Abstract
Signaling via the pre-T-cell receptor (pre-TCR), along with associated signals from Notch and chemokine receptors, regulates the β-selection checkpoint that operates on CD4− CD8− doubly negative (DN) thymocytes. Since many hematopoietic malignancies arise at the immature developmental stages of lymphocytes, understanding the signal integration and how specific signaling molecules and distal transcription factors regulate cellular outcomes is of importance. Here, a series of molecular and genetic approaches revealed that the ShcA adapter protein critically influences proliferation and differentiation during β-selection. We found that ShcA functions downstream of the pre-TCR and p56Lck and show that ShcA is important for extracellular signal-regulated kinase (ERK)-dependent upregulation of transcription factors early growth factor 1 (Egr1) and Egr3 in immature thymocytes and, in turn, of the expression and function of the Id3 and E2A helix-loop-helix (HLH) proteins. ShcA also contributes to pre-TCR-mediated induction of c-Myc and additional cell cycle regulators. Moreover, using an unbiased Saccharomyces cerevisiae (yeast) screen, we identified c-Abl as a binding partner of phosphorylated ShcA and demonstrated the relevance of the ShcA–c-Abl interaction in immature thymocytes. Collectively, these data identify multiple modes by which ShcA can fine-tune the development of early thymocytes, including a previously unappreciated ShcA–c-Abl axis that regulates thymocyte proliferation.
INTRODUCTION
The commitment of the bone marrow-derived T-cell progenitors to the α/β T-cell lineage is an orderly process, characterized by a series of developmental checkpoints in the thymus (1). The early progenitors differentiate into CD4− CD8− doubly negative thymocytes (DN), which can be further divided into four main developmental subsets (DN1 to DN4) based on the surface expression of c-kit, CD25, and CD44 (2). The successful rearrangement of the T-cell receptor beta (TCRβ) locus in immature DN3 thymocytes leads to expression of a functional pre-TCR that triggers signaling in conjunction with other cell surface receptors (3). Progress through the first checkpoint, also called β-selection, is associated with the survival, proliferation, and maturation of the DN thymocytes to CD4+ CD88+ (DP) thymocytes. The pre-TCR-initiated signaling, and its integration with other cues that mediate this developmental transition is still incompletely understood (4). Since many T-cell lymphomas arise from dysregulation at these developmental stages, a better understanding of this process and of the molecules regulating proliferation is clearly warranted.
Studies using genetic approaches have revealed the multiplicity of regulatory networks during β-selection. The complex consisting of pre-TCR and CD3 that transduces differentiation signals depends on the non-receptor tyrosine kinases implicated in TCR signaling. Knockout mouse studies for the tyrosine kinases p56Lck, ZAP-70, Syk, Tek, and c-abl have shown a defect in thymocyte development with a partial or complete block in the transition from DN3 to DN4 (5, 6). These tyrosine kinases recruit adaptor proteins such as LAT (7), SLP-76 (8), and ShcA (9) to coordinate the interactions between signaling pathways. Mouse models with either loss of these adapter proteins or expression of dominant-negative mutants show a developmental block in the DN3-to-DN4 transition (2). We previously demonstrated that ShcA-mediated signaling downstream of the pre-TCR is essential for the transition of DN3 to later stages of development and that ShcA contributes to nearly two-thirds of the activation of extracellular signal-regulated kinase 1 (ERK1) and ERK2 (referred to here as ERK) at this stage (10). Downstream targets of ERK, such as transcription factors early growth factor 1 (Egr1), Egr2, and Egr3, also contribute to early thymocyte differentiation, proliferation, and transition to the DP stage (11, 12). Those studies revealed the importance of the Ras/ERK pathway in thymocyte differentiation. However, the integration of ERK-independent signaling and parallel pathways is still incompletely understood.
ShcA is ubiquitously expressed as three isoforms: p46, p52, and p66. ShcA contains three conserved tyrosine residues (tyrosines 239, 240, and 317) that are phosphorylated by activated tyrosine kinases and serve as docking sites for Grb2-Sos (13). In T cells, ShcA becomes rapidly tyrosine phosphorylated upon T-cell receptor (TCR) stimulation, and this leads to the activation of the ERK pathway (9, 10). Mice with a global deletion of ShcA show early embryonic death, suggesting the importance of ShcA in development (14). To address the role of ShcA during T-cell development, we previously used two different genetic approaches: the inducible transgenic expression of a phosphorylation-defective dominant-negative mutant of ShcA (ShcFFF) and the conditional knockout of ShcA in thymocytes. Those studies revealed an essential and nonredundant role for ShcA during β-selection (15). ShcA also was required for CXCR4 function during β-selection (10, 16).
Proliferation is a significant step driven by pre-TCR/p56Lck/ERK signaling during β-selection. Activation of ERK kinases in early thymocytes contributes to the induction of Egr1 and Egr3 (early growth response factors) and promotes cell growth and differentiation (12). The rapid and transient expression of Egr1 and 3 leads to the induction of transcription regulator Id3, which inhibits the basic helix-loop-helix (bHLH) E proteins (17, 18). In early T-cell development, pre-TCR-induced Id3 protein expression supports cell proliferation, while the E-proteins promote cell cycle arrest, suggesting their opposing roles during β-selection (19). Abl family kinases are also involved in pre-TCR signaling, as the loss of abl1 and abl2 during β-selection leads to defects in thymocyte proliferation and differentiation (20). These findings indicate the importance of Erk-dependent and -independent signaling in the DN-to-DP transition.
Here, we addressed the importance of ShcA phosphorylation in controlling different outcomes downstream of pre-TCR signaling using transgenic expression of a phosphorylation-defective ShcFFF mutant in DN thymocytes in vivo (10). We find that ShcA is positioned downstream of the pre-TCR and p56Lck during β-selection. ShcA phosphorylation is specifically required for cellular proliferation through Egr transcription factors, ultimately affecting the expression of the Id3 and E2A HLH proteins. We also identified a new ShcA–c-Abl link that promotes cell proliferation and migration downstream of the pre-TCR and CXCR4. Collectively, these data reveal mechanisms by which the adapter protein ShcA can regulate outcomes of DN thymocytes during β-selection.
MATERIALS AND METHODS
Animals.
C57BL/6, D011.10, and Lck-Cre transgenic line mice and p53-deficient mice were from Jackson Laboratory (Bar Harbor, ME). The generation of the four different transgenic lines inducibly expressing wild-type ShcA or ShcA mutants has been described previously (10, 15). The LckF505 transgenic mouse line was obtained from Roger Perlmutter's laboratory. The Arg−/−, Lck-Cre Abl1fl/fl, and double knockout lines have been described previously (20). Mice were bred and maintained under specific-pathogen-free conditions at the University of Virginia animal facility according to IACUC-approved protocols.
Flow cytometry.
Thymocytes from 3- to 6-week-old mice were stained as described previously (10). Reagents used in the analysis included monoclonal antibodies (MAbs) to CD3ε (2C11), CD4 (RM4.5), CD8α (53.6-7), CD25 (PC61), CD44 (IM7), Thy1.2 (53-2.1), TCRβ (H57-597), Ter-119, Gr-1 (RB6-8C5), and B220 (RA3-6B2). Antibodies were purchased from eBiosciences or BD Biosciences. Flow cytometry was performed with BD FACSCanto II, and the results were analyzed with FlowJo software.
In vivo BrdU proliferation assay.
Mice were given intraperitoneal injection of 1 mg 5-bromo-2′-deoxyuridine (BrdU) in saline solution, and thymi were harvested 1 h postinjection. Single-cell suspensions of thymocytes were stained for CD4, CD8, CD3, CD44, and CD25 surface markers and a panel of hematopoietic lineage markers (CD11b, CD11c, B220, Ly6G, and Ter119). Staining for incorporated BrdU and for 7-aminoactinomycin D (7-AAD) was performed using a BrdU Flow kit (BD Biosciences). Proliferation was assessed within the DN and DP thymocyte subsets, based on specific surface marker expression.
T-cell differentiation on OP9-DL1 stromal cells.
OP9 stromal cells expressing the Notch delta-like-1 ligand (OP9-DL1) were maintained as described previously (21). DN3E thymocytes (small CD4− CD8− CD3− c-kit− CD44− CD25+) from ShcFFF or Lck-Cre/ShcFFF mice were sorted based on size after gating out of a panel of hematopoietic lineage markers was performed. Thymocytes were plated at 104 cells/well on a layer of nonconfluent OP9-DL-1. Thymocytes were collected on days 2, 4, and 6 and analyzed for Thy1.2, CD4, CD8, CD44, and CD25 surface expression by flow cytometry and counted using reference beads during cytometry (Spherotech). Any stromal cells carried over were gated out on the basis of their side scatter and green fluorescent protein (GFP) expression. Each coculture experiment was done in triplicate.
Retroviral gene transfer.
Retroviral constructs were generated by subcloning Egr1, Egr3, and c-AblPP cDNAs into an murine stem cell virus (MSCV)-based MigR-1 vector that permits bicistronic expression of the gene of interest and enhanced GFP (eGFP). Retroviral particles were used to transduce sorted DN3E thymocytes cultured for 24 h on OP9-DL1 cells. Thymocytes were then transduced with viral particles as described previously (22). Developmental progression of transduced cells was assessed by flow cytometry on days 2 and 6 of culture by gating on Thy1.2+ GFP+ events. For CellTrace violet labeling (Molecular Probes/Invitrogen), transduced OP9-DL1 coculture-derived DN3E cells from control and Lck-Cre ShcFFF mice were incubated with a 3 μM concentration of the dye at 107 cells/ml in phosphate-buffered saline (PBS)–0.1% bovine serum albumin (BSA) for 15 min at 37°C. Cells were washed twice with growth medium and plated on OP9-DL1 cells. Cell division was analyzed by flow cytometry via dye dilution on day 6.
RNA extraction and qPCR.
Total RNA from thymocyte subpopulations was obtained using a Qiagen RNeasy kit. cDNA were prepared using a SuperScript III kit (Invitrogen). The cDNA samples were subjected to quantitative PCRs (qPCRs) using TaqMan gene expression assays (Applied Biosystems) (data not shown). Each sample was amplified in duplicate and normalized to Gapdh mRNA and Hprt1 mRNA as internal controls. The relative levels of the target gene were calculated by the “comparative threshold cycle (CT) method” in StepOne v2.1 software (Applied Biosystems [ABI]). Standard deviation values were calculated after normalization of data from multiple experiments.
Intracellular staining for flow cytometry.
For intracellular staining of TCR Vβ chains, Bcl2, Bcl-xL, c-Myc, and E2A, 3 × 106 thymocytes were stained for CD4, CD8α, and CD3 and hematopoietic-lineage markers. Cells were then fixed and permeabilized with a BD Cytofix/Cytoperm kit (BD Biosciences). Antibodies specific to Vβ2, Vβ4, Vβ5.1, 5.2, Vβ6, Vβ10, Vβ12, and Vβ14 TCR were from BD Biosciences. Bcl2, Bcl-xL, and E2a antibodies were from eBiosciences. Nonspecific staining was excluded by the use of an isotype-matched control antibody in and from a fluorescence minus-one staining strategy. Cells were then analyzed by cytometry. Intracellular staining for Egr1, Egr3, c-Myc, and phosphoproteins was performed as previously described (10, 16). Egr1, Egr3, c-Myc antibodies, and blocking peptides were from Santa Cruz Biotechnology, and phospho-ERK, phospho-Zap70 (Y319), phospho-LAT (Y171), phospho-CrkL (Y207), and phospho-c-Abl (Y191) were from BD Bioscience and Cell Signaling Technology. A total of 106 events were collected by cytometry for each samples (FACSCanto II), and data were analyzed using FlowJo.
Chemotaxis assay.
Chemotaxis was performed as previously described (16). To determine the effect of inhibiting CXCR4 or c-Abl kinases, cells were pretreated with 0.5 μM AMD3100 or a 2 μM concentration of c-Abl inhibitor STI571 (Sigma) for 10 min at 37°C. Transwell assays were performed in duplicate for each condition with thymocytes from a pool of a minimum of three mice per genotype.
Immunoprecipitation and immunoblotting.
Thymocytes were lysed in a buffer containing 50 mM Tris (pH 7.6), 150 mM NaCl, 1 mM EDTA, 1% NP-40, 10 mM sodium pyrophosphate, 1 mM sodium orthovanadate, and protease inhibitors (Calbiochem). ShcA immunoprecipitation and immunoblotting to detect phospho-ShcA were performed as described previously (10). Immunoblots were developed using enhanced chemiluminescence (Pierce, Rockford, IL).
Yeast two-hybrid analysis.
Saccharomyces cerevisiae (yeast) strain HF7c (His negative [His−] Trp− Leu− Met−) was used to screen for protein-protein interactions between indicated constructs (Grb2 SH2 domain) cloned into pVP16 (containing the VP16 transactivation domain) and pGBT9 (containing the GAL4 DNA binding domain) carrying either the wild-type (WT) CH1 domain from ShcA or the CH1 domain with the three mutated tyrosines. A positive protein-protein interaction in the assay was defined as growth on selective dropout (DO) plates (Trp−, Leu−, and His− with 1 mM 3-amino-1,2,4-triazole [3AT]); growth on the nonselective plate (with His) was used as a viability control.
Statistical methods.
Results are expressed as means ± standard deviations. Statistical significance was estimated with a two-sided t test (α = 0.05). Statistical analyses were performed using the Microsoft Excel program.
RESULTS
The requirement for ShcA function during β-selection is T cell intrinsic and is not overcome by enhanced signaling via transgenic TCR.
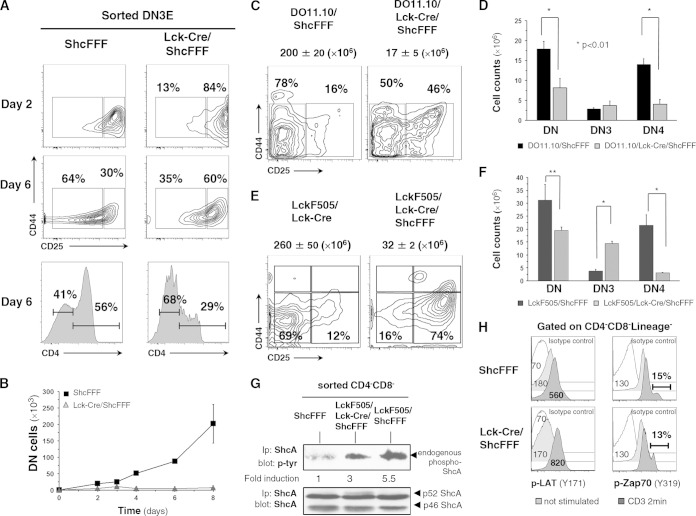
We have previously generated Cre-inducible ShcA transgenic mouse strains (15), where the transgenic ShcA proteins carry mutations in one or more of the three specific tyrosine residues (Y239F/Y240F, Y317F, and Y239F/Y240F/Y317F) (10). Crossing with the Lck-Cre mouse strain to induce expression of tyrosine-mutated ShcA proteins in DN thymocytes prior to β-selection revealed that the triply tyrosine-mutated ShcFFF transgenic mice displayed the most severe defect in T-cell development, with a complete arrest of the DN-to-DP transition (10). Among the three tyrosines, mutations of the Y239/Y240 tyrosines had a more profound effect than mutation of Y317. Here, we first tested whether the developmental block in transition of DN3 to DN4 (and subsequently to DP) observed in vivo in Lck-Cre/ShcFFF mice is intrinsic to the ShcFFF mice expressing thymocytes, using an ex vivo thymic differentiation assay on OP9-DL1 stromal cells (21). The DN3E thymocytes from the control mice showed CD25 downregulation from day 2, with DN4 and CD4+ cell thymocytes emerging by day 6 (Fig. 1A). Thymocytes from Lck-Cre/ShcFFF mice had very few DN4 cells and a 2-fold reduction in the percentage of CD4+ cells, with an apparent arrest at DN3 (Fig. 1A, right panel). The absolute numbers of DP (CD4+ CD88+) thymocytes were also markedly reduced in thymocytes expressing the ShcFFF transgene (Fig. 1B). Notably, overexpression of ShcFFF did not impair Notch1 expression in the various thymic subsets (data not shown), ruling out that possibility. These results suggested that the ShcFFF mutant-expressing thymocytes have an intrinsic defect in the pre-TCR-dependent signaling pathway.
FIG 1.
The requirement for ShcA is T cell intrinsic and not overcome by transgenic TCR. (A and B) DN3E thymocytes (sorted from control ShcFFF mice without Cre or Lck-Cre/ShcFFF mice) were seeded on OP9-DL1 stromal cells, and their maturation was analyzed on days 2 and 6 after initiation of coculture (n = 4 experiments). Data represent the absolute numbers of total thymocytes on days 2, 3, 4, 6, and 8. (C) Thymocytes from control DO11.10/ShcFFF doubly transgenic mice and DO11.10/Lck-Cre/ShcFFF triply transgenic mice were analyzed for the DN subsets (n = 4 experiments, with 6 mice analyzed per genotype). (D) Absolute numbers of gated KJ1-26-positive DN subpopulations from the DO11.10/ShcFFF and DO11.10/Lck-Cre/ShcFFF populations (n = 4). (E) Thymocytes from the indicated genotypes were analyzed in thymic subsets. Total thymocyte cellularity is indicated above each dot plot. (n = 3 independent experiments, with 6 mice per genotype). (F) The absolute numbers of total DN, DN3, and DN4 thymocytes in the different mouse strains (*, P < 0.05; **, P < 0.01). (G) Tyrosine phosphorylation of endogenous ShcA in DN thymocyte lysates in the different mouse strains. The densitometry of the signal for endogenous ShcA phosphorylation has been normalized to the ShcA expression level (n = 2). Ip, immunoprecipitation. (H) Histogram profiles show phospho-LAT (Y171) and phospho-ZAP70 (Y319) signal determined under unstimulated conditions or anti-CD3-stimulated conditions (2 min) in lineage-negative (Lin−) doubly negative (DN) (CD4− CD8−) subpopulations of thymocytes from control (ShcFFF) mice compared to those from Lck-Cre/ShcFFF mice. The data presented represent a pool of 3 and a pool of 4 mice, respectively, and are similar to data obtained from separate pools of control and Lck-Cre/ShcFFF mice (n > 3).
We next asked whether a rearranged/functional TCR transgene (DO11.10) could rescue the developmental block in Lck-Cre/ShcFFF mice (23). Thymocytes from DO11.10/Lck-Cre/ShcFFF triply transgenic mice were also arrested in their transition from DN to DP, essentially similarly to the Lck-Cre/ShcFFF mice (Fig. 1C). The absolute number of thymocytes from triply transgenic mice was reduced by almost 10-fold compared to the number from littermate DO11.10/ShcFFF mice (without Lck-Cre) (Fig. 1C). The DO11.10 transgenic TCR expression was confirmed in the DN thymocytes by staining with KJ1-26, a clonotypic antibody specific for the DO11.10 TCR (data not shown). There was an increase (from 9% to 48%) in the proportion of DN thymocytes in DO11.10/Lck-Cre/ShcFFF mice compared to control mice, with most of these DN thymocytes being DN3 and with a markedly reduced absolute number of DN4 cells (Fig. 1D). Although the control DO11.10 mice contain a higher-than-normal amount of DN thymocytes, these were mostly DN4 cells (as previously reported), due to faster progression to the DN4 stage as a result of the early expression of the rearranged TCR transgene. Thus, even in the presence of a rearranged TCR and the strong signaling known to occur through this transgenic TCR, the dominant-negative ShcFFF can substantially block differentiation at the DN3 stage.
ShcA is positioned downstream of p56Lck.
Current models propose p56Lck as the most proximal signaling molecule downstream of the pre-TCR, and mice with a disruption of the lck gene have a severe defect in thymocyte development, with a block at the DN3 stage (24). In vitro studies have demonstrated that p56Lck can mediate tyrosine phosphorylation of ShcA on the three tyrosine phosphorylation sites that were mutated in the ShcFFF mice (25). To determine the requirement of ShcA phosphorylation downstream of p56Lck during β-selection, we crossed the Lck-Cre/ShcFFF mice with LckF505 transgenic mice; the latter express the transgene encoding a constitutively active LckF505 mutant under the control of the lck proximal promoter (26). Previous studies have shown that this LckF505 transgene can drive the maturation of DN thymocytes to DP even in the context of Rag1−/− mice (26). Transgenic LckF505 expression could not overcome the block due to ShcFFF expression, as the thymic cellularity was still severely reduced in the LckF505/Lck-Cre/ShcFFF triply transgenic mice (Fig. 1E). Again, a large fraction of the DN thymocytes were arrested at the DN3 stage of development. In control LckF505/ShcFFF mice, the majority of the DN thymocytes were DN4 cells, due to the heightened signaling via the constitutively active LckF505 being perceived as a strong signal via the pre-TCR (Fig. 1F). This heightened LckF505 signal may also explain the less severe arrest due to ShcFFF at the DN3 stage in the triply transgenic mice compared to that seen in the Lck-Cre/ShcFFF mice (10). It is noteworthy that LckF505 was active at the DN stage in the LckF505/Lck-Cre/ShcFFF mice. LckF505-mediated silencing of TCRβ expression was affected (27), with 76% of the DN cells in the control mice being positive for intracellular TCRβ staining whereas only 6% of the DN cells were positive for expression of TCRβ in the LckF505/Lck-Cre/ShcFFF mice (data not shown).
LckF505 expression in thymocytes causes robust tyrosine phosphorylation of numerous proteins, including ShcA (data not shown). Compared with the level seen in transgenic mice carrying only LckF505, endogenous ShcA tyrosine phosphorylation was decreased in the LckF505/Lck-Cre/ShcFFF mice (Fig. 1G), which correlated with the block in thymic development in these mice. Of note, ShcFFF expression in DN thymocytes did not affect CD3-induced phosphorylation of ZAP-70 or LAT (Fig. 1H). Thus, ShcA phosphorylation in thymocytes occurs downstream of p56Lck, either as a direct or indirect substrate, and is critically required for at least some of the p56Lck-mediated functional effects during β-selection.
ShcA is dispensable for β allelic exclusion and thymocyte survival during β-selection.
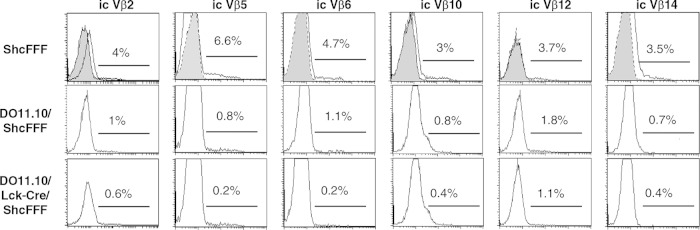
Signaling via the pre-TCR and Notch1 at the DN3 stage is coupled with at least four outcomes: proliferative expansion, differentiation to the early DP by downregulating IL-2-rα surface expression and maturation to the DP stage, allelic exclusion at the Tcrβ locus, and survival beyond that developmental checkpoint (22). In previous studies, the impaired pre-TCR signaling, such as the loss of the SLP-76 adapter protein, led to failure of allelic exclusion and continued rearrangement at the Tcrβ locus (28). To test whether ShcA affects signaling leading to allelic exclusion, we used DO11.10/Lck-Cre/ShcFFF transgenic mice, as a rearranged Tcrβ gene largely silences further rearrangement at the endogenous Tcrβ locus (29). In the DO11.10/Lck-Cre/ShcFFF mice, the profile of Vβ expression was essentially similar to that seen with the control DO11.10/ShcFFF mice (Fig. 2). Thus, allelic exclusion at the Tcrβ locus does not appear to be affected by ShcFFF expression.
FIG 2.
Allelic exclusion at the Tcrβ locus is not affected by ShcFFF transgene expression. Thymocytes from the ShcFFF, DO11.10/ShcFFF, or DO11.10/Lck-Cre ShcFFF mice were analyzed by intracellular (ic) staining with different Vβ-specific antibodies. Staining with six different Vβ chains within DN thymocytes is presented along with staining using an isotype-matched control antibody (gray) (n = 2).
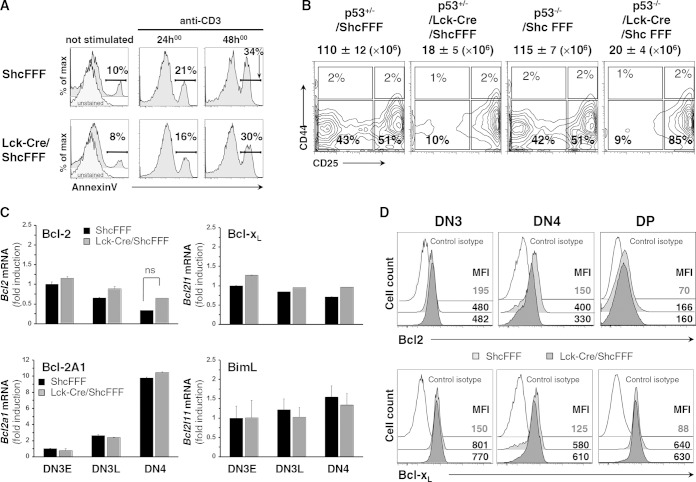
With respect to the potential role of ShcA in mediating survival signals during β selection, we did not detect any increase in the number of annexin V-positive thymocytes from Lck-Cre/ShcFFF mice, either immediately after harvesting the thymi or after incubation of thymocytes for various times in culture with or without anti-CD3 stimulation (Fig. 3A) (15). We also used a genetic approach to test this further. It has been previously observed that concurrent loss of the trp53 gene can partially restore the defect in thymic cellularity in Rag2−/− mice, depending on the age of the mice (30), by “rescuing” cells that would otherwise undergo apoptosis. We crossed the Lck-Cre/ShcFFF mice with trp53−/−mice and found there was no rescue of cell number or the DN4 population at 3 to 4 weeks of age (Fig. 3B), a time when trp53 loss has a substantial effect on thymic cell numbers in the Rag2−/− background. Since members of the Bcl-2 family proteins are known to influence DN cell survival (16, 31) and since ShcA has been linked to upregulation of Bcl-2 in mature T cells (32), we also assessed the expression of prosurvival Bcl2 family members in DN thymocytes. The control and Lck-Cre/ShcFFF mice displayed similar transcript levels for Bcl2, Bcl2l1, and the pre-TCR-inducible Bcl2a1 (Fig. 3C) as well as similar protein levels for the prosurvival targets (Fig. 3D). The levels of proapoptotic Bim (Bcl2l11), Bax, and Bak were also similar in control and Lck-Cre/ShcFFF mice (data not shown), suggesting no apparent role for ShcA in regulating the balance of proapoptotic and prosurvival factors. Thus, phosphorylation of ShcA likely does not influence survival signals downstream of the pre-TCR.
FIG 3.
ShcFFF expression does not affect apoptosis of DN thymocytes. (A) Thymocytes from the indicated mice were grown for 24 and 48 h with or without coated CD3 (10 μg/ml), and results of annexin V staining of DN thymocytes were measured by cytometry. Numbers adjacent to brackets indicate percent annexin V-positive cells. max, maximum. (B) DN thymocyte subsets from the indicated p53 heterozygous or homozygous null mice (3 weeks of age) in the Lck-Cre/ShcFFF context are presented, with total thymocyte numbers indicated on top of dot plots. Data are from 3 experiments performed with 3 animals per genotype. (C) Transcript inductions for Bcl2 (Bcl-2), Bcl2a1 (Bcl-2A1), Bcl2l1 (Bcl-xL), and Bcl2l11 (BimL) were measured by qPCR in purified DN3E, DN3L, and DN4 thymocytes from ShcFFF and Lck-Cre/ShcFFF mice. Standard deviation (SD) data were calculated from the measurement variations within 2 different experiments done in triplicate (n = 2, for a total of 6 animals per genotype). (D) Surface expression of Bcl2 and Bcl-XL in DN3, DN4, and DP thymic subsets from ShcFFF and Lck-Cre/ShcFFF mice, measured by intracytoplasmic staining (n = 3). MFI, mean fluorescence intensity.
ShcA is required for proliferation downstream of the pre-TCR.
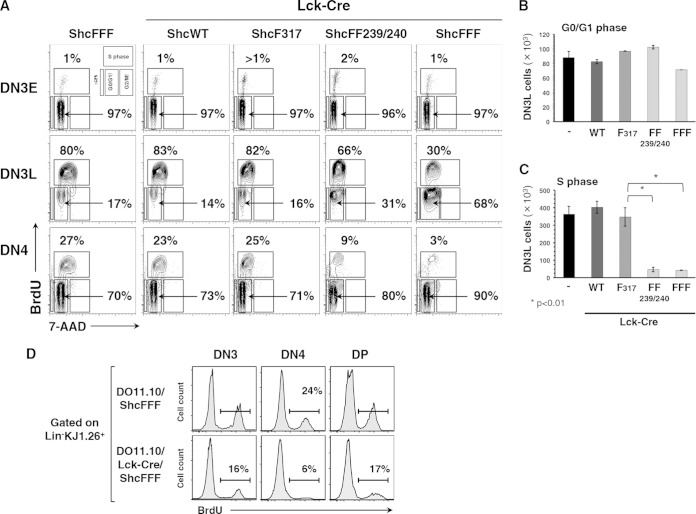
To test the effect of ShcA in proliferation downstream of the pre-TCR, we assessed BrdU incorporation and 7-AAD signal in the proliferating DN3 and DN4 cells in vivo in the different ShcA transgenic lines (10) (Fig. 4A). The DN3 population was gated into the smaller DN3E and the larger DN3L populations, based on cell size. We found that DN3E cells poorly proliferated, being mostly in the G0/G1 phase, and displayed no difference between control and the different ShcA mutant mice. Since DN3L cells grow larger and initiate pre-TCR signaling, they also enter into the S phase of cell cycle. Comparing the DN3L populations, 80% of the cells were proliferating within control mice, 83% in Lck-Cre/ShcWT mice, 82% in Lck-Cre/ShcF317 mice, 67% in Lck-Cre/ShcFF mice, and only 30% in the Lck-Cre/ShcFFF mice (Fig. 4A). Also, over multiple experiments, the absolute numbers of DN3L thymocytes from the Lck-Cre/ShcFF239/240 and the Lck-Cre/ShcFFF mice showed a significant decrease in S phase compared to the numbers seen with the control mice (P < 0.01) but no obvious defect in their numbers in G0/G1 phase (Fig. 4B and C).
FIG 4.
ShcA is required for pre-TCR-dependent proliferation. (A) Proliferation measured by determinations of BrdU uptake and 7-AAD signal levels in DN3, DN4, and DP thymic subsets from the different transgenic mouse strains. (B and C) The relative numbers of DN3L cells within each of the cell cycle-defective phases are plotted as the averages of the means of the numbers of cells ± SD. Data were obtained from 3- to 6-week-old littermates for each genotype. (D) Levels of proliferation of KJ1.26-positive DN3, DN4, and DP thymic subsets in DO11.10/ShcFFF and DO11.10/Lck-Cre/ShcFFF transgenic mice were assessed by BrdU uptake as described in Materials and Methods (n = 4).
Importantly, proliferation of the early DP subset was unaffected in Lck-Cre/ShcFFF mice, consistent with cells maturing to the DP stage in Lck-Cre/ShcFFF mice being essentially “normal” thymocytes/escapees that lack stop cassette deletion (data not shown) (15). The proliferative defect is maintained in the DN4 thymocytes, with an increased percentage of BrdU-negative cells having less than 2n DNA content in the Lck-Cre/ShcFFF mice. Thus, ShcFF- and ShcFFF-expressing DN3L cells fail to proliferate during progression through β-selection.
We next assessed if introducing DO11.10 transgenic TCR into the Lck-Cre/ShcFFF background could rescue or partially rescue the proliferation defect. However, proliferation of the DN3 and DN4 populations in the DO11.10/Lck-Cre/ShcFFF mice was also impaired compared to control levels (Fig. 4D). These data suggested that ShcA-mediated signaling specifically influences certain outcomes of pre-TCR signaling at the DN stage, such as differentiation and proliferation, and that ShcA signaling is dispensable for allelic exclusion and survival.
Impaired c-Myc expression in ShcA mutant mice.
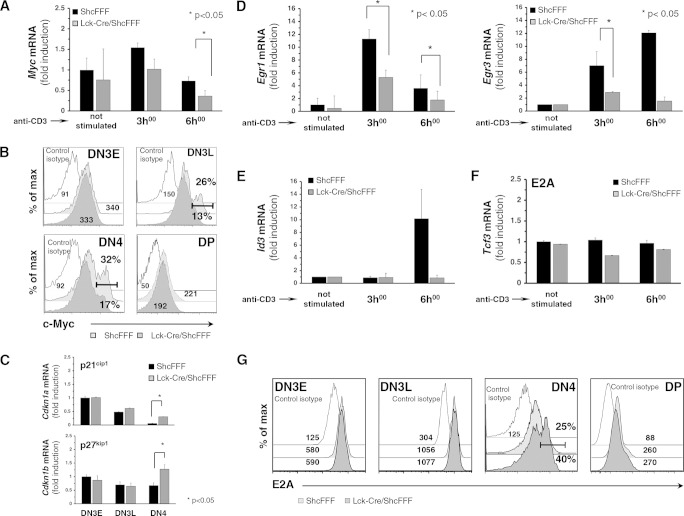
We next addressed the mechanism by which ShcA phosphorylation might regulate proliferation of DN thymocytes. A previous study has implicated c-Myc as one of the key regulators of proliferation by DN thymocytes upon pre-TCR signaling (33). Since ShcA was found to function upstream of c-Myc signaling in other cell types (34), we tested whether disruption of ShcA phosphorylation in DN thymocytes might interfere with pre-TCR-induced c-Myc expression. The induction of the c-Myc transcript was significantly lowered in DN4 thymocytes from Lck-Cre/ShcFFF mice compared to control mice (Fig. 5A). Analysis of c-Myc protein expression in DN4 thymocytes revealed that DN4 thymocytes from Lck-Cre/ShcFFF mice had fewer c-Myc-positive cells (17%) than DN4 thymocytes from control mice (32%) (Fig. 5B). c-Myc is known to transcriptionally regulate cell cycle inhibitors such as p21Cip1, p27kip1, and Gadd45α (35). A quantitative analysis in DN subsets from Lck-Cre/ShcFFF mice revealed a significant increase in transcript levels for cell cycle inhibitors Cdkn1a (p21Cip1) and Cdkn1b (p27Kip1) but not Gadd45a (Fig. 5C and data not shown). We also could not observe any defect in the transcript level for cyclinD3 (36) in any of the DN subpopulations of Lck-Cre/ShcFFF mice (data not shown). Thus, the loss of c-Myc expression downstream of pre-TCR may affect the usual downregulation of Cdkn1a and Cdkn1b transcripts during β-selection and may account for some of the proliferative defect observed in DN4 thymocytes from Lck-Cre/ShcFFF mice.
FIG 5.
ShcFFF expression causes decreased c-Myc expression and impaired Egr1 and Egr3 upregulation. (A) Induction of c-Myc transcript was measured by qPCR after anti-CD3 stimulation of purified DN4 thymocytes from ShcFFF and Lck-Cre/ShcFFF mice (n = 3 experiments, including 9 animals). (B) c-Myc protein expression in DN3, DN4, and DP subsets (n = 3). (C) qPCR analysis of Cdkn1a (p25cip1) and Cdkn1b (p27kip1) mRNA expression in purified DN3E, DN3L, and DN4 thymocytes from Lck-Cre/ShcFFF and control ShcFFF mice (n = 2 with 6 animals per genotype). (D) Induction of Egr1 and Egr3 transcripts was measured by qPCR upon anti-CD3 stimulation of purified DN4 thymocytes from ShcFFF and Lck-Cre/ShcFFF mice (n = 3 experiments with 9 animals per genotype). (E) qPCR analysis of CD3-induced Id3 mRNA expression in DN4 cells from ShcFFF and Lck-Cre/ShcFFF mice (n = 3). (F) Expression of E2A transcript measured by qPCR in CD3-stimulated DN4 thymocytes from ShcFFF and Lck-Cre/ShcFFF mice. (G) Expression of E2A protein measured by intracytoplasmic staining in DN3, DN4, and DP cells from ShcFFF (control) mice and Lck-Cre/ShcFFF mice (n = 2).
ShcA regulates ERK-dependent Egr1, Eg-3, and Id3 induction.
Upon pre-TCR signaling, Erk-1 and Erk-2 kinases modulate several genes affecting proliferation, including those encoding early growth factors 1 and 3 (Egr1 and Egr3) (37). Consistent with the reduced ERK phosphorylation in the Lck-Cre/ShcFFF mice, CD3-induced Egr1 and Egr3 transcriptional activation in DN4 thymocytes was decreased (by 4-fold) but was not completely eliminated (Fig. 5D). Protein levels for Egr1 and Egr3 were also affected in both DN3 and DN4 subpopulations from Lck-Cre/ShcFFF mice (data not shown), suggesting that the mutant ShcFFF leads to a defective de novo synthesis of cell proliferation regulators Egr1 and Egr3.
Id3 is a helix-loop-helix protein, and its gene is a target gene of Egr1. Id3 mainly acts as an inhibitor of the activity of E2A proteins, where E2A is necessary to prevent thymocyte differentiation and can negatively regulate cell proliferation (38, 39). In DN4 thymocytes from Lck-Cre/ShcFFF mice, we found defective induction of Id3 transcript upon CD3 stimulation compared to control mice (Fig. 5E). Although E2A mRNA levels (measured by quantitative PCR) were not affected within the DN3E, DN3L, and DN4 subsets in thymocytes from Lck-Cre/ShcFFF mice (Fig. 5F), increased E2A protein levels were found in DN4 subsets from Lck-Cre/ShcFFF mice (40%) compared to those from control mice (25%) (Fig. 5G).
Ectopic expression of Egr1 and Egr3 rescues the ShcFFF-induced developmental block.
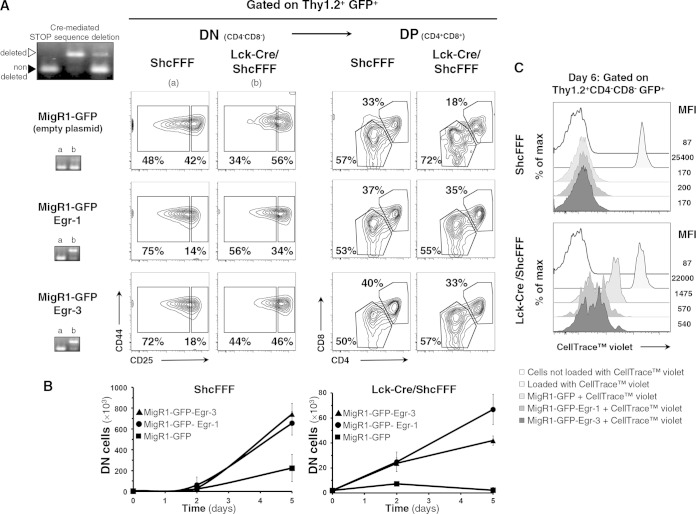
We next examined whether ectopic expression of Egr1 and Egr3 could rescue the developmental block observed in vivo in Lck-Cre/ShcFFF mice. Using retroviral vectors that coexpressed eGFP along with either Egr1 or Egr3 (40), we infected DN3E thymocytes from control and Lck-Cre/ShcFFF mice. After plating them on OP9-DL1 cultures, we tracked the emergence of DN4 and DP thymocytes among GFP-positive cells by flow cytometry on a time course of 5 days (Fig. 6A). Ectopic expression of Egr1 or Egr3 in thymocytes from wild-type mice accelerated the transition to the DN4 stage, in turn resulting in a 4-fold increase in the absolute number of thymocytes that progressed beyond the DN4 stage (Fig. 6B), although the ratio of DN to DP was minimally changed (Fig. 6B). In thymocytes from Lck-Cre/ShcFFF mice, the empty plasmid condition recapitulated the differentiation block at the DN3-to-DN4 transition (Fig. 6A). However, enforced expression of Egr1 or Egr3 in DN3E thymocytes from the Lck-Cre/ShcFFF mice rescued some differentiation beyond the β-selection checkpoint after 5 days of culture on OP9-DL1, with 56% of DN4 in Egr1 transduced thymocytes, compared to 34% in thymocytes transduced with empty plasmid (Fig. 6A, DN panel). Moreover, ectopic expression of Egr1 and Egr3 also resulted in a 10- to 20-fold increase in the absolute number of DN cells under the Lck-Cre/ShcFFF conditions (Fig. 6B) and also increased the ratio of DP cells to DN cells (data not shown). The absence of developmental progression in GFP-negative thymocytes indicates that retroviral expression of Egr1 or Egr3 was directly responsible for rescuing the developmental block due to the ShcA mutant expression during β-selection.
FIG 6.
Egr1 and Egr3 promote cell proliferation during β-selection in ShcFFF mice expressing thymocytes. (A) Sorted DN3E thymocytes (CD4− CD8− CD3− c-kit− CD44− CD25+) from control and Lck-Cre/ShcFFF mice retrovirally transduced with Egr1 or Egr3 or control vector expressing GFP were seeded on OP9-DL1 stromal cells, and differentiation to later stages was assessed on day 5. (B) Absolute numbers of total DN thymocytes in the coculture were determined at days 2 and 5 of coculture. (C) Proliferation of retrovirally infected ShcFFF and Lck-Cre/ShcFFF DN3E thymocytes labeled with CellTrace violet and cultured with OP9-DL1 stromal cells and assessed on day 5 by measuring the dilution of the violet dye by cytometry. Data are representative of the results of two experiments performed with 6 animals per genotype.
Next, we measured the effect of the forced expression of Egr1 or Egr3 on the ex vivo proliferation of DN thymocytes by labeling purified DN3E thymocytes with CellTrace Violet dye before seeding them on OP9-DL1 cells. DN thymocytes from control mice with or without transduction of Egr1 and Egr3 proliferated robustly, as measured by the dilution of the dye signal during successive proliferative steps (Fig. 6C, upper panel). The Egr1- or Egr3-transduced thymocytes from the Lck-Cre/ShcFFF mice displayed increased proliferation (Fig. 6C, lower panel). These data suggest that the overexpression of Egr1 or Egr3 proteins in thymocytes from the Lck-Cre/ShcFFF mice can partially rescue differentiation and proliferation. Collectively, these findings suggest that the phosphorylation of ShcA contributes to at least two distinct pathways regulating DN thymocyte proliferation: the Ras-ERK-Egr1-Id3-E2A pathway and c-Myc-dependent signaling.
c-Abl interacts with phospho-ShcA and promotes thymocyte differentiation and proliferation.
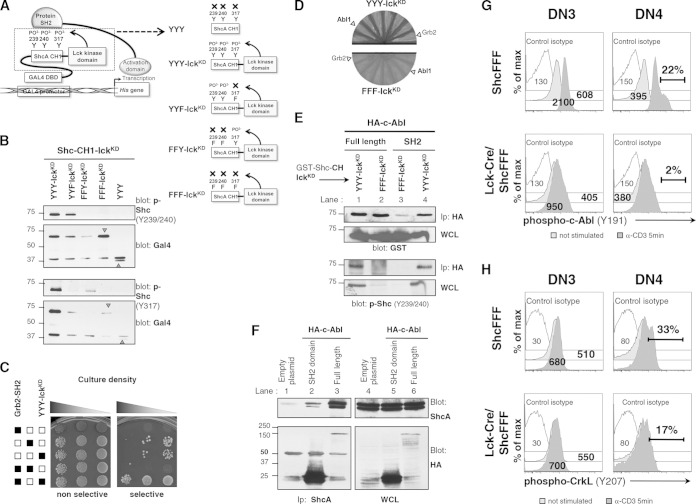
Since the ectopic expression of Egr1 and Egr3 transcription factors in ShcFFF mice expressing DN thymocytes did not fully rescue the defect, we considered the possibility that ShcA is also linked to other signaling networks. To address this issue, we designed a modified yeast two-hybrid assay to screen for new signaling proteins able to interact with the phosphorylated form of ShcA. We fused the coding region of the CH1 region of ShcA, which carries all three tyrosine residues, to the coding region of the isolated kinase domain of human p56Lck, with this construct being denoted Shc-CH1-lckKD (see schematic in Fig. 7A). This strategy was chosen to allow phosphorylation of ShcA tyrosines via Lck but avoid detecting proteins that might interact with the PTB or the SH2 domain of ShcA. As a control, we also created a Shc-CH1-FFF-Lck construct (with the three ShcA tyrosines mutated) to screen against proteins that may bind in a phosphotyrosine-independent manner to the ShcA CH1 domain.
FIG 7.
c-Abl can interact with phosphorylated ShcA and promote migration of immature thymocytes. (A) Schematic of modified yeast two-hybrid assay. Fusion of the p56Lck kinase domain (lckKD) to the CH1 region of ShcA allows p56Lck kinase to phosphorylate tyrosine residues Y239, Y240, and Y317 within the ShcA CH1 region. Fusion of the Gal4 DNA binding domain (DBD) to the ShcA CH1 region allows this protein to interact with the Gal4 promoter. Prey proteins, such as Grb2, can interact with the phosphorylated residues on CH1 domain from ShcA. This brings the Gal4 activation domain into proximity to activate transcription of the gene encoding His, thus rescuing the His auxotrophy, and permits growth of transformed yeast cells on selective medium. (B) The Grb2 SH2 domain interacts specifically with phosphorylated Shc-CH1 in yeast. HF7c yeast cells were transformed with the indicated constructs, including Grb2-SH2 pv16 as a positive control, to detect protein interactions. After transformation, yeast cells were plated on SD-LW plates (nonselective; selects only for the presence of plasmid) or SD-LWH plates with 10 mM 3-AT (selective; requires interaction of bait and prey proteins for growth). (C) HF7c yeast cells were transformed with the indicated Shc-CH1-lckKD variant. Yeast cell lysates were blotted for phospho-ShcY239/Y240 or for phospho-ShcY317 and for Gal4 expression. (D) HF7c yeast cells containing either the Shc-CH1 domain with the kinase domain of lck (Shc-CH1-lckKD; referred to here as YYY-lckKD) or tyrosine-mutated Shc-CH1 (Shc-CH1FFF-lckKD; referred to here as FFF-lckKD) transformed with positive clones from the screen. (E and F) 293T cells were transiently transfected with the indicated constructs. Cell lysates were immunoprecipitated with anti-HA–agarose beads and immunoblotted with anti-GST to detect Shc-CH1-lckKD proteins or with anti-phospho-ShcA (Y239/240) and anti-HA to detect c-Abl constructs. WCL, whole-cell lysates. (G) Phospho-c-Abl (Y191) signal from the unstimulated conditions or anti-CD3-stimulated conditions (5 min) in DN3 and DN4 subpopulations of thymocytes from control (ShcFFF) and Lck-Cre/ShcFFF mice. Data represent mean fluorescence values or percentages of phospho-c-Abl-positive cells. The data represent a pool of 3 mice and a pool of 4 mice, respectively, and are similar to data obtained from a separate pool of control and Lck-Cre/ShcFFF mice (n = 2). (H) CrkL phosphorylation upon CD3 stimulation in Lck-Cre/ShcFFF and control ShcFFF DN3 and DN4 thymocytes (3 mice per genotype).
As an initial test for whether these constructs and their phosphorylation occur as predicted, cell lysates from HF7c yeast cells transformed with the different Shc-CH1-lckKD constructs were probed with antibodies to either phospho-Y239/Y240-Shc or phospho-Y317 ShcA. The Shc-CH1-lckKD fusion proteins were phosphorylated, whereas no phosphorylation was observed in the Shc-CH1-FFF-Lck or CH1 domain with no Lck kinase domain fused (Fig. 7B). As a proof of principle, we tested whether the Shc-CH1-lckKD fusion protein can interact with the SH2 domain of Grb2, well known to bind to phosphorylated ShcA. Interestingly, cotransfection of yeast with Shc-CH1-lckKD and Grb2-SH2 allowed growth on selective medium. However, yeasts that were transformed individually with Grb2-SH2, Shc-CH1-lckKD, or Shc-CH1-FFF-lckKD grew equally on the nonselective medium, indicating no artificial toxicity of these proteins for yeast growth (Fig. 7C). This suggested that the phosphorylated Shc-CH1-lckKD fusion protein allows interaction of physiologically relevant target proteins in this context.
We then performed a yeast two-hybrid screen using the Shc-CH1-lckKD construct as bait against an expression library from the mouse embryo. There were approximately 180 positive hits in the initial screen, which upon further screening resulted in about 11 potential interacting proteins (data not shown). We further narrowed these hits based on those expressed in T cells and those linked to thymic development. One of the candidate binding partners that consistently tested positive in this yeast assay was the SH2 domain of c-Abl-1 and Abl-2 (Arg), comprising 6 of the 11 positive hits (Fig. 7D). Previously, c-Abl was linked to tonic signaling in the T-cell lineage and loss of c-Abl (along with its homologue Arg) was shown to be important for DN thymocyte development (20). Moreover, the binding between ShcA and the Bcr-Abl oncogenic version has been linked to proliferation of transformed cells (41). Therefore, we tested the relevance of the ShcA and c-Abl interaction during β-selection. To confirm the ShcA–c-Abl interaction in a mammalian system, we cotransfected 293T cells with glutathione S-transferase (GST)-tagged Shc-CH1-lckKD (or nonphosphorylatable Shc-CH1-FFF-lckKD) with full-length hemagglutinin (HA)-tagged c-Abl or the c-Abl SH2 domain alone. As expected, the Shc-CH1-lckKD protein was tyrosine phosphorylated whereas Shc-CH1-FFF-lckKD was not (Fig. 7E, lower panel). The c-Abl SH2 domain selectively coimmunoprecipitated Shc-CH1-lckKD but not Shc-CH1-FFF-lckKD, demonstrating the requirement for the phosphorylated tyrosine residues of Shc to interact with the c-Abl SH2 domain (Fig. 7E, lanes 3 and 4). Unexpectedly, we also found an association between full-length c-Abl protein and Shc-CH1-FFF-lckKD, suggesting the presence of a phosphorylation-independent interaction (Fig. 7E, lanes 1 and 2). It is not clear whether the full-length c-Abl was interacting with the CH1 region of ShcA or with the kinase domain of p56Lck. The full-length c-Abl protein contains an SH3 domain, which could mediate binding of PCCP motifs within the CH region of Shc. We also observed that the c-Abl SH2 domain alone and full-length c-Abl can coimmunoprecipitate with endogenous ShcA (Fig. 7F, lanes 2 and 3).
We next checked whether ShcFFF expression affected CD3-induced c-Abl phosphorylation in DN subsets. We performed in vitro CD3 stimulation for 5 min and assessed c-Abl phosphorylation of tyrosine 191 in DN3 and DN4 thymocytes. Expression of ShcFFF in the DN3 and DN4 subsets led to less phospho-c-Abl signal than in the DN3 and DN4 thymocytes from control mice (Fig. 7G). This inhibition correlated with a partial defect in the phosphorylation of c-Abl substrate CrkL in DN4 thymocytes from Lck-Cre/ShcFFF mice compared to control mice (33% versus 17%) (Fig. 7H). These data suggest that phosphorylated ShcA likely functions upstream of c-Abl and that impaired c-Abl phosphorylation in Lck-Cre/ShcFFF thymocytes may impact c-Abl function.
c-Abl can partially rescue the deficiency in ShcA-mediated signaling.
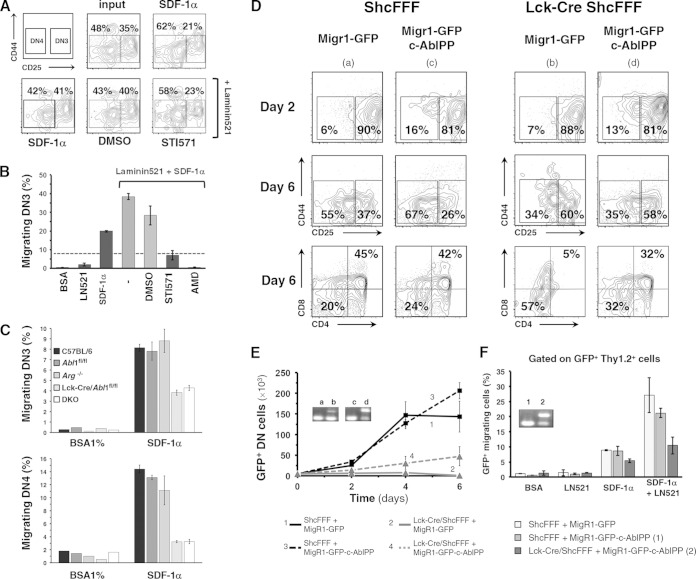
c-Abl is known to act downstream of pre-TCR and integrin receptors, promoting F-actin polymerization, thymocyte differentiation, and migration of thymocytes to a chemokine gradient. In our assays, pharmacological inhibition of c-Abl by STI571, a specific c-Abl activation inhibitor, partially blocked the migration of DN thymocyte to SDF-1α (Fig. 8A and B). We tested mice conditionally deleted for abl1, mice with a knockout for the c-Abl homologue Arg, or the double-knockout mice. We confirmed the previously observed requirement of abl1 for regulating DN thymocyte development and chemotaxis to CXCL12/SDF-1α (Fig. 8C). Loss of expression of Abl1 or of both Abl1 and Arg led to an aberrant distribution of CD25+ cells in the cortex (data not shown). These genetic data suggest that the phenotype caused by genetic loss of Abl1 is analogous to some of the phenotypes seen with impaired ShcA signaling (16).
FIG 8.
Expression of constitutively active c-Abl partially rescues thymocyte differentiation, proliferation, and migration. (A and B) Purified DN thymocytes from C57BL/6 mice were pretreated with STI571 or AMD3100 and assayed for migration to SDF-1α in a transwell assay. Bar graphs show the percentage of migrated DN3 cells as a fraction of total cell input ± SD. DMSO, dimethyl sulfoxide; AMD, AMD3100. (C) Thymocytes from control abl1fl/fl, arg−/−, Lck-Cre/abl1fl/fl, and double-knockout (DKO) mice (three mice for each group) were assayed for SDF-1α-dependent chemotaxis, and the fraction of migrated cells is depicted as a percentage of DN3 or DN4 thymocyte input. (D) Differentiation of sorted DN3E thymocytes from ShcFFF and Lck-Cre/ShcFFF mice that were retrovirally transduced with c-AblPP or with empty GFP-expressing control plasmid and seeded on OP9-DL1 stromal cells (the analysis was performed on day 5). Dot plots were first gated on Thy1.2+ GFP+ cells. (E) Absolute numbers of total DN thymocytes in the coculture on days 2, 4, and 6. Insets show stop sequence deletion (detected via PCR) from genomic DNA obtained from thymocytes after 6 days of coculture. (F) At day 6, GFP-positive thymocytes transduced with either empty plasmid or the c-AblPP plasmid construct were assayed for migration to SDF-1α. The fraction of migrated cells is depicted as a percentage of the thymocyte input. Insets show stop sequence deletion PCR amplicons from genomic DNA obtained from thymocytes that had migrated to the bottom well (n > 3) (see inset in Fig. 6A).
We hypothesized that if c-Abl were to interact with the phosphorylated form of ShcA, at least part of the c-Abl function would occur downstream of ShcA phosphorylation and, therefore, a constitutively active form of c-Abl might compensate for the defective signaling due to ShcFFF expression. To test this hypothesis, we retrovirally transduced purified DN3E thymocytes from control or Lck-Cre/ShcFFF thymocytes with a constitutively active form of c-Abl (referred to here as AblPP) and seeded them on OP9-DL1 cultures. Ectopic expression of AblPP in DN3E thymocytes from control mice led to a slight but not significant increase in the absolute number of cells recovered from day 6 of coculture on OP9-Dl1 (Fig. 8D and E). Interestingly, DN3E thymocytes from Lck-Cre/ShcFFF mice that were transduced with AblPP could resume differentiation beyond the β-selection checkpoint and also showed proliferation over the 6-day period. Even though the phenotypic rescue was only partial compared to the control condition, the cells collected from the coculture did have a deletion of the stop cassette (i.e., they were not escapees lacking ShcFFF transgene expression) (see the PCR insets in Fig. 6A and 8E). ShcA is also part of the CXCR4 signaling pathway, and impaired ShcA signaling blocks the DN migration to SDF-1α. AblPP expression in thymocytes from Lck-Cre/ShcFFF also improved the chemotactic efficiency with respect to SDF-1α (Fig. 8F).
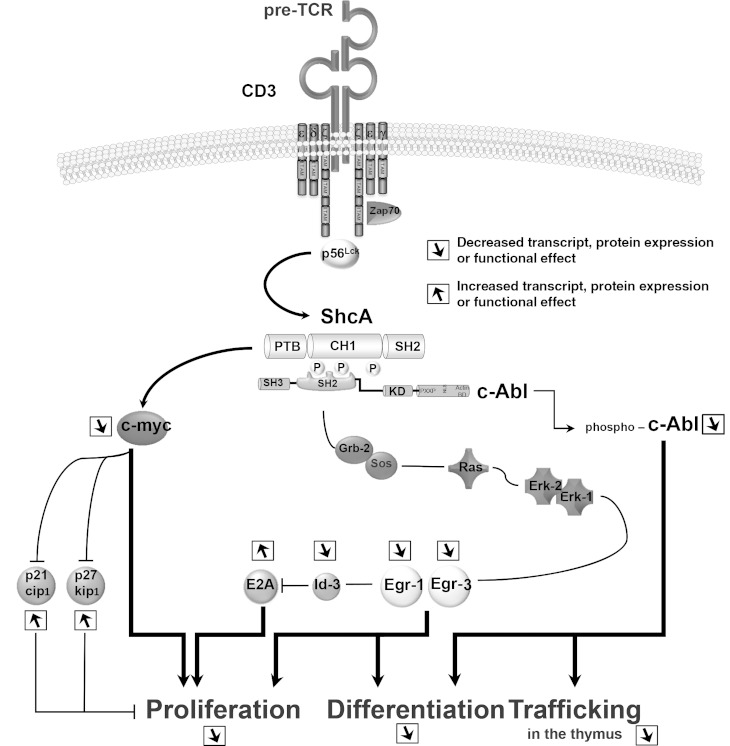
These data reveal that ShcA and c-Abl functionally interact and can contribute to signaling via the pre-TCR and CXCR4 during β-selection (Fig. 9).
FIG 9.
Effect of impaired ShcA-mediated signaling in phosphorylation-defective mutant ShcFFF. A schema depicting the role of ShcA downstream of pre-TCR-mediated signaling is shown. The functional properties of ShcA phosphorylation leading to ERK1/2 kinase-dependent and -independent pathways in regulating the differentiation, the proliferation, and the trafficking of immature thymocytes are indicated.
DISCUSSION
The integration of multiple signaling molecules downstream of the pre-TCR (along with Notch1 and CXCR4) is important for optimal thymic development and progression through the β-selection checkpoint. The data presented in this report suggest that the ShcA adapter protein plays a key role in regulating specific outcomes, in particular, in proliferation and differentiation. The similarities in phenotypes between the Lck-Cre/ShcFFF mice and the DO11.10/Lck-Cre/ShcFFF triply transgenic mice suggest that even expressing a rearranged TCR and the presumably robust signaling through such a receptor cannot overcome the developmental arrest at DN3 due to ShcFFF, which could not be rescued. This also confirms previous studies indicating that optimal signaling via ShcA at the DN3 stage is critical for maturation beyond this point (15).
Lck is recognized as a critical regulator of multiple events downstream of the pre-TCR, and yet the specific substrates and molecules that function downstream of p56Lck are not fully defined. The data obtained with LckF505/Lck-Cre/ShcFFF mice provide three important insights. First, endogenous ShcA is phosphorylated downstream of p56Lck in thymocytes, either as a direct substrate or indirectly, consistent with the phosphorylation of ShcA by p56Lck in T-cell lines in vitro (25). Second, increased phosphorylation of endogenous ShcA in LckF505 mice, coupled with the decrease in ShcA phosphorylation detected in the LckF505/Lck-Cre/ShcFFF mice, suggests that the transgenic dominant-negative ShcFFF protein, as intended, interferes with endogenous ShcA tyrosine phosphorylation in thymocytes. Moreover, the levels of tyrosine phosphorylation on a number of other proteins in the thymocyte lysates were comparable, suggesting that this is not a global effect. Third, while a constitutively active p56Lck would have been expected to activate multiple pathways in DN thymocytes, the potent decrease in the total thymocyte number in the LckF505/Lck-Cre/ShcFFF mice with a concomitant arrest at the DN stage suggests that optimal signaling via ShcA is critically required for maturation beyond β-selection. The nearly complete loss of TCRβ expression within the DN population in LckF505/Lck-Cre/ShcFFF mice suggested that the LckF505 was active at the DN stage. These data identify ShcA as a critical player downstream of p56Lck at the β-selection checkpoint.
In vivo BrdU pulse studies indicated a strong inhibition of DN3 and DN4 proliferation in the Lck-Cre/ShcFFF mice and the DO11.10/Lck-Cre/ShcFFF mice. This inhibition of proliferation by ShcFFF is the most likely reason for the large decrease in the absolute number of thymocytes found in Lck-Cre/ShcFFF mice and DO11.10/Lck-Cre/ShcFFF mice. Moreover, results of the studies using transgenic mice carrying mutations of specific tyrosine residues suggest that the Y239/Y240 tyrosines play a greater role in regulating proliferation at the β-selection checkpoint. While another reason for the decreased number of thymocytes in mice with impaired ShcA signaling could be reduced cell survival, our experiments do not support this possibility. We also found no obvious impairment of prosurvival factors Bcl-2 and Bcl-xL at the transcriptional and protein levels. Likewise, pre-TCR-inducible factor Bcl-2A1 was also unaffected by the expression of ShcFFF during β-selection. Additionally, we did not find a significant difference in any of the proapoptotic factors assessed (Bad, Bak, Bax, and Bim) in thymocytes from Lck-Cre/ShcFFF mice.
Impaired ShcA-mediated signaling does not seem to affect allelic exclusion at the Tcrβ locus. This is in contrast to the data determined with SLP-76, where optimal signaling via SLP-76 and PKC has been reported to be required for proper TCRβ allelic exclusion (42). This once again highlights the differential requirements of the different adapter proteins during thymic development.
Studies using knockout mice have provided evidence that induction of Egr1 and Egr3 transcription factors through the Ras/MEK/ERK pathway is selectively required for early thymocyte maturation and proliferation during β-selection (11, 43). Our data identify Egr1 and Egr3 as two major ERK-induced gene products transcriptionally downregulated in the ShcFFF mice expressing DN thymocytes. Even though this is a partial effect due to the complexity of the molecular cascade downstream of the pre-TCR, we can now position ShcA as a link from the pre-TCR and p56Lck to the primary ERK/Egr signaling cascade. Upon pre-TCR activation, the upregulation of Egr1 transcriptionally regulates expression of Id3 (38), which can negatively regulate E2A protein activity through the formation of stable heterodimers and eventually control proliferation in response to pre-TCR signal (12, 44). The block in Id3 transcript expression that we observed in DN4 cells from the Lck-Cre/ShcFFF mice could in part explain the increased E2A protein expression and the deficient proliferation during β-selection. Although E2A protein was upregulated in ShcFFF mice expressing DN4 thymocytes, we did not notice a significant effect on expression of E2A target gene products, such as intracytoplasmic TCRβ, Bcl2A1d, or cyclin D3 (45). This may be due to the absence of substantial degradation of E2A, which could explain why RORγt transcript was not affected in the ShcFFF mice expressing DN thymocytes compared to control mice. Another explanation could be a compensatory mechanism operating between the two E2A isoforms, E47 and E12, as described in a recent work from Xi et al. (12).
We also found that pre-TCR-dependent c-Myc expression is also altered in ShcFFF DN3L and DN4 thymocytes. c-Myc deficiency has been shown to be sufficient to affect cell growth but not cell survival during β-selection (33), and a few studies have linked ShcA phosphorylation with c-Myc-dependent mitogenic signaling in fibroblasts and B cells (46). In line with these observations, we found that the proliferative defect occurs in the G0/G1 transition to the S phase. Transcripts for p27kip1 and p21cip1, negative regulators of cell cycles, were expressed at a higher level in DN4 thymocytes from Lck-Cre/ShcFFF mice. This suggested that ShcA phosphorylation may be able to affect cell proliferation through both ERK- and c-Myc-dependent pathways.
Our rescue studies with Egr1 and Egr3 suggested that pathways other than Egr1 and Egr3 are also needed from ShcA to promote cell differentiation and/or proliferation. Using a yeast three-hybrid approach with the phosphorylated CH1 domain of Shc as bait, we identified Abl family members c-Abl and Arg as new SH2-containing proteins able to interact with phosphorylated ShcA. The Abl tyrosine kinases are known to regulate cell proliferation during β-selection. Moreover, recent studies have shown a decrease in CD3-induced Shc phosphorylation in thymocytes pretreated with imatinib or thymocytes lacking abl1 and arg expression. Our findings demonstrate that the ShcA CH1 domain binds c-Abl in a phosphotyrosine-dependent manner. Interestingly, lack of c-Abl expression also led to aberrant CD25+ thymocyte localization in the cortex and a block in their migration in response to a SDF-1α gradient. Similarities in these observations and the phenotype we described previously for thymocytes with impaired ShcA function or CXCR4 expression suggest that c-Abl may be a component of the cooperation between pre-TCR and CXCR4 and integrin signaling during β-selection. c-Abl is known to mediate Zap-70 (Y319), PLCγ-1, and LAT phosphorylation after TCR stimulation, leading to ERK activation, and thymocytes deficient for Abl1 and arg have decreased ERK activation. We found that CD3-induced ZAP-70 phosphorylation on tyrosine 319 is not affected by the expression of ShcFFF in DN thymocytes. Since ∼70% of ERK activation after pre-TCR signaling depends on ShcA, c-Abl may provide an alternative pathway for ERK activation through a ZAP-70/PLCγ1/LAT pathway, independently of ShcA. The constitutively active form AblPP allowed some of the ShcFFF thymocytes to resume proliferation and differentiation, suggesting that c-Abl may function downstream of ShcA. Interestingly, AblPP expression in ShcFFF-targeted thymocytes also improved the chemotactic response to SDF-1α, revealing a critical need for ShcA–c-Abl functional interaction in distinct properties of thymocytes. Collectively, these data suggest ShcA is a branch point for signals emanating from the pre-TCR via ERK-dependent and ERK-independent pathways, along with a previously unappreciated ShcA–c-Abl axis that regulates thymocyte proliferation at the β-selection step that we summarized in Fig. 9.
ACKNOWLEDGMENTS
We thank R. Perlmutter for the LckF505 mouse strain, J. C. Zuniga-Pflücker for the OP9-DL1 cells, David Wiest for the Egr1- and Egr3-containing LZRS constructs, Lisa Haney for her help with the animal colony, Jason Kinchen for help with immunofluorescence microscopy, and the FACS core facility at the University of Virginia (UVa) for assistance.
This work was supported by a grant from the National Institutes of Health (GM055761-11 [to K.S.R]), by the UVa Center for Cell Clearance (K.S.R), and by grant AI056266 (to A.M.P.).
P.C.T. designed and performed the experiments and wrote the manuscript; L.Z. did the experiments for Fig. 1C, E, and G; A.J.G. did the experiments for Fig. 5A to D; S.F.W. handled the molecular biology work and provided assistance with all the different migR1-GFP constructs and with proper expression in vitro; J.J.G. provided abl knockout mice and input on those experiments; A.M.P. provided access to the floxed c-Abl and the arg-deficient mouse strains and intellectual input; and K.S.R. provided overall coordination for the work and the writing of the manuscript.
We declare that we have no financial conflicts of interest.
REFERENCES
- 1.Petrie HT, Zuniga-Pflucker JC. 2007. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu Rev Immunol 25:649–679. doi: 10.1146/annurev.immunol.23.021704.115715. [DOI] [PubMed] [Google Scholar]
- 2.Kruisbeek AM, Haks MC, Carleton M, Michie AM, Zuniga-Pflucker JC, Wiest DL. 2000. Branching out to gain control: how the pre-TCR is linked to multiple functions. Immunol Today 21:637–644. doi: 10.1016/S0167-5699(00)01744-8. [DOI] [PubMed] [Google Scholar]
- 3.Aifantis I, Mandal M, Sawai K, Ferrando A, Vilimas T. 2006. Regulation of T-cell progenitor survival and cell-cycle entry by the pre-T-cell receptor. Immunol Rev 209:159–169. doi: 10.1111/j.0105-2896.2006.00343.x. [DOI] [PubMed] [Google Scholar]
- 4.Ciofani M, Zuniga-Pflucker JC. 2007. The thymus as an inductive site for T lymphopoiesis. Annu Rev Cell Dev Biol 23:463–493. doi: 10.1146/annurev.cellbio.23.090506.123547. [DOI] [PubMed] [Google Scholar]
- 5.Lucas JA, Felices M, Evans JW, Berg LJ. 2007. Subtle defects in pre-TCR signaling in the absence of the Tec kinase Itk. J Immunol 179:7561–7567. doi: 10.4049/jimmunol.179.11.7561. [DOI] [PubMed] [Google Scholar]
- 6.Palacios EH, Weiss A. 2004. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene 23:7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W, Sommers CL, Burshtyn DN, Stebbins CC, DeJarnette JB, Trible RP, Grinberg A, Tsay HC, Jacobs HM, Kessler CM, Long EO, Love PE, Samelson LE. 1999. Essential role of LAT in T cell development. Immunity 10:323–332. doi: 10.1016/S1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 8.Clements JL, Yang B, Ross-Barta SE, Eliason SL, Hrstka RF, Williamson RA, Koretzky GA. 1998. Requirement for the leukocyte-specific adapter protein SLP-76 for normal T cell development. Science 281:416–419. doi: 10.1126/science.281.5375.416. [DOI] [PubMed] [Google Scholar]
- 9.Ravichandran KS, Lee KK, Songyang Z, Cantley LC, Burn P, Burakoff SJ. 1993. Interaction of Shc with the zeta chain of the T cell receptor upon T cell activation. Science 262:902–905. doi: 10.1126/science.8235613. [DOI] [PubMed] [Google Scholar]
- 10.Trampont P, Zhang L, Ravichandran KS. 2006. ShcA mediates the dominant pathway to extracellular signal-regulated kinase activation during early thymic development. Mol Cell Biol 26:9035–9044. doi: 10.1128/MCB.00988-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xi H, Kersh GJ. 2004. Early growth response gene 3 regulates thymocyte proliferation during the transition from CD4-CD8- to CD4+CD88+. J Immunol 172:964–971. doi: 10.4049/jimmunol.172.2.964. [DOI] [PubMed] [Google Scholar]
- 12.Xi H, Schwartz R, Engel I, Murre C, Kersh GJ. 2006. Interplay between RORgammat, Egr3, and E proteins controls proliferation in response to pre-TCR signals. Immunity 24:813–826. doi: 10.1016/j.immuni.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 13.Ravichandran KS, Lorenz U, Shoelson SE, Burakoff SJ. 1995. Interaction of Shc with Grb2 regulates association of Grb2 with mSOS. Mol Cell Biol 15:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai KM, Pawson T. 2000. The ShcA phosphotyrosine docking protein sensitizes cardiovascular signaling in the mouse embryo. Genes Dev 14:1132–1145. [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Camerini V, Bender TP, Ravichandran KS. 2002. A nonredundant role for the adapter protein Shc in thymic T cell development. Nat Immunol 3:749–755. doi: 10.1038/ni820. [DOI] [PubMed] [Google Scholar]
- 16.Trampont PC, Tosello-Trampont AC, Shen Y, Duley AK, Sutherland AE, Bender TP, Littman DR, Ravichandran KS. 13 December 2009, posting date CXCR4 acts as a costimulator during thymic beta-selection. Nat Immunol doi: 10.1038/ni.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massari ME, Murre C. 2000. Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol 20:429–440. doi: 10.1128/MCB.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bain G, Cravatt CB, Loomans C, Alberola-Ila J, Hedrick SM, Murre C. 2001. Regulation of the helix-loop-helix proteins, E2A and Id3, by the Ras-ERK MAPK cascade. Nat Immunol 2:165–171. doi: 10.1038/84273. [DOI] [PubMed] [Google Scholar]
- 19.Rothenberg EV, Taghon T. 2005. Molecular genetics of T cell development. Annu Rev Immunol 23:601–649. doi: 10.1146/annurev.immunol.23.021704.115737. [DOI] [PubMed] [Google Scholar]
- 20.Gu JJ, Zhang N, He YW, Koleske AJ, Pendergast AM. 2007. Defective T cell development and function in the absence of Abelson kinases. J Immunol 179:7334–7343. doi: 10.4049/jimmunol.179.11.7334. [DOI] [PubMed] [Google Scholar]
- 21.Schmitt TM, Zuniga-Pflucker JC. 2002. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity 17:749–756. doi: 10.1016/S1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 22.Ciofani M, Schmitt TM, Ciofani A, Michie AM, Cuburu N, Aublin A, Maryanski JL, Zuniga-Pflucker JC. 2004. Obligatory role for cooperative signaling by pre-TCR and Notch during thymocyte differentiation. J Immunol 172:5230–5239. doi: 10.4049/jimmunol.172.9.5230. [DOI] [PubMed] [Google Scholar]
- 23.Murphy KM, Heimberger AB, Loh DY. 1990. Induction by antigen of intrathymic apoptosis of CD4+CD88+TCRlo thymocytes in vivo. Science 250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 24.Molina TJ, Kishihara K, Siderovski DP, van Ewijk W, Narendran A, Timms E, Wakeham A, Paige CJ, Hartmann KU, Veillette A, Davidson D, Mak TW. 1992. Profound block in thymocyte development in mice lacking p56lck. Nature 357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- 25.Walk SF, March ME, Ravichandran KS. 1998. Roles of Lck, Syk and ZAP-70 tyrosine kinases in TCR-mediated phosphorylation of the adapter protein Shc. Eur J Immunol 28:2265–2275. doi:. [DOI] [PubMed] [Google Scholar]
- 26.Mombaerts P, Anderson SJ, Perlmutter RM, Mak TW, Tonegawa S. 1994. An activated lck transgene promotes thymocyte development in RAG-1 mutant mice. Immunity 1:261–267. doi: 10.1016/1074-7613(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 27.Anderson SJ, Abraham KM, Nakayama T, Singer A, Perlmutter RM. 1992. Inhibition of T-cell receptor beta-chain gene rearrangement by overexpression of the non-receptor protein tyrosine kinase p56lck. EMBO J 11:4877–4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aifantis I, Feinberg J, Fehling HJ, Di Santo JP, von Boehmer H. 1999. Early T cell receptor beta gene expression is regulated by the pre-T cell receptor-CD3 complex. J Exp Med 190:141–144. doi: 10.1084/jem.190.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shinkai Y, Koyasu S, Nakayama K, Murphy KM, Loh DY, Reinherz EL, Alt FW. 1993. Restoration of T cell development in RAG-2-deficient mice by functional TCR transgenes. Science 259:822–825. doi: 10.1126/science.8430336. [DOI] [PubMed] [Google Scholar]
- 30.Haks MC, Krimpenfort P, van den Brakel JH, Kruisbeek AM. 1999. Pre-TCR signaling and inactivation of p53 induces crucial cell survival pathways in pre-T cells. Immunity 11:91–101. doi: 10.1016/S1074-7613(00)80084-9. [DOI] [PubMed] [Google Scholar]
- 31.Opferman JT. 2007. Life and death during hematopoietic differentiation. Curr Opin Immunol 19:497–502. doi: 10.1016/j.coi.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Lord JD, McIntosh BC, Greenberg PD, Nelson BH. 1998. The IL-2 receptor promotes proliferation, bcl-2 and bcl-x induction, but not cell viability through the adapter molecule Shc. J Immunol 161:4627–4633. [PubMed] [Google Scholar]
- 33.Dose M, Khan I, Guo Z, Kovalovsky D, Krueger A, von Boehmer H, Khazaie K, Gounari F. 2006. c-Myc mediates pre-TCR-induced proliferation but not developmental progression. Blood 108:2669–2677. doi: 10.1182/blood-2006-02-005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patrussi L, Savino MT, Pellegrini M, Paccani SR, Migliaccio E, Plyte S, Lanfrancone L, Pelicci PG, Baldari CT. 2005. Cooperation and selectivity of the two Grb2 binding sites of p52Shc in T-cell antigen receptor signaling to Ras family GTPases and Myc-dependent survival. Oncogene 24:2218–2228. doi: 10.1038/sj.onc.1208384. [DOI] [PubMed] [Google Scholar]
- 35.Cairo S, De Falco F, Pizzo M, Salomoni P, Pandolfi PP, Meroni G. 2005. PML interacts with Myc, and Myc target gene expression is altered in PML-null fibroblasts. Oncogene 24:2195–2203. doi: 10.1038/sj.onc.1208338. [DOI] [PubMed] [Google Scholar]
- 36.Sicinska E, Aifantis I, Le Cam L, Swat W, Borowski C, Yu Q, Ferrando AA, Levin SD, Geng Y, von Boehmer H, Sicinski P. 2003. Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell 4:451–461. doi: 10.1016/S1535-6108(03)00301-5. [DOI] [PubMed] [Google Scholar]
- 37.Haks MC, Lefebvre JM, Lauritsen JP, Carleton M, Rhodes M, Miyazaki T, Kappes DJ, Wiest DL. 2005. Attenuation of gammadeltaTCR signaling efficiently diverts thymocytes to the alphabeta lineage. Immunity 22:595–606. doi: 10.1016/j.immuni.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Engel I, Johns C, Bain G, Rivera RR, Murre C. 2001. Early thymocyte development is regulated by modulation of E2A protein activity. J Exp Med 194:733–745. doi: 10.1084/jem.194.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engel I, Murre C. 2001. The function of E- and Id proteins in lymphocyte development. Nat Rev Immunol 1:193–199. doi: 10.1038/35105060. [DOI] [PubMed] [Google Scholar]
- 40.Pear WS, Miller JP, Xu L, Pui JC, Soffer B, Quackenbush RC, Pendergast AM, Bronson R, Aster JC, Scott ML, Baltimore D. 1998. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood 92:3780–3792. [PubMed] [Google Scholar]
- 41.Tauchi T, Boswell HS, Leibowitz D, Broxmeyer HE. 1994. Coupling between p210bcr-abl and Shc and Grb2 adaptor proteins in hematopoietic cells permits growth factor receptor-independent link to ras activation pathway. J Exp Med 179:167–175. doi: 10.1084/jem.179.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michie AM, Soh JW, Hawley RG, Weinstein IB, Zuniga-Pflucker JC. 2001. Allelic exclusion and differentiation by protein kinase C-mediated signals in immature thymocytes. Proc Natl Acad Sci U S A 98:609–614. doi: 10.1073/pnas.98.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bettini M, Xi H, Milbrandt J, Kersh GJ. 2002. Thymocyte development in early growth response gene 1-deficient mice. J Immunol 169:1713–1720. doi: 10.4049/jimmunol.169.4.1713. [DOI] [PubMed] [Google Scholar]
- 44.Engel I, Murre C. 2004. E2A proteins enforce a proliferation checkpoint in developing thymocytes. EMBO J 23:202–211. doi: 10.1038/sj.emboj.7600017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ikawa T, Kawamoto H, Goldrath AW, Murre C. 2006. E proteins and Notch signaling cooperate to promote T cell lineage specification and commitment. J Exp Med 203:1329–1342. doi: 10.1084/jem.20060268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gotoh N, Toyoda M, Shibuya M. 1997. Tyrosine phosphorylation sites at amino acids 239 and 240 of Shc are involved in epidermal growth factor-induced mitogenic signaling that is distinct from Ras/mitogen-activated protein kinase activation. Mol Cell Biol 17:1824–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]