Abstract
Hair follicle (HF) morphogenesis relies on the coordinated exchange of signals between mesenchymal and epithelial compartments of embryonic skin. Chemokine receptor Cxcr4 expression was recently identified in dermal condensates (DCs) of nascent HFs, but its role in promoting HF morphogenesis remains unknown. Our analyses confirmed Cxcr4 expression in condensate cells, and additionally revealed transient Cxcr4 expression in incipient epithelial hair placodes. Placodal Cxcr4 appeared prior to detection in DCs, representing a switch of expression between epithelial and mesenchymal compartments. To explore the functional role of this receptor in both compartments for early HF formation, we conditionally ablated Cxcr4 with condensate-targeting Tbx18cre knock-in and epidermis-targeting Krt14-cre transgenic mice. Conditional knockouts for both crosses were viable throughout embryogenesis and into adulthood. Morphological and biochemical marker analyses revealed comparable numbers of HFs forming in knockout embryos compared to wild-type littermate controls in both cases, suggesting that neither dermal nor epithelial Cxcr4 expression is required for early HF morphogenesis. We conclude that Cxcr4 expression and chemokine signaling through this receptor in embryonic mouse skin is dispensable for HF formation.
Keywords: Dermal papilla cells, Stem cell niche, Hair follicle morphogenesis, Hair follicle stem cells, Mesenchymal-epithelial interactions
Background
Mesenchymal-epithelial interactions drive diverse morphogenetic events during embryogenesis, including development of the skin and its associated appendages (1–3). Hair follicle (HF) formation in particular is known to rely on signaling crosstalk between these two compartments, and is thought to involve specialized mesenchymal dermal condensate (DC) cells and epithelial placode cells (1). However the precise identity, timing and mechanisms of all exchanged signals remain unknown. In an effort to characterize potentially relevant signaling pathways involved in HF morphogenesis, we examined the unique expression patterns of chemokine receptor CXCR4 in both condensate and placode during early stages of initiation and downgrowth. CXCR4 is a well-studied receptor previously implicated in multiple developmental and disease paradigms including organogenesis, stem cell maintenance, cancer and HIV, wherein CXCR4 interactions with ligand CXCL12 promote biological processes such as directed cell migration, cell survival, and adhesion (4–6). In order to assess the functional role of CXCR4 in HF morphogenesis we used the cre/loxP recombination system in genetically modified mice to conditionally ablate this gene in both epithelial and mesenchymal compartments during embryogenesis and assessed HF development.
Questions addressed
Is Cxcr4 expression in placodes and dermal condensates required for hair follicle morphogenesis?
Experimental design
To investigate patterns of Cxcr4 expression in embryonic mouse skin we used immunofluorescence on sections and qRT-PCR on sorted cells. More detailed information is available in the supplementary materials and methods section.
Results
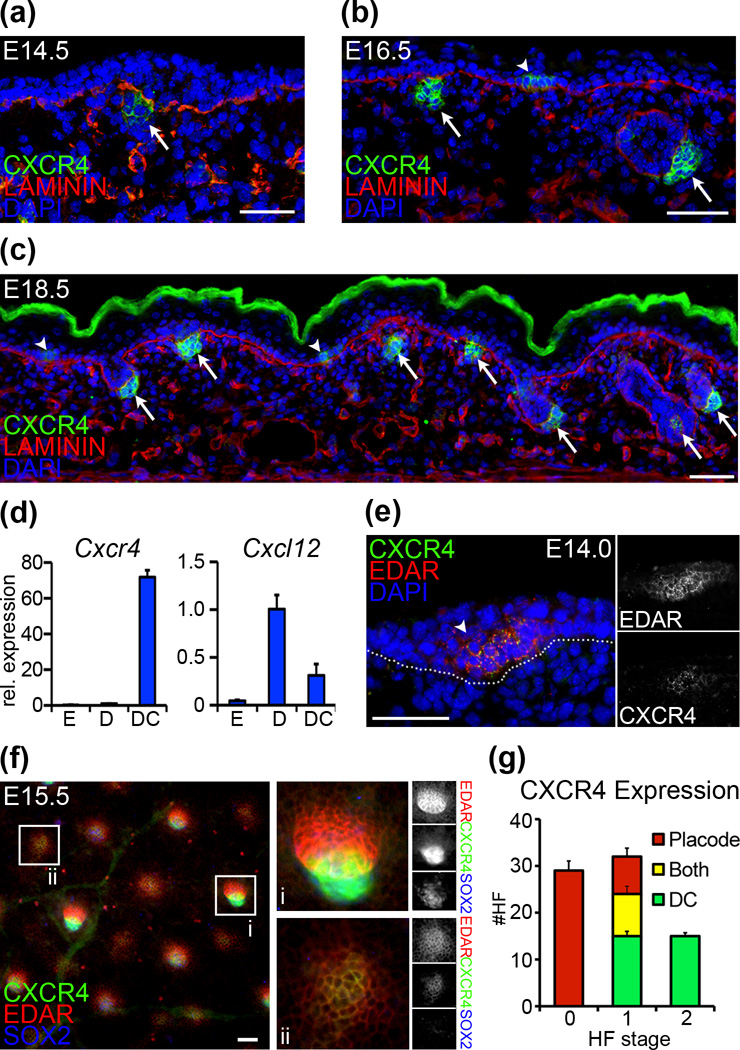
Chemokine signaling has been previously implicated in hair follicle (HF) morphogenesis, including recent descriptions of Cxcr4 expression in dermal condensates (DCs) of embryonic HFs (7, 8). To study the expression patterns of Cxcr4 in the HF mesenchyme in more detail, we used immunofluorescence staining on back skin sections from embryos aged 14.5 days (E14.5), E16.5 and E18.5 during the three main HF formation waves. In E14.5 sections we observed CXCR4 in condensates of stage 1 follicles (Fig. 1a, arrow). At E16.5, CXCR4 was present in DCs of downgrowing HFs and newly formed stage 1 follicles (Fig. 1b). At E18.5, we observed CXCR4 in condensates/dermal papillae of stage 1–5 follicles (Fig. 1c). High levels of Cxcr4 expression were confirmed by qRT-PCR analysis of FACS-isolated condensate cells at E14.5, compared to whole epidermis and dermis (Fig. 1d). The related chemokine ligand Cxcl12 is expressed in the embryonic dermis and DCs (Fig. 1d).
Figure 1. Cxcr4 expression in embryonic skin.
(a–c) Immunofluorescence staining for CXCR4 shows differential expression in epithelial placodes (arrowheads) and mesenchymal dermal condensates (arrows) of forming HFs at E14.5, E16.5, and E18.5. LAMININ labels basement membranes; Dapi highlights nuclei. (d) qRT-PCR of FACS sorted cells at E14.5 shows high Cxcr4 expression in dermal condensates (“DC”), compared to embryonic dermis (“D”) and epidermis (“E”). Cxcr4 ligand chemokine Cxcl12 is expressed in mesenchymal cells. (e) Immunofluorescence of E14.0 skin confirms CXCR4 expression in EDAR+ placodes of earliest forming HFs. (f) EDAR (placode marker) and SOX2 (condensate marker) expression highlight the stage- and compartment-specific localization of CXCR4 expression. In more advanced stage 2 follicles (i) CXCR4 co-localizes with SOX2. In early stage 0 follicles (ii) CXCR4 co-localizes with EDAR. (g) Quantification of CXCR4 localization in early forming HFs by stage at E15.0 (mean and SD of 3 embryos). CXCR4 expression switches from epithelial to mesenchymal cells. Dotted line denotes basement membrane. Scale bar = 50µm.
Through our initial immunofluorescence studies we uncovered CXCR4 staining also in epithelial placodes (Fig. 1b,c; arrowheads). With additional stainings we confirmed epithelial CXCR4 co-localization with placode marker EDAR at E16.5 and E18.5 (Fig. S1), indicating expression in stage 0 follicles confined to the epithelial compartment. Analyses of skin sections of earliest first-wave follicles at E14.0 corroborated CXCR4 co-localization with EDAR in nascent placodes (Fig. 1e). Finally, with whole-mount immunofluorescence stainings for CXCR4/EDAR of E15.5 Sox2GFP skin tissues, where GFP strongly labels condensates (9) and follicles are present in stages 0–2, we confirmed HF-stage dependent CXCR4 expression (Fig. 1f): larger advanced follicles with distinct elongated EDAR domains robustly stained for CXCR4 in condensates (Fig. 1f, insert i), while nascent follicles without obvious condensates displayed small circular EDAR domains that co-localized with CXCR4 (Fig. 1f, insert ii). Stage-specific quantification of compartmental CXCR4 localization revealed that epithelial expression is limited to stage 0 and few stage 1 follicles, whereas mesenchymal CXCR4 is found in stage 1 and stage 2 follicles. Collectively these results indicate that as follicles mature during morphogenesis, Cxcr4 expression switches from the epithelial to the mesenchymal HF domain.
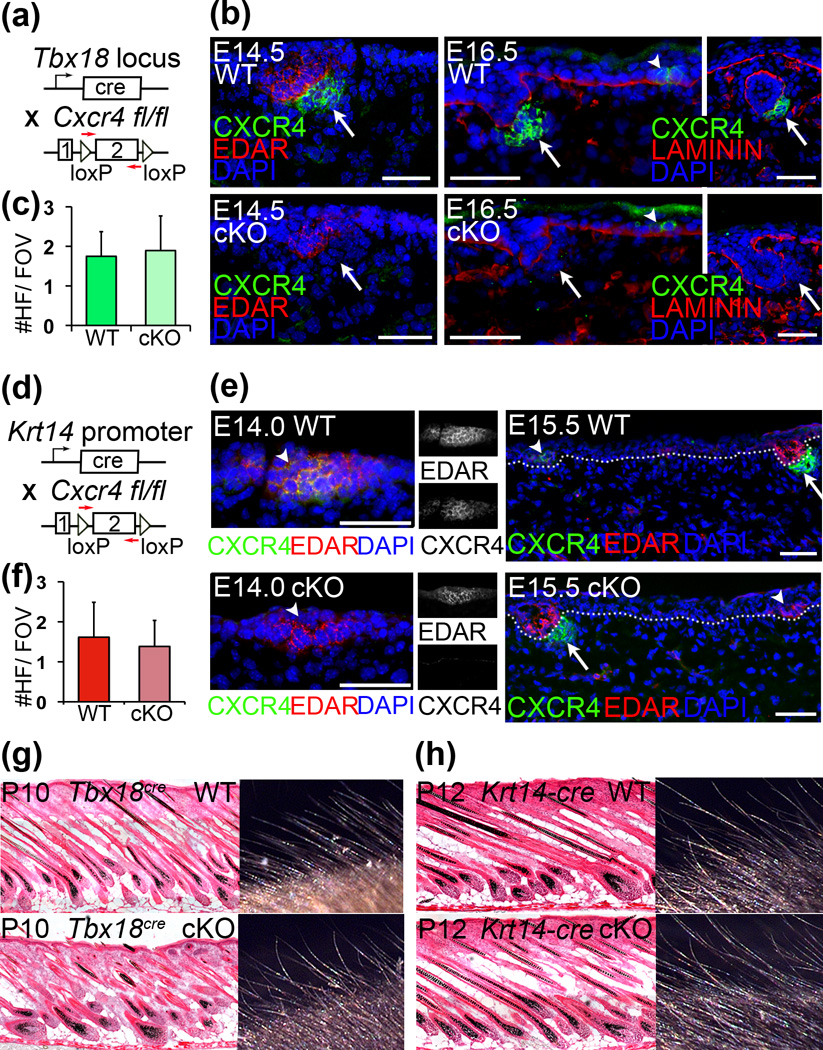
To explore the function of Cxcr4 in placodes and/or condensates of forming HFs, we used separate cre-loxP strategies to conditionally ablate expression in the epithelial and mesenchymal compartments. To target DCs, we crossed Tbx18cre (10) with Cxcr4 floxed mice (11) (Fig. 2a). Cxcr4 ablation in E14.5 conditional knockout (cKO) DCs was confirmed by immunofluorescence (Fig. 2b). Despite early and efficient Cxcr4 ablation, first-wave HFs formed normally in cKO skin and numbers were comparable to those found in wild-type (WT) littermates in both embryonic skin (Fig. S2a) and in postnatal P10 skin (Fig. 2c,g). To independently confirm that CXCR4 in DCs is dispensable for HF formation we ablated this gene also by crossing Cxcr4 floxed mice with the Prx1-cre transgenic line (Fig. S3a) (12). The Prx1 promoter is widely active in the ventral skin mesenchyme starting at E10.5, well before HF formation (13, 14). By E14.5 the entire ventral dermis is labeled with a cre reporter (Fig. S3b). Despite broad and early Cxcr4 ablation in the dermis, HFs were still induced in cKO ventral skin (Fig. S3c, SOX2+DCs in green), and HFs formed normally (data not shown). These data suggest that DC expression of Cxcr4 is not required for HF formation.
Figure 2. Targeted ablation of Cxcr4 in placodes or condensates does not affect HF formation.
(a) Schematic of Tbx18cre and Cxcr4fl/fl lines to target dermal condensates at E14.5 for Cxcr4 ablation. (b) Immunofluorescence staining confirms CXCR4 ablation at the protein level in condensates (arrows) at E14.5 and E16.5 stage 1–2 follicles, while epithelial CXCR4 expression remains intact (arrowheads). (c) Quantification of first-wave HF numbers per field of view (FOV). Comparable HF numbers form in P10 cKO skin compared to controls. (d–f) Krt14-cre targets epidermal cells including the placode for Cxcr4 ablation. (e) Immunofluorescence staining confirms absence of CXCR4 in placodes (arrowheads) as early as E14.0 in cKO skin. Note expression in condensates is still present (arrows). (f) Comparable numbers of first-wave follicles form in P12 cKO skin compared to controls. (g–h) Hematoxylin/eosin staining of skin sections and macroscopic view of external hair shafts. Hair follicles and shafts form normally in both Tbx18cre (g) and Krt14-cre (h) Cxcr4fl/flcKO pups. c,f: Data are mean and SD of 2 WT and 2 cKO sex-matched littermates (6–10 FOV). Dotted lines denote basement membranes. Scale bar = 50µm.
To investigate the role of Cxcr4 in the epithelial placode, we crossed Krt14-cre (15) with Cxcr4 floxed mice (Fig. 2d) and confirmed robust CXCR4 ablation in first wave HF placodes with immunofluorescence (Fig. 2e). Continued expression of CXCR4 in DCs highlighted the compartment-specific gene knockout (Fig. 2e). Despite the absence of CXCR4 expression in cKO placodes, embryonic HFs formed normally at E14.5 (Fig. S2b). Quantification of first-wave HFs at postnatal day P12 revealed formation in normal numbers in cKO skin compared to WT littermate controls (Fig. 2f,h). Collectively, these data suggest that Cxcr4 expression by either the epithelial or mesenchymal compartment is dispensable for normal HF morphogenesis to proceed.
Conclusions
Chemokine signaling has been previously implicated in HF development, and in this study we confirmed the expression of the chemokine receptor Cxcr4 in newly forming follicles and assessed its function using mouse genetics. We found Cxcr4 expression in nascent HFs within placodes, which switches to condensates in maturing follicles. Despite its uniquely localized expression, conditional gene ablation revealed that this receptor is dispensable for HF development. In general CXCR4 is thought to interact exclusively with ligand CXCL12 (16), suggesting that other chemokine receptors are unlikely to play a role in functionally compensating the absence of Cxcr4 through genetic redundancy. However in prior investigations of chemokine function in development, separate signaling cascades were found to cooperate with the Cxcr4-Cxcl12 axis to drive tissue morphogenesis (17). Therefore it might be interesting in future investigations to examine the gene expression profiles of conditional knockout skin for any compensatory gene changes.
Supplementary Material
Acknowledgements
Many thanks to our colleagues for sharing mice: Elena Ezhkova (Krt14-cre), Larysa Pevny (Sox2GFP) and Chen-Leng Cai (Tbx18cre). We also thank the personnel of the Flow Cytometry Core Facility. R.S. was supported by training grant T32GM008553 from NIH/NIGMS. M.R. was supported by grants from the NIH/NIAMS (R01AR059143; R01AR063151).
Footnotes
Author contributions
R.S. and M.R. conceived and designed the experiments, R.S., A.R., and K.D. performed experiments, C.C. contributed essential reagents and tools, R.S. and M.R. analyzed and interpreted the data, R.S. prepared figures, and R.S. and M.R. wrote the manuscript.
Conflict of interests
The authors declare no conflict of interest.
References
- 1.Sennett R, Rendl M. Mesenchymal-epithelial interactions during hair follicle morphogenesis and cycling. Semin Cell Dev Biol. 2012;23:917–927. doi: 10.1016/j.semcdb.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mikkola ML. Genetic basis of skin appendage development. Semin Cell Dev Biol. 2007;18:225–236. doi: 10.1016/j.semcdb.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118:216–225. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- 4.Miller RJ, Banisadr G, Bhattacharyya BJ. CXCR4 signaling in the regulation of stem cell migration and development. J Neuroimmunol. 2008;198:31–38. doi: 10.1016/j.jneuroim.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kucia M, Jankowski K, Reca R, et al. CXCR4-SDF-1 signaling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35:233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 6.Ratajczak MZ, Zuba-Surma E, Kucia M, et al. The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006;20:1915–1924. doi: 10.1038/sj.leu.2404357. [DOI] [PubMed] [Google Scholar]
- 7.Lefebvre S, Fliniaux I, Schneider P, et al. Identification of Ectodysplasin Target Genes Reveals the Involvement of Chemokines in Hair Development. J Invest Dermatol. 2012;132:1094–1102. doi: 10.1038/jid.2011.453. [DOI] [PubMed] [Google Scholar]
- 8.Belmadani A, Jung H, Ren D, et al. The chemokine SDF-1/CXCL12 regulates the migration of melanocyte progenitors in mouse hair follicles. Differentiation. 2009;77:395–411. doi: 10.1016/j.diff.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clavel C, Grisanti L, Zemla R, et al. Sox2 in the dermal papilla niche controls hair growth by fine-tuning BMP signaling in differentiating hair shaft progenitors. Dev Cell. 2012;23:981–994. doi: 10.1016/j.devcel.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grisanti L, Clavel C, Cai X, et al. Tbx18 Targets Dermal Condensates for Labeling, Isolation, and Gene Ablation during Embryonic Hair Follicle Formation. J Invest Dermatol. 2013;133:344–353. doi: 10.1038/jid.2012.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nie Y, Waite J, Brewer F, et al. The role of CXCR4 in maintaining peripheral B cell compartments and humoral immunity. J Exp Med. 2004;200:1145–1156. doi: 10.1084/jem.20041185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Logan M, Martin JF, Nagy A, et al. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33:77–80. doi: 10.1002/gene.10092. [DOI] [PubMed] [Google Scholar]
- 13.Lehman JM, Laag E, Michaud EJ, et al. An essential role for dermal primary cilia in hair follicle morphogenesis. J Invest Dermatol. 2009;129:438–448. doi: 10.1038/jid.2008.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woo W-M, Zhen HH, Oro AE. Shh maintains dermal papilla identity and hair morphogenesis via a Noggin-Shh regulatory loop. Genes Dev. 2012;26:1235–1246. doi: 10.1101/gad.187401.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dassule HR, Lewis P, Bei M, et al. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127:4775–4785. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- 16.Puchert M, Engele J. The peculiarities of the SDF-1/CXCL12 system: in some cells, CXCR4 and CXCR7 sing solos, in others, they sing duets. Cell Tissue Res. 2014;355:239–253. doi: 10.1007/s00441-013-1747-y. [DOI] [PubMed] [Google Scholar]
- 17.Vasyutina E, Stebler J. CXCR4 and Gab1 cooperate to control the development of migrating muscle progenitor cells. Genes Dev. 2005;19:2187–2198. doi: 10.1101/gad.346205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muzumdar MD, Tasic B, Miyamichi K, et al. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 19.Grisanti L, Rezza A, Clavel C, et al. Enpp2/Autotaxin in Dermal Papilla Precursors Is Dispensable for Hair Follicle Morphogenesis. J Invest Dermatol. 2013;133:2332–2339. doi: 10.1038/jid.2013.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai S-Y, Sennett R, Rezza A, et al. Wnt/β-catenin signaling in dermal condensates is required for hair follicle formation. Dev Biol. 2014;385:179–188. doi: 10.1016/j.ydbio.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.