Abstract
It has been postulated that gastroesophageal reflux plays a role in the etiology of head and neck squamous cell carcinomas (HNSCC) and contributes to complications after surgery or during radiotherapy. Antacid medications are commonly used in HNSCC patients for the management of acid reflux however their relationship with outcomes has not been well studied.
Associations between histamine receptor-2 antagonists (H2RAs) and proton pump inhibitors (PPIs) use and treatment outcomes were determined in 596 previously untreated HNSCC patients enrolled in our SPORE epidemiology program from 2003–2008 (median follow-up 55-month). Comprehensive clinical information was entered prospectively in our database. Risk strata were created based on possible confounding prognostic variables (age, demographics, socioeconomics, tumor stage, primary site, smoking status, HPV-16 status and treatment modality); correlations within risk strata were analyzed in a multivariable model.
Patients taking antacid medications had significantly better overall survival (PPI alone: p<0.001: H2RA alone, p=0.0479; both PPI+H2RA, p=0.0133). Using multivariable Cox models and adjusting for significant prognostic covariates, both PPIs and H2RAs use were significant prognostic factors for overall survival, but only H2RAs use for recurrence-free survival in HPV16-positive oropharyngeal patients. We found significant associations between use of H2RAs and PPIs, alone or in combination, and various clinical characteristics.
The findings in this large cohort study indicate that routine use of antacid medications may have significant therapeutic benefit in HNSCC patients. The reasons for this association remain an active area of investigation and could lead to identification of new treatment and prevention approaches with agents that have minimal toxicities.
Keywords: Head and neck squamous cell carcinoma (HNSCC), antacid medications, proton pump inhibitors (PPI), histamine receptor 2 antagonists (H2RA), clinical outcome, survival
Introduction
Pathological gastroesophageal reflux is a common condition in head and neck cancer patients [1–4]. There is evidence that acid reflux may play a role in the etiology of head and neck squamous cell cancer (HNSCC) and contribute to complications after surgery or during radiation and chemotherapy [2, 5–9]; acid reflux has been recently reported as an independent risk factor for squamous cancers of the pharynx and larynx [10]. Histamine receptor-2 antagonists (H2RA) and proton pump inhibitors (PPIs) are distinct groups of medications known for their similar ability to decrease and/or inhibit gastric acid production. At the University of Michigan (UM), these medications are commonly and regularly administered in HNSCC patients as part of their cancer treatment for the management of acid reflux and complications from conventional therapies. It is unknown if preventing acid reflux might prevent tumor recurrences and improve clinical outcome in HNSCC patients.
The objective of this study was to determine if clinical use of antacid drugs is associated with better clinical outcomes in a large retrospective cohort of 596 previously untreated patients enrolled in our Head and Neck Cancer Specialized Program of Research Excellence (SPORE) epidemiology program from 2003–2008. This is the first study to identify an association of the PPI and H2RA class of drugs with treatment outcomes and survival in patients with HNSCC. Elucidation of antacid drugs biologic effects on tumor progression could lead to new strategies for cancer prevention and treatment.
Materials and Methods
Patient population
Permission from the Institutional Review Board (IRB) for Human studies was granted to retrospectively analyze the patients that presented to the Department of Otolaryngology between 1/29/2003 and 11/7/2008 with head and neck squamous cell carcinoma (HNSCC) who were enrolled in our prospective Head and Neck SPORE epidemiology program. IRB approval was also granted for use of existing clinical health data regarding medication use from the patients’ medical records. All patients included provided informed and signed consent form.
The initial cohort of 884 unselected subjects prospectively completed longitudinal health surveys which collected health behaviors (tobacco and alcohol usage), quality of life measures, patient demographics (age, gender, race, marital status, US Armed Forces veteran status) and socioeconomic status (education level and median income from Census tract). The clinical and treatment outcome data were collected through SPORE data collection forms and health surveys. The investigators collected clinical and histopathological information (primary tumor site, TNM stage, HPV-16 status for oropharyngeal primaries), and follow-up information (type of treatment, duration of follow-up in months, incidence of recurrences, patterns of relapse, overall and cause-specific survival). Patient drug use was identified by retrospective chart review and data abstraction from patient electronic health records CareWeb using the University of Michigan’s EMERSE (Electronic Medical Record Search Engine) software. Using this custom designed software, we were able to create complex yet precise search queries to identify drugs taken and in which time periods (pre- or post- treatment), baseline demographics, clinical and histopathological data in this cohort. Data were independently collected by three investigators to minimize errors.
Computerized database (BioDBx)
The collected data was transferred to a clinical database (BioDBx) for analysis. Our Head and Neck SPORE has developed and instituted this powerful integrated database with an outstanding record of data collection, management, and analysis. BioBDx runs on a dedicated server, is firewall protected and supported by the UM Medical Center Information Technology department and Center of Advancement of Clinical Research. It is linked to the Health System clinical database (Careweb) for automatic download of clinical and demographic data and tracking of patient visits. Each patient entered in this database had identity protection through assignment of a unique identifying number. Categories of data entry included patient demographics, tumor site, tumor staging characteristics, health habits: tobacco use (cigarette smoking with average pack years: current, former [quit within 1 month v. > 1 month] and never; alcohol use (AUDIT score), and HPV-16 status for oropharyngeal primaries), treatment and detailed clinical follow-up. Our SPORE Program Tissue Core uses this same data management system for specimen tracking.
Data collection on various medications use
We searched for usage of all known members of each antacid class under their various generic and propriety names. Only usage documented after diagnosis date was counted. Within H2RA: Cimetidine (Tagamet), Ranitide (Zinetac, Zantac), Famotidine (Pepcidine, Pepcid); Nizatidine were included. Within PPIs: Omeprazole (Prilosec, Zegerid, Losec), Pantoprazole (Protonix, Somac, Zurcal), Esomeprazole (Nexium, Esotrex), Lansoprazole (Prevacid, Zoton, Levant); Rabeprazole (Zechin, Rabecid, AcipHex), Dexlansoprazole (Kapidex, Dexilant) were included.
Statistical analysis
We performed general survival analyses using Cox proportional hazards models to investigate which clinical factors and health behaviors measured by our SPORE Epidemiology project were associated with overall survival, disease-specific survival, time-to-recurrence, and patterns of relapse that included local recurrence, regional, or distant metastasis in these HNSCC patients. The development of second primary cancers was also assessed. These patients were censored at time of diagnosis of second primary in analyses of disease-specific survival, time-to-recurrence and patterns of relapse. We created multivariable models using available covariates such as age, clinical stage, primary disease site, treatment modality, smoking status, etc. We tested whether PPI and/or H2RA usage adds to the prognostic ability of our time-to-event models using a likelihood ratio test. Hazard ratios and their 95% confidence intervals were estimated to quantify the magnitude and direction of any associations.
Pairwise comparisons between PPI and H2RA use and other characteristics were explored. The following variables were analyzed for association with medication usage: gender, age, race, marital status, education, income, tumor site, stage, smoking and drinking history, primary treatment. Pearson Chi-square was used for categorical data and student’s t-test for continuous data. All p values reported correspond to two-sided comparisons.
Cox proportional hazard models were used for survival outcomes (including time to recurrences). Multivariable models using all covariates and also parsimonious analysis using only covariates which displayed significant relationships in bivariate analysis or were a priori determined to be scientifically important were performed. A subset analysis of PPI/H2RA use and outcomes according to HPV status was performed among patients with oropharyngeal cancers that had available tissues for HPV-16 testing. Survival time was defined as the time from diagnosis to death or last follow-up. Death from any cause was defined as an event for overall survival (OS), only death from cancer was defined as an event for disease specific survival (DSS). A recurrence event in the time to recurrence analysis was defined as any recurrence (local, regional, and/or distant). All statistical analyses were done in SAS version 9.2 (SAS Institute, Carey, NC). A two-tailed p value ≤0.05 was considered statistically significant.
Results
Cohort Characteristics
From an initial 884 cases enrolled in our Head and Neck SPORE epidemiology project, 706 were treated at UM hospital and eligible for this study of medication usage. After further review of the medical record, other reasons for exclusion included: withdrawn of consent (N=1), non-squamous cell cancer (N=2), unknown primary or nasal cavity primary (N=2), unresectable or palliation (N=25), incomplete clinical information (N=65), treatment for HNSCC prior to enrollment (N=5), cancer in situ (N=8), multiple primaries (N=2). Thus, our analyses for association between clinical data and use of various antacid medications was performed on a total of 596 previously untreated patients, diagnosed and treated at the University of Michigan for HNSCC between 1/29/2003 and 11/7/2008. The socio-demographics and clinic-pathological characteristics of this cohort are summarized in Table 1. The majority of cases were patients with advanced stage disease (Stage III or IV cases = 482, 81%); 244 cases (41%) were stage T0, T1, or T2; 305 cases (51.7%) T3 or T4; no T staging was possible in 44 cases (7.4%). The male/female ratio was 3:1 (448 males, 75% vs. 148 females, 25%), average age: 58 years (range 21–92); average age by gender: 59.4 (females) vs. 59.7 (males) years. By primary tumor sites: 150 cases (25%) of oral carcinomas, 251 cases (42%) of oropharyngeal carcinomas, 135 cases (23%) of hypopharynx and laryngeal carcinomas, and 58 cases (10%) in other head and neck sites (e.g. sinus, nasopharynx). The majority of patients had higher education (56%, with some college or more), 91% lived in counties with median income over 30K/year. There were 170 tumor recurrences and 222 deaths observed during follow-up; 28 patients presented with a second primary during follow-up (typically we consider a cancer a second primary if it is > 2cm from the original primary or it has been at least 3 years since the original primary was diagnosed). The Kaplan-Meier estimate for overall survival was 73% at 2 years and 59% at 5 years. Median follow-up time for overall survival was 55 months with a 95% CI of (50, 60) months. HNSCC conventional treatment was categorized according with standard treatment modalities: surgery only 68 cases (11%), radiation only 31 cases (5%), surgery +radiation 75 cases (13%), radiation+chemotherapy 246 cases (41 %), radiation+chemotherapy+surgery 176 cases (30%); there were no cases treated by chemotherapy alone, nor by a combination of surgery +chemotherapy.
Table 1.
Socio-demographic and clinico-pathologic characteristics of the HNSCC cohort.
The study included 596 previously untreated patients with head and neck squamous cell carcinomas (HNSCC) that were enrolled in the epidemiology program of the University of Michigan Head and Neck Cancer Specialized Program of Excellence in Research (SPORE) from 2003–2008.
| Numerical Measure | Mean (sd), Median | Range | |
|---|---|---|---|
| Age (years) | 57.9 years (11.2), 57 years | 21–92 | |
|
| |||
| Categorical Measures | N | % | |
|
| |||
| Gender | Male | 448 | 75% |
| Female | 148 | 25% | |
|
| |||
| Primary Tumor Subsite | OC | 150 | 25% |
| OP | 251 | 42% | |
| LA, HP | 135 | 23% | |
| Other | 58 | 10% | |
|
| |||
| Stage | Early (Cis, I, II) | 110 | 19% |
| Late (III, IV) | 482 | 81% | |
|
| |||
| T stage | 0,1,2 | 244 | 44% |
| 3,4 | 305 | 56% | |
| X,x | 44 | 7% | |
|
| |||
| Smoking | Never | 145 | 24% |
| Former (quit >1 month) | 226 | 38% | |
| Current (quit within 1 month) | 223 | 38% | |
|
| |||
| Race | European American/White | 560 | 94% |
| Non-white | 34 | 6% | |
|
| |||
| Married Yes/No | Married | 369 | 62% |
| Not Married | 223 | 38% | |
|
| |||
| Education | HS or less | 236 | 44% |
| Some college or more | 305 | 56% | |
|
| |||
| Treatment | Surgery only | 68 | 11% |
| Radiation only | 31 | 5% | |
| Surgery+Radiation | 75 | 13% | |
| Radiation+Chemotherapy | 246 | 41% | |
| Radiation, Chemotherapy and Surgery | 176 | 30% | |
Abbreviations: OC: oral cavity, OP: oropharynx, HP: hypopharynx, LA: larynx, NP: nasopharynx; the International Classification of Diseases for Oncology (ICD-9 codes) based on the Union for International Cancer Control (UICC) standard classification criteria for head and neck tumors were used; X: unknown; Cis: carcinoma in situ; HS: high school.
Pct may not add to 100% due to rounding.
Antacids usage and its impact on the clinical outcome of HNSCC patients
We defined users of antacid drugs in our association analyses as only those patients who had antacid usage documented after diagnosis date. Out of the 596 patients, 191 cases (32%) used only PPIs after diagnosis, 83 cases (14%) used only H2RAs; 136 cases (23%) used both (H2RA+ PPI) sometime after diagnosis (Table 2A). We also collected data on drug class use before diagnosis (recorded as “prior use”). Most patients with prior use continued to use PPIs after diagnosis but a small proportion of patients with prior use had no records of use after diagnosis date. Ten out of 16 patients with records of prior H2RA use did not have records of H2RA use within 2 years post-diagnosis and consequently were categorized as non-users for analysis. “Late-post use” was recorded when the first record of antacid use dated more than 2 years post-diagnosis and these patients were not included as PPI or H2RA users in our analysis. Frequencies of “prior” and “late post” users of antacid drug classes are summarized in Table 2B.
Table 2.
Antacid drug usage in 596 patients with head and neck squamous cell carcinomas (HNSCC).
The data collection on the administration of the drugs of interest was conducted independently by three investigators. Drug usage of all known members of each antacid class under their various generic and propriety names was identified using a custom designed software program EMERSE™ (Electronic Medical Record Search Engine) and users of antacid drugs in our association analyses were defined as only those patients who had antacid usage documented after diagnosis date.
| A: Drug usage documented after diagnosis date in this cohort of previously untreated HNSCC patients. | ||
|---|---|---|
| Family of Drugs | N | % (out of 596) |
| PPI alone | 191 | 32% |
| H2RA alone | 83 | 14% |
| PPI and H2RA | 136 | 23% |
| No record of usage | 186 | 31% |
| Total | 596 | 100% |
| B: Prior- and late-post drug usage in this cohort of previously untreated HNSCC patients. | |||
|---|---|---|---|
| Family of Drugs | Prior Use | Prior Use with no Post Use | Late-Post Use |
| PPI | 40 | 4 | 42 |
| H2RA | 16 | 10 | 26 |
| Combination of Both | 13 | 1 | 8 |
The analyses were done initially using any H2RA use and any PPI use separately as predictors. We then created a categorical variable combining the information from both drug classes into 4 categories: PPI use only, H2RA use only, PPI and H2RA use, and no antacid use. The bivariate demographic information of our cohort by these categories are summarized in Table 3.
Table 3.
Bivariate demographic information by patients’ intake of PPI and H2RA, alone and in combination.
The patients in PPI+ H2RA treatment group have been excluded from PPI alone and H2RA alone groups. All statistical analyses were done in SAS version 9.2 (SAS Institute, Carey, NC). A two-tailed p≤0.05 was considered statistically significant.
| Characteristic | Overall N=596 |
PPI alone N=191 |
H2RA alone N=83 |
PPI and H2RA N=136 |
None N=186 |
|
|---|---|---|---|---|---|---|
| Age (years) | Mean (sd), Median, Range among users | 58.3 (11.3), 57, 21–92 | 59.2 (10.4), 58,33–86 | 57.2 (12.2), 55,21–86 | 56.3 (11.2), 55, 22–85 | 58.2 (11.4), 57, 27–92 |
| p value | 0.06 | 0.56 | 0.05 | 0.72 | ||
|
| ||||||
| Overall |
PPI alone N (% users) |
H2RA alone N (% users) |
PPI+H2RA N (% users) |
None N (% users) |
||
|
| ||||||
| Gender | Male | 448 | 152 (34%) | 60(13%) | 98(22%) | 138(31%) |
| Female | 148 | 39(26%) | 23(16%) | 38(26%) | 48(32%) | |
| p value | 0.09 | 0.51 | 0.34 | 0.71 | ||
| Primary Tumor Site | OC | 150 | 32(21%) | 43(29%) | 43(29%) | 32(21%) |
| OP | 251 | 67(27%) | 29(12%) | 60(24%) | 95(38%) | |
| HP, LAR | 135 | 65(48%) | 8(6%) | 23(17%) | 39(29%) | |
| NP, Other, Unknown | 58 | 27(47%) | 2(3%) | 10(17%) | 19(33%) | |
| p value | <0.0001 | <0.0001 | 0.08 | 0.01 | ||
| Stage | Early | 110 | 42(38%) | 16(15%) | 19(17%) | 33(30%) |
| Late | 482 | 148(31%) | 66(14%) | 117(24%) | 151(31%) | |
| Missing | 4 | |||||
| p value | 0.13 | 0.82 | 0.12 | 0.79 | ||
| Tstage | 0,1,2 | 244 | 78(32%) | 31(13%) | 51(21%) | 84(34%) |
| 3,4 | 305 | 96(31%) | 48(16%) | 78(26%) | 83(27%) | |
| X,x | 44 | 17(39%) | 3(7%) | 7(16%) | 17(39%) | |
| Missing | 3 | |||||
| p value | 0.63 | 0.22 | 0.22 | 0.10 | ||
| Smoking | Never | 145 | 39(27%) | 17(12%) | 45(31%) | 44(30%) |
| Former | 226 | 77(34%) | 33(15%) | 45(20%) | 71(31%) | |
| Current-quit within 1 month | 223 | 75(34%) | 32(14%) | 46(21%) | 70(31%) | |
| Missing | 2 | |||||
| p value | 0.30 | 0.70 | 0.03 | 0.97 | ||
| Race | White | 560 | 178(32%) | 79(14%) | 131(23%) | 172(31%) |
| Non-White | 34 | 13(38%) | 3(9%) | 5(15%) | 13(38%) | |
| Missing | 2 | |||||
| p value | 0.43 | 0.39 | 0.24 | 0.36 | ||
| Married Yes/No | Married | 369 | 138(37%) | 49(13%) | 81(22%) | 101(27%) |
| Not Married | 223 | 53(24%) | 33(15%) | 54(24%) | 83(37%) | |
| Missing | 4 | |||||
| p value | 0.0006 | 0.60 | 0.52 | 0.01 | ||
| Education Some College | HS or less | 236 | 74(31%) | 42(18%) | 50(21%) | 70(30%) |
| Some college or more | 305 | 102(33%) | 34(11%) | 74(24%) | 95(31%) | |
| Missing | 55 | |||||
| p value | 0.61 | 0.03 | 0.40 | 0.71 | ||
| County Median Income from Census | 30K or Below | 55 | 16(29%) | 8(15%) | 7(13%) | 24(44%) |
| Above 30K | 541 | 175(32%) | 75(14%) | 129(24%) | 162(30%) | |
| p value | 0.62 | 0.89 | 0.06 | 0.04 | ||
| Treatment | Surgery only | 68 | 25(37%) | 18(26%) | 9(13%) | 16(24%) |
| Radiation only | 31 | 15(48%) | 1(3%) | 3(10%) | 12(39%) | |
| Surgery + Radiation | 75 | 24(32%) | 13(17%) | 16(21%) | 22(29%) | |
| Radiation + Chemotherapy | 246 | 79(32%) | 20(8%) | 50(20%) | 97(39%) | |
| Radiation + Chemotherapy + Surgery | 176 | 48(27%) | 31(18%) | 58(33%) | 39(22%) | |
| p value | 0.18 | <0.0003 | 0.001 | 0.002 | ||
1. Clinical significance of H2RA usage
Our analysis of H2RA usage and its potential therapeutic benefit identified 219 patients (37%) who received H2RAs within 2 years of diagnosis with HNSCC. These patients received Cimetidine (N=16), Ranitidine (N=215), Famotidine (N=37) (note: we did not find any Nizatidine usage).
1. A. Bivariate demographic
Our analysis indicated a statistically significant association (p<0.05) between H2RA usage and primary HNSCC tumor site, treatment modality, and patient education (Table 3).We observed higher H2RA use in patients with primary disease site in the oral cavity among all HNSCC sites, with higher education, and among those with trimodal (surgery, radiation and chemotherapy) treatment. H2RA usage was lowest among those treated with radiation only. We also observed more frequent H2RA usage in patients with higher T stage (48% in T3,4 vs. 31% in T0,1,2). Patients on H2RAs had a lower average age at diagnosis (57 vs. 59 years), but the distribution of ages across both groups was not notably different after closer look.
1. B. Patient survival and H2RA intake
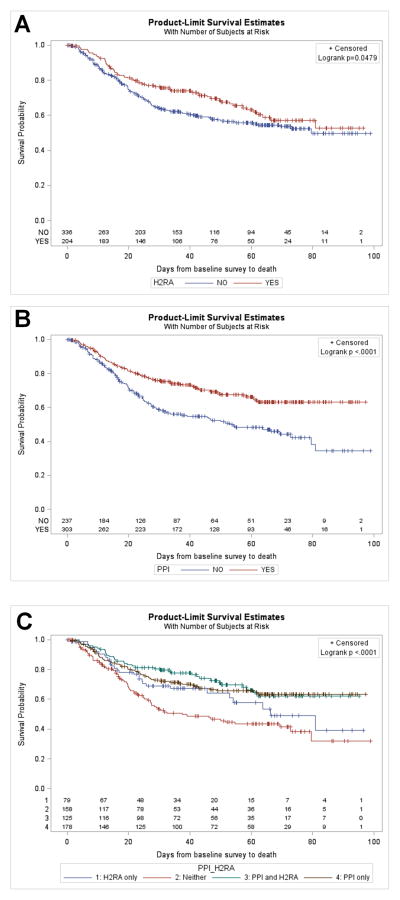
In univariate analysis, we observed that patients taking H2RA had significantly better overall survival OS (p=0.0479; Fig. 1A); when we considered drugs individually (Cimetidine, Ranitide, Famotidine) this association was not maintained for any one particular drug. The statistical significance of the association with overall survival proved stronger in multivariable analysis after controlling for potential confounding variables such as age, gender, tumor site, stage, smoking, socioeconomic status and treatment (p=0.02; HR(95%, CI): 0.67 (0.47, 0.95); Table 4). In addition, when a backward selection algorithm was used to choose a best multivariable prediction model, H2RA usage was consistently chosen as a significant predictor of survival along with age, primary tumor site and smoking status. In the whole cohort of patients, we did not find evidence of a benefit of H2RA use for recurrence-free survival.
Fig. 1.
Survival benefits according with antacids’ intake in patients with head and neck squamous cell carcinomas. Unadjusted overall survival in relation with usage of: Histamin 2 receptors antagonists class (A); Proton pump inhibitors class (B); Each antacid class alone and in combination vs. non-users (C). Median follow-up=55 months; 95% CI of (50, 60) months.
Table 4.
PPI and H2RA usage and patient overall survival and time to recurrence.
Overall survival and time to recurrence were analyzed using log-rank for univariate analysis and Cox proportional hazards models for multivariate analysis. All statistical analyses were done in SAS version 9.2 (SAS Institute, Carey, NC). A two-tailed p≤0.05 or less was considered statistically significant.
| Characteristic | |||||
|---|---|---|---|---|---|
|
| |||||
| OS univariate | OS Multivariable | Recur univariate | Recur multivariable | ||
| PPI usage | Yes | 0.55 (0.42, 0.73) | 0.55 (0.40, 0.74) | 0.83 (0.60, 1.14) | 0.71 (0.50, 1.01) |
| No | Ref. | Ref. | Ref. | Ref. | |
| p value | <0.0001 | <0.0001 | 0.39 | 0.06 | |
| H2RA usage | Yes | 0.74 (0.55, 1.00) | 0.67 (0.47, 0.95) | 1.02 (0.73, 1.42) | 0.90 (0.61, 1.34) |
| No | Ref. | Ref. | Ref. | Ref. | |
| p value | 0.0479 | 0.02 | 0.92 | 0.61 | |
| Gender | Male | 1.14 (0.82, 1.59) | 1.30 (0.90, 1.88) | 1.09 (0.74, 1.61) | 0.94 (0.62, 1.42) |
| Female | Ref. | Ref. | Ref. | Ref. | |
| p value | 0.42 | 0.16 | 0.68 | 0.75 | |
| Primary Tumor Site | OC | 1.43 (1.02, 2.00) | 2.44 (1.50, 3.96) | 1.26 (0.84, 1.87) | 1.90 (1.12, 3.23) |
| HP, LAR | 1.43 (0.99, 2.06) | 1.43 (0.94, 2.18) | 1.35 (0.90, 2.02) | 1.45 (0.92, 2.29) | |
| NP, Other, Unknown | 0.86 (0.50, 1.47) | 1.11 (0.62, 2.00) | 0.73 (0.38, 1.43) | 1.03 (0.50, 2.13) | |
| OP | Ref. | Ref. | Ref. | Ref. | |
| p value | 0.05 | 0.004 | 0.22 | 0.08 | |
| Stage | Late | 1.65 (1.09, 2.49) | 1.59 (0.97, 2.60) | 1.79 (1.10, 2.94) | 1.14 (0.65, 1.98) |
| Early | Ref. | Ref. | Ref. | Ref. | |
| p value | 0.02 | 0.07 | 0.02 | 0.65 | |
| Smoking Status | Never | 0.44 (0.30, 0.66) | 0.51 (0.33, 0.79) | 0.42 (0.26, 0.67) | 0.48 (0.29, 0.79) |
| Former | 0.72 (0.53, 0.98) | 0.62 (0.44, 0.88) | 0.80 (0.57, 1.14) | 0.73 (0.49, 1.07) | |
| Current-quit within 1 month | Ref. | Ref. | Ref. | Ref. | |
| p value | 0.0002 | 0.003 | 0.002 | 0.01 | |
| Education Some College | HS or less | Ref. | Ref. | Ref. | Ref. |
| Some college or more | 0.72 (0.54, 0.96) | 0.83 (0.61, 1.13) | 0.78 (0.56, 1.09) | 0.92 (0.64, 1.31) | |
| p value | 0.02 | 0.24 | 0.14 | 0.64 | |
| County Median Income from Census | 30K or Below | 1.61 (1.05, 2.47) | 1.16 (0.71, 1.90) | 1.70 (1.05, 2.75) | 1.36 (0.79, 2.33) |
| Above 30K | Ref. | Ref. | Ref. | Ref. | |
| p value | 0.03 | 0.55 | 0.03 | 0.27 | |
| Treatment | Surgery only | 0. 56 (0.33, 0.96) | 0.39 (0.20, 0.78) | 0.16 (0.06, 0.44) | 0.12 (0.04, 0.35) |
| Radiation only | 0.26 (0.08, 0.83) | 0.22 (0.06, 0.74) | 0.08 (0.01, 0.55) | 0.07 (0.01, 0.51) | |
| Surgery + Radiation | 1.13 (0.74, 1.73) | 0.56 (0.34, 0.93) | 0.67 (0.40, 1.25) | 0.44 (0.24, 0.80) | |
| Radiation + Chemotherapy | 1.05 (0.76, 1.45) | 0.96 (0.66, 1.39) | 0.71 (0.50, 1.01) | 0.75 (0.50, 1.12) | |
| Radiation + Chemotherapy+ Surgery | Ref. | Ref. | Ref. | Ref. | |
| p value | 0.03 | 0.009 | 0.0004 | <0.0001 | |
| Age (years) | 10 Year increase | 1.43 (1.26, 1.62) | 1.60 (1.38, 1.86) | 1.02 (1.01, 1.04) | 1.03 (1.01, 1.05) |
| p value | <0.0001 | <0.0001 | 0.006 | 0.0004 | |
Abbreviations: OS=Overall Survival. Recur=Time to Recurrence; OC: oral cavity, OP: oropharynx, HP: hypopharynx, LA: larynx, NP: nasopharynx; HS: high school. Provided are the hazard ratio followed by the 95% confidence interval values between parentheses.
Interestingly, subset analysis of the patients with oropharyngeal carcinomas and available HPV-16 status indicated H2RA usage as prognostic for better recurrence-free survival in multivariate analysis after controlling for HPV-16 (p = 0.03; HR (95%, CI) = 0.34 (0.12, 0.92)).
2. Clinical significance of PPI usage
Our analysis of PPI usage identified 327 patients who received PPI within 2 years of diagnosis of HNSCC (55% of the total 596 patients). These patients received Omeprazole (N=179, 30%), Lansoprazole (N=115, 19.3%), Esoprazole (N=104, 17.45%), Pantoprazole (N=127, 21.3%) and Rabeprazole (N=10, 1.7%). (Note: we did not find any Dexlansoprazole usage).
2. A. Bivariate demographic
Our analysis indicated statistically significant associations between PPI usage and primary HNSCC tumor site and marital status (Table 3). We observed higher PPI usage in patients with primary disease site in the oropharynx and in those who were married.
2. B. Patient survival and PPI intake
We observed in univariate analysis that patients taking PPI had significantly better overall survival OS (p<0.0001; Fig. 1B); this also was observed in multivariate analysis (p<0.0001; HR (95%, CI) = 0.55 (0.40, 0.74); Table 4). The statistical significance of the association proved stronger after controlling for potential confounding variables. Interestingly, when we considered drugs individually, this association with overall survival was maintained for omeprazole (p=0.0008) and esomeprazole (p=0.001); only a trend was noted for lansoprazole (p=0.06) while pantoprazole did not demonstrate a significant association (p=0.67). Univariate analysis failed to demonstrate an association or a trend between PPI use and unadjusted recurrence-free survival (p=0.39; HR (95%, CI) = 0.83 (0.60, 1.14); Table 4). However, there was a trend for better recurrence-free survival in PPI users in multivariate analysis after controlling for potential confounding variables such as age, gender, tumor site, stage, smoking, socioeconomic status and treatment (p=0.06; HR (95%, CI) = 0.71 (0.50, 1.01); Table 4). In addition, when a backward selection algorithm (with stay criteria alpha = 0.10) was used to choose a best multivariable prediction model, PPI usage was consistently chosen as a significant predictor of recurrence-free survival, along with age, smoking status and treatment.
3. Clinical significance of H2RA+PPI usage
Our analysis identified 136 patients who received both PPI and H2RA within 2 years of diagnosis of HNSCC (23% of the total 596 patients).
3. A. Bivariate demographic
Our analysis indicated a statistically significant association between H2RA+PPI usage and age, smoking and treatment modality. Higher incidence of combined H2RA+PPI was observed in those that quit within one month and those who received trimodal therapy. Only a trend was noted in relation with primary HNSCC tumor site (p=0.08) and median income level (p=0.06).
3. B. Patient survival and H2RA+PPI intake
We observed that patients taking H2RA+PPI had significantly better overall survival than patients taking no antacid at all (p<0.0001; Fig. 1C), and than those taking H2RA alone (p=0.05); we failed to find evidence that the combination was better than PPI alone (p=0.88) in univariate analysis. We did not find evidence of better recurrence-free survival in patients taking H2RA+PPI.
Discussion
To our knowledge, this is the first epidemiological study that indicates therapeutic benefit of common antacid medication intake in head and neck cancer patients. Our findings in this large epidemiologic cohort study indicate that clinical usage of the two classes of antacids (proton pump inhibitors and histamine 2 receptor antagonists) after diagnosis with head and neck squamous cell carcinomas (HNSCC) may have significant benefit by enhancing patient survival. It is known that antacid medications have the ability to decrease and/or inhibit the production of gastric acid and are commonly and chronically used in HNSCC patients for the management of their gastroesophageal reflux disease. However, the potential effects of antacid medications and any potential mechanisms for altering HNSCC progression and outcome are unknown. Identifying molecular mechanisms associated with HNSCC progression and metastasis is key to improving clinical outcomes.
HNSCC are marked by their aggressiveness and invasiveness [5]. HNSCC are known for poor clinical outcomes with mortality among the highest of all carcinomas mainly due to the development of metastatic disease [12]. The ability for cancer to metastasize seems to associate with the expression of endothelial adhesion molecules ligands by circulating tumor cells that allow them to bind to the endothelium lining the vasculature initiating extravasation [13, 14]. Sialyl Lewis X (sLeX) is an endothelial adhesion molecule known to play the key role in the initiation of the metastatic spread in gastro-intestinal cancers by initiating dissemination through direct interaction with E-selectin expressing endothelium [15]. In agreement with findings from other types of human cancer (e.g. gastric, breast, colon) [15, 17–19], our previous studies have shown that cimetidine, the prototypical drug of the histamine 2 receptor antagonists, may have an effect on E-selectin, a molecule with critical roles in cancer dissemination [20]. In addition, cimetidine seems to affect other players with important roles in tumor growth and progression (e.g. epithelial growth factor signaling pathway), and to prevent metastasis [21, 22, 23]. Our in vitro analysis of a well-characterized set of human cell lines derived from the most common locations of the HNSCC indicates that oral squamous cell carcinomas expressed higher sLeX, which it increases with advanced stage [16]. Our present study has identified the highest H2RA usage in patients with oral carcinomas. It is interesting to note, that in contrast to cimetidine, the most frequently prescribed H2RA drug in our cohort ranitidine, has not proven to have similar effects as cimetidine [23]; it is also known that the two also differ in molecular structure. In our patient cohort, cimetidine alone was used by only a few patients (16 out of 596) compared to ranitidine (215 out of 596). When analyzed per individual drug, despite the significant number of ranitidine users, our analysis failed to demonstrate the same benefit on patient survival as the entire H2RA class. Therefore, we postulate that H2RA drugs may differ in their mechanisms of action and may alter expression of other factors besides key endothelial adhesion molecules that could explain their clinical benefits in HNSCC patients.
Remarkably, our analysis identified H2RA class usage as significant prognostic factor for recurrence-free survival only in patients with oropharyngeal tumors positive for Human Papillomavirus (HPV-16). HPV has recently emerged as the primary etiologic factor for patients with tumors in the oropharynx that are also associated with younger age at diagnosis; 65–85% of the oropharyngeal cancers diagnosed this year in the US are HPV-related with 3-year failure rates of 30–36% [24–31]. Consequently, unique pathologic profiles have emerged that are consistent with the changing incidence of HNSCC [32–34]. Patients with HPV(+) head and neck cancer have a distinct risk profile, associated with a less remarkable history of tobacco and alcohol use [35, 36], a more beneficial micronutrient profile [10], improved cellular immunity [37, 38] and improved survival compared to those with HPV(−) tumors [39–42]. Notably, a significant subset (20–30%) of HPV(+) tumors fail to respond to therapy and recur principally as distant metastases. Studies conducted at the University of Michigan have made significant contributions to the understanding of the impact of HPV infection on the patho-biology of HNSCC and response to therapy [40–42]. Our present clinical findings have prompted laboratory studies to explore potential mechanisms of the correlations observed clinically using the HPV+ vs. HPV− carcinomas–derived cell lines from our large SPORE collection.
The major challenge in the management of HNSCC patients today is the development of evasive resistance to conventional therapies. Our recent evidence demonstrates that cancer stem cells (CSCs) play a critical role in the development of metastases in HNSCC and that sLex can help identify the metastatic CSC subset [16]. Malignant progression in cancer requires populations of CSCs endowed with unlimited self-renewal, survival under stress and low pH, and establishment of distant metastases. It is also known that increasing tumor mass leads to an acidic tumor micro-environment, while acidity contributes to both tumor progression and resistance to chemotherapy [43, 44]. Tumor cells are capable of maintaining a fine state of homeostasis with normal intracellular pH despite the acidic extracellular milieu because of proton pumps expressed in their plasma membranes. A key mechanism to counteract the cytosolic acidification is active proton extrusion by proton pumps. This causes intracellular alkalinization and extracellular acidification, which creates a pH gradient. Low pH of the extracellular microenvironment promotes the secretion and activation of proteolytic enzymes, and release of pro-angiogenic factors contributing to neo-vessels formation, cancer invasion and metastasis [45, 46]. This pH gradient also has been associated with multidrug resistance, likely from drug sequestration and neutralization in the acidic organelles or in the acidic extracellular environment [47, 48]. Although several pH regulatory mechanisms are operating in tumor cells (Na+/H+ exchangers, carbonic anhydrases, bicarbonate transporters, H+-linked mono-carboxylate transporters), the major mechanism is represented by the proton pumps such the vacuolar ATPase (V-ATPase) that are ubiquitously expressed on the plasma membrane of the tumor cells. Highly metastatic cells preferentially use V-ATPases, suggesting that the proton pumps are critical for acquisition of a more metastatic and invasive phenotype [48, 49]. Therefore, disruption of this pH gradient with proton pump inhibitors (PPIs) may be an important anti-metastatic mechanism.
Although the specific targets of PPIs are H+-ATPases contained within the lumen of gastric parietal cells, PPIs also inhibit the activity of V-ATPases, thus broadly blocking proton transport across membranes through the entire body. Our study identified that patients with HNSCC take PPIs, more often alone rather than in combination with H2RA, to treat symptoms that accompany conventional therapeutic regimens, and that their usage may lead to a better patient overall and free-recurrence survival with a higher ratio than with the H2RA use alone or of the combination of both. Interestingly, among the various class members, individual drug usage of only omeprazole and esomeprazole maintained the same survival benefit. At this time we do not fully understand the complex biological mechanisms by which antacid medications may influence patient outcome. Death from other causes and comorbidities is a major contributor to poor overall survival rates in patients with head and neck cancer, thus it is possible that PPIs and H2RAs influence deaths from other causes. Studies are currently underway in our laboratory to seek biological evidences (e.g. potential effects on tumor cells and stroma, modulation of microenvironment, effects on immunity etc) in support of the significant association with improved patient outcome observed in the clinical settings.
Elucidation of the novel link between the pathobiology of HNSCC and antacid medication use could lead to important new chemopreventive strategies for HNSCC patients, for whom the current preventive armamentarium is still limited. HNSCC are an ideal model for the study of chemoprevention because they follow a histopathological progression from normal tissue to hyperplasia to severe dysplasia to carcinoma in situ to invasive and metastatic carcinomas. Moreover, the phenomenon of field cancerization is well understood in HNSCC, having been characterized first in oral cancer [50]. Because of this retained risk for cancer development in the epithelium adjacent to primary disease, second primary tumors act as a possible target for secondary chemoprevention in patients previously diagnosed and treated for HNSCC; furthermore, oral premalignant lesions could also serve as prime targets for chemopreventive agents.
This is the first study to report an association of the PPI and H2RA class of drugs with treatment outcomes and survival in patients with HNSCC. Despite the current study’s limitations (absence of randomization), the intriguing associations observed in our cohort will deserve further validation in randomized prospective trials to provide comprehensive support for a novel therapeutic approach that could be readily translated into clinical benefit. Further elucidation of the mechanisms of action is necessary to determine if the beneficial effects might be extrapolated to other types of cancer. A series of focused clinical trials will be necessary to further evaluate the antacids anticancer potential in clinical settings, with the ultimate goal of improving the outcome of patients afflicted with HNSCC. If confirmed in prospective studies, new chemopreventive approaches may be possible with drugs that have a favorable therapeutic ratio and are readily available in the clinical settings.
Acknowledgments
This study was supported by the NCI/NIDCR P50 CA097248 (U-M Head and Neck Cancer Specialized Program of Research Excellence (SPORE, PI: Gregory Wolf, MD), the Research Scholar Grant RSG-13-103-01 – CCE from the American Cancer Society (PI: Silvana Papagerakis, MD/PhD) and Undergraduate Research Opportunity Program (UROP) at the University of Michigan Ann Arbor.
Footnotes
Conflict of interest: None.
References
- 1.Copper MP, Smit CF, Stanojcic LD, Devriese PP, Schouwenburg PF, Mathus-Vliegen LM. High incidence of laryngopharyngeal reflux in patients with head and neck cancer. Laryngoscope. 2000;110:1007–11. doi: 10.1097/00005537-200006000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Sato K, Umeno H, Chitose S, Nakashima T. Patterns of laryngopharyngeal and gastroesophageal reflux. J Laryngol Otol. 2009;(Supplement):42–7. doi: 10.1017/S0022215109005076. [DOI] [PubMed] [Google Scholar]
- 3.Toohill RJ, Kuhn JC. Role of refluxed acid in pathogenesis of laryngeal disorders. Amer J Med. 1997;103:100S–6S. doi: 10.1016/s0002-9343(97)00333-1. [DOI] [PubMed] [Google Scholar]
- 4.Ulualp SO, Roland PS, Toohill RJ, Shaker R. Prevalence of gastroesophagopharyngeal acid reflux events: an evidence-based systematic review. Amer J Otolaryngol. 2005;26:239–44. doi: 10.1016/j.amjoto.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Fennerty MB. The continuum of GERD complications. Review Cleve Clin J Med. 2003;70 (Suppl 5):S33–50. doi: 10.3949/ccjm.70.suppl_5.s33. [DOI] [PubMed] [Google Scholar]
- 6.Turcotte S, Duranceau A. Gastroesophageal reflux and cancer. Review Thorac Surg Clin. 2005;15(3):341–52. doi: 10.1016/j.thorsurg.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Pondugula K, Wani S, Sharma P. Barrett’s esophagus and esophageal adenocarcinoma in adults: long-term GERD or something else? Review Curr Gastroenterol Rep. 2007;9(6):468–74. doi: 10.1007/s11894-007-0061-9. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert EW, Luna RA, Harrison VL, Hunter JG. Barrett’s esophagus: a review of the literature. J Gastrointest Surg. 2011;15(5):708–18. doi: 10.1007/s11605-011-1485-y. [DOI] [PubMed] [Google Scholar]
- 9.Tae K, Jin BJ, Ji YB, Jeong JH, Cho SH, Lee SH. The role of laryngopharyngeal reflux as a risk factor in laryngeal cancer: a preliminary report. Clin Exp Otorhinolaryngol. 2011;4(2):101–4. doi: 10.3342/ceo.2011.4.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langevin SM, Michaud DS, Marsit CJ, Nelson HH, Birnbaum AE, Eliot M, et al. Gastric reflux is an independent risk factor for laryngopharyngeal carcinoma. Cancer Epidemiol, Biomarkers Prevention. 2013;22:1061–8. doi: 10.1158/1055-9965.EPI-13-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Cancer Society. Global Cancer Facts & Figures. 2011. [Google Scholar]
- 12.Shah J, Johnson NW, Batsakis JK. Oral Cancer. London: Martin Dunitz; 2003. SRC - GoogleScholar. [Google Scholar]
- 13.Haier J, Nicolson GL. The role of tumor cell adhesion as an important factor in formation of distant colorectal metastasis. Dis Colon Rectum. 2001;44:876–84. doi: 10.1007/BF02234713. [DOI] [PubMed] [Google Scholar]
- 14.Orr FW, Wang HH, Lafrenie RM, Scherbarth S, Nance DM. Interactions between cancer cells and the endothelium in metastasis. J Pathol. 2000;190:310–29. doi: 10.1002/(SICI)1096-9896(200002)190:3<310::AID-PATH525>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto S, Imaeda Y, Umemoto S, Kobayashi K, Suzuki H, Okamoto T. Cimetidine increases survival of colorectal cancer patients with high levels of sialyl Lewis-X and sialyl Lewis-A epitope expression on tumour cells. British J Cancer. 2002;86:161–7. doi: 10.1038/sj.bjc.6600048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Czerwinski MJ, Desiderio V, Shkeir O, Papagerakis P, Lapadatescu MC, Owen JF, et al. In vitro evaluation of sialyl Lewis X relationship with head and neck cancer stem cells. Otolaryngol - Head Neck Surg. 2013;149:97–104. doi: 10.1177/0194599813482879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bahra PS, Rainger GE, Wautier JL, Nash CB. Effects of pentoxifylline on the different steps during adhesion and transendothelial migration of flowing neutrophils. Cell Biochem Function. 2001;19:249–57. doi: 10.1002/cbf.922. [DOI] [PubMed] [Google Scholar]
- 18.Liu F-R, Jiang C-G, Li Y-S, Li J-B, Li F. Cimetidine inhibits the adhesion of gastric cancer cells expressing high levels of sialyl Lewis x in human vascular endothelial cells by blocking E-selectin expression. Int J Mol Med. 2011;27:537–44. doi: 10.3892/ijmm.2011.618. [DOI] [PubMed] [Google Scholar]
- 19.Tang N-H, Chen Y-L, Wang X-Q, Li X-J, Yin F-Z, Wang X-Z. Cooperative inhibitory effects of antisense oligonucleotide of cell adhesion molecules and cimetidine on cancer cell adhesion. World J Gastroenterol. 2004;10:62–6. doi: 10.3748/wjg.v10.i1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papagerakis S, Thornhill M. Therapeutic targets in oral cancer. Toxicol Pathol. 2006;34:1009–1009. [Google Scholar]
- 21.Fujikawa T, Shiraha H, Nakanishi Y, Takaoka N, Ueda N, Suzuki M, et al. Cimetidine inhibits epidermal growth factor-induced cell signaling. J Gastroenterol Hepatol. 2007;22:436–43. doi: 10.1111/j.1440-1746.2006.04541.x. [DOI] [PubMed] [Google Scholar]
- 22.Kubecova M, Kolostova K, Pinterova D, Kacprzak G, Bobek V. Cimetidine: an anticancer drug? Eur J Pharm Sci. 2011;42:439–44. doi: 10.1016/j.ejps.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi K, Matsumoto S, Morishima T, Kawabe T, Okamoto T. Cimetidine inhibits cancer cell adhesion to endothelial cells and prevents metastasis by blocking E-selectin expression. Cancer Res. 2000;60:3978–84. [PubMed] [Google Scholar]
- 24.Agrawal Y, Koch WM, Xiao W, Westra WH, Trivett AL, Symer DE, et al. Oral human papillomavirus infection before and after treatment for human papillomavirus 16-positive and human papillomavirus 16-negative head and neck squamous cell carcinoma. Clin Cancer Res. 2008;14:7143–50. doi: 10.1158/1078-0432.CCR-08-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. New England J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–9. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 27.D’Souza G, Agrawal Y, Halpern J, Bodison S, Gillison ML. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infectious Dis. 2009;199:1263–9. doi: 10.1086/597755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J National Cancer Inst. 2000;92:709–20. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 29.Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–9. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pannone G, Santoro A, Papagerakis S, Lo Muzio L, De Rosa G, Bufo P. The role of human papillomavirus in the pathogenesis of head and neck squamous cell carcinoma: an overview. Infect Agents Cancer. 2011;6:4. doi: 10.1186/1750-9378-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hausen H. Infections causing human cancer. Wiley-VCH Verlag; Weinheim, Germany: 2006. [Google Scholar]
- 32.Chenevert J, Seethala RR, Barnes EL, Chiosea SI. Squamous cell carcinoma metastatic to neck from an unknown primary: the potential impact of modern pathologic evaluation on perceived incidence of human papillomavirus-positive oropharyngeal carcinoma prior to 1970. Laryngoscope. 2012;122:793–6. doi: 10.1002/lary.21899. [DOI] [PubMed] [Google Scholar]
- 33.Vidal L, Gillison ML. Human papillomavirus in HNSCC: recognition of a distinct disease type. Hematol Oncol Clin North Am. 2008;22:1125–42. doi: 10.1016/j.hoc.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Tang A, Owen JH, Hauff S, Park J, Papagerakis S, Bradford C, et al. Head and neck cancer stem cells: the effect of HPV, an in vitro and mouse study. Otolaryngol Head Neck Surg. 2013 doi: 10.1177/0194599813486599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Applebaum KM, Furniss CS, Zeka A, Posner MR, Smith JF, Bryan J, et al. Lack of association of alcohol and tobacco with HPV16-associated head and neck cancer. J Nat Cancer Inst. 2007;99:1801–10. doi: 10.1093/jnci/djm233. [DOI] [PubMed] [Google Scholar]
- 36.Gillison ML, D’Souza G, Westra W, Sugar E, Xiao W, Begum S, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Nat Cancer Inst. 2008;100:407–20. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 37.Arthur AE, Duffy SA, Sanchez GI, Gruber SB, Terrell JE, Hebert JR, et al. Higher micronutrient intake is associated with human papillomavirus-positive head and neck cancer: a case-only analysis. Nutrition Cancer. 2011;63:734–42. doi: 10.1080/01635581.2011.570894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wansom D, Light E, Thomas D, Worden F, Prince M, Urba S, et al. Infiltrating lymphocytes and human papillomavirus-16--associated oropharyngeal cancer. Laryngoscope. 2012;122:121–7. doi: 10.1002/lary.22133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Nat Cancer Inst. 2008;100:261–9. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 40.Maxwell JH, Kumar B, Feng FY, Worden FP, Lee JS, Eisbruch A, et al. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010;16:1226–35. doi: 10.1158/1078-0432.CCR-09-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Worden FP, Kumar B, Lee JS, Wolf GT, Cordell KG, Taylor JM, et al. Chemoselection as a strategy for organ preservation in advanced oropharynx cancer: response and survival positively associated with HPV16 copy number. J Clin Oncol. 2008;26:3138–146. doi: 10.1200/JCO.2007.12.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cardonne RA, Casavola V, Reshkin SJ. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nature Rev Cancer. 2005;5:786–95. doi: 10.1038/nrc1713. [DOI] [PubMed] [Google Scholar]
- 43.De Milito A, Marino ML, Fais S. Rationale for the use of proton pump inhibitors as antineoplastic agents. Curr Pharm Des. 2012;18:1395–406. doi: 10.2174/138161212799504911. [DOI] [PubMed] [Google Scholar]
- 44.Martinez-Zaguilan R, Seftor EA, Seftor RE, Chu YW, Gillies RJ, Hendrix MJ. Acidic pH enhances the invasive behavior of human melanoma cells. Clin Exp Metastasis. 1996;14:176–86. doi: 10.1007/BF00121214. [DOI] [PubMed] [Google Scholar]
- 45.Rofstad EK, Mathiesen B, Kindem K, Galappathi K. Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res. 2006;66:6699–707. doi: 10.1158/0008-5472.CAN-06-0983. [DOI] [PubMed] [Google Scholar]
- 46.Raghunand N, Martínez-Zaguilán R, Wright SH, Gillies RJ. pH and drug resistance. Turnover of acidic vesicles and resistance to weakly basic chemotherapeutic drugs. Biochem Pharmacol. 1999;57:1047–58. doi: 10.1016/s0006-2952(99)00021-0. [DOI] [PubMed] [Google Scholar]
- 47.Tannock IF, Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989;49:4373–84. [PubMed] [Google Scholar]
- 48.Martinez-Zaguilan R, Raghunand N, Lynch RM. pH and drug resistance. Functional expression of plasmalemmal Vtype HATPase in drugresistant human breast carcinoma cell lines. Biochem Pharmacol. 1999;57:1037–46. doi: 10.1016/s0006-2952(99)00022-2. [DOI] [PubMed] [Google Scholar]
- 49.Sennoune SR, Bakunts K, Martínez GM, Chua-Tuan JL, Kebir Y, Attaya MN, et al. Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential: distribution and functional activity. Amer J Physiol Cell Physiol. 2004;286:1443–52. doi: 10.1152/ajpcell.00407.2003. [DOI] [PubMed] [Google Scholar]
- 50.Braakhuis BJ, Tabor MP, Leemans Cr, van der Waal I, Snow GB, Brakenhoff RH. Second primary tumors and field cancerization in oral and oropharyngeal cancer: molecular techniques provide new insights and definitions. Head Neck. 2002;24:198–206. doi: 10.1002/hed.10042. [DOI] [PubMed] [Google Scholar]