Abstract
Mice deficient in B-cells (μmT mice) were used to evaluate the role of antibody in enhanced chlamydial clearance and reduction of pathology afforded by vaccination with recombinant chlamydial protease-like activity factor (rCPAF). Enhanced, but comparable, chlamydial clearance was observed in μmT and wild type (WT) mice after rCPAF+CpG vaccination. Chlamydia-induced pathology was present in mock-immunized animals, but at significantly greater levels in μmT than WT mice, whereas vaccinated μmT and WT mice exhibited similar reductions in pathology. Thus, antibodies may play a role in protection against chlamydial pathology after primary infection, but were largely dispensable in rCPAF+CpG induced chlamydial clearance and reduction in pathology.
Introduction
Genital Chlamydia trachomatis infection in humans leads to pathological sequelae in the upper genital tract (UGT) including pelvic inflammatory disease, ectopic pregnancy and infertility (Morrison & Caldwell, 2002; Debattista et al., 2003; Brunham & Rey-Ladino, 2005). The incidence of genital chlamydial infection has continued to increase over the last decade despite the availability of efficacious antimicrobial therapy (Brunham & Rey-Ladino, 2005). A licensed preventive vaccine is thought to be the solution to this problem, but has yet to be developed (Morrison & Caldwell, 2002; Brunham & Rey-Ladino, 2005). Previously, we demonstrated the efficacy of recombinant chlamydial protease-like activity factor (rCPAF) (Zhong et al., 2001) vaccination in inducing robust antigen-specific IFN-γ, serum and vaginal antibody responses (Murthy et al., 2007) after immunization, and accelerated bacterial clearance and reduction of pathological sequelae following genital Chlamydia muridarum challenge (Murthy et al., 2006; Murthy et al., 2007; Cong et al., 2007).
Th 1 type cellular responses including IFN-γ production have been shown to play a predominant role in anti-chlamydial protective immunity (Rank et al., 1985; Rank et al., 1992; Su & Caldwell, 1995; Cotter et al., 1995; Johansson et al., 1997; Perry et al., 1997; Ito et al., 1999; Perry et al., 1999; Jupelli et al., 2008). We have shown that the protective immunity induced by rCPAF vaccination is dependent upon CD4+ T cells and IFN-γ produced locally in the genital tract (Murphey et al., 2006; Murthy et al., 2007; Li et al., 2008). The role of B cells and antibody in anti-chlamydial immunity is appreciated, but not well understood. The lack of antibody or Fc receptors has been shown not to significantly affect the kinetics of chlamydial clearance during primary genital infection (Moore et al., 2002; Moore et al., 2003). However, studies using depletion of CD4+ and/or CD8+ T cell compartments in μmT mice have revealed a predominant role for antibody in clearance of secondary genital chlamydial infection (Morrison et al., 2000; Morrison & Morrison, 2001; Morrison & Morrison, 2005). Additionally, mice deficient in activatory Fc receptors (FcR−/− mice) have been shown to display reduced resistance to secondary chlamydial challenge (Moore et al., 2002; Moore et al., 2003), further suggesting that the effects of antibody may occur via Fc receptor-dependent mechanisms. Collectively, antibody appears to play a role in chlamydial clearance. However, the role of antibody in the development of Chlamydia-induced UGT pathological sequelae has yet to be determined.
Given that vaccination with rCPAF+CpG induces accelerated chlamydial clearance as well as the reduction of UGT pathology, we studied the role of antibody in both aspects using mice deficient in the μ chain and antibody production (μmT mice). We observed that the absence of antibody did not alter the course of infection in either rCPAF+CpG vaccinated or mock (PBS) treated μmT mice, when compared to correspondingly treated C57BL/6 animals, suggesting that antibody was not required for primary chlamydial clearance. However, mock immunized μmT mice displayed significantly greater uterine horn pathology compared to similarly treated wild type mice, suggesting a role for antibody in reducing UGT pathology. rCPAF+CpG vaccinated C57BL/6 mice displayed significantly reduced UGT pathology when compared to mock wild type or μmT animals. Importantly, rCPAF+CpG vaccinated μmT mice displayed comparable reduced UGT pathology to similarly treated wild type animals, suggesting that vaccination was able to provide protection against pathology even in the total absence of antibody.
Materials and Methods
Bacteria
Chlamydia muridarum was grown on confluent HeLa cell monolayers as described previously (Murthy et al., 2004). Cells were lysed using a sonicator and elementary bodies (EBs) purified on Renograffin gradients. Aliquots of bacteria were stored at −70°C in sucrose–phosphate–glutamine (SPG) buffer.
Mice
Four-to-six week old female mice were used for all experiments. Wild type C57BL/6 mice were purchased from the National Cancer Institute (Bethesda, MD). C57BL/6 μmT (B cell deficient) mice were purchased from Jackson laboratories (Bar Harbor, ME). Animal care and experimental procedures were performed at the University of Texas at San Antonio in compliance with the Institutional Animal Care and Use Committee (IACUC) guidelines.
rCPAF and CpG
rCPAF from C. trachomatis L2 genome was cloned and expressed in a bacterial system as described previously (Murthy et al., 2007). Briefly, rCPAF constructs cloned from C. trachomatis L2 genome with a 6-Histidine tag (His) were cloned into pBAD vectors and expressed in Escherichia coli with L-Arabinose (Sigma Aldrich, St. Louis, MO) as an inducer. The fusion protein was purified using Ni-NTA agarose beads (Qiagen, Valencia, CA). The purified rCPAF was identified by Western blot analysis using a monoclonal anti-CPAF antibody (Murthy et al., 2007) and used as a source of protein for all experiments. PAGE-purified CpG deoxynucleotides (designated CpG in this study) synthesized with a sequence of 5′-TCC ATG ACG TTC CTG ACG TT-3′ and a completely phosphorothiolated backbone was obtained from Sigma Genosys (St. Louis, MO), and used as an adjuvant.
Intranasal immunization and vaginal C. muridarum challenge
Groups of 4–5 week old female μmT or C57BL6 mice were immunized i.n. with rCPAF (15 μg per mouse) and CpG (10 μg per mouse), or mock-immunzed with PBS, on day 0 with booster immunizations given on days 14 and 28, as described previously (Cong et al., 2007). Mice were rested for one month following the final booster immunization and challenged i.vag. with 5 × 104 IFU of C. muridarum. Ten and three days prior to challenge, mice were treated with 2.5 mg of Depo-Provera (Pharmacia Upjohn, Kalamazoo, MI) to synchronize the estrous cycles.
Antigen-specific splenocyte IFN-γ recall responses
Spleens were removed 14 days after primary vaccination and single cell suspensions prepared. The collected splenocytes (106/well) were incubated for 72 hr with 1 μg rCPAF/well, or with an equal concentration of the unrelated antigen hen egg lysozyme (HEL), in 96-well culture plates. Supernatants were assayed for levels of IFN-γ using BDOptEIA™ kits (BD Biosciences, San Diego, CA) per manufacturer’s instructions. Absorbance at 630 nm was measured using a μQuant ELISA microplate reader (Biotek Instruments, Winooski, VT).
Detection of antibody and isotype levels by ELISA
Ten days following final immunization, animals were bled, sera prepared and analyzed by ELISA as described previously (Murthy et al., 2006). Microtiter plates (96-well) were coated overnight with 10 μg rCPAF/ml sodium bicarbonate buffer (pH 9.5). Serially diluted serum samples were then added to the wells followed by either goat anti-mouse total Ig, IgG1, IgG2a, IgG2b, or IgA conjugated to horse radish perxoxidase (Southern Biotechnology Associates, Birmingham, AL). After washing, 3.3′, 5.5′-tetramethylbenzidine (TMB) substrate (BD Biosciences) was added and absorbance (O.D.) at 630 nm monitored using a μQuant ELISA microplate reader. Reciprocal serum dilutions corresponding to 50% maximal binding were used to obtain titers.
Determination of vaginal chlamydial shedding
The vaginal chlamydial shedding was monitored as described previously (Murthy et al., 2007). Vaginal swabs were obtained on the indicated days after vaginal challenge, followed by plating of swab material on HeLa cell monolayers grown on culture coverslips. Chlamydial inclusions were detected using a Chlamydia genus specific murine monoclonal primary antibody and goat anti-mouse IgG secondary antibody conjugated to Cy3 plus Hoescht nuclear stain. The number of inclusions was enumerated for each animal, and results expressed as percentage of animals in a group shedding Chlamydia at each time-point.
Determination of upper genital tract pathology
On day 80 post challenge, animals were euthanized, and genital tracts removed and examined. The gross appearance of the genital tract of each animal was photographed using a 6 megapixel F10 digital camera (Fujifilm, Tokyo, Japan) at a fixed distance. Images were saved at 6 MP resolution and photographs printed on an 8 × 11.25 inch sheet and oviduct cross-sectional diameter was measured. When multiple oviduct loops were present, the one with the greatest diameter was reported. For uterine horns, the average of the greatest cross-sectional diameter of each 5 mm longitudinal section was reported. Tissues were then embedded into paraffin blocks, sectioned (5 μm) and stained using hematoxylin and eosin (H&E). The stained sections were observed using a Zeiss Axiovision Research microscope (Carl Zeiss MicroImaging, Inc. Thornwood, NY) and images obtained using a Zeiss digital camera. Sections were scored for loss of epithelial architecture as follows: 0-normal epithelial folds; 1-loss of epithelial folds in one region of the uterine horn; 2-loss of epithelial folds in two non-contiguous regions of the uterine horn; 3-loss of epithelial folds in 3 non-contiguous regions of the uterine horn; 4-loss of epithelial folds in more than 3 non-contiguous regions or throughout the uterine horn. Scores for individual oviducts and the mean score ± SE per group are reported.
Statistics
Sigma Stat (Systat Software Inc., San Jose, CA) was used to perform all statistical tests. P < 0.05 was considered statistically significant. The student’s t test (data set passing normality test) or the Mann-Whitney Rank Sum test (data set failing normality test) was used for comparison between groups. For comparison of time taken for resolution of infection, the Kaplan-Meier Survival Curve analysis was employed. All data are representative of two independent experiments and each experiment was analyzed independently.
Results
Immune response after rCPAF and CpG vaccination in μmT mice
Groups of μmT and C57BL/6 mice were immunized i.n. with rCPAF+CpG (vaccinated) or PBS (mock) on day 0 and splenic IFN-γ reponses were measured 14 days after initial immunization. As shown in Fig. 1, splenocytes from vaccinated C57BL/6 mice displayed a high level of IFN-γ production upon stimulation with rCPAF, but not with the unrelated antigen HEL. Importantly, splenocytes from vaccinated μmT mice displayed significantly greater antigen-specific IFN-γ production when compared to those from vaccinated C57BL/6 mice. There was minimal IFN-γ production from splenocytes derived from mock μmT or C57BL/6 animals, and minimal IL-4 production in cells from all groups (data not shown). In parallel experiments, mice vaccinated on day 0 also received booster immunizations on days 14 and 28. Ten days after the last booster immunization, mice were bled, sera prepared, and analyzed for anti-CPAF antibody. As shown in Fig. 2, vaccinated C57BL/6 mice displayed high titers of anti-CPAF total Ab, IgG1, IgG2a, IgG2b, and IgA antibody. As expected, there was no detectable antibody in the sera from μmT mice. There also was no anti-CPAF antibody in mock C57BL/6 or μmT mice (data not shown). Collectively, these results suggest that rCPAF+CpG vaccination induces a robust cellular antigen-specific IFN-γ response in μmT mice that lack B cells and antibody production.
Fig. 1. Cellular IFN-γ response after rCPAF+CpG vaccination.
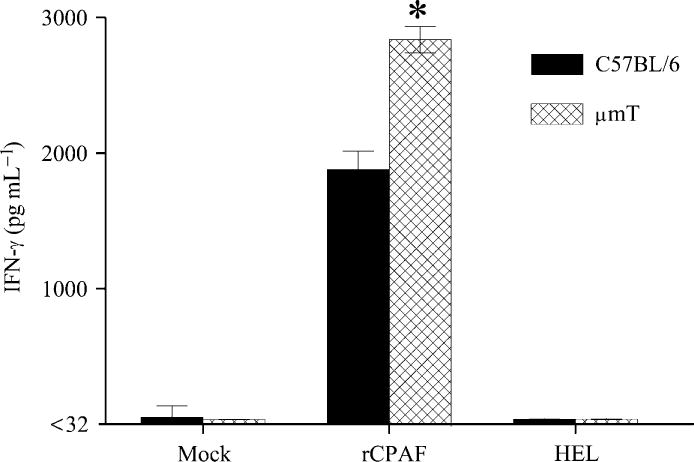
Groups (n=3) of C57BL/6 or μmT mice were vaccinated i.n. with rCPAF+CpG on day 0. On day 14, animals were euthanized and splenocytes were tested for rCPAF-induced IFN-γ production by ELISA. * Significant differences in IFN-γ secretion between vaccinated C57BL/6 and μmT mice (P = 0.004, student’s t test). Results are representative of two independent experiments.
Fig. 2. Humoral response after rCPAF+CpG vaccination.
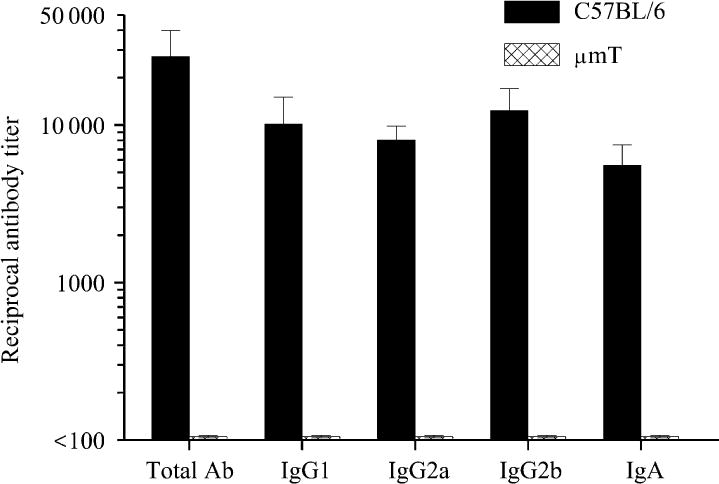
Groups (n=6) of C57BL/6 or μmT mice (n=5) were vaccinated i.n. with rCPAF+CpG on day 0 with booster immunizations given on days 14 and 28. Ten days following the last booster immunization, animals were bled and sera analyzed for anti-CPAF antibody by isotype ELISA. The mean ± SE of the reciprocal 50% maximal binding titers are reported. Results are representative of two independent experiments.
Resolution of vaginal C. muridarum infection in rCPAF+CpG vaccinated μmT mice
Vaccinated and mock mice were challenged i.vag. with 5 × 104 IFU of C. muridarum and vaginal chlamydial shedding monitored over a period of one month. As shown in Fig. 3, vaccinated C57BL/6 and μmT mice displayed reduced vaginal chlamydial shedding as early as day 8, with significant reductions at day 12 after challenge, when compared to corresponding groups of mock-immunized animals. Vaginal chlamydial clearance was exhibited in 17% of mock C57BL/6 mice by day 24 and 67% of mice by day 27, with all (100%) mice completely clearing the infection by day 30 after challenge. Mock μmT mice displayed comparable kinetics of vaginal chlamydial clearance to mock C57BL/6 animals. In contrast, chlamydial clearance was exhibited in 33% of vaccinated C57BL/6 mice as early as day 15, with 83% on day 18 and all (100%) mice on day 21 after challenge, which was significantly earlier than in mock C57BL/6 animals. Importantly, vaccinated μmT mice exhibited comparable resolution kinetics to vaccinated C57BL/6 animals with chlamydial clearance occurring in 20% of mice by day 15, 80% of mice by day 18, and all (100%) mice by day 21 after challenge. These results suggest that antibody may not contribute significantly to the rCPAF+CpG induced accelerated vaginal chlamydial clearance.
Fig. 3. Resolution of primary genital C. muridarum infection after rCPAF+CpG vaccination.
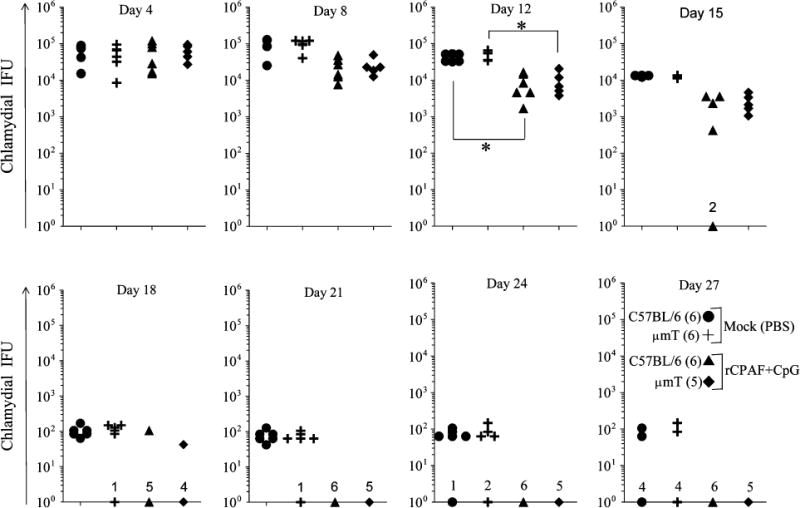
Groups (5–6 mice/group) of C57BL/6 or μmT mice were vaccinated with three doses of rCPAF+CpG or mock-immunized with PBS. One month following final vaccination, mice were challenged i.vag. with 5 × 104 IFU of C. muridarum. At the indicated days following challenge, chlamydial shedding was measured and the numbers of chlamydial inclusion forming units (IFU) recovered from vaginal swabs are shown. Each symbol represents an individual animal. The number of animals in each group that had resolved the infection is indicated on the X-axis. * Significant differences between indicated groups (P < 0.05, Kruskall-Wallis test). Kaplan Meier survival analyses indicated significant differences between vaccinated and mock C57BL/6 animals (P = 0.0015), and differences between vaccinated and mock μmT animals (P = 0.0009). Results are representative of three independent experiments.
Upper genital tract pathology in rCPAF+CpG vaccinated μmT mice
The pathology in the upper genital tract was examined on day 80 after chlamydial challenge. The development of oviduct dilatation and hydrosalpinx (Representative panel, Fig. 4-oviducts) are typical sequelae of genital C. muridarum infection in mice (Morrison & Caldwell, 2002; Brunham & Rey-Ladino, 2005). As shown in Fig. 5, most mock C57BL/6 mice exhibited oviduct dilatation (83% bilateral, 17% unilateral) at 80 days after genital chlamydial challenge. The diameter of the oviducts in these mice (2.29 ± 0.24 mm) was significantly greater than those in age-matched naïve mice (~0.5 mm, data not shown). A comparable number (83% bilateral) of mock μmT mice exhibited a similar degree of oviduct dilatation (1.86 ± 0.32 mm). In comparison, vaccinated C57BL/6 mice exhibited significantly lower oviduct diameters (1 ± 0.3 mm), with few (17% bilateral, 17% unilateral) mice displaying dilated oviducts. Vaccinated μmT mice exhibited oviduct diameters (1.1 ± 0.2 mm) that were comparable to vaccinated C57BL/6 animals (1 ± 0.3 mm) and significantly reduced compared to mock C57BL/6 animals (2.29 ± 0.24 mm). The oviduct dilatation in vaccinated μmT mice (1.1 ± 0.2 mm) was considerably reduced, although not significantly, compared to mock μmT mice (1.86 ± 0.32 mm).
Fig. 4. Gross and microscopic pathology in C. muridarum challenged mice.
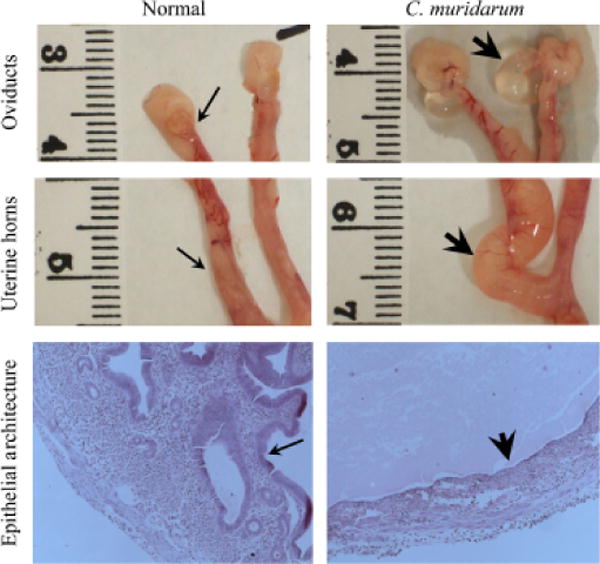
The histopathology was evaluated in mice on day 80 after C. muridarum or mock (PBS) challenge. The representative gross images from oviducts and uterine horns, and microscopic images (total magnification 25X) of uterine horn epithelium are shown. The normal (thin arrows) and dilated (thick arrows) oviducts and uterine horns are indicated. Additionally, the uterine epithelial architecture in H&E stained tissue sections displays abundant folds of the uterine epithelium in mock-challenged mice (thin arrow) versus a flattened, but continuous, epithelial lining (thick arrow) in C. muridarum challenged animals. Luminal exudate, with some inflammatory cells, is apparent in the tissues from infected mice.
Fig. 5. Oviduct dilatation following rCPAF+CpG vaccination/primary genital C. muridarum infection.
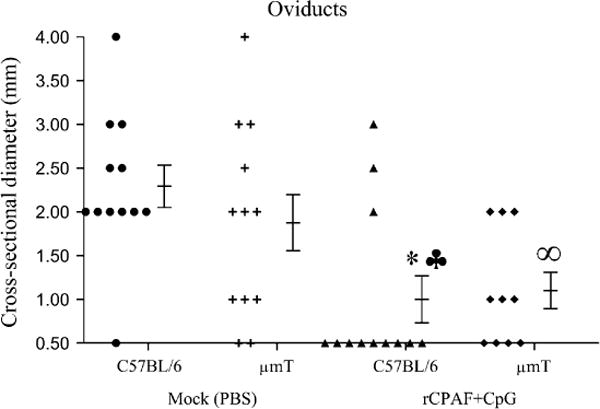
Groups (n= 5–6) of C57BL/6 or μmT mice were vaccinated intranasally with three doses of rCPAF+CpG (vaccinated) or treated with PBS (mock). One month following final vaccination, mice were challenged i.vag. with 5 × 104 IFU of C. muridarum. At day 80 after challenge, the mice were euthanized, genital tracts removed, photographed at a fixed distance, and oviduct diameters measured. The individual oviduct diameters and mean ± SE of all oviducts in a group are shown. *Significant differences between vaccinated and mock C57BL/6 animals (P = 0.009, Mann-Whitney Rank Sum test). ♣ Significant differences between vaccinated C57BL/6 mice and mock μmT animals (P = 0.03, Student’s t test). ∞Significant differences between vaccinated μmT mice and mock C57BL/6 animals (P = 0.002, Student’s t test). Results are representative of two independent experiments.
In addition to oviduct dilatation, C57BL/6 mice also develop uterine horn pathology after genital C. muridarum infection (Darville et al., 1997). Therefore, we compared the gross dilatation of uterine horns (Representative panel, Fig. 4-uterine horns) between vaccinated μmT and C57BL/6 mice. As shown in Fig. 6, the average diameters of uterine horns in all groups of mice were comparable, although the mock μmT mice (3.1 ± 0.3 mm) exhibited a relatively greater degree of dilatation compared to the mock C57BL/6 mice (2.4 ± 0.4 mm), or vaccinated μmT (2.6 ± 0.2 mm) and C57BL/6 mice (2.5 ± 0.5 mm). Given that uterine horn dilatation was present in short segments and a mean cross-sectional diameter may not fully represent the pathology, we also scored for loss of normal tissue architecture with regard to epithelial folds (Representative panel, Fig. 4-epithelial architecture). As shown in Fig. 7, the loss of epithelial folds in mock μmT mice (2.7 ± 0.5) was significantly greater than in mock C57BL/6 mice (0.7 ± 0.4). Vaccinated C57BL/6 mice (1.1 ± 0.5) exhibited comparable low scores to those of mock C57BL/6 mice (0.7 ± 0.4), but significantly lower scores than observed for mock μmT mice (2.7 ± 0.5). Vaccinated μmT mice (1.7 ± 0.6) exhibited lower pathology scores than mock μmT mice (2.7 ± 0.5), although the differences did not reach statistical significance. Moreover, the mean loss of epithelial architecture score in vaccinated μmT mice (1.7 ± 0.6) was marginally greater than in vaccinated C57BL/6 animals (1.1 ± 0.5). Collectively, these results suggest that B cells and antibody may play a role in protection against the upper genital pathological sequelae that follow C. muridarum challenge. However, rCPAF+CpG vaccination appears to induce non B cell mediated adaptive immunity that is able to substantially overcome the deficit of B cells and antibody to induce protection against C. muridarum-induced upper genital pathology.
Fig. 6. Uterine horn dilatation following rCPAF+CpG vaccination/primary genital C. muridarum infection.
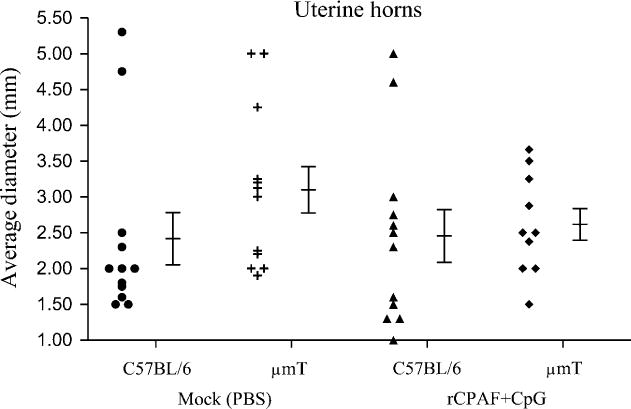
Groups (n= 5–6) of C57BL/6 or μmT mice were vaccinated intranasally with three doses of rCPAF+CpG (vaccinated) or treated with PBS (mock). One month following final vaccination, mice were challenged i.vag. with 5 × 104 IFU of C. muridarum. At day 80 after challenge, the mice were euthanized, genital tracts removed, photographed at a fixed distance, and the greatest uterine horn diameters for every 5 mm of longitudinal section was measured. The average diameter for individual uterine horns and the mean ± SE of uterine horn diameters in a group are shown. Results are representative of two independent experiments.
Fig. 7. Loss of uterine epithelial architecture following primary genital C. muridarum infection in rCPAF+CpG vaccinated mice.
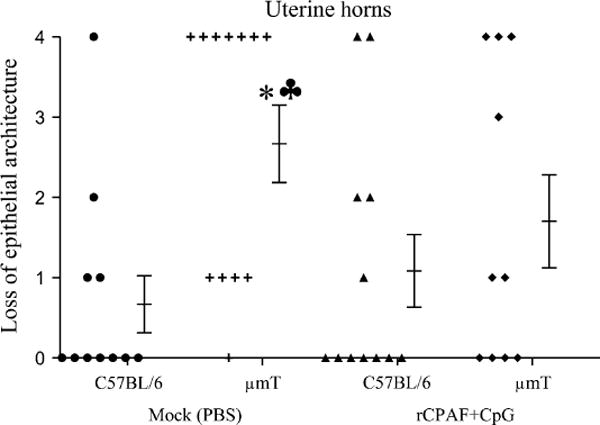
Groups (n= 5–6) of C57BL/6 or μmT mice were vaccinated intranasally with three doses of rCPAF+CpG (vaccinated) or treated with PBS (mock). One month following final vaccination, mice were challenged i.vag. with 5 × 104 IFU of C. muridarum. At day 80 after challenge, the mice were euthanized, genital tracts removed, embedded in paraffin, 5 μm sections made and stained with H&E. The H&E stained sections were observed under the microscope to score for the loss of uterine epithelial architecture. The individual scores for each uterine horn and the mean ± SE of all scores in a group are shown. *Significant differences between mock C57BL/6 and μmT mice (P = 0.005, Mann-Whitney Rank Sum test). ♣Significant differences between mock μmT mice and vaccinated C57BL/6 mice (P = 0.028, Mann-Whitney Rank Sum test). Results are representative of two independent experiments.
Discussion
There currently is no licensed vaccine against genital Chlamydia trachomatis infection, the leading cause of bacterial sexually transmitted disease worldwide. A comprehensive understanding of adaptive immune mechanisms against this pathogen will be important for design of an efficacious anti-Chlamydia trachomatis vaccine (Yang et al., 1999). To this end, Th1 CD4+ T cells (Igietseme et al., 1993; Perry et al., 1997), B cells and antibody (Morrison et al., 2000; Morrison & Morrison, 2001; Moore et al., 2002; Moore et al., 2003; Morrison & Morrison, 2005) have been shown to play a role in immunity against genital chlamydial infection. Previously, we have shown the efficacy of rCPAF vaccination in inducing enhanced resolution of genital chlamydial infection and reduced development of upper genital pathological sequelae (Murthy et al., 2006; Murthy et al., 2007; Cong et al., 2007), and that IFN-γ producing Ag-specific CD4+ T cells play an important role in inducing the protective immunity (Murphey et al., 2006; Li et al., 2008). Given that rCPAF-vaccination also induced robust systemic and mucosal anti-CPAF antibody responses, in this study, we evaluated directly the role of B cells and antibody in rCPAF-induced protective immunity.
B cells and antibody may contribute to protective immunity against bacterial infections in multiple ways: (A) Antibody can directly neutralize the antigen or fix complement and lead to bacterial killing (Lamm, 1997); (B) Antibody can enhance the priming of T cell responses via Fc receptor-mediated mechanisms (Moore et al., 2002; Moore et al., 2003) or B cells can act as sources of IFN-γ (Matsumoto et al, 2006); (C) Antibody can enhance opsonophagocytosis either via Fc receptor-mediated events or by activation of the classical complement activation pathway (Moore et al, 2002; Moore et al, 2003) and (D) Antibody can reduce antigenic load in tissues by immune exclusion, leading to regulated activation of cellular responses (Lamm, 1997). While all these general possibilities may play a role, the results of this study along with previous reports provide important insights into the contribution of antibody in the context of rCPAF+CpG vaccination-induced protective immunity against genital chlamydial infections.
First, lack of antibody did not alter the course of genital chlamdyial clearance in either mock or rCPAF+CpG vaccinated animals, suggesting that antibody was dispensable for neutralization of infectivity and chlamydial killing during the primary infection. This is supported by previous reports suggesting that primary chlamydial clearance may not be dependent upon antibody (Williams et al., 1987; Ramsey et al., 1988; Su et al., 1997), including the predominant mucosal antibody isotype IgA (Morrison & Morrison, 2005). However, this does not completely exclude a role for antibody in such events. A greater level of cellular IFN-γ response in μmT mice, compared to C57BL/6 mice, may have compensated for the lack of antibody. To this end, a role for anti-Chlamydia antibody in clearance of secondary genital infection has been revealed by the depletion of the CD4+ T cell compartment (Morrison et al., 2000; Morrison & Morrison, 2001; Morrison & Morrison, 2005). The efficacy of antibody in clearance of the primary infection is corroborated by the observation that administration of monoclonal anti-major outer membrane protein (MOMP) IgG or IgA via a backpack hybridoma tumor system induced enhanced clearance of the infection (Cotter et al., 1995). A deficiency of B cells also has been shown to result in greater chlamydial burden and mortality upon primary pulmonary chlamydial challenge (Yang & Brunham, 1998). Such a role in clearance is more likely for antibodies against surface proteins including MOMP, when compared to those against secreted proteins such as CPAF.
Second, there was greater induction of splenic CPAF-specific IFN-γ production in intranasally vaccinated μmT mice compared to C57BL/6 animals, suggesting that antibody was dispensable for priming effective T cell responses in this situation. To this end, Johansson et al. (Johansson & Lycke, 2001) have shown the induction of comparable T cell immunity and IFN-γ production in μmT and wild type mice after a primary genital C. trachomatis serovar D challenge. However, studies using C. muridarum infection of the lungs have demonstrated an involvement of antibody in the induction of robust Th1 type cellular cytokine response (Yang & Brunham, 1998). To explain this apparent discrepancy, it has been suggested previously that priming of T cell responses during pulmonary C. muridarum infection may involve B cells as antigen-presenting cells (Johansson & Lycke, 2001). A supporting role for Fc receptor mediated events in the priming of T cell responses against C. muridarum also has been reported (Moore et al., 2002; Moore et al., 2003). Given the host-specific immune evasion strategies employed by C. muridarum and C. trachomatis (Nelson et al., 2005), the enhancement of priming may be relevant to overcome such strategies in the respective hosts during an infection. However, such evasion strategies may not be particularly relevant in context of a subunit antigen administered with adjuvants (e.g., rCPAF+CpG).
Third, there was greater uterine horn pathology in mock-immunized μmT mice compared to similarly treated C57BL/6 animals, suggesting an involvement of antibody in protection against pathology. However, oviduct and uterine horn pathology was generally comparable, with marginally greater loss of uterine horn epithelial architecture in vaccinated μmT mice than vaccinated wild type animals, suggesting that antibody was largely dispensable in the protective immunity. Taken together, antibody was dispensable for chlamydial clearance but had an effect on pathology in mock-immunized animals, suggesting that the enhanced pathology in μmT mice may not be due to enhanced infection or presence of intact, replicating bacteria. It may be that antibody functions to reduce the antigenic load in tissues, thereby regulating the intensity of the cellular response. In this regard, immune exclusion is an important property of mucosal immunoglobulins, including IgA (Lamm, 1997; Murthy et al., 2006). We also have shown that mice deficient in IgA production display greater cellular infiltration and inflammatory cytokine production in the lungs after pulmonary C. muridarum challenge than corresponding wild type animals (Murthy et al., 2004). Additionally, antibody may influence the activation of CD4+CD25+ regulatory T cells (Yi et al., 2008), which have been proposed to play a protective role against Chlamydia-induced pathology (Yang et al., 1999; Johansson & Lycke, 2003; Brunham & Rey-Landino, 2005). μmT mice also have been shown to exhibit a greater frequency of splenic T cells and phagocytes compared to wild type animals (Su et al., 1997). Collectively, it appears that antibody may indirectly influence the development of chlamydial pathology by altering cellular composition or recruitment in tissues. We looked for differences in cellular infiltration in H&E stained sections of genital tract tissues, but the uterine horns of challenged mock-immunized μmT mice were dilated and thin walled and did not exhibit appreciable differences in the presence of inflammatory cellular infiltrates compared to mock C57BL/6 mice. A greater degree of cellular infiltration was apparent in rCPAF+CpG vaccinated μmT mice when compared to vaccinated wildtype animals (data not shown). A compensatory increase in cellular infiltration could be expected to induce deleterious effects in both mock and rCPAF+CpG vaccinated μmT mice after challenge. However, considerably greater pathology was seen only in the former situation, presumably due to predominant infiltration of non-immune cells that may not be as effective in chlamydial clearance as the immune cells in vaccinated animals, but may cause collateral damage. Nevertheless, the marginally greater loss of uterine horn epithelial architecture in vaccinated μmT versus C57BL/6 mice suggests that anti-CPAF antibody may have a limited protective, as opposed to any deleterious, role against pathology development in rCPAF+CpG vaccinated animals.
In summary, the results of this study demonstrate that antibody is largely dispensable for chlamydial clearance and the abrogation of UGT pathology in rCPAF+CpG vaccinated mice. However, it appears that anti-CPAF antibody may modulate the intensity of the rCPAF+CpG vaccination-induced cellular response and contribute to protective, rather than deleterious, immunity against Chlamydia-induced pathology. Taken together, our demonstration that rCPAF+CpG vaccination induces robust antigen-specific CD4+ T cells (Murphey et al., 2006), IFN-γ, and antibody production (Murthy et al., 2007), and that each component is likely to contribute to the protective immunity (Murthy et al., 2007; Li et al., 2008), suggests that the induction of both Th1 type CD4+ T cell and humoral responses remain the goal of efforts to develop an optimally effective Chlamydia trachomatis vaccine.
Acknowledgments
This work was supported by National Institutes of Health Grant SO6GM008194-24.
References
- Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5:149–161. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- Cong Y, Jupelli M, Guentzel MN, Zhong G, Murthy AK, Arulanandam BP. Intranasal immunization with chlamydial protease-like activity factor and CpG deoxynucleotides enhances protective immunity against genital Chlamydia muridarum infection. Vaccine. 2007;25:3773–3780. doi: 10.1016/j.vaccine.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter TW, Meng Q, Shen ZL, Zhang YX, Su H, Caldwell HD. Protective efficacy of major outer membrane protein-specific immunoglobulin A (IgA) and IgG monoclonal antibodies in a murine model of Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4704–4714. doi: 10.1128/iai.63.12.4704-4714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darville T, Andrews CW, Jr, Laffoon KK, Shymasani W, Kishen LR, Rank RG. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect Immun. 1997;65:3065–3073. doi: 10.1128/iai.65.8.3065-3073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debattista J, Timms P, Allan J, Allan J. Immunopathogenesis of Clamydia trachomatis infections in women. Fertil Steril. 2003;79:1273–1287. doi: 10.1016/s0015-0282(03)00396-0. [DOI] [PubMed] [Google Scholar]
- Igietseme JU, Ramsey KH, Magee DM, Williams DM, Kincy TJ, Rank RG. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific, Th1 lymphocyte clone. Reg Immunol. 1993;5:317–324. [PubMed] [Google Scholar]
- Ito JI, Lyons JM. Role of gamma interferon in controlling murine chlamydial genital tract infection. Infect Immun. 1999;67:5518–5521. doi: 10.1128/iai.67.10.5518-5521.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M, Lycke N. Immunological memory in B-cell-deficient mice conveys long-lasting protection against genital tract infection with Chlamydia trachomatis by rapid recruitment of T cells. Immunology. 2001;102:199–208. doi: 10.1046/j.1365-2567.2001.01167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M, Lycke N. A unique population of extrathymically derived alpha beta TCR+CD4−CD8− T cells with regulatory functions dominates the mouse female genital tract. J Immunol. 2003;170:1659–1666. doi: 10.4049/jimmunol.170.4.1659. [DOI] [PubMed] [Google Scholar]
- Johansson M, Schon K, Ward M, Lycke N. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect Immun. 1997;65:1032–1044. doi: 10.1128/iai.65.3.1032-1044.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupelli M, Guentzel MN, Meier PA, Zhong G, Murthy AK, Arulanandam BP. Endogenous IFN-gamma production is induced and required for protective immunity against pulmonary chlamydial infection in neonatal mice. J Immunol. 2008;180:4148–4155. doi: 10.4049/jimmunol.180.6.4148. [DOI] [PubMed] [Google Scholar]
- Lamm ME. Interaction of antigens and antibodies at mucosal surfaces. Annu Rev Microbiol. 1997;51:311–340. doi: 10.1146/annurev.micro.51.1.311. [DOI] [PubMed] [Google Scholar]
- Li W, Murthy AK, Guentzel MN, Seshu J, Forsthuber TG, Zhong G, Arulanandam BP. Antigen-specific CD4+ T cells produce sufficient IFN-gamma to mediate robust protective immunity against genital Chlamydia muridarum infection. J Immunol. 2008;180:3375–3382. doi: 10.4049/jimmunol.180.5.3375. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Kinoshita M, Ono S, Tsujimoto H, Majima T, Habu Y, Shinomiya N, Seki S. Cooperative IFN-gamma production of mouse liver B cells and natural killer cells stimulated with lipopolysaccharide. J Hepatol. 2006;45:290–298. doi: 10.1016/j.jhep.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Moore T, Ananaba GA, Bolier J, Bowers S, Belay T, Eko FO, Igietseme JU. Fc receptor regulation of protective immunity against Chlamydia trachomatis. Immunology. 2002;105:213–221. doi: 10.1046/j.0019-2805.2001.01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Ekworomadu CO, Eko FO, MacMillan L, Ramey K, Ananaba GA, Patrickson JW, Nagappan PR, Lyn D, Black CM, Igietseme JU. Fc receptor-mediated antibody regulation of T cell immunity against intracellular pathogens. J Infect Dis. 2003;188:617–624. doi: 10.1086/377134. [DOI] [PubMed] [Google Scholar]
- Morrison RP, Caldwell HD. Immunity to murine chlamydial genital infection. Infect Immun. 2002;70:2741–2751. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SG, Morrison RP. Resolution of secondary Chlamydia trachomatis genital tract infection in immune mice with depletion of both CD4+ and CD8+ T cells. Infect Immun. 2001;69:2643–2649. doi: 10.1128/IAI.69.4.2643-2649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SG, Morrison RP. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. J Immunol. 2005;175:7536–7542. doi: 10.4049/jimmunol.175.11.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SG, Morrison RP. The protective effect of antibody in immunity to murine chlamydial genital tract reinfection is independent of immunoglobulin A. Infect Immun. 2005;73:6183–6186. doi: 10.1128/IAI.73.9.6183-6186.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SG, Su H, Caldwell HD, Morrison RP. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4+ T cells but not CD8+ T cells. Infect Immun. 2000;68:6979–6987. doi: 10.1128/iai.68.12.6979-6987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey C, Murthy AK, Meier PA, Guentzel MN, Zhong G, Arulanandam BP. The protective efficacy of chlamydial protease-like activity factor vaccination is dependent upon CD4+ T cells. Cell Immunol. 2006;242:110–117. doi: 10.1016/j.cellimm.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy AK, Chambers JP, Meier PA, Zhong G, Arulanandam BP. Intranasal vaccination with a secreted chlamydial protein enhances resolution of genital Chlamydia muridarum infection, protects against oviduct pathology, and is highly dependent upon endogenous gamma interferon production. Infect Immun. 2007;75:666–676. doi: 10.1128/IAI.01280-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy AK, Cong Y, Murphey C, Guentzel MN, Forsthuber TG, Zhong G, Arulanandam BP. Chlamydial protease-like activity factor induces protective immunity against genital chlamydial infection in transgenic mice that express the human HLA-DR4 allele. Infect Immun. 2006;74:6722–6729. doi: 10.1128/IAI.01119-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy AK, Dubose CN, Banas JA, Coalson JJ, Arulanandam BP. Contribution of polymeric immunoglobulin receptor to regulation of intestinal inflammation in dextran sulfate sodium-induced colitis. J Gastroenterol Hepatol. 2006;21:1372–1380. doi: 10.1111/j.1440-1746.2006.04312.x. [DOI] [PubMed] [Google Scholar]
- Murthy AK, Sharma J, Coalson JJ, Zhong G, Arulanandam BP. Chlamydia trachomatis pulmonary infection induces greater inflammatory pathology in immunoglobulin A deficient mice. Cell Immunol. 2004;230:56–64. doi: 10.1016/j.cellimm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Nelson DE, Virok DP, Wood H, Roshick C, Johnson RM, Whitmire WM, Crane DD, Steele-Mortimer O, Kari L, McClarty G, Caldwell HD. Chlamydial IFN-gamma immune evasion is linked to host infection tropism. Proc Natl Acad Sci U S A. 2005;102:10658–10663. doi: 10.1073/pnas.0504198102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry LL, Feilzer K, Caldwell HD. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J Immunol. 1997;158:3344–3352. [PubMed] [Google Scholar]
- Perry LL, Su H, Feilzer K, Messer R, Hughes S, Whitmire W, Caldwell HD. Differential sensitivity of distinct Chlamydia trachomatis isolates to IFN-gamma-mediated inhibition. J Immunol. 1999;162:3541–3548. [PubMed] [Google Scholar]
- Ramsey KH, Soderberg LS, Rank RG. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infect Immun. 1988;56:1320–1325. doi: 10.1128/iai.56.5.1320-1325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank RG, Ramsey KH, Pack EA, Williams DM. Effect of gamma interferon on resolution of murine chlamydial genital infection. Infect Immun. 1992;60:4427–4429. doi: 10.1128/iai.60.10.4427-4429.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank RG, Soderberg LS, Barron AL. Chronic chlamydial genital infection in congenitally athymic nude mice. Infect Immun. 1985;48:847–849. doi: 10.1128/iai.48.3.847-849.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Caldwell HD. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect Immun. 1995;63:3302–3308. doi: 10.1128/iai.63.9.3302-3308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Feilzer K, Caldwell HD, Morrison RP. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infect Immun. 1997;65:1993–1999. doi: 10.1128/iai.65.6.1993-1999.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DM, Grubbs B, Schachter J. Primary murine Chlamydia trachomatis pneumonia in B-cell-deficient mice. Infect Immun. 1987;55:2387–2390. doi: 10.1128/iai.55.10.2387-2390.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Brunham RC. Gene knockout B cell-deficient mice demonstrate that B cells play an important role in the initiation of T cell responses to Chlamydia trachomatis (mouse pneumonitis) lung infection. J Immunol. 1998;161:1439–1446. [PubMed] [Google Scholar]
- Yang X, Brunham RC. In pursuit of a human chlamydial vaccine. Curr Opin Infect Dis. 1999;12:47–52. doi: 10.1097/00001432-199902000-00009. [DOI] [PubMed] [Google Scholar]
- Yang X, Gartner J, Zhu L, Wang S, Brunham RC. IL-10 gene knockout mice show enhanced Th1-like protective immunity and absent granuloma formation following Chlamydia trachomatis lung infection. J Immunol. 1999;162:1010–1017. [PubMed] [Google Scholar]
- Yi H, Zhang J, Zhao Y. The effects of antibody treatment on regulatory CD4+CD25+ T cells. Transpl Immunol. 2008;19:37–44. doi: 10.1016/j.trim.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Zhong G, Fan P, Ji H, Dong F, Huang Y. Identification of a chlamydial protease-like activity factor responsible for the degradation of host transcription factors. J Exp Med. 2001;193:935–942. doi: 10.1084/jem.193.8.935. [DOI] [PMC free article] [PubMed] [Google Scholar]


