Abstract
The Wnt/β-catenin pathway causes accumulation of β-catenin in the cytoplasm and its subsequent translocation into the nucleus to initiate the transcription of the target genes. Without Wnt stimulation, β-catenin forms a complex with axin (axis inhibitor), adenomatous polyposis coli (APC), casein kinase 1α (CK1α), and glycogen synthase kinase 3β (GSK3β) and undergoes phosphorylation-dependent ubiquitination. Phosphatases, such as protein phosphatase 2A (PP2A), interestingly, also are components of this degradation complex; therefore, a balance must be reached between phosphorylation and dephosphorylation. How this balance is regulated is largely unknown. Here we show that a heat shock protein, HSP105, is a previously unidentified component of the β-catenin degradation complex. HSP105 is required for Wnt signaling, since depletion of HSP105 compromises β-catenin accumulation and target gene transcription upon Wnt stimulation. Mechanistically, HSP105 depletion disrupts the integration of PP2A into the β-catenin degradation complex, favoring the hyperphosphorylation and degradation of β-catenin. HSP105 is overexpressed in many types of tumors, correlating with increased nuclear β-catenin protein levels and Wnt target gene upregulation. Furthermore, overexpression of HSP105 is a prognostic biomarker that correlates with poor overall survival in breast cancer patients as well as melanoma patients participating in the BRIM2 clinical study.
INTRODUCTION
Wnt signaling plays a crucial role in the regulation of cellular physiology, including cell proliferation, differentiation, survival, and self-renewal of stem cells (1). Abnormal activation of the pathway by perturbation of the levels of Wnt ligands, as well as altered activities of the pathway components, can result in defects during embryonic development or contribute to diverse diseases, including cancer, in adults (2, 3).
Wnt signaling regulates these diverse processes by promoting the stabilization of β-catenin and the activation of β-catenin-dependent transcription (1). In the absence of Wnt activation, cytoplasmic β-catenin protein interacts with a scaffolding protein, axin, which forms a complex with several other proteins, i.e., the tumor suppressor adenomatous polyposis coli (APC), casein kinase 1α (CK1α), and glycogen synthase kinase 3β (GSK3β) (4). CK1α and GSK3β sequentially phosphorylate the amino-terminal region of β-catenin, generating a phosphodegron recognized by the E3 ubiquitin ligase SCFβ-TRCP. β-Catenin is subsequently ubiquitinated and undergoes proteasome-dependent degradation (5, 6). This continual elimination of β-catenin prevents it from accumulating in the nucleus and represses the transcription of Wnt target genes (5).
In addition to kinases, protein phosphatase 2A (PP2A) has also been reported to positively regulate Wnt signaling (7, 8). PP2A is composed of a core catalytic subunit (PPP2CA), a structural subunit (PR65/A), and variable regulatory B subunits (9). Initially, PP2A was shown to be required for dorsal development, and the PP2A:B56ε complex was reported to function downstream of Wnt ligand and upstream of Dishevelled (DVL) (10). Later studies also suggested that PP2A can regulate Wnt signaling by directly regulating β-catenin. PR55α, a regulatory subunit, is required for PP2A to dephosphorylate β-catenin and positively activate the Wnt pathway (7). Furthermore, it has been shown that phospho-β-catenin not associated with APC is dephosphorylated by PP2A and is rescued from ubiquitination by SCFβ-TRCP (8). The coexistence of kinases and phosphatases in the β-catenin destruction complex suggests that a phosphorylation-dephosphorylation balance has to be reached and that disturbance of this delicate balance will possibly cause hyperactivation of β-catenin signaling.
Heat shock proteins are a highly conserved group of proteins that, when first discovered, were characterized by upregulation in response to stress induced by heat as well as chemical and physical perturbations (11). Subsequently, heat shock proteins have been identified as molecular chaperones that recognize and form complexes with proteins that are in nonnative conformations to (i) minimize the aggregation of the nonnative protein, (ii) target it for degradation and removal from the cell, (iii) assist in proper protein conformation, and (iv) assist in protein translocation across membranes to organelles (12, 13). Interestingly, members of the heat shock proteins have been shown to interact with kinases and phosphatases and to regulate their activities (14, 15).
Here we show that heat shock protein 105 (HSP105), a member of the HSP70 superfamily, is a component of the β-catenin degradation complex. The integrity of HSP105 in the β-catenin degradation complex is required for Wnt3a-induced β-catenin accumulation and Wnt target gene transcription. Mechanistically, HSP105 is required for recruiting the phosphatase PP2A to the β-catenin degradation complex to antagonize the phosphorylation of β-catenin by GSK3β, thus maintaining a phosphostatus balance of the β-catenin protein, leading to its accumulation or degradation based on the signaling cues.
MATERIALS AND METHODS
Plasmids, antibodies, and reagents.
Human HSP105-FLAG-Myc and axin1-FLAG-Myc were obtained from OriGene. FLAG-tagged proteins were detected with anti-FLAG antibody M2 from Sigma. Antibodies recognizing the following targets were obtained from commercial sources: HSP105 (Santa Cruz Biotechnology and Novus Biologicals); axin1, β-catenin, actin, pS33S37T41-β-catenin, CK1α, cleaved poly(ADP-ribose) polymerase (PARP), cleaved caspase-3, and HSP70 (Cell Signaling Technology); PPP2CA, GSK3 (Millipore), and Myc (USBiological); and axin1 (R&D Systems). Other reagents used were MG132 (Calbiochem) and Wnt3a (R&D Systems).
Proteomics and MS.
A total of 3 × 108 HEK293T cells transfected with 500 μg of a control vector, DVL3-FLAG, axin1-FLAG, or HSP105-FLAG for 48 h were lysed in 20 mM Tris-HCl (pH 7.8)–9 mM MgCl2–92 mM NaCl–0.1% Triton X-100. FLAG-tagged axin1 or HSP105 was affinity purified with anti-FLAG antibody M2-conjugated beads (Sigma) and was washed first in high-salt buffer (20 mM HEPES [pH 7.9], 1.5 mM MgCl2, 420 mM NaCl, 0.2 mM EDTA, 25% glycerol) for 10 min and then in low-salt buffer (20 mM Tris-HCl [pH 7.4], 300 mM NaCl, 0.2 mM EDTA, 20% glycerol, 0.1% NP-40) for 10 min (three repetitions) and given a final wash overnight. Complexes were eluted with 500 μg/ml 3×FLAG peptide (Sigma), reduced, separated by SDS-PAGE, and stained with SimplyBlue (Invitrogen). Protein bands were excised, destained, and subjected to in-gel trypsin digestion. Dried gel pieces were rehydrated with 10 ng/μl trypsin in 25 mM ammonium bicarbonate on ice for 1 h, and digestion was performed at 37°C overnight. Peptides were extracted with 50% acetonitrile (ACN)–0.1% trifluoroacetic acid (TFA) followed by 100% ACN and were dried to completion. Dried peptides were resuspended in 2% ACN–0.1% formic acid (FA) and were separated on a C18 reverse-phase column followed by tandem mass spectrometric (MS-MS) analysis in a linear trap quadrupole (LTQ) Orbitrap instrument (Thermo Fisher). Precursor ions were analyzed in the Fourier transform mass spectrometer (FTMS) at a resolution of 60,000; MS-MS was performed in the LTQ MS with the instrument operated in data-dependent mode, where the top 8 or 15 most abundant ions were subjected to fragmentation. MS-MS data were analyzed using the search algorithm Mascot (Matrix Science). Precursor and fragment ion mass tolerances were set to 50 ppm and 0.8 Da, respectively. Variable modifications included oxidized methionine (+15.9949 Da) and an acrylamide (+71.04 Da) or carbamidomethyl (+57.0215) adduct for cysteine residues. Peptide assignments were filtered at a false discovery rate (FDR) of 1%, and data were summarized to the protein level using an in-house program, Trestles (Genentech). Alternatively, data were compiled using Scaffold (Proteome Software).
Cell culture, transfection, and luciferase assays.
293T and 293 cells stably transfected with a TOPbrite firefly luciferase Wnt reporter and pRL-SV40 constitutively expressing Renilla luciferase (Promega) were cultured in Dulbecco's modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS; Sigma). SW480, SW403, and LS1034 cells were cultured in RPMI 1640 medium with 10% FBS. Plasmids were transfected with Fugene 6 (Promega) or Lipofectamine 2000 (Invitrogen). Small interfering RNAs (siRNAs; from Dharmacon or Ambion) were transfected using Lipofectamine RNAiMax (Invitrogen).
RNA-Seq data processing.
Both TCGA (The Cancer Genome Atlas) and Genentech in-house Cancer Genome Project (CGP) samples were used in the analysis. The raw transcriptome sequencing (RNA-Seq) data (Illumina FASTQ sequencing files) were aligned using GSNAP software (16). The expression of each gene was measured as total RNA-Seq reads uniquely mapped to the gene-coding regions. Gene expression was then normalized across samples using the Bioconductor DESeq package (17). Gene expression levels, expressed as RPKMs (reads per kilobase transcript per million reads), were calculated based on normalized read counts. The expression values shown in Fig. 6A and B are on a log2 scale of normalized counts, calculated as log2(count + 1).
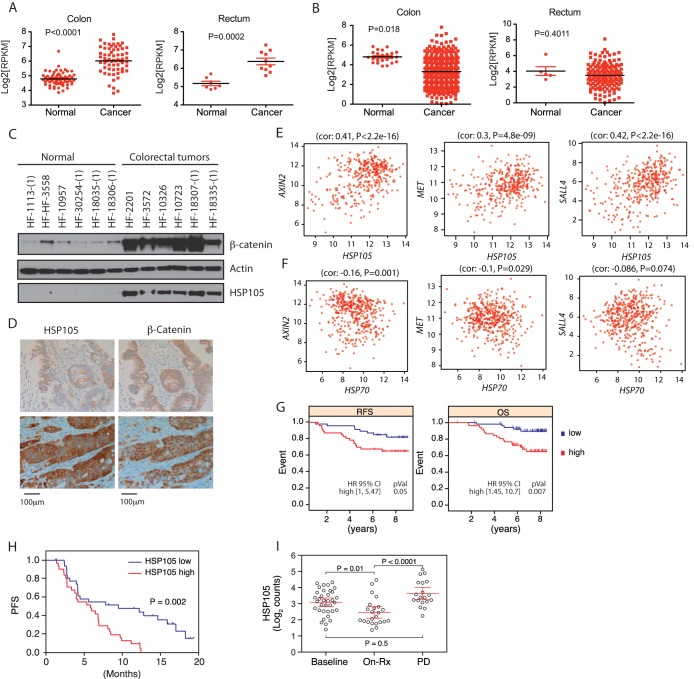
FIG 6.
HSP105 is associated with a poor prognosis for cancer patients. (A and B) Transcript abundance of HSP105 (A) or HSP70 (B) measured by RNA-Seq in human colon and rectal cancers as well as normal tissues included in The Cancer Genome Atlas (TCGA) database. P values were derived from Student's t test for differences between cancer and normal samples. (C) Western blot analysis of HSP105 and β-catenin in human colorectal tumor samples and noncancerous colon tissue samples. (D) Representative immunohistochemical staining for HSP105 and β-catenin in human colorectal tumors (n = 66). β-Catenin staining is seen primarily in the membrane in HSP105 low-staining samples (top) and in the nucleus in HSP105 high-staining samples (bottom). (E and F) In colorectal tumor samples, HSP105 (E) but not HSP70 (F) mRNA levels show correlations (cor) with the transcription of Wnt target genes AXIN2, MET, and SALL4. (G) Kaplan-Meier curves representing the relapse-free survival (RFS) and overall survival (OS) rates of breast cancer patients with high or low HSP105 expression. pVal, P value. (H) Kaplan-Meier curve representing the progression-free survival (PFS) rates of patients in the BRIM2 study with high and low HSP105 mRNA expression. (I) HSP105 mRNA abundance in melanoma samples from the BRIM2 study as measured by NanoString software. Samples were collected prior to vemurafenib treatment (Baseline), at day 15 of treatment (On-Rx), or at disease progression (PD). P values were derived from a Tukey test comparing all groups.
Kaplan-Meier overall survival (OS) curve for breast cancer patients.
The normalized gene expression data of 159 breast cancer tissue samples were imported from the GEO website (GSE1456). High and low expression levels of the HSP105 gene were defined based on ranking (the top 30% of samples have high expression, and the bottom 30% have low expression). Survival analysis was then done using Kaplan-Meier curves, and the P value was calculated based on the Cox proportional-hazards model.
IHC.
Immunohistochemistry (IHC) was performed on 4-μm-thick formalin-fixed, paraffin-embedded (FFPE) tissue sections mounted on glass slides. All IHC steps were carried out on the Ventana Discovery XT (Ventana Medical Systems, Tucson, AZ) autostainer platform. Pretreatment was carried out for the standard time with Cell Conditioner 1. The primary antibody, anti-HSP105 (rabbit monoclonal antibody EPR4577; Abcam, Cambridge, MA), was used at a concentration of 1.29 μg/ml and was incubated on slides for 60 min at room temperature. Then the slides were incubated with a Ventana OmniMap horseradish peroxidase (HRP)-conjugated anti-rabbit antibody (Ventana Medical Systems, Tucson, AZ). Ventana diaminobenzidine (DAB) and hematoxylin II were used for chromogenic detection and counterstaining.
In vivo and in vitro interaction assays.
In most cases, whole-cell lysates were cleared by centrifugation at 16,000 × g for 30 min. Protein A, protein G, or anti-FLAG M2 beads were then incubated for 2 h with the soluble lysate. Beads were washed 5 times with lysis buffer and were eluted in SDS-PAGE LDS (lithium dodecyl sulfate) sample buffer with boiling.
Recombinant PPP2CA and HSP105 proteins were generated with an SP6 high-yield wheat germ protein expression system (Promega). The recombinant PPP2CA was mixed with recombinant axin1 from a baculoviral expression system in NP-40 buffer (1% NP-40, 120 mM NaCl, 50 mM Tris-HCl [pH 7.4], 1 mM EDTA) and was immunoprecipitated (IP) with an anti-axin1 antibody (R&D Systems) overnight. Protein G-Sepharose (GE Healthcare) was used to pull down the complex.
Most Western blot experiments were performed as described previously (18). Cytosolic β-catenin levels were determined after depletion of cadherin-associated β-catenin with concanavalin A (ConA)-Sepharose (GE Healthcare). HRP-conjugated TrueBlot antibodies (eBioscience) were used if the IP antibody and the primary antibody were from the same species.
Cell proliferation assay.
The cells were plated in 96-well plates overnight and were transfected with HSP105 siRNA (s21240; Ambion) or control siRNA. The cells were transfected again after 3 days for a total of 6 days. The cells were permeabilized with 4% paraformaldehyde (50 μl/well) for 15 min and were stained with Syto 60 (1:5,000 dilution in PBS) for 1 h at room temperature. The cells were washed 3 times with PBS after each step. Finally, 50 μl of water was added to each well, and plates were read on the Li-Cor Odyssey scanner.
Patients and RNA analysis from tumor tissues.
BRIM2 (NCT00949702) was a single-arm phase 2 study in which patients with previously treated metastatic melanoma carrying a BRAFV600E/K mutation received vemurafenib (19). The data cutoff used in the analyses here was February 2012. Pretreatment (archival or baseline) tissue blocks were available from 64 of 132 (48%) patients from BRIM2. Tissue biopsy blocks taken on day 15 of continuous vemurafenib treatment were available from 23 patients, and tissue biopsy blocks taken after documented disease progression were available from 19 patients. Patient consent was obtained for exploratory research conducted on all tissues.
mRNA was prepared from FFPE sections of tumor tissues, and gene expression was measured using NanoString software. Data were normalized to the geometric mean for all 800 genes measured. The effect of baseline HSP105 expression was determined using a Cox proportional-hazards model.
RESULTS
HSP105 is a component of the β-catenin destruction complex.
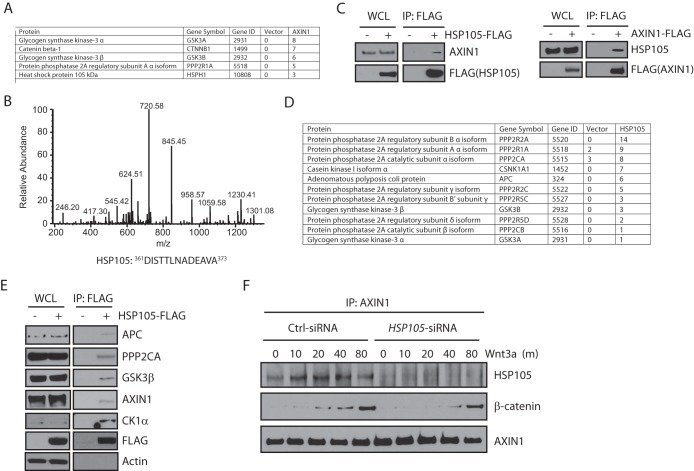
To identify targets of Wnt signaling that could potentially be inhibited with drugs, we characterized the proteins interacting with axin1, a key scaffolding protein of the β-catenin degradation complex. FLAG-tagged axin1 was overexpressed in HEK293T cells and was purified by anti-FLAG immunoprecipitation. The purified proteins were digested with trypsin and were analyzed by liquid chromatography (LC)–MS-MS. In addition to some previously identified interacting proteins, such as β-catenin, GSK3α, GSK3β, and phosphatase 2A regulatory subunit A isoform α (PPP2R1A) (Fig. 1A), we also identified a heat shock protein, HSP105, that specifically interacted with axin1 (Fig. 1A and B). Endogenous HSP105 or axin1 also coimmunoprecipitated with ectopically expressed axin1-FLAG or HSP105-FLAG, respectively (Fig. 1C). These data suggest that HSP105 may be a novel component of the β-catenin degradation complex.
FIG 1.
HSP105 is a component of the β-catenin destruction complex. (A) Proteins coimmunoprecipitated with axin1-FLAG from HEK293T cells and identified by mass spectrometry. Numbers indicate the numbers of peptides detected in FLAG immunoprecipitates from cells transfected with a vector alone or with axin1-FLAG. (B) Representative fragmentation spectrum of HSP105 identified by axin1 pulldown. (C) Western blot analysis of endogenous axin1 or HSP105 coimmunoprecipitated with ectopically expressed HSP105-FLAG or axin1-FLAG. 293T cells were transfected with HSP105-FLAG or axin1-FLAG for 48 h. WCL, whole-cell lysates. (D) Proteins coimmunoprecipitated with HSP105-FLAG from HEK293T cells and identified by mass spectrometry. Numbers indicate the numbers of peptides detected in FLAG immunoprecipitates from cells transfected with a vector alone or with HSP105-FLAG. (E) Western blot analysis of endogenous APC, PPP2CA, GSK3β, axin1, and CK1α coimmunoprecipitated with HSP105-FLAG in 293T cells transfected with a vector or with HSP105-FLAG for 48 h. (F) 293T cells were transfected with control (Ctrl) or HSP105 siRNA for 72 h and were then treated with 100 ng/ml Wnt3a for the indicated times (in minutes). Whole-cell lysates were immunoprecipitated with an anti-axin1 antibody and were immunoblotted with the indicated antibodies.
In agreement with this concept, mass spectrometry analysis suggested that overexpressed HSP105-FLAG in HEK293T cells pulled down endogenous APC, GSK3β, and casein kinase 1α (CK1α), all of which are essential components of the β-catenin degradation complex (Fig. 1D). This was confirmed by a coimmunoprecipitation experiment showing that endogenous APC, GSK3β, axin1, and CK1α associated with ectopically expressed HSP105-FLAG (Fig. 1E). Furthermore, endogenous axin1 also interacted with HSP105 (Fig. 1F). Taken together, these data suggest that HSP105 is a component of the β-catenin degradation complex.
Interestingly, in the HSP105 immunoprecipitation/mass spectrometry experiment, we also identified peptides from the catalytic subunit (PPP2CA) and several regulatory subunits of phosphatase PP2A (Fig. 1D). This is consistent with the observation that components of PP2A also interacted with axin1 (Fig. 1A). Indeed, PPP2CA, the catalytic subunit of PP2A, was confirmed to interact with HSP105 by the coimmunoprecipitation experiment (Fig. 1E).
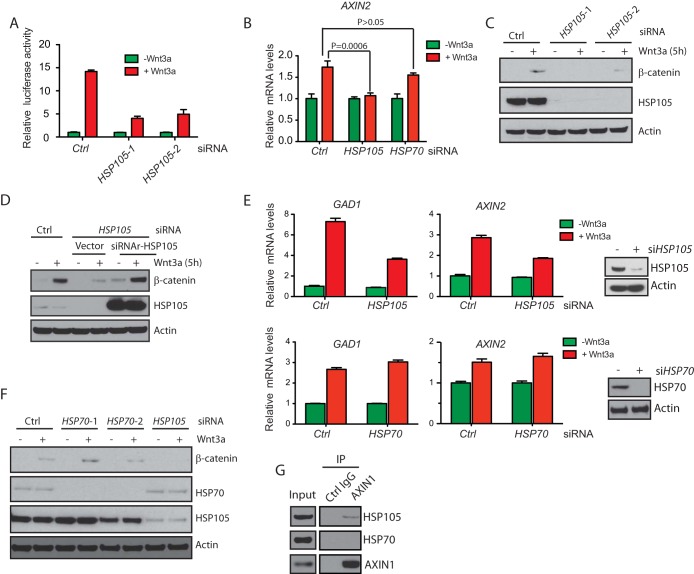
HSP105 is required for canonical Wnt signaling.
Wnt signaling stabilizes cytosolic β-catenin, driving its nuclear translocation and interaction with the TCF (T cell factor)/LEF (lymphoid-enhancing factor) transcription factors to promote target gene transcription (20–22). Since HSP105 interacted with components of the β-catenin destruction complex, we investigated whether HSP105 also plays a role in canonical Wnt signaling. Using HEK293 cells that harbor a Wnt-dependent TOPbrite luciferase reporter (23, 24), we found that knockdown of HSP105 by two siRNAs drastically decreased Wnt-dependent luciferase activity (Fig. 2A). Depletion of HSP105, but not depletion of another chaperone, HSP70, also blocked Wnt3a-induced upregulation of AXIN2 transcription (Fig. 2B). This is likely because HSP105 knockdown caused 293T cells to have decreased cytosolic β-catenin accumulation upon Wnt3a treatment (Fig. 2C). Importantly, when siRNA-resistant HSP105 was cotransfected, the Wnt3a-induced accumulation of β-catenin was recovered (Fig. 2D). In addition, in human PA-1 teratocarcinoma cells, knockdown of HSP105, but not knockdown of HSP70, significantly compromised Wnt3a-induced transcriptional upregulation of Wnt target genes, such as AXIN2 and GAD1 (24) (Fig. 2E). HSP105 has been shown to interact with HSP70 (25). However, the regulation of Wnt signaling by HSP105 seemed to be independent of HSP70, since depletion of HSP70 did not affect Wnt3a-induced upregulation of target genes (Fig. 2B and E) or accumulation of β-catenin (Fig. 2F). Furthermore, HSP105, but not HSP70, interacted with axin1 (Fig. 2G). Collectively, these data suggest that HSP105 is an effector required for canonical Wnt signal transduction.
FIG 2.
HSP105 is required for Wnt signaling. (A) Expression of a TOPbrite luciferase reporter in HEK293 cells transfected with 2 siRNAs against HSP105 for 67 h, followed by treatment with 100 ng/ml Wnt3a for 5 h. Error bars represent standard errors of the means for three individual experiments. (B) Expression of AXIN2 in HEK293T cells transfected with siRNAs against HSP105 or HSP70 for 66 h, followed by treatment with 100 ng/ml Wnt3a for 10 h. Error bars represent standard errors of the means of triplicate measurements. (C) β-Catenin protein abundance in HEK293T cells transfected with 2 siRNAs against HSP105 for 67 h, followed by treatment with 100 ng/ml Wnt3a for 5 h. (D) HEK293T cells transfected with control (Ctrl) or HSP105 siRNA for 16 h, followed by transfection of a vector or siRNA-resistant HSP105 (siRNAr-HSP105) for 48 h. Membrane β-catenin-depleted whole-cell lysates were immunoblotted with the indicated antibodies. (E) Relative expression of GAD1 and AXIN2 in PA-1 cells transfected with control or HSP105 siRNA for 60 h, followed by treatment with 100 ng/ml Wnt3a for 16 h. Relative mRNA levels were determined by TaqMan reverse transcription-quantitative PCR. Error bars represent standard errors of the means of triplicate measurements. (F) β-Catenin protein levels in HEK293T cells transfected with siRNAs against HSP70 or HSP105 for 67 h, followed by treatment with 100 ng/ml Wnt3a for 5 h. (G) Western blot analysis of endogenous HSP105 and HSP70 coimmunoprecipitated with axin1 in 293T cells.
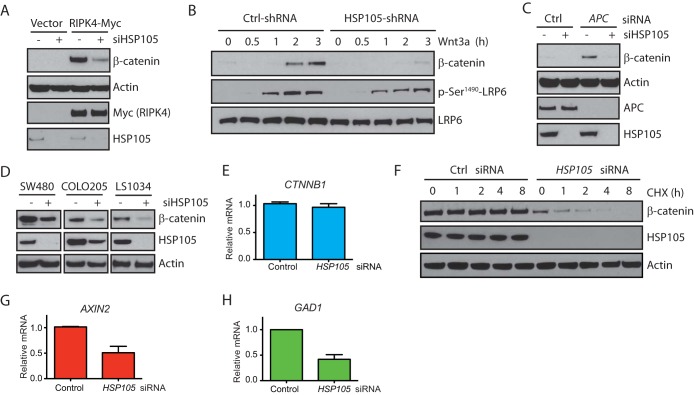
Knockdown of HSP105 compromises β-catenin accumulation in APC mutant CRC cell lines.
To investigate how HSP105 regulates Wnt signaling, we monitored the accumulation of cytosolic β-catenin after perturbations that activate the Wnt pathway in the absence or presence of HSP105. Receptor-interacting serine/threonine protein kinase 4 (RIPK4) has been shown to activate the Wnt pathway by phosphorylating DVL proteins and facilitating DVL signalosome formation (24). Knockdown of HSP105 drastically attenuated RIPK4-induced β-catenin accumulation (Fig. 3A). Similarly, knockdown of HSP105 also blocked β-catenin accumulation induced by overexpression of LRP6 (low-density lipoprotein receptor-related protein 6) or DVL2 proteins (data not shown). In addition, knockdown of HSP105 did not affect the phosphorylation of LRP6 upon Wnt3a stimulation (Fig. 3B), suggesting that HSP105 functions at a level downstream of receptors. APC is an essential component of the β-catenin degradation complex, and loss of function of APC causes the activation of Wnt signaling and the accumulation of cytosolic β-catenin (26). Indeed, APC knockdown induced robust accumulation of β-catenin in 293T cells, which was abrogated when HSP105 was absent (Fig. 3C), suggesting that HSP105 functions either at the same level as APC or downstream of APC. These results suggest that HSP105 plays a role in maintaining β-catenin protein levels even when the β-catenin degradation complex is disrupted, prompting us to investigate if knockdown of HSP105 could decrease β-catenin protein levels and therefore impair proliferation in colorectal cancer (CRC) cell lines with mutated APC and high levels of active β-catenin.
FIG 3.
Knockdown of HSP105 decreases β-catenin accumulation. (A) 293T cells were transfected with control or HSP105 siRNA for 16 h, followed by transfection of a vector or RIPK4-Myc for 48 h. Membrane β-catenin-depleted whole-cell lysates were immunoblotted with the indicated antibodies. (B) 293T cells were transfected with control (Ctrl) or HSP105 siRNA for 72 h and were then treated with 100 ng/ml Wnt3a for the indicated times. Whole-cell lysates were immunoblotted with the indicated antibodies. (C) 293T cells were transfected with control or APC siRNA in the absence or presence of HSP105 siRNA for 72 h. Membrane β-catenin-depleted whole-cell lysates were immunoblotted with the indicated antibodies. (D) SW480, COLO205, or LS1034 cells were transfected with control or HSP105 siRNA for 72 h. Membrane β-catenin-depleted whole-cell lysates were immunoblotted with the indicated antibodies. (E) CTNNB1 gene expression in SW480 cells transfected with HSP105 siRNA for 72 h. (F) SW480 cells were first transfected with control (Ctrl) siRNA or HSP105 siRNA for 72 h, then treated with 10 μg/ml cycloheximide (CHX), and finally harvested at the indicated times. Membrane β-catenin-depleted whole-cell lysates were immunoblotted with the indicated antibodies. (G and H) Relative expression of AXIN2 (G) and GAD1 (H) in SW480 cells transfected with control or HSP105 siRNA for 72 h. Levels are expressed relative to those in control siRNA-transfected cells. Error bars represent standard errors of the means of triplicate measurements.
We used SW480, COLO205, and LS1034 colon cancer cell lines, all of which have a mutant APC gene and constitutive β-catenin accumulation (27). Indeed, depletion of HSP105 significantly reduced the β-catenin protein levels in all cell lines tested (Fig. 3D). The decrease in β-catenin protein was unlikely to be at the transcription level, since the mRNA level of CTNNB1 did not change when HSP105 was knocked down (Fig. 3E). Furthermore, although β-catenin is fairly stable in SW480 cells, knockdown of HSP105 decreased the half-life of β-catenin to ∼2 h (Fig. 3F), which correlated with reductions in the mRNA levels of Wnt target genes, such as AXIN2 and GAD1 (Fig. 3G and H; also data not shown).
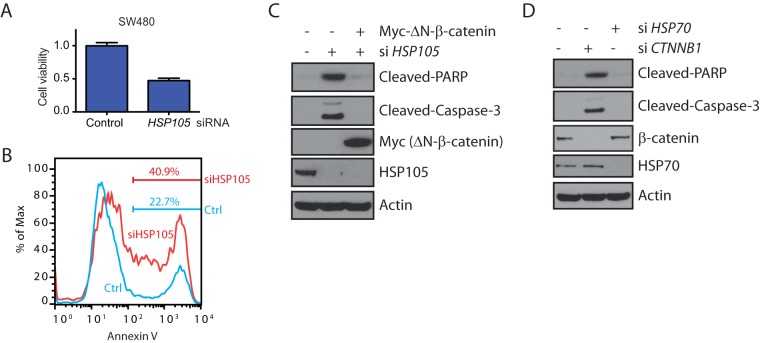
Dominant negative TCF1/TCF4 or depletion of β-catenin slowed down cell proliferation in long-term assays and inhibited tumor formation in xenograft models (28, 29). Similarly, knockdown of HSP105 compromised the proliferation of SW480 cells, as measured by a Syto 60 proliferation assay, relative to that with a nontargeting control siRNA (Fig. 4A). This was likely due to apoptosis induced by knockdown of HSP105, as shown by increased numbers of cells staining positive for annexin V (Fig. 4B). Furthermore, depletion of either HSP105 or β-catenin, but not HSP70 depletion, caused cleavage of PARP and caspase-3 (Fig. 4C and D). Importantly, the cleavage of PARP and caspase-3 induced by HSP105 depletion could be blocked by the transfection of nondegradable ΔN-β-catenin (Fig. 4C), suggesting that the apoptotic effect likely occurs by reducing β-catenin protein levels. These data are consistent with a previous study showing that HSP105 knockdown compromised cancer cell proliferation and induced apoptosis (30).
FIG 4.
HSP105 knockdown induces colorectal cancer cells to undergo apoptosis. (A) Viability of SW480 cells transfected with control or HSP105 siRNA for 144 h, as measured by Syto 60 nucleic acid staining. Viability was plotted relative to that of cells transfected with control siRNA. Error bars represent standard errors of the means of triplicate measurements. (B) SW480 cells were transfected with control (Ctrl) or HSP105 siRNA for 144 h. Cells were then collected and were stained with annexin V. (C) SW480 cells were transfected with control or HSP105 siRNA for 96 h, followed by transfection with a vector or Myc-ΔN-β-catenin for 48 h. Whole-cell lysates were immunoblotted with the indicated antibodies. (D) SW480 cells were transfected with control siRNA or with siRNA against HSP70 or CTNNB1 for 144 h. Whole-cell lysates were immunoblotted with the indicated antibodies.
HSP105 is required for PP2A to interact with the β-catenin degradation complex.
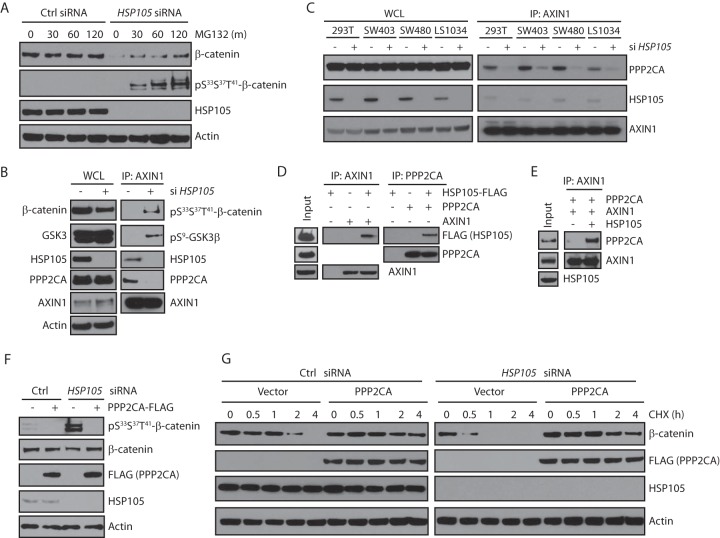
Previous studies have documented that β-catenin is sequentially phosphorylated by CK1α and GSK3β to generate a phosphodegron and undergo ubiquitination-dependent degradation (31, 32). The data mentioned above, showing that HSP105 knockdown decreased β-catenin protein levels but not CTNNB1 gene transcript levels, suggest that HSP105 may regulate the phosphorylation of β-catenin. Indeed, in the SW480 cell line, which has stable β-catenin due to defective APC, HSP105 depletion significantly decreased β-catenin levels (Fig. 3D and F), a decrease that could be reversed when the cells were treated with MG132, a proteasome inhibitor, to block β-catenin degradation (Fig. 5A). Importantly, MG132 treatment also significantly increased phospho-β-catenin levels, which were negligible when the cells were transfected with control siRNA (Fig. 5A), suggesting that HSP105 loss induced the phosphorylation of β-catenin and its ensuing degradation. This was further confirmed in 293T cells: although there was less β-catenin in whole-cell lysates when HSP105 was depleted, there was much more phospho-β-catenin interacting with axin1 (Fig. 5B). Interestingly, we also detected more pSer9-GSK3β interacting with axin1 (Fig. 5B), suggesting that other components of the β-catenin complex could also be phosphorylated at higher levels when HSP105 is depleted.
FIG 5.
HSP105 is required for the interaction of PP2A with the destruction complex. (A) SW480 cells were transfected with control or HSP105 siRNA for 72 h and were incubated with 25 μM MG132 for the indicated times (in minutes) prior to harvest. Western blots are shown for the indicated proteins. (B) HEK293T cells were transfected with control or HSP105 siRNA for 72 h. Whole-cell lysates were immunoprecipitated with an anti-axin1 antibody, and Western blots for the indicated proteins are shown. (C) HEK293T, SW403, SW480, and LS1034 cells were transfected with control or HSP105 siRNA for 72 h. Whole-cell lysates were immunoprecipitated with an anti-axin1 antibody, and Western blots for the indicated proteins are shown. (D) HSP105 protein was mixed with axin1 or PPP2CA, immunoprecipitated with anti-axin1 or anti-PPP2CA, and analyzed by Western blotting. (E) PPP2CA was mixed with axin1 in the absence or presence of recombinant HSP105 protein overnight, immunoprecipitated with an anti-axin1 antibody, and analyzed by Western blotting. (F) 293T cells were transfected with control or HSP105 siRNA for 16 h, followed by transfection of a vector control or PPP2CA for 48 h. Cells were treated with 25 μM MG132 for 4 h before harvest. Whole-cell lysates were immunoblotted with the indicated antibodies. (G) 293T cells were transfected with control or HSP105 siRNA for 16 h, followed by transfection of a vector or PPP2CA for 48 h. Cells were then treated with 10 μg/ml cycloheximide (CHX) and were harvested at the times indicated. Membrane β-catenin-depleted whole-cell lysates were immunoblotted with the indicated antibodies.
Under basal conditions, β-catenin is subject to a delicate balance of phosphorylation and dephosphorylation by CK1α, GSK3β, and PP2A (4). Since HSP105 is also a component of the β-catenin degradation complex (Fig. 1) and HSP105 interacts with PPP2CA (Fig. 1E), potential explanations for the increased phosphorylation of the β-catenin complex upon HSP105 depletion could be that (i) HSP105 is required for PP2A to interact with the β-catenin degradation complex or (ii) HSP105 is required for PP2A to be active. In the above-described experiment, we found that HSP105 knockdown also caused PPP2CA, the catalytic subunit of PP2A, to dissociate from axin1 (Fig. 5B). This was not restricted to 293T cells, since knockdown of HSP105 also caused PPP2CA to dissociate from axin1 in the SW480, SW403, and LS1034 colon cancer cell lines (Fig. 5C), suggesting that HSP105 likely behaves as a bridging molecule for PPP2CA to associate with the β-catenin degradation complex to dephosphorylate β-catenin. Indeed, using recombinant purified proteins, we found that both axin1 and PPP2CA interacted with HSP105 (Fig. 5D). Importantly, HSP105 seemed to be required for the association between PPP2CA and axin1, since in an in vitro interaction assay, we found that although recombinant axin1 and PPP2CA proteins weakly interacted with each other, the addition of HSP105 protein drastically increased this interaction (Fig. 5E). Furthermore, ectopically expressed PPP2CA could override the effect of HSP105 depletion in 293T cells, causing β-catenin to be dephosphorylated even when HSP105 was knocked down (Fig. 5F). Finally, although HSP105 depletion significantly reduced the half-life of β-catenin in HEK293T cells, β-catenin was stabilized to a level comparable to that with control siRNA when PPP2CA was overexpressed (Fig. 5G).
HSP105 is associated with a poor prognosis for cancer patients.
Abnormal activation of Wnt signaling is found in various types of human cancers (3, 33, 34). This is especially relevant for colorectal cancer patients, the majority of whom have genetic disorders on APC (∼80%) or β-catenin itself (∼10%), or translocation of the R-spondin gene (3, 34). Since HSP105 is required for cytosolic β-catenin accumulation and Wnt target gene transcription upon Wnt stimulation (Fig. 2), we examined human tumors for overexpression of HSP105. The RNA-sequencing data reviewed showed that mRNA levels of HSP105 but not HSP70 were increased in many colon and rectum tumors (Fig. 6A and B). To confirm this, HSP105 expression in human colorectal adenocarcinomas was investigated by immunoblotting, and all samples contained more HSP105 protein than normal cells, correlating with significantly upregulated cytosolic β-catenin protein levels; β-catenin is less abundant, and HSP105 is barely detectable, in most noncancerous tissues (Fig. 6C). To exclude the possibility of contamination from noncancer cells, we performed immunohistochemical (IHC) staining of additional colon adenocarcinoma samples (n = 66). The IHC results also showed that increased HSP105 levels correlated with increases in nuclear/cytosolic but not membrane β-catenin levels in tumor samples. (P < 0.001) (Fig. 6D). Furthermore, we demonstrated that in colon cancer samples, the expression of HSP105 but not HSP70 was significantly correlated with mRNA levels of AXIN2 (Spearman's rank correlation coefficient, 0.41; P, <2.2 × 10−16), MET (Spearman's rank correlation coefficient, 0.3; P, 4.8 × 10−9), and SALL4 (Spearman's rank correlation coefficient, 0.42; P, <2.2 × 10−16), all of which are Wnt target genes (Fig. 6E and F) (35, 36).
Interrogation of public expression databases showed that elevated HSP105 mRNA levels in tumors were significantly associated with a poor prognosis for patients with breast cancer. Patients expressing lower HSP105 mRNA levels had a significantly better relapse-free survival (RFS) (P = 0.05), or overall survival (OS) (P = 0.007) rate (Fig. 6G). Furthermore, in BRIM2, a study of the BRAF inhibitor vemurafenib in metastatic melanoma (19), higher baseline mRNA expression of the HSP105 gene was associated with a shorter duration of progression-free survival (PFS) (P = 0.002), with a hazard ratio (HR) of 2.58 (95% confidence interval, [95% CI],1.42 to 4.8; P < 0.002) (Fig. 6H). Interestingly, HSP105 mRNA expression was lower in tumor biopsy specimens taken while patients were on vemurafenib treatment and rebounded at the disease progression (PD) stage (Fig. 6I). Taken together, our findings indicate that the expression of HSP105 is of prognostic relevance for several human cancers.
DISCUSSION
PP2A has been shown to dephosphorylate and stabilize β-catenin and therefore to positively regulate Wnt signaling (7, 8). Here we show that this regulation of β-catenin by PP2A is also dependent on HSP105. HSP105 is a component of the β-catenin degradation complex by virtue of its direct association with axin (Fig. 1 and 5). Importantly, this interaction is indispensable for the association of PP2A with the degradation complex and the dephosphorylation of β-catenin, facilitating a fine balance between the phosphorylation and dephosphorylation of β-catenin. When HSP105 is depleted, PPP2CA, the catalytic subunit of PP2A, falls off from the β-catenin degradation complex, and the balance is skewed to the phosphorylation of β-catenin; thus, more β-catenin is degraded (Fig. 5). This function of HSP105 is important not only for normal Wnt signaling but also in cancer cell models, since knockdown of HSP105 impairs β-catenin transcriptional activities in both settings (Fig. 2 and 3). Furthermore, HSP105 seems to regulate the phosphorylation of other proteins in the β-catenin complex as well, since more GSK3β was phosphorylated when HSP105 was knocked down (Fig. 5B and data not shown). Interestingly, the phosphorylation of GSK3 decreases its kinase activity, whereas we still detected more phospho-β-catenin upon HSP105 depletion. This may be because phosphorylation only attenuates, but does not abrogate, the kinase activity of GSK3. Therefore, any phosphorylated β-catenin will still be quickly degraded if it is not dephosphorylated by PP2A. How HSP105 regulates the interaction of PP2A with axin1 is still unknown. Heat shock proteins interact with a large group of substrate proteins, or “clients,” promoting their correct folding and function (12). It is possible that HSP105 regulates the proper conformation of PP2A in order for PP2A to associate with axin1. This has been demonstrated for the small heat shock protein HSP20, which interacts with protein phosphatase 1 (PP1) and enhances sarcoplasmic reticulum calcium cycling (14). Furthermore, HSP105 could simply behave as an adaptor protein in order for PP2A to bind with axin1.
Heat shock proteins are highly expressed in a wide range of human cancer cells and are essential for those tumor cells to proliferate and differentiate (37–39). Though seldom treated as informative predictive diagnostic biomarkers, the expression levels of heat shock proteins are useful prognostic biomarkers for tumorigenesis in some cancers (38, 39). For example, the expression level of HSP27 is associated with a poor prognosis in gastric, liver, and prostate carcinomas (40–42). It has also been shown that HSP105 is one of the most highly upregulated proteins in melanoma and colon cancer patients, an upregulation that is correlated with hyperactivation of Wnt signaling (43–45). However, it was not completely clear what benefit tumor cells might gain from overexpression of HSP105. Here we provide an explanation of a potential benefit for those tumors. We found that overexpression of HSP105 is correlated with upregulated β-catenin protein levels and transcription of Wnt target genes (Fig. 6). Importantly, this upregulation of β-catenin is independent of transcription (Fig. 3E). Furthermore, only nuclear/cytosolic β-catenin levels, not membrane β-catenin levels, correlate with HSP105 expression levels in colorectal tumor samples (Fig. 6D). Correspondingly, the transcription of Wnt target genes is downregulated in cell line models upon HSP105 depletion (Fig. 2) and is upregulated upon overexpression of HSP105 in cancer patients (Fig. 6). Abnormally activated Wnt signaling may cause cancer, providing an explanation of a possible mechanism of action for overexpression of HSP105.
The implication of heat shock proteins in tumorigenesis has led to the development of successful inhibitors for cancer therapy. For example, the involvement of HSP90 in multiple oncogenic pathways makes it an ideal target for anticancer agents. Geldanamycin, a benzoquinone ansamycin antibiotic that inhibits the function of HSP90 by fitting into the ADP/ATP-binding pocket of the protein, induces the degradation of proteins that are mutated in tumor cells, such as BCR-ABL and p53 (46, 47), both of which are crucial players in many tumors, supporting further research into the development of novel HSP90 inhibitors (48). Here we show that knockdown of HSP105 downregulates cytosolic/nuclear β-catenin protein levels, even when APC is depleted or mutated (Fig. 3C and D). Furthermore, siRNA knockdown of HSP105 inhibits cell proliferation in multiple colon cancer cell lines that harbor APC mutations (Fig. 4 and data not shown). These data establish HSP105 as a potential target for the treatment of colon cancer patients, the majority of whom have APC mutations and active β-catenin signaling (3). Furthermore, like many other heat shock proteins, HSP105 has an ATPase domain at its N terminus (49, 50). It will be interesting to see if the weak ATPase activity of HSP105 is required for its regulation of Wnt signaling and therefore has potential as a novel target for anticancer therapy.
ACKNOWLEDGMENTS
We thank Jim Nonomiya for providing purified recombinant axin1 protein and Mingjian Fei for proofreading the manuscript.
REFERENCES
- 1.MacDonald BT, Tamai K, He X. 2009. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell 17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clevers H, Nusse R. 2012. Wnt/β-catenin signaling and disease. Cell 149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Polakis P. 2000. Wnt signaling and cancer. Genes Dev 14:1837–1851. doi: 10.1101/gad.14.15.1837. [DOI] [PubMed] [Google Scholar]
- 4.Stamos JL, Weis WI. 2013. The β-catenin destruction complex. Cold Spring Harb Perspect Biol 5:a007898. doi: 10.1101/cshperspect.a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C, Kato Y, Zhang Z, Do VM, Yankner BA, He X. 1999. β-Trcp couples β-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc Natl Acad Sci U S A 96:6273–6278. doi: 10.1073/pnas.96.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winston JT, Strack P, Beer-Romero P, Chu CY, Elledge SJ, Harper JW. 1999. The SCFβ-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκBα and β-catenin and stimulates IκBα ubiquitination in vitro. Genes Dev 13:270–283. doi: 10.1101/gad.13.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang W, Yang J, Liu Y, Chen X, Yu T, Jia J, Liu C. 2009. PR55α, a regulatory subunit of PP2A, specifically regulates PP2A-mediated β-catenin dephosphorylation. J Biol Chem 284:22649–22656. doi: 10.1074/jbc.M109.013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su Y, Fu C, Ishikawa S, Stella A, Kojima M, Shitoh K, Schreiber EM, Day BW, Liu B. 2008. APC is essential for targeting phosphorylated β-catenin to the SCFβ-TrCP ubiquitin ligase. Mol Cell 32:652–661. doi: 10.1016/j.molcel.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Kalev P, Sablina AA. 2011. Protein phosphatase 2A as a potential target for anticancer therapy. Anticancer Agents Med Chem 11:38–46. doi: 10.2174/187152011794941172. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Wu J, Tan C, Klein PS. 2003. PP2A:B56ε is required for Wnt/β-catenin signaling during embryonic development. Development 130:5569–5578. doi: 10.1242/dev.00762. [DOI] [PubMed] [Google Scholar]
- 11.Lindquist S, Craig EA. 1988. The heat-shock proteins. Annu Rev Genet 22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 12.Feder ME, Hofmann GE. 1999. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- 13.Georgopoulos C, Welch WJ. 1993. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol 9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- 14.Qian J, Vafiadaki E, Florea SM, Singh VP, Song W, Lam CK, Wang Y, Yuan Q, Pritchard TJ, Cai W, Haghighi K, Rodriguez P, Wang HS, Sanoudou D, Fan GC, Kranias EG. 2011. Small heat shock protein 20 interacts with protein phosphatase-1 and enhances sarcoplasmic reticulum calcium cycling. Circ Res 108:1429–1438. doi: 10.1161/CIRCRESAHA.110.237644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taipale M, Krykbaeva I, Koeva M, Kayatekin C, Westover KD, Karras GI, Lindquist S. 2012. Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell 150:987–1001. doi: 10.1016/j.cell.2012.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu TD, Nacu S. 2010. Fast and SNP-tolerant detection of complex variants and splicing in short reads. Bioinformatics 26:873–881. doi: 10.1093/bioinformatics/btq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anders S, Huber W. 2010. Differential expression analysis for sequence count data. Genome Biol 11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang X, Summers MK, Pham V, Lill JR, Liu J, Lee G, Kirkpatrick DS, Jackson PK, Fang G, Dixit VM. 2011. Deubiquitinase USP37 is activated by CDK2 to antagonize APCCDH1 and promote S phase entry. Mol Cell 42:511–523. doi: 10.1016/j.molcel.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 19.Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, McArthur GA, Hutson TE, Moschos SJ, Flaherty KT, Hersey P, Kefford R, Lawrence D, Puzanov I, Lewis KD, Amaravadi RK, Chmielowski B, Lawrence HJ, Shyr Y, Ye F, Li J, Nolop KB, Lee RJ, Joe AK, Ribas A. 2012. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacDonald BT, Yokota C, Tamai K, Zeng X, He X. 2008. Wnt signal amplification via activity, cooperativity, and regulation of multiple intracellular PPPSP motifs in the Wnt co-receptor LRP6. J Biol Chem 283:16115–16123. doi: 10.1074/jbc.M800327200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunner E, Peter O, Schweizer L, Basler K. 1997. pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature 385:829–833. doi: 10.1038/385829a0. [DOI] [PubMed] [Google Scholar]
- 22.Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destrée O, Clevers H. 1996. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell 86:391–399. doi: 10.1016/S0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Appleton BA, Wiesmann C, Lau T, Costa M, Hannoush RN, Sidhu SS. 2009. Inhibition of Wnt signaling by Dishevelled PDZ peptides. Nat Chem Biol 5(4):217–219. doi: 10.1038/nchembio.152. [DOI] [PubMed] [Google Scholar]
- 24.Huang X, McGann JC, Liu BY, Hannoush RN, Lill JR, Pham V, Newton K, Kakunda M, Liu J, Yu C, Hymowitz SG, Hongo JA, Wynshaw-Boris A, Polakis P, Harland RM, Dixit VM. 2013. Phosphorylation of Dishevelled by protein kinase RIPK4 regulates Wnt signaling. Science 339:1441–1445. doi: 10.1126/science.1232253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamagishi N, Ishihara K, Hatayama T. 2004. Hsp105α suppresses Hsc70 chaperone activity by inhibiting Hsc70 ATPase activity. J Biol Chem 279:41727–41733. doi: 10.1074/jbc.M407947200. [DOI] [PubMed] [Google Scholar]
- 26.Rubinfeld B, Souza B, Albert I, Müller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P. 1993. Association of the APC gene product with β-catenin. Science 262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- 27.Rowan AJ, Lamlum H, Ilyas M, Wheeler J, Straub J, Papadopoulou A, Bicknell D, Bodmer WF, Tomlinson IP. 2000. APC mutations in sporadic colorectal tumors: a mutational “hotspot” and interdependence of the “two hits.” Proc Natl Acad Sci U S A 97:3352–3357. doi: 10.1073/pnas.97.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H. 2002. The β-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111:241–250. doi: 10.1016/S0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 29.Scholer-Dahirel A, Schlabach MR, Loo A, Bagdasarian L, Meyer R, Guo R, Woolfenden S, Yu KK, Markovits J, Killary K, Sonkin D, Yao YM, Warmuth M, Sellers WR, Schlegel R, Stegmeier F, Mosher RE, McLaughlin ME. 2011. Maintenance of adenomatous polyposis coli (APC)-mutant colorectal cancer is dependent on Wnt/β-catenin signaling. Proc Natl Acad Sci U S A 108:17135–17140. doi: 10.1073/pnas.1104182108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosaka S, Nakatsura T, Tsukamoto H, Hatayama T, Baba H, Nishimura Y. 2006. Synthetic small interfering RNA targeting heat shock protein 105 induces apoptosis of various cancer cells both in vitro and in vivo. Cancer Sci 97:623–632. doi: 10.1111/j.1349-7006.2006.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernández AR, Klein AM, Kirschner MW. 2012. Kinetic responses of β-catenin specify the sites of Wnt control. Science 338:1337–1340. doi: 10.1126/science.1228734. [DOI] [PubMed] [Google Scholar]
- 32.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. 2002. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108:837–847. doi: 10.1016/S0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 33.Polakis P. 2007. The many ways of Wnt in cancer. Curr Opin Genet Dev 17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Seshagiri S, Stawiski EW, Durinck S, Modrusan Z, Storm EE, Conboy CB, Chaudhuri S, Guan Y, Janakiraman V, Jaiswal BS, Guillory J, Ha C, Dijkgraaf GJ, Stinson J, Gnad F, Huntley MA, Degenhardt JD, Haverty PM, Bourgon R, Wang W, Koeppen H, Gentleman R, Starr TK, Zhang Z, Largaespada DA, Wu TD, de Sauvage FJ. 2012. Recurrent R-spondin fusions in colon cancer. Nature 488:660–664. doi: 10.1038/nature11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Böhm J, Sustmann C, Wilhelm C, Kohlhase J. 2006. SALL4 is directly activated by TCF/LEF in the canonical Wnt signaling pathway. Biochem Biophys Res Commun 348:898–907. doi: 10.1016/j.bbrc.2006.07.124. [DOI] [PubMed] [Google Scholar]
- 36.Boon EM, van der Neut R, van de Wetering M, Clevers H, Pals ST. 2002. Wnt signaling regulates expression of the receptor tyrosine kinase Met in colorectal cancer. Cancer Res 62:5126–5128. [PubMed] [Google Scholar]
- 37.Cornford PA, Dodson AR, Parsons KF, Desmond AD, Woolfenden A, Fordham M, Neoptolemos JP, Ke Y, Foster CS. 2000. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res 60:7099–8105. [PubMed] [Google Scholar]
- 38.van't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH. 2002. Gene expression profiling predicts clinical outcome of breast cancer. Nature 415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 39.van de Vijver MJ, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van der Velde T, Bartelink H, Rodenhuis S, Rutgers ET, Friend SH, Bernards R. 2002. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 40.Kapranos N, Kominea A, Konstantinopoulos PA, Savva S, Artelaris S, Vandoros G, Sotiropoulou-Bonikou G, Papavassiliou AG. 2002. Expression of the 27-kDa heat shock protein (HSP27) in gastric carcinomas and adjacent normal, metaplastic, and dysplastic gastric mucosa, and its prognostic significance. J Cancer Res Clin Oncol 128:426–432. doi: 10.1007/s00432-002-0357-y. [DOI] [PubMed] [Google Scholar]
- 41.King KL, Li AF, Chau GY, Chi CW, Wu CW, Huang CL, Lui WY. 2000. Prognostic significance of heat shock protein-27 expression in hepatocellular carcinoma and its relation to histologic grading and survival. Cancer 88:2464–2470. doi:. [DOI] [PubMed] [Google Scholar]
- 42.Bostwick DG. 2000. Immunohistochemical changes in prostate cancer after androgen deprivation therapy. Mol Urol 4(3):101–107. [PubMed] [Google Scholar]
- 43.Kai M, Nakatsura T, Egami H, Senju S, Nishimura Y, Ogawa M. 2003. Heat shock protein 105 is overexpressed in a variety of human tumors. Oncol Rep 10:1777–1782. doi: 10.3892/or.10.6.1777. [DOI] [PubMed] [Google Scholar]
- 44.Skrzypczak M, Goryca K, Rubel T, Paziewska A, Mikula M, Jarosz D, Pachlewski J, Oledzki J, Ostrowski J. 2010. Modeling oncogenic signaling in colon tumors by multidirectional analyses of microarray data directed for maximization of analytical reliability. PLoS One 5:e13091. doi: 10.1371/journal.pone.0013091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Talantov D, Mazumder A, Yu JX, Briggs T, Jiang Y, Backus J, Atkins D, Wang Y. 2005. Novel genes associated with malignant melanoma but not benign melanocytic lesions. Clin Cancer Res 11:7234–7242. doi: 10.1158/1078-0432.CCR-05-0683. [DOI] [PubMed] [Google Scholar]
- 46.Gorre ME, Ellwood-Yen K, Chiosis G, Rosen N, Sawyers CL. 2002. BCR-ABL point mutants isolated from patients with imatinib mesylate-resistant chronic myeloid leukemia remain sensitive to inhibitors of the BCR-ABL chaperone heat shock protein 90. Blood 100:3041–3044. doi: 10.1182/blood-2002-05-1361. [DOI] [PubMed] [Google Scholar]
- 47.Blagosklonny MV, Toretsky J, Neckers L. 1995. Geldanamycin selectively destabilizes and conformationally alters mutated p53. Oncogene 11:933–939. [PubMed] [Google Scholar]
- 48.Whitesell L, Santagata S, Lin NU. 2012. Inhibiting HSP90 to treat cancer: a strategy in evolution. Curr Mol Med 12:1108–1124. doi: 10.2174/156652412803306657. [DOI] [PubMed] [Google Scholar]
- 49.Andréasson C, Fiaux J, Rampelt H, Druffel-Augustin S, Bukau B. 2008. Insights into the structural dynamics of the Hsp110-Hsp70 interaction reveal the mechanism for nucleotide exchange activity. Proc Natl Acad Sci U S A 105:16519–16524. doi: 10.1073/pnas.0804187105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andréasson C, Fiaux J, Rampelt H, Mayer MP, Bukau B. 2008. Hsp110 is a nucleotide-activated exchange factor for Hsp70. J Biol Chem 283:8877–8884. doi: 10.1074/jbc.M710063200. [DOI] [PubMed] [Google Scholar]