Abstract
Purpose
The primary objective of this study was to determine if strength loss and recovery following eccentric contractions is impaired in healthy and dystrophic female mice with low levels of ovarian hormones.
Methods
Female C57BL/6 (wildtype) or mdx mice were randomly assigned to ovarian-intact (Sham) and ovariectomized (Ovx) groups. Anterior crural muscles were tested for susceptibility to injury from 150 or 50 eccentric contractions in wildtype and mdx mice, respectively. An additional experiment challenged mdx mice with a 2-wk treadmill running protocol followed by an eccentric contraction injury to posterior crural muscles. Functional recovery from injury was evaluated in wildtype mice by measuring isometric torque 3, 7, 14, or 21 days following injury.
Results
Ovarian hormone deficiency in wildtype mice did not impact susceptibility to injury as the ~50% isometric torque loss following eccentric contractions did not differ between Sham and Ovx mice (p=0.121). Similarly in mdx mice, hormone deficiency did not affect percent of pre injury isometric torque lost by anterior crural muscles following eccentric contractions (p=0.952), but the percent of pre injury torque in posterior crural muscles was lower in Ovx compared to Sham mice (p=0.014). Recovery from injury in wildtype mice was affected by hormone deficiency. Sham mice recovered pre injury isometric strength by 14 days (96 ± 2%) while Ovx mice maintained deficits at 14 and 21 days post injury (80 ± 3% and 84 ± 2%; p<0.001)
Conclusion
Ovarian hormone status did not impact the vulnerability of skeletal muscle to strength loss following eccentric contractions. However, ovarian hormone deficiency did impair the recovery of muscle strength in female mice.
Keywords: eccentric contraction, estrogen, mdx, muscle function, skeletal muscle
INTRODUCTION
Sex-based differences in contraction-induced skeletal muscle injury is a controversial topic as exemplified in the Point:Counterpoint exchange [14, 33]. Implicated in the possible male-female differences are ovarian hormones, with a particular focus on estrogens. However, the specific effects of these sex hormones and the mechanisms by which they function in skeletal muscle injury and recovery are not well understood. One method used to characterize contraction-induced muscle injury is by the level creatine kinase activity in the blood. Based on this marker there is some indication that sex differences exist and are mediated by ovarian hormones in studies on rodents. For example, female rats have smaller increases in serum creatine kinase activity in response to exercise than do male rats [2, 3], and ovariectomized female rodents have higher serum creatine kinase activity following muscle injury induced by exercise compared to those treated with estradiol [3, 30].
Creatine kinase activity and other indirect markers of muscle injury (e.g., range of motion, soreness, and muscle histopathology) do not correlate well with muscle function [36]. Therefore, measures of functional strength are considered the most direct quantification of muscle injury [36]. A type of contraction-induced muscle injury frequently studied is that which occurs following exercise biased toward eccentric contractions because the high forces generated during this type of contraction results in a loss of strength which is usually reversible over days to weeks (e.g., [35]). Whether or not this physiological type of muscle injury is affected by the presence or absence of ovarian hormones is unclear. Several studies in which muscle strength was evaluated immediately following eccentric or eccentrically-biased contractions in rodents do not support the contention that ovarian hormones are protective against injury [20, 37, 38]. Whereas another study showed that estrogen was protective against submaximal but not maximal force loss in soleus muscles of rats 0 and 72 h following downhill running [29]. Recently it was shown that long-term treatment with an estrogenic compound was protective against deterioration of muscle structure and function in diseased muscle of male mice following repetitive contractions [26]. Based on these disparate reports, the first objective of this study was to test the hypothesis that muscle deficient of circulating ovarian hormones has greater strength loss in response to eccentric contractions compared to muscle that has been exposed to normal circulating levels of ovarian hormones. In addition to using healthy female mice to address our first objective, female mice modeling a muscular dystrophy (mdx) were also used because muscle lacking dystrophin is known to be highly susceptible to contraction-induced injury.
Ovarian hormones, primarily estrogens, have been reported to influence major post injury events that contribute to the recovery of muscle strength, such as inflammation and protein balance. Tiidus and co-workers showed inflammation is blunted by estrogen in exercise-injured muscle of rats between 1 h and 7 days post injury, as indicated by reduced infiltration by neutrophils and macrophages [8, 31, 34]. The presence of ovarian hormones has also been shown to expedite the inflammatory response in injured rat muscle [18]. The functional consequences of these ovarian hormone-related inflammatory responses on muscle strength are not known. Following the inflammatory phase, the balance between protein synthesis and degradation rates becomes important and contributes to strength gains and deficits observed in the latter stages of recovery [35]. Estrogens have been shown to promote increased protein synthesis and decreased protein degradation in bovine satellite cell cultures [15]. In rats, those that were ovariectomized failed to recover muscle mass following hindlimb unloading in the same time period as ovary-intact rats [19, 28]. Presumably, reduced muscle mass equated to reduced muscle function, but it was not measured in those studies. Thus, the effects of ovarian hormones on inflammation and protein balance suggest hormones play a role in long-term recovery of muscle strength following injury, but this has not been directly tested. Therefore, we hypothesized that ovarian hormone-deficient muscle has impaired recovery of strength following contraction-induced injury.
METHODS
Animals and study design
Eight-wk old female C57BL/6 (wildtype) mice were acquired from Jackson Laboratories (Bar Harbor, ME). Female mdx mice were bred on-site with breeders from Jackson Laboratories and experiments began when mice were 8 wk of age. Mice were housed in groups of 3-5 and had access to phytoestrogen-free food (Harlan-Teklad; #2019) and water ad libitum. The room was maintained on a 12:12 light:dark cycle. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Minnesota and complied with guidelines set by the American College of Sports Medicine.
Acute strength loss in wildtype mice
The aim of the first experiment was to determine the extent to which ovarian hormone deficiency impacted strength loss in response to contraction-induced injury. C57BL/6 mice underwent a bilateral ovariectomy (Ovx) or sham (Sham) surgery (n=61 total). Ovarian hormone deficiency was confirmed by vaginal cytology ~3 wk following surgery and again immediately prior to contraction-induced injury. Two Ovx mice were removed from experiments based on vaginal cytology indicative of unsuccessful ovariectomy. Three to five wk after surgery, anterior crural muscles were subjected to an in vivo eccentric injury protocol with maximal isometric torque of those muscles assessed 3 min prior to (pre) and 5 min after (post) 150 eccentric contractions.
Acute strength loss in dystrophic mice
Mdx mice lack dystrophin and as a result their skeletal muscle is highly susceptible to injury from forced exercise and eccentric contractions. Therefore, these mice were utilized in attempt to further unmask effects of ovarian hormones on strength loss. Eight wk-old female mdx mice underwent Sham or Ovx surgeries (n=29 total). Approximately 4 wk later, experiments were performed but only mdx mice with vaginal cytology consistent with normal estrogen cycling in the Sham group were included (two mdx mice in this group were excluded due to abnormal cytology). All Ovx mdx mice demonstrated two consecutive days of diestrous; no mice in this group were excluded. We completed two experiments on mdx mice to assess both anterior and posterior crural muscle susceptibility to injury.
The first experiment on mdx mice was done to evaluate the effects of ovarian hormones on contraction-induced injury in anterior crural muscles (Sham, n=4; Ovx, n=5). The experimental design was the same as that described for the wildtype mice except that only 50 eccentric contractions were done due to the vulnerability of mdx mouse muscle to injury from eccentric contractions. Immediately after the post isometric contraction, mice were euthanized by an overdose of pentobarbital sodium (200 mg/kg body mass [BM]).
The second experiment on mdx mice was done to evaluate 1) the effects of ovarian hormones on contraction-induced injury in posterior crural muscles and 2) the effect of a treadmill running injury protocal in dystrophic mice. Mdx mice were randomly subdivided into sedentary (Sed; n=4 Sham Sed and n=4 Ovx Sed) and treadmill (Run; n=5 Sham Run and n=5 Ovx Run) groups. The Sed mice maintained normal cage activities for 4 wk. Run mice were subjected to a standardized, forced treadmill exercise twice a week for 4 wk (2-3 day breaks between exercise bouts) as described by Radly-Crabbe et al [25] in attempt to cause exercise-induced injury to hindlimb muscles. Twenty-four h following the final treadmill run, sedentary and run mdx mice were anesthetized and blood was drawn, then serum prepared for measuring creatine kinase activity. Next posterior crural muscles were subjected to an in vivo eccentric injury protocol, including pre and post maximal isometric torque measurements. In the treadmill mdx experiment, the posterior muscles were targeted because a) the gastrocnemius muscle is more affected by disease in mdx mice than is the tibialis anterior (TA) muscle [11] and b) the gastrocnemius muscle is recruited to a greater extent than is the TA muscle during running in rodents [32, 39]. Immediately after the post isometric torque measurement, mice were further anesthetized (50 mg sodium pentobarbitol/kg BM, i.p.), gastrocnemius muscles were dissected, weighed, either frozen in optimal cutting temperature compound or liquid nitrogen and stored at -80 °C. The uterus of each mouse was dissected and weighed and then mice were euthanized by exsanguination.
Recovery of strength from contraction-induced injury in wildtype mice
A preliminary experiment was first conducted on ovarian-intact, wildtype, female C57BL/6 mice (n =6) to determine the time course of recovery from eccentric contractions. Anterior crural muscles of all mice were subjected to the in vivo 150 eccentric contraction protocol as in the previously described for acute injury loss in wildtype mice. Maximal isometric torque was reassessed in the same mice 3, 7, 14, and 21 days later. After the final torque measurement, mice were euthanized by an overdose of pentobarbital sodium (200 mg/kg BM).
For the second part of the experiment on recovery, wildtype mice were randomized into groups that were reassessed for maximal isometric torque at one of four time points: 3d (n =14), 7d (n =16), 14d (n=14), or 21d (n=15) post injury to evaluate the impact of ovarian hormones on functional recovery of the anterior crural muscles. Immediately after recovery torque was measured at the given time point, mice were further anesthetized (100 mg sodium pentobarbitol/kg BM, i.p.) and TA and EDL muscles were dissected and weighed. TA muscles were cut in half at the mid-belly, with the proximal half frozen in optimal cutting compound for histological assessment and the distal half frozen in liquid nitrogen, and stored at -80 °C. EDL muscles were dissected, weighed, frozen in liquid nitrogen, and stored at -80 °C. EDL muscles from the 14d cohort were homogenized and evaluated for total, myofibrillar, and soluble protein content to determine if ovarian hormonal influence on functional or mass recovery was related to protein content of the muscle. The uterus of each mouse was dissected and weighed and then mice were euthanized by an overdose of pentobarbital sodium (200 mg/kg BM).
Ovariectomy and sham surgeries
Ovariectomy and sham operations on mdx and wildtype mice were conducted as previously described [21]. Briefly, mice were anesthetized with 1.75% isoflurane at a flow rate of 200ml/min. Two dorsal abdominal incisions were made between the iliac crest and lower ribs. Both ovaries were excised in Ovx mice, and in Sham mice the ovaries were located and placed back into the body. The abdominal wall was sutured and skin incisions closed with 7-mm wound clips. Prior to and after surgery mice are given a subcutaneous injection of 0.15 μg Bupronorphine while placed on a heating pad. Successful removal of ovarian tissue was confirmed by vaginal cytology with two consecutive days of cells that were predominantly leukocytes, which is consistent with diestrus and, subsequently, low levels of circulating of estrogens [23]. Uterine mass served as a final confirmation of successful ovariectomy. Among wildtype mice, uterine mass was 8-fold greater in Sham than OVX mice (74±2 and 11±3 mg, respectively; p<0.001). Uterine masses for Sham and Ovx mdx mice were 92±7 and 14±1 mg, respectively (p<0.001).
In vivo torque measurements and protocols
Anterior crural muscles
Maximal isometric torque by the anterior crural muscles was measured in vivo in anesthetized C57BL/6 mice as previously described [1]. With the left knee stabilized by a clamp and the foot secured in a plate attached to the shaft of the servomotor (300B-LR; Aurora Scientific, Aurora, Ontario, Canada), the peroneal nerve was stimulated via platinum subdermal needle electrodes attached to a stimulator and stimulus isolation unit (models E2-12, S48, and SIU5, respectively; Grass Telefactor, Warwick, Rhode Istand). Parameters on the stimulator were set for a 200-ms contraction duration consisting of 0.5-ms square-wave pulses at 300 Hz to produce maximal isometric tetanic torque at the ankle. The voltage on the stimulator started at 3.0 V and was adjusted between 1.0 and 8.0 V until torque no longer increased. The same procedure was used for initial and repeated testing of strength recovery. The same investigator conducted all testing of the anterior crural muscles and was blinded to the treatment groups (Sham or Ovx).
Posterior crural muscles
Maximal isometric torque by the posterior crural muscles was measured in vivo in anesthetized mdx mice as described by Baltgalvis et al [1]. In brief, after severing the left peroneal nerve and securing the foot 90° with respect to the tibia, electrodes were inserted to stimulate the sciatic nerve. Stimulation parameters were 250 Hz of 0.15-ms square wave pulses with voltage adjusted between 3-8 V as necessary to elicit maximal torque. The same investigator conducted all testing of the posterior crural muscles and was blinded to the treatment groups (Sham or Ovx, Sed or Run).
Eccentric contraction-induced injury protocol
In vivo eccentric contractions commenced 3 min following the last pre isometric contraction. For the anterior crural muscles of the C57BL/6 mice, a total of 150 eccentric contractions were performed with 10 s of rest between contractions [1]. The servomotor passively dorsiflexed the foot 20° and then the peroneal nerve was stimulated while the foot was plantarflexed 40° at a velocity of -2000 °/s. After stimulation, the foot was passively dorsiflexed 20° back to its original resting position. For the posterior crural muscles of the mdx mice, the peroneal nerve was severed and 50 eccentric contractions were performed with 10 s of rest between contractions [5]. The servomotor passively plantarflexed the foot 19° and then the sciatic nerve was stimulated while the foot was dorsiflexed 38° at a velocity of 2000 °/s. The foot was then passively plantarflexed back to its original position. For the anterior crural muscles of the mdx mice, the protocol was the same as for C57BL/6 except that only 50 contractions were performed. The number of eccentric contractions performed by the mdx mice was decreased because of their exaggerated susceptibility to strength loss from eccentric contractions.
Treadmill injury protocol for mdx mice
A treadmill protocol previously shown to be injurious to muscle of male mdx mice was used (Protocol B, [25]) first, followed by in vivo eccentric contraction injury to posterior crural muscles. For the treadmill protocol, mice were run in groups of 3-4, approximately divided between Sham and Ovx in each group, with each mouse having its own lane. During each of the eight exercise bouts, mice were given a 2 min period to become acquainted with the environment while the treadmill was stationary and then were acclimated to the moving tread starting at 4 m/min and then 8 m/min for a total warm-up duration of 8 min. The treadmill injury protocol followed and consisted of 30 min of running at 12 m/min with 0° incline (Exer-6M Treadmill, Columbus Instruments, Columbus, OH).
Muscle analyses
Protein assays
Total, soluble, and myofibrillar protein content were determined in uninjured and injured EDL muscles of the 14d cohort, as previously performed with slight modifications [6, 22]. Briefly, individual EDL muscles were homogenized in homogenization buffer (250mM Sucrose, 100mM KCl, 200mM MOPS, 0.5M EDTA, pH 6.8). A portion was saved for analysis of total protein content and the remaining homogenate was centrifuged at 5000g for 15 min at 4°C. Supernatant was separated and saved for analysis of soluble protein content. The pellet was re-homogenized in re-suspension buffer (150mM KCl and 200mM MOPS, pH 7.0) and saved for analysis of myofibrillar protein content. Homogenates were then assayed in triplicate using the bicinchoninic acid (BCA) protein assay and albumin standards (Pierce Biotechnology, Rockford IL).
Serum Creatine Kinase
Blood was collected via the facial vein prior to administration of anesthesia for muscle functional testing. Serum was prepared and stored at -80 °C until time of analysis. Samples were diluted 1:1 with PBS and CK activity was analyzed per manufacturer instructions using a kinetic assay (Creatine Kinase, C7512-300, Pointe Scientific, Inc., Canton, MI) and a Spectramax Plus 384 spectrophotometer with Softmax Pro v5 software (Molecular Devices, Sunnyvale, CA).
Histology
TA and gastrocnemius muscles were sectioned on a cryostat (10 μM thick) and hematoxylin and eosin-phloxine staining was performed. These sections were used to count the percentage of fibers with central nuclei using the methods previously described [1]. Additional TA muscle sections from 3 and 7 day cohorts were assessed for Mac-1 expression, a marker for leukocytes. To do this, sections were fixed in acetone, blocked using a Streptavidin/Biotin Blocking Kit (SP-2002, Vector Laboratories, Burlingame, CA), incubated in rat anti-mouse CD11b primary antibody (550282, BD Biosciences, San Jose, CA) or IgG2b (559478, BD Biosciences) for controls, followed by a biotin goat anti-rat Ig secondary antibody (559286, BD Biosciences). Antigen-antibody interactions were visualized using DAB (550880, BD Biosciences). For each slide, ten digital images were acquired at 40X magnification (Leica DM2000 and QImaging Micropublisher RTV 5.0). A single, blinded investigator assessed the % area of positive staining per image using thresholding (Image J software, National Institutes of Health, Bethesda, MD). The average % of muscles’ cross-sectional area stained positive from the 10 images per muscle section was calculated and used for statistical analyses.
Statistical analyses
Torque at every 10th eccentric contraction as a percent of the first contraction was analyzed by a two-way ANOVA (treatment [Sham versus Ovx] x eccentric contraction #). Isometric torque data were analyzed by a two-way ANOVA (treatment x time since injury), with the exception of the mdx treadmill experiment, which utilized a two-way ANOVA (treatment x exercise intervention [Sed versus Run]). Isometric torque data from the preliminary, recovery experiment on wildtype mice were analyzed by one-way repeated measures ANOVA. Protein content data were analyzed by a two-way ANOVA (treatment x side [uninjured versus injured]) for wildtype studies and a two-way ANOVA (treatment x exercise intervention) for mdx treadmill experiment. Creatine kinase data were analyzed by a two-way ANOVA (treatment x exercise intervention). Muscles masses, central nucleated fibers (CNF), and Mac-1 expression were analyzed with two-way ANOVAs (treatment x time). Holm-Sidak post-hoc testing was used as a post-hoc analysis for significant interactions and main effects when needed. All analyses were conducted using Sigma Stat (version 3.05; Systat Software) and significance was accepted at the α ≤0.05 level. All values are reported as means ± SE.
RESULTS
Influence of ovarian hormones on strength loss in female wildtype mice
Eccentric torque produced by the anterior crural muscles (TA and EDL muscles) was analyzed for every tenth contraction, relative to the first contraction. There was a main effect of contraction number, with contractions 20 through 150 being 15-50% lower than contractions 1 and 10 (Figure 1a; p<0.001), regardless of treatment. There was also a main effect of treatment, with Ovx maintaining slightly more torque than Sham (by the end of the eccentric protocol, Ovx torque was 53±1 versus Sham torque of 50±1%; p=0.001).
Figure 1.
Anterior crural muscle eccentric torque (a) of sham-operated (Sham) and ovariectomized (Ovx) wildtype mice over 150 in vivo eccentric contractions. Data for every tenth contraction are presented relative to the first eccentric contraction (means ±SE). Isometric torque (b) of anterior crural muscles in Sham and Ovx wildtype mice pre and post eccentric injury. Data were analyzed by two-way ANOVA (treatment x eccentric number and treatment x time for a and b, respectively). *Significantly different than contraction #1; ‡Significantly different than Sham; †Significantly different than Pre. Means ±SE.
Similar to the minimal impact of ovarian hormones on eccentric torque loss, contraction-induced injury evaluated by maximal isometric torque of the anterior muscles immediately after the eccentric contractions (post) did not differ between Sham and Ovx mice (Figure 1b; p =0.121). Isometric torque declined to ~40% of pre injury values and this percent strength loss also did not differ between Sham and Ovx mice (p=0.179). These data indicate that in wildtype mice ovarian hormones did not affect susceptibility of muscle to contraction-induced injury.
Influence of ovarian hormones on strength loss in female mdx mice
Anterior muscle torque loss
Eccentric torque produced by the anterior crural muscles in mdx mice was analyzed for every tenth contraction, relative to the first contraction. As expected there was a significant main effect of contraction number on eccentric torque, with contractions 10-50 bein~32-65% lower than the first contraction (Figure 2a; p<0.001). There was also a main effect of treatment with Ovx mice losin~2-12% more torque than Sham over 50 contractions (Figure 2a; p=0.029). Maximal isometric torque following eccentric injury (post) was significantly lower than pre isometric torque for both Ovx and Sham (Figure 2b; p=0.001). There was also a main effect of treatment, with muscle of Ovx mice having less torque than that of Sham mice (Figure 2b; p=0.020). However, the isometric torque as a percent of pre injury did not show an effect of treatment (p=0.952), with Sham and Ovx mice maintaining 49.5±4.7% and 49.9 ±5.1% of pre-injury isometric torque following the eccentric contractions, respectively. These results indicate that anterior crural muscles of mdx Ovx mice were weaker than Sham mice, but were not more susceptible to contraction-induced injury.
Figure 2.
Eccentric torque (a) of anterior crural muscles in sham-operated (Sham) and ovariectomized (Ovx) mdx mice over 50 in vivo eccentric contractions and isometric torque (b) pre and post eccentric injury. Data were analyzed by two-way ANOVA (treatment x eccentric number and treatment x time for a and b, respectively). *Significantly different than contraction #1; ‡Significantly different than Sham; †Significantly different than Pre. Means ±SE.
Posterior muscle torque loss
A second experiment was performed with the mdx mouse model to further probe the impact of ovarian hormones on susceptibility to contraction-induced injury. In this experiment an established treadmill intervention was implemented in attempt to induce muscle injury. Maximal isometric torque generation by the posterior crural muscles (gastrocnemius and soleus muscles) was not significantly affected by the treadmill intervention, that is, there was no difference between Sed and Run mdx mice (Figure 3a; p=0.125). There was also no main effect of ovarian hormones on maximal isometric torque of this muscle group (Figure 3a; p=0.977). Although the treadmill intervention did not result in exercise-induced injury to the posterior crural muscles as determined by strength loss, the subsequent eccentric contraction protocol did. The percent decrement in eccentric torque from the 1st to the 50th contraction was not affected by the treadmill intervention or by ovarian hormone status (p≥0.056). There was also no main effect of treadmill intervention on post isometric torque following the eccentric contractions (Figure 3b; p=0.516). However, a significant main effect of treatment on post isometric torque occurred, with mdx Ovx mice having more torque loss than Sham mice (Figure 3b; p=0.014). Similarly, when post injury isometric torque was expressed as a percentage of pre, Ovx mice had greater decrements in posterior crural muscle strength than did Sham mice (Figure 5c; p<0.001), regardless of previous treadmill intervention (p=0.480).
Figure 3.
Isometric torque of posterior crural muscles in sedentary (Sed) and treadmill (Run) groups of sham-operated (Sham) and ovariectomized (Ovx) mdx mice. (a) Isometric torque before in vivo injury from eccentric contractions (Pre) (b) Isometric torque immediately after eccentric contractions (Post) (c) Post isometric torque expressed as a percentage of pre. All data were analyzed by a two-way ANOVA (treatment x treadmill intervention [Sed versus Run]). *Significantly different than Sham. Means ±SE.
Figure 5.
Recovery of isometric torque by anterior crural muscles following in vivo eccentric injury in groups of ovarian-intact (Sham) and ovarian-deficient (Ovx) mice. Data were analyzed by a two-way ANOVA (treatment x time) and a significant interaction was detected. *Among Sham, significantly different than Pre; †Among Ovx, significantly different than Pre; ‡Within a time point, significantly different between treatments. Means ±SE.
Markers of injury and posterior muscle morphology
Secondary markers of injury were assessed to further probe the susceptibility of female mdx mice to the treadmill-induced muscle injury and to examine any impact of ovarian hormone status. Serum creatine kinase activity was not affected by treadmill running (p=0.384) and was not different between Sham and Ovx groups (890 ± 112 and 946 ± 122 U/L, respectively; p=0.758). The percentage of fibers in gastrocnemius muscles that contained central nuclei was not affected by treadmill intervention or treatment (p≥0.382), with the average for all groups of mdx mice being 69.5 ± 4.0%. Wet mass gastrocnemius muscles were not affected by the treadmill intervention (p=0.781) or ovarian hormone status (p=0.117). However, protein content of gastrocnemius muscle was affected by ovarian hormone status Ovx mice had 11% less total protein than Sham mice (p=0.019).
Recovery of strength in female wildtype mice
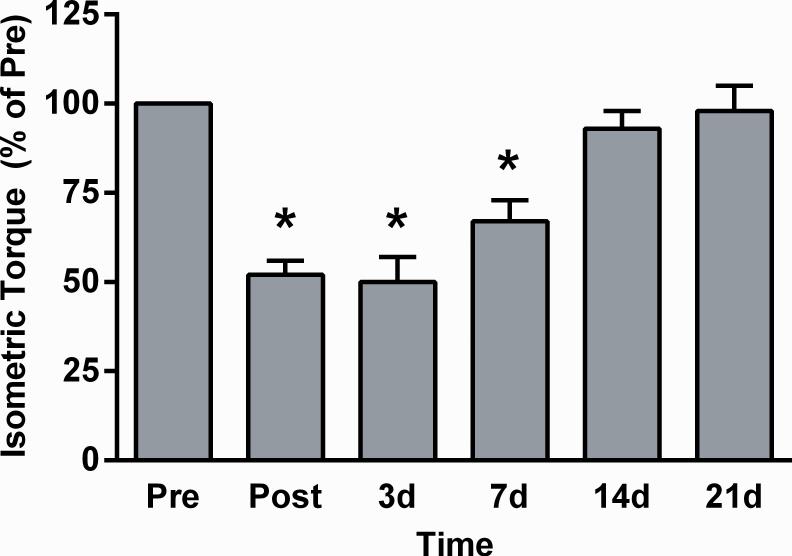
As a first step to determine if ovarian hormones affect recovery of strength following contraction-induced injury, a longitudinal experiment was conducted to establish the time course of strength recovery from our eccentric contraction protocol. Maximal isometric torque of the anterior crural muscles averaged 1.9±0.07 N•mm prior to contraction-induced injury. When re-measured in the same group of mice following injury, there was a significant effect of time (Figure 4; p<0.001). Isometric torque immediately post, 3d, 7d after injury was 48-33% less than at pre injury. By 14d and 21d post injury, isometric torque had returned to pre injury values indicating complete recovery of strength from the in vivo eccentric contraction-induced injury. With this time course established for ovarian-intact, wildtype mice we next sought to determine the impact of ovarian hormone deficiency on the recovery of strength.
Figure 4.
Anterior crural muscle isometric torque of ovarian-intact mice presented as a percentage of pre injury torque in a repeated measure study. Data were analyzed by a one-way, repeated measures ANOVA (time). *Significantly different than Pre. Means ±SE.
Influence of ovarian hormones on strength recovery
The presence of ovarian hormones improved the recovery of torque following contraction-induced injury but was dependent on time since injury (interaction between treatment and time, p=0.001). At 3d and 7d post injury, isometric torque expressed as a percentage of pre injury torque was low in both Sham and Ovx mice (Figure 5). By 14d and 21d post injury, isometric torque had returned to pre injury levels in Sham mice, indicating full recovery of strength. However, isometric torque of Ovx mice at 14d and 21d remained significantly lower than pre injury and Sham mice (Figure 5). At 14d post injury, Ovx mice only recovered to 80±2% of pre-injury while Sham mice had recovered 96±2% of it pre injury strength, indicating that ovarian hormone deficiency impairs recovery of strength from contraction-induced injury. Isometric torque of the anterior crural muscles normalized to body mass also showed an interaction between treatment and time with Sham being greater than Ovx at pre, 14d, and 21d time points with Sham, but not Ovx, having 14d and 21d torques values equivalent to pre injury (Table 1).
Table 1.
Body masses and in vivo torque and masses of the anterior crural muscles before and following contraction-induced injury in groups of wildtype mice that were sham-operated (Sham) or ovariectomized (Ovx).
| Time | †Pre | Post | 3d | 7d | 14d | 21d | Main effect of Treatment |
Main effect of Time |
Inter-action p-value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Sham | Ovx | Sham | Ovx | Sham | Ovx | Sham | Ovx | Sham | Ovx | Sham | Ovx | |||
| Body mass (g) | 20.1±0.2 | 21.9±0.3 | - | - | 19.7±0.7 | 21.2±0.3 | 20.1±0.5 | 21.9±0.4 | 20.6±0.1 | 22.4±0.5 | 21.1±0.3 | 23.1±0.6 | 0.001 | 0.009 | 0.980 |
| Isometric torque (N*mm) | 2.16±0.05 | 2.22±0.06 | 0.86±0.03 | 0.94±0.05 | 0.50±0.12 | 0.59±0.08 | 1.13±0.15 | 1.19±0.156 | 2.01±0.05 | 1.81±0.08 | 2.16±0.12 | 1.89±0.11 | 0.576 | <0.001# | 0.160 |
| Isometric torque (N*mm/k g BM) | 107.7±2.3 | 101.4±2.5* | 42.7±1.6 | 42.9±2.2 | 24.4±5.1 | 28.0±3.7 | 55.7±7.0 | 54.7±7.0 | 97.4±2.5 | 80.8±3.6* | 102.7±4.8 | 81.6±4.0* | 0.004 | <0.001 | 0.020 |
| TA mass (mg) | 37.5±0.7 | 37.6±0.8 | - | - | 38.7±1.5 | 39.2±0.9 | 35.2±0.9 | 32.3±1.2 | 39.0±1.2 | 35.1±1.8 | 42.7±1.0 | 41.3±0.9 | 0.060 | <0.001** | 0.285 |
| EDL mass (mg) | 8.6±0.2 | 8.9±0.2 | - | - | 8.6±0.4 | 8.8±0.3 | 7.8±0.3 | 7.6±0.1 | 7.7±0.3 | 8.0±0.3 | 8.6±0.3 | 8.2±0.3 | 0.863 | <0.001## | 0.644 |
For TA and EDL muscle masses, “Pre” data are from the uninjured, contralateral muscles.
21d is not different from Pre or 14d with all other time points differing from one another.
Significantly different than Sham at corresponding time.
7d differs from pre, 3d & 21d, and 21d differs from pre and 14d.
7d differs from pre & 3d and 14d differs from pre.
Data are means ± SE.
Anterior muscle morphology
To determine if Ovx mice had low dorsiflexion torque 14-21 days post injury due to atrophied TA and EDL muscles, muscle masses were analyzed (Table 1). The mass of both injured TA and EDL muscles varied as a function of time since injury (p<0.001). Injured TA and EDL muscle masses at 7d were lower than pre and 3d values, but were back to pre injury mass by 14 or 21 days (Table 1). There was not a significant main effect of treatment on either TA or EDL muscle mass (p≥0.060) indicating that ovarian hormone deficiency did not elicit muscle atrophy.
Even though muscle mass had recovered 14-21 days post injury in Sham and Ovx mice, strength had not recovered in the Ovx mice. Therefore, protein contents of 14d EDL muscles were analyzed as a representative anterior crural muscle. There were trends for EDL muscles that had been injured from eccentric contractions to contain less total and soluble protein compared to uninjured muscles (p≥0.054); however, there was no effect of treatment on those protein fractions (p≥0.340). Myofibrillar protein content was not affected by injury or treatment (p≥0.175).
As expected, the percent of fibers with central nuclei in 14d TA muscles was greater than in uninjured muscles (p<0.001). Those uninjured TA muscles averaged 2±0.3% CNFs, whereas injured muscles had 17±4% CNFs. There was no effect of treatment on the percentage of CNFs (p=0.516).
To begin to probe for possible ovarian hormone impact on the post injury inflammatory response, Mac-1 was assessed in TA muscles. There was a significant interaction between treatment and time since injury on the percent of muscle cross-sectional area that was positive for this leukocyte marker (p=0.020). At 3d post injury, only 2.1±0.3% of TA muscle from Sham mice was positive for Mac-1 staining while Ovx muscle had significantly more at 2.9 ± 0.5%. By 7d both Sham and Ovx muscle demonstrated <1% Mac-1 staining indicating that inflammation in terms of leukocyte presence was not affected by ovarian hormones at this later time point.
DISCUSSION
The first objective of our study was to determine the impact of ovarian hormones on muscle strength loss following contraction-induced injury. We hypothesized that mice deficient of ovarian hormones would demonstrate greater susceptibility to injury, as indicated by decrements in in vivo torque, compared to mice with normal circulating ovarian hormones. Most of the results do not support this hypothesis. The in vivo eccentric contraction injury protocol was effective in producing substantial loss of strength but with minimal difference between Sham and Ovx mice in the percentage of eccentric torque loss (Figures 1a and 2a). Similarly, maximal isometric torque after injurious eccentric contractions was lower than pre-injury, but there was no difference in the percent isometric torque lost by anterior crural muscles in wildtype or mdx mice. Only in the posterior crural muscles of mdx mice was the percent of isometric torque loss greater in Ovx than Sham mice (Figure 3c) indicating that the presence of ovarian hormones was modestly protective of gastrocnemius and soleus muscle strength from contraction-induced injury in dystrophin-deficient mice.
Results of our experiments on anterior crural muscles (TA and EDL) in wildtype and mdx mice do not support the contention that ovarian hormones are protective against strength loss by contraction-induced injury, similar to other reports in rodents. Muscles evaluated in previous studies which did not support acute ovarian hormone protection against strength loss included soleus [20] and anterior crural muscles [13, 37, 38]. In contrast, the presence of ovarian hormones was protective in posterior crural muscles (gastrocnemius and soleus) in mdx mice (Figure 3a and b) and estradiol treatment administered to ovariectomized rats partially protected soleus muscle contractility following eccentrically-biased downhill running [29]. However, the overall evidence that ovarian hormones are protective against muscle strength loss is much less convincing than evidence that ovarian hormones can be protective against intramuscular protein efflux, such as creatine kinase, in response to contraction-induced injury [2, 3, 16, 30].
To probe the question further, we used the mdx mouse model in attempt to discern whether ovarian hormones can impact the extent of functional injury to muscle. The mdx mouse lacks the cytoskeletal protein, dystrophin, and a robust phenotype of this mouse is its exaggerated susceptibility to strength loss from eccentric contractions. In one experiment on mdx female mice we attempted to induce strength loss via a standardized treadmill protocol. Eight bouts of treadmill running, however, did not cause a loss of function in the posterior crural muscles. This was surprising given the substantial amount of morphological and biochemical evidence that this treadmill intervention has on male mdx mice [25]. This discrepency may be explained by our use of female mdx mice whose muscle may not respond to injurious treadmill running the same as males due to sex differences. However, in female mdx mice the eccentric contraction protocol was affective in inducing strength loss and we show no protective effect of ovarian hormones from strength loss of the anterior crural muscles with a minimal protective effect on the posterior muscles. Dorchies et.al. administered tamoxifen, an estrogen agonist in skeletal muscle, to mdx mice and found muscle contractile properties of the diaphragm and triceps muscles were improved [7]. The phytoestrogen, genistein, has also been shown to improve muscle strength in mdx mice [4]. While injury protocols were not enacted in these two studies, the results are still relevant to our study when considering mdx mouse muscle exists in a chronically injured state. Hourde et. al. [13] compared eccentric contraction-induced strength loss in TA muscles between male and female, young and old mdx mice. Young female mdx mice had less strength loss than young males. However, the female protection against strength loss was not extended to the old, female mdx mice or estradiol-treated old, female mdx mice [13]. Collectively these data indicate estrogenic compounds may be beneficial to young mdx muscle function but not necessarily protective against strength loss from contractions.
The second objective of our study was to test the hypothesis that mice with low circulating ovarian hormones would have impaired recovery of strength. In our initial experiment, we used a repeated measures study design to learn that strength was restored by 14 days post injury in ovarian-intact mice (Figure 4). Therefore, in our next experiment we a priori selected time points out to 21 days in order to investigate how ovarian hormone deficiency via ovariectomy would impact the recovery of strength. Results from control, sham-operated mice in this cross-sectional experiment were consistent with the longitudinal experiment in that pre-injury strength was restored by day 14 (Figure 5). However, mice that were ovarian deficient had only recovered ~80% of pre injury strength at days 14 and 21 (Figure 5), supporting our hypothesis that ovarian hormones promote recovery of muscle function following injury.
A study by Rader et. al. [24] lend support to our results that ovarian hormones can impact the recovery of muscle strength following injury. In that study, maximum isometric force was measured at 1 hour, 3 days, 1 month, and 2 months following an eccentric protocol similar to that used in the current experiments. The injurious contractions elicited ~75% loss of isometric strength in both young and aged female mice 1 hour post injury. The aged mice were 25-29 months old and thus far beyond the age of ~16-20 months when ovaries naturally fail [10, 12], rendering them deficient of ovarian hormones. The aged mice did not recover strength by 2 months post injury when the young mice demonstrated full-recovery of strength [24], suggesting ovarian hormone deficiency may affect long-term recovery of muscle function following injury.
Studies on ovarian hormones and muscle injury in human subjects are limited and have largely focused on sex differences. Considering females may demonstrate fluctuations in muscle strength with changes in sex hormone levels during the menstrual cycle suggests that sex studies may not capture specific estrogenic effects. A study by Sipavicience et. al. [27] investigated the effect of fluctuating estrogen levels on muscle function following 100 stretch-shortening jumps. This study suggested potential effects of estrogens on recovery of maximal voluntary quadriceps strength as well as involuntary electrical induced contractions at 20 and 100 Hz within 72 hours of injury. Subjects in the ovulatory phase (high level of estradiol) appeared to have greater strength gains beginning at 48 hours post injury with return to pre injury level by 72 hours. Whereas those in the follicular phase (relatively low estradiol) exhibited significant differences compared to baseline levels at all time points. While the mechanism of injury remained dissimilar from injuries evoked in the aforementioned rodent studies, the results parallel findings that ovarian hormones augment recovery from injury in females.
Mechanisms underlying impaired recovery in muscle deficient of ovarian hormones are speculative at this point. Specific for eccentric contraction-induced injury in mouse muscle, strength loss during the first 3-5 days post injury is primarily caused by a failure to activate the force generating structures (i.e., excitation-contraction problems) [35]. The frank loss of contractile protein begins to contribute to strength loss at day 3 and is the primary determinant at time points past 14 days [17, 35]. Therefore, we speculated that the extra strength loss in ovariectomized compared to sham-operated female mice following eccentric contractions could have been a result of muscle atrophy or reduced protein content in the injured muscle. Three days post injury there was no difference in TA and EDL muscle masses compared to uninjured contralateral muscles, and 7 day post injury masses were significantly lower. Lower muscle masses at 7 days post injury reflected muscle atrophy and explain some of the strength decrement at that time point (Table 1). Muscle masses, however, were restored by 14 days (TA) and 21 days (EDL) with no differences detected between muscles from Sham and Ovx mice at any one time point (Table 1). Similarly, while there were trends for total and soluble protein contents to be reduced in EDL muscles 14 days post injury, at no time point was total, myofibrillar, or soluble protein contents different between Sham and Ovx mice indicating ovarian hormones did not impact protein balance following contraction-induced injury. This is somewhat in contrast to other models of muscle perturbation in which ovariectomy diminished the recovery of muscle mass following hindlimb unloading [18, 19, 28], suggesting that the type of muscle insult can modulate ovarian hormone effects on mass. For contraction-induced injury, the lack of Sham-Ovx difference in contractile protein content and muscle masses indicate that muscle atrophy did not contribute to deficits in the recovery of strength following injury in the Ovx mice. This suggests the quality of muscle contractile structures as opposed to the quantity of muscle was implicated in strength deficits observed in Ovx mice following injury.
The impact of ovarian hormones on inflammatory and regenerative processes following muscle injury likely contributed to the poor recovery of strength in the Ovx mice. Our analyses of centrally nucleated fibers did not reveal a difference between muscles from Sham and Ovx mice suggesting that the magnitude of regeneration processes had occurred similarly up to 14 days post injury. However, CNFs is a general marker of regeneration and subsequently lacks the discrimination to distinguish inflammatory versus proliferative processes. . Therefore, we also examined a specific inflammatory marker (Mac-1), which indicated a greater inflammatory response in Ovx mouse muscles 3 days post injury. Despite the presence of regenerative processes (CNF) and an enhanced inflammatory response (Mac-1), these observations did not translate to function as Ovx mice demonstrated strength deficits 14 days after injury at a time when Sham mice had completely recovered. Other players in muscle regeneration have been shown to be affected by ovarian hormones and could explain the functional impact. For example, satellite cells are critical to regeneration and decrements in their activation and proliferation have been demonstrated in estrogen-deficient rodents [8, 9]. Additional studies will need to explore these inflammatory and regenerative processes further to better understand the mechanism behind the functional deficits observed in Ovx mice following contraction-induced injury.
The mechanisms associated with susceptibility to injury are important to distinguish because they can directly affect the time course and extent of muscle's functional recovery. From a clinical standpoint, practitioners are often consulted following injury and therefore the approach is generally rehabilitative versus preventative. However, the importance of muscle injury prevention is not to be discounted as the fields of community health and preventative medicine are rapidly growing to alleviate the considerable rise in health care costs. Therefore identification of populations susceptible to muscle injury, and consequently at risk for frailty, falls, and morbidity, will be important when considering allocation and focus of resources for injury prevention. Understanding the interaction between susceptibility to injury, the acute injury itself and subsequent recovery of function will be important to develop therapeutic strategies to prevent, lessen, regain and finally improve muscle strength in females with low levels of circulating ovarian hormones.
ACKNOWLDEGEMENTS
The authors thank the following University of Minnesota Doctorate of Physical Therapy students for their contributions: J. Drewitz, D. Erlandson, K. Gedicke, P. Glatt, L. Grosskreutz, K. Hanten, J. Hoffman, A. Holleran, Z. Horning, and B. Kramer, as well as Lillehei Institute Summer Scholar, Emily Kvasnicka. This work was supported by National Institutes of Health (NIH) grants R01 AG031743 (DAL), K02 AGA036827 (DAL), T32-AG029796 (TLM), and T32-AR07612 (SAN, KAB). Results of the present study do not constitute endorsement by the American College of Sports Medicine, and the authors disclose no conflicts of interest.
Footnotes
The authors disclose no conflicts of interest.
REFERENCES
- 1.Baltgalvis KA, Call JA, Nikas JB, Lowe DA. Effects of prednisolone on skeletal muscle contractility in mdx mice. Muscle Nerve. 2009;40(3):443–54. doi: 10.1002/mus.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bar PR, Amelink GJ. Protection against muscle damage exerted by oestrogen: hormonal or antioxidant action? Biochemical Society transactions. 1997;25(1):50–4. doi: 10.1042/bst0250050. [DOI] [PubMed] [Google Scholar]
- 3.Bar PR, Amelink GJ, Oldenburg B, Blankenstein MA. Prevention of exercise-induced muscle membrane damage by oestradiol. Life Sci. 1988;42(26):2677–81. doi: 10.1016/0024-3205(88)90243-3. [DOI] [PubMed] [Google Scholar]
- 4.Bitto A, Marini H, Burnett BP, et al. Genistein aglycone effect on bone loss is not enhanced by supplemental calcium and vitamin D3: a dose ranging experimental study. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2011;18(10):879–86. doi: 10.1016/j.phymed.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Call JA, Eckhoff MD, Baltgalvis KA, Warren GL, Lowe DA. Adaptive strength gains in dystrophic muscle exposed to repeated bouts of eccentric contraction. J Appl Physiol. 2011;111(6):1768–77. doi: 10.1152/japplphysiol.00942.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Call JA, Voelker KA, Wolff AV, et al. Endurance capacity in maturing mdx mice is markedly enhanced by combined voluntary wheel running and green tea extract. J Appl Physiol. 2008;105(3):923–32. doi: 10.1152/japplphysiol.00028.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorchies OM, Reutenauer-Patte J, Dahmane E, et al. The anticancer drug tamoxifen counteracts the pathology in a mouse model of duchenne muscular dystrophy. The American journal of pathology. 2013;182(2):485–504. doi: 10.1016/j.ajpath.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Enns DL, Iqbal S, Tiidus PM. Oestrogen receptors mediate oestrogen-induced increases in post-exercise rat skeletal muscle satellite cells. Acta Physiologica. 2008;194(1):81–93. doi: 10.1111/j.1748-1716.2008.01861.x. [DOI] [PubMed] [Google Scholar]
- 9.Enns DL, Tiidus PM. Estrogen influences satellite cell activation and proliferation following downhill running in rats. J Appl Physiol. 2008;104(2):347–53. doi: 10.1152/japplphysiol.00128.2007. [DOI] [PubMed] [Google Scholar]
- 10.Felicio LS, Nelson JF, Finch CE. Longitudinal studies of estrous cyclicity in aging C57BL/6J mice: II. Cessation of cyclicity and the duration of persistent vaginal cornification. Biol Reprod. 1984;31(3):446–53. doi: 10.1095/biolreprod31.3.446. [DOI] [PubMed] [Google Scholar]
- 11.Garlich MW, Baltgalvis KA, Call JA, Dorsey LL, Lowe DA. Plantarflexion contracture in the mdx mouse. Am J Phys Med Rehabil. 2010;89(12):976–85. doi: 10.1097/PHM.0b013e3181fc7c9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greising SM, Carey RS, Blackford JE, Dalton LE, Kosir AM, Lowe DA. Estradiol treatment, physical activity, and muscle function in ovarian-senescent mice. Exp Gerontol. 2011;46(8):685–93. doi: 10.1016/j.exger.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hourde C, Joanne P, Noirez P, Agbulut O, Butler-Browne G, Ferry A. Protective effect of female gender-related factors on muscle force-generating capacity and fragility in the dystrophic mdx mouse. Muscle & nerve. 2013;48(1):68–75. doi: 10.1002/mus.23700. [DOI] [PubMed] [Google Scholar]
- 14.Hubal MJ, Clarkson PM. Counterpoint: Estrogen and sex do not significantly influence post-exercise indexes of muscle damage, inflammation, and repair. Journal of applied physiology. 2009;106(3):1012–4. doi: 10.1152/japplphysiol.90848.2008a. discussion 1014, 1022. [DOI] [PubMed] [Google Scholar]
- 15.Kamanga-Sollo E, White ME, Hathaway MR, Weber WJ, Dayton WR. Effect of Estradiol-17beta on protein synthesis and degradation rates in fused bovine satellite cell cultures. Domestic animal endocrinology. 2010;39(1):54–62. doi: 10.1016/j.domaniend.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Koot RW, Amelink GJ, Blankenstein MA, Bar PR. Tamoxifen and oestrogen both protect the rat muscle against physiological damage. The Journal of steroid biochemistry and molecular biology. 1991;40(4-6):689–95. doi: 10.1016/0960-0760(91)90292-d. [DOI] [PubMed] [Google Scholar]
- 17.Lowe DA, Warren GL, Ingalls CP, Boorstein DB, Armstrong RB. Muscle function and protein metabolism after initiation of eccentric contraction-induced injury. J Appl Physiol. 1995;79(4):1260–70. doi: 10.1152/jappl.1995.79.4.1260. [DOI] [PubMed] [Google Scholar]
- 18.McClung JM, Davis JM, Carson JA. Ovarian hormone status and skeletal muscle inflammation during recovery from disuse in rats. Exp Physiol. 2007;92(1):219–32. doi: 10.1113/expphysiol.2006.035071. [DOI] [PubMed] [Google Scholar]
- 19.McClung JM, Davis JM, Wilson MA, Goldsmith EC, Carson JA. Estrogen status and skeletal muscle recovery from disuse atrophy. J Appl Physiol. 2006;100(6):2012–23. doi: 10.1152/japplphysiol.01583.2005. [DOI] [PubMed] [Google Scholar]
- 20.Moran AL, Nelson SA, Landisch RM, Warren GL, Lowe DA. Estradiol replacement reverses ovariectomy-induced muscle contractile and myosin dysfunction in mature female mice. J Appl Physiol. 2007;102(4):1387–93. doi: 10.1152/japplphysiol.01305.2006. [DOI] [PubMed] [Google Scholar]
- 21.Moran AL, Warren GL, Lowe DA. Removal of ovarian hormones from mature mice detrimentally affects muscle contractile function and myosin structural distribution. J Appl Physiol. 2006;100(2):548–59. doi: 10.1152/japplphysiol.01029.2005. [DOI] [PubMed] [Google Scholar]
- 22.Moran AL, Warren GL, Lowe DA. Soleus and EDL muscle contractility across the lifespan of female C57BL/6 mice. Exp Gerontol. 2005;40(12):966–75. doi: 10.1016/j.exger.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE. A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol Reprod. 1982;27(2):327–39. doi: 10.1095/biolreprod27.2.327. [DOI] [PubMed] [Google Scholar]
- 24.Rader EP, Faulkner JA. Effect of aging on the recovery following contraction-induced injury in muscles of female mice. J Appl Physiol. 2006;101(3):887–92. doi: 10.1152/japplphysiol.00380.2006. [DOI] [PubMed] [Google Scholar]
- 25.Radley-Crabb H, Terrill J, Shavlakadze T, Tonkin J, Arthur P, Grounds M. A single 30min treadmill exercise session is suitable for ‘proof-of concept studies’ in adult mdx mice: A comparison of the early consequences of two different treadmill protocols. Neuromuscul Disord. 2011;22(2):170–82. doi: 10.1016/j.nmd.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Ruegg UT. Pharmacological prospects in the treatment of Duchenne muscular dystrophy. Current opinion in neurology. 2013;26(5):577–84. doi: 10.1097/WCO.0b013e328364fbaf. [DOI] [PubMed] [Google Scholar]
- 27.Sipaviciene S, Daniuseviciute L, Kliziene I, Kamandulis S, Skurvydas A. Effects of estrogen fluctuation during the menstrual cycle on the response to stretch-shortening exercise in females. BioMed research international. 2013;2013:243572. doi: 10.1155/2013/243572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sitnick M, Foley AM, Brown M, Spangenburg EE. Ovariectomy prevents the recovery of atrophied gastrocnemius skeletal muscle mass. J Appl Physiol. 2006;100(1):286–93. doi: 10.1152/japplphysiol.00869.2005. [DOI] [PubMed] [Google Scholar]
- 29.Sotiriadou S, Kyparos A, Albani M, et al. Soleus muscle force following downhill running in ovariectomized rats treated with estrogen. Appl Physiol Nutr Metab. 2006;31(4):449–59. doi: 10.1139/h06-008. [DOI] [PubMed] [Google Scholar]
- 30.Sotiriadou S, Kyparos A, Mougios V, Trontzos C, Sidiras G, Matziari C. Estrogen effect on some enzymes in female rats after downhill running. Physiol Res. 2003;52(6):743–8. [PubMed] [Google Scholar]
- 31.St Pierre Schneider B, Correia LA, Cannon JG. Sex differences in leukocyte invasion in injured murine skeletal muscle. Research in nursing & health. 1999;22(3):243–50. doi: 10.1002/(sici)1098-240x(199906)22:3<243::aid-nur6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 32.Thota AK, Watson SC, Knapp E, Thompson B, Jung R. Neuromechanical control of locomotion in the rat. J Neurotrauma. 2005;22(4):442–65. doi: 10.1089/neu.2005.22.442. [DOI] [PubMed] [Google Scholar]
- 33.Tiidus PM, Enns DL. Point:Counterpoint: Estrogen and sex do/do not influence post-exercise indexes of muscle damage, inflammation, and repair. J Appl Physiol. 2009;106(3):1010–2. doi: 10.1152/japplphysiol.90848.2008. discussion 1014-15, 1021. [DOI] [PubMed] [Google Scholar]
- 34.Tiidus PM, Holden D, Bombardier E, Zajchowski S, Enns D, Belcastro A. Estrogen effect on post-exercise skeletal muscle neutrophil infiltration and calpain activity. Can J Physiol Pharmacol. 2001;79(5):400–6. [PubMed] [Google Scholar]
- 35.Warren GL, Ingalls CP, Lowe DA, Armstrong RB. What mechanisms contribute to the strength loss that occurs during and in the recovery from skeletal muscle injury? J Orthop Sports Phys Ther. 2002;32(2):58–64. doi: 10.2519/jospt.2002.32.2.58. [DOI] [PubMed] [Google Scholar]
- 36.Warren GL, Lowe DA, Armstrong RB. Measurement tools used in the study of eccentric contraction-induced injury. Sports Med. 1999;27(1):43–59. doi: 10.2165/00007256-199927010-00004. [DOI] [PubMed] [Google Scholar]
- 37.Warren GL, Lowe DA, Inman CL, et al. Estradiol effect on anterior crural muscles-tibial bone relationship and susceptibility to injury. J Appl Physiol. 1996;80(5):1660–5. doi: 10.1152/jappl.1996.80.5.1660. [DOI] [PubMed] [Google Scholar]
- 38.Willems ME, Stauber WT. Force deficits after repeated stretches of activated skeletal muscles in female and male rats. Acta Physiologica Scandinavica. 2001;172(1):63–7. doi: 10.1046/j.1365-201X.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- 39.Wu H, Rothermel B, Kanatous S, et al. Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway. Embo J. 2001;20(22):6414–23. doi: 10.1093/emboj/20.22.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]