Abstract
A scaffold obtained from whole-genome shotgun sequencing of Paenibacillus popilliae ATCC 14706T shares partial homology with plasmids found in other strains of P. popilliae. PCR and sequencing for gap enclosure indicated that the scaffold originated from a 15,929-bp circular DNA. The restriction patterns of a plasmid isolated from P. popilliae ATCC 14706T were identical to those expected from the sequence; thus, this circular DNA was identified as a plasmid of ATCC 14706T and designated pPOP15.9. The plasmid encodes 17 putative open reading frames. Orfs 1, 5, 7, 8, and 9 are homologous to Orfs 11, 12, 15, 16, and 17, respectively. Orf1 and Orf11 are annotated as replication initiation proteins. Orf8 and Orf16 are homologs of KfrA, a plasmid-stabilizing protein in Gram-negative bacteria. Recombinant Orf8 and Orf16 proteins were assessed for the properties of KfrA. Indeed, they formed multimers and bound to inverted repeat sequences in upstream regions of both orf8 and orf16. A phylogenetic tree based on amino acid sequences of Orf8, Orf16 and Kfr proteins did not correlate with species lineage.
Keywords: Entomopathogenic bacteria, Whole-genome shotgun sequencing, Multimer formation, DNA binding capacity
Highlights
-
•
A 15.9 kb plasmid of P. popilliae was identified and completely sequenced.
-
•
The plasmid was predicted to encode 17 putative open reading frames.
-
•
Recombinant KfrA proteins formed multimers and bound upstream of the kfrA genes.
-
•
Phylogenetic analysis suggests that kfrA genes were horizontally transferred.
Introduction
Paenibacillus popilliae (formerly Bacillus popilliae) is a rod-shaped, endospore-forming bacterium with variable Gram stain reactivity (Pettersson et al., 1999, Tanada and Kaya, 1992). The bacterium infects the Japanese beetle Popillia japonica and related species, causing milky disease. The name “milky disease” refers to the clinical condition in which the larval hemolymph becomes so clouded with bacterial cells or spores that it appears milky (Steinkraus and Tashiro, 1967). Like other bacteria, P. popilliae carry plasmids, some of which have been sequenced (Dingman, 1994, Faust et al., 1979, Longley et al., 1997). Dingman (1994) isolated three plasmids from P. popilliae: pBP68, pBP88, and pBP94. Hybridization analysis suggested that pBP88 may be a chimera of pBP68 and pBP94 (Dingman, 1994). A plasmid isolated from P. popilliae strain 17, pBP614, encodes homologs of replication proteins for rolling-circle plasmids, suggesting that pBP614 also replicates by a rolling-circle mechanism (Longley et al., 1997).
Plasmids mediate important biological and pathogenic functions such as antibiotic resistance in Salmonella spp. (Miriagou et al., 2006), toluene degradation in Pseudomonas putida (Jutkina et al., 2013), EPEC adherence factor (EAF) in enteropathogenic Escherichia coli (Levine et al., 1985), and the toxins of Bacillus thuringiensis subsp. israelensis (Berry et al., 2002). The role of the plasmid in P. popilliae remains unknown. We previously published a draft genome analysis (Iiyama et al., 2013), but found it difficult to characterize the plasmid sequence because of the number of contigs (583 contigs). Subsequent sequencing analysis revealed the scaffold originated from a plasmid encoding 17 putative proteins, including two KfrA homologs.
The first kfrA was described in broad host range IncP plasmid RK2, where the gene product mediates plasmid maintenance (Thomas et al., 1990). Transcription of kfrA is repressed by the products of korA and korF, also encoded on RK2. KfrA is a DNA-binding protein with a long, alpha-helical, coiled-coil tail. Although most KfrA homologs have been found in Gram-negative bacteria, some have been identified in Gram-positive bacteria such as Virgibacillus halodenitrificans (CDQ36163) and Mycobacterium abscessus (YP_006316029). The role of KfrA in Gram-positive bacteria, however, has not been defined. The present study focused on the multimer formation and specific DNA-binding capacity of recombinant KfrA from the P. popilliae plasmid.
Materials and methods
Bacterial strain, culture, DNA extraction, and primers
P. popilliae ATCC 14706T was cultured in MYPGP (Costilow and Coulter, 1971), and genomic DNA was extracted (Iiyama et al., 2013). Plasmid DNA was extracted with a LaboPass Mini kit (Hokkaido System Science Co., Ltd, Sapporo, Japan) according to manufacturer instructions. Primers are described in Table 1.
Table 1.
Plasmids and primers used in this study.
| Plasmid or oligonucleotide | Relevant features or sequence | Source, reference or purpose |
|---|---|---|
| Plasmids | ||
| pET20b | Expression vector, pelB leader sequence for potential periplasmic localization, AmpR | Laboratory stock |
| pOrf8H | orf8 in pET20b, expression for Orf8 (8×His-tagged) fused to pelB leader sequence, AmpR | This study |
| pOrf8F | orf8 in pET20b, expression for Orf8 (FLAG-tagged) fused to pelB leader sequence, AmpR | This study |
| pOrf16H | orf8 in pET20b, expression for Orf8 (8×His-tagged) fused to pelB leader sequence, AmpR | This study |
| pOrf16F | orf8 in pET20b, expression for Orf8 (FLAG-tagged) fused to pelB leader sequence, AmpR | This study |
| pOrf8H2 | Plasmid which removed pelB leader sequence from pOrf8H, AmpR | This study |
| pOrf8F2 | Plasmid which removed pelB leader sequence from pOrf8F, AmpR | This study |
| pOrf16H2 | Plasmid which removed pelB leader sequence from pOrf16H, AmpR | This study |
| pOrf16F2 | Plasmid which removed pelB leader sequence from pOrf16F, AmpR | This study |
| pBBR1MCS2 | Cloning vector, KmR | Kovach et al. (1995) |
| pIR1 | IR1 in pBBR1MCS2 at SacI and Asp718 sites, KmR | This study |
| pIR2 | IR2 in pBBR1MCS2 at SacI and Asp718 sites, KmR | This study |
| pIR3 | IR3 in pBBR1MCS2 at SacI and Asp718 sites, KmR | This study |
| pIR4 | IR4 in pBBR1MCS2 at SacI and Asp718 sites, KmR | This study |
| Oligonucleotides | ||
| 652R | CGCTAAAGTTGAAAGGCAGA | Gap closure |
| 5036F | ATATCCCTCCTTTGGTTTGG | Gap closure |
| 7489R | TGGTTACTCTCGGTTTGCTC | Gap closure |
| 14243F | GGGACCCCTATAAATCCTCA | Gap closure |
| 20bf | CATGGCCATCGCCGGCTGGG | Linearization of pET20b |
| 20br-8His | CACCACCACCACCACCACCACCACTGAGAT | Linearization of pET20b |
| 20br-FLAG | GATTACAAGGATGACGACGATAAGTGAGATCCGGCTGCTAACAAA | Linearization of pET20b |
| orf8f(-327) | CATCCGTCTGTGGGAGGTAG | Amplification of orf8 |
| orf8r1692 | GCTGACATCGTTATAGACAACACA | Amplification of orf8 |
| orf16f(-233) | ATTGCTGCTCGTCTCCAGTT | Amplification of orf16 |
| orf16r1549 | CGGGACCCCTATAAATCCTC | Amplification of orf16 |
| orf8F | CCGGCGATGGCCATGGCAGGAGTAGCAAAAATCACG | Amplification for In-Fusion substrate |
| orf8-16RHis | GTGGTGGTGGTGGTGTGACTTTTCCTCACCCTTATTAATAGACTT | Amplification for In-Fusion substrate |
| orf16F | CCGGCGATGGCCATGGCAGGAGTAGCACGGATTAC | Amplification for In-Fusion substrate |
| orf8-16RFlag | GTCATCCTTGTAATCTGACTTTTCCTCACCCTTATTAATAGACTT | Amplification for In-Fusion substrate |
| orf8Fpeldel | ATGGCAGGAGTAGCAAAAATCAC | Removal of pelB leader sequence |
| orf16Fpeldel | ATGGCAGGAGTAGCACGG | Removal of pelB leader sequence |
| orf8-16Rpeldel | ATGTATATCTCCTTCTTAAAGTTAAACAAAATTATTTC | Removal of pelB leader sequence |
| orf8(-476)f | CGCGCTGGAGAGATTAAGG | Amplification of orf8 upstream region |
| orf16(-570)f | GGGGTGCTATATGTCGAGGA | Amplification of orf16 upstream region |
| orf8-16(71)r | TTACCCTCTTGTAACAACTTTTCTG | Amplification of orf8 and orf16 upstream regions |
| IR1f | CATGAAAAAATCATG | Creation of IR1 |
| IR1r | GTACCATGATTTTTTCATGAGCT | Creation of IR1 |
| IR2f | CATATTGTAGTATG | Creation of IR2 |
| IR2r | GTACCATACTACAATATGAGCT | Creation of IR2 |
| IR3f | CATACTACATATTACATATTACATACTACATACTACAATATGGAGGGTG | Creation of IR3 |
| IR3r | GTACCACCCTCCATATTGTAGTATGTAGTATGTAATATGTAATATGTAGTATGAGCT | Creation of IR3 |
| IR4f | CATACTACATACAATATACAACATACTACAATATGGAGGGTG | Creation of IR4 |
| IR4r | GTACCACCCTCCATATTGTAGTATGTTGTATATTGTATGTAGTATGAGCT | Creation of IR4 |
| FITC-UnivF | (FITC)-CGCCAGGGTTTTCCCAGTCACGAC | Preparation of FITC-labeled fragment |
| UnivR | TCACACAGGAAACAGCTATGAC | Preparation of FITC-labeled fragment |
Genome sequence, assembly, BLAST search, gap filling, and annotation
Genomic DNA of P. popilliae ATCC 14706T was sheared into 3-kb fragments using HydroShear (Digilab Inc., MA, USA) to produce fragments for a paired-end library. Whole genome sequencing was performed on a 454 GS FLX system with a GS FLX Titanium sequencing kit (Roche Diagnostics, Tokyo, Japan). Reads were de novo assembled with 454 Newbler (version 2.7).
In order to identify the putative plasmid sequence, we used NCBI BLAST (version 2.2.25 +) to search for sequences with homology to pBP68 (accession number DQ925488, Dingman, 1994) and pBP614 (U78608, Longley et al., 1997) harbored by P. popilliae strains KLN 4 and strain 7, respectively.
To ascertain whether the scaffold (designated scaffold 8) is comprised of circular DNA, PCR was performed with PrimeSTAR GXL DNA polymerase (TAKARA BIO INC., Shiga, Japan) and appropriate primers (652R/14243F, 5036F/7489R) specific to the ends of scaffold 8 and the neighboring gap region. Genomic and plasmid DNA were used as templates. After electrophoresis, PCR products were gel-purified (Wizard SV Gel and PCR Clean-Up system, Promega KK, Tokyo, Japan) and the DNA sequence was determined without cloning by Sanger sequencing. In order to procure highly accurate data, the reads obtained by sequencing on a HiSeq 2000 (Iiyama et al., 2013) were mapped to the circular DNA originated from scaffold 8 with Bowtie2 (version 2.1.0). The sequence was annotated with the Microbial Genome Annotation Pipeline under default settings (MiGAP; Sugawara et al., 2009). The plasmid map was created with PlasmaDNA (Angers-Loustau et al., 2007) and graphically modified.
Secondary structure prediction
The secondary structure of Orf8 and Orf16 was predicted with PSIPRED v3.3 (Jones, 1999). KfrA proteins in Thiothrix nivea, Methylophaga frappieri, pMCBF1 of P. putida and broad-host-range plasmid RK2 were analyzed for comparison. Amino acid sequences were aligned with ClustalX (Thompson et al., 1997). The secondary structural information was overlaid on the alignment.
Construction of recombinant protein expression vectors
pET20b was linearized by PCR with primer pairs 20bf/20br-8His and 20bf/20br-FLAG to construct plasmids for His- and FLAG-tagged protein expression. The orf8 fragment was amplified from plasmid DNA extracted from P. popilliae ATCC 14706T with primers orf8f(-327)/orf8r1692 and PrimeSTAR GXL DNA polymerase. Nested PCR was performed with primers orf8F/orf8-16RHis and orf8F/orf8-16RFlag for His- and FLAG-tagged protein expression. Similarly, orf16 was amplified with orf16f(-233)/orf16r1549, orf16F/orf8-16RHis and orf16F/orf8-16RFlag primer pairs. Four expression plasmids were constructed with In-Fusion reagents (TAKARA BIO, Shiga, Japan): pOrf8H (His-tagged Orf8), pOrf8F (FLAG-tagged Orf8), pOrf16H (His-tagged Orf16), and pOrf16F (FLAG-tagged Orf16).
To eliminate the pelB leader sequence for potential periplasmic localization from pOrf8H and pOrf8F, PCR products generated with primers orf8Fpeldel/orf8-16Rpeldel were circularized by intramolecular ligation with T4 DNA ligase to yield pOrf8H2 and pOrf8F2, respectively. The same procedure was used to construct pOrf16H2 and pOrf16F2 with orf16Fpeldel/orf8-16Rpeldel from pOrf16H and pOrf16F.
Expression and purification of recombinant Orf8 and Orf16
E. coli BL21 DE3 (pLysS) harboring an expression vector including pOrf8H, pOrf8F, pOrf16H, pOrf16F, pOrf8H2, pOrf8F2, pOrf16H2, or pOrf16F2 was cultured in LB broth containing 50 μg/mL ampicillin and 34 μg/mL chloramphenicol at 37 °C overnight. A 30-μL aliquot of seed culture was transferred to 50 mL fresh broth and cultured at 37 °C. After 3.5 h, gene expression was induced with 0.4 mM IPTG at 37 °C for 3 h. Cells were harvested by centrifugation (2000 × g, 4 °C, 30 min) and frozen at − 80 °C.
To investigate production and cellular localization of recombinant proteins, cells were lysed with complete Lysis-B (Roche Diagnostics K.K., Tokyo, Japan) and the lysate was centrifuged (20, 630 × g, 4 °C, 5 min). An equal volume of 2 × Laemmli sample buffer was added to the supernatant (soluble fraction) and the pellet was solubilized with 1 × Laemmli sample buffer (insoluble fraction). Whole cell lysis was performed with 1 × Laemmli sample buffer (total cell fraction). A 10-μL culture equivalent of each sample was analyzed by 10% SDS-PAGE.
His-tagged proteins in the insoluble fraction were dissolved with a small volume of binding buffer containing 8 M urea, 0.5 M NaCl, 5 mM imidazole, and 20 mM NaH2PO4–Na2HPO4 (pH 7.4). Target proteins were purified by standard affinity chromatography using HisTrap HP (GE Healthcare Japan Corporation, Tokyo, Japan). After washing with binding buffer, the target proteins were eluted with a gradient of 5 mM to 250 mM imidazole. Fractions containing the target proteins were pooled and dialyzed against 2 mM NaH2PO4–Na2HPO4 buffer (pH 7.4) supplemented with 137 mM NaCl. Aliquots of the retentate were stored at − 20 °C until use. FLAG-tagged proteins in the soluble fraction were dialyzed without purification and the aliquots were stored at − 20 °C.
Homomultimer formation of recombinant Orf8 and Orf16
Homomultimer formation of recombinant protein under various pH conditions was assayed by cross-linking and ultrafiltration methods.
Cross-linking method
Recombinant proteins (5 μg) were incubated at 30 °C for 2 h in 50-μL reactions containing 137 mM NaCl and 20 mM buffer. Buffers were citric acid–sodium citrate (pH 2.5–3.5), acetic acid–sodium acetate (pH 4.0–5.5), NaH2PO4–Na2HPO4 (pH 6.0–8.0), boric acid–NaOH (pH 8.5–9.0), and NaOCO3–NaOH (pH 9.5–10.0). Glutaraldehyde (2.5 μL of 2.5%) was added and the samples were incubated at 30 °C for 1 h. The cross-linking reaction was quenched by adding 5 μL of 1 M Tris–HCl (pH 8.0). The samples were added to an equal volume of 2 × Laemmli sample buffer and analyzed by 7.5% SDS-PAGE.
Ultrafiltration method
Recombinant proteins (20 μg) were incubated in 200-μL reactions containing 137 mM NaCl and 20 mM buffer at 30 °C for 2 h. Buffers were citric acid–sodium citrate (pH 2.5) and NaH2PO4–Na2HPO4 (pH 7.5). After ultrafiltration on a 100 K Nanosep device (20 °C, 14,000 × g, 5 min, Pall Life Sciences, MI, USA), the permeate (< ca. 100 kDa) was collected and added to an equal volume of 2 × Laemmli sample buffer. The retentate (> ca. 100 kDa) was diluted with 200 μL of the same buffer and centrifuged to eliminate the remaining low-molecular-weight protein. This washing step was repeated five times. The final retentate was collected and brought to a 200-μL volume with water. The samples were added 200 μL of 2 × Laemmli sample buffer. Permeate and retentate fractions were analyzed by 10% SDS-PAGE.
In order to investigate the pH stability of homomultimer formation, recombinant proteins (1 μg/10 μL) were incubated under various pH conditions as described above. The samples were added to 90 μL of 137 mM NaCl and 22.2 mM secondary buffer. NaH2PO4–Na2HPO4 buffers of pH 8.0 and pH 7.5 were used for the samples previously incubated at pH 2.5–3.5 and pH 4.0–10.0, respectively. In a preliminary experiment, we confirmed that addition of the secondary buffer restored the reaction pH to 7–8, a suitable range for cross-linking by glutaraldehyde. After incubation at 30 °C for 2 h, 5 μL of 2.5% glutaraldehyde was added for cross-linking. The reaction was terminated and analyzed by 7.5% SDS-PAGE. Since protein concentrations were low in this pH stability test, the proteins were visualized by silver staining.
Molecular interaction between recombinant Orf8 and Orf16
Preliminary testing revealed that the recombinant proteins formed monomers in 8 M urea, 20 mM NaH2PO4–Na2HPO4, and 137 mM NaCl. FLAG-tagged Orf8 and His-tagged Orf16 (6 μg each) were co-incubated in 100 μL buffer at room temperature for 2 h to promote monomer formation. FLAG-tagged Orf8 alone was used as a control. Multimer formation was initiated by transferring to 20 mM NaH2PO4–Na2HPO4 (pH 7.4), and 137 mM NaCl using a 10 K Nanosep device. The retentate was incubated at 30 °C for 2 h, added to 20 μL of 50% slurry of equilibrated COSMOGEL His-Accept (Nacalai Tesque, Kyoto, Japan), and mixed with mild rotation at 4 °C overnight. The mixtures were transferred to a 0.45-μm Nanosep MF device. After centrifugation (20 °C, 1 min, 3000 × g), the flow-through fraction was collected and washed five times with 500 μL of 20 mM NaH2PO4–Na2HPO4 buffer (pH7.4), 0.5 M NaCl, and 5 mM imidazole. The bound proteins were eluted with the same buffer containing 250 mM imidazole. The proteins in the flow-through and eluent fractions were separated by 10% SDS-PAGE. For immunoblot analysis, the proteins were electrotransferred to nitrocellulose membranes (BioTrace NT, Pall Corporation, NY, USA) by using a semidry transfer system (2 mA/cm2, 90 min). The blot was blocked with 0.5% Alkali-soluble Casein (Merck Millipore, MA, USA) in Tris-buffered saline containing 0.1% Tween 20 (TBS-T) for 1 h. Antibody to FLAG tag (1:1000 dilution, Anti-DDDDK-tag, MBL, Aichi, Japan) and Goat Anti-Rabbit IgG (Fc), AP conjugate (1:7500 dilution, Promega KK, Tokyo, Japan) were used as primary and secondary antibodies. At each step, unbound antibody was washed away with three 10-min washes of TBS-T. The blot was developed using Western Blue™ Stabilized Substrate for Alkaline Phosphatase (Promega KK, Tokyo, Japan).
Electrophoresis mobility shift assays
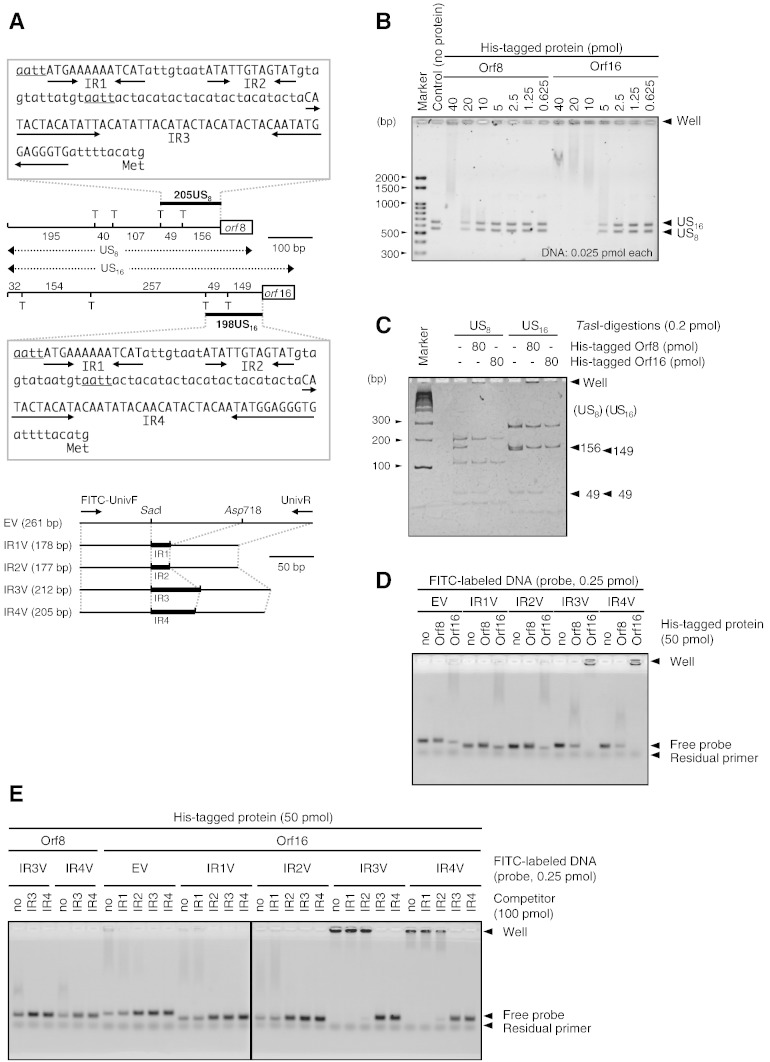
Electrophoresis mobility shift assays (EMSAs) were performed to investigate the DNA-binding capacities of Orf8 and Orf16. DNA substrates were prepared as described below (Fig. 6A). A 547-bp fragment containing a 476-bp upstream segment of orf8 (US8) was amplified from the plasmid with primers orf8(-476)f and orf8-16(71)r and Phusion DNA polymerase (New England Biolabs Japan Inc., Tokyo, Japan). Similarly, a 641-bp fragment containing a 570-bp upstream segment of orf16 (US16) was amplified with primers orf16(-570)f and orf8-16(71)r. The purified PCR products (US8 and US16) and their TasI digests were used as substrates for EMSAs. For further EMSA, synthetic double stranded DNA, IR1, IR2, IR3 and IR4 were created with appropriate oligonucleotides, IR1f–IR4r (Table 1). These dsDNA, IR1, IR2, IR3 and IR4 were ligated into SacI–Asp718 sites of pBBR1MCS2 to construct pIR1, pIR2, pIR3 and pIR4, respectively. PCR was carried out using universal primers, FITC-UnivF and UnivR to label the insert DNA of the plasmids with FITC. The PCR products were purified to remove unreacted primers. Since the PCR products contained vector sequences, the labeled DNA fragments were termed IR1V, IR2V, IR3V, and IR4V to avoid confusion with IR1–IR4.
Fig. 6.
DNA binding capacity of Orf8 and Orf16 by electrophoretic mobility shift assays (EMSAs). Preparation of DNA substrate is graphically shown in panel A. US8 and US16 indicate upstream regions of orf8 and orf16 genes. T indicates TasI site, and the numbers beside TasI map mean length of fragment in bp. 205US8 and 198US16 indicate 205-bp upstream region of orf8 and 195-bp upstream region of orf16, respectively. Sequences of 205US8 and 198US16 are boxed. In the sequences, underlined letters and arrows indicate TasI sites and inverted repeat sequences, respectively. FITC-UnivF and UnivR show primers for FITC labeling. US8, US16 (panel B), TasI digested US8, US16 (panel C) and FITC-labeled EV and IR1V–IR4V (panels D and E) were used as DNA substrates. Panel E shows the EMSA result for non-labeled IR1–IR4 competitors. The ethidium bromide and FITC signals were detected in panels A, B and C, D, respectively.
DNA substrates (US8, US16, their TasI digests, and IR1V–IR4V) were incubated with appropriate amounts of His-tagged Orf8 or His-tagged Orf16 in binding buffer (10 mM Tris–Cl, 1 mM EDTA, 100 mM KCl, 0.1 mM DTT, 5% glycerol, 10 μg/mL BSA, pH 8.0) at 30 °C for 30 min. When required to clarify binding-specificity, IR1–IR4 were added as competitors. The reactions were analyzed by 1.5% agarose or 12% polyacrylamide gel electrophoreses. Signal of FITC-labeled DNA was detected using a molecular imager FX (Bio-Rad Laboratories, Inc., CA, USA). Non-labeled DNA was stained with ethidium bromide and detected by visualization under UV light.
Phylogenetic analysis of KfrA proteins
The amino acid sequences of KfrA proteins from various bacteria were aligned in ClustalX (Thompson et al., 1997). The phylogenetic tree was visualized with an unrooted NJ-plot (Perrière and Gouy, 1996).
Results and discussion
Sequencing and identification of open reading frames in pPOP15.9
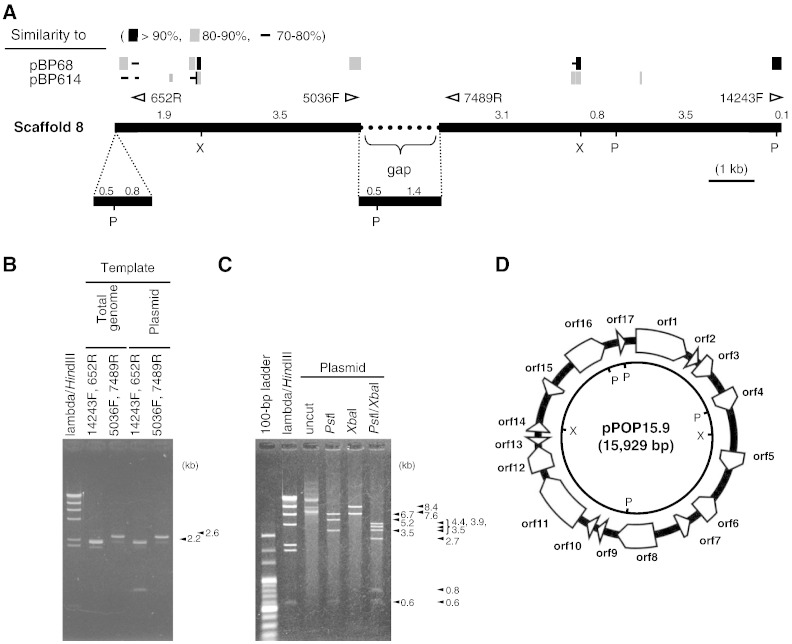
A total of 407,478 reads was assembled and 16 scaffolds of 3,973,017 nucleotides with an N50 of 681.8 kb were generated. The mean coverage was 25.2-fold. BLAST search indicated that pBP68 and pBP614 shared partial homology with scaffold 8 (Fig. 1A). The length of scaffold 8 is 14.5 kb of linear DNA with a 1.7-kb assembly gap. PCR products were amplified using appropriate primers from genomic and plasmid DNA templates (Fig. 1B). The results indicated that scaffold 8 is a circular DNA. After sequencing, the length of the circular DNA was determined to be 15,929 bp. Mapping of HiSeq 2000 reads to the sequence yielded a map ratio and read depth of 3.286% and 7760.6. The plasmid extracted from P. popilliae was digested with PstI and/or XbaI. The digestion profiles were consistent with expectations for the circular DNA originated from scaffold 8 (Fig. 1C), indicating that this circular DNA is a plasmid, designated pPOP15.9.
Fig. 1.
Plasmid pPOP15.9 found in P. popilliae ATCC 14706T. Similarity of scaffold 8 to pBP68 and pBP614 (A). Open triangles indicate primers for gap enclosure. Restriction nuclease sites are shown as P (PstI) and X (XbaI). The numbers on the bold line for scaffold 8 represent DNA fragment length (kb). PCR was performed for gap closure of scaffold 8 (B). Plasmid was extracted and digested with PstI, XbaI, or both (C). Seventeen open reading frames were predicted. The inner circle represents the restriction map.
MiGAP predicted 17 open reading frames (orfs) on pPOP15.9 (Fig. 1D; Table 2). Amino acid sequence alignment indicated that Orf1, Orf5, Orf7, Orf8, and Orf9 are homologous with Orf11, Orf12, Orf15, Orf16, and Orf17, respectively, with ≥ 40% similarity. One or two domains were identified in Orf1, Orf6–8, Orf11, and Orf15–16 (Table 2). Putative domains in Orf1, Orf11 and Orf6, Orf15 are found in replication initiator proteins and transcription regulators, respectively. Interestingly Orf8 and Orf16 are closely related to proteins found in Gram-negative bacteria (KfrA proteins). Some KfrA-like protein sequences in Gram-positive bacteria are listed in the NCBI database. The phylogenetic analysis based on the amino acid sequences of KfrA proteins is described in Section 3.7.
Table 2.
Putative open reading frames (orf) found in pPOP15.9 and their homologs.
| Orf | Length (aa) |
Mass (kDa) |
Putative domain a |
Homolog |
|||||
|---|---|---|---|---|---|---|---|---|---|
| pPOP15.9b |
Other organismsc |
||||||||
| Domain (ID) | E-value | Orf | Identity/similarity | Protein (bacterial species) | Locus | E-value | |||
| Orf1 | 461 | 55.5 | Replication initiation factor (pfam02486) | 2.14e − 04 | Orf11 | 90.5/95.9 | Hypothetical protein (Ppol) | WP_016818384 | 3e − 180 |
| Replication initiation protein (Bthu) | WP_001135316 | 1e − 123 | |||||||
| Replication initiation protein (Bcer) | WP_001141237 | 5e − 122 | |||||||
| Orf2 | 72 | 8.19 | No hit | – | No hit | – | No hit | – | – |
| Orf3 | 143 | 16.7 | No hit | – | No hit | – | No hit | – | – |
| Orf4 | 168 | 18.2 | No hit | – | No hit | – | Hypothetical protein (Palv) | WP_005543610 | 3e − 04 |
| Orf5 | 167 | 18.5 | No hit | – | Orf12 | 29.9/43.4 | Hypothetical protein (Psp.) | WP_017687016 | 3e − 29 |
| Hypothetical protein (Pgin) | WP_019535067 | 4e − 25 | |||||||
| Hypothetical protein (Psp.) | WP_016818383 | 3e − 22 | |||||||
| Orf6 | 184 | 22.0 | MerR HTH family regulatory protein (pfam13411) | 1.01e − 05 | No hit | – | Hypothetical protein (Blat) | WP_003338843 | 5e − 31 |
| Hypothetical protein (Blat) | WP_003335127 | 2e − 20 | |||||||
| DNA-binding protein (Bcer) | WP_002167222 | 3e − 20 | |||||||
| Orf7 | 111 | 12.6 | Helix-turn-helix XRE-family like proteins (cd00093) | 5.30e − 15 | Orf15 | 45.0/63.3 | Hypothetical protein (Pbar) | WP_016311381 | 1e − 21 |
| Transcriptional regulator (Ppeo) | WP_010349619 | 2e − 21 | |||||||
| Transcriptional regulator (Pter) | YP_005073849 | 2e − 21 | |||||||
| Orf8 | 327 | 36.0 | Plasmid replication region DNA binding N-term (pfam11740) | 1.44e − 15 | Orf16 | 95.1/97.9 | KfrA protein (Pput, pMCBF1) | AAY97967 | 2e − 29 |
| 5′ nucleotidase family (cl17687) | 7.11e − 03 | KfrA protein (Tniv) | WP_002706521 | 2e − 27 | |||||
| KfrA protein (Mfra) | YP_006297578 | 3e − 27 | |||||||
| Orf9 | 61 | 7.08 | No hit | – | Orf17 | 87.1/90.3 | No hit | – | – |
| Orf10 | 70 | 8.22 | No hit | – | No hit | – | Hypothetical protein (Ppol) | WP_016822061 | 1e − 06 |
| Hypothetical protein (Bsp.) | WP_019123583 | 2e − 04 | |||||||
| Hypothetical protein (Psp.) | WP_009673026 | 0.001 | |||||||
| Orf11 | 462 | 55.7 | Replication initiation factor (pfam02486) | 1.28e − 5 | Orf1 | 90.5/95.9 | Hypothetical protein (Ppol) | WP_016818384 | 1e − 179 |
| Replication initiation protein (Bthu) | WP_001135316 | 7e − 124 | |||||||
| Replication initiation protein (Bcer) | WP_001141237 | 9e − 122 | |||||||
| Orf12 | 206 | 22.9 | No hit | – | Orf5 | 29.9/43.4 | Hypothetical protein (Palv) | WP_021254164 | 5e − 13 |
| Orf13 | 63 | 7.39 | No hit | – | No hit | – | No hit | – | – |
| Orf14 | 89 | 9.77 | No hit | – | No hit | – | No hit | – | – |
| Orf15 | 110 | 12.8 | Helix-turn-helix XRE-family like proteins (cd00093) | 7.42e − 13 | Orf7 | 45.0/63.3 | Hypothetical protein (Bmas) | WP_019156383 | 8e − 41 |
| Predicted transcriptional regulators (COG1396) | 8.01e − 06 | Transcriptional regulator (Pter) | YP_005073849 | 6e − 33 | |||||
| Transcriptional regulator (Ppol) | YP_003872652 | 2e − 30 | |||||||
| Orf16 | 326 | 35.8 | Plasmid replication region DNA binding N-term (pfam11740) | 7.77e − 17 | Orf8 | 95.1/97.9 | KfrA protein (Pput, pMCBF1) | AAY97967 | 2e − 29 |
| Hypothetical protein (Tter) | WP_021249357 | 5e − 28 | |||||||
| KfrA protein (un-b) | NP_598100 | 1e − 27 | |||||||
| Orf17 | 61 | 7.27 | No hit | – | Orf9 | 87.1/90.3 | No hit | – | – |
Putative domains were searched against NCBI's Conserved domain database (Marchler-Bauer et al., 2011).
An end-to-end pairwise alignment of nucleotide and amino acid sequences was performed using the EMBOSS Needle provided by the European Bioinformatics Institute (http://www.ebi.ac.uk/Tools/psa/). Similarity less than 40% is indicated as “no hit”.
Each amino acid sequence was analyzed using NCBI's BLASTP with E-value cutoff at 0.1. When four or more hits were obtained, the top three are indicated. Abbreviations: Bcer, Bacillus cereus; Blat, Bacillus laterosporus; Bmas, Bacillus massiliosenegalensis; Bsp., Brevibacillus sp.; Bthu, Bacillus thuringiensis; Mfra, Methylophaga frappieri; Palv, Paenibacillus alvei; Pbar, Paenibacillus barengoltzii; Pgin, Paenibacillus ginsengihumi; Ppeo, Paenibacillus peoriae; Ppol, Paenibacillus polymyxa; Pput, Pseudomonas putida; Psp., Paenibacillus sp.; Pter, Paenibacillus terrae; Tniv, Thiothrix nivea; Tter, Thauera terpenica; un-b, uncultured bacterium
In most strains of B. thuringiensis, the delta endotoxin-encoding cry gene is located on a plasmid (Dean, 1984). Although P. popilliae also carries a cry gene (Zhang et al., 1997), it is not encoded on pPOP15.9, nor are other virulence factors such as toxins; therefore, this plasmid does not contribute to P. popilliae pathogenesis. Since the significance of the P. popilliae plasmid remains unknown, pPOP15.9 is considered a cryptic plasmid.
Prediction of secondary structure of Orf8 and Orf16
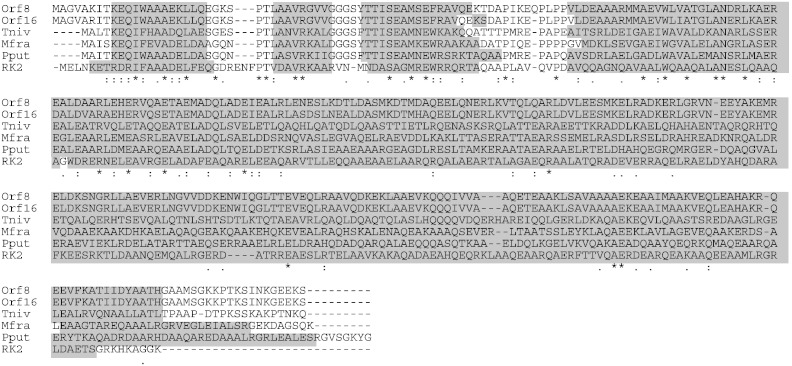
The secondary structures of Orf8 and Orf16 were compared to those of KfrA proteins of T. nivea, Methylophaga sp., P. putida, and broad-host-range plasmid RK2 (Fig. 2). Orf8, Orf16, and other KfrA proteins are highly α-helical and share nearly identical predicted secondary structures, with an N-terminal globular domain and a long, coiled-coil α-helical tail (Adamczyk et al., 2006, Jagura-Burdzy and Thomas, 1992). Addition of FLAG- or His tags to the carboxyl termini of Orf8 and Orf16 had no effect on the secondary structure (data not shown).
Fig. 2.
Secondary structure of Orf8, Orf16, and other KfrA proteins. Tniv, Mfr, Pput, and RK2 represent the KfrA proteins of Thiothrix nivea, Methylophaga frappieri, pMCBF1 of Pseudomonas putida, and RK2 plasmid. The gray regions indicate alpha helix structures predicted by PSIPRED v3.3.
Expression of recombinant Orf8 and Orf16 proteins
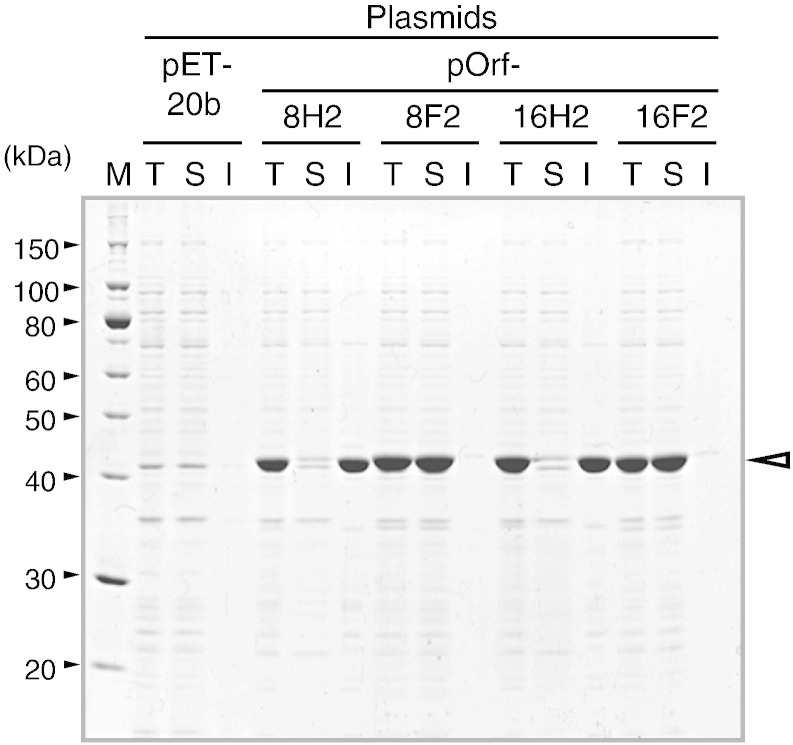
pOrf8H, pOrf8F, pOrf16H, and pOrf16F yielded small amounts of expressed protein that mainly localized to the cytoplasm (data not shown), suggesting failure of periplasmic export; the pelB leader peptide remained in almost all of the proteins. Extraction of recombinant protein from the periplasmic space was difficult because the contaminating cytoplasm could not be eliminated. The pelB leader sequence was deleted to create pOrf8H2, pOrf8F2, pOrf16H2, and pOrf16F2, all of which expressed abundant recombinant proteins (Fig. 3). FLAG-tagged proteins localized in the cytoplasm as soluble proteins and His-tagged proteins were detected in the insoluble fraction. The His-tagged proteins were purified as described in Materials and methods section (data not shown).
Fig. 3.
Cellular localization of recombinant Orf8 and Orf16 proteins. M, T, S, and I indicate molecular weight marker, total cell, soluble, and insoluble fractions. An equivalent of 10-μL culture per lane was analyzed. Open triangle indicates the recombinant protein band.
Homomultimer formation of recombinant proteins
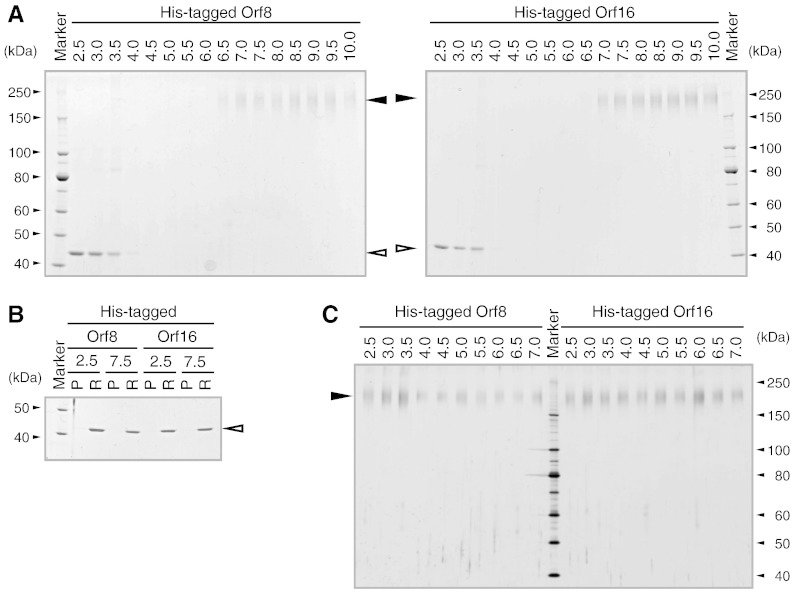
Broad bands of 180 kDa were detected when His-tagged proteins were incubated at pH 6.5 or higher, indicating multimer formation (Fig. 4A). A previous report of RK2-encoded KfrA cross-linking yielded a clear dimer band and a faint tetramer band that was difficult to reproduce (Jagura-Burdzy and Thomas, 1992). We did not observe the dimer band in this study. Although the detected bands may suggest tetramer formation, low resolution in the high molecular mass range and the observed broad band could not rule out other levels of multimerization; thus, we adopted the term “multimer” in this manuscript.
Fig. 4.
Effect of pH on homomultimer formation of His-tagged Orf8 and Orf16 proteins. The recombinant proteins were incubated at various pH values and cross-linked with glutaraldehyde. The samples (250 ng/lane) were electrophoresed and CBB stained (A). The recombinant proteins were incubated at pH 2.5 and pH 7.5. After ultrafiltration (100-kDa cutoff), permeate (P) and retentate (R) fractions were electrophoresed and CBB stained (B). To ascertain pH stability, the recombinant proteins were incubated at various pH values, diluted, and shifted to pH 7–8. After cross-linking, the samples (25 ng/lane) were electrophoresed and silver stained (C). In all panels, large closed and open triangles indicate multimers and monomers, respectively.
No signals were detected in the pH 4.5–6.0 samples, in which His-tagged proteins likely aggregated and formed macromolecules with glutaraldehyde, which could not enter the polyacrylamide gel. A monomer band was detected at pH 2.5–4.0, with clearer signals at lower pH. Glutaraldehyde reacts with the amino group over a wide pH range and the reaction increases with increasing pH (Okuda et al., 1991). Therefore, the monomer band detected at low pH may have been due to the absence of cross-linking. To verify this, His-tagged proteins incubated at pH 2.5 and 7.5 were subjected to ultrafiltration (Fig. 4B). Recombinant proteins were detected only in the retentate fractions of samples treated at pH 7.5 and pH 2.5. It is unlikely that the proteins could fold properly at low pH; they likely aggregated under these conditions. Homomultimer formation was restored to proteins treated at pH 2.5–7.0 when they were shifted to pH 7–8 (Fig. 4C). Thus, His-tagged proteins formed homomultimers at pH 6.5–10.0; multimerization was retained over a pH range of 2.5–10.0 at 30 °C for 2 h. Similar results were obtained for the FLAG-tagged proteins (Supplemental Fig. S1), which differed from the His-tagged proteins only in homomultimer formation at pH 6.0. The hydrophilic nature of the FLAG tag prevents aggregation. The difference in tag hydrophilicity may also have affected cellular localization in E. coli (Fig. 3).
Orf8 and Orf16 multimerized at pH 6.5 or higher. Although the proteins seemed to aggregate at low pH, the ability to form multimers was not completely lost and was reversible. In E. coli, the cytoplasmic pH fell within 10 to 20 s to pH 5.6 to 6.5 when the external pH was lowered from pH 7.5 to 5.5. Rapid recovery, however, occurred until about 30 s after HCl addition and was followed by slower recovery over the next 5 min (Wilks and Slonczewski, 2007). If it is hypothesized that P. popilliae has a similar pH homeostasis, and that multimerization is indispensable to the function of Orf8 and Orf16, thus, pH stability enables multimerization after a shift from acidic to neutral pH.
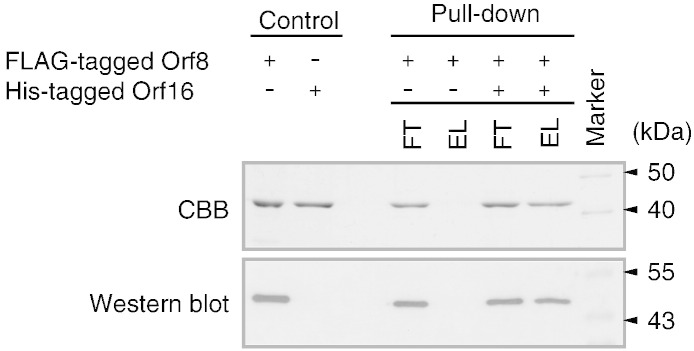
Molecular interaction between recombinant Orf8 and Orf16
When FLAG-tagged Orf8 alone was applied to the pull-down assay as a control, it was detected in the flow-through fraction but not in the eluent (Fig. 5). Co-incubation with His-tagged Orf16, however, brought the FLAG-tagged Orf8 into the flow-through and elution fractions. Western blot analysis indicated the FLAG-tagged Orf8 interacted with His-tagged Orf16, suggesting heteromultimer formation.
Fig. 5.
Pull-down assay to test interaction between recombinant Orf8 and Orf16 proteins. Total proteins were detected by CBB staining (upper panel). FLAG-tagged Orf8 was detected with a FLAG antibody.
In this study, the number of subunits per multimer and the function of the proteins were not determined. If the proteins form a tetramer, three heterotetramer combinations (1:3, 2:2, 3:1 in Orf8:Orf16) are possible. After having solved these remaining problems, it is necessary to determine whether the heteromultimers are functional.
Electrophoresis mobility shift assays (EMSAs)
EMSAs were performed to evaluate the interaction between recombinant proteins and DNA substrates. In all EMSAs, it was difficult to detect the protein–DNA complex as anything but a smeared band; otherwise, the complex remained in the sample well because of charge, molecular mass, or some unexplained reason. Therefore, we evaluated binding capacity based on the decrease or disappearance of free DNA.
In the first EMSA with upstream regions of orf8, US8, and orf16, US16 (Fig. 6A), DNA bands were not detected after co-incubation of 40 pmol His-tagged proteins with 0.025 pmol DNA substrates (Fig. 6B). The decrease in DNA signal intensity correlated with an increase in protein dose. Although His-tagged Orf8 did not cause a loss of signal intensity at 10 pmol, His-tagged Orf16 caused a clear reduction at this dose, suggesting that Orf16 has a greater DNA-binding affinity than Orf8. Next, TasI-digested US8 and US16 (Fig. 6A) were used as substrates to more closely identify the binding site. The second EMSA indicated that Orf8 bound to a 156-bp fragment of US8 and a 149-bp fragment of US16 (Fig. 6C). On the other hand, Orf16 bound to these DNA fragments and to 49-bp fragments of both US8 and US16. This result suggested that Orf8 and Orf16 could bind to sequence(s) 205 bp upstream of orf8 (205US8) and 198 bp upstream of orf16 (198US16).
KfrA, encoded on the broad-host-range RK2 plasmid, specifically binds to an inverted repeat sequence upstream of kfrA (Jagura-Burdzy and Thomas, 1992). Three putative inverted repeat sequences were found in 205US8 (designated as IR1, IR2 and IR3) and 198US16 (IR1, IR2 and IR4). IR1 and IR2 were common to both regions (Fig. 6A). These fragments were labeled with FITC (IR1V, IR2V, IR3V and IR4V) and used as EMSA substrates. Free IR3V and IR4V were reduced by co-incubation with Orf8. Other free substrates were not reduced by Orf8 (Fig. 6D). On the other hand, Orf16 induced loss of free IR1V and IR2V, as well as IR3V and IR4V. However, a decrease of free insert-less DNA fragment (EV) was also induced by Orf16 (Fig. 6D). This unexpected result suggests that Orf16 could also bind to the vector sequence. Therefore, to clarify the sequence-specific binding capacity, EMSA was performed with non-labeled competitors (IR1, IR2, IR3, IR4). The competitors did not contain vector sequences (Fig. 6A). Binding of Orf8 to IR3V or IR4V was completely inhibited by excess IR3 or IR4 (Fig. 6E). Interactions of Orf16 with EV and IR1V were inhibited by IR2, IR3, and IR4, but not completely by IR1. This result suggests that Orf16 interacts with the vector sequence in IR1V. The Orf16–IR2V complex was dissociated by IR2, IR3, and IR4. IR1 did not cause this dissociation. Binding of Orf16 to IR3V and IR4V was completely inhibited by IR3 and IR4. IR2 partially inhibited this binding. IR1 did not affect this interaction.
The EMSA results demonstrate binding of His-tagged Orf8 and Orf16 to IR3 (upstream of orf8) and IR4 (upstream of orf16). Orf16 also weakly bound to IR2 (upstream of both genes).
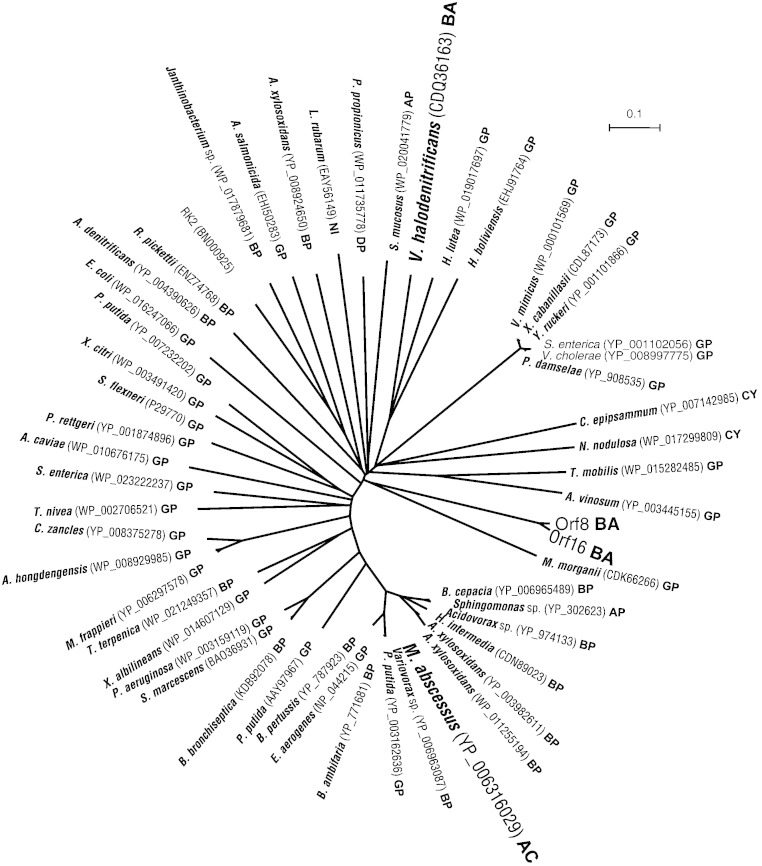
Phylogenetic analysis of KfrA proteins
The KfrA phylogenetic tree clearly differed from the species tree (Fig. 7). For example, KfrA proteins of Gram-positive bacteria are scattered and do not form a phylogenetic clade in the tree, suggesting that the kfrA gene was horizontally transferred from other bacteria. The kfrA gene was first described in an IncP-1 plasmid RK2 (Thomas et al., 1990). The IncP-1 plasmids can replicate and are stably maintained in almost all Gram-negative bacteria and may be transferred by conjugation to Gram-positive bacteria, yeasts, and eukaryotic cell lines (Adamczyk and Jagura-Burdzy, 2003). The kfrA genes in pPOP15.9 may have been obtained from a broad-host-range plasmid like RK2. The host range of pPOP15.9 remains unresolved.
Fig. 7.
Unrooted phylogenetic tree based on amino acid sequences of Orf8, Orf16, and other KfrA proteins. Abbreviations of class names (AC, Actinobacteria; BA, Bacilli; AP, Alphaproteobacteria; BP, Betaproteobacteria; GP, Gammaproteobacteria; DP, Deltaproteobacteria; CY, Cyanophyceae; NI, Nitrospira) are shown in bold letters with the exception of RK2. Gram-positive bacteria are indicated in larger letters.
In this study, Orf8 and Orf16 encoded on pPOP15.9 formed a multimer and bound to upstream regions of both orf8 and orf16. However, the function of these proteins and the contribution of pPOP15.9 to bacterial host behavior were not investigated and remain to be clarified in the future.
Accession number
The nucleotide sequence of pPOP15.9 has been deposited in DDBJ/EMBL/GenBank under accession number AB931111.
The following are the supplementary related to this article.
Homomultimer formation of FLAG-tagged Orf8 and Orf16 proteins at varying pH values. The recombinant proteins were incubated at various pH values and cross-linked with glutaraldehyde. The samples (250 ng/lane) were electrophoresed and stained with CBB (A). The recombinant proteins were incubated at pH 2.5 and pH 7.5. After ultrafiltration (100-kDa cutoff), permeate (P) and retentate (R) fractions were electrophoresed and stained with CBB (B). Recombinant proteins were incubated at various pH values, diluted, and then incubated at pH 7–8. After cross-linking, the samples (25 ng/lane) were electrophoresed and silver stained (C). In all panels, large closed and open triangles indicate multimers and monomer, respectively.
Acknowledgments
The cost of publication was supported in part by a Research Grant for Young Investigators from the Faculty of Agriculture, Kyushu University. We would like to thank Editage (www.editage.jp) for English language editing.
Contributor Information
Kazuhiro Iiyama, Email: iiyama@grt.kyushu-u.ac.jp.
Hiroaki Mon, Email: mhiro@agr.kyushu-u.ac.jp.
Kazuki Mori, Email: kmori917@grt.kyushu-u.ac.jp.
Takumi Mitsudome, Email: t.mitsudome@agr.kyushu-u.ac.jp.
Jae Man Lee, Email: jaemanle@agr.kyushu-u.ac.jp.
Takahiro Kusakabe, Email: kusakabe@agr.kyushu-u.ac.jp.
Kousuke Tashiro, Email: ktashiro@grt.kyushu-u.ac.jp.
Shin-ichiro Asano, Email: sangaku@abs.agr.hokudai.ac.jp.
Chisa Yasunaga-Aoki, Email: yasunaga@grt.kyushu-u.ac.jp.
References
- Adamczyk M., Jagura-Burdzy G. Spread and survival of promiscuous IncP-1 plasmids. Acta Biochim. Pol. 2003;50:425–453. [PubMed] [Google Scholar]
- Adamczyk M., Dolowy P., Jonczyk M., Thomas C.M., Jagura-Burdzy G. The kfrA gene is the first in a tricistronic operon required for survival of IncP-1 plasmid R751. Microbiology. 2006;152:1621–1637. doi: 10.1099/mic.0.28495-0. [DOI] [PubMed] [Google Scholar]
- Angers-Loustau A., Rainy J., Wartiovaara K. PlasmaDNA: a free, cross-platform plasmid manipulation program for molecular biology laboratories. BMC Mol. Biol. 2007;8:77. doi: 10.1186/1471-2199-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C., O'Neil S., Ben-Dov E., Jones A.F., Murphy L., Quail M.A., Holden M.T., Harris D., Zaritsky A., Parkhill J. Complete sequence and organization of pBtoxis, the toxin-coding plasmid of Bacillus thuringiensis subsp. israelensis. Appl. Environ. Microbiol. 2002;68:5082–5095. doi: 10.1128/AEM.68.10.5082-5095.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costilow R.N., Coulter W.H. Physiological studies of an oligosporogenous strain of Bacillus popilliae. Appl. Microbiol. 1971;22:1076–1084. doi: 10.1128/am.22.6.1076-1084.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D.H. Biochemical genetics of the bacterial insect-control agent Bacillus thuringiensis: basic principles and prospects for genetic engineering. Biotechnol. Genet. Eng. Rev. 1984;2:341–346. doi: 10.1080/02648725.1984.10647804. [DOI] [PubMed] [Google Scholar]
- Dingman D.W. Physical properties of three plasmids and the presence of interrelated plasmids in Bacillus popilliae and Bacillus lentimorbus. J. Invertebr. Pathol. 1994;63:235–243. [Google Scholar]
- Faust R.M., Spizizen J., Gage V., Travers R.S. Extrachromosomal DNA in Bacillus thuringiensis var. kurstaki, var. finitimus, var. sotto, and in Bacillus popilliae. J. Invertebr. Pathol. 1979;33:233–238. [Google Scholar]
- Iiyama K., Mori K., Mon H., Chieda Y., Lee J.M., Kusakabe T., Tashiro K., Asano S., Yasunaga-Aoki C., Shimizu S. Draft genome sequence of Paenibacillus popilliae ATCC 14706T. J. Insect Biotechnol. Sericology. 2013;82:45–48. [Google Scholar]
- Jagura-Burdzy G., Thomas C.M. kfrA gene of broad host range plasmid RK2 encodes a novel DNA-binding protein. J. Mol. Biol. 1992;225:651–660. doi: 10.1016/0022-2836(92)90392-w. [DOI] [PubMed] [Google Scholar]
- Jones D.T. Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 1999;292:195–202. doi: 10.1006/jmbi.1999.3091. [DOI] [PubMed] [Google Scholar]
- Jutkina J., Hansen L.H., Li L., Heinaru E., Vedler E., Jõesaar M., Heinaru A. Complete nucleotide sequence of the self-transmissible TOL plasmid pD2RT provides new insight into arrangement of toluene catabolic plasmids. Plasmid. 2013;70:393–405. doi: 10.1016/j.plasmid.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Kovach M.E., Elzer P.H., Hill D.S., Robertson G.T., Farris M.A., Roop R.M., II, Peterson K.M. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- Levine M.M., Nataro J.P., Karch H., Baldini M.M., Kaper J.B., Black R.E., Clements M.L., O'Brien A.D. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J. Infect. Dis. 1985;152:550–559. doi: 10.1093/infdis/152.3.550. [DOI] [PubMed] [Google Scholar]
- Longley M., MacDonald R., Poulter T.M. Characterization of pBP614, a putative rolling-circle plasmid from Bacillus popilliae. Plasmid. 1997;37:15–21. doi: 10.1006/plas.1996.1275. [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A., Lu S., Anderson J.B., Chitsaz F., Derbyshire M.K., DeWeese-Scott C., Fong J.H., Geer L.Y., Geer R.C., Gonzales N.R., Gwadz M., Hurwitz D.I., Jackson J.D., Ke Z., Lanczycki C.J., Lu F., Marchler G.H., Mullokandov M., Omelchenko M.V., Robertson C.L., Song J.S., Thanki N., Yamashita R.A., Zhang D., Zhang N., Zheng C., Bryant S.H. CDD: a conserved domain database for the functional annotation of proteins. Nucleic Acids Res. 2011;39:D225–D229. doi: 10.1093/nar/gkq1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miriagou V., Carattoli A., Fanning S. Antimicrobial resistance islands: resistance gene clusters in Salmonella chromosome and plasmids. Microbes Infect. 2006;8:1923–1930. doi: 10.1016/j.micinf.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Okuda K., Urabe I., Yamada Y., Okada H. Reaction of glutaraldehyde with amino and thiol compounds. J. Ferment. Bioeng. 1991;71:100–105. [Google Scholar]
- Perrière G., Gouy M. WWW-query: an on-line retrieval system for biological sequence banks. Biochimie. 1996;78:364–369. doi: 10.1016/0300-9084(96)84768-7. [DOI] [PubMed] [Google Scholar]
- Pettersson B., Rippere K.E., Yousten A.A., Priest F.G. Transfer of Bacillus lentimorbus and Bacillus popilliae to the genus Paenibacillus with emended descriptions of Paenibacillus lentimorbus comb. nov. and Paenibacillus popilliae comb. nov. Int. J. Syst. Bacteriol. 1999;49:531–540. doi: 10.1099/00207713-49-2-531. [DOI] [PubMed] [Google Scholar]
- Steinkraus K.H., Tashiro H. Milky disease bacteria. Appl. Microbiol. 1967;15:325–333. doi: 10.1128/am.15.2.325-333.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara H., Ohyama A., Mori H., Kurokawa K. Abstr. 20th Int. Conf. Genome Informatics, Kanagawa, Japan. 2009. Microbial genome annotation pipeline (MiGAP) for diverse users, abstr S-001-1-2. [Google Scholar]
- Tanada Y., Kaya H.K. Academic Press; San Diego, CA: 1992. In Insect Pathology; pp. 83–146. [Google Scholar]
- Thomas C.M., Theophilus B.D.M., Johnston L., Jagura-Burdzy G., Schilf W., Lurz R., Lanka E. Identification of a seventh operon on plasmid RK2 regulated by the korA gene product. Gene. 1990;89:29–35. doi: 10.1016/0378-1119(90)90202-3. [DOI] [PubMed] [Google Scholar]
- Thompson J.D., Gibson T.J., Plewniak F., Jeanmougin F., Higgins D.G. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks J.C., Slonczewski J.L. pH of the cytoplasm and periplasm of Escherichia coli: rapid measurement by green fluorescent protein fluorimetry. J. Bacteriol. 2007;189:5601–5617. doi: 10.1128/JB.00615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Hodgman T.C., Krieger L., Schnetter W., Schairer H.U. Cloning and analysis of the first cry gene from Bacillus popilliae. J. Bacteriol. 1997;179:4336–4341. doi: 10.1128/jb.179.13.4336-4341.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Homomultimer formation of FLAG-tagged Orf8 and Orf16 proteins at varying pH values. The recombinant proteins were incubated at various pH values and cross-linked with glutaraldehyde. The samples (250 ng/lane) were electrophoresed and stained with CBB (A). The recombinant proteins were incubated at pH 2.5 and pH 7.5. After ultrafiltration (100-kDa cutoff), permeate (P) and retentate (R) fractions were electrophoresed and stained with CBB (B). Recombinant proteins were incubated at various pH values, diluted, and then incubated at pH 7–8. After cross-linking, the samples (25 ng/lane) were electrophoresed and silver stained (C). In all panels, large closed and open triangles indicate multimers and monomer, respectively.