Abstract
Background
Recent data suggest a role for the intestinal microbiota in the pathogenesis of functional bowel disorders (FBD). Probiotic studies in FBD generated inconsistent results suggesting a strain and product specific effect.
Aim
To investigate the clinical efficacy of Lactobacillus acidophilus NCFM (L-NCFM) and Bifidobacterium lactis Bi-07 (B-LBi07) in non-constipation FBD.
Methods
A double-blind, placebo-control clinical trial of the probiotic bacteria L-NCFM and B-LBi07 bid (2×1011cfu/day) vs. placebo over 8-weeks. Primary endpoints: global relief of GI symptoms and satisfaction with treatment. Secondary endpoints: Change in symptoms severity, well being, and quality of life. Microbiology effect was assessed by qPCR on fecal samples.
Results
Sixty subjects (probiotic n=31; placebo n=29), 72% females, 84% whites, mean age 37 years. Abdominal bloating improved in the probiotics compared to the placebo group at 4 weeks (4.10 vs. 6.17, p=0.009; change in bloating severity p=0.02) and 8 weeks (4.26 vs. 5.84, p=0.06; change in bloating severity p<0.01). Analyses on the IBS subgroup (n=33) showed similar results.
Conclusions
L-NCFM and B-LBi07 BID improve symptoms of bloating in patients with FBD. This data supports the role of intestinal bacteria in the pathophysiology of FBD and the role for probiotic bacteria in the management of these disorders.
Keywords: Functional bowel disorders, Irritable Bowel Syndrome, Probiotics, Intestinal microbiota
Introduction
Functional bowel disorders (FBD) are the most common gastrointestinal disorders seen in primary care and gastroenterology clinics in the western world (1, 2). These disorders result in major patient disability, impaired quality of life (3, 4, 5), and a significant economical burden for society (6). FBD have been traditionally viewed as disorders of altered intestinal motility and visceral hypersensitivity, in which the clinical presentation and the severity of the symptoms can be influenced by various psychosocial factors (7). Despite intensive research over the past 15 years the etiology of these disorders is still poorly understood. Additionally, effective treatments are limited, provide only partial or transient symptomatic relief, and are often associated with considerable side effects and costs (8).
The emerging epidemiologic, physiologic, and clinical data over the past few years have provided evidence that the intestinal microbiota play an important role in maintaining normal GI function and that alteration in the composition of the intestinal microbiota can contribute to the development of functional GI symptoms (9, 10). Furthermore, recent clinical data indicate that manipulation of the intestinal microbiota by probiotics and/or antibiotics may be effective in alleviating these symptoms (11, 12, 13, 14, 15). Most of the early studies examining the effect(s) of probiotics in functional GI disorders focused on irritable bowel syndromes (IBS) and have shown mixed results. These mixed results may relate to considerable methodological limitations, the use of different probiotic bacteria, and the use of heterogeneous and not clearly defined study population (11, 16, 17, 18). However, more recent data coming from studies using more sound methodologies demonstrated some positive clinical and physiological effects (9). Taken together, the data coming from probiotic studies with IBS subjects has demonstrated that not all probiotics are the same, and that the effect of a specific probiotic cannot be extrapolated to another probiotic strain. These studies also emphasize the need to test the beneficial effect(s) of each probiotic product separately for specific clinical conditions. The aim of our investigator-initiated study was to investigate the clinical effect, safety and tolerability of a blend of two probiotic bacteria, Lactobacillus acidophilus NCFM (L-NCFM) and Bifidobacterium animalis subsp. lactis Bi-07 (B-LBi07), in a well-defined group of patients with non-constipation FBD.
Methods
Study population
Study population included male and female subjects aged 18 – 65 years who met the Rome III criteria (2) for non-constipation-IBS, functional diarrhea, or functional bloating. In addition, subjects were included if they had active symptoms for at least two weeks prior to participation in the study despite current therapy. Subjects were not included if they had any of the following: history of chronic inflammation or structural abnormality of the digestive tract (e.g. inflammatory bowel disease, duodenal or gastric ulcer, intestinal obstruction, or symptomatic cholelithiasis); serious, unstable medical condition; insulin-dependent diabetes mellitus; major psychiatric diagnosis; abnormal laboratory results at baseline; a diagnosis of lactase deficiency or if symptoms resolved with lactose-free diet or a diagnosis of celiac disease (in patients with chronic diarrhea). In addition, subjects who received antibiotic treatment or intentionally consumed probiotic products on a daily basis during the last 8 weeks prior to participating in the study were also excluded (a washout period of 8 weeks was required if these subjects consumed antibiotics or probiotics); pregnant or lactating subjects and subject with predisposition to infection (i.e. compromised immune system, rheumatic heart disease, artificial heart valve, history of bacterial endocarditis, or active infectious diseases).
All subjects were recruited from the general population by advertising. Study period was January 2006 through January 2007. The study was approved by the University of North Carolina at Chapel Hill (UNC-CH) institutional review board and all subjects signed a consent form prior to participation in the study. The study is registered at ClinicalTrials.gov number NCT00618904.
Study design
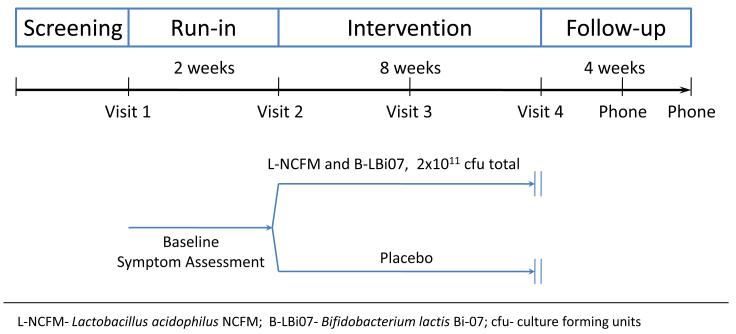
Eligible subjects were enrolled into a prospective, double-blind, placebo-controlled clinical trial. Randomization was carried out by the UNC-CH Investigational Drug Service. The investigators, study coordinators and statisticians were blinded with regard to the type of intervention throughout the duration of the study and the data analysis. Subjects were randomized into two parallel intervention arms, an active probiotic arm and a placebo arm. The study included a two-week run-in period to evaluate for baseline symptoms and their severity, and an eight-week intervention period during which participants received either the study probiotic product or a placebo. The study product was provided twice daily for 8 weeks in the form of a pill containing either a mixture of equivalent amounts of two probiotic bacteria (L-NCFM and B-LBi07- 1×1011 CFU in each pill), or placebo material composed of microcrystalline cellulose. A four-week follow-up period after discontinuation of intervention was used to evaluate post- intervention effect(s) (Figure 1).
Figure 1.
Study design
Concomitant stable (>1 month) medications were allowed during the study. Subjects were specifically asked to avoid any change in their stable medications and to avoid taking new medications during their participation in the study. Changes in medications during the study period were review and recorded in each study visit. Compliance with the probiotic intervention was assessed by counting unused pills at each study visit.
Assessment for clinical effect
Patients were evaluated in clinic 4 times during the course of the study: at baseline, end of run-in period (randomization visit), and at 4 and 8 weeks of the intervention period. Phone interviews were carried out at 2 and 4 weeks of the follow-up period to assess post-intervention effects (Figure 1). The study primary endpoints were two subjective global end points; global relief of GI symptoms assessed by a single question comparing the change in subjects’ bowel symptoms scored on a seven-point scale from ‘substantially worse’ to ‘substantially improved’ (19), and satisfaction with treatment as assessed by a Satisfaction Survey (20, 21, 22). Secondary endpoints included change in specific functional GI symptoms including abdominal pain, abdominal bloating and post-prandial symptoms (measured on a 10 points Likert scale), stool frequency (by number of bowel movements per day), stool consistency (using the stool Bristol Score) (23), symptoms severity (irritable bowel severity scoring system) (24), global well being (assessed by a single question rating the subject’s general well-being and scored on a five-point Likert scale from 1 ‘poor’ to 5 ‘excellent’) (25), and Health Related Quality of Life (IBS-QOL) (26).
Tolerability and safety
Tolerability and safety were assessed by recording all reported adverse events and laboratory blood tests at baseline, 4 (mid intervention period) and 8 weeks (end of intervention period). Blood tests included erythrocyte sedimentation rate (ESR), complete blood counts with platelets and differential, electrolytes (sodium, chloride, potassium, calcium, phosphorus), kidney and liver function tests [alanine aminotransferase (ALT), aspartate aminotransferase (AST), Blood urea nitrogen (BUN) and serum creatinine], total protein and albumin, and thyroid-stimulating hormone (TSH). In addition, fecal calprotectin and plasma mononuclear elastase (PMN elastase), early markers for inflammatory response and neutrophil degranulation (27, 28) were assessed as markers for inflammation in all subjects. Subjects with abnormal laboratory blood tests or positive pregnancy tests at baseline were excluded from the study.
Fecal microbiology analysis
Fecal samples were collected from subjects at four time-points during the study: at enrollment, following a two week run-in period (prior to probiotic intervention), at four weeks of probiotic consumption, and at the end of eight weeks of probiotic consumption. Fresh fecal samples were homogenized, separated into three-gram aliquots and stored in −80°C until analyzed using DNA-based methods.
DNA from fecal samples was extracted using a phenol/chloroform extraction method combined with physical disruption of bacterial cells and a DNA clean-up kit (Qiagen DNeasy® Blood and Tissue extraction kit). Briefly, approximately 100-200 mg of frozen feces was suspended in 750 μl of sterile bacterial lysis buffer (200 mM NaCl, 100 mM Tris [pH 8.0], 20 mM EDTA, 20 mg/ml lysozyme) and incubated at 37°C for 30 min. Next, 40 μl of proteinase K (20 mg/ml) and 85 μl of 10% SDS was added to the mixture and incubated at 65°C for 30 min. 300 mg of 0.1 mm zirconium beads (BioSpec Products) were added and the mixture was then homogenized in a bead beater (BioSpec Products) for 2 min. The homogenized mixture was cooled on ice and then centrifuged at 14,000 rpm for 5 min. The supernatant was transferred to a new 1.5 ml microfuge tube and fecal DNA was further extracted by phenol/chloroform/iso-amyl alcohol (25:24:1) and then chloroform/iso-amyl alcohol (24:1). DNA in the supernatant was precipitated by absolute ethanol at −20°C for 1 hour. The precipitated DNA was suspended in DNase free H2O and then cleaned using the DNeasy® Blood and Tissue extraction kit (Qiagen) from step 3 as per the manufacturer’s instructions. Purified fecal DNA was eluted in 200 μl volumes and diluted 1:10 for qPCR analysis.
Quantitative real time PCR (qPCR) was performed with QuantiTect SYBR® Green (Qiagen) using fecal DNA as template and specific primers that recognize the CRISPR region of L-NCFM (forward, 5′-TCGGTACCCAGGATCACC-3′; reverse, 5′-ATTGTCCGCTTTAACGTATTTTC-3′) (29) and the 16S-23S region of B-LBi07 (30) (forward, 5′-CACACCACACAATCCAATAC-3′; reverse, 5′-GCATGTTGCCAGCGGGT-3′) (31). Each assay was conducted in 96-well plates on an Eppendorf Realplex2 mastercycler thermocycler (Eppendorf). Each PCR was carried out in a final volume of 12 μl and contained the following: 1 × QuantiTect SYBR® Green, 0.5 μM of each primer and approximately 50 ng of purified fecal DNA. PCR conditions were as follows: 10 min at 95°C, followed by 40 cycles of 95°C for 15 s, 20 s at 50°C, and 72°C for 60 s. Each plate included duplicate reactions per DNA sample and the appropriate set of standards. Serially diluted genomic DNA from L. acidophilus NCFM and Bifidobacterium animalis ssp. lactis Bi-07 were used as standards for the L-NCFM and B-LBi07 assays, respectively. Thus, the concentration of each probiotic was expressed as the genome copy number per μg of fecal DNA. Melting curve analysis of the PCR products was conducted following each assay to confirm that the fluorescent signal originated from specific PCR products and not from primer-dimers or other artifacts. All qPCR assays included a ‘no template’ negative control for each primer set to ensure the specificity of each primer set. All microbiology analyses of fecal samples were performed blindly, without knowledge of the subjects’ clinical data.
Data analysis
Analyses of differences between the two study groups in mean values for continuous and ordinal were conducted by using two-group t-tests. Group differences in categorical variables were assessed by Fisher’s exact test. An alpha level of p=0.05 was used in all analyses as the threshold of statistical significance. Primary analyses were conducted without knowledge of the nature of the intervention for each group.
Results
I. Study Population
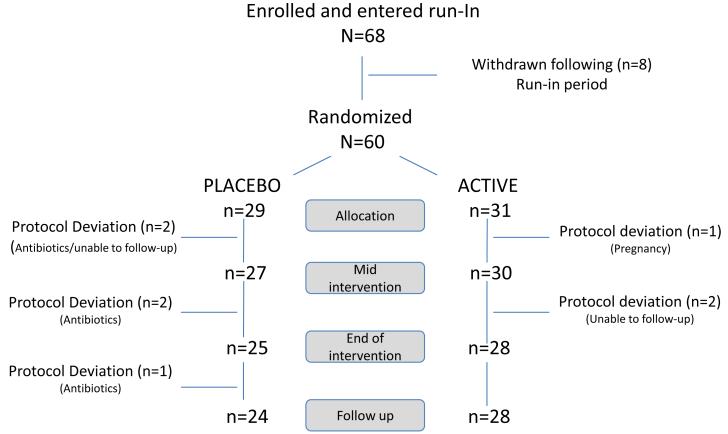
A total of 68 patients were enrolled. Eight patients did not meet the inclusion criteria at the end of the run-in period and were withdrawn prior to randomization. Out of the 60 patients who were randomized 53 completed the intervention and had complete data for analysis. Dropouts during the intervention phase were due to use of antibiotics (n=3, from the placebo arm), inability to follow-up (n=2, one from each of the study arms) and pregnancy (n=1, from the active arm). Additional two subjects were excluded during the follow-up period due to use of antibiotics (n=2, both from the placebo arm). Thirty one patients received the probiotic blend of L-NCFM and B-LBi07 and 29 received placebo. The study population consisted of 72% females, 84% whites with a mean age of 37 years. Baseline demographics and disease characteristics were similar in the two study groups (Table 1).
Table 1.
Patient Baseline Demographics and Disease Characteristics
| Characteristic | Probiotic Blend | Placebo |
|---|---|---|
| Mean age (SD) | 36.0 (10.9) | 37.0 (14.7) |
| Gender (% female) | 72.4 | 70.4 |
| Race -- n (%) | ||
| White | 25 (83.3) | 23 (85.2) |
| Black | 5 (16.7) | 1 (3.7) |
| Asian | 0 (0) | 2 (7.4) |
| Other | 0 (0) | 1 (3.7) |
| Education level (% with college degree) |
66.6 | 51.8 |
| Marital status (% married or co-habiting) |
46.7 | 39.7 |
| Diagnosis (n of patients) | ||
| IBS | 17 | 16 |
| FBD | 12 | 9 |
| FD | 1 | 2 |
| Baseline mean overall bowel symptom severity (SD) |
261.7 (68.5) | 261.9 (100.3) |
| Baseline mean abdominal pain and discomfort score (SD) |
5.8 (2.1) | 5.9 (2.3) |
| Baseline mean bloating score (SD) |
5.7 (1.9) | 5.4 (3.1) |
| Bloating at baseline -- n (%) | 23 (79) | 17 (70) |
| Loose/watery stools ≥ 25% of bowel movements -- % (SD) |
93.3 (25.4) | 76.9 (43.0) |
| Hard/lumpy stools > 25% of bowel movements -- % (SD) |
43.3 (50.4) | 50.0 (51.0) |
| Health-related QOL (IBS-QOL) – score (SD) |
76.5 (16.4) | 75.8 (21.8) |
| Depression scores -- mean (SD) | 7.2 (8.5) | 8.7 (9.4) |
II. Clinical Effects
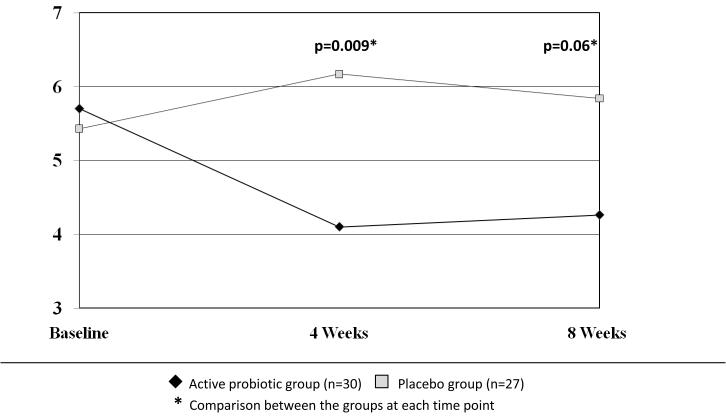
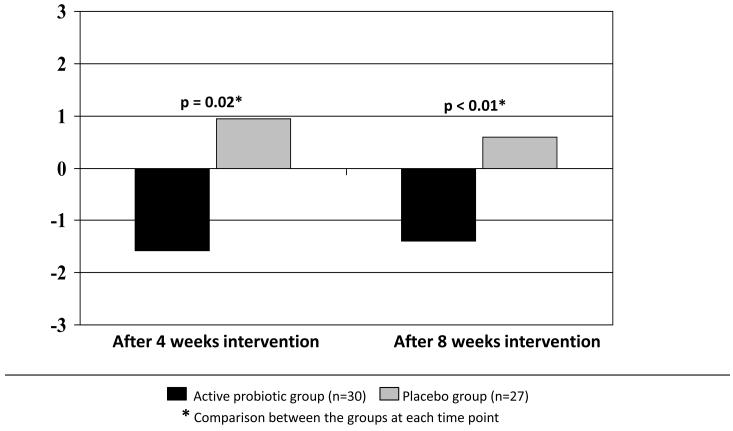
Global relief of GI symptoms, satisfaction with treatment, and HR-QOL improved in all subjects, however the level of improvement did not differ between the active and the placebo groups. Nevertheless, between-group analysis of the effect of the probiotic mix on individual functional bowel symptoms showed a significant beneficial effect on bloating and distention scores. Bloating symptoms were significantly less severe in the probiotic group compared to the placebo group at 4 weeks (4.10±3 vs. 6.17±3, p=0.009 respectively), and showed a strong trend in a similar direction at 8 weeks (4.26 ±3 vs. 5.84±3, p=0.06 respectively) (Figure 3). Analysis of the pre- to post- intervention change (calculated by subtracting the pre-intervention from the post-intervention scores) in bloating severity scores confirmed a significant improvement (i.e., a decrease in bloating symptoms) in the probiotic group compared to the placebo group at 4 weeks (−1.58 vs. 0.95, p=0.02 respectively) and at 8 weeks (−1.4 vs. 0.6, p<0.01 respectively) (Figure 4). This mean pre- to post- intervention change represent a 15% reduction in clinical bloating symptoms in the intervention group.
Figure 3.
Bloating severity at baseline, 4 weeks and 8 weeks of intervention
Figure 4.
Change in bloating severity from baseline to 4 weeks and from baseline to 8 weeks of intervention
Secondary analyses on the subgroup of subjects with IBS (n=33) showed a similar effect with significantly lower bloating severity scores in the probiotic group (n=17) compared to the placebo (n=16) group (4.24 ±3 vs. 6.73±3, p=0.03 respectively) at 4 weeks and a trend in a similar direction at 8 weeks (1.58 vs. −0.69, p=0.19). The pre- to post- intervention change in bloating severity scores indicate a significant improvement in bloating symptoms in the probiotic group compared to the placebo group at 4 weeks (−0.79 vs. 2.8 respectively, p=0.03) and an effect in a similar direction at 8 weeks, although the latter did not reach a statistical significance (−0.7 vs. 1.6 respectively, p=NS).
Both groups showed a significant improvement in pain scores between baseline to 8 weeks (3.33 vs. 2.46, respectively; p=0.03 for the probiotic group and 3.7 vs. 2.48; p=0.01 for the placebo group). However, these effects were not significantly different in between-group analysis.
There were no differences between the groups in any of the study endpoints during the follow-up period.
III. Safety and Tolerability
The most common adverse events were cold symptoms (n=8) (the study was done during winter time), fatigue (n=4), abdominal pain (n=2), sinus infection (n=2). There were no differences in these reported adverse events between the intervention and the placebo groups. No significant adverse events or changes in blood tests between baseline to mid- and post-intervention periods were recorded. In addition, analysis of fecal samples for markers of inflammation (fecal calprotectin and PMN elastase) did not reveal differences between the groups both at baseline and at the end of intervention (Table 2).
Table 2.
Mean levels of fecal markers for intestinal inflammation in the placebo and probiotic groups.
| Calprotectin mg/g feces |
PMN elastase mg/g feces |
|
|---|---|---|
| Placebo | ||
| Baseline | 0.7 | 5.5 |
| Post Intervention | 0.8 | 5.7 |
|
| ||
| Probiotics | ||
| Baseline | 0.8 | 6.4 |
| Post Intervention | 0.8 | 5.8 |
IV. Fecal microbiology analysis
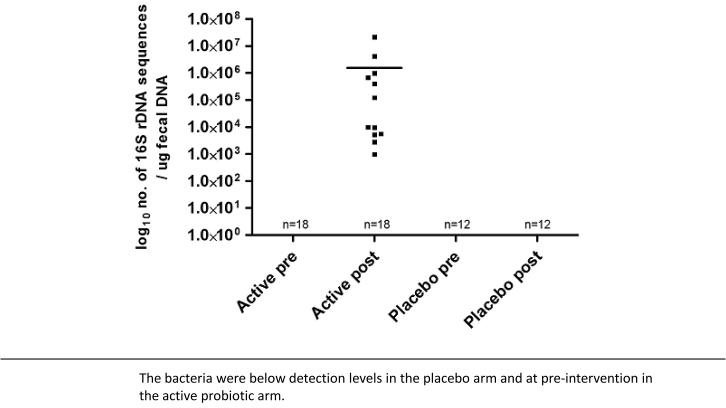
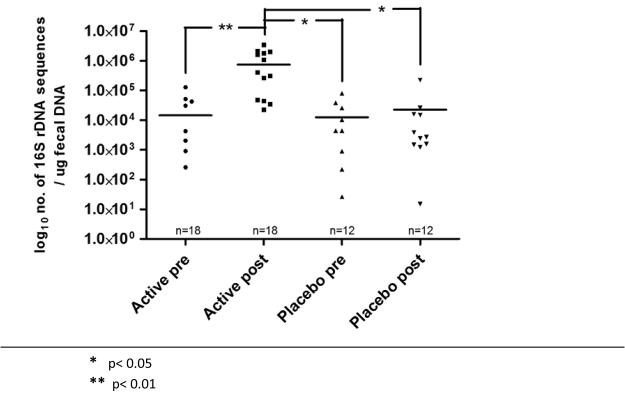
DNA was extracted from fecal sample of 30 subjects enrolled in this study (active arm, n=18; placebo arm, n=12). The qPCR assay for L-NCFM demonstrated that genetic sequences associated with this probiotic was detected only in fecal DNA from ‘post-probiotic intervention’ samples obtained from subjects on the active arm of the study (Figure 5). The qPCR assay for B-LBi07 demonstrated that genetic sequences associated with these probiotic bacteria was detected in the majority of fecal DNA samples tested in both the active and placebo arms. However, the concentration of these B-LBi07 associated sequences were significantly higher in ‘post-probiotic intervention’ samples obtained from subjects on the active arm compared to their corresponding ‘pre-probiotic intervention’ samples (P<0.01), and ‘pre-’ and ‘post-probiotic intervention’ samples obtained from subjects on the placebo arm of the study (P<0.05) (Figure 6).
Figure 5.
Concentration of Lactobacillus acidophilus NCFM in fecal samples from subjects pre- and post- intervention
Figure 6.
Concentration of Bifidobacterium lactis Bi07 in fecal samples from subjects pre- and post- intervention
Discussion
Our study intervention of daily consumption of L-NCFM and B-LBi07 (2×1011 CFU per day) did not show a significant improvement over placebo on the study’s two primary endpoints, global relief of GI symptoms and satisfaction with treatment. However, the study findings indicate a significant beneficial effect of this probiotic blend on bloating symptoms in patients with functional bowel disorders. The significant pre- to post-intervention decrease in the severity of bloating symptoms in the probiotic group compared to the placebo group (between group analysis) both at 4 weeks and 8 weeks of the intervention and the fact that the differences between the groups continued to hold statistical significance in the secondary analysis on a smaller subgroup of patients with IBS (n=33) suggest a considerable effect of this intervention on bloating symptoms.
Being the first prospective study using these probiotic strains in patients with FBDs the present study was designed as a pilot study to look for signals of effect(s), evaluate the tolerability and safety, and help directing future studies in regard to targeted populations and power calculations. As such, the study included a relatively broad population of patients with bowel symptoms including pain, diarrhea, and bloating. However, based on a hypothesis that this probiotic blend may be more effective in patients with diarrhea symptoms we a priori decided to exclude patients with constipation. Our study findings do not support this hypothesis as we did not see significant changes in bowel habits or a decrease in diarrhea symptoms with the consumption of these probiotic strains. Furthermore, based on recent epidemiological data indicating that bloating symptoms are significantly more prevalent in patients with constipation (32) it is possible that our study results would display a more significant effect if we included (or focused on) FBD patients with constipation.
Bloating-related symptoms (including bloating, distention, and gas) are commonly seen in clinical practice and their presence is often associated with a significant impact and reduced quality of life. A recent population-based study demonstrated a prevalence of bloating in approximately 20% of the white population in the US (33). A recent study of 337 IBS patients in the US has shown that bloating symptoms are reported in the majority (82.5%) of the patients. Additionally, this study found that bloating is the second most bothersome symptom, after abdominal pain, and the third (of 14) most important reason to seek medical care (32). This study has also shown that bloating symptoms are associated with increased healthcare utilization and, the use of medications, and reduced quality of life. Despite the high prevalence and impact of these symptoms in patients with FBD the underlying pathophysiological mechanisms of these symptoms are not completely understood leading to a lack of available effective treatments (34). Several studies targeting the intestinal microbiota for treatment of functional GI symptoms reported a prominent beneficial effect of these interventions on bloating symptoms.
Two small randomized controlled trials used a probiotic blend of eight different probiotic bacteria (VSL#3) for 8 weeks in patients with D-IBS (35) and IBS associated with bloating (36). Similar to our findings, the results of the first study found no difference between the probiotic intervention group and the placebo group in regard to global relief of symptoms or change in bowel functions. However, the probiotic group had a significant post-intervention reduction in abdominal bloating (p= 0.046); whereas, the placebo group did not (p = 0.54). However, no between-group comparisons were reported in this study (35). The second study reported a significant reduction in flatulence with probiotic intervention compared to placebo; although the effect on bloating was not statistically significant in this study (36). Other probiotic studies have also shown some effect on bloating although the magnitude of these effects was relatively modest (37, 38, 39, 40, 41).
Two studies using the intestinal non-absorbable antibiotic rifaximin have shown similar beneficial effects in patients with IBS and bloating (12, 13). A larger and more recent study using rifaximin in patients with D-IBS demonstrated a significant and sustained improvement in global IBS symptoms and IBS-associated bloating (14). This study also identified daily bloating at baseline as a predictor of clinical response (15). Together with these studies, our study results further support the hypothesis that the main beneficial effect of manipulation of the intestinal microbiota in patients with FBD may be the effects on bloating symptoms. However, the role of probiotics and/or antibiotics in the management of patients with IBS and the mechanism(s) by which they induce their beneficial effect/s need further investigation.
Although, the probiotics used in this study showed a statistically significant reduction in bloating symptoms in our study population, the clinical benefit of this intervention needs further investigation. This is emphasized by the fact that we were not able to show beneficial effects on other study outcomes including the two primary outcomes (global relief of GI symptoms and satisfaction with treatment), and overall well being and IBS-QOL. However, the lack of an overall clinical benefit in this study may relate to its inherent limitations as a pilot study including the small number of subjects tested which may lead to lack of statistical power (type 2 error) and the inclusion of a relatively heterogeneous patient population; and the exclusion of a subgroup of patients (FBD with constipation) which, post factum, were likely to benefit most from the study intervention. Additional studies focusing on bloating symptoms and targeting the appropriate population are needed.
The study product was well tolerated. There were no reports of severe adverse events and no differences in reported adverse events between the groups. Moreover, the lack of increase in fecal markers of intestinal inflammation suggests no inflammatory response to the product.
With regard to the effect of the study intervention on the intestinal microbiota, we demonstrated significant changes in fecal concentration of L-NCFM and B-LBi07. Both bacteria were significantly increased in the post-intervention fecal samples from subjects in the active arm while no such increase was found in the samples collected from subjects in the placebo arm. The considerations and methodologies for assessing the microbiology effects of probiotics on the intestinal microbiota are discussed in detail elsewhere (42). However, one aspect related to the specificity of the primers available to detect the consumed probiotic bacteria is demonstrated in figures 6. Using specific primers to detect L-NCFM we were able to demonstrate within and between-groups differences in pre- and post-intervention samples (Figure 5). However, since the available primers to detect B-LBi07 are less specific for this bacterial strain and can amplify genetic sequences from other closely related bacteria that are found indigenously in the human GI tract the separation between the different conditions (pre vs. post intervention) and groups (active vs. placebo) are less prominent (Figure 6).
In conclusion, our study provides additional support to the hypothesis that manipulation of the intestinal microbiota may be beneficial in patients with FBD. Our findings suggest that the combination of probiotic bacteria used in this study may be helpful in alleviating symptoms of bloating in patients with functional GI disorders. The clinical benefit of this intervention needs further investigation. In view of the high prevalence of bloating symptoms in patients with FBD, their overall impact on the patients’ health and well being, and the lack of effective treatment, this probiotic combination may be an important and useful addition to the management of patients with these symptoms.
Figure 2.
Flowchart of study enrollment, intervention and follow-up Enrolled and entered run-In
Acknowledgments
Supported by K23 DK075621, RR00046, and Danisco USA Inc.
Abbreviations
- (FBD)
Functional bowel disorders
- (IBS)
Irritable Bowel Syndrome
- (GI)
Gastrointestinal
Footnotes
Authors’ declaration of personal interests: Dr. Ringel has received research funding and consultation honorarium from Danisco USA Inc. Dr. Ringel-Kulka has received educational/research funding from Danisco USA Inc. Dr. Leyer is an employee of Danisco USA, Inc. Ms. Maier, Dr. Carroll, Dr. Galanko, and Dr. Palsson are employees of UNC Chapel Hill and have no conflict of interest to declare.
ClinicalTrials.gov number NCT00618904.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saito YA, Schoenfeld P, Locke GR., 3rd The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol. 2002;97:1910–1915. doi: 10.1111/j.1572-0241.2002.05913.x. [DOI] [PubMed] [Google Scholar]
- 2.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. In: Drossman DA, Corazziari E, Delvaux M, et al., editors. Rome III: the functional gastrointestinal disorders. 3rd edition Vol. 9. Degnon Associates; McLean (VA): 2006. pp. 487–555. [Google Scholar]
- 3.Hungin AP, Chang L, Locke GR, et al. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharamacol Ther. 2005;21:1365–1375. doi: 10.1111/j.1365-2036.2005.02463.x. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB, Olden K, Bjorkaman D. Health-related quality of life among persons with irritable bowel syndrome: a systematic review. Aliment Pharmacol Ther. 2002;16:1171–1185. doi: 10.1046/j.1365-2036.2002.01290.x. [DOI] [PubMed] [Google Scholar]
- 5.Spigel BM. The burden of IBS: looking at metrics. Curr Gastroenterol Rep. 2009;11(4):265–9. doi: 10.1007/s11894-009-0039-x. [DOI] [PubMed] [Google Scholar]
- 6.Maxion-Bergemann S, Thielecke F, Abel F, Bergemann R. Costs of irritable bowel syndrome in the UK and US. Pharmacoeconomics. 2006;24(1):21–37. doi: 10.2165/00019053-200624010-00002. [DOI] [PubMed] [Google Scholar]
- 7.Ringel Y, Drossman DA. Irritable bowel syndrome. In: Runge MS, Greganti MA, editors. Netter’s textbook of internal medicine. 2nd edition Vol. 59. Saunders Elsevier; Philadelphia: 2008. pp. 419–25. [Google Scholar]
- 8.Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, Spiegel BM, Talley NJ, Quigley EM. American College of Gastroenterology Task Force on Irritable Bowel Syndrome. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104(Suppl 1):S1–35. doi: 10.1038/ajg.2008.122. [DOI] [PubMed] [Google Scholar]
- 9.Ringel Y, Carroll I. Alternations of Intestinal Microbiota and Functional Bowel Symptoms. In “Gastrointestinal Motility and Neurogastroenterology”. Gastrointest Endosc Clin N Am. 2009;19(1):141–50. doi: 10.1016/j.giec.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Arebi N, Gurmany S, Bullas D, Hobson A, Stagg A, Kamm M. Review article: the psychoneuroimmunology of irritable bowel syndrome – an exploration of interactions between psychological, neurological and immunological observations. Aliment Pharmacol Ther. 2008;28:830–840. doi: 10.1111/j.1365-2036.2008.03801.x. [DOI] [PubMed] [Google Scholar]
- 11.Brenner DM, Moeller MJ, Chey WD, Schoenfeld PS. The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review. Am J Gastroenterol. 2009;104(4):1033–49. doi: 10.1038/ajg.2009.25. [DOI] [PubMed] [Google Scholar]
- 12.Pimentel M, Park S, Mirocha J, Kane SV, Kong Y. The effect of nonabsorbed oral antibiotic (rifamixin) on the symptoms of the irritable bowel syndrome: a randomized trial. Ann Intern Med. 2006;145:557–563. doi: 10.7326/0003-4819-145-8-200610170-00004. [DOI] [PubMed] [Google Scholar]
- 13.Sharara AI, Aoun E, Abdul-Baki H, Mounzer R, Sidani S, Elhajj I. A randomized double-blind placebo-controlled trial of rifamixin in patients with abdominal bloating and flatulence. Am J Gastroenterol. 2006;101:326–333. doi: 10.1111/j.1572-0241.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 14.Lembo A, Zakko S, Ferreira N, et al. Rifaximin for the treatment of diarrhea-associated irritable bowel syndrome: short term treatment leading to long term sustained response. Gastroenterology. 2008;134(4 Suppl 1):A545. [Google Scholar]
- 15.Ringel Y, Zakko S, Ferreira N, et al. Predictors of clinical response from a phase 2 multi-center efficacy trial using rifaximin, a gut-selective, nonabsorbed antibiotic for the treatment of diarrhea-associated irritable bowel syndrome. Gastroenterology. 2008;134(4 Suppl 1):A550. [Google Scholar]
- 16.Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein A, Brandt L, Quigley E. The efficacy of probiotics in the therapy of irritable bowel syndrome: a systematic review. Gut. 2008 doi: 10.1136/gut.2008.167270. [DOI] [PubMed] [Google Scholar]
- 17.Spiller R. Review article: probiotics and prebiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:385–396. doi: 10.1111/j.1365-2036.2008.03750.x. [DOI] [PubMed] [Google Scholar]
- 18.Jonkers D, Stockbrügger R. Review article: Probiotics in gastrointestinal and liver diseases. Aliment Pharmacol Ther. 2007;26(Suppl 2):133–48. doi: 10.1111/j.1365-2036.2007.03480.x. [DOI] [PubMed] [Google Scholar]
- 19.Irvine EJ, Whitehead EW, Chey WD, et al. Design of Treatment Trials for Functional Gastrointestinal Disorders. In: Drossman DA, Corazziari E, Delvaux M, et al., editors. Rome III: The Functional Gastrointestinal Disorders. 3rd ed Vol. 15. Degnon Associates; McLean,VA: 2006. pp. 798–811. [Google Scholar]
- 20.Toner BB, Segal ZV, Emmott S, et al. Cognitive behavior group therapy for patients with irritable bowel syndrome. Int J Group Psychother. 1998;48(2):215–243. doi: 10.1080/00207284.1998.11491537. [DOI] [PubMed] [Google Scholar]
- 21.Drossman DA, Toner BB, Whitehead WE, et al. Cognitive-behavioral therapy verses education and desipramine verses placebo for moderate to severe functional bowel disorders. Gastroenterol. 2003;125:19–31. doi: 10.1016/s0016-5085(03)00669-3. [DOI] [PubMed] [Google Scholar]
- 22.Weinland SR, Morris CB, Dalton C, Hu Y, Whitehead WE, Toner BB, Diamant N, Leserman J, Bangdiwala SI, Drossman DA. Cognitive Factors Affect Treatment Response to Medical and Psychological Treatments in Functional Bowel Disorders. Am J Gastroenterol. 2010 Jan 19; doi: 10.1038/ajg.2009.748. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–24. doi: 10.3109/00365529709011203. the stool Bristol Score. [DOI] [PubMed] [Google Scholar]
- 24.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 25.Drossman DA, Li Z, Leserman J, Toomey TC, Hu Y. Health status by gastrointestinal diagnosis and abuse history. Gastroenterology. 1996;110:999–1007. doi: 10.1053/gast.1996.v110.pm8613034. [DOI] [PubMed] [Google Scholar]
- 26.Patrick DL, Drossman DA, Frederick IO, et al. Quality of life in persons with irritable bowel syndrome. Development and validation of a new measure. Dig Dis Sci. 1998;43:400–411. doi: 10.1023/a:1018831127942. [DOI] [PubMed] [Google Scholar]
- 27.Otten CM, Kok L, Witteman BJ, Baumgarten R, Kampman E, Moons KG, de Wit NJ. Diagnostic performance of rapid tests for detection of fecal calprotectin and lactoferrin and their ability to discriminate inflammatory from irritable bowel syndrome. Clin Chem Lab Med. 2008;46(9):1275–80. doi: 10.1515/CCLM.2008.246. [DOI] [PubMed] [Google Scholar]
- 28.Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008;103(1):162–9. doi: 10.1111/j.1572-0241.2007.01556.x. [DOI] [PubMed] [Google Scholar]
- 29.Rousseaux C, Thuru X, Gelot A, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nature Med. 2007;13(1):35–7. doi: 10.1038/nm1521. [DOI] [PubMed] [Google Scholar]
- 30.Barrangou R, Briczinski EP, Traeger LL, et al. Comparison of the complete genome sequences of Bifidobacterium animalis subsp. lactis DSM 10140 and Bl-04. J Bacteriol. 2009;191(13):4144–4151. doi: 10.1128/JB.00155-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ventura M, Reniero R, Zink R. Specific identification and targeted characterization of Bifidobacterium lactis from different environmental isolates by a combined multiplex-PCR approach. Appl Environ Microbiol. 2001;67(6):2760–5. doi: 10.1128/AEM.67.6.2760-2765.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ringel Y, Williams RE, Kalilani L, et al. The prevalence, characteristics and impact of bloating symptoms in patients with irritable bowel syndrome. Clinical Gastroenterology and Hepatology. 2009;7:68–72. doi: 10.1016/j.cgh.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Jiang X, Locke GR, 3rd, Choung RS, et al. Prevalence and risk factors for abdominal bloating and visible distention: a population- based study. Gut. 2008;57:756–763. doi: 10.1136/gut.2007.142810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simren M. Bloating and Abdominal Distention: Not So Poorly Understood Anymore! Gastroenterology. 2009;136:1487–1489. doi: 10.1053/j.gastro.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 35.Kim HJ, Camilleri M, McKinzie S, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17:895–904. doi: 10.1046/j.1365-2036.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- 36.Kim HJ, Roque MI Vazquez, Camilleri M, et al. A randomized controlled trial of a probiotic combination VSL#3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17:687–696. doi: 10.1111/j.1365-2982.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 37.O’Mahoney L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 38.Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581–1590. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 39.Agrawal A, Houghton LA, Morris J, Reilly B, Guyonnet D, Feuillerat N Goupil, Schlumberger A, Jakob S, Whorwell PJ. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173-010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2008;29:104–114. doi: 10.1111/j.1365-2036.2008.03853.x. [DOI] [PubMed] [Google Scholar]
- 40.Kajander K, Myllyluoma E, Rajilić-Stojanović M, Kyrönpalo S, Rasmussen M, Järvenpää S, Zoetendal EG, de Vos WM, Vapaatalo H, Korpela R. Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther. 2008 Jan 1;27(1):48–57. doi: 10.1111/j.1365-2036.2007.03542.x. [DOI] [PubMed] [Google Scholar]
- 41.Guandalini S, Magazzù G, Chiaro A, La Balestra V, Di Nardo G, Gopalan S, Romano C, Canani R Berni, Lionetti P, Setty M. VSL#3 improves symptoms in children with irritable bowel syndrome. An international, randomized, placebo-controlled, double-blinded, cross-over study. J Pediatr Gastroenterol Nutr. 2010;51:24–30. doi: 10.1097/MPG.0b013e3181ca4d95. [DOI] [PubMed] [Google Scholar]
- 42.Carroll MI, Ringel-Kulka T, Ringel Y. Quantification and Identification of Probiotic Organisms in Humans. In: Floch MH, Kim AS, editors. Probiotics: A Clinical Guide. 1st edition SLACK Incorporated; New Jersey: 2010. [Google Scholar]