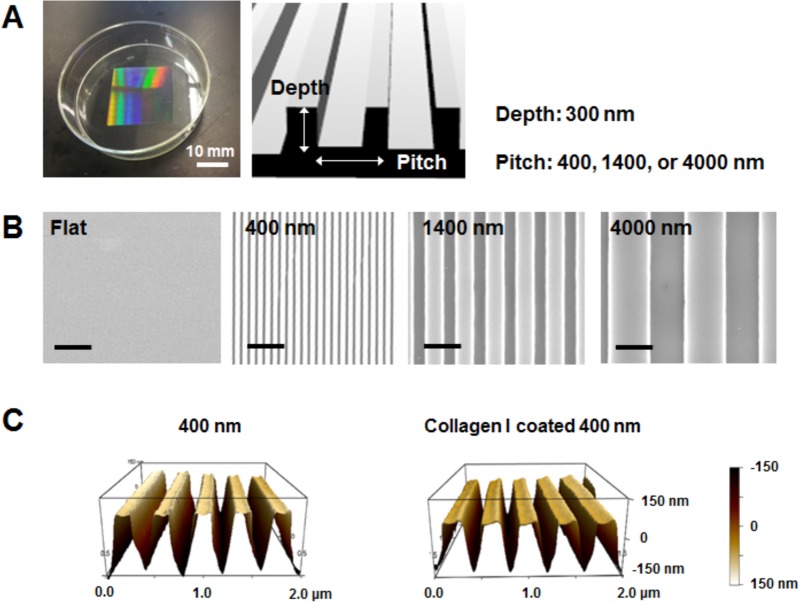
Figure 2A shows the representative photographic image of patterned substrate. The sizes of the pitch in the resulting patterns (depth, 300 nm) ranged from 400 to 1400 nm. SEM images in Figure 2B show highly uniform groove and ridge nanostructure patterns on the fabricated substrates. The dimensions of the nanostructured surface mimicked collagen fibrils that range in size from 10 to 300 nm and fibers that can extend up to several microns.15
Figure 2.
Characterization of topographically patterned substrates. (A) Photograph image of nanotopography on polystyrene substrate and nanoscale pitch/depth pattern with pitch sizes from 400 to 4000 nm. (B) Scanning electron microscopic (SEM) images of flat and patterned substrates (400, 1400, and 4000 nm). Scale bars = 2 μm. (C) Atomic force microscope (AFM) analysis of the 400 nm pitch topographic features before and after collagen I coating demonstrating the preservation of features after coating.
It has been recognized that nanotopographic cues can significantly affect cellular phenotype through contact guidance and integrin-mediated intracellular tension.33−36 To test effects of topographic cues on hepatocytes, these cells were cultured in hepatic medium (containing fetal bovine serum) on 400, 1400, 4000 nm pitch patterned surfaces, and planar controls with and without preadsorption of collagen type I. AFM analysis presented in Figure 2C revealed that preadsorption of monomeric collagen I did not change nanotopography features.
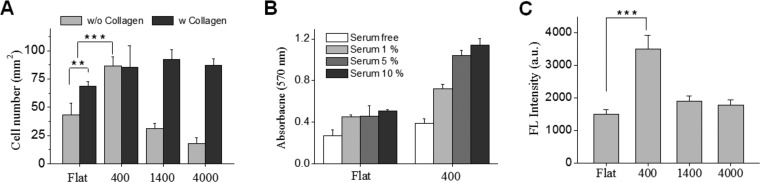
As shown in Figure 3A, hepatocytes exhibited differential adhesion, depending on topographic feature size and preadsorption of collagen I. In the absence of collagen preadsorption, significantly fewer hepatocytes adhered to flat control, 1400 and 4000 nm pitch. Interestingly, hepatocyte adhesion was markedly increased on 400 nm pitch patterned surfaces even in the absence of collagen preadsorption. Cell density on 400 nm pitch patterned substrates without collagen coating was 86 cells/mm2. This density is comparable to flat, 400, 1400, 4000 nm pitches, and TCPS (68, 86, 92, 87, and 81 cells/mm2, respectively) that had been precoated with collagen. One explanation for attachment of hepatocytes without collagen may be that small scale topographic features modulate the adsorption and conformation of serum proteins promoting cell adhesion. There have been several reports suggesting high protein adsorption on nanostructured surfaces and altered conformation of adsorbed proteins.37−41
Figure 3.
Effects of collagen I precoating and different fetal bovine serum (FBS) concentration on the adhesion of primary rat hepatocytes. (A) Quantification of cell adhesion on flat and nanotopography substrates with and without collagen I precoating. Number of cells attached on collage-coated tissue culture polystyrene (TCPS) was 81 cells/mm2. (B) MTT assay of hepatocytes on flat and 400 nm nanotopography substrates in a different FBS concentration. (C) Fluorescence intensity of rhodamine -fibronectin physically adsorbed onto the flat and nanopatterned surfaces. (*** p ≤ 0.001, ** 0.001 < p ≤ 0.01).
To investigate the relationship between the serum adhesion protein and cell adhesion on topographically patterned substrates, we analyzed hepatocytes adhesion using an MTT assay on planar and 400 nm pitch substrates in different serum concentrations (Figure 3B). Under serum-free conditions, limited cell attachment was observed on flat and 400 nm pitch patterned substrates. However, as serum concentration was increased, cell attachment on topographically patterned substrates improved while remaining poor on flat surfaces.
To further analyze the effects of topographic cues on the adsorption of adhesive protein, we incubated flat and topographically patterned substrates with rhodamine-labeled fibronectin. This cell-adhesive protein has been reported to be present in bovine serum.42,43 As shown in Figure 3C and Figure S1 in the Supporting Information, 400 nm pitch patterned surfaces evidenced more than 2 times higher fluorescence compared to flat surfaces. The increase in fluorescence is comparable to the surface area enhancement due to nanostructures. These results are reasonable given that surface area enhancement was 2.5 times for 400 nm pitch patterned surfaces. Therefore, we postulate that a likely reason for attachment of hepatocytes onto 400 nm pitch substrates in the absence of collagen is a large number of other cell-adhesive proteins adsorbing from serum. What these proteins are remains to be determined in the future studies.
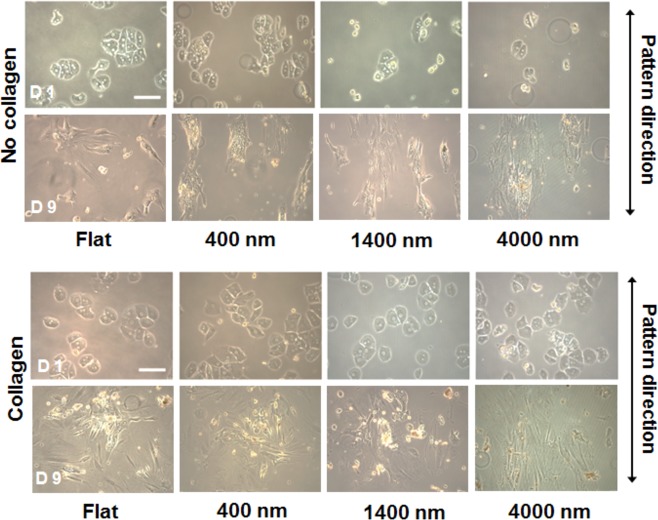
Figure 4 shows representative images of hepatocytes cultured on various substrates with or without collagen coating at day 1 and 9. The image fields contain approximately equivalent cell numbers and nonclustered cells were selected to monitor the change in cell morphology over cell cultivation. On topographically patterned substrates in the absence of collagen precoating, some hepatocytes were observed to be aligned parallel to the long axis of the underlying topographic features. On the other hand, for all size scales of nanotopographic features with collagen precoating, hepatocytes lacked alignment and had a more spread out morphology. We have carried out AFM studies and verified that adsorption of monomeric collagen did not appreciably affect nanotopography (see Figure 2C). As seen from images in Figure 4, hepatocytes cultured on all collagen-coated substrates and collagen-free flat substrates spread out and acquired extended fibroblastic morphology at day 9, indicative of dedifferentiation and loss of the epithelial phenotype. On the other hand, hepatocytes cultured on collagen-free 400 nm pitch substrates became more aggregated morphology at day 9.
Figure 4.
Morphology of primary rat hepatocytes cultured on flat and nanotopography substrates with and without collagen I coating at day 1 and day 9. Scale bar = 200 μm.
We next quantified cell spreading by measuring projected surface area after 24 h of incubation. Cell shape has been recognized as an important parameter in regulating cell fate.44 It has been reported that increased hepatocyte spreading is related to a reduction of liver-specific function.45−47 Cell area on collagen-free substrates was significantly lower compared to collagen precoated substrates (see Figure S2 in the Supporting Information). In the absence of collagen, cells on all topographically patterned substrates were less spread out compared to cells on flat controls.
A more in-depth analysis of hepatic phenotype as a function of nanotopographic cues and collagen adsorption was undertaken and is presented in Figure 5. Albumin production is a reflection of the ability of hepatocytes to synthesize liver-specific serum proteins and is a benchmark of hepatic function in vitro. Albumin ELISA results in Figure 5A show that hepatocytes on all collagen-coated substrates exhibited significantly lower levels of albumin secretion compared to cells on collagen-free substrates. Importantly, the highest level of albumin synthesis was observed on 400 nm pitch patterned substrates with hepatic albumin production remaining high at day 10 culture. In contrast, with cells cultured on larger scale topographic features as well as planar substrates, the albumin levels declined over the course of 10 days documenting a loss of function and possibly indicating dedifferentiation. Because there were no statistically significant differences in substrate stiffness between planar and topographically patterned surfaces (data not shown), the scale nanotopographic features may be the main factor determining hepatocyte function. Similar to ELISA data, intracellular staining for albumin and E-cadherin, another marker of epithelial phenotype and characteristic of differentiated hepatocytes, revealed high expression levels in hepatocytes cultured on 400 nm collagen-free compared to collagen-coated 400 nm pitch and collagen free flat substrates (Figure 5B). This phenotype analysis fits in with our observations that cells on 400 nm pitch collagen-free substrates appeared more epithelial and differentiated than cells on flat collagen-coated substrates. This observation, however, may be somewhat counterintuitive as collagen I coated substrates are commonly used for hepatocyte cultivation. One explanation may be that the use of collagen I is dictated by the need to promote attachment of hepatocytes in vitro and by the ready availability of this protein. There are reports in the literature suggesting that monomeric collagen I coating contributes to dedifferentiation of hepatocytes.16 Therefore, while coating of substrates with collagen I promotes hepatocyte attachment it may inhibit hepatocyte function. Our data suggest that hepatocytes attach and function well on small scale nanotopographically patterned substrates in the absence of adhesion-promoting surface protein coating. Given the cost, batch to batch variability and xenogeneic source of collagen, its exclusion from hepatocyte culture protocols may be a significant step toward providing improved and better defined hepatocyte cultivation procedures.
Figure 5.
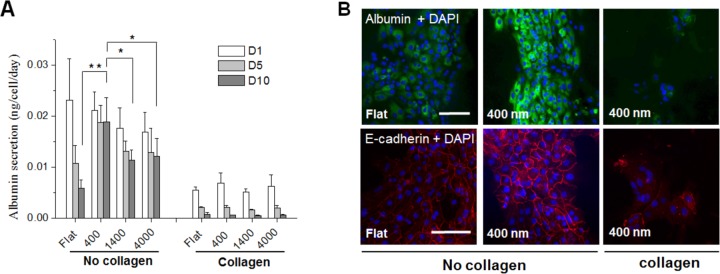
Hepatocyte function is modulated by topographic feature size and collagen I precoating. (A) ELISA analysis of albumin secretion by primary hepatocytes cultured on flat, topographically patterned with and without collagen I adsorption. It can be seen that in the absence of collagen precoating, the presence of topographic cues prevents the rapid decline in albumin secretion over time. The incorporation of collagen precoating diminished the impact of topographic cues in this regard. (B) Intracellular albumin and E-cadherin expression in hepatocytes cultured on flat control and 400 nm pitch with and without collagen I adsorption at day 7. Scale bar = 100 μm. (** 0.001 < p ≤ 0.01, * p < 0.05) In the absence of collagen precoating, the smallest scale features in the biomimetic range produced the greatest expression of albumin and E-cadherin in cultured hepatocytes.
In summary, our studies revealed that hepatocyte attachment on collagen-free 400 nm pitch patterned substrates was comparable to that observed for collagen-coated substrates. Moreover, albumin production and E-cadherin expression of hepatocytes on collagen-free 400 nm pitch patterned substrates was higher than on collagen-coated surfaces. Therefore, 400 nm pitch patterns were chosen for further experiments related to the integration of micron scale hydrogels.
3.3. Incorporating Hydrogel Microstructures Across the Surface of 400 nm Pitch Patterned Substrates
To further increase complexity of the cellular microenvironment, microstructures composed of bioactive heparin gels were fabricated across the surface of 400 nm pitch patterned substrates. The patterned hydrogel (1) provided a constrained environment to allow for simultaneous presentation of biophysical and biochemical cues, (2) provided matrix associated biochemical cues because of the intrinsic composition of the heparin hydrogel, and (3) when seeded with fibroblasts, enabled delivery of soluble cytoactive factors from the embedded fibroblasts. Heparin hydrogels have been shown by us and others to sequester growth factors and to release these molecules in a controlled manner.8,48−50 Furthermore, heparin hydrogels are excellent matrices for encapsulation of functional cells.8,25
Optical microscopic and SEM images in Figure 6 show heparin hydrogel microstructures 100 μm in width with 100 μm spacing fabricated across the surface of a 400 nm pitch patterned substrate. Toluidine blue staining was used to visualize presence of heparin in the hydrogel stripes (Figure 6B).
Figure 6.
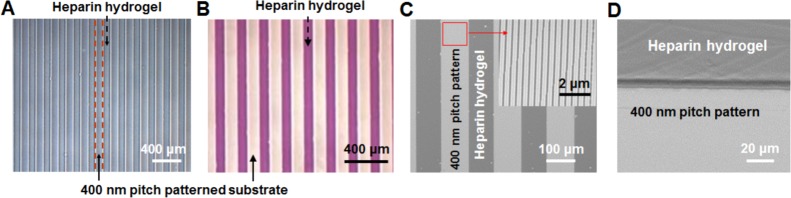
Characterization of biomimetically inspired scaffolds enable the simultaneous presentation of biophysical and biochemical cues. (A) Optical microscopic images of the 100 μm heparin hydrogel patterns integrated across the surface of 400 nm pitch patterned substrates. (B) Optical microscopic image after toluidine blue (TB) staining. (C) Scanning electron microscopic (SEM) images of the 100 μm heparin hydrogel patterns integrated across the surface of 400 nm pitch patterned substrates. Inset: Magnified SEM images of 400 nm pitch patterned substrates. (D) Tilted SEM image showing heparin hydrogel patterns fabricated across the surface of 400 nm pitch patterned substrates.
Phenotype of primary hepatocytes is enhanced by cocultivation with fibroblasts stromal cells that secrete cytoactive factors.20,51 In this study, we sought to integrate stromal cells, 3T3 fibroblasts, into the hydrogel microstructures. Cells were incorporated into prepolymer solution and successfully encapsulated into hydrogel microstructures as shown in Figure S3A in the Supporting Information. To examine the biocompatibility and proliferation of fibroblasts inside 3D heparin hydrogel structures, Live/Dead staining, and MTT assay were conducted. Most fibroblasts inside heparin hydrogel microstructures were found to be alive at 14 days in culture, indicating high cell viability (∼95%) (see Figure S3B in the Supporting Information). The proliferation of fibroblasts was additionally evaluated using the MTT assay (see Figure S3C in the Supporting Information). As expected, the proliferation rate of fibroblasts encapsulated inside heparin hydrogels was significantly lower than that of fibroblasts cultured on TCPS. The low proliferation rate of cells encapsulated inside hydrogels might originate from contact inhibition.52 These findings suggest that even though 3T3 fibroblasts did not proliferate highly inside heparin hydrogels, the majority of the embedded cells remained viable after 14 days in culture.
After molding the cell-carrying hydrogel microstructures onto nanopatterned surfaces, the substrates were incubated with hepatocytes, which attached on 400 nm pitch patterned regions between hydrogel lanes (Figure 7). Three types of hydrogels were used in these experiments that were all molded as alternating ridges and grooves over the surface of the 400 nm pitch topographically patterned substrates: (1) PEG hydrogel (biologically inert), (2) bioactive heparin hydrogel (without fibroblasts), and (3) heparin hydrogel with embedded fibroblasts. As seen from Figure 7C, hepatocytes on 400 nm pitch patterned surfaces in the proximity of encapsulated fibroblasts exhibited a less-elongated cell morphology and clear cell–cell borders, markers of the hepatic phenotype, whereas cells on other substrates lost these markers.
Figure 7.
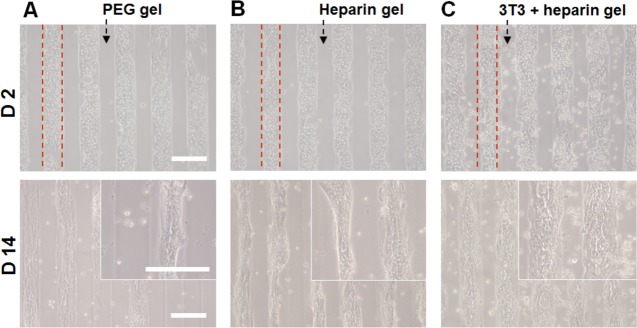
Bright-field microscopic images of hepatocytes monocultured on (A) PEG hydrogel microstructure and (B) heparin gel microstructure, (without integrated cells) molded on surface of 400 nm pitch patterned substrate, (C) cocultured on heparin gel microstructure molded with long axis of micropatterned hydrogel parallel to long axis of underlying 400 nm pitch patterned substrate. Images were obtained at day 2 and 14. Scale bar = 200 μm. Primary rat hepatocytes were cultured on three kinds of hydrogel 100 μm microstructure patterned 400 nm pitch for mono- and coculture. In coculture, hepatocytes were cultured on 400 nm pitch patterned substrates and 3T3 fibroblasts were cultured within the overlying heparin gel 100 μm micropatterns.
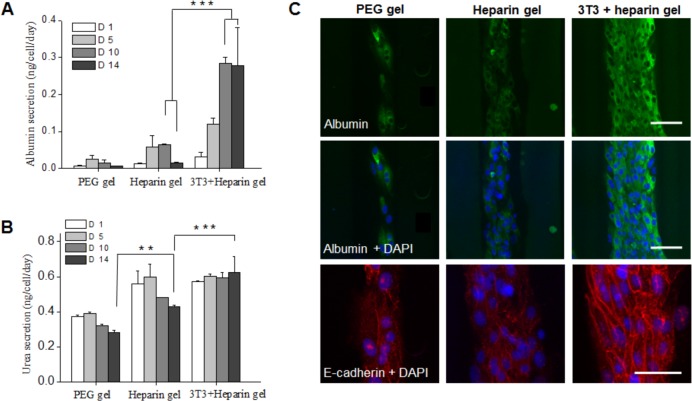
ELISA results shown in Figure 8A highlight differences in albumin production by hepatocytes cultured in different microenvironments. For example at day 10, hepatocytes cultured within the grooves of the heparin hydrogel produced 4 times more albumin than the cells cultured within the grooves of PEG only hydrogels. This observation is consistent with our previous reports suggesting that heparin gels are bioactive even in the absence of encapsulated trophic factors or cells.9 Hepatocytes cultured in the presence of inert PEG gel ridges and grooves produced similar amount of albumin (on a per cell basis) compared to hepatocytes cultured on nanopatterned surfaces without gel microstructures. The highest level of albumin function was observed in hepatocytes cultured next to heparin hydrogel with embedded stromal cells. These hepatocytes were 4.5 times more functional than cells next to heparin gel stripes without fibroblasts and 18 times more functional than hepatocytes cultures adjacent to the PEG only hydrogel. This demonstrates that confinement of hepatocytes in the grooves created by the hydrogel molded across the surface of the substrates did not play an appreciable role in phenotype enhancement but the presence of heparin was enabling. Furthermore, embedding fibroblasts within heparin hydrogels significantly promoted the maintenance of hepatocyte phenotype.
Figure 8.
Liver-specific functions of primary hepatocytes at mono- and coculture. (A) ELISA analysis of albumin secretion and (B) urea synthesis by hepatocytes at different time points during 14 days of culture. Error bars indicate standard deviation of the mean for n = 3 samples. (C) Intracellular albumin and expression of E-cadherin in hepatocytes monocultured on PEG hydrogel microstructure, heparin gel microstructure and cocultured on heparin gel microstructure at day 10. Green fluorescence was intracellular albumin and blue fluorescence was DAPI staining of nuclei. Red fluorescence is E-cadherin. Scale bar = 50 μm. (*** p ≤ 0.001, ** 0.001 < p ≤ 0.01).
Additionally, although urea synthesis was less pronounced in comparison with albumin secretion on all surfaces, the highest level of urea secretion was observed when hepatocytes were cultured between fibroblast embedded heparin hydrogels (Figure 8B). Immunofluorescence staining for albumin and E-cadherin corroborated findings of the albumin ELISA and urea secretion results (Figure 8C). The highest albumin and E-cadherin levels were observed for hepatocytes cocultured with heparin gels containing embedded fibroblasts were molded across the surface of the 400 nm pitch substrates. In aggregate, the data suggest that hepatocyte phenotype expression on 400 nm pitch patterned substrates was further enhanced by the simultaneous presentation of biochemical cues coming from heparin hydrogel microstructures and encapsulated stromal cells.
4. Conclusions
In this study, we describe a system that provides both biophysical and soluble biochemical signals for cultivation of primary hepatocytes. We considered three major factors to develop our biomaterial scaffold: (1) creating nanotopographically patterned substrates to guide cell–substrate interactions; (2) adding heparin gel microstructures to the nanopatterned substrates; (3) embedding stromal cells into heparin gel structures for production and release of soluble cytoactive factors.
We demonstrated that 400 nm pitch patterned surfaces elicited the highest level of hepatocyte function compared with larger scale topographic cues (1400 and 4000 nm pitch) and planar substrates. Hepatocyte function on 400 nm pitch patterned surfaces was enhanced by introducing heparin gel microstructures, and was elevated further by incorporating fibroblasts into the gel. These data have relevance to the development of cellular microenvironments with improved functionality.
Acknowledgments
The authors thank Mr Yow-Ren Chang for technical assistance. This study was funded by grants from National Institutes of Health: 2R01EY016134 awarded to C.J.M. and R01DK079977 awarded to A.R.
Supporting Information Available
Photograph image of topographically patterned substrate on polystyrene, fluorescent images of rhodamine fibronectin physically adsorbed onto the flat and patterned surfaces, analysis of cell area of hepatocytes cultured on flat and topographically patterned substrates, alive/dead staining and MTT assay of 3T3 fibroblasts encapsulated inside heparin gels. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Trappmann B.; Gautrot J. E.; Connelly J. T.; Strange D. G. T.; Li Y.; Oyen M. L.; Stuart M. A. C.; Boehm H.; Li B. J.; Vogel V.; Spatz J. P.; Watt F. M.; Huck W. T. S. Extracellular-matrix Tethering Regulates Stem-Cell Fate. Nat. Mater. 2012, 11, 642–649. [DOI] [PubMed] [Google Scholar]
- Lienemann P. S.; Karlsson M.; Sala A.; Wischhusen H. M.; Weber F. E.; Zimmermann R.; Weber W.; Lutolf M. P.; Ehrbar M. A Versatile Approach to Engineering Biomolecule-Presenting Cellular Microenvironments. Adv. Healthcare Mater. 2013, 2, 292–296. [DOI] [PubMed] [Google Scholar]
- Albrecht D. R.; Underhill G. H.; Wassermann T. B.; Sah R. L.; Bhatia S. N. Probing The Role of Multicellular Organization In Three-Dimensional Microenvironments. Nat. Methods 2006, 3, 369–375. [DOI] [PubMed] [Google Scholar]
- Gasiorowski J. Z.; Liliensiek S. J.; Russell P.; Stephan D. A.; Nealey P. F.; Murphy C. J. Alterations in Gene Expression of Human Vascular Endothelial Cells Associated with Nanotopographic Cues. Biomaterials 2010, 31, 8882–8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. T.; Wood J. A.; Shah N. M.; Hughbanks M. L.; Russell P.; Barakat A. I.; Murphy C. J. Integration of Basal Topographic Cues and Apical Shear Stress in Vascular Endothelial Cells. Biomaterials 2012, 33, 4126–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watari S.; Hayashi K.; Wood J. A.; Russell P.; Nealey P. F.; Murphy C. J.; Genetos D. C. Modulation of Osteogenic Differentiation in hMSCs Cells by Submicron Topographically-Patterned Ridges and Grooves. Biomaterials 2012, 33, 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J.; Park S. A.; Shin D. S.; Patel D.; Raghunathan V. K.; Kim M.; Murphy C. J.; Tae G.; Revzin A. Characterizing the Effects of Heparin Gel Stiffness on Function of Primary Hepatocytes. Tissue Eng., Part A 2013, 19, 2655–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.; Lee J. Y.; Jones C. N.; Revzin A.; Tae G. Heparin-Based Hydrogel as a Matrix for Encapsulation and Cultivation of Primary Hepatocytes. Biomaterials 2010, 31, 3596–3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S. S.; Kim M.; Cahill-Thompson K.; Tae G.; Revzin A. Micropatterning of Bioactive Heparin-Based Hydrogels. Soft Matter 2011, 7, 3133–3140. [Google Scholar]
- You J.; Shin D. S.; Patel D.; Gao Y.; Revzin A. Multilayered Heparin Hydrogel Microwells for Cultivation of Primary Hepatocytes. Adv. Healthcare Mater. 2013, 3, 126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. H.; Lee H.; Lee Y. K.; Nam J. M.; Levchenko A. Biomimetic Nanopatterns as Enabling Tools for Analysis and Control of Live Cells. Adv. Mater. 2010, 22, 4551–4566. [DOI] [PubMed] [Google Scholar]
- Bettinger C. J.; Langer R.; Borenstein J. T. Engineering Substrate Topography at the Micro- and Nanoscale to Control Cell Function. Angew. Chem., Int. Ed. 2009, 48, 5406–5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonca G.; Mendonca D. B.; Aragao F. J.; Cooper L. F. Advancing Dental Implant Surface Technology--from Micron- to Nanotopography. Biomaterials 2008, 29, 3822–3835. [DOI] [PubMed] [Google Scholar]
- Hewitt N. J.; Lechon M. J.; Houston J. B.; Hallifax D.; Brown H. S.; Maurel P.; Kenna J. G.; Gustavsson L.; Lohmann C.; Skonberg C.; Guillouzo A.; Tuschl G.; Li A. P.; LeCluyse E.; Groothuis G. M.; Hengstler J. G. Primary Hepatocytes: Current Understanding of the Regulation of Metabolic Enzymes and Transporter Proteins, and Pharmaceutical Practice for the Use of Hepatocytes in Metabolism, Enzyme Induction, Transporter, Clearance, and Hepatotoxicity Studies. Drug Metab. Rev. 2007, 39, 159–234. [DOI] [PubMed] [Google Scholar]
- Flemming R. G.; Murphy C. J.; Abrams G. A.; Goodman S. L.; Nealey P. F. Effects of Synthetic Micro- and Nano-Structured Surfaces on Cell Behavior. Biomaterials 1999, 20, 573–88. [DOI] [PubMed] [Google Scholar]
- Hansen L. K.; Wilhelm J.; Fassett J. T. Regulation of Hepatocyte Cell Cycle Progression and Differentiation by Type I Collagen Structure. Curr. Top. Dev. Biol. 2006, 72, 205–236. [DOI] [PubMed] [Google Scholar]
- Aufderheide E.; Chiquet-Ehrismann R.; Ekblom P. Epithelial-Mesenchymal Interactions in the Developing Kidney Lead to Expression of Tenascin in the Mesenchyme. J. Cell Biol. 1987, 105, 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia S. N.; Balis U. J.; Yarmush M. L.; Toner M. Microfabrication of Hepatocyte/Fibroblast Co-Cultures: Role of Homotypic Cell Interactions. Biotechnol. Prog. 1998, 14, 378–387. [DOI] [PubMed] [Google Scholar]
- Houssaint E. Differentiation of the mouse hepatic primordium. I. An Analysis of Tissue Interactions in Hepatocyte Differentiation. Cell Differ. 1980, 9, 269–279. [DOI] [PubMed] [Google Scholar]
- Bhatia S. N.; Balis U. J.; Yarmush M. L.; Toner M. Probing Heterotypic Cell Interactions: Hepatocyte Function in Microfabricated Co-Cultures. J. Biomater. Sci., Polym. Ed. 1998, 9, 1137–1160. [DOI] [PubMed] [Google Scholar]
- Lee J. Y.; Shah S. S.; Yan J.; Howland M. C.; Parikh A. N.; Pan T.; Revzin A. Integrating Sensing Hydrogel Microstructures into Micropatterned Hepatocellular Cocultures. Langmuir 2009, 25, 3880–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revzin A.; Rajagopalan P.; Tilles A. W.; Berthiaume F.; Yarmush M. L.; Toner M. Designing a Hepatocellular Microenvironment with Protein Microarraying and Poly(ethylene glycol) Photolithography. Langmuir 2004, 20, 2999–3005. [DOI] [PubMed] [Google Scholar]
- Underhill G. H.; Chen A. A.; Albrecht D. R.; Bhatia S. N. Assessment of Hepatocellular Function within PEG hydrogels. Biomaterials 2007, 28, 256–270. [DOI] [PubMed] [Google Scholar]
- Cruise G. M.; Scharp D. S.; Hubbell J. A. Characterization of Permeability and Network Structure of Interfacially Photopolymerized Poly(Ethylene Glycol) Diacrylate Hydrogels. Biomaterials 1998, 19, 1287–1294. [DOI] [PubMed] [Google Scholar]
- Tae G.; Kim Y. J.; Choi W. I.; Kim M.; Stayton P. S.; Hoffman A. S. Formation of a Novel Heparin-Based Hydrogel in the Presence of Heparin-Binding Biomolecules. Biomacromolecules 2007, 8, 1979–1986. [DOI] [PubMed] [Google Scholar]
- Karuri N. W.; Liliensiek S.; Teixeira A. I.; Abrams G.; Campbell S.; Nealey P. F.; Murphy C. J. Biological Length Scale Topography Enhances Cell-Substratum Adhesion of Human Corneal Epithelial Cells. J. Cell Sci. 2004, 117, 3153–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin D.; Xia Y.; Whitesides G. M. Soft Lithography for Micro- and Nanoscale Patterning. Nat. Protoc. 2010, 5, 491–502. [DOI] [PubMed] [Google Scholar]
- Liliensiek S. J.; Wood J. A.; Yong J. A.; Auerbach R.; Nealey P. F.; Murphy C. J. Modulation of Human Vascular Endothelial Cell Behaviors by Nanotopographic Cues. Biomaterials 2010, 31, 5418–5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liliensiek S. J.; Campbell S.; Nealey P. F.; Murphy C. J. The Scale of Substratum Topographic Features Modulates Proliferation of Corneal Epithelial Cells and Corneal Fibroblasts. J. Biomed. Mater. Res., Part A 2006, 79A, 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee C. T.; Raghunathan V. K.; Nealey P. F.; Russell P.; Murphy C. J. Topographic Modulation of the Orientation and Shape of Cell Nuclei and Their Influence on the Measured Elastic Modulus of Epithelial Cells. Biophys. J. 2011, 101, 2139–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N.; Kiick K. L. Polysaccharide-Poly(Ethylene Glycol) Star Copolymer as a Scaffold for the Production of Bioactive Hydrogels. Biomacromolecules 2005, 6, 1921–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. C.; Yarmush M. L.; Koebe H. G.; Tompkins R. G. Hepatocyte Function and Extracellular Matrix Geometry: Long-Term Culture in a Sandwich Configuration. FASEB J. 1989, 3, 174–177. [DOI] [PubMed] [Google Scholar]
- Kim D. H.; Provenzano P. P.; Smith C. L.; Levchenko A. Matrix Nanotopography as a Regulator of Cell Function. J. Cell Biol. 2012, 197, 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira A. I.; Abrams G. A.; Bertics P. J.; Murphy C. J.; Nealey P. F. Epithelial Contact Guidance on Well-Defined Micro- and Nanostructured Substrates. J. Cell Sci. 2003, 116, 1881–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara L. E.; McMurray R. J.; Biggs M. J.; Kantawong F.; Oreffo R. O.; Dalby M. J. Nanotopographical Control of Stem Cell Differentiation. J. Tissue Eng. 2010, 1, 120623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs M. J.; Richards R. G.; Dalby M. J. Nanotopographical Modification: a Regulator of Cellular Function Through Focal Adhesions. Nanomedicine. 2010, 6, 619–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis A.; Wilkinson C. Topographical Control of Cells. Biomaterials 1997, 18, 1573–1583. [DOI] [PubMed] [Google Scholar]
- Galli C.; Coen M. C.; Hauert R.; Katanaev V. L.; Groning P.; Schlapbach L. Creation of Nanostructures to Study the Topographical Dependency of Protein Adsorption. Colloids Surf., B 2002, 26, 255–267. [Google Scholar]
- Galli C.; Coen M. C.; Hauert R.; Katanaev V. L.; Wymann M. P.; Groning P.; Schlapbach L. Protein Adsorption on Topographically Nanostructured Titanium. Surf. Sci. 2001, 474, L180–L184. [Google Scholar]
- Tsai W. B.; Lin J. H. Modulation of Morphology and Functions of Human Hepatoblastoma Cells by Nano-Grooved Substrata. Acta Biomater. 2009, 5, 1442–1454. [DOI] [PubMed] [Google Scholar]
- Alvarez S. D.; Derfus A. M.; Schwartz M. P.; Bhatia S. N.; Sailor M. J. The Compatibility of Hepatocytes with Chemically Modified Porous Silicon with Reference to in Vitro Biosensors. Biomaterials 2009, 30, 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid T.; Kenney C.; Waring G. O. Isolation and Characterization of Fibronectin from Bovine Aqueous Humor. Invest. Ophthalmol. Visual Sci. 1982, 22, 57–61. [PubMed] [Google Scholar]
- Hayman E. G.; Ruoslahti E. Distribution of Fetal Bovine Serum Fibronectin and Endogenous Rat Cell Fibronectin in Extracellular Matrix. J. Cell Biol. 1979, 83, 255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhvi R.; Kumar A.; Lopez G. P.; Stephanopoulos G. N.; Wang D. I. C.; Whitesides G. M.; Ingber D. E. Engineering Cell-Shape and Function. Science 1994, 264, 696–698. [DOI] [PubMed] [Google Scholar]
- Benzeev A.; Robinson G. S.; Bucher N. L. R.; Farmer S. R. Cell-Cell and Cell-Matrix Interactions Differentially Regulate the Expression of Hepatic and Cytoskeletal Genes in Primary Cultures of Rat Hepatocytes. Proc. Natl. Acad. Sci. U.S.A. 1988, 85, 2161–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney D.; Hansen L.; Vacanti J.; Langer R.; Farmer S.; Ingber D. Switching from Differentiation to Growth in Hepatocytes - Control by Extracellular-Matrix. J. Cell. Physiol. 1992, 151, 497–505. [DOI] [PubMed] [Google Scholar]
- Carlisle E. S.; Mariappan M. R.; Nelson K. D.; Thomes B. E.; Timmons R. B.; Constantinescu A.; Eberhart R. C.; Bankey P. E. Enhancing Hepatocyte Adhesion by Pulsed Plasma Deposition and Polyethylene Glycol Coupling. Tissue Eng. 2000, 6, 45–52. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Cai S.; Shu X. Z.; Shelby J.; Prestwich G. D. Release of Basic Fibroblast Growth Factor from a Crosslinked Glycosaminoglycan Hydrogel Promotes Wound Healing. Wound Repair Regen. 2007, 15, 245–251. [DOI] [PubMed] [Google Scholar]
- Tae G.; Kornfield J. A.; Hubbell J. A. Sustained Release of Human Growth Hormone from in Situ Forming Hydrogels Using Self-Assembly of Fluoroalkyl-Ended Poly(Ethylene Glycol). Biomaterials 2005, 26, 5259–5266. [DOI] [PubMed] [Google Scholar]
- Choi W. I.; Kim M.; Tae G.; Kim Y. H. Sustained Release of Human Growth Hormone from Heparin-Based Hydrogel. Biomacromolecules 2008, 9, 1698–1704. [DOI] [PubMed] [Google Scholar]
- Bhandari R. N.; Riccalton L. A.; Lewis A. L.; Fry J. R.; Hammond A. H.; Tendler S. J.; Shakesheff K. M. Liver Tissue Engineering: a Role for Co-Culture Systems in Modifying Hepatocyte Function and Viability. Tissue Eng. 2001, 7, 345–357. [DOI] [PubMed] [Google Scholar]
- Kobayashi A.; Yamakoshi K.; Yajima Y.; Utoh R.; Yamada M.; Seki M. Preparation of Stripe-Patterned Heterogeneous Hydrogel Sheets Using Microfluidic Devices for High-Density Coculture of Hepatocytes and Fibroblasts. J. Biosci. Bioeng. 2013, 116, 761–767. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.