Abstract
IMPORTANCE
Normal-tension glaucoma (NTG) is a common cause of vision loss.
OBJECTIVE
To investigate the role of TANK binding kinase1(TBK1) gene duplications in NTG to gain insights into the causes of glaucoma that occurs at low intraocular pressure (IOP).
DESIGN, SETTING, AND PARTICIPANTS
In this multicenter case-control study, we investigated patients who met the criteria for NTG, including glaucomatous optic neuropathy, visual field defects, and maximum recorded untreated IOP of 21 mm Hg or less, and matched controls. Participants (N = 755) were recruited from Southampton, United Kingdom (180 patients and 178 controls), Rochester, Minnesota (65 patients and 12 controls), New York, New York (96 patients and 16 controls), and Iowa City, Iowa (208 controls).
MAIN OUTCOMES AND MEASURES
Detection of TBK1 gene duplications and comparison of the extent of the identified DNA that is duplicated with prior TBK1 copy number variations associated with NTG.
RESULTS
A TBK1 gene duplication was detected in 1 of 96 patients (1.0%) from New York and none of the controls. Analysis of duplication borders with comparative genome hybridization demonstrated that this patient has a novel duplication that has not been previously reported. No gene duplications were detected in any of the other cohorts of patients or controls.
CONCLUSIONS AND RELEVANCE
Duplication of the TBK1 gene is a rare cause of NTG. The identification of another case of NTG attributed to TBK1 gene duplication strengthens the case that this mutation causes glaucoma.
The genetic basis of primary open-angle glaucoma (POAG) is complex. Recent large population-based studies have identified numerous genetic factors related to POAG, including CAV1/CAV2,1, 2 CDKN2B-AS1,3–7 ATOH7,4, 5 SIX1/SIX6,4 TMCO1,3, 8 TLR4,9 SRBD1,10 and ELOVL5.10 These glaucoma risk factors are observed in healthy individuals; however, they are more commonly detected in patients with POAG than in healthy controls. Each of these genetic factors contributes a small risk for POAG, and although none may cause the disease on their own, in combination they may lead to the development of glaucoma.11
Studies of familial POAG have led to the identification of several genes that cause glaucoma with simple Mendelian inheritance patterns. These cases of POAG, with autosomal dominant inheritance, are caused primarily by individual genes acting alone. Mutations in MYOC (OMIM 601652)12 or OPTN (OMIM 602432)13 can cause POAG with minimal influence from other genes or environmental factors. Mutations in MYOC cause 3% to 4% of POAG cases worldwide.14 Patients with MYOC-related glaucoma typically have markedly elevated intraocular pressure (IOP) and early-onset disease.15 Mutation of OPTN is associated with POAG that occurs at lower IOP (ie, normal-tension glaucoma [NTG]).13 OPTN mutations have been linked to 1% to 2% of NTG cases.16,17 Overall, the known single-gene causes of POAG are responsible for approximately 5% of cases of POAG.11
More recently, a third glaucoma gene, TBK1, that is associated with NTG has been identified.18 Prior studies18,19 have found that several African American, white, and Asian patients with NTG have duplications on chromosome 12q14 that span the TBK1 gene. TBK1 encodes a kinase protein that directly interacts with and phosphorylates OPTN,20,21 the protein encoded by the only other known NTG gene.13 TBK1 is the only gene encompassed by all known chromosome 12q14 duplications in NTG patients.18,19 Moreover, TBK1 is specifically expressed within the ocular tissue most affected by NTG, the retinal ganglion cell layer, and duplication of the TBK1 gene leads to a significant increase in its transcription level.18 The sum of these data strongly suggest that duplication of TBK1 causes 0.4% to 1.3% of NTG cases.18,19 However, animal and/or functional studies will be required to definitively prove that chromosome 12q14 duplications cause NTG by altering the function of TBK1 rather than through effects on other neighboring genes.
The discovery that TBK1 is a glaucoma gene suggests biological pathways that may be important in the pathogenesis of NTG. Both known NTG genes, TBK1 and OPTN, function in nuclear factor–κB (NF-κB) signaling pathways,22,23 which have been previously connected with apoptosis and cell death. TBK1 and OPTN also have essential functions in autophagy, a pathway for eliminating damaged or accumulating intracellular materials. Prior studies20,24 have also indicated that TBK1 colocalizes with OPTN and that TBK1 phosphorylates OPTN as part of a cascade of events that ultimately leads to activation of autophagy. Autophagy can protect cells in times of nutrient deprivation or serve as a means to degrade accumulating intracellular proteins, dysfunctional organelles (eg, damaged mitochondria), or intracellular pathogens. Excessive autophagy may also lead to cell death in retinal ganglion cells.25,26 Previous investigations demonstrated that TBK1 gene duplications in NTG patients lead to increased transcription of TBK1 messenger RNA,18 which may lead to retinal ganglion cell death by activation of autophagy or altering NF-κB signaling. In this report, we investigated the role of TBK1 gene duplication in 3 additional NTG patient populations to further explore the role of the TBK1 gene in NTG.
METHODS
All participants provided written informed consent, and research was conducted with the approval of the institutional review board of the University of Iowa. All participants were examined by a fellowship-trained glaucoma specialist. Criteria for diagnosis of NTG included typical glaucomatous optic nerve damage and visual field loss with a maximum recorded IOP of 21 mm Hg or less, as previously described.15,18,19 Three cohorts of patients and controls were enrolled from Southampton, United Kingdom (180 patients and 178 controls), Rochester, Minnesota (65 patients and 12 controls), and New York, New York (96 patients and 16 controls). An additional 208 controls from Iowa were also enrolled. None of the patients or controls in the current report were included in previous studies of TBK1.
DNA from NTG patients and controls was examined for TBK1 gene duplications using a quantitative polymerase chain reaction assay (TaqMan Number Assay; Applied Biosystems) as previously described.18,19 Positive quantitative polymerase chain reaction results were confirmed, and duplication borders were defined with comparative genome hybridization (CGH) using whole genome microarrays (NimbleGen 720 000 microarray; Roche NimbleGen) following the manufacturer’s protocol. The borders and extent of detected TBK1 gene duplications were compared with previously reported TBK1 gene duplications in other NTG patients using the current build of the human genome (hg19).18,19
RESULTS
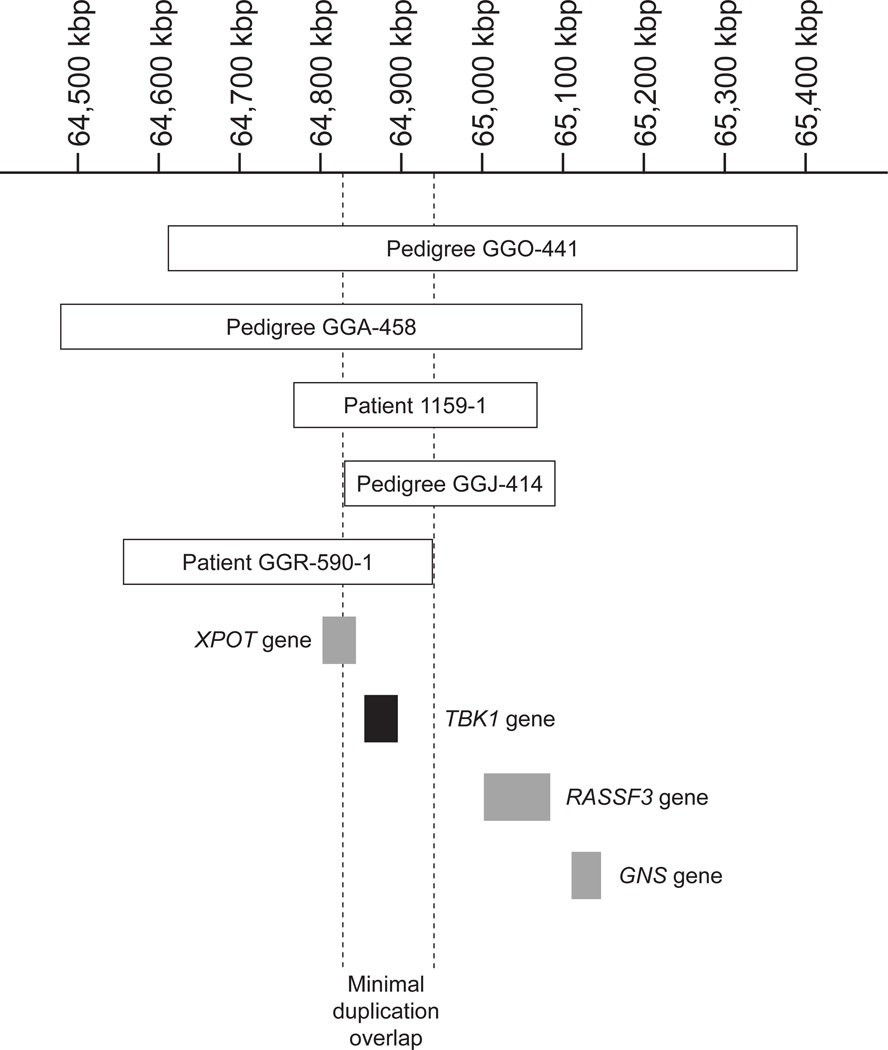
A total of 755 participants from 3 populations (Southampton, United Kingdom; Rochester, Minnesota; and New York, New York) were tested for duplication of the TBK1 gene using a quantitative polymerase chain reaction assay. A TBK1 gene duplication was detected in 1 (patient GGR-590-1) of 96 patients (1.0%) from New York. No gene duplication was detected in any of the controls or in the other NTG cohorts. The extent of the chromosome 12q14 duplication in patient GGR-590-1 was determined by examination with a CGH microarray. The duplication encompasses 370 kilobase pairs (kbp), extends from 64 563 to 64 933 kbp, and spans the TBK1 gene and part of the XPOT gene (Figure 1).
Figure 1. TBK1 gene duplications.
The position and extent of each of the detected TBK1 gene duplications and their relationship to flanking genes are shown using the current human genome build (hg19).18,19 Duplications in normal-tension glaucoma pedigrees GGO-441, GGA-416, GGA-1159, and GGJ-414 were previously reported (using the hg18 genome build). kbp indicates kilobase pair.
Case Report
Patient GGR-590-1 is a 65-year-old white woman who was diagnosed as having NTG at 47 years of age with maximum recorded IOP of 16 mm Hg in both eyes, progressive visual field damage (left eye greater than right eye), and glaucomatous cup-to-disc ratios. As part of her evaluation, magnetic resonance imaging ruled out nonglaucomatous optic neuropathy. She had no known family history of glaucoma. Argon laser trabeculoplasty was performed in both eyes at 48 years of age.
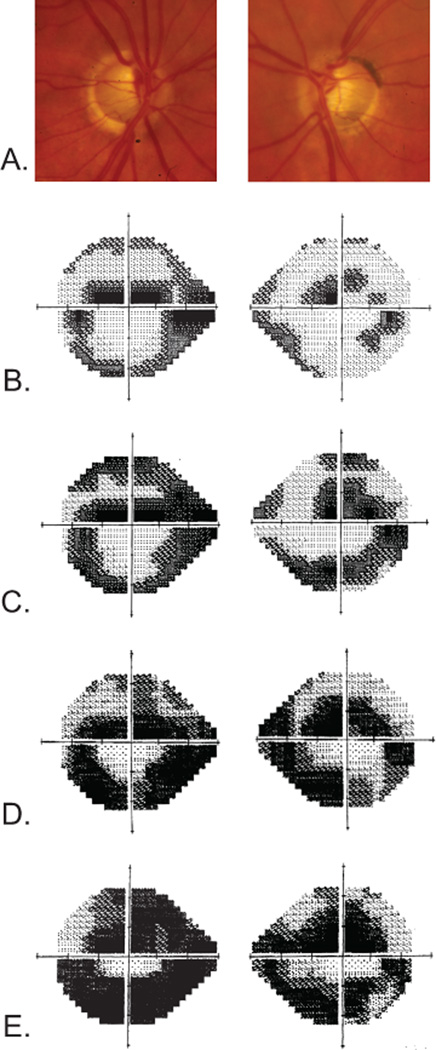
She was first seen by one of us (R.R.) at 53 years of age. At that time her medications consisted of topical timolol, dorzolamide hydrochloride, brimonidine tartrate, and travoprost for both eyes. She denied a history of migraine, Raynaud phenomenon, or cardiac arrhythmia but stated that she had chronic low blood pressure. A complete eye examination revealed 20/20 visual acuity in both eyes, IOPs of 10 mm Hg in both eyes, and thin corneas. Gonioscopy revealed grade IV open angles with trace pigmentation. Her cup-to-disc ratios were 0.8 OD and 0.9 OS in 2002. Optic nerve head cupping progressively worsened in both eyes (Figure 2A), and the cup-to-disc ratio was 0.99 OU by 2011. Automated perimetry (24-2 Swedish Interactive Thresholding Algorithm Standard) in 2002 revealed superior and inferior arcuate scotomas with a superior paracentral defect in both eyes. The loss was greater in the left eye (Figure 2B). Visual field loss also progressed from 2002 to 2012 (Figure 2, C–E) despite maintaining an IOP of 12 mm Hg or less in both eyes.
Figure 2. Patient GGR-590-1 clinical data.
A, Disc photographs at 56 years of age that demonstrate significant cupping. Humphrey visual field tests (24-2 Swedish Interactive Thresholding Algorithm Standard) performed at 53 years of age (B), 56 years of age (C), 59 years of age (D), and 64 years of age (E) demonstrate progressive glaucomatous visual field loss despite maximum intraocular pressure of 12 mm Hg in both eyes.
Subsequent 24-hour blood pressure monitoring revealed persistent nocturnal dipping between 12:00 and 4:00 AM. The lowest readings reported were 98 mm Hg systolic, 44 mm Hg diastolic, and 62 mm Hg mean arterial pressure. The nocturnal mean arterial pressure was 30% to 40% lower than the diurnal mean arterial pressure. Findings of magnetic resonance imaging of the brain with and without contrast were unremarkable except for optic nerve thinning.
Analysis of TBK1 Gene Duplications
Three different chromosome 12q14 duplications spanning the TBK1 gene were previously detected in 1 African American NTG pedigree and 2 white NTG pedigrees (Figure 1).18 A fourth TBK1 gene duplication was later detected in a Japanese NTG pedigree, GGJ-414,19 but the borders of this duplication were not reported. Using CGH, we found that the borders of the TBK1 gene duplication in this Japanese NTG pedigree span 267 kbp of DNA on chromosome 12q14, from 64 830 to 65 096 kbp. These data indicate that NTG patients in pedigree GGJ-414 have a novel TBK1 mutation (Figure 1).
Each of the 5 known TBK1 gene duplications was detected in unrelated NTG pedigrees. These duplications are novel and appear to have arisen independently. No evidence of a founder effect or common ancestry was detected for those carrying these copy number variations. Moreover, no repetitive DNA sequences were identified in the region that might predispose patients to relatively frequent development of copy number variations.
DISCUSSION
TBK1 gene duplications were recently reported to be associated with 0.4% to 1.3% of NTG cases in white and Asian populations.18,19 We report identification of an additional case of TBK1 gene duplication in 1 of 96 NTG patients (1.0%) from New York. These data further confirm the role of TBK1 gene dosage in the pathogenesis of NTG and are consistent with prior reports that suggest approximately 1 in 100 NTG patients may carry a TBK1 gene duplication. When the data from all of the populations studied in the prior 2 reports of TBK1 and NTG18,19 are combined with the data from the current report, 5 of 803 NTG patients (0.62%) were found to carry TBK1 gene duplications, whereas no such mutations were identified in 1116 controls. TBK1 duplications have been detected in African American, white, and Asian NTG patients, suggesting that although these mutations are rare, they may be found worldwide. Moreover, each of the 5 duplications differ from each other in the extent of duplicated DNA, which does not support a founder effect and suggests that each mutation arose independently.
Patients with NTG that is associated with TBK1 gene duplications have some characteristic clinical features. Patient GGR-590-1 and other previously described patients with TBK1-associated NTG had early onset of severe disease.18,19 Many patients have a strong family history of NTG18,19; however, patient GGR-590-1 reported no history of NTG in her family. Similarly, many but not all NTG patients have thin central corneas.18,19 Finally, 2 NTG patients were evaluated for low blood pressure, and nocturnal hypotension was detected in 1 patient (patient GGR-590-1). Unfortunately, nocturnal blood pressure data were not available from the other patients with TBK1-associated NTG. Low blood pressure has been studied as a contributor to the pathophysiology of NTG27,28; however, its role in TBK1-related disease is unclear.
The specific mechanism by which TBK1 gene duplications lead to NTG is not known. However, the known functions of TBK1 and other NTG genes (OPTN and TLR4) suggest that mutations of TBK1 may cause NTG through abnormal activation of autophagy. TBK1, OPTN, and TLR4 each encode proteins that interact to activate autophagy. On the basis of these data, we hypothesize that mutations (such as a TBK1 gene duplication) may abnormally activate autophagy and lead to retinal ganglion cell death and glaucoma. TBK1 also regulates the immune response and inflammation (ie, noncanonical NF-κB signaling). Consequently, the role of NF-κB signaling and other downstream pathways cannot be ruled out as an alternative for causing TBK1-associated NTG. Interestingly, TBK1 may also mediate crosstalk between the autophagic and NF-κB pathways.29–31 This type of crosstalk has been found with other innate immunity signaling pathways, such as activation of NOD2 by intracellular pathogens, that also result in activation of both autophagy and NF-κB.32 These findings suggest a high degree of coordination between these pathways. They also suggest that pathogenesis of NTG caused by defects in one particular branch of the pathway could be mediated by other pathways connected by crosstalk.
CONCLUSIONS
This report provides additional evidence that defects in the TBK1 gene (ie, gene duplication) may cause 0.4% to 1.3% of NTG cases in numerous populations. Future studies of TBK1 function using a range of approaches and resources, including human donor eyes, transgenic mice, and cell culture, may clarify the role of autophagy and/or NF-κB signaling in the pathogenesis of NTG. These investigations may provide new insights into the mechanisms by which TBK1, OPTN, and TLR4 gene defects lead to glaucoma and may suggest new methods of intervention.
ACKNOWLEDGMENTS
This research was conducted with support in part from NIH EY R01018825, Robert and Sharon Wilson, Optegra, UK, Eire Glaucoma Society, International Glaucoma Association and the T F C Frost Charitable Trust. None of these funding organizations or sponsors had any roles in following: the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication. The corresponding author, John H. Fingert, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
All authors (Robert Ritch, Ben Darbro, Geeta Menon, Cheryl L. Khanna, Frances Solivan-Timpe, Ben R. Roos, Mansoor Sarfarzi, Kazuhide Kawase, Tetsuya Yamamoto, Alan L. Robin, Andrew J. Lotery, and John H. Fingert) have no conflict of interests or financial disclosures relevant to this research.
REFERENCES
- 1.Thorleifsson G, Walters GB, Hewitt AW, et al. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat Genet. 2010;42(10):906–909. doi: 10.1038/ng.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiggs JL, Kang JH, Yaspan BL, et al. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma in Caucasians from the USA. Hum Mol Genet. 2011;20(23):4707–4713. doi: 10.1093/hmg/ddr382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burdon KP, MacGregor S, Hewitt AW, et al. Genome-wide association study identifies susceptibility loci for open angle glaucoma at TMCO1 and CDKN2B-AS1. Nat Genet. 2011;43(6):574–578. doi: 10.1038/ng.824. [DOI] [PubMed] [Google Scholar]
- 4.Ramdas WD, van Koolwijk LME, Lemij HG, et al. Common genetic variants associated with open-angle glaucoma. Hum Mol Genet. 2011;20(12):2464–2471. doi: 10.1093/hmg/ddr120. [DOI] [PubMed] [Google Scholar]
- 5.Fan BJ, Wang DY, Pasquale LR, Haines JL, Wiggs JL. Genetic variants associated with optic nerve vertical cup-to-disc ratio are risk factors for primary open angle glaucoma in a US Caucasian population. Invest Ophthalmol Vis Sci. 2011;52(3):1788–1792. doi: 10.1167/iovs.10-6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiggs JL, Yaspan BL, Hauser MA, et al. Common variants at 9p21 and 8q22 are associated with increased susceptibility to optic nerve degeneration in glaucoma. PLoS Genet. 2012;8(4):e1002654. doi: 10.1371/journal.pgen.1002654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao D, Jiao X, Liu X, et al. CDKN2B polymorphism is associated with primary open-angle glaucoma (POAG) in the Afro-Caribbean population of Barbados, West Indies. PLoS ONE. 2012;7(6):e39278. doi: 10.1371/journal.pone.0039278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma S, Burdon KP, Chidlow G, et al. Association of genetic variants in the TMCO1 gene with clinical parameters related to glaucoma and characterization of the protein in the eye. Invest Ophthalmol Vis Sci. 2012;53(8):4917–4925. doi: 10.1167/iovs.11-9047. [DOI] [PubMed] [Google Scholar]
- 9.Shibuya E, Meguro A, Ota M, et al. Association of Toll-like receptor 4 gene polymorphisms with normal tension glaucoma. Invest Ophthalmol Vis Sci. 2008;49(10):4453–4457. doi: 10.1167/iovs.07-1575. [DOI] [PubMed] [Google Scholar]
- 10.Writing Committee for the Normal Tension Glaucoma Genetic Study Group of Japan Glaucoma Society. Meguro A, Inoko H, Ota M, Mizuki N, Bahram S. Genome-wide association study of normal tension glaucoma: common variants in SRBD1 and ELOVL5 contribute to disease susceptibility. Ophthalmology. 2010;117(7):1331.e5–1338.e5. doi: 10.1016/j.ophtha.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Fingert JH. Primary open-angle glaucoma genes. Eye. 2011;25(5):587–595. doi: 10.1038/eye.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stone EM, Fingert JH, Alward WL, et al. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275(5300):668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- 13.Rezaie T, Child A, Hitchings R, et al. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. 2002;295(5557):1077–1079. doi: 10.1126/science.1066901. [DOI] [PubMed] [Google Scholar]
- 14.Fingert JH, Héon E, Liebmann JM, et al. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet. 1999;8(5):899–905. doi: 10.1093/hmg/8.5.899. [DOI] [PubMed] [Google Scholar]
- 15.Alward WL, Fingert JH, Coote MA, et al. Clinical features associated with mutations in the chromosome 1 open-angle glaucoma gene (GLC1A) N Engl J Med. 1998;338(15):1022–1027. doi: 10.1056/NEJM199804093381503. [DOI] [PubMed] [Google Scholar]
- 16.Alward WLM, Kwon YH, Kawase K, et al. Evaluation of optineurin sequence variations in 1,048 patients with open-angle glaucoma. Am J Ophthalmol. 2003;136(5):904–910. doi: 10.1016/s0002-9394(03)00577-4. [DOI] [PubMed] [Google Scholar]
- 17.Aung T, Ebenezer ND, Brice G, et al. Prevalence of optineurin sequence variants in adult primary open angle glaucoma: implications for diagnostic testing. J Med Genet. 2003;40(8):e101. doi: 10.1136/jmg.40.8.e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fingert JH, Robin AL, Stone JL, et al. Copy number variations on chromosome 12q14 in patients with normal tension glaucoma. Hum Mol Genet. 2011;20(12):2482–2494. doi: 10.1093/hmg/ddr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawase K, Allingham RR, Meguro A, et al. Confirmation of TBK1 duplication in normal tension glaucoma. Exp Eye Res. 2012;96(1):178–180. doi: 10.1016/j.exer.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morton S, Hesson L, Peggie M, Cohen P. Enhanced binding of TBK1 by an optineurin mutant that causes a familial form of primary open angle glaucoma. FEBS Lett. 2008;582(6):997–1002. doi: 10.1016/j.febslet.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 21.Weidberg H, Elazar Z. TBK1 mediates crosstalk between the innate immune response and autophagy. Sci Signal. 2011;4(187):pe39. doi: 10.1126/scisignal.2002355. [DOI] [PubMed] [Google Scholar]
- 22.Pomerantz JL, Baltimore D. NF-kappaB activation by a signaling complex containing TRAF2, TANK and TBK1, a novel IKK-related kinase. EMBO J. 1999;18(23):6694–6704. doi: 10.1093/emboj/18.23.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu G, Wu C-J, Zhao Y, Ashwell JD. Optineurin negatively regulates TNFalpha-induced NF-kappaB activation by competing with NEMO for ubiquitinated RIP. Curr Biol. 2007;17(16):1438–1443. doi: 10.1016/j.cub.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 24.Wild P, Farhan H, McEwan DG, et al. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science. 2011;333(6039):228–233. doi: 10.1126/science.1205405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park HYL, Kim JH, Park CK. Activation of autophagy induces retinal ganglion cell death in a chronic hypertensive glaucoma model. Cell Death Dis. 2012;3:e290. doi: 10.1038/cddis.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piras A, Gianetto D, Conte D, Bosone A, Vercelli A. Activation of autophagy in a rat model of retinal ischemia following high intraocular pressure. PLoS ONE. 2011;6(7):e22514. doi: 10.1371/journal.pone.0022514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham SL, Drance SM, Wijsman K, Douglas GR, Mikelberg FS. Ambulatory blood pressure monitoring in glaucoma. The nocturnal dip. Ophthalmology. 1995;102(1):61–69. doi: 10.1016/s0161-6420(95)31053-6. [DOI] [PubMed] [Google Scholar]
- 28.Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114(11):1965–1972. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 29.Kang R, Tang D, Lotze MT, Zeh HJ. RAGE regulates autophagy and apoptosis following oxidative injury. Autophagy. 2011;7(4):442–444. doi: 10.4161/auto.7.4.14681. [DOI] [PubMed] [Google Scholar]
- 30.Criollo A, Chereau F, Malik SA, et al. Autophagy is required for the activation of NFκB. Cell Cycle. 2012;11(1):194–199. doi: 10.4161/cc.11.1.18669. [DOI] [PubMed] [Google Scholar]
- 31.Niso-Santano M, Criollo A, Malik SA, et al. Direct molecular interactions between Beclin 1 and the canonical NFκB activation pathway. Autophagy. 2012;8(2):268–270. doi: 10.4161/auto.8.2.18845. [DOI] [PubMed] [Google Scholar]
- 32.Billmann-Born S, Lipinski S, Böck J, Till A, Rosenstiel P, Schreiber S. The complex interplay of NOD-like receptors and the autophagy machinery in the pathophysiology of Crohn disease. Eur J Cell Biol. 2011;90(6–7):593–602. doi: 10.1016/j.ejcb.2010.10.015. [DOI] [PubMed] [Google Scholar]