Abstract
Endoplasmic reticulum (ER) stress is recognized as a common theme in the development of metabolic syndrome and other diseases. Chronic liver diseases develop ER stress and also show decreased capacity of drug metabolism. The pregnane X receptor (PXR) is a master regulator of genes involved in drug elimination. This study was performed to determine whether ER stress condition decreases the expression of PXR and whether the decrease alters the induction of cytochrome P450 3A4 (CYP3A4). Human primary hepatocytes and HepG2 cell line (human hepatocellular carcinoma) were treated with brefeldin A and thapsigargin, 2 well-established ER stressors. Without exceptions, both stressors significantly decreased the expression of PXR. The decrease led to reduced induction of CYP3A4. Reporter dissection study, electrophoretic mobility shift assay, and chromatin immunoprecipitation located in the PXR promoter region 2 adjacent elements recognized by hepatocyte nuclear factor-4α (HNF-4α) and cytidine-cytidine-adenosine-adenosine-thymidine enhanced binding proteins (C/EBPs), respectively. Additional studies demonstrated that HNF-4α was down-regulated during ER stress but the expression of C/EBPβ varied depending on a particular form of C/EBPβ. Liver-enriched activator protein (LAP) was down-regulated but liver-enriched inhibitory protein (LIP) was highly induced. Nevertheless, over-expression of HNF-4α or LAP restored the expression of PXR. Interestingly, the very same sequence also responded to interleukin-6 (IL-6), and primary hepatocytes treated with thapsigargin significantly increased the level of IL-6 mRNA. These findings establish a functional interconnection between ER stress and signaling of proinflammatory cytokines in the regulation of PXR expression.
Keywords: PXR, ER stress, CYP3A4, proinflammatory cytokines, STAT3
The endoplasmic reticulum (ER) is an organelle involved in diverse cellular functions including protein synthesis/ transportation, membrane generation, calcium concentration regulation, and xenobiotic metabolism (Johnson et al., 2013; Li et al., 2012). Therefore, ER homeostasis is critical in maintaining the overall cellular functions. On the other hand, many factors such as oxidative stress disrupt ER homeostasis, leading to ER stress (Adolph et al., 2012; Calì et al., 2011; Xu and Zhu, 2012). While the precise mechanisms of ER stress remain to be determined, one of the outcomes is the accumulation of unfolded proteins in the ER (Benbrook and Long, 2012; Haeri and Knox, 2012). The unfolded protein response (UPR), occurring at the initial stage of ER stress, is triggered to slow down protein synthesis, improve protein folding capacity, and enhance degradation of unfolded proteins (Lin et al., 2012). Nevertheless, persistent ER stress has been linked to the development of various conditions such as type 2 diabetes and chronic liver diseases (Back and Kaufman, 2012; Bock and Bock-Hennig, 2010; Pagliassotti, 2012; Santoro et al., 2007).
The liver is the largest internal organ and plays the primary role in drug metabolism (Bock and Bock-Hennig, 2010; Santoro et al., 2007; Villarroya et al., 2010). The prevalence of hepatic dysfunction is high and it affects more than 10% of Americans (Liver foundation, 2009). Worldwide, liver cancer and chronic liver diseases are the seventh leading cause of death (IPA, 2007). Many liver diseases are accompanied with ER stress and exhibit decreased capacity of drug metabolism and detoxication. Steatotic livers, for example, were found to have significant decreases in the activity of cytochrome P450 3A4 (CYP3A) (Kolwankar et al., 2007), the most robust catalytic enzyme in the oxidative metabolism of drugs and other xenobiotics. In cultured primary hepatocytes, lipid loading significantly decreased the expression of CYP3A4 (Donato et al., 2007). Furthermore, livers from diabetic patients showed significantly lower expression of CYP3A4 (Dostalek et al., 2011).
The expression of CYP3A4 is regulated by several major transcription factors. Among these proteins, the pregnane X receptor (PXR) has been established to play the primary role (Ihunnah et al., 2011). This receptor forms heterodimer with the retinoid X receptor-α and binds to PXR response elements that contain a half-site AG(G/T)TCA or related sequence. We and other investigators have functionally characterized 4 PXR elements in the CYP3A4 gene and some of the elements operate in a coordinate manner (Goodwin et al., 1999; Liu et al., 2008; Song et al., 2004; Toriyabe et al., 2009). While PXR regulates the expression of CYP3A4 and many other drug-eliminating genes, we have demonstrated that the expression of PXR was altered by drugs and disease mediators (Ma et al., 2005; Shi et al., 2010; Yang et al., 2010). Importantly, the expression of PXR directly affects CYP3A induction. Dexamethasone, a synthetic glucocorticoid, induces PXR and synergistically induces CYP3A (Shi et al., 2010). Likewise, clofibrate, the lipid-lowering agent, causes super-induction of CYP3A23 (Ma et al., 2005). Conversely, interleukin-6 (IL-6), a proinflammatory cytokine, decreases PXR expression and reduce CYP3A4 induction (Yang et al., 2010).
This study was performed to determine whether ER stress condition decreases the expression of PXR and whether the decrease alters the induction of CYP3A4. Brefeldin A (BFA) and thapsigargin (Thaps), 2 well-established ER stressors (Nickel, 2010; Ri et al., 2012; Salido et al., 2009), significantly decreased the expression of PXR in both primary hepatocytes and HepG2 cell line (human hepatocellular carcinoma). The decrease led to reduced induction of CYP3A4. The decrease of PXR expression was achieved by transcriptional repression via 2 adjacent elements recognized by hepatocyte nuclear factor-4α (HNF-4α) and cytidine-cytidine-adenosine-adenosine-thymidine enhanced binding proteins (C/EBPs), respectively. Over-expression of either protein restored the expression of PXR. Interestingly, the adjacent elements also responded to IL-6, suggesting a functional interconnection between ER stress and signaling of proinflammatory cytokines.
MATERIALS AND METHODS
Chemicals and supplies
IL-6 and Thaps were from R&D Systems (Minneapolis, Minnesota). BFA, Hanks balanced salt solution, and the antibody against glyceradehyde-3-phosphate dehydrogenase (GAPDH) were from Sigma (St Louis, Missouri). Dulbecco’s Modified Eagle’s Medium (DMEM) and high fidelity Platinum Taq DNA polymerase were from Life Technology (Carlsbad, California). The antibodies against HNF4α or C/EBPβ were from Abcam Inc (Cambridge, Massachusetts). The goat anti-rabbit IgG conjugated with horseradish peroxidase was from Pierce (Rockford, Illinois). Plated human primary hepatocytes were obtained from the Liver Tissues Procurement and Distribution System (University of Minnesota) or CellzDirect (Pittsboro, North Carolina). Nitrocellulose membranes were from Bio-Rad (Hercules, California). Expression constructs were from OriGene Technologies Inc (Rockville, Maryland). Unless otherwise specified, all other reagents were purchased from Fisher Scientific (Fair Lawn, New Jersey).
Reverse transcription-quantitative polymerase chain reaction
Total RNA (1 µg) was subjected to the synthesis of the first strand cDNA as described previously (Xiao et al., 2013). cDNAs were then diluted 8 times and reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was conducted with TaqMan Gene Expression Assay (Applied Biosystems, Foster City, California). The TaqMan probes were: PXR, Hs00243666_m1; HNF4α, Hs00230853_m1; C/EBPβ, Hs00942496_s1; IL-6, Hs00985639_m1; and GAPDH, 4352934E; and RNA polymerase II, Hs00172187_m1. The normalization of RT-qPCR was performed primarily based on the signal of GAPDH mRNA and selective samples were analyzed for the level of RNA polymerase II to confirm the normalization. The PCR amplification was conducted in a total volume of 20 µl containing universal PCR master mixture (10 µl), gene-specific TaqMan assay mixture (1 µl), and cDNA template (6 µl). The mRNA levels were normalized according to the level of GAPDH and the normalization of selected samples was confirmed based on the signal of RNA polymerase II. Amplification and quantification were done with the Applied Biosystems 7500 Real-Time PCR System.
Reporter constructs and cotransfection assays
PXR promoter reporters were prepared to contain various lengths of PXR genomic sequence. All promoter reporters were subcloned from the PXR-1285-Luc reporter through Mlu I and Xho I sites. All cloning and subcloning were performed by PCR with high fidelity Platinum Taq DNA polymerase. To prepare reporters with a disruption of the element HNF4α, C/EBPβ, or both, oligonucleotides with the wild type or mutant sequences were synthesized and annealed. The resultant double-stranded oligonucleotides were ligated to the pGL3 promoter vector through Nhe I and Xho I sites. The sequences of oligonucleotides for the reporters are shown in Table 1. All reporter constructs were subjected to sequence analysis. To determine the reporter activities, cotransfection in HepG2 cells was performed. Transfection mixtures contained 100 ng of a reporter plasmid and 0.2 ng of CMV-Renilla luciferase plasmid. In some cases, an expression construct was used including HNF4α and C/EBPβ in the transfection mixtures. The corresponding vector was used to equalize the total amount of plasmid DNA in transfection. Typically, cells were transfected for 12 h and the medium was replaced with fresh medium supplemented with 1% fetal bovine serum. The treatment lasted for 24 h and the cells were washed once with phosphate-buffered saline and collected by scraping. The reporter enzyme activities were assayed with a Dual-Luciferase Reporter Assay System as described previously (Yang et al., 2011).
TABLE 1.
Sequences of Oligonucleotides
| Oligonucleotide | Sequence |
|---|---|
| Native promoter reporters (numbered according to Kurose et al. [2005]) | |
| PXR-1285-MluI | 5′-tcctagccctagcagaatcccatgtggata-3′ |
| PXR-505-MluI | 5′-gttctgagatcaaagtggtggggtcacatt-3′ |
| PXR-204-MluI | 5′-atttgccactctcttcccct-3′ |
| PXR-104-MluI | 5′-atttgctagttcaagtgctg-3′ |
| PXR-56-MluI | 5′-gcttagtgcctacatctgac-3′ |
| PXR+14-BamH1 | 5′-gacaagattgtctcatatccggggaaat-3′ |
| Element reporters | |
| PXR-wild type | 5′-gctagttcaagtgctggacttgggacttaggaggggcaatgg-3′ |
| PXR-HNF4α mutant | 5′-gctagttcaagtgctggaaccgggacttaggaggggcaatgg-3′ |
| PXR-C/EBP mutant | 5′-gctagttcaagtgctggacttgggacttaggattttaccggg-3′ |
| PXR-double mutant | 5′-gctagttcaagtgctggaaccgggacttaggattttaccggg-3′ |
| EMSA | |
| PXR-HNF4α | 5′-tagttcaagtgctggacttgggacttagga-3′ |
| PXR-HNF4α (mutant) | 5′-tagttcaagtgctggaaccgggacttagga-3′ |
| PXR-C/EBP | 5′-cttgggacttaggaggggcaatggagccgcttag-3′ |
| PXR-C/EBP(mutant) | 5′-cttgggacttaggattttaccgggagccgcttag-3′ |
| ChIP | |
| Element sense | 5′-gcggatatttgccactctctt-3′ |
| Element reverse | 5′-cggatatgagacaatcttgtc-3′ |
| Non-element sense | 5′-gagtcttttcattgctacctc-3′ |
| Non-element reverse | 5′-tggatgcagagacacagaatg-3′ |
| Semi-quantitative PCR | |
| XBP1-sense | 5′-ttacgagagaaaactcatggcc-3′ |
| XBP1-reverse | 5′-gggtccaagttgtccagaatgc-3′ |
| GAPDH-sense | 5′-agggctgcttttaactctggt-3′ |
| GAPDH-reverse | 5′-ccccacttgattttggaggga-3′ |
Electrophoretic mobility shift assay
The electrophoretic mobility shift assay (EMSA) experiment was performed as described previously (Liu et al., 2008; Yang et al., 2012). Nuclear extracts of HepG2 cells treated with Thaps (10 nM) for 24 h were prepared with the nuclear and cytoplasmic extraction kit (Pierce, Rockford, Illinois). The sense and antisense oligonucleotides (Table 1) were annealed by heating at 94°C for 5 min followed by gradually cooling to room temperature. The sense strand was synthesized as labeled or non-labeled form (for competition). Nuclear protein (5 μg) was incubated with a double-stranded biotinylated probe (0.1 pmol) at room temperature for 20 min. In competition assays, nuclear extracts were first incubated with an unlabeled probe at a 25× or 100× excess for 5 min before addition of the labeled probe. For antibody-disruption assay, the nuclear extracts were first incubated with an antibody against HNF4α or C/EBPβ on ice for 20 min and then with the labeled probe. The protein-DNA complexes were resolved by non-denaturing polyacrylamide gel electrophoresis (5%) and transferred onto a Biodyne nylon membrane. The biotinylated probe was detected with streptavidin-conjugated horseradish peroxidase and chemiluminescent substrate (PIERCE, Rockford, Illinois). The chemiluminescent signal was captured by GelLogic 2200 PRO Imager (Carestream Health, Inc, Rochester, New York).
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) experiment was performed, essentially described previously (Liu et al., 2008). HepG2 Cells were treated with DMSO or Thaps (10 nM) for 24 h, washed and underwent cross-linking for 15 min by 1.0% formaldehyde at room temperature, and the cross-linking was terminated with glycine (final concentration of 125 mM). The soluble chromatins were prepared as described previously (Chen et al., 2012). For ChIP experiment, chromatins were precleared for 2 h at 4°C with protein G beads pretreated with herring sperm DNA (0.2 mg/ml) and BSA (0.5 mg/ml). A fraction of the precleared chromatins was stored at −80°C for later use as an input. An antibody against HNF4α or C/EBPβ was added into the precleared chromatins, and an overnight incubation at 4°C was performed. As a negative control, incubation was performed with pre-immune IgG. The antibody-bound chromatins and DNA input were analyzed by PCR for the presence of the genomic fragments containing the HNF4α or C/EBPβ-bound element with primers shown in Table 1. The PCR was performed with Platinum Taq DNA polymerase for a total of 32 cycles at 94°C for 30 s, 58°C for 30 s, and 68°C for 60 s. A 3-min initial denaturation was performed.
Other analyses
Protein concentrations were determined with BCA assay (Pierce) based on albumin standard. Western blotting was performed as described previously (Shi et al., 2011) and the preparation of the antibody against PXR was described elsewhere (Sachdeva et al., 2003). Data are presented as mean ± SD of at least 3 separate experiments, except where results of blots are shown in which case a representative experiment is depicted in the figures. Statistical significance between 2 means was made according to 1-way ANOVA followed by a DUNCAN’s multiple comparison test (P < 0.05).
RESULTS
Down-regulation of PXR by ER Stressors
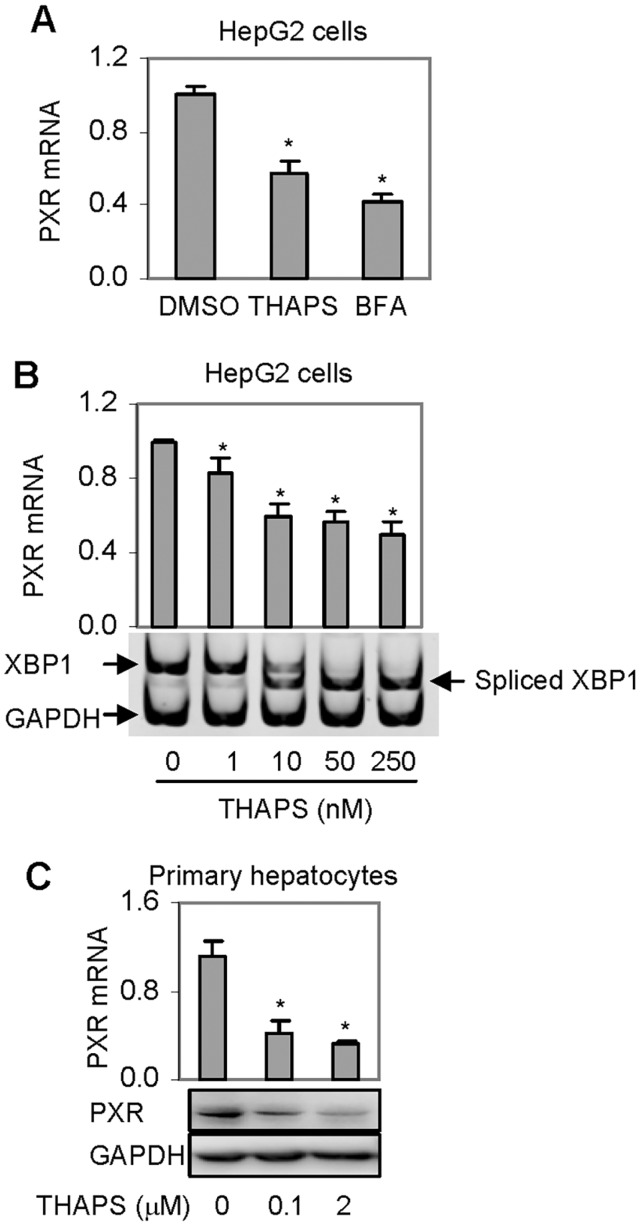
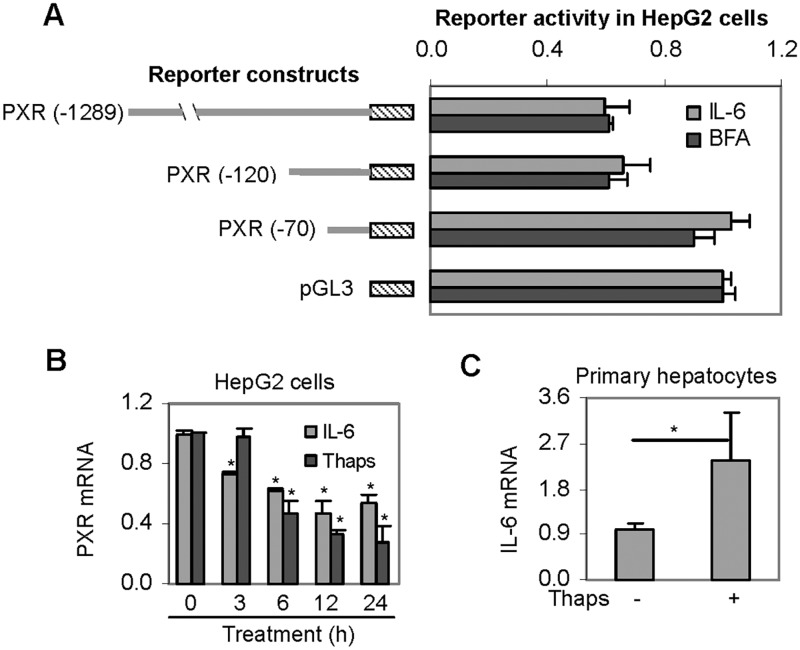
ER stress is a phenomenon in various chronic diseases and many PXR-target genes are down-regulated by disease conditions associated with ER stress (Cali et al., 2011; Johnson et al., 2013). To test whether the expression of PXR itself is decreased during ER stress, HepG2 cells were treated with Thaps and BFA. Thaps induces ER stress by depleting calcium in the ER (Salido et al., 2009), whereas BFA by retrograde-transporting proteins from the Golgi apparatus to the ER (Nickel, 2010). As shown in Figure 1A, treatment with either stressor significantly decreased the level of PXR mRNA. BFA was slightly more potent than Thaps (Fig. 1A). Next, we determined the decrease of PXR mRNA as a function of the amount of Thaps. As shown in Figure 1B, Thaps at 1 nM caused a 20% decrease of PXR mRNA and at 10 nM caused a 40% decrease. Further increased concentrations of Thaps up to 250 nM caused only a 10% additional decrease (Fig. 1B). To ascertain the cellular ER stress level, semi-quantitative PCR was performed to detect the presence of spliced X-box binding protein 1 (XBP1 gene) mRNA, a widely used marker for ER stress (Ri et al., 2012). As shown in Figure 1B (bottom), little spliced XBP1 mRNA was detected in cells treated with solvent or 1 nM Thaps. Comparable levels of spliced and non-spliced XBP1 mRNA were detected in cells treated at 10 nM (Fig. 1B). In contrast, cells treated at 25 or 250 nM exhibited the presence of spliced XBP1 mRNA only. To gain in vivo relevance, primary hepatocytes were treated with Thaps, and the expression of PXR was determined. As shown in Figure 1C, Thaps decreased PXR at both mRNA and protein levels, and the decrease was even greater than that in HepG2 cells (Figs. 1A and 1B).
FIG. 1.
Effect of Thaps or BFA on the expression of PXR. A, Suppression of PXR mRNA by BFA and Thaps in HepG2 cell line. Cells were treated with Thaps and BFA at 1 µM or DMSO for 24 h. The level of PXR mRNA was determined by RT-qPCR. The level of PXR mRNA was normalized according to the level of GAPDH mRNA. Asterisk signs indicate statistical significance from the DMSO control (P < 0.05). B, Suppression of PXR mRNA as a function of Thaps HepG2 cells were treated with Thaps at 0, 1, 10, 50, or 250 nM and the level of PXR mRNA was determined. Asterisk signs indicate statistical significance from the solvent control (P < 0.05). To ascertain the magnitude of ER stress, the presence of spliced XBP1 mRNA was determined by semi-quantitative RT-PCR with the level of GAPDH mRNA as a control. The PCR amplification was performed with 2 pairs of primers designed to target XBP1 and GAPDH. Preliminary study established that these primers did not interfere with each other. C, Suppression of PXR expression in primary hepatocytes Human primary hepatocytes (n = 4) were treated with Thaps at 0.1 or 2 µM for 24 h. Total RNA was analyzed for the level of PXR mRNA by RT-qPCR. Cell lysates (25 µg) from pooled samples were analyzed by Western blotting for the level of PXR protein. Asterisk signs indicate statistical significance from the solvent control (P < 0.05).
Transcriptional Repression of PXR by Thaps
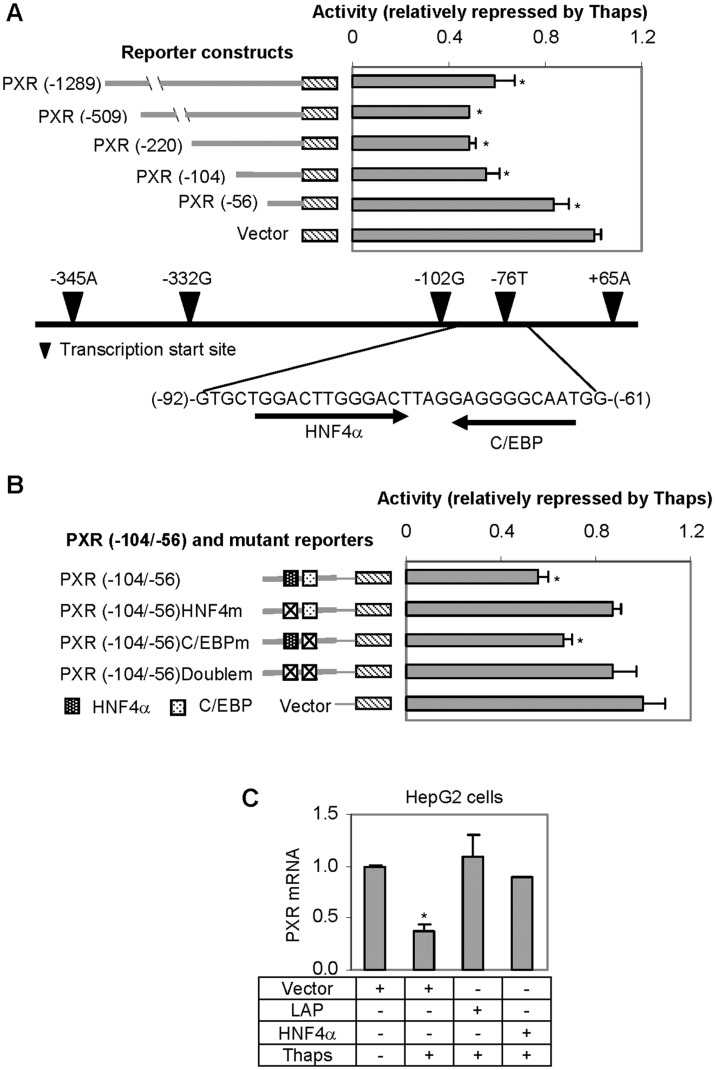
The decreases in PXR mRNA pointed 2 possibilities: ER stressors enhanced PXR mRNA degradation and/or reduced PXR transactivation. To shed light on the second possibility, various PXR reporters containing the promoter or along with up-stream regulatory sequences at varying length were tested for the repression in response to Thaps. As shown in Figure 2A, all PXR reporters, compared with the vector control, were significantly repressed. However, the reporter PXR-56Luc, compared with PXR-104Luc, was repressed to a significantly less extent, suggesting that the DNA segment from −104 to −56 nt is critical for the repression. Based on element predication with computer program, this DNA segment contains an HNF4α and a C/EBP binding site. These 2 elements are spaced by 3 nt (Fig. 2A). It should be noted that as many as 5 transcription start sites (filled triangles in Fig. 2A) are located in the PXR promoter region (Kurose et al., 2005; Tompkins et al., 2008; Zhang et al., 2001).
FIG. 2.
Repression of PXR reporters and reversal of Thaps suppression of PXR expression. A, Repression of PXR promoter reporters HepG2 cells were transiently transfected by FuGene HD with a mixture containing 50 ng of a reporter, or the vector along with 5 ng of the null-Renilla luciferase plasmid. The transfected cells were then treated with DMSO or Thaps at 50 nM for 24 h. Luciferase activities were determined with a Dual-Luciferase Reporter Assay System and the signals were expressed as percentages of the normalized luciferase activity (Thaps over solvent). Below is the diagram with reported transcription start sites numbered according to Kurose et al. (2005). Asterisk signs indicate statistical significance from the vector control (P < 0.05). B, Repression of PXR element reporters HepG2 cells were transfected with an element reporter (wild type of a mutant) and treated as described above. Once gain, the luciferase activity was expressed after normalization. Asterisk signs indicate statistical significance from the vector control (P < 0.05). C, Reversal of Thaps-suppression of PXR by LAP or HNF4α. HepG2 cells were transfected with an expression construct (LAP or HNF4α) or the corresponding vector. After an overnight incubation, the transfected cells were treated with Thaps at 50 nM for 24 h. Cells were collected and total RNA was isolated. The level of PXR mRNA was determined by RT-qPCR. The level of PXR in vector-transfected and DMSO-treated cells was expressed as 100%. Asterisk signs indicate statistical significance from the vector (P < 0.05).
To test whether these 2 elements support Thaps-repression, element reporters were prepared to contain this segment or segment with one or both elements disrupted. The resultant reporters were tested for the abolished response to Thaps. HepG2 cells were transfected with the wild-type or a mutant PXR reporter, treated with DMSO or Thaps at 50 nM for 24 h, and then reporter activity was determined. As shown in Figure 2B, all mutant reporters (disrupted HNF4α, C/EBP, or both elements) showed less repression by Thaps than the wild-type reporter. Specifically, the wild-type reporter was repressed by 45%, the HNF4α-mutant reporter by 14%, the C/EBP mutant by 34%, and the double mutant by 13%. These results suggested that both elements were important for Thaps-repression with the HNF4α element being more efficacious than the C/EBP mutant.
To test whether proteins binding to these elements alter the repressive effect of Thaps on PXR mRNA, HepG2 cells were transfected with HNF4α or liver-enriched activator protein (LAP: a form of C/EBPβ), treated with Thaps, and detected for the level of PXR mRNA. The C/EBP element is known to support the binding for multiple proteins including LAP, a major form of C/EBPβ (Tsukada et al., 2011). Importantly, C/EBPβ proteins have been reported to alter their functionality during ER stress (Arensdorf and Rutkowski, 2013; Li et al., 2008). As shown in Figure 2C, transfection of HNF4α or LAP reversed the decrease of PXR mRNA in Thaps-treated cells (columns 3 and 4 vs column 2). The transfection experiment pointed to 2 important conclusions: both HNF4α and LAP are positive regulators (eg, transactivators) of the PXR gene and they can function independently of each other.
Effect of Thaps on the Expression of HNF4α and C/EBP (LAP and LIP)
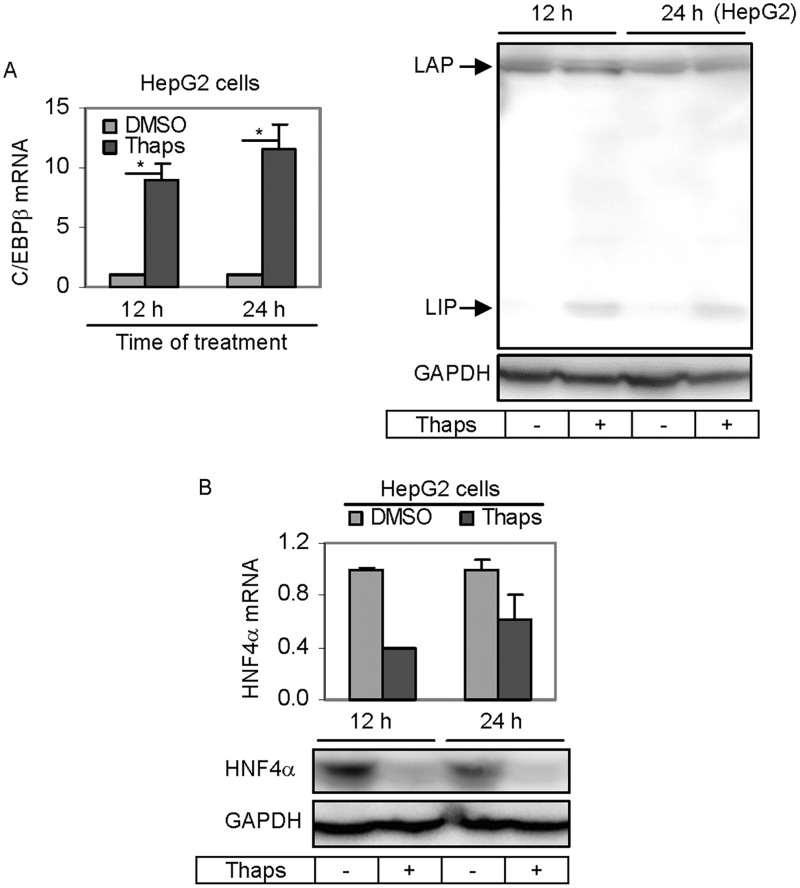
Next we tested whether Thaps decreases PXR expression by down-regulating HNF4α and C/EBPβ. Cells were treated with Thaps and the expression of HNF4α and C/EBPβ was determined by RT-qPCR and Western blotting. As shown in Figure 3A, Thaps surprisingly caused a 9- to 11-fold induction of C/EBPβ mRNA. It has been well established that C/EBPβ mRNA produces several in-frame polypeptides including LAP* (38 kDa), LAP (35 kDa), and liver-enriched inhibitory protein (LIP: 20 kDa) (Tsukada et al., 2011). Importantly, these polypeptides are functionally distinct with LAP* and LAP being activators and LIP being an inhibitor. To determine whether the increased C/EBPβ mRNA by Thaps differentially increases these polypeptides, Western blotting was performed. As shown in Figure 3A (Right), little changes were detected on the levels of LAP and LAP*. In contrast, LIP was markedly increased. These results established that induction of C/EBPβ mRNA by Thaps increased the production of the inhibitor LIP but not the activators LAP* and LAP. The level of HNF4α mRNA, in contrast to the level of C/EBPβ mRNA, was significantly decreased by Thaps (Fig. 3B) and the decrease was less with prolonged treatment. The 12 h time-point showed a 60% decrease whereas the 24 h time-point showed a 40% decrease (Fig. 3B). Consistent with the decrease in HNF4α mRNA, the level of HNF4α protein was drastically decreased (Fig. 3B).
FIG. 3.
Effect of Thaps on the expression of HNF4α and C/EBPβ (LAP*, LAP, and LIP). A, Effect of Thaps on the expression of C/EBPβ (LAP*, LAP, and LIP) HepG2 cells were treated with Thaps at 50 nM for 12 or 24 h. Cells were collected, total RNA was isolated, and lysates were prepared. The level of C/EBPβ mRNA was determined by RT-qPCR (Left). Lysates (20 µg) were resolved by 7.5% SDS-PAGE and transferred electrophoretically to nitrocellulose membranes. The blots were incubated with a carboxylesterase antibody and developed with chemiluminescent substrate and re-probed by GAPDH antibody. The signal was captured by Carestream 2200 PRO Imager. Asterisk signs indicate statistical significance (P < 0.05). B, Effect of Thaps on the expression of HNF4α HepG2 cells was treated and samples were processed as described above. RT-qPCR was performed to determine the level of HNF4α mRNA whereas Western blotting was performed to determine the level of HNF4α protein. Asterisk signs indicate statistical significance (P < 0.05).
Occupancy of the PXR Promoter by HNF4α and C/EBPβ
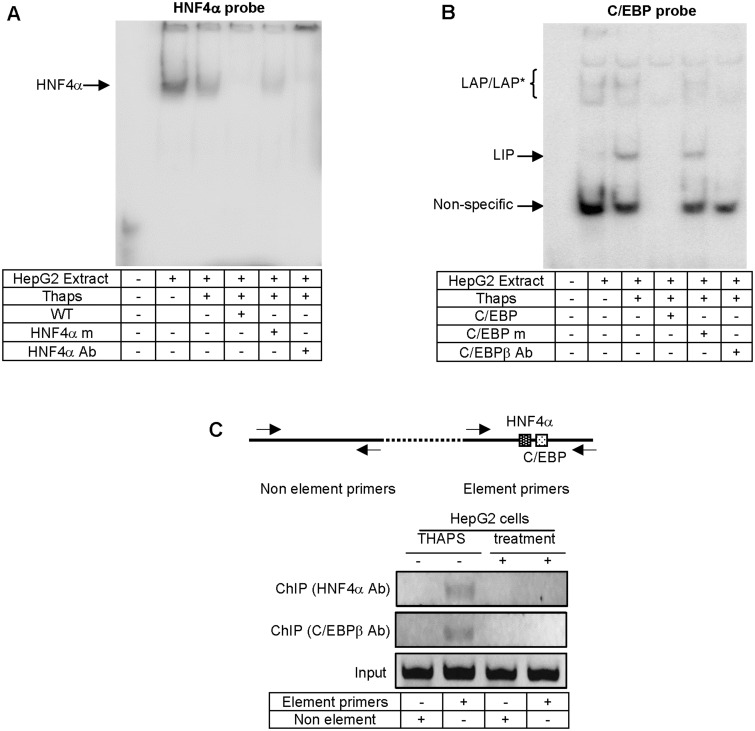
The reporter and expression studies collectively suggested that the PXR promoter is targeted by HNF4α and C/EBPβ. To directly test this possibility, EMSA and ChIP experiments were performed. The EMSA experiment was performed with 2 probes: one containing the putative HNF4α site and the other the C/EBP site. As shown in Figure 4A, incubation with the HNF4α probe led to the detection of a major shifted band (Fig. 4A). The intensity of this band was weaker when incubation was performed with nuclear extracts from Thaps-treated cells, consistent with the fact that Thaps down-regulated HNF4α. This band was competed by non-labeled probe and abolished by the antibody against HNF4α. Addition of the antibody also intensified the band on the top of the gel (Fig. 4A). Incubation with the C/EBPβ probe, on the other hand, led to the detection of several bands (Fig. 4B). Incubation with extracts from Thaps-treated cells produced a new shifted band (probably bound by LIP) and decreased the intensities of 2 shifted bands (arrowed in column 3). All shifted bands except the top one were competed by non-labeled probe but not by the corresponding non-labeled probe with disrupted C/EBP-binding site. Nonetheless, the putative LAP- and LIP-binding bands were abolished by the antibody against C/EBPβ (Fig. 3).
FIG. 4.
Characterization of HNF4α and C/EBP elements by EMSA and ChIP. A, EMSA analysis of HNF4α element. Nuclear extracts (5 μg) from HepG2 cells treated with DMSO or Thaps (50 nM) for 24 h were incubated with a biotinylated HNF4α probe for 20 min. In the competition assay, nuclear extracts were preincubated with the unlabeled element (×100), for 5 min, and then incubated with the biotinylated probe. In the disruption assay, nuclear extracts were incubated first with an antibody against HNF4α on ice for 20 min and then with the biotinylated probe. The protein-DNA complexes were electrophoretically resolved, transferred to a Biodyne nylon membrane and located with streptavidin-conjugated horseradish peroxidase and chemiluminescent substrate. B, EMSA analysis of C/EBPβ element. Incubations were performed as characterization of the HNF4α element. However, the C/EBP probe and an antibody against C/EBPβ were used. C, ChIP analysis HepG2 cells were treated with DMSO or Thaps at 50 nM for 24 h, washed and underwent cross-linking for 15 min by 1% formaldehyde, and the cross-linking was terminated with 125 mM glycine. The soluble chromatins were prepared, precleared with protein G beads, and incubated with an antibody against HNF4α or C/EBPβ. As a control, the antibody was replaced with preimmune IgG. The antibody-bound chromatins and DNA input (1/20 of the antibody-bound chromatins) were analyzed by PCR for the presence of the genomic fragment containing the HNF4α and C/EBPβ adjacent elements. The location of the primers is shown in the diagram and the sequences of primers are shown in Table 1. All experiments in this figure were performed 3 times.
The EMSA experiment established that the PXR promoter contained HNF4α and C/EBP-binding site. Next we tested whether both proteins occupy the PXR promoter. ChIP experiment was performed in cells treated with solvent or Thaps. In addition to HNF4α and C/EBP element-containing segment, a segment of the PXR gene containing either element was subjected to PCR-amplification as a control. As shown in Figure 4C, PCR detected the amplification of both segments with input DNA. However, PCR detected the HNF4α-C/EBP but not the control segment with ChIPed-DNA. The amplification was observed with ChIPed DNA from control but not Thaps-treated cells (Fig. 4C). It should be noted that preimmune IgG for ChIP experiment did not yield any amplification.
Interconnection between Thaps and IL-6 in the Suppression of PXR
We have previously showed that PXR was down-regulated by the proinflammatory cytokine IL-6 (Yang et al., 2010). To determine whether Thaps and IL-6 use similar genomic sequence in the down-regulation, HepG2 cells were transfected with various PXR reporters, treated with IL-6 as shown, and detected for luciferase activity. BFA, another commonly used ER stressor, was also included in this study. As predicted, both IL-6 and BFA produced a similar responding pattern as Thaps among these reporters (Figs. 2A and 5A). Two additional experiments were performed to shed light on the mechanistic connection between ER stress and IL-6. Firstly, the suppression of PXR by IL-6 and Thaps was determined as a function of the time of treatment. Secondly, the expression of IL-6 was determined in Thaps-treated primary hepatocytes. As shown in Figure 5B, both IL-6 and Thaps significantly decreased PXR mRNA. However, the decrease by IL-6 occurred sooner than that by Thaps (Fig. 5B). We next tested whether human primary hepatocytes treated with Thaps actually support the induction of IL-6. As shown in Figure 5C, treatment with Thaps significantly increased IL-6 mRNA (Fig. 5C).
FIG. 5.
Regulated expression of PXR by IL-6. A, Repression of PXR promoter reporters by IL-6 and BFA HepG2 cells were transiently transfected as described in the legend of Figure 2. The transfected cells were then treated with IL-6 (10 ng/ml), BFA (1μM), or the corresponding solvent for 24 h. Luciferase activities were determined with a Dual-Luciferase Reporter Assay System and the signals were expressed as percentages of the normalized luciferase activity of the vector reporter. B, Suppression of PXR mRNA as a function of treatment time by Thaps and IL-6 HepG2 cells were treated with Thaps at 50 nM, IL-6 at10 ng/ml, or the corresponding solvent for 0–24 h. The level of PXR mRNA was determined by RT-qPCR. Asterisk signs indicate statistical significance from the corresponding zero-time point (P < 0.05). C, Effect of Thaps at the level of IL-6 mRNA. Primary hepatocytes (n = 4) were treated with Thaps at 0.1 µM for 24 h. The level of IL-6 mRNA was determined. Asterisk signs indicate statistical significance from the solvent control (P < 0.05).
Effect of ER Stress on CYP3A4 Induction
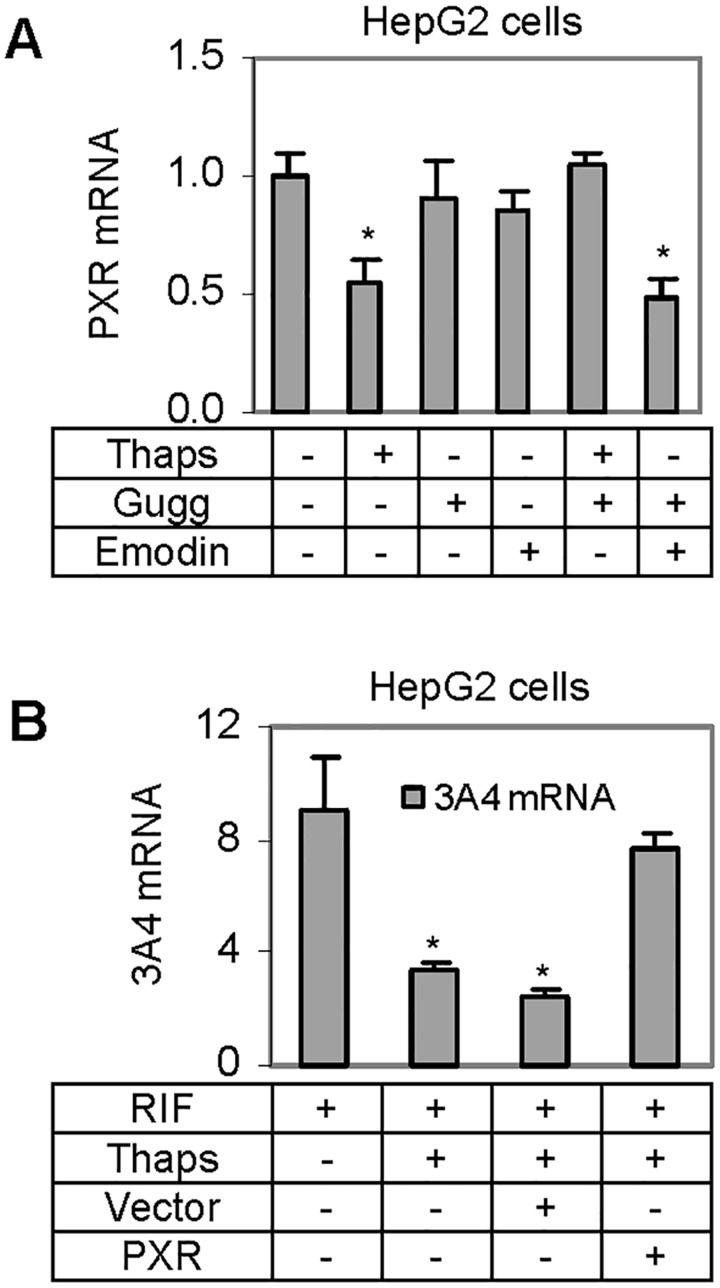
The enhanced production of IL-6 by Thaps suggested that IL-6 is a contributor to Thaps-mediated down-regulation of PXR. It is well established that signal transducer and activator of transcription-3 (STAT3) supports the activity of IL-6 (Bode et al., 2012). We next tested whether Z-guggulsterone, a blocker of STAT3 (Leeman-Neill et al., 2009), antagonizes Thaps in down-regulating PXR. On the other hand, Z-guggulsterone is a known antioxidant (Chen et al., 2012), therefore, emodin was included in this study as a control for antioxidant property (Shia et al., 2010). HepG2 cells were treated with Thaps, Z-guggulsterone, emodin, or in combination, and then the level of PXR mRNA was determined. As expected, Thaps significantly decreased PXR mRNA (Fig. 6A). The decrease, however, was almost completely reversed by Z-guggulsterone but not by emodin. It should be noted that Z-guggulsterone and emodin alone showed no effect on the level of PXR mRNA (Fig. 6A).
FIG. 6.
Interplay of Thaps with antioxidants and rifampicin. A, Reversal of Thaps-mediated suppression of PXR by Z-guggulsterone HepG2 cells were treated Thaps at 50 nM, Z-guggulsterone (10µM), emodin (10µM), or in various combinations. The treatment lasted for 24 h and the expression of PXR mRNA was determined by RT-qPCR. The results were from 3 individual experiments in triplicate. Asterisk signs indicate statistical significance from the solvent control (P < 0.05). B, Effect of PXR transfection on the reduced induction of CYP3A4 by Thaps HepG2 cells were treated with rifampicin (10µM), Thaps (50 nM). Both transfected and non-transfected cells were used. The transfection was performed with a PXR expression construct or the corresponding vector. The treatment lasted for 24 h, cells were collected and total RNA was isolated. The level of CYP3A4 mRNA was determined. The results were expressed as fold of induction. Asterisk signs indicate statistical significance from nontransfected and RIF-treated cells (P < 0.05).
Next we tested whether overexpression of PXR itself reverses the effect of Thaps in terms of the induction of CYP3A4. Both transfected and non-transfected HepG2 cells were used, and the transfection was performed with a PXR expression construct or the corresponding vector. The cells were treated with Thaps, rifampicin, or both for 24 h and analyzed for the mRNA level of CYP3A4, a prototypical target of PXR (Klein and Zanger, 2013). The results were expressed as fold of induction. As shown in Figure 6B, Thaps significantly decreased the induction of CYP3A4 in both vector- and nontransfected cells. However, the decrease was reversed in PXR transfected cells.
DISCUSSION
ER stress is recognized as a common theme in the development of metabolic syndrome and other diseases (Back and Kaufman, 2012; Flamment et al., 2012; Johnson et al., 2013; Lin et al., 2012; Pagliassotti, 2012) and emerging evidence has pointed to decreased capacity of metabolism in liver diseases associated with ER stress (Flamment et al., 2012; Pagliassotti, 2012). PXR is a master regulator of genes in xenobiotic elimination. In this study, we have shown that Thaps and BFA, 2 well-characterized ER stressors significantly decreased the expression of PXR. The decrease was mediated through transcriptional repression and led to reduced induction of CYP3A4, a prototypical target gene of PXR (Klein and Zanger, 2013). The decrease of PXR expression by Thaps was reversed by Z-guggulsterone, an active ingredient of the hypolipidemic herb guggul (Yang et al., 2012).
It is likely that the reversal by Z-guggulsterone was achieved by blocking STAT3 activity. Several lines of evidence support this possibility. Firstly, Z-guggulsterone is an antioxidant and many antioxidants reportedly protect against ER stress (Ding et al., 2013; Li et al., 2012), however, emodin (an antioxidant) showed no reversal activity on the Thaps-mediated downregulation of PXR (Fig. 6A, Harlev et al., 2012), excluding an involvement of the antioxidant property in the reversal of PXR downregulation. Secondly, we have shown that IL-6 and Thaps targeted the same regulatory sequence (Figs. 2A and 5A) and IL-6 is known to activate the STAT3 signaling pathway (Bode et al., 2012). Thirdly, treatment with Thaps induced the expression of IL-6 (Fig. 5C), suggesting that increased expression of IL-6 at least in part plays a role in Thaps-mediated downregulation of PXR. On the other hand, it remains to be determined whether increased expression of IL-6 by Thaps represents a general phenomenon among ER stressors and diseases associated with ER stress. The connection between Thaps and IL-6, nevertheless, provides a mechanistic understanding of how ER stress conditions may exert differential effect on the expression of PXR depending on the increased secretion of cytokines such as IL-6.
STAT3 is a DNA-sequence-specific transcription factor (Bode et al., 2012). However, the PXR promoter regulatory sequence targeted by Thaps and IL-6 does not harbor a consensus STAT3 element. Instead, this sequence contains 2 adjacent elements that were recognized by HNF4α and C/EBP proteins, respectively. It is therefore assumed that STAT3 decreases the expression of PXR by regulating the expression of HNF4α, C/EBPs, or both. While it is not clear whether STAT3 down-regulates HNF4α, it was reported that STAT3 up-regulated the expression of C/EBPβ (Anastasov et al., 2010). Furthermore, STAT3 was shown to interact directly with C/EBPβ. Given the fact that cotransfection of LAP increased PXR expression (Fig. 2C), the STAT3-C/EBPβ complex likely exerts repressive activity. Alternatively, such complex no longer acts on the PXR promoter, thus functioning as a dominant negative in comparison with LAP. The C/EBP family has several members and they all bind to same or similar DNA elements (Tsukada et al., 2010). It is conceivable that other C/EBP members likely participate in the regulated expression of PXR during ER stress.
One of the interesting findings in this study is the unique interplay between C/EBPβ and HNF4α. In the reporter experiment, disruption of the HNF4α element completely eliminated the repressive activity in response to Thaps (Fig. 2B). In contrast, disruption of the C/EBP element diminished the repression to a much lesser extent (Fig. 2B). These observations suggested that HNF4α played an essential or a greater role than a C/EBP protein (probably C/EBPβ) in supporting the expression of PXR. However, transfection of HNF4α surprisingly caused less increases of PXR mRNA than cotransfection of LAP (an active form of C/EBPβ) (Fig. 2C). One explanation is that LAP functioned as a transactivator of HNF4α and/or LAP enhanced the activity of HNF4α. In support of the last possibility, LAP was shown to increase nuclear translocation of HNF4α (Shen et al., 2000).
C/EBPβ mRNA produces several in-frame translated polypeptides including LAP*, LAP, and LIP. Under normal conditions, LAP is the most abundant form. While LAP* and LAP are transactivators, LIP acts as a transcriptional repressor (Tsukada et al., 2010). It is generally accepted that the repressive activity of LIP is achieved by forming non-functional dimmer with C/EBP activating members and/or a DNA-binding dominant negative. It is also accepted that the relative abundance of various C/EBPβ forms (eg, LAP vs LIP) largely depends on the relative efficiency of the initiation codons for translation. Interestingly, Thaps treatment caused an 11-fold increase of C/EBPβ mRNA (Fig. 3A), and yet the increase in proteins was detected on LIP but not LAP or LAP* (Fig. 3A). One explanation is that the initiation codon for LIP was more efficient under ER stress condition induced by Thaps. It has been reported that LAP can be converted into LIP (LAP is bigger than LIP) through proteolytic digestion through an unknown protease. It is likely that such a protease(s) is up-regulated and/or activated by Thaps (Welm et al., 1999). Alternatively, LIP was relatively more stable than LAP in the presence of Thaps (Li et al., 2008).
Nevertheless, EMSA experiment detected increases in DNA binding, apparently by LIP (Fig. 4B). In contrast, the intensity of the shifted bands by LAP and LAP* was slightly decreased in nuclear extracts of cells treated with Thaps. Based on ChIP experiment, however, the increased LIP did not lead to increases in the occupancy of the C/EBP element in the PXR promoter, although the same antibody was used in both EMSA and ChIP experiments. One explanation is that chromatin-bound LIP (ChIP) posed a configuration that hided the epitope from being recognized by this antibody. Alternatively, LIP normally does not bind to the C/EBP element in the PXR promoter under the native condition (ie, cell), although it did so under non-cellular context (ie, EMSA). Nonetheless, cotransfection of LIP indeed conferred potent repressive activity toward the PXR promoter reporter (data not shown).
In summary, our study presents several important conclusions. Firstly, ER stressors decreased the expression of PXR and the induction of CYP3A4, pointing to the possibility of reduced capacity of drug metabolism and detoxication during ER stress condition. Secondly, the decreased expression of PXR was a sequence-specific event through adjacent HNF4α-C/EBP elements. Cotransfection of HNF4α or LAP restored PXR expression, suggesting that factors, altering the activity of these transcription factors, likely affect the expression of PXR and its target genes. Thirdly, ER stressors and IL-6 targeted the same element in repressing PXR, establishing a novel functional link. This is particularly of significance as such connection suggests that ER stress conditions may vary in suppressing PXR expression depending on the enhanced secretion of cytokines such as IL-6.
FUNDING
The National Institutes of Health (grants R01GM061988, R01ES007965, and R15AT007705 to B.Y.).
REFERENCES
- Adolph T. E., Niederreiter L., Blumberg R. S., Kaser A. (2012). Endoplasmic reticulum stress and inflammation. Dig. Dis. 30, 341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasov N., Bonzheim I., Rudelius M., Klier M., Dau T., Angermeier D., Duyster J., Pittaluga S., Fend F., Raffeld M., Quintanilla-Martinez L. (2010). C/EBPβ expression in ALK-positive anaplastic large cell lymphomas is required for cell proliferation and is induced by the STAT3 signaling pathway. Haematologica 95, 760–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arensdorf A. M., Rutkowski D. T. (2013). Endoplasmic reticulum stress impairs IL-4/IL-13 signaling through C/EBPβ-mediated transcriptional suppression. J. Cell Sci. 126(Pt 17), 4026–4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back S. H., Kaufman R. J. (2012). Endoplasmic reticulum stress and type 2 diabetes. Annu. Rev. Biochem. 81, 767–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrook D. M., Long A. (2012). Integration of autophagy, proteasomal degradation, unfolded protein response and apoptosis. Exp. Oncol. 34, 286–297. [PubMed] [Google Scholar]
- Bock K. W., Bock-Hennig B. S. (2010). UDP-glucuronosyltransferases (UGTs): from purification of Ah-receptor-inducible UGT1A6 to coordinate regulation of subsets of CYPs, UGTs, and ABC transporters by nuclear receptors. Drug Metab. Rev. 42, 6–13. [DOI] [PubMed] [Google Scholar]
- Bode J. G., Albrecht U., Häussinger D., Heinrich P. C., Schaper F. (2012). Hepatic acute phase proteins—regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-κB-dependent signaling. Eur. J. Cell Biol. 91, 496–505. [DOI] [PubMed] [Google Scholar]
- Calì T., Ottolini D., Brini M. (2011). Mitochondria, calcium, and endoplasmic reticulum stress in Parkinson’s disease. Biofactors 37, 228–240. [DOI] [PubMed] [Google Scholar]
- Chen Y. Z., Shi D., Yang D., Yan B. (2012). Antioxidant sulforaphane and sensitizer trinitrobenzene sulfonate induce carboxylesterase-1 through a novel element transactivated by nuclear factor-E2 related factor-2. Biochem. Pharmacol. 84, 864–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Dai X., Jiang Y., Zhang Z., Bao L., Li Y., Zhang F., Ma X., Cai X., Jing L., et al. (2013). Grape seed proanthocyanidin extracts alleviate oxidative stress and ER stress in skeletal muscle of low-dose streptozotocin- and high-carbohydrate/high-fat diet-induced diabetic rats. Mol. Nutr. Food Res. 57, 365–369. [DOI] [PubMed] [Google Scholar]
- Donato M. T., Jiménez N., Serralta A., Mir J., Castell J. V., Gómez-Lechón M. J. (2007). Effects of steatosis on drug-metabolizing capability of primary human hepatocytes. Toxicol. in vitro 21, 271–276. [DOI] [PubMed] [Google Scholar]
- Dostalek M., Court M. H., Yan B., Akhaghi F. (2011). Significantly reduced cytochrome P4503A4 expression and activity in liver from human with diabetes mellitus. Br. J. Pharmacol. 163, 937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamment M., Hajduch E., Ferré P., Foufelle F. (2012). New insights into ER stress-induced insulin resistance. Trends Endocrinol. Metab. 23, 381–390. [DOI] [PubMed] [Google Scholar]
- Goodwin B., Hodgson E., Liddle C. (1999). The orphan human pregnane X receptor mediates the transcriptional activation of CYP3A4 by rifampicin through a distal enhancer module. Mol. Pharmacol. 56, 1329–1339. [DOI] [PubMed] [Google Scholar]
- Haeri M., Knox B. E. (2012). Endoplasmic reticulum stress and unfolded protein response pathways: potential for treating age-related retinal degeneration. J. Ophthalmic Vis. Res. 7, 45–59. [PMC free article] [PubMed] [Google Scholar]
- Harlev E., Nevo E., Lansky E. P., Ofir R., Bishayee A. (2012). Anticancer potential of aloes: antioxidant, antiproliferative, and immunostimulatory attributes. Planta Med. 78, 843–852. [DOI] [PubMed] [Google Scholar]
- Ihunnah C. A., Jiang M., Xie W. (2011). Nuclear receptor PXR, transcriptional circuits and metabolic relevance. Biochim. Biophys. Acta 1812, 956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPA. (2007). Top 20 Causes of Mortality Throughout the World. Available at: http://www.infoplease.com/ipa/A0779147.html.
- Johnson N., Powis K., High S. (2013). Post-translational translocation into the endoplasmic reticulum. Biochim. Biophys. Acta 1833, 2403–2409. [DOI] [PubMed] [Google Scholar]
- Klein K., Zanger U. M. (2013). Pharmacogenomics of cytochrome P450 3A4: recent progress toward the “missing heritability” problem. Front. Genet. 4, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolwankar D., Vuppalanchi R., Ethell B., Jones D. R., Wrighton S. A., Hall S. D., Chalasani N. (2007). Association between nonalcoholic hepatic steatosis and hepatic cytochrome P-450 3A activity. Clin. Gastroenterol. Hepatol. 5, 388–393. [DOI] [PubMed] [Google Scholar]
- Kurose K., Koyano S., Ikeda S., Tohkin M., Hasegawa R., Sawada J. (2005). 5′ diversity of human hepatic PXR (NR1I2) transcripts and identification of the major transcription initiation site. Mol. Cell Biochem. 273, 79–85. [DOI] [PubMed] [Google Scholar]
- Leeman-Neill R. J., Wheeler S. E., Singh S. V., Thomas S. M., Seethala R. R., Neill D. B., Panahandeh M. C., Hahm E. R., Joyce S. C., Sen M., et al. (2009). Guggulsterone enhances head and neck cancer therapies via inhibition of signal transducer and activator of transcription-3. Carcinogenesis 30, 1848–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Wang L., Huang K., Zheng L. (2012). Endoplasmic reticulum stress in retinal vascular degeneration: protective role of resveratrol. Invest. Ophthalmol. Vis. Sci. 53, 3241–3249. [DOI] [PubMed] [Google Scholar]
- Li Y., Bevilacqua E., Chiribau C. B., Majumder M., Wang C., Croniger C. M., Snider M. D., Johnson P. F., Hatzoglou M. (2008). Differential control of the CCAAT/enhancer-binding protein beta (C/EBPbeta) products liver-enriched transcriptional activating protein (LAP) and liver-enriched transcriptional inhibitory protein (LIP) and the regulation of gene expression during the response to endoplasmic reticulum stress. J. Biol. Chem. 283, 22443–22456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S., Sun S., Hu J. (2012). Molecular basis for sculpting the endoplasmic reticulum membrane. Int. J. Biochem. Cell Biol. 44, 1436–1443. [DOI] [PubMed] [Google Scholar]
- Liu F., Yang D., Song X., Deng R., Yan B. (2008). The far and distal enhancers in the CYP3A4 gene coordinates the proximal promoter in responding to the pregnane X receptor similarly but differentially to hepatocyte nuclear factor-4α. Biochem. J. 409, 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liver Foundation. (2009). More than 30 million people in the U.S. have liver disease - or 1 in 10 Americans. Available at: http://www.liverfounda tion.org/chapters/lam2009.
- Ma Y., Song X., Sachdeva K., Liu J., Li Y., Yang D., Deng R., Chichester C. O., Yan B. (2005). Clofibrate and perfluorodecanoate both up-regulate the expression of the pregnane X receptor but only clofibrate enhances its ligand-dependent induction of cytochrome P4503A23. Biochem. Pharmacol. 69, 1363–1371. [DOI] [PubMed] [Google Scholar]
- Nickel W. (2010). Pathways of unconventional protein secretion. Curr. Opin. Biotechnol. 21, 621–626. [DOI] [PubMed] [Google Scholar]
- Pagliassotti M. J. (2012). Endoplasmic reticulum stress in nonalcoholic fatty liver disease. Annu. Rev. Nutr. 32, 17–33. [DOI] [PubMed] [Google Scholar]
- Ri M., Tashiro E., Oikawa D., Shinjo S., Tokuda M., Yokouchi Y., Narita T., Masaki A., Ito A., Ding J., et al. (2012). Identification of Toyocamycin, an agent cytotoxic for multiple myeloma cells, as a potent inhibitor of ER stress-induced XBP1 mRNA splicing. Blood Cancer J. 2, e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva K., Yan B., Chichester C. O. (2003). Lipopolysaccharide and cecal ligation/puncture differentially affect the subcellular distribution of the pregnane X receptor but consistently cause suppression of its target gene CYP3A. Shock 19, 470–475. [DOI] [PubMed] [Google Scholar]
- Salido G. M., Sage S. O., Rosado J. A. (2009). Biochemical and functional properties of the store-operated Ca2+ channels. Cell Signal. 21, 457–461. [DOI] [PubMed] [Google Scholar]
- Santoro A., Mancini E., Ferramosca E., Faenza S. (2007). Liver support systems. Contrib. Nephrol. 156, 396–404. [DOI] [PubMed] [Google Scholar]
- Shen C. N., Slack J. M., Tosh D. (2000). Molecular basis of transdifferentiation of pancreas to liver. Nat. Cell Biol. 2, 879–887. [DOI] [PubMed] [Google Scholar]
- Shi D., Yang D., Yan B. (2010). Dexamethasone transcriptionally increases the expression of the pregnane X receptor and synergistically enhances pyrethroid deltamethrin in the induction of cytochrome P450 3A23. Biochem. Pharmacol. 80, 1274–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D., Yang D., Prinssen E. P., Davies B. E., Yan B. (2011). Surge in expression of carboxylesterase-1 during the post-natal stage enables a rapid gain of the capacity to activate the anti-influenza prodrug oseltamivir. J. Infect. Dis. 203, 937–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shia C. S., Hou Y. C., Tsai S. Y., Huieh P. H., Leu Y. L., Chao P. D. (2010). Differences in pharmacokinetics and ex vivo antioxidant activity following intravenous and oral administrations of emodin to rats. J. Pharm. Sci. 99, 2185–2195. [DOI] [PubMed] [Google Scholar]
- Song X., Xie M., Zhang H., Li Y., Sachdeva K., Yan B. (2004). The pregnane X receptor binds to response elements in a genomic context-dependent manner and its activator rifampicin selectively alters the bindings among target genes. Drug Metab. Dispos. 32, 35–42. [DOI] [PubMed] [Google Scholar]
- Tompkins L. M., Sit T. L., Wallace A. D. (2008). Unique transcription start sites and distinct promoter regions differentiate the pregnane X receptor (PXR) isoforms PXR 1 and PXR 2. Drug Metab. Dispos. 36, 923–929. [DOI] [PubMed] [Google Scholar]
- Toriyabe T., Nagata K., Takada T., Aratsu Y., Matsubara T., Yoshinari K., Yamazoe Y. (2009). Unveiling a new essential cis element for the transactivation of the CYP3A4 gene by xenobiotics. Mol. Pharmacol. 75, 677–684. [DOI] [PubMed] [Google Scholar]
- Tsukada J., Yoshida Y., Kominato Y., Auron P. E. (2011). The CCAAT/enhancer (C/EBP) family of basic-leucine zipper (bZIP) transcription factors is a multifaceted highly-regulated system for gene regulation. Cytokine 54, 6–19. [DOI] [PubMed] [Google Scholar]
- Villarroya F., Domingo P., Giralt M. (2010). Drug-induced lipotoxicity: lipodystrophy associated with HIV-1 infection and antiretroviral treatment. Biochim. Biophys. Acta 801, 392–399. [DOI] [PubMed] [Google Scholar]
- Welm A. L., Timchenko N. A., Darlington G. J. (1999). C/EBPalpha regulates generation of C/EBPbeta isoforms through activation of specific proteolytic cleavage. Mol. Cell Biol. 19, 1695–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D., Yang D., Charpentier M., Yan B. (2013). Regulation of carboxylesterase-2 expression by p53 family proteins and enhanced anticancer activities among 5-fluorouracil, irinotecan and doxazolidine prodrug. Br. J. Pharmacol. 168, 1989–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Zhu X. P. (2012). Endoplasmic reticulum stress and prion diseases. Rev. Neurosci. 23, 79–84. [DOI] [PubMed] [Google Scholar]
- Yang J., Hao C., Yang D., Shi D., Song X., Luan X., Hu G., Yan B. (2010). Pregnane X receptor is required for interleukin-6-mediated down-regulation of cytochrome P450 3A4 in human hepatocytes. Toxicol. Lett. 197, 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Shi D., Yang J., Deng R., Yan B. (2011). Scoparone potentiates transactivation of the bile salt export pump gene and this effect is enhanced by cytochrome P450 metabolism but abolished by a PKC inhibitor. Br. J. Pharmacol. 164, 1547–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Yang J., Shi D., Black C., Deng R., Yan B. (2012). The hypolipidemic agent Z-guggulsterone: metabolism interplays with induction of cholesteryl ester hydrolase CES1 and bile salt export pump. J. Lipid Res. 53, 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Kuehl P., Green E. D., Touchman J. W., Watkins P. B., Daly A., Hall S. D., Maurel P., Relling M., Brimer C., et al. (2001) The human pregnane X receptor: genomic structure and identification and functional characterization of natural allelic variants. Pharmacogenetics 11, 555–572. [DOI] [PubMed] [Google Scholar]