Abstract
As part of an ongoing project to explore filamentous fungi for anticancer and antibiotic leads, eleven compounds were isolated and identified from an organic extract of the fungus Scytalidium album (MSX51631) using bioactivity-directed fractionation against human cancer cell lines. Of these, eight were a series of sorbicillinoid analogues (1–8), of which four were new [scalbucillin A (2), scalbucillin B (3), scalbucillin C (6), and scalbucillin D (8)], two were phthalides (9–10), and one was naphthalenone (11). Compounds (1–11) were tested in the MDA-MB-435 (melanoma) and SW-620 (colon) cancer cell lines. Compound 1 was the most potent with IC50 values of 1.5 and 0.5 μM, respectively, followed by compound 5, with IC50 values of 2.3 and 2.5 μM at 72 h. Compound 1 showed a 48-h IC50 value of 3.1 μM when tested against the lymphocytic leukemia cell line OSU-CLL, while the nearly identical compound 5 had almost no activity in this assay. Compounds 1 and 5 showed selective and equipotent activity against Aspergillus niger with minimum inhibitory concentration values of 0.05 and 0.04 μg/ml (0.20 and 0.16 μM), respectively. The in vitro hemolytic activity against sheep erythrocytes of compounds 1 and 5 was investigated and were found to provoke 10% hemolysis at 52.5 and 45.0 μg/ml, respectively, indicative of a promising safety factor.
Keywords: cytotoxicity, fungus, antifungal, hemolysis, Scytalidium, sorbicillinoid analogues
INTRODUCTION
Bioactivity-directed purification studies of filamentous fungi from the Mycosynthetix library, representing over 55,000 accessions, have yielded structurally diverse cytotoxic secondary metabolites.1–6 As part of these, the organic extract of a solid phase culture of a fungus identified as Scytalidium album (MSX51631), which was isolated in 1990 from Cobb County, Georgia, USA, showed promising cytotoxic activities against the SW-620 (colon) and MDA-MB-435 (melanoma) cancer cell lines. Bioactivity-directed fractionation resulted in the isolation and identification of eight sorbicillinoid analogues (1–8), of which four were new [scalbucillin A (2), scalbucillin B (3), scalbucillin C (6) and scalbucillin D (8)] and four were known [5'-formyl-2'-hydroxyl-4'-methoxy-(E,E)-sorbophenone (1), 1-(2'-hydroxy-4'-methoxy-5'-methylphenyl)-2,4-E,E-hexadien-1-one (4), 5′-formyl-2′-hydroxy-4′-methoxy-(E)-4-hexenophenone (5), and 1-(2'-hydroxy-4'-methoxy-5'-hydroxymethylphenyl)-E-4-hexen-1-one (7)]. Also isolated were two phthalides [5,7-dimethoxyphthalide (9) and 4,6-dimethoxyphthalide (10)] and one naphthalenone [isosclerone (11)]. Compounds 1–11 were evaluated for cytotoxicity against human cancer cell lines, and compounds 1 and 5 were further evaluated for growth inhibitory activity against a B-lymphocytic leukemia cell line, OSU-CLL.7 The antimicrobial activities of all isolates were also evaluated in an array of bacteria and fungi. The hemolytic activity of compounds 1, 3, and 5 was measured against sheep red blood cells, so as to probe a safety factor with respect the promising anti-Aspergillus activity of these three compounds.
RESULTS AND DISCUSSION
Two solid-substrate large-scale cultures of the fungus Scytalidium album were extracted with 1:1 CHCl3-MeOH and partitioned with water. The vacuum-dried organic extract was then defatted with 1:1 CH3CN/MeOH-hexane. The resulting CH3CN/MeOH-soluble extract showed cytotoxic activity against the SW-620 and MDA-MB-435 cancer cell lines (~83% and 71% inhibition of cell growth when tested at 20 μg/ml, respectively) and was subjected to fractionation using flash chromatography. Active fractions on the two cancer cell lines were purified using reversed-phase preparative and semi-preparative HPLC to yield compounds 1–11 with >95% purity as evidenced by UPLC (Supplementary Figure S1).
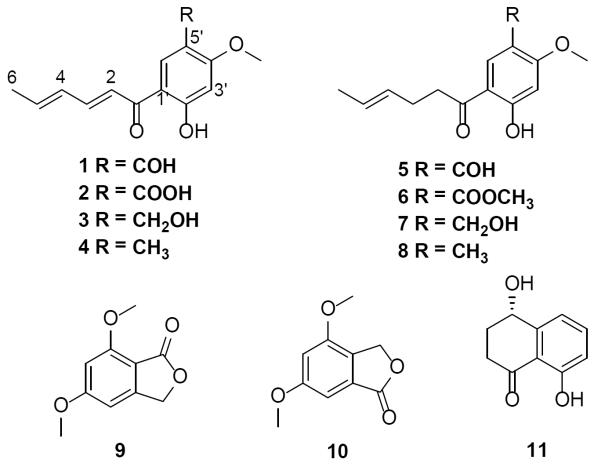
A series of eight sorbicillinoid analogues (1–8), of which four were new, all possessing the carbon skeleton of the known antibiotic, sorbicillin,8 were isolated and identified in this study (Figure 1). The NMR, HRMS, and UV data identified 1 (13.87 mg) and 5 (3.25 mg) as the known 5'-formyl-2'-hydroxyl-4'-methoxy-(E,E)-sorbophenone and 5′-formyl-2′-hydroxy-4′-methoxy-(E)-4-hexenophenone, respectively. Both of these were isolated in 1973 from the fungus Scytalidium sp. FY strain.9 In an analogous manner, compounds 4 (5.89 mg) and 7 (8.21 mg) were identified as the known 1-(2'-hydroxy-4'-methoxy-5'-methylphenyl)- E,E-2,4-hexadien-1-one, and 1-(2'-hydroxy-4'-methoxy-5'-hydroxymethylphenyl)-E-4-hexen-1-one, respectively. Both 4 and 7 were isolated in 2006 from a fungicolous isolate of the genus Phaeoacremonium.10 Characterization data for these known isolates compared favorably with the literature (See Supplementary Figures S2, S5, S6, and S8 for 1H and 13C NMR spectra of compounds 1, 4, 5, and 7, respectively).
Figure 1.
Structure of compounds (1 – 11) isolated from S. album.
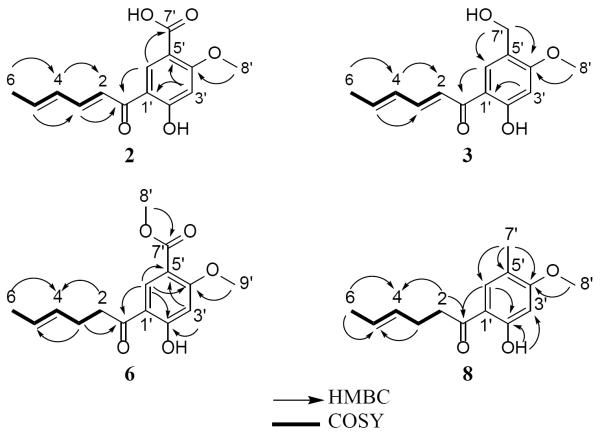
Four new sorbicillinoid analogues (2, 3, 6, and 8) were isolated as well, and their structures were identified by comparison of HRMS and NMR data with each other and with structurally related known compounds. Compound 2 (1.77 mg) was obtained as a yellow powder. The molecular formula was determined as C14H14O5 via HRESIMS along with 1H, 13C, and edited-HSQC NMR data (Tables 1 and 2, Supplementary Figure S3), establishing an index of hydrogen deficiency of 8. Inspection of the HRMS and NMR data suggested 2 as a sorbicillinoid analogue with structural similarity with 1. For example, compound 2 showed the characteristic C1'–C6' sorbyl side chain, as noted by two conjugated double bonds with coupling constants consistent with an E, E configuration (JH-2/H-3 and JH-4/H-5 = 14.6 Hz) that were conjugated with a ketone carbonyl (δC 192.8) that was further conjugated with an aryl group. Additional similarities included NMR signals characteristic of six aromatic carbons and two singlet aromatic protons, suggesting a 1,2,4,5-tetrasubstituted benzene ring with two aromatic protons para to each other, a methoxy group, and a hydrogen-bonded phenolic proton. All of the benzene ring substituents were confirmed by HMBC correlations (Figure 2). Key differences between compounds 1 and 2 were the replacement of the singlet aldehyde proton and the carbonyl carbon in 1 (δH/δC 10.27/187.7) by a broad exchangeable proton and a carboxylic acid carbon in 2 (δH/δC 10.05/164.3). These data, along with an extra oxygen atom in 2 relative to 1, as supported by a 16 amu difference in the HRMS data, suggested that the aldehyde substituent at C-5' of the benzene ring in 1 was replaced by a carboxylic acid group in 2. The trivial name scalbucillin A was ascribed to 2.
Table 1.
1H NMR data for compounds 2, 3, 6 and 8 (in CDC13, 700 MHz for 2 and 3 and 500 MHz for 6 and 8, chemical shifts in ppm, coupling constants in Hz)
| Position | 2 | 3 | 6 | 8 |
|---|---|---|---|---|
|
| ||||
| δH, Mult. (J in Hz) | δH, Mult. (J in Hz) | δH, Mult. (J in Hz) | δH, Mult. (J in Hz) | |
| 1 | — | — | — | — |
| 2 | 7.05, d (14.6) | 6.95, d (15.0) | 3.02, dd (7.5, 6.9) | 2.95, dd (8.0, 7.5) |
| 3 | 7.54, dd (14.6, 9.9) | 7.49, dd (15.0, 10.6) | 2.41, ddd (7.5, 6.9, 6.3) | 2.40, ddd (8.0, 7.5, 5.2) |
| 4 | 6.39, m | 6.34, m | 5.49, dtq (15.5, 6.3, 1.2) | 5.49, dtq (15.5, 5.2, 0.6) |
| 5 | 6.38, dq (14.6, 5.1) | 6.31, dq (15.0, 6.4) | 5.50, dq (15.5, 6.3) | 5.50, dq (15.5, 5.2) |
| 6 | 1.94, d (5.1) | 1.92, dd (6.4, 0.6) | 1.65, dd (6.3, 1.2) | 1.65, dd (5.2, 0.6) |
| 1' | — | — | — | — |
| 2' | — | — | — | — |
| 3' | 6.58, s | 6.46, s | 6.47, s | 6.37, s |
| 4' | — | — | — | — |
| 5' | — | — | — | — |
| 6' | 8.74, s | 7.71, s | 8.37, s | 7.44, s |
| 7' | — | 4.64, d (6.1) | — | — |
| 8' | 4.12, s | 3.90, s | 3.88, s | 3.84, s |
| 9' | — | — | 3.93, s | — |
| 2'-OH | 13.87, s | 13.58, s | 13.05, s | 12.79, s |
| 7'-OH | 10.05, bs | 2.02, t (6.1) | — | — |
Abbreviations: Mult., multiplicity.
Table 2.
13C NMR data for compounds 2, 3, 6 and 8 (in CDC13, 175 MHz for 2 and 3, and 125 MHz for 6 and 8, chemical shifts in ppm)
| Position | 2 | 3 | 6 | 8 |
|---|---|---|---|---|
|
| ||||
| δ C | δ C | δ C | δ C | |
| 1 | 192.8 | 192.4 | 204.7 | 204.2 |
| 2 | 120.6 | 121.4 | 38.0 | 38.0 |
| 3 | 146.9 | 145.1 | 27.2 | 27.5 |
| 4 | 130.4 | 130.4 | 129.2 | 129.6 |
| 5 | 143.5 | 141.8 | 126.6 | 126.2 |
| 6 | 19.1 | 19.0 | 18.0 | 18.0 |
| 1′ | 115.1 | 113.3 | 112.9 | 112.7 |
| 2′ | 169.6 | 166.5 | 167.9 | 164.0 |
| 3′ | 100.5 | 99.7 | 100.5 | 99.0 |
| 4′ | 163.1 | 163.8 | 165.5 | 164.3 |
| 5′ | 109.2 | 120.7 | 111.6 | 118.1 |
| 6′ | 137.2 | 129.9 | 135.9 | 131.1 |
| 7′ | 164.3 | 61.2 | 165.4 | 15.8 |
| 8′ | 57.2 | 55.8 | 52.2 | 55.8 |
| 9' | — | — | 56.5 | — |
Figure 2.
Key HMBC and COSY correlations of compounds 2, 3, 6, and 8.
Compound 3 (1.10 mg) was obtained as a yellow powder with a molecular formula of C14H16O4 as determined by HRESIMS and analysis of 1H NMR, 13C NMR, and edited-HSQC NMR data (Tables 1 and 2, Supplementary Figure S4). Inspection of the NMR data suggested compound 3 as a sorbicillinoid analogue with structural similarity to 7. However, compound 3 lacked the four aliphatic protons H2-2 and H2-3 (δH 2.98 and 2.41), which were replaced by two olefinic protons (δH 6.95 and 7.49) with a coupling constant consistent with an E configuration (JH-2/H-3 = 15.0 Hz). Conjugation of the C2–C3 double bond with the ketone carbonyl resulted in an upfield shift of the chemical shift of C1 in 3 relative to 7. Further examination of the NMR data, including COSY and HMBC spectra (Figure 2), yielded the structure of 3, which was ascribed the trivial name scalbucillin B.
Compound 6 (9.97 mg) was obtained as a white powder. The chemical formula was determined as C15H18O5 by HRESIMS and analysis of 1H, 13C, and edited-HSQC NMR data (Tables 1 and 2, Supplementary Figure S7). Examining the NMR data suggested a sorbicillinoid analogue with structural similarity to 5 (Table 1). As in 5, compound 6 showed NMR signals consistent with a side chain containing two olefinic protons with a coupling constant that supported an E configuration (JH-4/H-5 = 15.5 Hz). A chemical shift of the ketone carbonyl of 204.7 ppm indicated the lack of conjugation with the double bond. Furthermore, the presence of a three proton doublet of doublets (δH-6 1.65, dd, J = 6.3, 1.2 Hz) with a chemical shift and coupling constants consistent with an allylic position established the side chain of compound 6 (Figure 1). Additional similarities included NMR data characteristic of a 1,2,4,5-tetrasubstituted benzene ring with two sharp singlet aromatic protons, indicating positions para to each other. A sharp singlet proton (δH 13.05), which did not show single bond coupling to a carbon as evidenced from the edited HSQC NMR experiment, indicated a phenolic proton ortho to the side chain and hydrogen-bonded with the carbonyl carbon. Key differences between compounds 5 and 6 include replacement of the aldehyde proton in 5 (δH 10.26) by a methoxy functionality in 6 (δH/δC 3.88/52.2) and replacement of the aldehyde carbon (δC 187.5) by an ester functionality (δC 165.4). These data, along with a 30 amu difference in the HRMS data of compounds 5 and 6, indicated replacement of the aldehyde in 5 by a methyl ester in 6. The trivial name scalbucillin C was ascribed to compound 6.
Compound 8 (2.47 mg), which was obtained as a white powder, had a molecular formula of C14H18O3 as determined by HRESIMS and analysis of 1H, 13C, and edited-HSQC NMR data (Tables 1 and 2, Supplementary Figure S9). The NMR data of 8 indicated a sorbicillinoid analogue with structural similarity to 4. However, 8 lacked the H-2 and H-3 olefinic protons, which were replaced by four aliphatic protons (δH 2.95 and 2.40 for H2-2 and H2-3, respectively). This resulted in a downfield shift of C1 in 8 (δC 204.2), relative to that in 4 (δC 192.3), since the latter conjugates with the C2-C3 double bond. Further analysis of the NMR data, including COSY and HMBC experiments (Figure 2), yielded the structure of 8, which was ascribed the trivial name scalbucillin D.
In addition to compounds 1–8, two known phthalides [5,7-dimethoxyphthalide (9)11, 12 and 4,6-dimethoxyphthalide (10)13, 14], and one known naphthalenone [isosclerone (11)]15 were isolated and identified by comparison of NMR, HRMS, and optical rotation data (for 11) to the literature. This is the first report of 10 from a fungal source.
The cytotoxicity of compounds (1–11) was measured against MDA-MB-435 and SW-620 cancer cell lines. Compound 1 was the most potent with IC50 values (concentrations that inhibit growth by 50% relative to untreated cells) of 1.5 and 0.5 μM, respectively, followed by compound 5, with IC50 values of 2.3 and 2.5 μM, respectively (Table 3). Testing of closely related analogues permitted preliminary conclusions regarding structure-activity relationships, particularly the importance of the aldehyde group at 5′. Replacing the aldehyde with a carboxylic acid rendered the compounds inactive, as in 2. A second important structural feature was the requirement of the intact sorbyl side chain for biological activity. Compound 5, with a reduced C2-C3 double bond, was less active by 1.5 and 5 fold in comparison with 1 when measured against the MDA-MB-435 and SW-620 cancer cell lines, respectively. This latter observation was supported by moderate growth inhibitory activity with compounds 3 and 4 that was not present in compounds 7 and 8, which differ only by reduction of the same double bond. Interestingly, compound 1 was similarly active against the B-lymphocytic leukemia cell line OSU-CLL as compared to the solid tumor cell lines (48-h IC50 of 3.1 μM), but compound 5 lacked significant activity in this context. Compound 1, which was isolated previously from the fungus Scytalidiun album,9, 16 displayed growth inhibitory activity when tested against 9KB as reported by the National Cancer Institute (NSC 174259), with ED50 (dose that inhibits growth to 50% of the control growth by 3 days after addition of the drug) value of 0.3 μg/ml.16 Neither the researchers' own activity data of compound 1 nor the NCI data were published (Personal communication with Dr. David Newman, National Cancer Institute).
Table 3.
Activity of compounds 1–11 against two human tumor cell lines
Compounds 2 and 6–11 were inactive.
IC50 values were determined as the concentration required to inhibit growth to 50% of control with a 72 h incubation.
Positive control was vinblastine tested at concentration of 1 nM in MDA-MB-435 cells and 10 nM in SW620 cells, which had 48% and 30% viable cells, respectively.
The antimicrobial activity of the isolated compounds (1–11) was evaluated against a panel of bacteria and fungi (Tables 4 and S1). Compounds 1 and 5 showed selective and equipotent activity against the filamentous fungus Aspergillus niger with MIC values of 0.05 and 0.04 μg/ml (0.20 and 0.16 μM), respectively, followed by compound 3 with an MIC value of 0.60 μg/ml (2.42 μM). Relative to amphotericin B, a clinically used agent that was used as a positive control, compounds 1 and 5 were more potent by more than two orders of magnitude. A hemolytic assay was used to assess the toxicity of compounds 1, 3, and 5 towards sheep red blood cells in vitro. Compounds 1, 3, and 5 resulted in 10% hemolysis of sheep red blood cells at 52.5, 37.5, and 45.0 μg/ml, respectively, with safety factors (calculated as the 10% hemolysis concentration over MIC) of 1050, 62.5, and 1125, respectively (Table 4). Antifungal activities in disk assays against A. flavus and F. verticillioides of compounds 1 and 7 were reported previously by Reátegui et al.10 In the current study, compound 7 showed no activity against the tested fungal strains.
Table 4.
Antifungal and hemolytic activity of compounds 1, 3, and 5
| Minimal inhibitory concentration (μg/ml) |
10% Hemolysis (μg/ml)a | Safety factorb | |||
|---|---|---|---|---|---|
| Compound | C. albicans | C. neoformans | A. niger | ||
| 1 | >53 | >53 | 0.05 | 52.5 | 1050 |
| 3 | >38 | >38 | 0.60 | 37.5 | 62.5 |
| 5 | >45 | >45 | 0.04 | 45.0 | 1125 |
| Amphotericin Bc | 16 | 8 | 31 | 62.5 | 2.0 |
| Triton X-100c | NT | NT | NT | 0.40 | NA |
Hemolytic activity is the concentration required to provoke 10% hemolysis
Safety factor was calculated as the concentration inducing 10% hemolysis over MIC against A niger.
Positive controls.
Abbreviations: NT. not tested. NA. not available.
CONCLUSIONS
A series of eight sorbicillinoid analogues (1–8), of which four were new, along with two phthalides (9–10), and one naphthalenone (11), were isolated from the fungus Scytalidium album (MSX51631). Although the sorbicillinoid family encompasses over 50 members, only 4 sorbicillin-type phenolics, as described by Harned et al,8 have been isolated previously from natural sources. This study served to double that to eight related analogues; it is not clear, however, if these compounds are produced by the same biosynthetic route that leads to sorbicillin.8
This report expands significantly upon the structure-activity relationship of these compounds in cytotoxicity assays, establishing the importance of the aldehyde moiety at the 5' position and the conjugated sorbyl side chain. Whether the aldehyde was oxidized to the carboxylic acid (such as compound 2), or reduced to the primary alcohol (such as compound 3), the growth inhibitory activity was diminished, suggesting the reaction of the aldehyde-containing compounds with DNA, RNA, or proteins to form Schiff base adducts.17, 18 Similarly, when the aldehyde was retained but the sorbyl side chain was reduced, as in compound 5 versus 1, the growth inhibitory activity was reduced by 1.5–5 fold in solid tumor cell lines, and at least ten-fold in a B-lymphocytic cell line (IC50 of 5 not reached in those cells). This is in clear contrast to the activities of compounds 1 and 5 against A. niger, in which they are effectively equipotent. This difference suggests that the saturated sorbyl side chain is important for the biologic activity of compound 1 only in mammalian cells. This interesting observation may not only provide clues as to mechanism of action of this class of agents, it also suggests that compound 5 may be a potential candidate for development as an antifungal, as it may not elicit substantial negative effects in normal human cells. This hypothesis was supported by the relatively high safety factor of compound 5 (as well as 1) with regards to hemolysis of sheep red blood cells. In addition to compounds 1 and 5, compound 3 also showed selective potency against A. niger, with a similarly low toxicity toward sheep red blood cells in vitro but a lower safety factor (i.e., higher MIC).
The discovery of strong, narrow-spectrum anti-A. niger activity underscores the utility of subjecting natural products to as wide a screen as possible to detect biological activity. Since Aspergillus species are highly invasive to human beings and continue to present safety challenges for agricultural products and food, the exploration of novel structures for potential application in these settings is a high priority.19–21 The exceptional potency and apparent selectivity of several of the compounds isolated here strongly supports further exploration of this class of secondary metabolites.
METHODS
General
UV spectra were obtained using a Varian Cary 100 Bio UV-Vis spectrophotometer (Varian Inc., Walnut Creek, CA, USA). NMR data were collected using either a JEOL ECA-500 NMR spectrometer operating at 500 MHz for 1H and 125 MHz for 13C, or a JEOL ECS-400 NMR spectrometer operating at 400 MHz for 1H and 100 MHz for 13C and equipped with a high sensitivity JEOL Royal probe and a 24-slot autosampler (both from JEOL Ltd., Tokyo, Japan), or an Agilent 700 MHz NMR spectrometer (Agilent Technologies, Inc., Santa Clara, CA, USA), equipped with a cryoprope, operating at 700 MHz for 1H and 175 MHz for 13C. Residual solvent signals were utilized for referencing. High resolution mass spectra (HRMS) were obtained using a Thermo LTQ Orbitrap XL mass spectrometer equipped with an electrospray ionization source (Thermo Fisher Scientific, San Jose, CA, USA). A Waters Acquity UPLC system (Waters Corp., Milford, MA, USA) utilizing a Waters BEH C18 column (1.7 μm; 50 × 2.1 mm) was used to check the purity of the isolated compounds with data collected and analyzed using Empower software. Phenomenex Gemini-NX C18 analytical (5 μm; 250 × 4.6 mm), preparative (5 μm; 250 × 21.2 mm), and semipreparative (5 μm; 250 × 10.0 mm) columns (all from Phenomenex, Torrance, CA, USA) and a YMC ODS-A analytical (5 μm; 150 × 4.6 mm), preparative (5 μm; 250 × 20 mm), and semipreparative (5 μm; 250 × 10.0 mm) columns (all from YMC America, Inc., Allentown, PA, USA) were used on a Varian Prostar HPLC system equipped with ProStar 210 pumps and a Prostar 335 photodiode array detector (PDA), with data collected and analyzed using Galaxie Chromatography Workstation software (version 1.9.3.2, Varian Inc.). Flash chromatography was conducted on a Teledyne ISCO CombiFlash Rf using Silica Gold columns and monitored by UV and evaporative light-scattering detectors (both from Teledyne Isco, Lincoln, NE, USA).
Fungal strain isolation and identification
Mycosynthetix fungal strain MSX51631 was collected in 1990 from Cobb County, Georgia, USA. Molecular techniques were used to identify the strain by sequencing the nuclear internal transcribed spacers and intervening 5.8S gene22 along with the D1/D2 regions of the adjacent nuclear ribosomal large subunit.23, 24 DNA extraction, PCR amplification, sequencing, and phylogenetic analyses were conducted as described previously.2, 3, 25–27 The ITS region was used for species-level identification using the pairwise sequence alignment option on the Fungal Barcoding webpage (http://www.fungalbarcoding.org/).24 This function allows performing online alignment between an input (query) sequence and the available sequences in the database, which comprise different curated global fungal databases, including, Q-bank Fungi database (http://www.q-bank.edu/Fungi); CBS-KNAW Fungal Biodiversity Centre (http://www.cbs.knaw.nl/Collections); Molecular Mycology Research Laboratory (http://www.mycologylab.org/); Portuguese Yeast Culture Collection (http://pycc.bioaware.com/); and NCBI GenBank. The search returns the best-matching piecewise (local) or global alignments of two query sequences. Identifications were performed in a monophasic way; in which sequences were compared to produce a global similarity or probability coefficient between the unknown sequence and the reference sequence database (http://www.fungalbarcoding.org/). Based on the results of the pairwise sequence alignment, the closest genus and species that matched the ITS sequence of the strain MSX51631 were representatives of Scytalidium album (isotype sequence; CBS 372.65) (identities = 482/485 (99.3%), gaps = 0), the other closest matches included those of Ascomycota sp. olrim237 (AY787735; identities = 470/470 (100%), gaps = 0), Ascomycota sp. olrim349 (HM036638; identities = 454/454 (100%), gaps = 0). Pairwise sequence alignment was also performed with combined ITS-LSU region, which suggested that the closest match of the query sequence was Scytalidium album (isotype sequence; CBS 372.65) (identities = 927/942 (98%), gaps = 6 (0.7%).
A combined ITS-LSU rDNA complete linkage tree was generated using pairwise sequence alignment search on the Fungal Barcoding webpage (Supplementary Figure S13). Therefore, the strain MSX51631 was identified as having affinities to Scytalidium album L. Beyer & Klingström. (Helotiales, Leotiomycetidae, Leotiomycetes, Pezizomycotina, Ascomycota). The combined ITS-LSU sequence was deposited in the GenBank (accession no KJ772505).
Fermentation, extraction and isolation
Storage conditions, fermentation, and extraction procedure of the fungal strain MSX51631 were as described previously.3, 5, 28 Briefly, cultures of strain MSX51631 were grown in 2.8-l Fernbach flasks (Corning, Inc., Corning, NY, USA) containing 150 g rice and 300 ml H2O. A seed culture grown in YESD medium was used as an inoculum. Following incubation at 22 °C for 14 d, the solid culture was extracted by adding a 500 ml mixture of 1:1 MeOH-CHCl3. The culture was cut into small pieces using a spatula and shaken for about 16 h at ~125 rpm at rt, followed by vacuum filtration. The remaining residues were washed with 100 ml of 1:1 MeOH-CHCl3. To the filtrate, 900 ml CHCl3 and 1500 ml H2O were added; the mixture was stirred for 30 min and then transferred into a separatory funnel. The bottom layer was drawn off and evaporated to dryness. The dried organic extract was re-constituted in 100 ml of 1:1 MeOH-CH3CN and 100 ml of hexanes. The biphasic solution was shaken vigorously and then transferred to a separatory funnel. The MeOH-CH3CN layer was drawn off and evaporated to dryness under vacuum. The defatted material (~750 mg) was dissolved in a mixture of CHCl3-MeOH, adsorbed onto Celite 545, and fractionated via flash chromatography using a gradient solvent system of hexane-CHCl3-MeOH at a 30 ml/min flow rate and 61.0 column volumes over 34.1 min to afford five fractions. Fraction 2 (~315.97 mg) was subjected to preparative reversed phase HPLC over a Phenomenex Gemini-NX C18 preparative column using a gradient system of 50:50 to 70:30 over 50 min to 100:00 of CH3CN-H2O (acidified with 0.1% formic acid) at a flow rate of 21 ml/min to yield thirteenth sub-fractions. Sub-fractions 2 and 7 yielded compounds 11 (0.46 mg) and 3 (0.55 mg), which eluted at ~6.5 and ~27.5 min, respectively. Sub-fractions 8–11 were subjected to further purifications. Sub-fraction 8 (3.23 mg) was subjected to semi-preparative HPLC purification over a Phenomenex Gemini-NX C18 column using a gradient system of 50:50 to 60:40 of CH3CN-H2O (acidified with 0.1% formic acid) over 15 min at a flow rate of 4.72 ml/min to yield compounds 2 (0.75 mg) and 7 (1.65 mg), which eluted at ~8.2 and ~10.5 min, respectively. Sub-fraction 9 (9.14 mg) was subjected to semi-preparative HPLC purification using a Phenomenex Gemini-NX C18 and a gradient system of 70:30 to 80:20 of CH3OH-H2O (acidified with 0.1% formic acid) over 20 min at a flow rate of 4.72 ml/min to yield compound 1 (3.10 mg) that eluted at ~12.5 min. Sub-fraction 10 (14.2 mg) was subjected to preparative HPLC purification using a YMC ODS-A column and an isocratic solvent system of 60:40 of CH3OH-H2O (acidified with 0.1% formic acid) at a flow rate of 15 ml/min to yield compounds 1 (5.21 mg), 5 (4.16 mg), and 6 (1.83 mg), which eluted at ~80.2, ~91.5, and ~106.3 min, respectively. Sub-fraction 11 (146.52 mg) was subjected to preparative HPLC purification using a Phenomenex Gemini-NX C18 and a gradient system of 60:40 to 100:00 of CH3CN-H2O (acidified with 0.1% formic acid) over 30 min at a flow rate of 21.24 ml/min to yield compounds 1 (3.33 mg) and 4 (3.10 mg), which eluted at ~11.0 and ~16.3 min, respectively. To obtain purity over 95% as measured by UPLC, compounds 5 and 6 were further purified using semipreparative reversed phase HPLC.
Another two large scale cultures of MSX51631 were extracted, combined, and fractionated as described above. The MeOH-CH3CN fraction (1.77 g) was subjected to fractionation using flash chromatography and a gradient solvent system of hexane-CHCl3-MeOH at a 40 ml/min flow rate and 53.3 column volumes over 63.9 min to afford four fractions. Fraction 2 (~868.95 mg) was subjected to extensive purifications using preparative and semipreparative reversed phase HPLC and yielded additional amounts of compounds 1–7, which were isolated from the first processed large scale of MSX51631, along with compounds 8 (2.47 mg), 9 (5.54 mg), and 10 (7.91 mg).
Scalbucillin A (2)
Compound 2 was isolated as a yellow solid (1.77 mg); UV (MeOH) λmax (log ε) 349 (3.57), 309 (3.64), 247 (3.51), 222 (3.47) nm; 1H NMR (CDCl3, 700 MHz) and 13C NMR (CDCl3, 175 MHz), see Tables 1 and 2 and Supplementary Figure S3; HRESIMS m/z 263.0910 [M + H]+ (calcd for C14H15O5 263.0914).
Scalbucillin B (3)
Compound 3 was isolated as a yellow solid (1.10 mg); UV (MeOH) λmax (log ε) 349 (3.22), 310 (3.31), 231 (3.22) nm; 1H NMR (CDCl3, 700 MHz) and 13C NMR (CDCl3, 175 MHz), see Tables 1 and 2 and Supplementary Figure S4; HRESIMS m/z 249.1115 [M + H]+ (calcd for C14H17O4 249.1121).
Scalbucillin C (6)
Compound 6 was isolated as a white solid (9.97 mg); UV (MeOH) λmax (log ε) 312 (3.56), 275 (3.71), 246 (3.71) nm; 1H NMR (CDCl3, 500 MHz) and 13C NMR (CDCl3, 125 MHz), see Tables 1 and 2 and Supplementary Figure S7; HRESIMS m/z 279.1221 [M + H]+ (calcd for C15H19O5 279.1227).
Scalbucillin D (8)
Compound 8 was isolated as a white solid (2.47 mg); UV (MeOH) λmax (log ε) 324 (3.61), 275 (3.65), 237 (3.62) nm; 1H NMR (CDCl3, 500 MHz) and 13C NMR (CDCl3, 125 MHz), see Tables 1 and 2 and Supplementary Figure S9; HRESIMS m/z 235.1323 [M + H]+ (calcd for C14H18O3 235.1329).
Cytotoxicity assays
Compounds (1–11) were tested against the MDA-MB-43529 human melanoma (HTB-129, ATCC) and the SW-62030 human colorectal adenocarcinoma (CCL-227, ATCC) cell lines, both were purchased from the American Type Culture Collection (Manassas, VA), as described previously.31 Briefly, the lines were propagated at 37 °C in an atmosphere of 5% CO2 in air in RPMI 1640 supplemented with fetal bovine serum (10%), penicillin (100 units/ml), and streptomycin (100 μg/ml). Cells in log phase growth were harvested by trypsinization and washed twice with PBS to remove all traces of enzyme. A total of 5,000 cells were seeded per well of a 96-well clear, flat-bottom plate (Microtest 96®, Falcon) and incubated overnight (37 °C in 5% CO2). Samples dissolved in DMSO were then diluted and added to the appropriate wells (concentrations: 25 μg/ml, 5 μg/ml, 1 μg/ml, 0.2 μg/ml, 0.04 μg/ml; total volume: 100 μl; DMSO: 0.5%). The cells were incubated in the presence of test substance for 72 h at 37 °C and evaluated for viability with a commercial absorbance assay (CellTiter 96® AQueous One Solution Cell Proliferation Assay, Promega Corp, Madison, WI). Activity was expressed by molarity relative to the negative (solvent) control. The positive control was vinblastine tested at 1 nM in MDA-MB-435 cells (48% viability) and 10 nM in SW-620 cells (30% viablility). Compounds 1 and 5 were also tested against the B-lymphocytic cell line OSU-CLL7 using CellTiter 96 reagents according to the manufacturer's instructions (Promega, Madison WI, USA). The 48-h IC50 of compound 1 was calculated using Prism software (GraphPad, La Jolla, CA, USA).
Antimicrobial assay
The antimicrobial activity of compounds (1–11) against a panel of bacteria and fungi were measured as described previously.31 Briefly, strains of Escherichia coli strain C (ATCC 13706), Staphylococcus aureus (ATCC 6538) and Mycobacterium smegmatis (ATCC 607) were obtained from the American Type Culture Collection (ATCC). Saccharomyces cerevisiae, Candida albicans, Cryptococcus neoformans, Aspergillus niger and Micrococcus luteus strains were obtained from the Virginia Tech Microbiology teaching culture collection. Colonies of E. coli, S. aureus, M. luteus, S. cerevisiae, C. albicans and C. neoformans were grown on 1/10-strength Brain Heart Infusion Broth (BBL Microbiology Systems, Cockeysville, MD, USA) containing 0.2% (w/v) sucrose (BHIB+S) and 1.5% (w/v) agar. M. smegmatis was grown on Middlebrook 7H10 agar (BBL Microbiology Systems) and A. niger on potato dextrose agar (PDA; BBL Microbiology Systems). Streaked plates were incubated at 37 °C for 3–7 days, except for that of A. niger, which was incubated in the dark at 30 °C. A single colony for each microbe, except A. niger, was used to inoculate 5 ml of 1/10-strength BHIB+S (E. coli, M. luteus, and S. aureus), Middlebrook 7H9 broth (M. smegmatis) or yeast extract peptone maltose broth (S. cerevisiae, C. albicans and C. neoformans) and incubated at 37 °C (S. cerevisiae and M. luteus at 30 °C) for 4–7 days. After growth, the resulting broth cultures were diluted with buffered saline gelatin [BSG; gelatin (0.1 g/l), NaCl (8.5 g/l), KH2PO4 (0.3 g/l), Na2HPO4 (0.6 g/l)] to equal the turbidity of a No. 1 McFarland standard. Broth cultures were streaked on plate count agar (BBL Microbiology Systems) and incubated for 3–4 days at 37 °C, so as to check for viability and contamination. Plates for M. luteus and S. cerevisiae were grown at 30 °C. Spores of A. niger were scraped from the surface of the PDA and suspended in 5 ml of 1/10-strength BHIB+S, and that suspension was transferred to a sterile test tube. The turbidity was adjusted to be equivalent to that of a No. 1 McFarland standard by dilution with BSG. Those spore suspensions were streaked on PDA and incubated at 37 °C for 3–4 days, so as to confirm viability and lack of contamination.
The minimal inhibitory concentrations (MICs) of the isolated compounds were measured by broth microdilution in 96-well microtitre plates. A 2-fold dilution series of the compounds was prepared in 96-well microtitre plates in a 50 μl volume of 1/10-strength BHIB+S, and the dilution series was inoculated with 50 μl of each cell suspension. The resulting inoculated dilution series were incubated at either 30 or 37 °C (same as growth temperature), and growth, as noted by turbidity, was scored visually and recorded on the fourth day. MIC of each compound was measured in triplicate and was defined as the lowest concentration of drug resulting in absence of turbidity compared with the drug-free control.
Hemolytic assay
Hemolytic activity of compounds 1, 3, and 5 was measured against sheep red blood cells as described elsewhere.32
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by program project grant P01 CA125066 from the National Cancer Institute/National Institutes of Health, Bethesda, MD, USA. The high resolution mass spectrometry data were acquired at the Triad Mass Spectrometry Laboratory at the University of North Carolina at Greensboro. Sequence data was generated at the Mycology laboratory of Dr. Andrew N. Miller, Illinois Natural History Survey, University of Illinois at Urbana-Champaign.
References
- 1.El-Elimat T, Figueroa M, Ehrmann BM, Cech NB, Pearce CJ, Oberlies NH. High-resolution MS, MS/MS, and UV database of fungal secondary metabolites as a dereplication protocol for bioactive natural products. J. Nat. Prod. 2013;76:1709–1716. doi: 10.1021/np4004307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Elimat T, et al. Waol A, trans-dihydrowaol A, and cis-dihydrowaol A: polyketide-derived gamma-lactones from a Volutella species. Tetrahedron Lett. 2013;54:4300–4302. doi: 10.1016/j.tetlet.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Elimat T, et al. Benzoquinones and terphenyl compounds as phosphodiesterase-4B inhibitors from a fungus of the order Chaetothyriales (MSX 47445) J. Nat. Prod. 2013;76:382–387. doi: 10.1021/np300749w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Elimat T, et al. Chemical diversity of metabolites from fungi, cyanobacteria, and plants relative to FDA-approved anticancer agents. ACS Med. Chem. Lett. 2012;3:645–649. doi: 10.1021/ml300105s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Figueroa M, et al. Cytotoxic epipolythiodioxopiperazine alkaloids from filamentous fungi of the Bionectriaceae. J. Antibiot. 2012;65:559–564. doi: 10.1038/ja.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sy-Cordero AA, Pearce CJ, Oberlies NH. Revisiting the enniatins: A review of their isolation, biosynthesis, structure determination, and biological activities. J. Antibiot. 2012;65:541–549. doi: 10.1038/ja.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hertlein E, et al. Characterization of a new chronic lymphocytic leukemia cell line for mechanistic in vitro and in vivo studies relevant to disease. Plos One. 2013;8:e76607. doi: 10.1371/journal.pone.0076607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harned AM, Volp KA. The sorbicillinoid family of natural products: Isolation, biosynthesis, and synthetic studies. Nat. Prod. Rep. 2011;28:1790–1810. doi: 10.1039/c1np00039j. [DOI] [PubMed] [Google Scholar]
- 9.Geigert J, Stermitz FR, A SH. Two new natural substituted hexenophenones from fungus Scytalidium. Tetrahedron. 1973;29:2343–2345. [Google Scholar]
- 10.Reátegui RF, Wicklow DT, Gloer JB. Phaeofurans and sorbicillin analogues from a fungicolous Phaeoacremonium species (NRRL 32148) J. Nat. Prod. 2006;69:113–117. doi: 10.1021/np0504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Optiz L, Hänsel R. Phthalide aus Helichrysum italicurn. Arch. Pharm. 1971;304:228–230. doi: 10.1002/ardp.19713040312. [DOI] [PubMed] [Google Scholar]
- 12.Yoshikawa K, Kokudo N, Hashimoto T, Yamamoto K, Inose T, Kimura T. Novel phthalide compounds from Sparassis crispa (Hanabiratake), hanabiratakelide A-C, exhibiting anti-cancer related activity. Biol. Pharm. Bull. 2010;33:1355–1359. doi: 10.1248/bpb.33.1355. [DOI] [PubMed] [Google Scholar]
- 13.Nakano Y, Takashima T. Extractives of Albizzia julibrissin Durazz. heartwood. Mokuzai Gakkaishi. 1975;21:577–580. [Google Scholar]
- 14.dos Santos ED, Beatriz A, de Lima DP, Marques MR, Leite CB. Synthesis of resorcinolic lipids bearing structural similarities to cytosporone A. Quim. Nova. 2009;32:1856–1859. [Google Scholar]
- 15.Evidente A, et al. Regiolone and isosclerone, two enantiomeric phytotoxic naphthalenone pentaketides: computational assignment of absolute configuration and its relationship with phytotoxic activity. Eur. J. Org. Chem. 2011:5564–5570. [Google Scholar]
- 16.Stermitz FR, Adamovics JA, Geigert J. Synthesis and photoreactions of sorbophenones: A photochemical synthesis of flavone. Tetrahedron. 1975;31:1593–1595. [Google Scholar]
- 17.Ichihashi K, Osawa T, Toyokuni S, Uchida K. Endogenous formation of protein adducts with carcinogenic aldehydes: Implications for oxidative stress. J. Biol. Chem. 2001;276:23903–23913. doi: 10.1074/jbc.M101947200. [DOI] [PubMed] [Google Scholar]
- 18.Wang M, McIntee EJ, Cheng G, Shi Y, Villalta PW, Hecht SS. A Schiff base is a major DNA adduct of crotonaldehyde. Chem. Res. Toxicol. 2001;14:423–430. doi: 10.1021/tx000234w. [DOI] [PubMed] [Google Scholar]
- 19.Klich MA. Health effects of Aspergillus in food and air. Toxicol. Ind. Health. 2009;25:657–667. doi: 10.1177/0748233709348271. [DOI] [PubMed] [Google Scholar]
- 20.Perrone G, et al. Biodiversity of Aspergillus species in some important agricultural products. Stud. Mycol. 2007:53–66. doi: 10.3114/sim.2007.59.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lass-Florl C, Roilides E, Loffler J, Wilflingseder D, Romani L. Minireview: host defence in invasive aspergillosis. Mycoses. 2013;56:403–413. doi: 10.1111/myc.12052. [DOI] [PubMed] [Google Scholar]
- 22.Schoch CL, et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc. Natl. Acad. Sci. U.S.A. 2012;109:6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu KL, Porras-Alfaro A, Kuske CR, Eichorst SA, Xie G. Accurate, rapid taxonomic classification of fungal large-subunit rRNA genes. Appl. Environ. Microbiol. 2012;78:1523–1533. doi: 10.1128/AEM.06826-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eberhardt U. Methods for DNA Barcoding of Fungi. In DNA Barcodes. In: Kress WJ, Erickson DL, editors. Methods in Molecular Biology. Humana Press; New York, NY, USA: 2012. pp. 183–205. [DOI] [PubMed] [Google Scholar]
- 25.Figueroa M, et al. Peptaibols, tetramic acid derivatives, isocoumarins, and sesquiterpenes from a Bionectria sp (MSX 47401) J. Nat. Prod. 2013;76:1007–1015. doi: 10.1021/np3008842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raja HA, Oberlies NH, El-Elimat T, Miller AN, Zelski SE, Shearer CA. Lindgomyces angustiascus, (Lindgomycetaceae, Pleosporales, Dothideomycetes), a new lignicolous species from freshwater habitats in the USA. Mycoscience. 2013;54:353–361. [Google Scholar]
- 27.Raja HA, et al. Freshwater Ascomycetes: Minutisphaera (Dothideomycetes) revisited, including one new species from Japan. Mycologia. 2013;105:959–976. doi: 10.3852/12-313. [DOI] [PubMed] [Google Scholar]
- 28.Ayers S, et al. Resorcylic acid lactones with cytotoxic and NF-kB inhibitory activities and their structure-activity relationships. J. Nat. Prod. 2011;74:1126–1131. doi: 10.1021/np200062x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rae J, Creighton C, Meck J, Haddad B, Johnson M. MDA-MB-435 cells are derived from M14 Melanoma cells-a loss for breast cancer, but a boon for melanoma research. Breast Cancer Res. Treat. 2007;104:13–19. doi: 10.1007/s10549-006-9392-8. [DOI] [PubMed] [Google Scholar]
- 30.Leibovitz A, Stinson JC, McCombs WB, McCoy CE, Mazur KC, Mabry ND. Classification of human colorectal adenocarcinoma cell lines. Cancer Res. 1976;36:4562–4569. [PubMed] [Google Scholar]
- 31.Alali FQ, et al. Bioactive withanolides from Withania obtusifolia. Phytochem. Lett. 2014;9:96–101. [Google Scholar]
- 32.Lin ZJ, et al. Burkholdines from Burkholderia ambifaria: Antifungal agents and possible virulence factors. J. Nat. Prod. 2012;75:1518–1523. doi: 10.1021/np300108u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.