Abstract
Objective. The aim of this study was to determine whether biologic-free remission of RA is possible with discontinuation of abatacept.
Methods. Japanese RA patients in 28-joint DAS with CRP (DAS28-CRP) remission (<2.3) after >2 years of abatacept treatment in a phase II study and its long-term extension entered this 52 week, multicentre, non-blinded, prospective, observational study. At enrolment, the patients were offered the option to continue abatacept or not. The primary endpoint was the proportion of patients who remained biologic-free at 52 weeks after discontinuation. Clinical, functional and structural outcomes were compared between those who continued and those who discontinued abatacept.
Results. Of 51 patients enrolled, 34 discontinued and 17 continued abatacept treatment. After 52 weeks, 22 of the 34 patients (64.7%) remained biologic-free. Compared with the continuation group, the discontinuation group had a similar remission rate (41.2% vs 64.7%, P = 0.144) although they had a significantly higher mean DAS28-CRP score at week 52 (2.9 vs 2.0, P = 0.012). The two groups were also similar with regard to mean HAQ Disability Index (HAQ-DI) score (0.6 for both, P = 0.920), mean change in total Sharp score (ΔTSS; 0.80 vs 0.32, P = 0.374) and proportion of patients in radiographic remission (ΔTSS ≤ 0.5) at the endpoint (64.3% vs 70.6%, P = 0.752). Those attaining DAS28-CRP < 2.3 or < 2.7 without abatacept at the endpoint had significantly lower HAQ-DI score and/or CRP at enrolment. Non-serious adverse events occurred in three patients who continued or resumed abatacept.
Conclusion. Biologic-free remission of RA is possible in some patients after attaining clinical remission with abatacept. Lower baseline HAQ-DI or CRP may predict maintenance of remission or low disease activity after discontinuation of abatacept.
Trial registration: UMIN Clinical Trials Registry, http://www.umin.ac.jp/ctr/ (UMIN000004137).
Keywords: rheumatoid arthritis, abatacept, biologic-free remission, observational study
Introduction
RA is a systemic inflammatory disease characterized by polyarthritis and progressive joint destruction. In RA, synovial monocyte-/macrophage-like cells and dendritic cells serve as antigen-presenting cells (APCs) due to their expression of antigen–MHC class II complexes and co-stimulatory molecules such as CD80 and CD86 [1]. Activated CD4+ T cells expressing CD28 significantly infiltrate into the synovial membrane of affected joints and exacerbate synovitis and joint destruction by secreting inflammatory cytokines and activating synovial cells and osteoclasts [2–4]. The activation of CD4+ T cells is therefore an important stage in the development of rheumatic synovitis, with the CD28-mediated co-stimulatory signal being required for full T cell activation and playing a major role in the immunopathological process of RA.
Abatacept is a genetically engineered humanized fusion protein consisting of the extracellular domain of human cytotoxic T lymphocyte-associated molecule 4 (CTLA-4) connected to a modified Fc region (hinge-CH2-CH3 domain) of human immunoglobulin G-1. Abatacept is a novel anti-rheumatic drug that acts by modulating the activation of naive T cells through the competitive binding of co-stimulation molecules expressed on APCs (CD80 and CD86) and blockade of CD4+ T cell co-stimulation via CD28 [5].
Abatacept has been reported to control disease activity, prevent or delay joint destruction and improve quality of life [6–12]. Further, abatacept exhibits similar efficacy in Japanese MTX-intolerant patients with active RA, achieving clinical remission [28-joint DAS with CRP (DAS28-CRP) <2.6] in 24.6% of patients after 24 weeks [7]. Due to the high cost of biologic DMARDs and concerns regarding their long-term safety, the potential for biologic-free remission has been identified as an issue for further investigation [13, 14]. No previous studies have addressed this potential therapeutic application of abatacept despite evidence of its ability to suppress CD4+ T cell activation in autoimmune diseases such as RA.
Thus we conducted the present study in Japanese RA patients who had completed a phase II study of abatacept [7] and its long-term extension in order to determine whether clinical remission attained with the drug was sustained following its discontinuation.
Methods
Before enrolment in this study, written informed consent was obtained from each participating patient according to the Declaration of Helsinki (updated 2008). Prior to the start of the study, the institutional review board of each centre reviewed and approved the study.
Study design and patients
In the previous phase II study [7], 194 Japanese RA patients received double-blind treatment with abatacept or placebo for 24 weeks in addition to prior MTX therapy and 174 of them entered its long-term extension and received open-label abatacept for a mean of 37.7 months (range 3.6–45.1). Those who had completed the phase II study [7] and its long-term extension were eligible for this multicentre, non-blinded, prospective, observational study if they were in clinical remission (DAS28-CRP < 2.3) and not receiving any other biologic therapy at enrolment. Inclusion criteria for the phase II study were age ≥20 years; fulfilment of the 1987 ACR criteria for the diagnosis of RA with a functional status of class I, II or III; previous treatment with MTX at 6–8 mg/week for at least 12 weeks and one or more of the following: ≥10 swollen joints (66-joint count), ≥12 tender joints (68-joint count) or CRP ≥ 1.0 mg/dl.
Procedures
At enrolment, patients were offered the option to continue or discontinue abatacept during the study. Those who discontinued abatacept treatment (discontinuation group) were periodically followed up for disease activity. Those who chose to continue abatacept (continuation group) were treated with the drug every 4 weeks at its approved dosage and received similar follow-up. Abatacept could be restarted at a fixed dose of 10 mg/kg in response to a sign of relapse (DAS28-CRP > 2.7 at two consecutive visits) or at the investigator’s discretion. If restarted after an interval of ≤12 weeks, administration was every 4 weeks, whereas if started after an interval of >12 weeks, the first two doses were administered every 2 weeks and subsequent doses every 4 weeks.
During the study, dose modifications of non-biologic DMARDs (e.g. MTX) and glucocorticoids were allowed at the investigator’s discretion. Concomitant administration of NSAIDs was permitted, but that of biologic agents was not.
Efficacy outcomes
The primary outcome measure of this study was the proportion of patients who remained biologic-free at 52 weeks after discontinuation of abatacept. Secondary and tertiary outcomes were efficacy and safety, respectively.
RA disease activity was assessed in terms of DAS28-CRP and DAS28-ESR at weeks 0, 4, 12, 24, 36 and 52. If a patient resumed abatacept treatment, this assessment was made at the time of resumption as well as after 12 and 24 weeks.
In accordance with DAS28-CRP scores, disease activity was classified as remission ( < 2.3), low (≤2.3 to <2.7), moderate (≤2.7 to <4.1) or high (≥4.1) [15]. The proportion of patients in each disease activity class at each specified time and the proportion of patients in DAS28-CRP remission (<2.3) at week 52 were calculated.
Similarly, disease activity was classified by DAS28-ESR as remission (<2.6), low (LDA; ≤2.6 to <3.2), medium (MDA; ≤3.2 to <5.1) or high (HAD; ≥5.1) [15]. To assess disease impact on a patient’s level of functional ability, the HAQ Disability Index (HAQ-DI) was determined at weeks 0, 4, 12, 24, 36 and 52.
Radiographic progression of joint destruction was assessed in terms of van der Heijde–modified total Sharp score (mTSS) [16, 17] at weeks 0 and 52 or at the time of withdrawal from the study, where possible. Changes from baseline in TSS (ΔTSS), joint erosion (ΔJE) score and joint space narrowing (ΔJSN) score at week 52 were determined. The proportion of patients with no (ΔTSS ≤ 0), little (ΔTSS ≤ 0.5; defined as radiographic remission) and rapid radiographic progression (RRP; ΔTSS ≥5) [18] was calculated.
Time to abatacept treatment resumption
The mean time to resumption of abatacept treatment was determined in the discontinuation group.
Safety
Patients remaining on abatacept were monitored for adverse events (AEs) throughout the study period. In the discontinuation group, AE monitoring was done only if and after abatacept was resumed following relapse. To investigate the relationship between the immunogenicity of abatacept and its tolerability, the anti-abatacept antibody titre in blood was measured at the time of discontinuation, time of resumption and 24 weeks after resumption of abatacept, if applicable.
Statistical analysis
Missing data were imputed by linear extrapolation (radiographic assessments) or last observation carried forward (LOCF) (other efficacy variables). Continuous metric data were summarized in terms of descriptive statistics and were expressed as the mean (s.d.). Data between the two groups were compared using Wilcoxon’s rank sum test (demographic and baseline characteristics, DAS28, HAQ-DI, ΔTSS, ΔJE and ΔJSN) or Fisher’s exact test (proportion of patients in DAS28-CRP remission at week 52 and the proportions of patients with ΔTSS ≤0, ≤0.5 and ≥5).
Results
Patient disposition and baseline characteristics
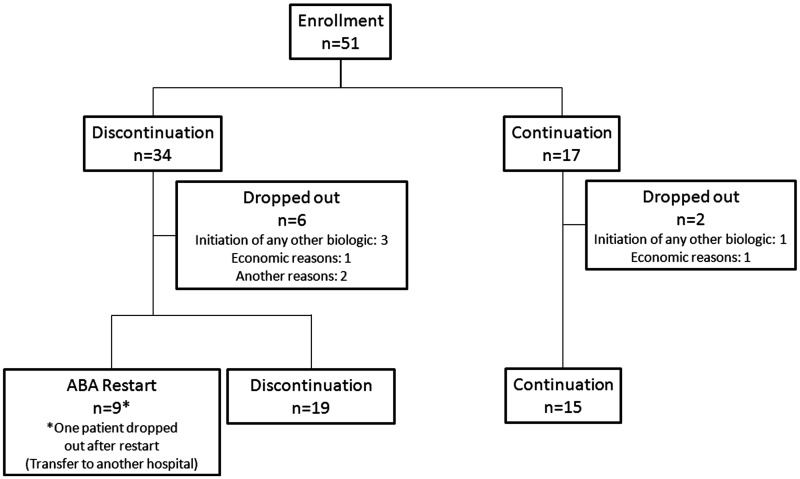
Fifty-one consenting patients were enrolled and chose to either discontinue (n = 34) or continue (n = 17) abatacept. Nine of the 34 patients from the discontinuation group restarted abatacept at the investigator’s discretion (n = 8) or due to relapse (n = 1). Six patients from the discontinuation group (with an additional patient withdrawn after resumption) and two from the continuation group dropped out of the study, leaving a total of 28 and 15 patients, respectively. Nineteen patients from the discontinuation group remained biologic-free at week 52 (Fig. 1). The demographic and baseline characteristics of the 51 patients enrolled are summarized in Table 1. The two groups had comparable baseline characteristics, except for significantly shorter disease duration and significantly less joint damage in terms of JSN and TSS in those who discontinued abatacept at enrolment (P < 0.05 for all comparisons).
Fig. 1.
Patient disposition
Table 1.
Patient characteristics
| Discontinuation (n = 34) | Continuation (n = 17) | P-value | |
|---|---|---|---|
| Age, mean (s.d.), years | 56.9 (11.4) | 60.9 (9.5) | 0.195a |
| Male, n (%) | 5 (14.7) | 4 (23.5) | 0.443b |
| Female, n (%) | 29 (85.3) | 13 (76.5) | |
| RA disease duration, mean (s.d.), years | 9.6 (5.2) | 15.3 (10.5) | 0.018a |
| DAS28-CRP, mean (s.d.) | 1.8 (0.4) | 1.7 (0.5) | 0.803a |
| Tender joint count (0–28), mean (s.d.) | 0.3 (0.6) | 0.1 (0.5) | 0.788a |
| Swollen joint count (0–28), mean (s.d.) | 0.5 (0.8) | 0.6 (0.9) | 0.429a |
| HAQ-DI, mean (s.d.) | 0.5 (0.5) | 0.5 (0.5) | 0.356a |
| CRP, mean (s.d.), mg/dl | 0.3 (0.5) | 0.2 (0.2) | 0.285a |
| ESR, mean (s.d.), mm/h | 18.7 (9.5) | 17.6 (8.5) | 0.790a |
| DAS28-ESR, mean (s.d.) | 2.4 (0.5) | 2.3 (0.6) | 0.705a |
| MMP-3, mean (s.d.), ng/ml | 79.5 (63.3)c | 75.3 (46.3)d | 0.707a |
| RF, mean (s.d.), IU/ml | 72.8 (128.5)c | 50.7 (76.1)e | 0.822a |
| RF positive, n (%) | 14 (48.3)c | 6 (60.0)e | 0.394b |
| PGA (0–100 mm VAS), mean (s.d.) | 12.7 (10.7) | 17.4 (15.2) | 0.363a |
| Erosion, mean (s.d.) | 29.9 (37.9)f | 62.0 (58.4) | 0.015a |
| Joint space narrowing, mean (s.d.) | 28.6 (27.2)f | 55.5 (41.2) | 0.020a |
| TSS (0–448), mean (s.d.) | 58.5 (64.1)f | 117.5 (97.7) | 0.016a |
| Concomitant use of MTX, n (%) | 19 (55.9) | 12 (70.6) | 1.000a |
| MTX dose, mean (s.d.), mg/week | 6.7 (2.2)g | 8.7 (2.3)h | 0.211a |
| Concomitant use of PSL, n (%) | 12 (35.3) | 8 (47.1) | 0.372a |
| PSL dose, mean (s.d.), mg/day | 4.0 (2.8)i | 3.9 (2.8)j | 0.538a |
PGA: patient’s global assessment of disease activity; VAS: visual analogue scale; RF: rheumatoid factor; TSS: total Sharp score; PSL: prednisolone. aWilcoxon’s rank sum test; bFisher's exact test; cn = 29; dn = 14; en = 10; fn = 28; gn = 17; hn = 12; in = 9; jn = 8.
Efficacy outcomes
Of the 34 patients who discontinued abatacept at enrolment, 22 patients from an intention-to-treat (ITT) analysis (64.7%) remained biologic-free after 52 weeks. While the mean DAS28-CRP score remained constant in the continuation group, it gradually increased over time in the discontinuation group, leading to a significant difference between the groups at week 52 (2.9 vs 2.0, P = 0.012).
This was also true when the subgroup of discontinuing patients who remained in the study and never restarted abatacept (n = 19) were compared with the continuing patients remaining in the study (n = 15; 2.8 vs 2.1, P = 0.036).
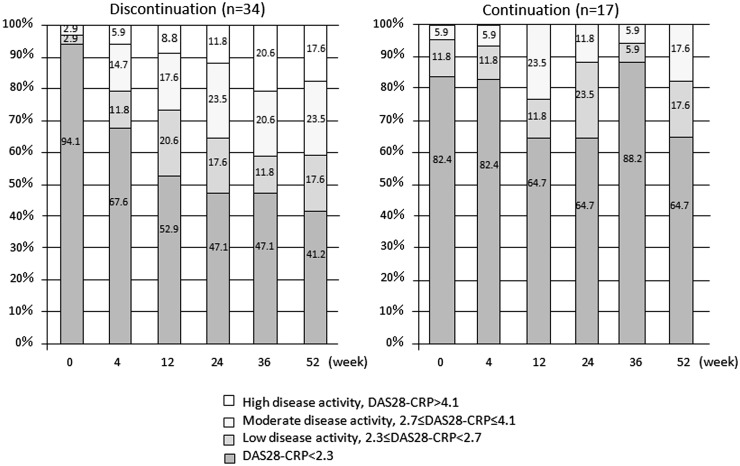
Fig. 2 shows the proportion of patients in each RA disease activity class at specified times. In the discontinuation group there was a tendency towards a decrease in the proportion of patients in DAS28-CRP remission and an increase in the proportion of those with HDA as follow-up progressed. At week 52 (LOCF), the proportion of patients in remission was 41.2% in the discontinuation group compared with 64.7% in the continuation group (P = 0.144). Sixteen of the 17 continuing patients (94.1%) experienced no disease flare (DAS28-CRP < 2.7), while 20 of the 34 discontinuing patients (58.8%) were in remission or maintained LDA. Compared with the 14 patients who failed to do so, these 20 patients had significantly lower baseline HAQ-DI scores and CRP (P = 0.036 and P = 0.048, respectively). Of the 19 patients who went without abatacept for 52 weeks, 7 were in remission at the endpoint and 12 were not. These two subgroups had comparable baseline characteristics, except that more patients in remission than not in remission at the endpoint were in functional remission (HAQ-DI ≤ 0.5) at enrolment (100% vs 41.7%, P = 0.016). The mean time-averaged DAS28-CRP (TA-DAS28-CRP) [19, 20] was 1.9 (s.d. 0.4) for those who maintained LDA compared with 3.0 (s.d. 0.7) for those who failed to do so (P < 0.0001).
Fig. 2.
Proportion of disease activity
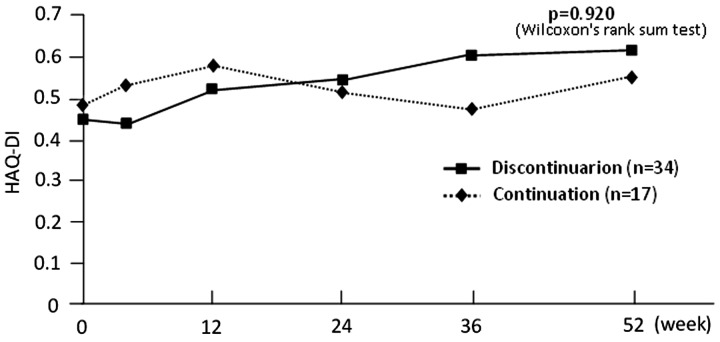
In contrast to consistently low (<2.6) scores in the continuation group, the mean DAS28-ESR score in the discontinuation group increased slightly, from 2.4 at baseline to 2.7 at week 4, 3.1 at week 12, 3.3 at week 24, 3.5 at week 36 and 3.6 at week 52. According to the endpoint DAS28-ESR scores, 24.2% of the discontinuing vs 47.1% of the continuing patients were in remission, 30.3% vs 35.3% had LDA, 27.3% vs 17.6% had MDA and 18.2% vs 0% had HDA. The mean HAQ-DI scores for the two groups followed similar time courses and were 0.6 for both groups at week 52 (P = 0.920; Fig. 3).
Fig. 3.
Transition diagram of HAQ-DI
DI: Disability Index.
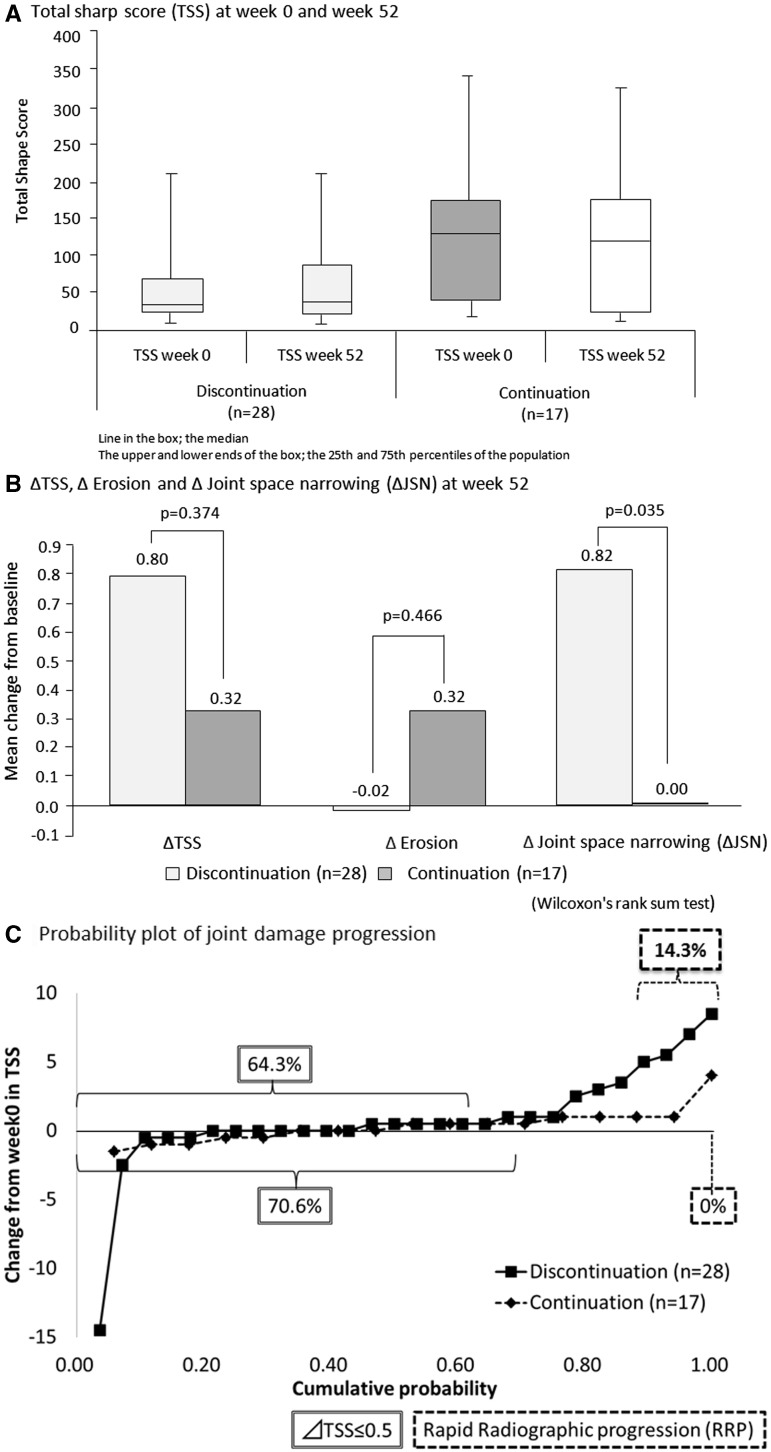
The TSS at weeks 0 and 52 was similar in the discontinuation and continuation groups, but the baseline TSS was higher for the continuation group (Fig. 4A). Mean ΔTSS (0.80 vs 0.32, P = 0.374) and ΔJE (−0.02 vs 0.32, P = 0.466) were similar for the two groups, while mean ΔJSN was significantly greater in the discontinuation group (0.82 vs 0, P = 0.035; Fig. 4B). After correction by linear extrapolation, the proportion of patients in radiographic remission (ΔTSS ≤ 0.5) was 64.3% in the discontinuation group compared with 70.6% in the continuation group (P = 0.752; Fig. 4C). No radiographic progression was seen in 42.9% and 47.1% of patients, while RRP was seen in 14.3% and 0% of patients in the discontinuation and continuation groups, respectively (Fig. 4C). The four patients who showed RRP after discontinuation had significantly higher CRP at enrolment in this study and lower RF in the previous phase III study compared with the 24 patients who did not show RRP in this group (P = 0.034 and P = 0.020, respectively).
Fig. 4.
Total Sharp score
In the discontinuation group, 10 of the 14 patients in DAS28-CRP remission at week 52 were evaluable for ΔTSS, of whom 7 (70%) were in radiographic remission. In the continuation group, all 11 patients in DAS28-CRP remission at week 52 were evaluable for ΔTSS and 7 (63.6%) were in radiographic remission.
Resumption of abatacept treatment
Nine patients resumed abatacept treatment after a mean interval of 149.6 days (s.d. 34.5). After resumption, the mean DAS28-CRP score steadily decreased, from 5.0 (s.d. 1.1) to 3.7 (s.d. 1.6) at 12 weeks and to 3.7 (s.d. 1.7) at 24 weeks, as was observed in the previous phase II/III study [from 4.8 (s.d. 0.8) at baseline to 3.0 (s.d. 0.9) at week 12 and to 2.8 (s.d. 0.9) at week 24; not significant by Wilcoxon’s rank sum test].
In the previous study, time to remission in those who resumed (n = 9) and did not resume (n = 25) abatacept was similar (P = 0.643; log rank test); clinical remission was achieved in 2 of 9 (22.2%) vs 13 of 25 (52.0%) patients at week 24 and in 88.9% vs 96.0% of patients at the endpoint, respectively. The two populations also had comparable demographic and baseline characteristics.
Safety
Non-serious AEs occurred in one patient who resumed abatacept (acute upper respiratory tract infection) and two patients who continued the drug (acute bronchitis in one and low back pain, cystitis, constipation, common cold and left scapulohumeral periarthritis in the second). No serious AEs were reported. Anti-abatacept antibody titre was measured in 26 of the 34 patients upon discontinuation of abatacept, as well as in 7 of 9 and 6 of 9 patients immediately and at 24 weeks after resumption. Positive titres were recorded in four patients (15.4%) upon discontinuation, in two patients (28.6%) immediately after resumption and in no patients at 24 weeks after resumption. Two of the four patients with positive titres upon discontinuation restarted abatacept. Both patients had positive titres again upon resumption, but not after 24 weeks. None of the patients with positive anti-abatacept antibody titre developed AEs or responded poorly to abatacept.
Discussion
Accumulating evidence suggests that CD4+ T cells play a key role in RA-associated inflammation [21–23], although the extent to which they contribute to this disease is not fully understood. Abatacept, which blocks a T cell co-stimulation pathway, has been shown to have favourable efficacy and tolerability profiles in Japanese and non-Japanese MTX-intolerant, TNFinhibitor-intolerant or MTX-naive [early (<2 years)] RA patients [7–12].
The ACR and European League Against Rheumatism treatment recommendations propose that remission or LDA should be the primary target for treatment of RA [24]. Combined therapy with currently available biologic and non-biologic DMARDs can help attain current treatment targets in the majority of RA patients. Nonetheless, the high costs of biologic agents have encouraged ongoing efforts to reduce the economic burden upon patients, including trials to discontinue biologic therapy in patients in sustained clinical remission. While existing data support the potential for biologic-free remission following intensive treatment with TNFinhibitors [25–28], definitive evidence for this potential following discontinuation of abatacept is limited. One study suggested that there was no further radiographic or MRI progression of joint destruction after discontinuation of abatacept in patients with undifferentiated inflammatory arthritis or very early RA [29]. Here we determined the potential of abatacept in promoting biologic-free remission in RA patients already in clinical remission.
At week 52, 64.7% of the patients who discontinued abatacept in an ITT population remained biologic-free (primary endpoint). In a drug-free follow-up of 102 RA patients (mean disease duration 5.9 years) who attained LDA with infliximab [25], 55% of the patients maintained LDA and 39 of the 83 patients (47%) who had achieved remission (DAS28 < 2.6) at enrolment remained in remission for 1 year. In a similar study for adalimumab [28], 14 of 22 patients (64%) maintained LDA (DAS28-CRP < 2.7) without the drug for 1 year. On comparison with these TNF inhibitors, abatacept seems to have a similar potential in the induction of biologic-free remission.
After discontinuation of abatacept, the mean DAS28-CRP score gradually increased and reached a level significantly higher than in the continuation group at week 52. This was also true when the mean endpoint DAS28-CRP score was compared between the 19 patients who went without abatacept and the 15 patients who continued the drug for 52 weeks. In the discontinuation group, the number of patients in DAS28-CRP remission decreased and the number of patients with HDA increased. HAQ-DI and CRP are two baseline parameters that were significantly different between those with (n = 20) and without (n = 14) LDA at week 52. In addition, HAQ-DI is the only baseline parameter that was significantly different between those in remission (n = 7) and those not in remission (n = 12) without abatacept at week 52. These findings suggest that the HAQ-DI or CRP immediately before discontinuation of abatacept may predict the probability of subsequent maintenance of remission or LDA. According to TA-DAS28-CRP data, those with LDA at the endpoint maintained LDA throughout the period of follow-up. Comparison between the discontinuation and continuation groups showed similar proportions of patients in clinical remission at week 52 and similar changes in the HAQ-DI over time, indicating that the effects of abatacept on clinical and functional outcomes are durable even after discontinuation.
In RA, joint destruction progresses over time, causing significant disability, which imposes an enormous social burden. Although the recently introduced biologic agents, including abatacept, can prevent or delay joint destruction in a proportion of patients, it is not known if they prevent disease relapse following discontinuation. In the present study, radiographic assessment of joint destruction showed no significant difference between those who discontinued and those who continued abatacept with regard to mean ΔTSS or the percentage of patients with ΔTSS ≤0, ≤0.5 or ≥5. These data confirm that abatacept exerts a sustainable effect in preventing or delaying joint damage and thus keeps patients in radiographic remission even after discontinuation. These radiographic benefits of abatacept appear to be comparable to those of infliximab and adalimumab (in early RA), as evidenced by 67% [25] and 81% [27] of patients with LDA remaining in radiographic remission after discontinuation of those drugs.
As a proportion of RA patients have to suspend their biologic therapy for economic or other reasons, we also assessed the efficacy and safety of re-treatment with abatacept after relapse. Re-treatment with abatacept was effective in controlling disease activity but may be less effective than the initial treatment with abatacept, which was evaluated in the previous phase II study [7].
Abatacept was well tolerated after resumption and during extended use, with only non-serious AEs being reported in three patients. Regarding the immunogenicity of abatacept, two of the limited number of patients assessed were positive for anti-abatacept antibody at the resumption of treatment but were negative after 24 weeks. The disappearance of anti-abatacept antibody after resumption of abatacept treatment may reflect the immunomodulatory effect of the drug.
The present study has several limitations. First, this was an exploratory study about the possibility of biologic-free remission after attaining clinical remission with abatacept. This study had no hypothesis to be tested because no data were available about this possibility with any other biologic DMARDs when we planned this study. Second, this was a small, non-randomized, observational study. Only Japanese RA patients who had completed a phase II study of abatacept [7] and its long-term extension and were in DAS28-CRP remission (<2.3) were enrolled, and for ethical reasons they were offered the option to continue abatacept or not at enrolment. As an expected consequence, the two groups were not well matched at baseline; those who chose to discontinue the drug were at an earlier stage of RA and had less progressive joint damage. Therefore data comparing the two groups should be interpreted cautiously. Third, we imputed missing data for non-radiographic efficacy variables using LOCF, a less favoured method than multiple imputation. This might introduce uncertainly about the reliability of the disease activity data and compromise their interpretation. Despite these limitations, the results are informative, as they indicate that the clinical remission achieved after abatacept treatment is potentially maintained following discontinuation of the drug in some of the patients, particularly in those who have also achieved a low HAQ-DI score and/or low CRP after the treatment. Given that the decision to continue or discontinue abatacept after attaining clinical remission was made by individual patients and their physicians, this finding will also be helpful for implementing the treat-to-target principle in RA practice.
Rheumatology key messages.
The effects of abatacept on clinical, functional and structural outcomes in RA continue after its discontinuation.
Biologic-free remission of RA can be maintained after attaining sustained clinical remission with abatacept.
Lower HAQ DI or CRP may predict maintenance of RA remission or low disease activity after discontinuation of abatacept.
Acknowledgements
We are grateful to all patients participating in this study as well as the following investigators and sites: M. Iwahashi, Higashi-Hiroshima Memorial Hospital; T. Ishii, Tohoku University Hospital; T. Sumida, Tsukuba University Hospital; R. Matsumura, National Hospital Organization Chiba-East Hospital; T. Tsuru, PS Clinic; T. Atsumi, Hokkaido University Hospital; Y. Munakata, Taihaku Sakura Hospital; T. Mimura, Saitama Medical School Hospital; Y. Yoshida, Kitasato University Kitasato Institute Medical Center Hospital; M. Matsushita, National Hospital Organization Osaka Minami Medical Center; K. Saito and S. Hirata, University of Occupational and Environmental Health, Japan; S. Ohta, Oasis Clinic; E. Tanaka, Institute of Rheumatology, Tokyo Women's Medical University; Y. Kaneko, Keio University Hospital and K. Kikuchi, T. Abe and L. Lin, Keio Center for Clinical Research.
Funding: This work was supported by Bristol-Myers K.K.
Disclosure statement: Y.T. has received consulting fees, speaking fees, and/or honoraria from Mitsubishi Tanabe, Eisai, Chugai, Abbott, Astellas, Daiichi Sankyo, AbbVie, Janssen, Pfizer, Takeda, AstraZeneca, Eli Lilly, GlaxoSmithKline, Quintiles, MSD and Asahi Kasei and research grants from Bristol-Myers, Mitsubishi Tanabe, AbbVie, MSD, Chugai, Astellas and Daiichi Sankyo. S.T. has received grants/research support from Mitsubishi Tanabe, Astellas, Chugai and Abbott. T.T. has received grants from Abbott, Astellas, Bristol-Myers, Chugai, Daiichi Sankyo, Eisai, Mitsubishi Tanabe, Pfizer, Sanofi, Santen, Takeda, Teijin, AbbVie, Asahi Kasei and Taisho Toyama; lecture fees from Abbott, Bristol-Myers, Chugai, Eisai, Janssen, Mitsubishi Tanabe, Pfizer, Takeda, Astellas and Daiichi Sankyo and consulting fees from AstraZeneca, Eli Lilly, Novartis, Mitsubishi Tanabe, Asahi Kasei, AbbVie and Daiichi Sankyo. H.Y. has received lecture fees from AbbVie, Chugai, Daiichi Sankyo, Eisai, Mitsubishi Tanabe, Pfizer, Takeda and Teijin and research grants from AbbVie, Asahi Kasei, Astellas, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Eisai, GlaxoSmithKline, Janssen, Mitsubishi Tanabe, MSD, Nippon Kayaku, Pfizer, Santen, Taisho Toyama, Takeda and Teijin. K.A. has received research grants from Chugai and speaking fees from AbbVie, Astellas, Bristol-Myers, Eisai, Chugai, Pfizer and Mitsubishi Tanabe. M.M. has received speaking fees from Pfizer, Mitsubishi Tanabe, Janssen and Novartis and chair fees from Eisai, Taisho Toyama, AbbVie and Astellas. T.M. has received speaking fees from Pfizer Japan and Janssen Pharmaceutical and research grants from Nippon Kayaku, Pfizer Japan, Bristol-Myers Squibb, Otsuka Pharmaceutical, Quintiles Transnational Japan, Janssen Pharmaceutical, Astellas Pharma, Takeda Chemical Industries, Eli Lilly Japan, Mitsubishi Tanabe Pharma, AstraZeneca, Eisai, Santen Pharmaceutical and Daiichi Sankyo. N.M. has received research grants from AbbVie Japan, Astellas Pharma, Bristol-Myers Squibb, Chugai Pharmaceutical, Dainihon-Sumitomo Pharma, Daiichi-Sankyo, Eisai, Mitsubishi-Tanabe Pharma, Novartis Pharma, Takeda Pharmaceutical and Teijin Pharma and received consulting fees or honoraria from AbbVie, Bristol-Myers Squibb, Janssen Pharmaceutical and Otsuka Pharmaceutical. S.O. has received speaking fees from Mitsubishi Tanabe, Pfizer, Takeda, Eisai, AbbVie, Chugai, Janssen, Astellas and Bristol-Myers Squibb.
References
- 1.Ranheim EA, Kipps TJ. Elevated expression of CD80 (B7/BB1) and other accessory molecules on synovial fluid mononuclear cell subsets in rheumatoid arthritis. Arthritis Rheum. 1994;37:1637–46. doi: 10.1002/art.1780371113. [DOI] [PubMed] [Google Scholar]
- 2.Verwilghen J, Corrigall V, Pope RM, Rodrigues R, Panayi GS. Expression and function of CD5 and CD28 in patients with rheumatoid arthritis. Immunology. 1993;80:96–102. [PMC free article] [PubMed] [Google Scholar]
- 3.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–52. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 4.Isaacs JD. Therapeutic T-cell manipulation in rheumatoid arthritis: past, present and future. Rheumatology. 2008;47:1461–8. doi: 10.1093/rheumatology/ken163. [DOI] [PubMed] [Google Scholar]
- 5.Moreland LW, Alten R, Van den Bosch F, et al. Costimulatory blockade in patients with rheumatoid arthritis: a pilot, dose-finding, double-blind, placebo-controlled clinical trial evaluating CTLA-4Ig and LEA29Y eighty-five days after the first infusion. Arthritis Rheum. 2002;46:1470–9. doi: 10.1002/art.10294. [DOI] [PubMed] [Google Scholar]
- 6.Genant HK, Peterfy CG, Westhovens R, et al. Abatacept inhibits progression of structural damage in rheumatoid arthritis: results from the long-term extension of the AIM trial. Ann Rheum Dis. 2008;67:1084–9. doi: 10.1136/ard.2007.085084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeuchi T, Matsubara T, Nitobe T, et al. Phase II dose-response study of abatacept in Japanese patients with active rheumatoid arthritis with an inadequate response to methotrexate. Mod Rheumatol. 2013;23:226–35. doi: 10.1007/s10165-012-0668-z. [DOI] [PubMed] [Google Scholar]
- 8.Kremer JM, Genant HK, Moreland LW, et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2006;144:865–76. doi: 10.7326/0003-4819-144-12-200606200-00003. [DOI] [PubMed] [Google Scholar]
- 9.Schiff M, Keiserman M, Codding C, et al. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis. 2008;67:1096–103. doi: 10.1136/ard.2007.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genovese MC, Becker JC, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353:1114–23. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 11.Bathon J, Robles M, Ximenes AC, et al. Sustained disease remission and inhibition of radiographic progression in methotrexate-naive patients with rheumatoid arthritis and poor prognostic factors treated with abatacept: 2-year outcomes. Ann Rheum Dis. 2011;70:1949–56. doi: 10.1136/ard.2010.145268. [DOI] [PubMed] [Google Scholar]
- 12.Matsubara T, Yamana S, Tohma S, et al. Tolerability and efficacy of abatacept in Japanese patients with rheumatoid arthritis: a phase I study. Mod Rheumatol. 2013;23:634–45. doi: 10.1007/s10165-012-0722-x. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka Y. Next stage of RA treatment: is TNF inhibitor-free remission a possible treatment goal? Ann Rheum Dis. 2013;23:226–35. doi: 10.1136/annrheumdis-2012-202350. [DOI] [PubMed] [Google Scholar]
- 14.Nishimoto N, Amano K, Hirabayashi Y, et al. Drug free REmission/low disease activity after cessation of tocilizumab (Actemra) Monotherapy (DREAM) study. Mod Rheumatol. 2014;24:17–25. doi: 10.3109/14397595.2013.854079. [DOI] [PubMed] [Google Scholar]
- 15.Inoue E, Yamanaka H, Hara M, Tomatsu T, Kamatani N. Comparison of Disease Activity Score (DAS)28- erythrocyte sedimentation rate and DAS28- C-reactive protein threshold values. Ann Rheum Dis. 2007;66:407–9. doi: 10.1136/ard.2006.054205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Heijde DM. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol. 2000;27:261–3. [PubMed] [Google Scholar]
- 17.van der Heijde DM, van Leeuwen MA, van Riel PL, et al. Biannual radiographic assessments of hands and feet in a three-year prospective followup of patients with early rheumatoid arthritis. Arthritis Rheum. 1992;35:26–34. doi: 10.1002/art.1780350105. [DOI] [PubMed] [Google Scholar]
- 18.Vastesaeger N, Xu S, Aletaha D, St Clair EW, Smolen JS. A pilot risk model for the prediction of rapid radiographic progression in rheumatoid arthritis. Rheumatology. 2009;48:1114–21. doi: 10.1093/rheumatology/kep155. [DOI] [PubMed] [Google Scholar]
- 19.Smolen JS, van der Heijde DM, Keystone EC, et al. Association of joint space narrowing with impairment of physical function and work ability in patients with early rheumatoid arthritis: protection beyond disease control by adalimumab plus methotrexate. Ann Rheum Dis. 2013;72:1156–62. doi: 10.1136/annrheumdis-2012-201620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kameda H, Kanbe K, Sato E, et al. A merged presentation of clinical and radiographic data using probability plots in a clinical trial, the JESMR study. Ann Rheum Dis. 2013;72:310–2. doi: 10.1136/annrheumdis-2012-201804. [DOI] [PubMed] [Google Scholar]
- 21.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 22.Li NL, Zhang DQ, Zhou KY, et al. Isolation and characteristics of autoreactive T cells specific to aggrecan G1 domain from rheumatoid arthritis patients. Cell Res. 2000;10:39–49. doi: 10.1038/sj.cr.7290034. [DOI] [PubMed] [Google Scholar]
- 23.Klimiuk PA, Yang H, Goronzy JJ, Weyand CM. Production of cytokines and metalloproteinases in rheumatoid synovitis is T cell dependent. Clin Immunol. 1999;90:65–78. doi: 10.1006/clim.1998.4618. [DOI] [PubMed] [Google Scholar]
- 24.Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631–7. doi: 10.1136/ard.2009.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka Y, Takeuchi T, Mimori T, et al. Discontinuation of infliximab after attaining low disease activity in patients with rheumatoid arthritis: RRR (remission induction by Remicade in RA) study. Ann Rheum Dis. 2010;69:1286–91. doi: 10.1136/ard.2009.121491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nawata M, Saito K, Nakayamada S, Tanaka Y. Discontinuation of infliximab in rheumatoid arthritis patients in clinical remission. Mod Rheumatol. 2008;18:460–4. doi: 10.1007/s10165-008-0089-1. [DOI] [PubMed] [Google Scholar]
- 27.Kavanaugh A, Fleischmann RM, Emery P, et al. Clinical, functional and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26-week results from the randomised, controlled OPTIMA study. Ann Rheum Dis. 2013;72:64–71. doi: 10.1136/annrheumdis-2011-201247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harigai M, Takeuchi T, Tanaka Y, et al. Discontinuation of adalimumab treatment in rheumatoid arthritis patients after achieving low disease activity. Mod Rheumatol. 2012;22:814–22. doi: 10.1007/s10165-011-0586-5. [DOI] [PubMed] [Google Scholar]
- 29.Emery P, Durez P, Dougados M, et al. Impact of T-cell costimulation modulation in patients with undifferentiated inflammatory arthritis or very early rheumatoid arthritis: a clinical and imaging study of abatacept (the ADJUST trial) Ann Rheum Dis. 2010;69:510–6. doi: 10.1136/ard.2009.119016. [DOI] [PMC free article] [PubMed] [Google Scholar]