Abstract
Protein-energy wasting is highly prevalent in hemodialysis patients, and it contributes to patient morbidity and mortality. The ubiquitin-proteasome system is the major pathway for intracellular protein degradation and it is involved in the regulation of basic cellular processes. However, the role of this system in the determination of nutritional status is largely unknown. To examine a relationship between protein-energy wasting and the ubiquitin-proteasome system, a cross-sectional study of 76 hemodialysis patients was performed. Plasma concentrations of 20S proteasome were studied to evaluate its association with muscle and fat mass, which were investigated by abdominal muscle and fat areas measured using computed tomography and by creatinine production estimated using the creatinine kinetic model. Plasma 20S proteasome concentrations significantly and negatively correlated with abdominal muscle areas and creatinine production (rho = -0.263, P < 0.05 and rho = -0.241, P < 0.05, respectively), but not abdominal subcutaneous and visceral fat areas. Multiple regression analyses showed that 20S proteasome was a significant independent predictor of abdominal muscle area (P < 0.05). In conclusion, plasma 20S proteasome concentrations were independently associated with abdominal muscle mass in hemodialysis patients. Our findings indicate a relationship between circulating 20S proteasomes and muscle metabolism in these patients.
Trial Registration
UMIN Clinical Trials Registry UMIN000012341
Introduction
Protein-energy wasting (PEW) is a condition associated with chronic kidney disease (CKD), and it is characterized as decreased body stores of muscle and fat mass [1]. PEW is present in a large proportion of advanced CKD patients, and it is related to increased morbidity and mortality [2]. Inadequate nutrition, inflammation, perturbations of appetite-controlling hormones, insulin resistance, and metabolic acidosis may contribute to the pathogenesis of PEW [3, 4].
Protein degradation via the ubiquitin-proteasome system (UPS) is the major pathway of the non-lysosomal proteolysis, and this system plays important roles in a variety of fundamental cellular processes, such as the regulation of cell cycle progression, apoptosis, sodium channel function, fibrosis, and the modulation of inflammatory responses [5, 6]. UPS also plays a critical role in muscle atrophy during various catabolic states, including sepsis, burn injuries, cancer, diabetes and uremia [7]. The 20S proteasome is a central element of intracellular UPS [8].
Recently, a growing body of evidence demonstrated the presence of 20S proteasome both intracellularly and in the extracellular space, where it may exert physiological functions [9]. Furthermore, 20S proteasomes are physiologically present in the human circulation, and increased concentrations were demonstrated in diverse pathological states, such as autoimmune diseases [10], sepsis, acute respiratory distress syndrome [11] and several cancers [12–14]. However, the role of circulating 20S proteasomes has not been investigated in patients with CKD.
This study reports cross-sectional data from a well-characterized cohort of patients undergoing maintenance HD. We also assessed associations between circulating 20S proteasomes and several nutritional markers in these patients.
Materials and Methods
Subjects
Seventy-six patients (50 men, 26 women) who had been undergoing HD for at least three consecutive months at Iwata City Hospital (Shizuoka, Japan) were enrolled in this cross-sectional study. All patients were subjected to regular HD for 4–5 hours three times per week at a blood flow rate of 180–240 mL/min. All patients used bicarbonate dialysate (Kindaly AF-2E, Fuso, Osaka, Japan) at a dialysate flow rate of 500 mL/min.
The institutional ethics committee approved the study protocol, and all patients provided informed consent before participation. This study was also registered with the Clinical Trial Registry of the University Hospital Medical Information Network (http://www.umin.ac.jp/, study number: UMIN000012341).
Anthropometric measurements
Body weight was measured before and after each dialysis session, and the post-dialysis body weight of each patient was used as his or her dry weight (DW). Body mass index (BMI, kg/m2) was calculated by dividing the DW (kg) by the squared height (m).
Blood sampling and laboratory examinations
Blood samples of patients were drawn at the beginning and end of the first dialysis session of the week, following a 2-day interval. As a control, blood samples from 5 healthy adults were also drawn. Plasma samples were separated immediately and stored at -80°C until analyzed. Serum electrolytes, urea nitrogen, creatinine (Cr), albumin, cholesterol, triglyceride, and C-reactive protein (CRP) levels were measured using standard laboratory techniques with an auto-analyzer. Plasma 20S proteasome and interleukin-6 (IL-6) concentrations were measured using enzyme-linked immunosorbent assays (ELISA, Proteasome ELISA kit: Enzo Life Sciences, Farmingdale, NY, USA and Ultra-sensitive human IL-6 immunoassay kit: R&D Systems, Minneapolis, MN, USA, respectively).
Evaluation of 20S proteasome concentrations using Western blot analysis
Equal amounts of plasma samples (0.8 μL) after dilution were loaded for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as described previously [15]. The primary antibody was a mouse monoclonal anti-20S proteasome (alpha6 subunit, clone MCP20, Enzo Life Sciences, Farmingdale, NY, USA), whish was also used as the capture antibody in ELISA described above. A 20S Proteasome Stock Solution (0.05 μg/mL, Enzo Life Sciences) was used as a standard. Band intensities were quantified using NIH-IMAGE, which is a public domain planimetry program available from the National Institutes of Health (written by Wayne Rasband, The National Institutes of Health, Bethesda, MD, USA), and compared with a standard of 20S proteasomes.
Measurements of abdominal muscle and fat areas using computed tomography
Abdominal computed tomography (CT) scans were performed during a patient’s periodic check-up. The CT scan and measurement of Cr production were performed within 3 months of each other. Each patient was examined in the supine position and the thickness of each slice was 10 mm. Axial CT images for muscle and fat mass evaluations were obtained at the level of the third lumber spine. Radiographic images were digitally scanned for analyses on a personal computer. The adipose-tissue-free abdominal muscle area (AMA), abdominal subcutaneous fat area (ASFA) and abdominal visceral fat area (AVFA) were measured using NIH-IMAGE [16].
Normalized protein equivalent of nitrogen appearance and evaluation of hemodialysis dose
The normalized protein equivalent of nitrogen appearance (nPNA) was calculated using the formula published by the K/DOQI Hemodialysis Adequacy Work Group [17]. Data collected during a beginning-of-the-week dialysis session were used for these calculations. HD dose was evaluated using the following formula:
where Kt/V urea is single-pool Kt/V urea, R is the ratio of post-dialysis to pre-dialysis serum urea nitrogen, t is time of dialysis in hours, UF is the amount of ultrafiltration in liters, and W is post-dialysis body weight in kilograms. nPNA was evaluated using the following formula:
where C0 is the pre-dialysis concentration of serum urea nitrogen in mg per deciliter.
Geriatric nutritional risk index
The geriatric nutritional risk index (GNRI) was calculated from the patient’s serum albumin and body weight by using the equation developed by Bouillanne et al. [18] as follows:
Estimation of creatinine production using the creatinine kinetic model
The Cr production rate was estimated using the pre- and post-dialysis Cr concentrations at the first dialysis session of the week based on the Cr kinetic model developed by Shinzato et al. [19] with slight modification, according to the following equation:
where
where Cs (mg/dL) is the pre-dialysis Cr concentration, Ce (mg/dL) is the post-dialysis Cr concentration, ΔBW (kg) is the body weight decrease resulting from dialysis, IBW (kg) is the ideal body weight and Td (hour) is the dialysis duration.
Statistical analysis
Data are expressed as the means ± standard deviation (SD) for continuous variables with normal distributions or the median and interquartile range (25th to 75th percentiles) for data with skewed distributions. The threshold for statistical significance was set at P < 0.05. Comparisons between two groups were performed using the Mann-Whitney U-test. Spearman’s rank-order correlation analysis was used to evaluate potential associations between the 20S proteasome and the selected parameters. Multivariate regression analyses assessed the independent predictors of AMA and AMA standardized for height. All statistical analyses were performed using StatView 5 (SAS Institute Inc., Cary, NC, USA) or IBM SPSS statistical software, version 19.0 (IBM SPSS, Tokyo, Japan).
Results
Causes of end-stage kidney diseases
The causes of end-stage kidney diseases in this study population were primary kidney diseases, such as chronic glomerulonephritis and nephrosclerosis in 63 patients (82%), overt diabetic nephropathy in 8 patients (11%) and polycystic kidney disease in 5 patients (7%).
Plasma levels of 20S proteasome
Plasma levels of 20S proteasome measured with ELISA and Western blot analysis were significantly correlated each other (P < 0.05), but the ELISA values were slightly higher than Western blot analysis (1.34 ± 1.12 μg/mL and 1.33 ± 0.53 μg/mL, respectively, Table 1). Two patients demonstrated extremely high 20S proteasome levels when plasma samples were measured with ELISA (110.90 μg/mL and 70.10 μg/mL). Furthermore, the laddered bands, which might be included as levels when the samples were measured with ELISA, were present under the specific bands of 20S proteasome (Fig. 1). Therefore, we used the data measured with Western blot analysis in subsequent analyses.
Table 1.
Patient Characteristics.
| Variables | Total (n = 76) | Male (n = 50) | Female (n = 26) | P |
|---|---|---|---|---|
| Age, years | 67.0 (60.0 to 73.3) | 67.5 (60.3 to 74.0) | 65.5 (59.8 to 71.5) | 0.493 |
| Dialysis vintage, months | 142.5 (42.3 to 269.8) | 136.0 (39.3 to 267.0) | 157.5 (85.8 to 282.5) | 0.536 |
| Height, m | 1.60 ± 0.09 | 1.64 ± 0.06 | 1.52 ± 0.08 | <0.001 |
| Dry weight, kg | 50.3 ± 9.7 | 53.8 ± 8.9 | 43.5 ± 7.4 | <0.001 |
| BMI, kg/m2 | 20.3 ± 2.9 | 20.7 ± 2.8 | 19.6± 3.0 | 0.057 |
| Systolic blood pressure, mmHg | 157.3 ± 19.3 | 156.2 ± 22.1 | 159.5 ± 12.6 | 0.243 |
| Diastolic blood pressure, mmHg | 85.2± 13.0 | 82.8± 13.1 | 89.8 ± 11.8 | <0.05 |
| Hemoglobin, g/dL | 11.4 ± 1.1 | 11.4 ± 1.1 | 11.4 ± 1.3 | 0.665 |
| Total protein, g/dL | 6.4 ± 0.4 | 6.4 ± 0.5 | 6.4 ± 0.4 | 0.965 |
| Serum albumin, g/dL | 3.7 ± 0.4 | 3.7 ± 0.4 | 3.7 ± 0.4 | 0.252 |
| Total cholesterol, mg/dL | 152.4 ± 32.5 | 145.1 ± 31.0 | 166.3 ± 31.1 | <0.05 |
| LDL choresterol, mg/dL | 86.0 ± 20.8 | 83.4 ± 21.2 | 90.8 ± 19.6 | 0.114 |
| Blood urea nitrogen, mg/dL | 62.1 ± 12.7 | 64.2 ± 10.8 | 58.2 ± 15.1 | <0.05 |
| Serum creatinine, mg/dL | 10.7 ± 2.7 | 11.1 ± 3.0 | 9.8 ± 1.7 | <0.05 |
| Sodium, mEq/L | 139.3 ± 2.8 | 138.7 ± 2.7 | 140.4 ± 2.7 | <0.05 |
| Potassium, mEq/L | 4.6 ± 0.5 | 4.7 ± 0.5 | 4.5 ± 0.6 | 0.277 |
| Calcium, mg/dL | 9.5 ± 0.9 | 9.3 ± 0.9 | 9.7 ± 0.8 | 0.116 |
| Phosphate, mg/dL | 5.0 ± 1.3 | 5.1 ± 1.4 | 4.8 ± 1.2 | 0.437 |
| Intact PTH, pg/mL | 101.3 ± 107.1 | 88.0 ± 53.4 | 127.0 ± 166.6 | 0.978 |
| Beta2-microglobulin, mg/L | 26.5 ± 6.6 | 26.1 ± 6.6 | 27.3 ± 6.7 | 0.669 |
| Kt/V urea | 1.6 ± 0.3 | 1.5 ± 0.2 | 1.9 ± 0.3 | <0.001 |
| nPNA, g/kg/day | 0.96± 0.17 | 0.97± 0.15 | 0.94 ± 0.21 | 0.266 |
| GNRI | 91.8± 7.7 | 92.1 ± 7.7 | 91.2 ± 7.8 | 0.547 |
| CRP, mg/dL | 0.1 (0.0 to 0.3) | 0.1 (0.1 to 0.4) | 0.0 (0.0 to 0.1) | <0.01 |
| IL-6, pg/mL | 5.10 (3.10 to 10.45) | 5.12(3.51 to 10.25) | 4.65 (2.75 to 10.17) | 0.529 |
| 20S Proteasome, ELISA | 1.34 ± 1.12 | 1.26 ± 1.11 | 1.51 ± 1.13 | 0.233 |
| 20S Proteasome, Western blot | 1.33 ± 0.53 | 1.28 ± 0.47 | 1.46 ± 0.66 | 0.244 |
| AMA, cm2 | 96.5 (81.4 to 119.0) | 114.6 (90.0 to 128.0) | 78.7 (69.0 to 91.3) | <0.001 |
| AMA standardized for height | 61.1 (50.9 to 73.4) | 68.9 (56.4 to 76.8) | 51.6 (43.7 to 59.2) | <0.001 |
| ASFA, cm2 | 70.6 (49.0 to 108.6) | 67.2(46.1 to 106.0) | 75.1 (58.8 to 121.6) | 0.178 |
| ASFA standardized for height | 45.1 (31.9 to 64.7) | 42.6 (27.9 to 62.3) | 50.2 (36.8 to 77.9) | 0.090 |
| AVFA, cm2 | 47.8(22.1 to 87.2) | 54.1 (22.7 to 110.8) | 34.5 (21.9 to 58.8) | 0.090 |
| AVFA standardized for height | 31.2 (14.0 to 54.0) | 34.5 (13.8 to 69.8) | 23.3 (14.1 to 38.1) | 0.168 |
| Creatinine production rate, g/day | 0.83 (0.69 to 1.08) | 0.99 (0.72 to 1.19) | 0.74 (0.61 to 0.86) | <0.01 |
All variables are expressed as the means ± SD or the medians and interquartile range (25th to 75th percentiles).
Abbreviations: AMA, abdominal muscle area; ASFA, abdominal subcutaneous fat area; AVFA, abdominal visceral fat area; BMI, body mass index; CRP, C-reactive protein; GNRI, geriatric nutritional risk index; IL-6, interleukin-6; Kt/V urea, amount of dialysis delivered to each patient per treatment; LDL, low-density lipoprotein; nPNA, normalized protein equivalent of nitrogen appearance; PTH, parathyroid hormone.
Fig 1. Western blot analysis of 20S proteasome in 11 patients.

Bands of the 20S proteasome are detected (arrow head). Laddered bands were also present under the specific bands (asterisk).
A comparison of 20S proteasome levels between patients and healthy subjects
In patients undergoing hemodialysis, plasma levels of 20S proteasome were relatively higher than those in healthy controls (1.33 ± 0.53 μg/mL vs. 0.95 ± 0.13 μg/mL, respectively, P = 0.110).
Clinical profiles
Table 1 presents the characteristics of the study population. The median age was 67.0 years (the 25th to 75th percentile ranged from 60.0 to 73.3 years). The median dialysis vintage was 142.5 months (range, 42.3 to 269.8 months), and the mean BMI was 20.3 ± 2.9 kg/m2.
No significant sex differences were observed with respect to age, dialysis vintage, BMI, or serum albumin levels of the study participants. On the other hand, the patient height, dry weight (DW), serum Cr levels, AMA, AMA standardized for height and Cr production rate were significantly greater in men than women. No significant differences in IL-6 and 20S proteasome levels were observed between men and women.
Correlations between 20S proteasome and clinical parameters
Significant negative correlations were observed between the 20S proteasome levels and dry weight (P < 0.05), phosphate (P < 0.001), intact PTH (P < 0.05), AMA (P < 0.05, Fig. 2), AMA standardized for height (P < 0.05) and the Cr production rate (P < 0.05). Conversely, the 20S proteasome levels were not correlated with age, gender, dialysis vintage, height, nPNA, GNRI, log-transformed IL-6, ASFA, ASFA standardized for height, AVFA and AVFA standardized for height (Table 2).
Fig 2. Association between abdominal muscle areas and 20S proteasome levels.
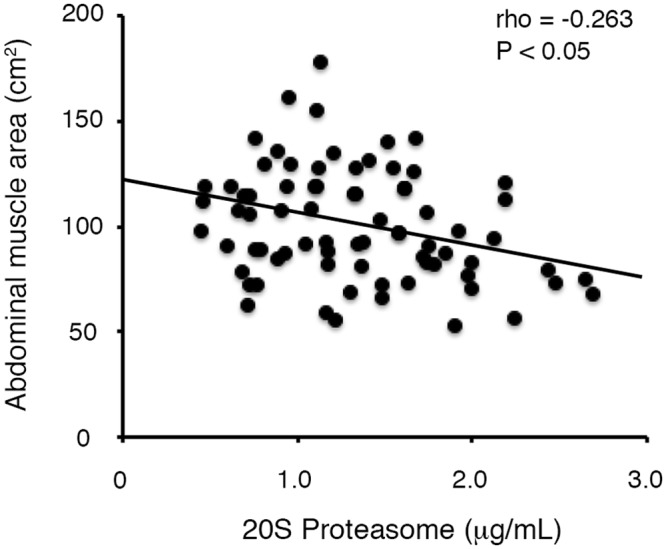
Table 2.
Correlations between 20S Proteasome Concentrations and Clinical Variables.
| 20S Proteasome | ||
|---|---|---|
| Correlation coefficient | P | |
| Age | 0.133 | 0.252 |
| Gender, male | -0.135 | 0.246 |
| Dialysis vintage | −0.146 | 0.207 |
| Height | −0.154 | 0.183 |
| Dry weight | −0.242 | <0.05 |
| BMI | −0.190 | 0.101 |
| Systolic blood pressure | 0.157 | 0.177 |
| Diastolic blood pressure | −0.046 | 0.694 |
| Total protein | 0.072 | 0.535 |
| Serum albumin | 0.077 | 0.511 |
| Total cholesterol | 0.240 | <0.05 |
| LDL cholesterol | 0.144 | 0.214 |
| Blood urea nitrogen | −0.004 | 0.971 |
| Serum creatinine | −0.185 | 0.110 |
| Calcium | 0.019 | 0.873 |
| Phosphate | −0.391 | <0.001 |
| Intact PTH | −0.227 | <0.05 |
| Beta 2-microglobulin | 0.024 | 0.835 |
| Kt/V urea | 0.202 | 0.080 |
| nPNA | 0.049 | 0.675 |
| GNRI | −0.107 | 0.358 |
| Log IL-6 | −0.144 | 0.216 |
| AMA | −0.263 | <0.05 |
| AMA standardized for height | −0.266 | <0.05 |
| ASFA | −0.034 | 0.771 |
| ASFA standardized for height | −0.018 | 0.874 |
| AVFA | −0.038 | 0.745 |
| AVFA standardized for height | −0.034 | 0.768 |
| Creatinine production rate | −0.241 | <0.05 |
Abbreviations: AMA, abdominal muscle area; ASFA, abdominal subcutaneous fat area; AVFA, abdominal visceral fat area; BMI, body mass index; GNRI, geriatric nutritional risk index; IL-6, interleukin-6; Kt/V urea, amount of dialysis delivered to each patient per treatment; LDL, low-density lipoprotein; nPNA, normalized protein equivalent of nitrogen appearance; PTH, parathyroid hormone.
Determinants of abdominal muscle mass
Multiple regression analyses revealed that 20S proteasome was independently associated with AMA when 20S proteasome, age, gender, dialysis vintage, height, log-transformed IL-6 (model 1) and nPNA (model 2) were adjusted as independent variables (Table 3). The analyses also revealed that 20S proteasome was independently associated with AMA standardized for height when 20S proteasome, age, gender, dialysis vintage, log-transformed IL-6 and nPNA were adjusted as independent variables (P = 0.036).
Table 3.
Multiple Regression Analysis using Abdominal Muscle Area (AMA) as the Dependent Variable and 20S Proteasome, Age, Sex, Dialysis Vintage, Height, Log IL-6 and nPNA as Independent Variables.
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Beta | P | Beta | P | |
| Dependent variable: Abdominal muscle area (AMA) | ||||
| 20S Proteasome | −0.190 | 0.037 | −0.185 | 0.043 |
| Age | −0.224 | 0.058 | −0.229 | 0.054 |
| Gender, male | 0.464 | 0.001 | 0.468 | 0.001 |
| Dialysis vintage | −0.094 | 0.274 | −0.077 | 0.379 |
| Height | 0.155 | 0.289 | 0.163 | 0.266 |
| Log IL-6 | −0.135 | 0.140 | −0.136 | 0.138 |
| nPNA | - | - | −0.083 | 0.342 |
Abbreviations: IL-6, interleukin-6; nPNA, normalized protein equivalent of nitrogen appearance.
Furthermore, plasma 20S proteasome levels significantly and inversely decreased when the levels were divided into 2 groups according to AMA (1.49 ± 0.58 μg/mL in lower AMA group and 1.18 ± 0.54 μg/mL in higher AMA group, respectively, P < 0.05) and the Cr production rate (1.46 ± 0.56 μg/mL in lower Cr production rate group and 1.22 ± 0.51 μg/mL in higher Cr production rate group, respectively, P < 0.05).
Discussion
The primary finding of this study is that plasma 20S proteasome levels are associated with abdominal muscle mass in HD patients, even after adjusting for potential confounding variables. In addition, 20S proteasome levels are significantly and negatively correlated with dry weight, serum phosphate levels and the Cr production rate. To our knowledge, this report is the first study to demonstrate a link between circulating 20S proteasome levels and muscle metabolism of patients with advanced CKD.
CKD patients often suffer from nutritional problems that are associated with increased morbidity and mortality [20]. PEW is a proposed term to describe the state of decreased body stores of protein and energy that occurs in CKD and is diagnosed if three characteristics are present in four categories: (1) serum chemistry (low serum levels of albumin, prealbumin, or cholesterol), (2) reduced body mass (low BMI, weight loss, or low body fat percentage), (3) reduced muscle mass (muscle wasting, reduced mid-arm muscle circumference, or creatinine appearance), and (4) reduced dietary intake (low intake of protein or energy) [1]. In fact, HD patients exhibit lower BMIs than age- and sex-matched control subjects from the general population [21] and a previous study showed that increased BMI contributed to survival advantages in dialysis patients [22]. Furthermore, increased serum Cr levels are associated with improved survival, whereas lower serum Cr levels are associated with increased mortality [23]. This finding suggests that low serum Cr levels, as a marker of low muscle mass, could be related to poorer outcomes. Carrero et al. [24] also reported that muscle wasting measured by subjective global assessment (SGA) was associated with increased mortality. These observations suggest that muscle mass is an important predictor of mortality in CKD patients.
The relationship between UPS and muscle wasting has been demonstrated. For example, treatment with a proteasome inhibitor, N-benzyloxycarbonyl-Ile-Glu-(O-t-butyl)-Ala-leucinal (PSI), prevented sepsis-induced muscle atrophy in an animal model [25]. In addition, the levels of messenger RNAs encoding several 20S proteasome subunits increase under catabolic conditions despite a reduction in muscle total RNA contents [26]. Du et al. [27] excellently demonstrated that conditions such as hyperglycemia and uremia accelerate the degradation of myofibrillar proteins (myosin and actin) that compose 60 to 70 percent of muscle protein. These findings indicate that UPS plays a critical role in muscle wasting of catabolic patients, including CKD patients.
Recently, the presence of extracellular and circulating 20S proteasomes was identified. Zoeger et al. [28] reported that purified 20S proteasomes from the plasma of healthy donors and patients with autoimmune diseases were intact and functional 20S particles using morphological and biochemical techniques. Henry et al. [29] also confirmed that circulating 20S proteasomes contain the same subunits as intracellular proteasomes using proteomic analysis. Therefore, circulating 20S proteasomes may exert physiological functions [9].
Clinically, elevated concentrations of 20S proteasomes were demonstrated in patients with cancer of the breast, stomach, kidney, colon, lung, ovary and skin [12–14]. Jakob et al. [13] also reported that circulating 20S proteasome concentrations were an independent prognostic factor for survival in patients with multiple myeloma. On the other hand, significantly elevated concentrations of 20S proteasomes were found in non-cancerous patients, including burn injury, sepsis and systemic autoimmune diseases [10, 11]. Notably, these concentrations were correlated well with disease activities of autoimmune diseases [10]. Taken together, these observations suggest that circulating 20S proteasomes play a role in these pathological conditions.
One of the key findings in the present study is that the negative association of 20S proteasome levels with abdominal muscle mass remains significant even after adjusting for nPNA (Table 3). Our result indicates that the 20S proteasome level is associated with abdominal muscle mass independent of dietary protein intake, because we adjusted for nPNA as a marker of protein intake levels to clarify the role of circulating 20S proteasome in dialysis patients. Further studies are needed to clarify the effect of circulating 20S proteasomes on muscle mass of CKD patients.
The origin of circulating 20S proteasomes is not known in our cohort, but several possibilities are speculated. First, circulating 20S proteasomes may be released passively from the muscle as a result of cellular damage [30]. This passive release would allow the 20S proteasome to be used as a surrogate marker for muscle wasting in hemodialysis patients. However, the fact that its concentration appears to be independent of LDH and CPK (rho = 0.080, P = 0.494 and rho = 0.059, P = 0.614, respectively) suggests that circulating 20S proteasomes do not derive from muscle breakdown. Second, microparticles, small membrane enclosed vesicles, are a possible source of circulating 20S proteasomes [31]. Interestingly, Bochmann et al. [31] have reported that in vitro generated microparticles derived from T lymphocytes released 20S proteasomes after the incubation with sphingomyelinase. Third, exosomes, which are 50–100 nm vesicular structures, are also a potential source of circulating 20S proteasomes [32]. Recently, Lai et al. [32] have reported that the exosomes derived from the endosomal compartment of mesenchymal stem cells contained 20S proteasomes and might have a cardio-protective effect. Taken together, microparticles or exosomes derived from the muscle may actively release 20S proteasomes into the circulation, as reported in such particles derived from T lymphocytes or the endosomal compartment of mesenchymal stem cells, respectively. Fourth, circulating 20S proteasomes may be secreted from immuno-competent cells, including thrombocytes, dendritic cells and other antigen-presenting cells, as suggested in patients with autoimmune diseases [10]. If so, the 20S proteasomes secreted from those cells may act as immuno-proteasomes and play a role in antigen presentation, possibly for the destruction of muscle proteins [33]. Further studies are needed to clarify the origin and the role of circulating 20S proteasomes in CKD patients.
Our study has several limitations. First, a longitudinal causal relationship could not be established between the changes in plasma 20S proteasome levels and alterations in muscle mass because of the cross-sectional study design. Second, the generalizability of our conclusions is not clear because of the relatively small number of patients in our single-center cohort. The variation of plasma 20S proteasome levels is also high. Therefore, larger sample sizes and validation in different cohorts are needed to confirm our results. Third, while the 20S proteasome levels in CKD patients had a tendency to increase compared with those in healthy subjects, no statistical significance was observed. The possible reason for this statistical result is that the number in healthy subjects was small. Fourth, the muscle function such as grip strength or gait speed has not been evaluated in this study. Further studies are needed to clarify the relationship between plasma 20S proteasome levels and the muscle function.
In conclusion, plasma 20S proteasome levels are significantly and negatively associated with abdominal muscle mass in HD patients. Our findings suggest that circulating 20S proteasomes and muscle metabolism are related. Future longitudinal observations and interventional studies are warranted to establish whether this link is causal in nature.
Acknowledgments
We thank Ms. Kozue Sakuma for measuring 20S proteasome concentrations and abdominal muscle areas.
Data Availability
All relevant data are within the paper.
Funding Statement
Support was provided by the Japanese Association of Dialysis Physicians (JADP Grant 2011-08, to HF) [http://touseki-ikai.or.jp/]. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73: 391–398. [DOI] [PubMed] [Google Scholar]
- 2. Stenvinkel P, Heimburger O, Paultre F, Diczfalusy U, Wang T, Berglund L, et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55: 1899–1911. [DOI] [PubMed] [Google Scholar]
- 3. Mak RH, Cheung W, Cone RD, Marks DL. Mechanisms of disease: Cytokine and adipokine signaling in uremic cachexia. Nat Clin Pract Nephrol. 2006;2: 527–534. [DOI] [PubMed] [Google Scholar]
- 4. Carrero JJ, Nakashima A, Qureshi AR, Lindholm B, Heimburger O, Barany P, et al. Protein-energy wasting modifies the association of ghrelin with inflammation, leptin, and mortality in hemodialysis patients. Kidney Int. 2011;79: 749–756. 10.1038/ki.2010.487 [DOI] [PubMed] [Google Scholar]
- 5. Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82: 373–428. [DOI] [PubMed] [Google Scholar]
- 6. Fukasawa H. The role of the ubiquitin-proteasome system in kidney diseases. Clin Exp Nephrol. 2012;16: 507–517. 10.1007/s10157-012-0643-1 [DOI] [PubMed] [Google Scholar]
- 7. Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med. 1996;335: 1897–1905. [DOI] [PubMed] [Google Scholar]
- 8. Tanaka K. The proteasome: overview of structure and functions. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85: 12–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sixt SU, Dahlmann B. Extracellular, circulating proteasomes and ubiquitin—incidence and relevance. Biochim Biophys Acta. 2008;1782: 817–823. 10.1016/j.bbadis.2008.06.005 [DOI] [PubMed] [Google Scholar]
- 10. Egerer K, Kuckelkorn U, Rudolph PE, Ruckert JC, Dorner T, Burmester GR, et al. Circulating proteasomes are markers of cell damage and immunologic activity in autoimmune diseases. J Rheumatol. 2002;29: 2045–2052. [PubMed] [Google Scholar]
- 11. Roth GA, Moser B, Krenn C, Roth-Walter F, Hetz H, Richter S, et al. Heightened levels of circulating 20S proteasome in critically ill patients. Eur J Clin Invest. 2005;35: 399–403. [DOI] [PubMed] [Google Scholar]
- 12. Lavabre-Bertrand T, Henry L, Carillo S, Guiraud I, Ouali A, Dutaud D, et al. Plasma proteasome level is a potential marker in patients with solid tumors and hemopoietic malignancies. Cancer. 2001;92: 2493–2500. [DOI] [PubMed] [Google Scholar]
- 13. Jakob C, Egerer K, Liebisch P, Turkmen S, Zavrski I, Kuckelkorn U, et al. Circulating proteasome levels are an independent prognostic factor for survival in multiple myeloma. Blood. 2007;109: 2100–2105. [DOI] [PubMed] [Google Scholar]
- 14. Heubner M, Wimberger P, Dahlmann B, Kasimir-Bauer S, Kimmig R, Peters J, et al. The prognostic impact of circulating proteasome concentrations in patients with epithelial ovarian cancer. Gynecol Oncol. 2011;120: 233–238. 10.1016/j.ygyno.2010.10.014 [DOI] [PubMed] [Google Scholar]
- 15. Yoshida T, Kumagai H, Suzuki A, Kobayashi N, Ohkawa S, Odamaki M, et al. Relaxin ameliorates salt-sensitive hypertension and renal fibrosis. Nephrol Dial Transplant. 2012;27: 2190–2197. 10.1093/ndt/gfr618 [DOI] [PubMed] [Google Scholar]
- 16. Kaizu Y, Ohkawa S, Kumagai H. Muscle mass index in haemodialysis patients: a comparison of indices obtained by routine clinical examinations. Nephrol Dial Transplant. 2002;17: 442–448. [DOI] [PubMed] [Google Scholar]
- 17. Kdoqi. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis. 2007;49: S12–154. [DOI] [PubMed] [Google Scholar]
- 18. Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82: 777–783. [DOI] [PubMed] [Google Scholar]
- 19. Shinzato T, Nakai S, Miwa M, Iwayama N, Takai I, Matsumoto Y, et al. New method to calculate creatinine generation rate using pre- and postdialysis creatinine concentrations. Artif Organs. 1997;21: 864–872. [DOI] [PubMed] [Google Scholar]
- 20. Bergstrom J. Nutrition and mortality in hemodialysis. J Am Soc Nephrol. 1995;6: 1329–1341. [DOI] [PubMed] [Google Scholar]
- 21. Kopple JD, Zhu X, Lew NL, Lowrie EG. Body weight-for-height relationships predict mortality in maintenance hemodialysis patients. Kidney Int. 1999;56: 1136–1148. [DOI] [PubMed] [Google Scholar]
- 22. Cabezas-Rodriguez I, Carrero JJ, Zoccali C, Qureshi AR, Ketteler M, Floege J, et al. Influence of body mass index on the association of weight changes with mortality in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8: 1725–1733. 10.2215/CJN.10951012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park J, Mehrotra R, Rhee CM, Molnar MZ, Lukowsky LR, Patel SS, et al. Serum creatinine level, a surrogate of muscle mass, predicts mortality in peritoneal dialysis patients. Nephrol Dial Transplant. 2013;28: 2146–2155. 10.1093/ndt/gft213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carrero JJ, Chmielewski M, Axelsson J, Snaedal S, Heimburger O, Barany P, et al. Muscle atrophy, inflammation and clinical outcome in incident and prevalent dialysis patients. Clin Nutr. 2008;27: 557–564. 10.1016/j.clnu.2008.04.007 [DOI] [PubMed] [Google Scholar]
- 25. Fischer D, Gang G, Pritts T, Hasselgren PO. Sepsis-induced muscle proteolysis is prevented by a proteasome inhibitor in vivo. Biochem Biophys Res Commun. 2000;270: 215–221. [DOI] [PubMed] [Google Scholar]
- 26. Price SR, England BK, Bailey JL, Van Vreede K, Mitch WE. Acidosis and glucocorticoids concomitantly increase ubiquitin and proteasome subunit mRNAs in rat muscle. Am J Physiol. 1994;267: C955–960. [DOI] [PubMed] [Google Scholar]
- 27. Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B, et al. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest. 2004;113: 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zoeger A, Blau M, Egerer K, Feist E, Dahlmann B. Circulating proteasomes are functional and have a subtype pattern distinct from 20S proteasomes in major blood cells. Clin Chem. 2006;52: 2079–2086. [DOI] [PubMed] [Google Scholar]
- 29. Henry L, Lavabre-Bertrand T, Douche T, Uttenweiler-Joseph S, Fabbro-Peray P, Monsarrat B, et al. Diagnostic value and prognostic significance of plasmatic proteasome level in patients with melanoma. Exp Dermatol. 2010;19: 1054–1059. 10.1111/j.1600-0625.2010.01151.x [DOI] [PubMed] [Google Scholar]
- 30. Wada M, Kosaka M, Saito S, Sano T, Tanaka K, Ichihara A. Serum concentration and localization in tumor cells of proteasomes in patients with hematologic malignancy and their pathophysiologic significance. J Lab Clin Med. 1993;121: 215–223. [PubMed] [Google Scholar]
- 31. Bochmann I, Ebstein F, Lehmann A, Wohlschlaeger J, Sixt SU, Kloetzel PM, et al. T lymphocytes export proteasomes by way of microparticles: a possible mechanism for generation of extracellular proteasomes. J Cell Mol Med. 2014;18: 59–68. 10.1111/jcmm.12160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lai RC, Tan SS, Teh BJ, Sze SK, Arslan F, de Kleijn DP, et al. Proteolytic Potential of the MSC Exosome Proteome: Implications for an Exosome-Mediated Delivery of Therapeutic Proteasome. Int J Proteomics. 2012;2012: 971907 10.1155/2012/971907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bellavista E, Santoro A, Galimberti D, Comi C, Luciani F, Mishto M. Current understanding on the role of standard and immunoproteasomes in inflammatory/immunological pathways of multiple sclerosis. Autoimmune Dis. 2014;2014: 739705 10.1155/2014/739705 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.


