Abstract
OBJECTIVES: Basosquamous carcinoma (BSC) is a rare tumor entity, and the most common onset is in the head and neck region (BSC-HN). The data on diagnosis, treatment, and especially risk assessment concerning disease course and outcome are deficient or inconsistent. This study aimed to evaluate risk factors for local relapse (LR) and lymph node metastasis (LNM) and their impact on progression-free survival (PFS). MATERIALS AND METHODS: In a retrospective monocentric study, patients with BSC-HN treated between 1999 and 2011 were analyzed regarding clinical and histologic characteristics. Prognostic parameters for LR, LNM, and PFS were evaluated. In total, 89 patients (55 male, 34 female, mean age of 71.8 years) with a mean follow-up time of 47.7 months (range 12-112) were included. RESULTS: LR occurred in four patients (4.5%), LNM occurred in five patients (5.6%). Patients with LNM had a significantly shorter PFS time (16.1 months) compared with patients without LNM (154.2 months; P < .001). Tumor depth and size (T classification), incomplete resection, localization at the ear, deep maximal vertical infiltration, muscle and vessel invasion all showed significant (P < .05) associations with LR, LNM, and shorter PFS time. BSC showed more histologic features of basal cell carcinoma (BCC), especially with regard to BerEP4 expression. CONCLUSION: While histology shows some typical characteristics of BCC, the biologic behavior and aggressiveness of BSC are similar to those of cutaneous squamous cell carcinoma. This is the first study to show that LR and, especially, LNM indicate a higher risk of an unfavorable outcome.
Introduction
Basosquamous carcinoma (BSC) is an uncommon skin cancer entity. As a rare variant or subtype of basal cell carcinoma (BCC), it features characteristics of both BCC (especially histologic characteristics) and squamous cell carcinoma (SCC; mainly clinical behavior) [1], [2], [3], [4], [5]. First described by MacCormac in 1910 [6], BSC is a rare tumor with an incidence of less than 2% of all non-melanoma skin cancers and is predominant in men [5], [7], [8], [9]. Its etiology is multifactorial, but UV radiation, aging, and tobacco exposure seem to play key roles in the onset of BSC [1], [5], [10], [11]. BSCs mainly occur in older Caucasian males and are usually located in the head and neck area (BSC-HN) or in other sun-exposed areas [5], [7], [12]. In addition, rare cases have also been detected in the upper aerodigestive tract, such as the base of the tongue, hypopharynx and larynx, and in less common sites of origin, such as the esophagus, lungs, or genitals [13], [14], [15], [16]. BSC-HN often simulate BCCs clinically and morphologically, but BSC-HN is more aggressive and metastasizes in up to 17.9% of cases [2], [7], [17], [18], [19]. BSC-HN appearance can vary and can include BCC signs (e.g., telangiectasia) or SCC characteristics or can appear as a palpable mass with or without ulceration. The symptoms of BSC-HN are not specific and depend on tumor location [1], [5], [20], [21], [22]. The histogenesis of BSC-HN is not yet clear, but it is thought that the tumor originates from totipotent cells in the basal layer [2], [3], [23].
Standard therapy for BSC-HN includes Mohs micrographic surgery with wider excision margins than for BCC or SCC, and careful follow-up is obligatory [8], [19], [24]. Concerning dissemination and risk factors for nodal and distant metastasis (DM) in BSC, in general, and BSC-HN, especially, data are insufficient. Therefore, the need for lymph node management in BSC-HN has not been sufficiently elucidated previously. To date, it is unclear to what extent clinical or histologic parameters can aid in risk assessment in BSC-HN.
The purpose of this study was to analyze characteristics and outcomes in patients with BSC-HN and to evaluate which variables may be useful for prognostication of local recurrence, nodal dissemination, and progression-free survival (PFS).
Material and Methods
Patients
Patients treated between 1999 and 2011 for BSC in the head and neck region (BSC-HN) were identified in a retrospective manner from our institutional database. Correct classification as histologically proven BSC was confirmed by the histologic analysis described below. Only patients with a minimum follow-up of 12 months were included in this study. Tumor excision was performed using strictly histographic surgery in all operated cases. This study included 89 patients with BSC-HN (55 male and 34 female, ratio 1.6:1).
The local ethics committee (Ethical Committee of the Westphalian Wilhelms-University, Münster, Germany; Approval No. 2014-528-f-S) approved this study. The study was conducted in accordance with the Guidelines for Good Clinical Practice and in compliance with the Declaration of Helsinki. Patients gave written informed consent for the analysis of their data and tumor specimens.
Histologic Analysis
A tumor biopsy was cut into 2- to 4-μm sections after being fixed in formalin and embedded in paraffin. In addition to hematoxylin/eosin (HE) staining, immunohistochemistry was performed using standard immunoperoxidase techniques. The sections were dewaxed in xylol (Merck, Darmstadt, Germany) and rehydrated in serial dilutions of ethanol. The DAKO autostainer was used in combination with the Chem-Mate Detection Kit by DAKO (LSAB-KIT, DAKO, Hamburg, Germany). All biopsies were reviewed independently by two experts in dermatopathology (C.H. and H.-J.S.). Histopathologic pictures were captured with a Zeiss Axioskop 2 plus microscope using a Zeiss Axiocam HRC camera (Zeiss, Jena, Germany)
We used the following strict histologic criteria for the diagnosis of BSC: loss of BerEP4 staining in portions of the tumor, loss of palisading, increased nuclear atypia, significant squamous differentiation, and loss of basaloid cytology in portions of the tumor (Figure 1). We excluded patients with composite tumors of SCC and BCC as well as adnexal tumors.
Figure 1.
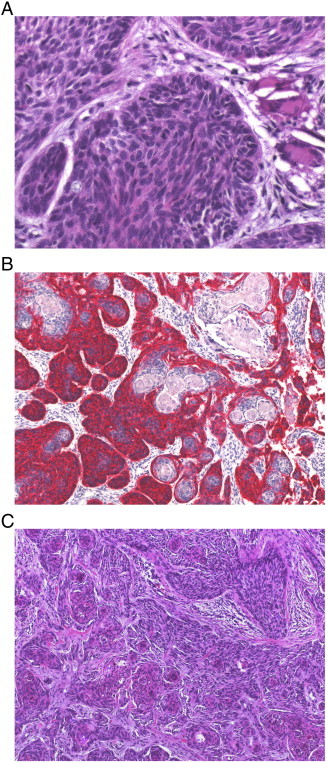
Histologic characteristics of BSC. In conventional histology (A; HE, × 400), BSC shows nuclear pleomorphia, with many mitotic cells showing atypical mitosis, and a loss of palisades, which are usually typical for BCC. Immunohistochemical staining with BerEP4 (B; BerEP4, × 100) illustrates irregular mixture of BCC-typical areas (red) and dedifferentiated areas with more similarities to SCC. Conventional histology of the same tumor (C; HE, × 100) depicts the aforementioned BSC characteristics.
Data Acquisition
We retrospectively assessed the following clinical and outcome variables: gender, age at first diagnosis, comorbidities, primary tumor site, primary tumor, regional lymph node involvement, distant metastatic spread classification (according to 7th edition of the Union Internationale Contre le Cancer for skin cancers), R classification (resection status: R0 = negative margins, R1 = microscopically positive margins, R2 = macroscopically positive margins), number of operations necessary for tumor resection (resection number), ulceration (yes/no), preexisting skin damage [chronic actinic damage (CAD), scar, and radioderm], local relapse (LR, yes/no), occurrence of regional lymph node metastasis (LNM, yes/no), DM (yes/no), and PFS.
Furthermore, we analyzed the following histopathologic characteristics: tumor depth (TD), according to Breslow (in mm); maximal vertical infiltration (MVI, S = superficial: dermis, M = medium: subcutis, D = deep: fascia, muscle, cartilage, or bone); vessel invasion (VI, yes/no); muscle invasion (MI, yes/no); and bone infiltration (BI, yes/no).
Statistical Analysis
All statistical analyses were performed by a statistician using the Statistical Package for Social Sciences, version 18.0 (SPSS Inc, Chicago, IL). Significance was defined as the probability of type one error of < 5% (P < .05).
Categorical variables were analyzed using the chi-square test and Fisher exact test. For continuous variables, the Mann-Whitney U test was used as a non-parametric test for abnormally distributed variables (age), and an independent t test was used to analyze normally distributed variables (TD). PFS was defined as the time from first diagnosis to local relapse or nodal or distant dissemination, and the data on patients without these events were censored for the last follow-up period. PFS was calculated using the Kaplan-Meier method, and group differences were analyzed using the log-rank test.
Results
The mean age at first diagnosis was 71.8 years (median 74.1, SD 12.5, range 23.8-92.6). The most common primary tumor site was the nose (31.5%), followed by the ear and periauricular region (20.2%), the forehead and scalp (13.5%), the periorbital region (10.1%), the cheek (9.0%), the lips and perioral region (7.9%), and the neck (6.7%). In one case of a tumor that extended over several anatomic regions, the region of first onset was indeterminable. The mean follow-up time was 47.7 months (SD 26.9, range 12-112). We observed local relapse in four patients (4.5%). Five patients (5.6%) developed LNM (one initial nodal disease and four during follow-up), of these two (2.2%) experienced distant dissemination and tumor-dependent death. Seven patients (7.9%) developed disease progression (local relapse, nodal or distant dissemination), resulting in a mean PFS time of 147.4 months [95% confidence interval (CI): 138.4-156.3] for the total study population.
The characteristics of the study population with regard to nodal disease are shown in Table 1. The analysis of differences between patients with and without LNM revealed that increased TD (P = .002), localization at the ear or periauricular region (P = .004), MVI (P = .008), resection status [R0 vs R1/R2: P = .017, OR (odds ratio) = 12.3 (95% CI: 1.8-83.9)], MI [P = .026, OR = 9.9 (95% CI: 1.5-66.4)], VI [P = .032, OR = 9.0 (95% CI: 1.4-59.6)], and pT classification [T1/T2 vs T3/T4: P = .054, OR = 6.9 (95% CI: 1.1-44.9)] were associated with increased risk for nodal disease.
Table 1.
Demographic and Clinical Characteristics of the Study Population with regard to Nodal Disease (LNM)
| Parameter | LNM |
Significance (P Value) | ||
|---|---|---|---|---|
| No | Yes | |||
| Gender | (1.000) | |||
| Male | n (%) | 52 (94.5) | 3 (5.5) | |
| Female | n (%) | 32 (94.1) | 2 (5.9) | |
| Age | (.776) | |||
| Mean | Years | 71.7 | 72.4 | |
| SD | Years | 12.7 | 9.2 | |
| Comorbidities | ||||
| Immunosuppression (yes/no) | n | 1/83 | 0/5 | (1.000) |
| Renal insufficiency (yes/no) | n | 7/77 | 1/4 | (.383) |
| Cardiovascular disease | n | 14 | 2 | (.187) |
| Diabetes mellitus | n | 7 | – | (1.000) |
| Thyroidal disease | n | 3 | – | (1.000) |
| Primary tumor site | (.004)** | |||
| Nose | n (%) | 27 (96.4) | 1 (3.6) | |
| Ear and periauricular | n (%) | 16 (88.9) | 2 (11.1) | |
| Scalp and forehead | n (%) | 12 (100.0) | (0.0) | |
| Periorbital/eyelids | n (%) | 9 (100.0) | (0.0) | |
| Cheek | n (%) | 8 (100.0) | (0.0) | |
| Perioral/lips | n (%) | 6 (85.7) | 1 (14.3) | |
| Neck | n (%) | 6 (100.0) | (0.0) | |
| More than one region | n (%) | (0.0) | 1 (100.0) | |
| pT classification | (.097) | |||
| pT1 | n (%) | 54 (98.2) | 1 (1.8) | |
| pT2 | n (%) | 15 (93.8) | 1 (6.3) | |
| pT3 | n (%) | 12 (85.7) | 2 (14.3) | |
| pT4 | n (%) | 3 (75.0) | 1 (25.0) | |
| Skin damage | (.908) | |||
| CAD | n (%) | 22 (95.7) | 1 (4.3) | |
| Scar | n (%) | 8 (100.0) | (0.0) | |
| CAD and scar | n (%) | 2 (100.0) | (0.0) | |
| Radioderm | n (%) | 1 (100.0) | (0.0) | |
| None | n (%) | 51 (92.7) | 4 (7.3) | |
| R classification (resection status) | (.017)* | |||
| R0 (in sano) | n (%) | 75 (97.4) | 2 (2.6) | |
| R1/R2 (non in sano) | n (%) | 9 (75.0) | 3 (25.0) | |
| Resection number | (.379) | |||
| Mean | 1.5 | 1.6 | ||
| SD | 0.8 | 0.5 | ||
| Median | 1 | 2 | ||
| PFS time | (< .001)*** | |||
| Mean | Months | 154.2 | 16.1 | |
| 95% CI | Months | 147.8-160.6 | 8.1-24.1 | |
P < .05.
P < .01.
P < .001.
Patients with local relapse had VI, MI, BI, and advanced T classification of T3 or T4 significantly more often than patients without local relapse (all P < .001). Furthermore, MVI (P = .001), TD (P = .009), and R classification (P = .029) showed significant associations with local tumor recurrence.
MVI, VI, MI, BI, and T and R classifications also showed significant associations with PFS time. Figures 2 and 3 illustrate the associations of PFS time with MVI and T classification.
Figure 2.
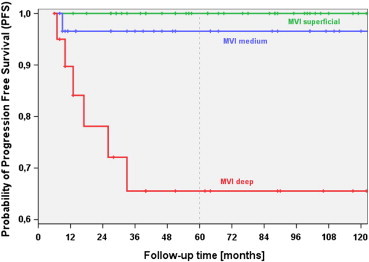
PFS with regard to maximal vertical invasion (MVI) of BSC of the head and neck (BSC-HN). BSC-HN patients with deep MVI had significantly more unfavorable outcomes, as indicated by PFS, compared to patients with medium MVI (P = .010) and superficial MVI (P < .001). The difference in PFS between patients with superficial MVI and medium MVI was not significant (P = .252).
Figure 3.
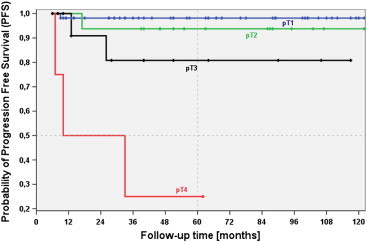
PFS with regard to pT classification of BSC-HN. Patients with advanced BSC-HN with pT4 classification had significantly shorter PFS than those with pT3 (P = .024), pT2 (P = .001), and pT1 (P < .001) classifications. The unfavorable outcome rate, indicated by PFS, was significantly greater for BSC-HN cases classified as pT3 than for those classified as pT1 (P = .026); however, differences between BSC-HN cases classified as pT3 and pT2 (P = .307) and between those classified as pT2 and pT1 (P = .397) were not significant.
Discussion
Due to its rare occurrence, diagnostics including immunohistochemistry, therapy, and prognostic prediction for BSC, especially BSC-HN, remain controversial [5], [18], [22], [24]. Due to increasing incidence of non-melanoma skin cancer, the incidence of BSC can be expected to rise, and clinicians and researchers need valuable data for improving the management of these patients. Most BSCs arise in the head and neck, so our study provides important insights into this tumor entity. Our study included 89 patients, constituting one of the largest cohorts in published studies of BSC, and our study specifically included only primary BSC-HN cases. Only Leibovitch et al. presented follow-up data for a larger BSC sample (n = 98) [8]. In terms of gender and age distribution and primary tumor location, our results are in line with those published previously [1], [2], [5], [7], [8], [9], [12], [25], [26], [27].
As in most tumor entities of the head and neck, crucial events for unfavorable outcomes with limited PFS and disease specific survival include local tumor relapse, occurrence of nodal metastases, and distant dissemination. In BSC, possible parameters of prognostic relevance for these events and PFS have not been sufficiently analyzed before.
In our study, we observed a low local recurrence rate of only 4.5% (n = 4), which can be attributed to the usage of histographic Mohs tumor resection. Older studies that did not use histographic controlled Mohs tumor resection presented recurrence frequencies between 12% and 51% [25], [27]. Performing histographic surgery (Mohs micrographic surgery) diminishes this rate to 4% to 9% [8], [19], [24]. As described previously, incomplete tumor excision (R classification), TD, and tumor size (T classification) influenced local relapse in the current study. Furthermore, we found significant associations between local recurrence and maximal vertical infiltration (MVI), BI, muscle invasion (MI), and VI. These parameters had not been explicitly analyzed by previous studies.
BSC has a much higher potential to metastasize than BCC and the dissemination rate for BSC is more similar to that of cutaneous SCC (cSCC) [5], [22]. While histologic and immunohistochemical characteristics (especially the expression of BerEP4 and epithelial membrane antigen) showed similarities between BSC and BCC, the biologic behavior and aggressiveness of BSC are more comparable to cSCC. Only a few studies have systematically analyzed nodal and distant dissemination within a defined follow-up period. Table 2 summarizes currently available data on this topic. Nodal metastases occurred in 4.4% of all BSC patients and the risk of DM was approximately 2.5%. No study had evaluated potential risk factors for LNM previously. This study was the first that analyzed associations between MVI, BI, MI, and VI and nodal disease in BSC and was the first to show a significant association between these parameters, T and R classification, TD, and localization at the ear and LNM. Our results may help define BSC-HN patients with high risk of metastasis in the future. In these high-risk cases, elective surgical lymph node management (e.g., sentinel lymph node biopsy, as described by Yoshida et al. [28], or elective lymph node dissection), intensified follow-up care, including ultrasonography and radiologic assessments (computed tomography, magnetic resonance imaging), and even adjuvant therapies, such as irradiation of the lymph drainage area, can be considered. The presently available data do not allow for the definition and evaluation of a well-defined lymph node management protocol; thus, further prospective studies are necessary (Table 3).
Table 2.
Histologic Features of the Study Population with regard to Nodal Disease (LNM)
| Parameter | LNM |
Significance (P Value) | ||
|---|---|---|---|---|
| No | Yes | |||
| Ulceration | (.658) | |||
| No | n (%) | 46 (95.8) | 2 (4.2) | |
| Yes | n (%) | 38 (92.7) | 3 (7.3) | |
| TD | (.002)** | |||
| Mean | mm | 3.5 | 14.0 | |
| SD | mm | 2.9 | 6.0 | |
| 95% CI | mm | 2.9-4.2 | 6.6-21.4 | |
| Maximal vertical infiltration (MVI) | (.008)** | |||
| Superficial | n (%) | 29 (96.7) | 1 (3.3) | |
| Medium | n (%) | 38 (100.0) | (0.0) | |
| Deep | n (%) | 17 (81.0) | 4 (19.0) | |
| Vessel invasion (VI) | (.032)* | |||
| No | n (%) | 72 (97.3) | 2 (2.7) | |
| Yes | n (%) | 12 (75.0) | 3 (25.0) | |
| Muscle invasion (MI) | (.026)* | |||
| No | n (%) | 73 (97.3) | 2 (2.7) | |
| Yes | n (%) | 11 (78.6) | 3 (21.4) | |
| Bone infiltration (BI) | (.095) | |||
| No | n (%) | 76 (96.2) | 3 (3.8) | |
| Yes | n (%) | 8 (80.0) | 2 (20.0) | |
⁎⁎⁎P < .001.
P < .05.
P < .01.
Table 3.
Overview of the Studies Presenting Data Concerning Metastatic BSC
| Author (Year) | Patients [n (%)] | LNM [n (%)] | DM [n (%)] | Total (LNM + DM) [n (%)] |
|---|---|---|---|---|
| Betti et al. (2003) [5] | 35 | n.s. | 1 (2.9) | 1 (2.9) |
| Borel et al. (1973) [27] | 35 | n.s. | 3 (8.6) | 3 (8.6) |
| Bowman et al. (2003) [7] | 27 | n.s. | 2 (7.4) | 2 (7.4) |
| Leibovitch et al. (2005)⁎[8] | 98 | 2 (2.0) | (0.0) | 2 (2.0) |
| Martin et al. (2000) [2] | 28 | 5 (17.9) | 1 (3.6) | 5 (17.9) |
| Schuller et al. (1979) [25] | 33 | n.s. | 2 (6.1) | 2 (6.1) |
| Skaria (2010) [19] | 56 | (0.0) | (0.0) | (0.0) |
| Wermker et al. (2014) | 89 | 5 (5.6) | 2 (2.2) | 5 (5.6) |
| Total LNM | 271 | 12 (4.4) | ||
| Total DM | 401 | 11 (2.7) | ||
| Total LNM + DM | 401 | 20 (5.0) |
n.s. = not specified.
Leibovitch et al. [8] presented in total 178 BSC cases, but data concerning metastasization were only published for 98 patients who completed a 5-year follow-up period.
Some limitations of this study should be considered. Due to the rarity of this tumor entity, the number of patients included in this study was relatively small, especially within even smaller subgroups. Furthermore, the retrospective nature of our analysis generates the risks of poor data quality and selection bias due to monocentricity. In contrast, the strength of our analysis was that we presented a well-defined and relatively homogeneous sample of BSC cases in the head and neck area.
Conclusion
While the histologic characteristics of BSC tend to define it as a subtype of BCC, its biologic and clinical behaviors suggest that BSC is its own type of skin cancer with disease courses similar to cSCC. Risk factors for LNM are of special interest for the management of BSC-HN patients. In particular, incomplete tumor resection (R classification), increased tumor size (T classification), TD, maximal vertical infiltration, muscle invasion, and VI indicate a higher risk for LNM in BSC-HN.
Acknowledgements
We thank Margret Leygraf and her colleagues for their skilled technical assistance.
Footnotes
Funding: None.
Conflict of interest statement: None declared. All authors declare that no competing interests exist.
No prior or subsequent publication: Neither the submitted article nor any similar manuscript, in whole or in part, has been submitted or published or is in press elsewhere.
References
- 1.Lehnerdt G, Manz D, Jahnke K, Schmitz KJ. Cutaneous basosquamous cell carcinoma. HNO. 2008;56(3):306–311. doi: 10.1007/s00106-007-1559-z. [DOI] [PubMed] [Google Scholar]
- 2.Martin RC, Edwards MJ, Cawte TG, Sewell CL, McMasters KM. Basosquamous carcinoma: analysis of prognostic factors influencing recurrence. Cancer. 2000;88(6):1365–1369. doi: 10.1002/(sici)1097-0142(20000315)88:6<1365::aid-cncr13>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 3.Lopes de Faria J, Nunes PH. Basosquamous cell carcinoma of the skin with metastases. Histopathology. 1988;12(1):85–94. doi: 10.1111/j.1365-2559.1988.tb01919.x. [DOI] [PubMed] [Google Scholar]
- 4.Jones MS, Helm KF, Maloney ME. The immunohistochemical characteristics of the basosquamous cell carcinoma. Dermatol Surg. 1997;23(3):181–184. doi: 10.1111/j.1524-4725.1997.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 5.Betti R, Crosti C, Ghiozzi S, Cerri A, Moneghini L, Menni S. Basosquamous cell carcinoma: a survey of 76 patients and a comparative analysis of basal cell carcinomas and squamous cell carcinomas. Eur J Dermatol. 2013;23(1):83–86. doi: 10.1684/ejd.2012.1890. [DOI] [PubMed] [Google Scholar]
- 6.MacCormac H. The relation rodent ulcer to squamous cell carcinoma of the skin. Arch Middx Hosp. 1910;19:172–183. [Google Scholar]
- 7.Bowman PH, Ratz JL, Knoepp TG, Barnes CJ, Finley EM. Basosquamous carcinoma. Dermatol Surg. 2003;29(8):830–832. doi: 10.1046/j.1524-4725.2003.29217.x. [discussion 833] [DOI] [PubMed] [Google Scholar]
- 8.Leibovitch I, Huilgol SC, Selva D, Richards S, Paver R. Basosquamous carcinoma: treatment with Mohs micrographic surgery. Cancer. 2005;104(1):170–175. doi: 10.1002/cncr.21143. [DOI] [PubMed] [Google Scholar]
- 9.Tarallo M, Cigna E, Frati R, Delfino S, Innocenzi D, Fama U, Corbianco A, Scuderi N. Metatypical basal cell carcinoma: a clinical review. J Exp Clin Cancer Res. 2008;27:65. doi: 10.1186/1756-9966-27-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strom SS, Yamamura Y. Epidemiology of nonmelanoma skin cancer. Clin Plast Surg. 1997;24(4):627–636. [PubMed] [Google Scholar]
- 11.Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146(Suppl. 61):1–6. doi: 10.1046/j.1365-2133.146.s61.2.x. [DOI] [PubMed] [Google Scholar]
- 12.Beer TW, Shepherd P, Theaker JM. Ber EP4 and epithelial membrane antigen aid distinction of basal cell, squamous cell and basosquamous carcinomas of the skin. Histopathology. 2000;37(3):218–223. doi: 10.1046/j.1365-2559.2000.00999.x. [DOI] [PubMed] [Google Scholar]
- 13.Ioannidis O, Cheva A, Paraskevas G, Kotronis A, Papadimitriou N, Chatzopoulos S, Konstantara A, Makrantonakis A, Sakkas A, Kakoutis E. Basosquamous cell carcinoma of the anus. J Gastrointest Cancer. 2012;43(1 Suppl):184–186. doi: 10.1007/s12029-012-9392-3. [DOI] [PubMed] [Google Scholar]
- 14.Kimball KJ, Straughn JM, Conner MG, Kirby TO. Recurrent basosquamous cell carcinoma of the vulva. Gynecol Oncol. 2006;102(2):400–402. doi: 10.1016/j.ygyno.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 15.Belding RC, Sabet Z. Basosquamous carcinoma of the supraglottic larynx with sudden death from asphyxia. South Med J. 2002;95(7):765–767. [PubMed] [Google Scholar]
- 16.Sancho Martín I, Alvarez Montero OL, Félix Muñiz J, Alonso Alonso L, Muñoz Colado M, Coca Menchero S. Basosquamous carcinoma of the floor of the mouth. Acta Otorrinolaringol Esp. 2000;51(8):743–747. [PubMed] [Google Scholar]
- 17.Kazantseva IA, Khlebnikova AN, Babaev VR. Immunohistochemical study of primary and recurrent basal cell and metatypical carcinomas of the skin. Am J Dermatopathol. 1996;18(1):35–42. doi: 10.1097/00000372-199602000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Kececi Y, Argon A, Kebat T, Sir E, Gungor M, Vardar E. Basosquamous carcinoma: is it an aggressive tumor? J Plast Surg Hand Surg. 2014:1–5. doi: 10.3109/2000656X.2014.944188. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 19.Skaria AM. Recurrence of basosquamous carcinoma after Mohs micrographic surgery. Dermatology. 2010;221(4):352–355. doi: 10.1159/000320127. [DOI] [PubMed] [Google Scholar]
- 20.Lima NL, Verli FD, de Miranda JL, Marinho SA. Basosquamous carcinoma: histopathological features. Indian J Dermatol. 2012;57(5):382–383. doi: 10.4103/0019-5154.100489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giacomel J, Lallas A, Argenziano G, Reggiani C, Piana S, Apalla Z, Ferrara G, Moscarella F, Longo C, Zalaudek I. Dermoscopy of basosquamous carcinoma. Br J Dermatol. 2013;169(2):358–364. doi: 10.1111/bjd.12394. [DOI] [PubMed] [Google Scholar]
- 22.Garcia C, Poletti E, Crowson AN. Basosquamous carcinoma. J Am Acad Dermatol. 2009;60(1):137–143. doi: 10.1016/j.jaad.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 23.Maloney ML. What is basosquamous carcinoma? Dermatol Surg. 2000;26(5):505–506. doi: 10.1046/j.1524-4725.2000.00057.x. [DOI] [PubMed] [Google Scholar]
- 24.Allen KJ, Cappel MA, Killian JM, Brewer JD. Basosquamous carcinoma and metatypical basal cell carcinoma: a review of treatment with Mohs micrographic surgery. Int J Dermatol. 2014;53(11):1395–1403. doi: 10.1111/ijd.12587. [DOI] [PubMed] [Google Scholar]
- 25.Schuller DE, Berg JW, Sherman G, Krause CJ. Cutaneous basosquamous carcinoma of the head and neck: a comparative analysis. Otolaryngol Head Neck Surg. 1979;87(4):420–427. doi: 10.1177/019459987908700405. [DOI] [PubMed] [Google Scholar]
- 26.De Faria J. Basal cell carcinoma of the skin with areas of squamous cell carcinoma: a basosquamous cell carcinoma? J Clin Pathol. 1985;38(11):1273–1277. doi: 10.1136/jcp.38.11.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borel DM. Cutaneous basosquamous carcinoma. Review of the literature and report of 35 cases. Arch Pathol. 1973;95(5):293–297. [PubMed] [Google Scholar]
- 28.Yoshida Y, Shiomi T, Tahira M, Yamamoto O. Metastatic basosquamous carcinoma detected by sentinel lymph node biopsy. J Dermatol. 2013;40(8):635–637. doi: 10.1111/1346-8138.12204. [DOI] [PubMed] [Google Scholar]


