Abstract
Introduction:
Post-prostatectomy incontinence (PPI) is a potentially highly significant complication of a common urological procedure. Pathophysiology may be multifactorial but most commonly involves urinary sphincter weakness. The gold standard treatment for severe incontinence is artificial urinary sphincter but multiple alternatives exist. The growing incidence of PPI has led to the development of a specialized regional service dedicated to management
Patients and methods:
In 2004 a regional referral protocol for PPI was established with a dedicated clinic at a single centre for assessment and management including videourodynamics, pelvic floor rehabilitation, biofeedback and a consultant with a specialist interest in PPI surgery. Data regarding all in-house and tertiary referrals to this clinic between 2004 and 2011 were analysed with patients categorized by symptom severity.
Results:
A total of 267 patients were referred to the post-prostatectomy service (mean age 66.6, range 49–83 years) with numbers increasing year on year. Two-thirds of these were tertiary referrals: 27.7% of referrals were for mild symptoms, 35.2% moderate and 33.3% severe. One-third of referrals were made within 2 years of the primary procedure. Just over half of referred patients underwent invasive treatment including 24.3 artificial sphincter (24.3%) and male slings (22.8%). 7.5% patients were managed with medication, 14.6% were managed conservatively with containment therapy only. One-fifth remain under assessment or have deferred treatment.
Conclusion:
PPI is of increasing personal and societal impact which should be identified early and supported. Investigation and management can be standardized and intervention at a high volume centre achieved by early specialist referral.
Keywords: bladder neck stenosis, post-prostatectomy incontinence, radical prostatectomy, urinary incontinence
Introduction
Post-prostatectomy incontinence (PPI) is a relatively common complication after radical prostatectomy and is also seen occasionally following transurethral resection and other prostate surgery for benign disease. It is a symptom which can have devastating effects on patients’ quality of life, result in significant psychological morbidity, and require specialist treatment [Doherty and Almallah, 2012].
The true incidence of PPI is difficult to determine owing to the lack of a single definition of what represents continence after radical prostatectomy. The European Association of Urology (EAU) guidelines define continence following radical prostatectomy as either total control with no leakage or pad usage, no pad use but loss of a few drops of urine, or use of up to one ‘safety’ pad per day [Schroder et al. 2010]. Radical prostatectomy is the commonest cause of stress urinary incontinence in men [Shamliyan et al. 2009] and recent studies on the effects of population screening for prostate cancer have estimated that 14–20% of men who undergo radical prostatectomy require the use of absorbent pads long term to manage incontinence [Carlsson et al. 2011]. With the exponential rise in the number of radical prostatectomies performed since the advent of widespread prostate-specific antigen (PSA) testing and the recent emergence of minimally invasive surgical techniques, PPI undoubtedly represents a growing clinical problem facing urologists.
The pathophysiology of PPI is often multifactorial and may include reduced bladder compliance, detrusor overactivity, bladder neck contracture or anastomotic stenosis [King and Almallah, 2012] as well as deficiency in the sphincter mechanism itself. Thus, thorough patient assessment and investigation is required in order to ensure the best outcome from treatment [Doherty and Almallah, 2012]. In terms of treatment options, these have expanded in recent years. The artificial urinary sphincter (AUS) remains the best established surgical option for the treatment of severe stress incontinence; however, it has now been joined by the less-invasive male slings which may represent a better option in mild to moderate cases [Zeif and Almallah, 2010].
The growing incidence of PPI, the expansion of surgical options for management and the increasing expectations of patients for high-quality treatment has led to the development of a specialized regional service dedicated to the management of PPI. In this paper we review the referral characteristics and the management outcomes for patients managed by this service since its inception.
Patients and methods
Establishing regional referral protocols
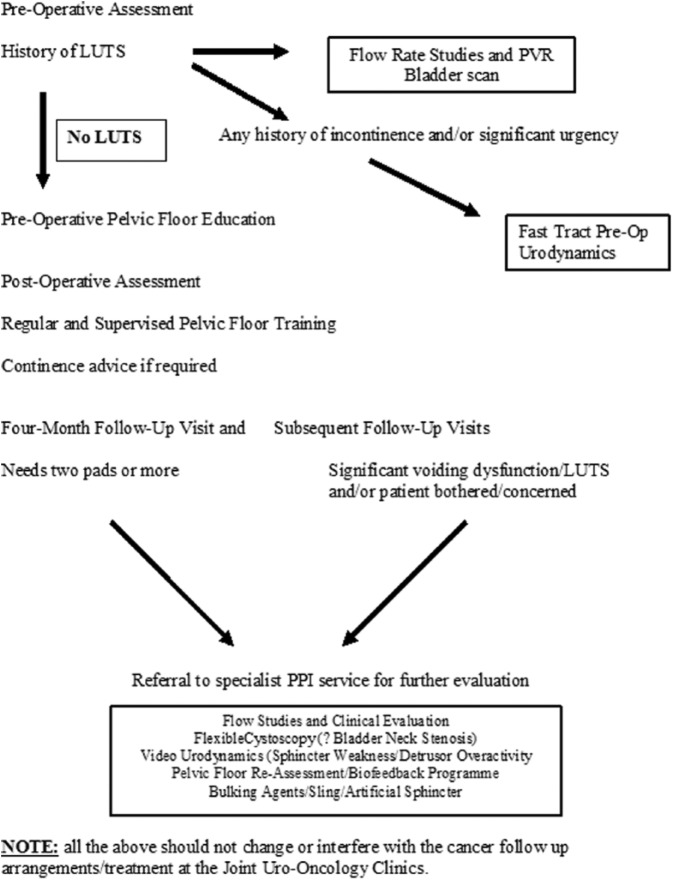
Before 2004 and the inception of the specialist PPI service in the West Midlands, UK, there was a lack of consistency in how patients with PPI were managed and when they were considered for specialist treatment. Key goals in the establishment of the service were to develop protocols for the preoperative and postoperative assessment of all patients undergoing radical prostatectomy with regard to urinary incontinence and to create guidelines for referral into the service which could be applied consistently and would run alongside oncological follow up. The current referral pathway is illustrated in Figure 1. Preoperatively, patients are assessed for a history of lower urinary tract symptoms and are taught pelvic floor exercises. Those with a prior history of incontinence and or significant urgency are referred to undergo preoperative urodynamics. While such patients are relatively few, prior assessment allows better informed choice of primary treatment options. Such patients are also made aware of potential incontinence treatments preoperatively as part of that decision making process. Postoperatively, patients are advised to continue pelvic floor exercises and given advice and support as necessary locally.
Figure 1.
Current referral protocols.
In the postoperative period, it is recommended that patients with severe incontinence, defined as the use of four or more pads per day (PPD), at 4–6 months follow up, are referred to the PPI specialist service for further assessment (Figure 1). Referral of patients with moderate PPI (2–4 PPD) is recommended at 6–12 months of follow up and patients with mild PPI (two or fewer PPD and/or bothersome symptoms) at 12–18 months of follow up.
The initial assessment included history and clinical examination, flow studies and flexible cystoscopy if bladder neck stenosis is suspected. Patients with PPI of less than 12 months are usually offered targeted and machine-assisted pelvic floor muscle training (PFMT) as well as bio-feedback techniques in a specialized nurse-led clinic. Patients with moderate and severe PPI and or patients with more than 12 months of symptoms are offered videourodynamics (VUDS) to confirm and quantify the diagnosis with a view to more invasive treatment. VUDS is also used to identify significant detrusor overactivity as a contribution. Furthermore, establishing a normal detrusor function with normal voiding pattern may be essential in patients who are candidate for insertion of male sling to avoid serious complications of voiding dysfunction and retention of urine. However, one can argue that patients with severe stress urinary incontinence with previous normal voiding pattern before radical prostatectomy can progress to having the AUS without the real need for urodynamics.
PFMT and bio-feedback clinic
In 2004 we established a specialised nurse-led clinic for the initial assessment of patients with PPI of less than 12 months. Those patients, if appropriate, would be offered a total of four sessions (45 minutes every 3 weeks) of targeted PFMT with bio-feedback techniques to enhance their continence recovery. The Phenix 2 programme with anal probes (Genesis Medical, London, UK) was used. Simple lifestyle advice regarding fluid intake was also given.
The clinic is run by a senior advanced nurse practitioner together with a senior continence advisor. Both nurses are highly experienced in urology and have undergone the appropriate training and certifications for pelvic floor rehabilitation. The clinic is supervised by a consultant urologist specialised in male incontinence. The clinic accepts direct referrals from regional urology clinics that follow up patients following radical prostatectomy.
Patients with PPI who do not respond to PFMT or for whom it was inappropriate are either seen by the consultant or referred directly for urodynamics.
Analysis
Patient data were recorded prospectively. For all patients referred between April 2004 and December 2011, data regarding demographics, severity of incontinence, timing of both initial prostatectomy and subsequent referral to the service, as well as modality of treatment provided were analysed. In addition, International Consultation on Incontinence Questionnaire (ICIQ) scores, both at initial consultation and after subsequent treatment, were reviewed. Severity of incontinence was classified according to daily pad usage with use >4 pads/day classified as severe incontinence, 3–4 as moderate, and 2 or fewer as mild. Incontinence grading of referrals was used to direct choice of therapeutic intervention and give chance for mild PPI to be treated conservatively and/or give time for spontaneous improvement.
Results
Between April 2004 and December 2011, 267 patients were seen by the PPI service. The mean age at first clinic appointment was 66.6 years (range 49–83). Overall referrals have been increasing year on year, particularly since 2007 (Figure 2), from only seven in 2004 to 79 in 2011. Within this generalised increase, tertiary referrals constitute a growing proportion of the total patients seen. Overall, tertiary referrals represent 65.1% of patients seen. In 2011, 64.5% of patients seen were tertiary referrals (27 in-house, 51 tertiary), an increase from 22% (14 in-house, four tertiary) in 2004–2005.
Figure 2.
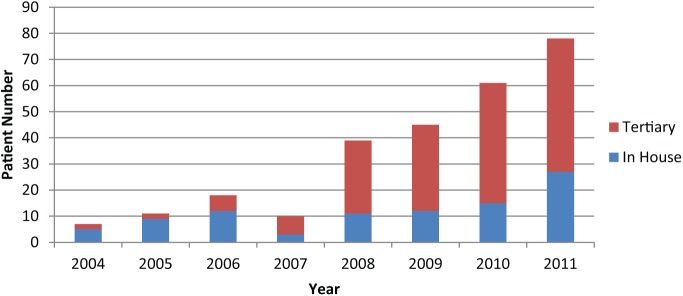
Referrals to post-prostatectomy incontinence clinic by year.
Overall, mild PPI accounted for 74 (27.7%) referrals, 94 (35.2%) were moderate and 89 (33.3%) were severe (Table 1). In 2007, mild and moderate cases represented 33% of tertiary referrals, this rose to 57.7% in 2008 and was 69.2% in 2011 (Figure 3(a)). The proportion of mild, moderate and severe incontinence seen amongst in-house referrals has remained relatively fixed over time (Figure 3(b)) and probably represents complete capture of the PPI cases generated at our institution.
Table 1.
Yearly referrals to post-prostatectomy incontinence (PPI) clinic by severity.
| Severity of PPI |
||||
|---|---|---|---|---|
| Year of clinic | Mild | Moderate | Severe | Not recorded |
| 2004 | 4 | 3 | ||
| 2005 | 4 | 4 | 2 | 1 |
| 2006 | 5 | 6 | 6 | 1 |
| 2007 | 1 | 3 | 5 | 1 |
| 2008 | 8 | 17 | 12 | 2 |
| 2009 | 12 | 13 | 18 | 2 |
| 2010 | 20 | 11 | 25 | 2 |
| 2011 | 24 | 36 | 18 | 1 |
| Total | 74 | 94 | 89 | 10 |
| Total % | 27.7% | 35.2% | 33.3% | 3.7% |
Figure 3.
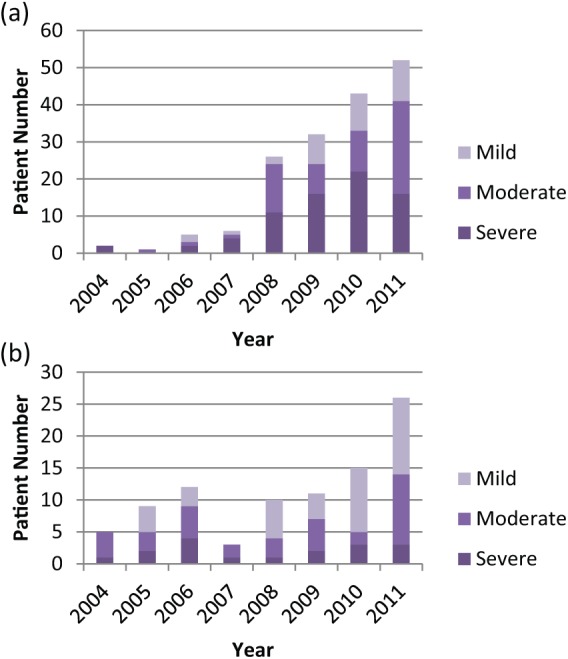
(a) Tertiary referrals by severity. (b) In-house referrals by severity.
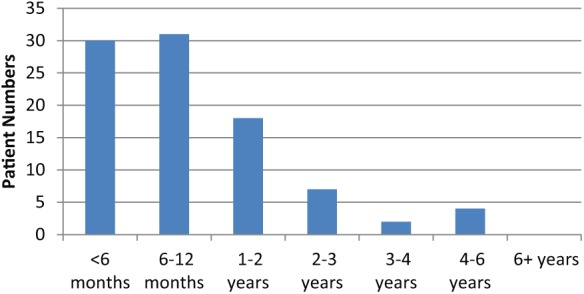
The vast majority, 235 (88.0%), of patients were referred with incontinence following radical prostatectomy, the remainder were post-TURP 23 (8.6%), laser three (1.1%) or radio/cryotherapy five (1.9%). One-third (90, 33.7%) of referrals were made within 2 years of the primary procedure and the majority (153, 57.4%) within 3 years (Figure 4). However, over 40% were not referred until at least 3 years had elapsed since their surgery. Earlier referral was more pronounced from our own establishment with significant numbers of tertiary referrals (16%) occurring 6 years or more post-procedure. Only nine patients waited more than 10 years for referral.
Figure 4.
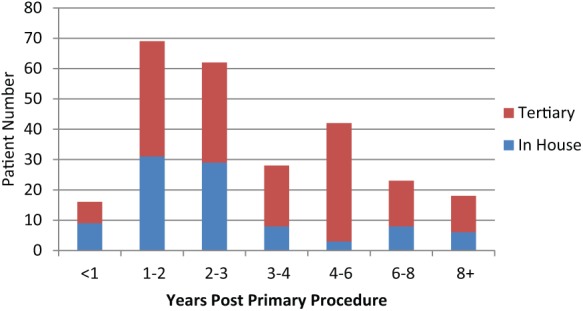
Time from primary procedure to post-prostatectomy incontinence referral.
A total of 150 (56.1%) referred patients had had some form of invasive treatment or were awaiting surgery at the time of their last follow up. Choice of invasive management was tailored to degree of incontinence, as assessed by the patient and by urodynamics, as well as patient preference. A total of 65 (24.3%) patients underwent implantation of AUS, 61 (22.8%) had male slings, 13 (4.9%) received urethral bulking injections and 20 (7.5%) bladder neck incision, while 10 (3.7%) patients who had significant DO, identified on urodynamics, underwent intravesical injection of botulinum toxin. Several patients had multiple procedures e.g. botulinum and AUS (five patients), and bladder neck incision followed by AUS (12 patients).
Of the remainder who had not undergone invasive treatment, 20 (7.5%) were managed with medication (anticholinergics or duloxetine) and PFMT with or without biofeedback. A total of 39 (14.6%) were managed conservatively with containment measures (pads, external collection devices, etc.) and 52 (21.0%) patients were undergoing further assessment or had deferred intervention at latest follow up. Six patients (2.2%) self-discharged from PPI clinic follow up (Figure 5).
Figure 5.
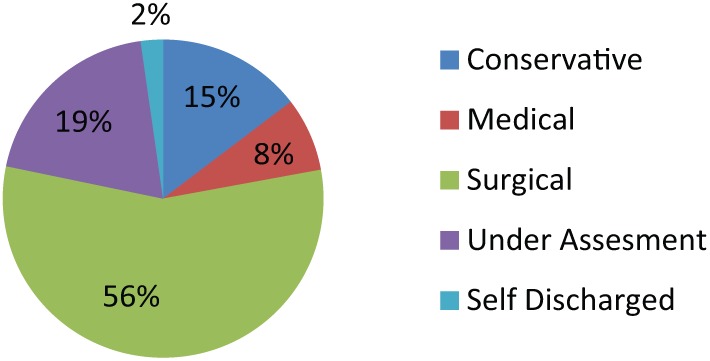
Final management of post-prostatectomy incontinence clinic patients.
The final management of patients varied with the severity of incontinence at initial referral (Figure 6). Invasive management was utilized in 48.4% with mild symptoms, 75.7% with moderate and 85.1% with severe symptoms. AUS was used in 55.2% of those with severe symptoms versus 25.7% moderate and 7.4% mild (Figure 6). The majority (66.3%) of patients undergoing invasive procedures underwent them within 1 year of first review (Figure 7), and a further 19.6% within the second year.
Figure 6.
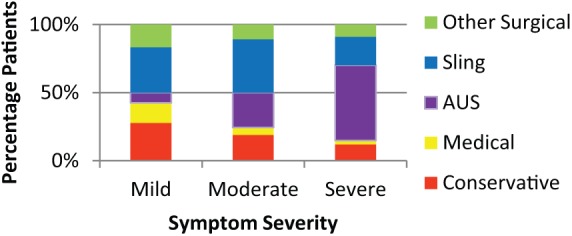
Treatment for mild, moderate and severe post-prostatectomy incontinence patients.
Figure 7.
Time from first review to invasive treatment.
Discussion
In this paper we have audited the first 8 years of a regional tertiary subspecialist service for PPI. The principle requirements include a dedicated specialist nurse with specific training in PFMT, easy access to investigations such as VUDS and a consultant with a subspecialist interest in PPI surgery. The putative advantages of such a service are the standardization of management and improved outcomes in a specialized and growing complication of a common procedure.
The ethos of our tertiary service was: ‘we have cured or potentially cured those men from prostate cancer so let’s give them the best quality of life and rid them of incontinence pads’. Delay from time of prostatectomy to referral for PPI assessment can have a significant impact on the wellbeing of those men, who are often active and working.
Several trends are apparent over the first 8 years of this service. A steady rise in referrals and an increased proportion from regional centres represent a growing awareness throughout the region of the service and, it is hoped, its good outcomes. At the time of writing, patients within the region are routinely referred to this tertiary centre if they would consider surgery for PPI, with patients who do not wish to be considered for surgery continue their follow up at their initial treatment centre. Increasing numbers of radical prostatectomies and recognition of PPI and PPI surgery are likely to be the reasons for the increased referral rate. Also, the increased proportion of referrals with mild or moderate severity PPI probably reflects an increased awareness of the impact of PPI as well as the availability of procedures less invasive than the AUS. Of note, referrals for mild and moderate incontinence increased significantly from 2008 onwards, when NICE guidance on prostate cancer was published. Assessment of severity of incontinence has been principally by pad use numbers in this study. Pad weight has been assessed as part of this clinic since 2011 and will be included in future analyses. However, we are encouraging future referral with history of bother rather than pad numbers only.
The natural history of PPI sees the high initial incidence falling dramatically during the first 12 months after operation [Van Kampen et al. 1997], with little spontaneous recovery thereafter, Hence, it is appropriate that the highest referral rates are 1–3 years post-primary procedure. However, the fact that a significant proportion of tertiary referrals occur after 3 years is probably not ideal and all patients should be offered the opportunity to discuss further treatment if their PPI is bothersome.
This service has been principally set up for post-surgical patients although a small number of men are seen who have developed incontinence after primary radiotherapy. Nevertheless, there is probably an under-representation of patients with PPI who have undergone salvage radiotherapy post-prostatectomy. It may be that such patients are not referred due to concerns about complications and worse outcome of PPI surgery in irradiated patients. Including such patients in the service in future may involve assessment and counselling before and after radiotherapy treatment.
It is not surprising that the need for invasive intervention increases with severity of symptoms, and much greater use of the AUS in men with severe symptoms reflects its position as the gold standard of male incontinence treatment [Montague, 2012] and its superiority in severe symptoms on Cochrane analysis [Silva et al. 2011]. A possible niche for sling surgery exists in mild–moderate PPI, however, randomized, controlled trials and more long-term results are still needed to inform a clear surgical policy that ensures that individual men get the best treatment for them. While several patients progressed through multiple treatments, with progression through PFMT, biofeedback and pharmacotherapy before surgery, it is reassuring that the vast majority of patients who did undergo invasive procedures did so within 2 years of initial referral. Outliers usually represented an early decision to defer therapy or a less invasive therapy initially being successful but subsequently losing efficacy. A significant proportion of referrals were still under review at the time of latest follow up and further analysis after all data have matured is required.
Finally, pharmacological treatment for PPI appears to be lacking behind the advances in surgical intervention. Few small studies looked at the off-license use of duloxetine in PPI with variable results [Neff et al. 2013]. However, in our experience, the use of this medication in men with PPI was generally disappointing and led to a significant side effect of nausea and drowsiness. These clinical impressions are not dissimilar to the experience in women with SUI. Nevertheless, some studies showed that other pharmacological agents like the administration of phosphodiesterase type 5 inhibitors could improve urinary continence recovery in patients treated with nerve-sparing radical prostatectomy and such role need to be explored further in future studies [Gandaglia et al. 2013; Gacci et al. 2010].
Conclusion
PPI is a complication of increasing personal and societal impact which should be identified early and managed actively. Investigation and management can be standardized and early intervention at a high-volume centre can be achieved by early referral for specialist care. A tertiary centre requires established referral and assessment protocols as well as timely access to specialist surgical procedures.
Within this report, success of the service is evidenced by the steady increase in the referral rate to the service.
Acknowledgments
The authors would like to thank Michele Miletic and Fran Harries for running PFMT clinics and their invaluable help with our patients; and Ellen Ward Lindley and Jennifer Whale for their help with data collection and analysis.
Footnotes
Conflict of interest statement: Zaki Almallah is an advisor for the National Institute for Health and Clinical Excellence (NICE) and the NHS Health Technology Assessment (HTA) Programme and user/trainer of various male anti-incontinence devices manufactured by American Medical Systems.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Contributor Information
Y. Zaki Almallah, Department of Urology, Queen Elizabeth Hospital, University Hospital Birmingham NHS Foundation Trust, Edgbaston, Birmingham B15 2TH, UK.
Samuel J.S. Grimsley, Department of Urology, The Queen Elizabeth Hospital, University Hospital Birmingham NHS Foundation Trust, Edgbaston, Birmingham, UK
References
- Carlsson S., Berghahl S., Khatami A., Lodding P., Stranne J., Hugosson J. (2011) The excess burden of side-effects from treatment in men allocated to screening for prostate cancer. The Göteborg randomised population-based prostate cancer screening trial. Eur J Cancer 47: 545–553. [DOI] [PubMed] [Google Scholar]
- Doherty R., Almallah Y. (2012) Urinary incontinence after treatment for prostate cancer long term morbidity can be minimized by early referral to specialist centres. BMJ 344: 12. [Google Scholar]
- Gacci M., Ierardi A., Rose A.D., et al. (2010) Vardenafil can improve continence recovery after bilateral nerve sparing prostatectomy: results of a randomized, double blind, placebo-controlled pilot study. J Sex Med 7: 234–243. [DOI] [PubMed] [Google Scholar]
- Gandaglia G., Albersen M., Suardi N., et al. (2013) Postoperative phosphodiesterase type 5 inhibitor administration increases the rate of urinary continence recovery after bilateral nerve-sparing radical prostatectomy. Int J Urol 20: 413–419. [DOI] [PubMed] [Google Scholar]
- King T., Almallah Y. (2012) Post-radical-prostatectomy urinary incontinence: the management of concomitant bladder neck contracture. Adv Urol 2012: 295798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague D. (2012) Artificial urinary sphincter: long-term results and patient satisfaction. Adv Urology 2012: 835290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff D., Guise A., Guralnick M., et al. (2013) Duloxetine for the treatment of post-prostatectomy stress urinary incontinence. Can Urol Assoc J 7(5–6): E260–E262 DOI: 10.5489/cuaj.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder A., Abrams P., Andersson K., et al. (2010) Guidelines on Urinary Incontinence European Association of Urology: 5. [Google Scholar]
- Shamliyan T., Wyman J., Ping R., et al. (2009) Male urinary incontinence: prevalence, risk factors, and preventive interventions. Rev Urol 11: 145–165. [PMC free article] [PubMed] [Google Scholar]
- Silva L., Andriolo R., Atallah A., da Silva E. (2011) Surgery for stress urinary incontinence due to presumed sphincter deficiency after prostate surgery. Cochrane Database Syst Rev CD008306. [DOI] [PubMed] [Google Scholar]
- Van Kampen M., De Weerdt W., Van Poppel H., Baert L. (1997) Urinary incontinence following transurethral, transvesical and radical prostatectomy. Retrospective study of 489 patients. Acta Urol Belg 65(4): 1–7. [PubMed] [Google Scholar]
- Zeif H., Almallah Z. (2010) The male sling for post-radical prostatectomy urinary incontinence: urethral compression versus urethral relocation or what is next? Br J of Medical and Surgical Urology 3(4): 134–143. [Google Scholar]