Abstract
Background
Abnormal glucose metabolism (AGM) and metabolic syndrome (MS) are individually associated with a poor cardiovascular outcome in kidney transplant recipients. We prospectively studied the relationship between AGM and MS in non-diabetic kidney transplant recipients early after transplantation.
Methods
A total of 203 de novo kidney transplant recipients underwent standard 2-hr glucose tolerance test 10 weeks after transplantation. Demographic and clinical characteristics were collected. AGM was defined as impaired fasting glucose, impaired glucose tolerance, and new onset diabetes after transplant according to the WHO criteria, and MS was defined according to the National Cholesterol Education Expert Panel criteria.
Results
Overall, 97 patients (47.8%) met the diagnosis of AGM and 98 patients (48.3%) met the criteria of MS. AGM and MS are highly associated (χ2, P<0.001). Fasting plasma glucose levels before the transplant are independent predictors common for AGM and MS. Age predicts AGM with and without MS, whereas body mass index before transplant predicts MS. Patients with impaired glucose tolerance and new-onset diabetes after transplant displayed significant worsening of their fasting plasma glucose levels during the 10-week observational period. MS and the components of MS, but not AGM, were associated with reduced transplant renal function (P=0.002).
Conclusion
The early screening of AGM and MS should be emphasized, and the role of early therapeutic interventions aimed at both conditions explored. The long-term follow-up of these patients will yield more insight on the significance of such findings.
Keywords: Kidney transplant, Abnormal glucose metabolism, Metabolic syndrome
Abnormal glucose metabolism (AGM) is common early after kidney transplantation and is a risk factor for new-onset diabetes after transplant (NODAT) (1, 2). The majority of NODAT occurs early after kidney transplantation, and it is associated with increased cardiovascular events and mortality (3–5). Metabolic syndrome (MS) is a constellation of metabolic and nonmetabolic disorders that occurs frequently in kidney transplant recipients as well, and it is associated with impaired long-term renal allograft function and poor patient survival (6, 7). In addition, various components of MS are shown to be risk factors for the development of AGM and NODAT (1, 8). It is unclear what relationship exists between AGM and MS early after kidney transplantation.
Current guidelines recommend using fasting plasma glucose or oral glucose tolerance to diagnose AGM (9–11). However, in the kidney transplant population, it has been shown that the oral glucose tolerance test (OGTT) has a superior sensitivity in diagnosing AGM when compared with fasting glucose levels alone (12). Furthermore, the OGTT can clearly define a subgroup of patients who demonstrate an impaired glucose tolerance (IGT) that can be a precursor of type 2 diabetes mellitus and a predictor of mortality in the general population (13, 14). Thus, the early detection of AGM and MS after transplantation could be of great importance because it will allow for a more timely intervention to prevent or reduce the long-lasting deleterious effects of both conditions on patient and allograft survival.
We performed a prospective observational study investigating the relationship between AGM and MS in de novo kidney transplant recipients. Here, we report the findings at 10 weeks after kidney transplantation.
MATERIALS AND METHODS
Patients and Laboratory Studies
This is a prospective observational study of glucose metabolism in previously non-diabetic solitary kidney trans-plant recipients. The study was approved by the institutional review board.
Between October 2006 and September 2008, all non-diabetic de novo kidney transplant recipients were recommended to have a standard 2-hr OGTT 10 weeks after kidney transplantation as per the institutional standard protocol. Their demographic characteristics were collected at the time of kidney transplantation. The fasting plasma glucose levels were collected at different time points: before transplant, first 3 days in the hospital, 4 and 10 weeks after transplant. At the tenth week (from 8 to 16 weeks), a standard 2-hr OGTT was performed at the central laboratory of our institution. In brief, patients were instructed to have at least 8 hr of overnight fasting the day before their routinely scheduled clinical visits. After drawing a fasting blood sample, patients were given 75 g of anhydrous glucose dissolved in 250 mL of water to drink over a period of 5 min. A second blood sample was obtained 2 hr later for the measurement of glucose level. In addition, a comprehensive chemistry panel, including fasting lipid panel, uric acid, and creatinine, was obtained from the fasting blood sample at the time of the OGTT. The blood pressure measurement was taken during their clinical visit that occurred between 10 and 12 weeks after transplant. Body mass index (BMI) was calculated as weight (kilograms) over squared height (meter square) at the time of transplant and 10 weeks after transplant. Renal function, expressed as estimated glomerular filtration rate (eGFR), was calculated using abbreviated modification of diet in renal disease formula (15).
Definitions
AGM including impaired fasting glucose (IFG), IGT, and NODAT were defined based on the results of 2-hr OGTT according to the American Diabetic Association (ADA) and the World Health Organization (WHO) criteria: normal with fasting plasma glucose less than 100 and 2-hr glucose less than 140 mg/dL, IFG with a fasting plasma glucose between 100 and 125 mg/dL and IGT with 2-hr glucose between 140 and 199 mg/dL, and NODAT with a fasting plasma glucose more than or equal to126 mg/dL or 2-hr glucose more than or equal to 200 mg/dL (10, 11).
MS was defined according to the National Cholesterol Education Expert Panel (NCEP) criteria, but BMI was used instead of waist circumference (16–18). A patient was classified as having MS if at least three of the following criteria were present: BMI of 30 kg/m2 or greater, plasma fasting glucose levels of 100 mg/dL or greater, presence of hypertension (130/85 mm Hg or greater, or on an anti-hypertensive medication), fasting triglyceridemia of 150 mg/dL or greater, or a high-density lipoprotein cholesterol of 40 mg/dL or lower for men and 50 mg/dL or lower for women.
Immunosuppression
Immunosuppression was provided according to the institutional standard protocols. Induction was given selectively to patients with high immunologic risks using rabbit antithymocyte globulin or an anti-IL2 receptor antibody (basiliximab). All patients were started on a triple-drug regimen that included a calcineurin inhibitor (CNI), cyclosporine (CsA) or tacrolimus (Tac), an antiproliferative agent, mycophenolate moftile or sirolimus (Rap), and corticosteroids (prednisone). The dose of CNIs was determined by trough concentrations. The 12-hr trough levels were maintained between 150 and 300 ng/mL for CsA and 5 and 15 ng/mL for Tac through the first 10 to 12 weeks after transplantation. Prednisone was tapered according to the protocol, and the dose was tapered to 10 mg at 8 weeks after transplant. Acute rejection was diagnosed by biopsy, and treatment was tailored according to the severity of the rejection based on the updated Banff classification (19, 20).
Statistical Analyses
The statistical software SAS 9.1 was used to perform all the analyses. Continuous and categorical variables were compared using the ANOVA technique and chi-square test between the groups. Cochran-Mantel-Haenszel statistics were used to test association between AGM and MS. Multivariate multinomial logistic regression was performed to identify the risk factors for AGM and MS. Multivariate linear regression analysis was used to determine the association of blood uric acid levels and eGFR with AGM and MS. Multivariate generalized linear regression analysis for repeat measures was used to assess the evolution of fasting glucose levels over the period of 10 weeks after kidney transplantation.
RESULTS
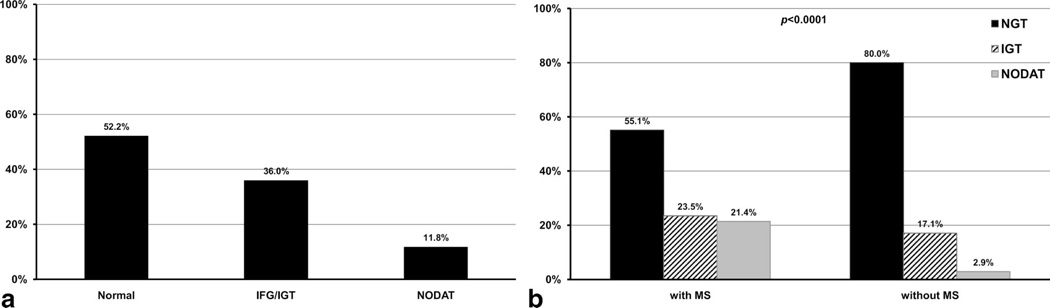
A total of 203 de novo kidney transplant recipients were included in this observational study. Overall, 97 patients (47.8%) displayed various degrees of AGM at 10 weeks (median 10.3 weeks, range 8.0 –18.6 weeks) after transplantation defined by the abnormal fasting plasma glucose or 2-hr oral glucose tolerance levels (Fig. 1a). Applying ADA and WHO criteria using fasting and 2-hr OGTT values, 97 patients had AGM. Among them, 32 (15.8%), 41 (20.2%), and 24 (11.8%) patients met diagnostic criteria for IFG, IGT, and NODAT, respectively. Applying NCEP criteria, 98 (48.3%) patients met the definition of MS. AGM and MS were closely related (Fig. 1b, P<0.001). In fact, 66 patients met the definition for both condition (MS/AGM; Table 1). Patients with either or both conditions were significantly older and heavier and had higher fasting glucose levels prior to the transplantation and during the first 3 days after transplant. Positive family history of type 2 diabetes mellitus was also seen more frequently in patients with MS or AGM, although not statistically different. Multivariate multinomial logistic regression analysis showed that fasting plasma glucose levels before transplant are independent predictors for the presence of MS or AGM 10 weeks after kidney transplantation. For every 1 mg/dL increase in pretransplant fasting plasma glucose levels (between 60 and 120 mg/dL), the risk of having MS or AGM is approximately 10% to 13% greater. Age is the risk factor for AGM with or without MS but not MS alone. Indeed, for every 1 year increase in age, there is approximately 8% greater risk to have AG Mat 10 weeks after transplant (Table 2). Not surprisingly, elevated BMI before transplantation predicts MS at 10 week after transplantation, a 17% increase in risk for each unit of BMI elevation. In this model, the use of Tac was associated with greater risk for AGM but not for MS, although the wide confidence interval suggests the effect of relatively few patients taking this medication. In a multivariate linear regression model, patients taking Tac had on average of 25.6 mg/dL higher 2-hr glucose levels than patients taking CsA (P=0.03) without significantly affecting the fasting glucose levels (data not shown). On the other hand, the positive family history of type 2 diabetes mellitus was associated with the presence of MS or AGM (OR, 1.4 –3.5), but it did not reach statistical significance in this model, likely related to small sample size.
Figure 1.
Distribution in glucose metabolism (a) and relationship between glucose metabolism and metabolic syndrome (b) 10 weeks after kidney transplantation.
TABLE 1.
Demographic and baseline characteristics of study population
| Normal (N=74) |
MS alone (N=32) |
AGM alone (N=31) |
MS/AGM (N=66) |
P | |
|---|---|---|---|---|---|
| Age (yr) | 41.4 (14.2) | 42.7 (13.7) | 51.5 (12.5) | 54.8 (12.0) | <0.001 |
| Male (%) | 44 (59.5) | 21 (65.6) | 23 (74.2) | 40 (60.6) | 0.51 |
| African American (%) | 16 (21.6) | 6 (18.8) | 8 (25.8) | 10 (15.2) | 0.62 |
| Living transplant (%) | 43 (58.1) | 11 (34.4) | 16 (51.6) | 28 (42.4) | 0.09 |
| First transplant (%) | 61 (82.4) | 27 (84.4) | 27 (87.1) | 54 (81.8) | 0.17 |
| Positive HCV serology (%) | 1 (1.4) | 4 (12.5) | 4 (12.9) | 4 (6.1) | 0.03 |
| Family DM history (%) | 13 (17.6) | 10 (31.3) | 9 (29.0) | 17 (25.8) | 0.37 |
| Smoking history | 0.05 | ||||
| None (%) | 48 (64.9) | 20 (60.5) | 11 (35.5) | 33 (50.0) | — |
| Former (%) | 15 (20.3) | 6 (18.8) | 14 (45.2) | 25 (37.9) | — |
| Current (%) | 11 (14.8) | 6 (18.7) | 6 (19.3) | 8 (12.1) | — |
| Delayed graft function (%) | 6 (8.1) | 5 (15.6) | 6 (19.4) | 8 (12.1) | 0.40 |
| Acute rejection (%) | 6 (8.2) | 1 (3.1) | 2 (6.7) | 7 (11.1) | 0.67 |
| BMI at transplant (kg/m2) | 26 (5) | 30 (6) | 25 (4) | 29 (5) | <0.001 |
| FBG at transplant (mg/dL) | 81 (7) | 86 (8) | 88 (9) | 90 (10) | <0.001 |
| FBG first 3 d (mg/dL) | 134 (24) | 124 (16) | 137 (15) | 137 (22) | 0.02 |
| CNIs | 0.28 | ||||
| CsA (%) | 67 (93.1) | 27 (87.1) | 25 (80.7) | 56 (86.2) | — |
| Tac (%) | 5 (6.9) | 4 (12.9) | 6 (19.3) | 9 (13.9) | — |
| Cumulative prednisone (mg/person) | 1998 (243) | 1858 (254) | 1999 (244) | 1985 (387) | 0.17 |
The SD values are given in parentheses.
BMI, body mass index; FBG, fasting blood glucose; CNIs, calcineurin inhibitors; MS, metabolic syndrome; AGM, abnormal glucose metabolism; CsA, cyclosporine; Tac, tacrolimus; HCV, hepatitis C virus; DM, diabetes mellitus.
TABLE 2.
Predictors for metabolic syndrome, abnormal glucose metabolism, or both
| Predictors | Odds ratio | 95% CI | P |
|---|---|---|---|
| Age (1 yr increase) | |||
| MS alone | 1.009 | 0.972–1.048 | 0.63 |
| AGM alone | 1.086 | 1.042–1.133 | <0.001 |
| MS/AGM | 1.085 | 1.048–1.123 | <0.001 |
| BMI at transplant (1 kg/m2 increase) | |||
| MS alone | 1.166 | 1.068–1.272 | <0.001 |
| AGM alone | 0.876 | 0.773–0.992 | 0.04 |
| MS/AGM | 1.055 | 0.967–1.151 | 0.23 |
| FGB at transplant (1 mg/dL increase) | |||
| MS alone | 1.100 | 1.031–1.173 | 0.004 |
| AGM alone | 1.104 | 1.029–1.184 | 0.006 |
| MS/AGM | 1.129 | 1.063–1.199 | <0.001 |
| CNIs usage (Tac vs. CsA) | |||
| MS alone | 2.510 | 0.486–12.968 | 0.27 |
| AGM alone | 7.220 | 1.328–39.249 | 0.02 |
| MS/AGM | 2.016 | 0.377–10.772 | 0.41 |
BMI, body mass index; FBG, fasting blood glucose; CNIs, calcineurin inhibitors; MS, metabolic syndrome; AGM, abnormal glucose metabolism; CsA, cyclosporine; Tac, tacrolimus.
Because plasma glucose levels and BMI at 10 weeks were two of the five components defining the presence of MS, we reanalyzed our data in a multivariate multinomial model that did not include plasma glucose levels and BMI before transplantation. Among all baseline variables, only age and the use of Tac remained as independent predictors for AGM with and without MS (data not shown).
At 10 weeks, patients with MS or AGM or both conditions had worse metabolic and cardiovascular risk profile as given in Table 3. In particular, patients with MS had higher levels of blood uric acid and reduced renal function. Multivariate linear regression analyses, adjusting for important confounding factors, demonstrated that MS, not AGM, was associated with elevated blood uric acid levels and inferior transplant renal function, expressed as eGFR. In fact, patients with MS have on average 0.5 mg/dL higher blood uric acid levels and 9.2 mL/min lower eGFR compared with patients without MS(P=0.03 and P=0.002, respectively). In addition, each component of MS contributes to 4.5 mL/min reduction in eGFR (P=0.002). Another factor associated with reduced renal function is episodes of acute rejection. On the other hand, female sex and African American race were associated with higher renal function 10 weeks after kidney transplantation (Table 4).
TABLE 3.
Metabolic and cardiovascular risk profile at 10 wk
| Normal (N=74) |
MS alone (N=32) |
AGM alone (N=31) |
MS/AGM (N=66) |
P | |
|---|---|---|---|---|---|
| BMI (kg/m2) | 27 (4) | 30 (7) | 25 (4) | 29 (5) | <0.001 |
| FBG (mg/dL) | 87 (7) | 88 (7) | 98 (10) | 111 (18) | <0.001 |
| HA1c (mg/dL) | 5.1 (0.5) | 5.2 (0.6) | 5.4 (0.5) | 5.8 (0.7) | <0.001 |
| Total cholesterol (mg/dL) | 215 (50) | 221 (51) | 208 (48) | 223 (50) | 0.52 |
| Triglyceride (mg/dL) | 167 (81) | 261 (133) | 141 (60) | 236 (103) | <0.001 |
| HDL cholesterol (mg/dL) | 53 (11) | 40 (9) | 59 (16) | 44 (10) | <0.001 |
| LDL cholesterol (mg/dL) | 128 (44) | 130 (37) | 121 (38) | 133 (38) | 0.61 |
| Statin usage (%) | 7 (9.5) | 3 (9.4) | 4 (12.9) | 11 (16.7) | 0.59 |
| SBP (mm Hg) | 134 (18) | 134 (13) | 138 (18) | 136 (18) | 0.78 |
| DBP (mm Hg) | 77 (10) | 80 (11) | 77 (13) | 74 (12) | 0.10 |
| PP (mm Hg) | 58 (16) | 54 (12) | 60 (11) | 62 (18) | 0.09 |
| HTN medication | 1.6 (0.9) | 2.0 (0.9) | 1.9 (1.1) | 2.2 (0.8) | 0.003 |
| RAS blockade (%) | 4 (5.4) | 4 (12.5) | 5 (16.1) | 10 (15.2) | 0.18 |
| Uric acid (mg/dL) | 6.2 (1.3) | 7.1 (2.0) | 6.3 (1.6) | 6.8 (1.6) | 0.02 |
| Allopurinol usage (%) | 6 (8.1) | 2 (6.3) | 4 (12.9) | 7 (10.6) | 0.76 |
| Thiazide usage (%) | 1 (1.4) | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| eGFR (mL/min) | 67 (15) | 59 (18) | 73 (33) | 57 (18) | <0.001 |
| Trough level (ng/mL) | |||||
| CsA | 174 (61) | 172 (44) | 176 (44) | 172 (52) | 0.99 |
| Tac | 8.2 (2.3) | 8.5 (1.0) | 8.5 (1.6) | 9.0 (2.7) | 0.91 |
The SD values are given in parentheses.
BMI, body mass index; FBG, fasting blood glucose; PP, pulse pressure; MS, metabolic syndrome; AGM, abnormal glucose metabolism; CsA, cyclosporine; Tac, tacrolimus; HDL, high-density lipoprotein; LDL, low-density lipoprotein; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; HTN, hypertension; RAS, renin angiotensin system.
TABLE 4.
Factors associated with transplant renal function at 10 wk
| Changes in eGFRa, mL/min |
P | |
|---|---|---|
| MS (yes vs. no) | −9.2 | 0.002 |
| Component of MS (each one) | −4.5 | 0.002 |
| Acute rejection (yes vs. no) | −12.4 | 0.020 |
| Gender (women vs. men) | 7.0 | 0.017 |
| Race (African American vs. others) | 8.6 | 0.016 |
eGFR by abbreviate modification of diet in renal disease formula.
eGFR, estimated glomerular filtration rate; MS, metabolic syndrome.
We further studied the evolution of the fasting plasma glucose levels through the first 10 weeks after kidney transplant. With the exception of the fasting plasma glucose levels immediately after kidney transplantation, the patients on average had a slow and progressive increase in fasting plasma glucose levels through the first 10 weeks after transplantation (P<0.001). There is a strong interaction between the time and the fasting glucose levels at 10 weeks after transplant (Fig. 2, P<0.001). Age, BMI (obese vs. no obese), and the use of CNI (Tac vs. CsA) were positively correlated with the changes in the fasting plasma glucose levels after transplantation (P=0.02, P=0.01, and P=0.002, respectively).
Figure 2.
Change in fasting plasma glucose levels during the first 10 weeks after kidney transplantation.
DISCUSSION
Our study showed that AGM and MS are prevalent early after kidney transplantation in a previously non-diabetic patient population. Furthermore, the two conditions were closely associated and share some common risk factors. Increased fasting plasma glucose levels before transplant, although within the non-diabetic range, are a common predictor for both conditions. The increased fasting plasma glucose levels seen shortly after kidney transplant, when all patients received high doses of steroids, failed to predict either condition 10 weeks later. Furthermore, our study showed that having either condition is associated with impaired metabolic and cardiovascular risk profile, and MS, not AGM, was associated with significantly higher blood uric acid levels and lower renal function early after transplantation.
NODAT and MS are both associated with poor patient and graft outcome (4, 7, 21). It is striking to see that the presence of MS or the individual components of MS early after kidney transplantation were associated with reduced renal function, which likely will lead to worsening kidney graft survival and increased cardiovascular death over the years as evidenced by previous studies (22, 23). Thus, early detection of the conditions will likely identify patients at risk for future deterioration in their transplant outcome. Although, fasting plasma glucose levels are generally recommended to diagnose AGM, they underestimate the incidence or prevalence of AGM in the kidney transplant population when used alone (12, 24). The importance of detecting prediabetes conditions has been shown by several studies in the general population as well as in the kidney transplant population (2, 25). It has been also shown that IGT but not impaired fasting plasma glucose levels predicts the progression toward type 2 diabetes and leads to an increased incidence of cardiovascular events in the general population (13, 14). In addition, based on our findings, screening for other components of MS before transplant may have even greater effect than fasting glucose levels alone as each component seemed to contribute to the reduced renal function. Thus, there is a potential for intervention, such as encouraging weight loss, early use of statins and even insulin sensitizers (26). Kidney transplant patients, even without preexistent diabetes, are considered high-risk patients for cardiovascular events, aggressive early screening for AGM and MS should be encouraged (27, 28).
One of the major risk factors for AGM is the use of various immunosuppressive medications for the purpose of kidney transplantation. In fact, CNIs, CsA or Tac, and steroids induce AGM through different mechanisms (29–31). Our study confirms the differential effects of CsA and Tac on glucose metabolism even in the early period after kidney transplantation implicating a greater β-cell toxicity by the later (32). The risk seems time dependent as the incidence of NODAT was highest shortly after kidney transplant and tapered off gradually to a stable level later on, likely a reflection of the practice pattern with the use of immunosuppressive medications (3, 33). It has been shown that a simple reduction in the daily dose of steroids could lead to improvement of glucose tolerance and differential effect of two CNIs become less striking in the late posttransplant period (33, 34).
The strength of our study included a clear description of the relationship between AGM and MS, demonstration of the progression of fasting glucose levels and the risk factors associated with the progression, and negative association of MS with reduced renal function early posttransplant in a prospective fashion. The results of our study suggest the need for detection of not only AGM but also the various components of MS early posttransplant, or event before transplant, so appropriate intervention could be instituted.
There are several limitations related to this study. First, we did not perform screening for either condition before kidney transplantation, thus we are not able to exclude the possibility that either of two conditions could be present. The fact that elevated BMI and fasting blood glucose before transplantation predicted the presence of MS or AGM 10 weeks after kidney transplantation suggests that both conditions and their individual components could well be present before transplantation. On the other hand, the result of this study placed emphasis on the importance and benefit of such screening before transplantation so early intervention targeting such risk components could be instituted. Second, the duration of observation is too short as 10 weeks after transplant may not yet represent a stable phase in the use of the immunosuppressive agents, thus a longer duration may alter the distribution of AGM or MS. However, previous studies have demonstrated that AGM early posttransplant was a risk factor for NODAT during the follow-up years and the incidence of MS increases with time after kidney transplantation (2, 7). Finally, because this is an observational study, all patients received routine care as per institutional protocols. The underutilization of statins and renin angiotensin system (RAS) blockers reflects the current practice pattern of initiating those medications mostly in the third month posttransplantation for the concern of drug-drug interaction and adverse effects. With the findings presented here, such practice should be reconsidered.
In conclusion, both AGM and MS are common early after kidney transplantation. MS is associated with reduced renal function. Longer follow-up of these patients will allow a better understanding of the evolution in both conditions and real impact on patient and graft survival. Early screening for both conditions and possible therapeutic intervention may represent the best opportunity to prevent or reduce morbidity caused by AGM or MS.
Footnotes
Portions of this work were presented in the poster session at American Transplant Congress, 2008, Toronto, Canada.
F.L.L. participated in study design, data collection and analyses, manuscript preparation and revision; L.J.S. participated in data collection, manuscript preparation, and revision; A.O.O. participated in study design and manuscript preparation.
REFERENCES
- 1.Hjelmesaeth J, Hartmann A, Midtvedt K, et al. Metabolic cardiovascular syndrome after renal transplantation. Nephrol Dial Transplant. 2001;16:1047. doi: 10.1093/ndt/16.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Kuypers DR, Claes K, Bammens B, et al. Early clinical assessment of glucose metabolism in renal allograft recipients: Diagnosis and prediction of post-transplant diabetes mellitus (PTDM) Nephrol Dial Transplant. 2008;23:2033. doi: 10.1093/ndt/gfm875. [DOI] [PubMed] [Google Scholar]
- 3.Kasiske BL, Snyder JJ, Gilbertson D, et al. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3:178. doi: 10.1034/j.1600-6143.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 4.Hjelmesaeth J, Hartmann A, Leivestad T, et al. The impact of early-diagnosed new-onset post-transplantation diabetes mellitus on survival and major cardiac events. Kidney Int. 2006;69:588. doi: 10.1038/sj.ki.5000116. [DOI] [PubMed] [Google Scholar]
- 5.Cosio FG, Kudva Y, van der Velde M, et al. New onset hyperglycemia and diabetes are associated with increased cardiovascular risk after kidney transplantation. Kidney Int. 2005;67:2415. doi: 10.1111/j.1523-1755.2005.00349.x. [DOI] [PubMed] [Google Scholar]
- 6.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 7.Porrini E, Delgado P, Bigo C, et al. Impact of metabolic syndrome on graft function and survival after cadaveric renal transplantation. Am J Kidney Dis. 2006;48:134. doi: 10.1053/j.ajkd.2006.04.078. [DOI] [PubMed] [Google Scholar]
- 8.Roland M, Gatault P, Doute C, et al. Immunosuppressive medications, clinical and metabolic parameters in new-onset diabetes mellitus after kidney transplantation. Transpl Int. 2008;21:523. doi: 10.1111/j.1432-2277.2008.00640.x. [DOI] [PubMed] [Google Scholar]
- 9.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 10.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29(suppl 1):S43. [PubMed] [Google Scholar]
- 12.Sharif A, Moore RH, Baboolal K. The use of oral glucose tolerance tests to risk stratify for new-onset diabetes after transplantation: An under-diagnosed phenomenon. Transplantation. 2006;82:1667. doi: 10.1097/01.tp.0000250924.99855.42. [DOI] [PubMed] [Google Scholar]
- 13.Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: An analysis of six prospective studies. Diabetes. 1997;46:701. doi: 10.2337/diab.46.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glucose tolerance and mortality: Comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet. 1999;354:617. [PubMed] [Google Scholar]
- 15.Poge U, Gerhardt T, Palmedo H, et al. MDRD equations for estimation of GFR in renal transplant recipients. Am J Transplant. 2005;5:1306. doi: 10.1111/j.1600-6143.2005.00861.x. [DOI] [PubMed] [Google Scholar]
- 16.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143. [PubMed] [Google Scholar]
- 17.Meigs JB, Wilson PW, Nathan DM, et al. Prevalence and characteristics of the metabolic syndrome in the San Antonio Heart and Framingham Offspring Studies. Diabetes. 2003;52:2160. doi: 10.2337/diabetes.52.8.2160. [DOI] [PubMed] [Google Scholar]
- 18.Ford ES, Mokdad AH, Giles WH. Trends in waist circumference among U.S. adults. Obes Res. 2003;11:1223. doi: 10.1038/oby.2003.168. [DOI] [PubMed] [Google Scholar]
- 19.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 20.Racusen LC, Colvin RB, Solez K, et al. Antibody-mediated rejection criteria—An addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3:708. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 21.de Vries AP, Bakker SJ, van Son WJ, et al. Metabolic syndrome is associated with impaired long-term renal allograft function not all component criteria contribute equally. Am J Transplant. 2004;4:1675. doi: 10.1111/j.1600-6143.2004.00558.x. [DOI] [PubMed] [Google Scholar]
- 22.Hariharan S, McBride MA, Cherikh WS, et al. Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int. 2002;62:311. doi: 10.1046/j.1523-1755.2002.00424.x. [DOI] [PubMed] [Google Scholar]
- 23.Meier-Kriesche HU, Baliga R, Kaplan B. Decreased renal function is a strong risk factor for cardiovascular death after renal transplantation. Transplantation. 2003;75:1291. doi: 10.1097/01.TP.0000061602.03327.E2. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong KA, Prins JB, Beller EM, et al. Should an oral glucose tolerance test be performed routinely in all renal transplant recipients? Clin J Am Soc Nephrol. 2006;1:100. doi: 10.2215/CJN.00090605. [DOI] [PubMed] [Google Scholar]
- 25.Unwin N, Shaw J, Zimmet P, et al. Impaired glucose tolerance and impaired fasting glycaemia: The current status on definition and intervention. Diabet Med. 2002;19:708. doi: 10.1046/j.1464-5491.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 26.Fujioka K. Metabolic syndrome treatment strategies. Pharmacotherapy. 2006;26:222S. doi: 10.1592/phco.26.12part2.222S. [DOI] [PubMed] [Google Scholar]
- 27.Kasiske BL, Chakkera HA, Roel J. Explained and unexplained ischemic heart disease risk after renal transplantation. J Am Soc Nephrol. 2000;11:1735. doi: 10.1681/ASN.V1191735. [DOI] [PubMed] [Google Scholar]
- 28.Standards of medical care in diabetes–2006. Diabetes Care. 2006;29(suppl 1):S4. [PubMed] [Google Scholar]
- 29.Hjelmesaeth J, Hartmann A, Kofstad J, et al. Glucose intolerance after renal transplantation depends upon prednisolone dose and recipient age. Transplantation. 1997;64:979. doi: 10.1097/00007890-199710150-00008. [DOI] [PubMed] [Google Scholar]
- 30.Duijnhoven EM, Boots JM, Christiaans MH, et al. Influence of tacrolimus on glucose metabolism before and after renal transplantation: A prospective study. J Am Soc Nephrol. 2001;12:583. doi: 10.1681/ASN.V123583. [DOI] [PubMed] [Google Scholar]
- 31.Menegazzo LA, Ursich MJ, Fukui RT, et al. Mechanism of the diabetogenic action of cyclosporin A. Horm Metab Res. 1998;30:663. doi: 10.1055/s-2007-978954. [DOI] [PubMed] [Google Scholar]
- 32.van Duijnhoven EM, Christiaans MH, Boots JM, et al. Glucose metabolism in the first 3 years after renal transplantation in patients receiving tacrolimus versus cyclosporine-based immunosuppression. J Am Soc Nephrol. 2002;13:213. doi: 10.1681/ASN.V131213. [DOI] [PubMed] [Google Scholar]
- 33.Luan FL, Zhang H, Schaubel DE, et al. Comparative risk of impaired glucose metabolism associated with cyclosporine versus tacrolimus in the late posttransplant period. Am J Transplant. 2008;8:1871. doi: 10.1111/j.1600-6143.2008.02328.x. [DOI] [PubMed] [Google Scholar]
- 34.Hjelmesaeth J, Hartmann A, Kofstad J, et al. Tapering off prednisolone and cyclosporin the first year after renal transplantation: The effect on glucose tolerance. Nephrol Dial Transplant. 2001;16:829. doi: 10.1093/ndt/16.4.829. [DOI] [PubMed] [Google Scholar]