Abstract
Aims
Despite multiple studies investigating the environmental controls on CH4 fluxes from arctic tundra ecosystems, the high spatial variability of CH4 emissions is not fully understood. This makes the upscaling of CH4 fluxes from plot to regional scale, particularly challenging. The goal of this study is to refine our knowledge of the spatial variability and controls on CH4 emission from tundra ecosystems.
Methods
CH4 fluxes were measured in four sites across a variety of wet-sedge and tussock tundra ecosystems in Alaska using chambers and a Los Gatos CO2 and CH4 gas analyser.
Results
All sites were found to be sources of CH4, with northern sites (in Barrow) showing similar CH4 emission rates to the southernmost site (ca. 300 km south, Ivotuk). Gross primary productivity (GPP), water level and soil temperature were the most important environmental controls on CH4 emission. Greater vascular plant cover was linked with higher CH4 emission, but this increased emission with increased vascular plant cover was much higher (86 %) in the drier sites, than the wettest sites (30 %), suggesting that transport and/or substrate availability were crucial limiting factors for CH4 emission in these tundra ecosystems.
Conclusions
Overall, this study provides an increased understanding of the fine scale spatial controls on CH4 flux, in particular the key role that plant cover and GPP play in enhancing CH4 emissions from tundra soils.
Keywords: Arctic, Climate change, Permafrost, Greenhouse gas emission, Vegetation control
Introduction
Global warming in the Arctic is occurring at nearly twice the global average rate (IPCC 2013), resulting in increased temperatures, permafrost degradation, decreased snow-cover duration, changes in the hydrological cycle and changes in vegetation composition (Callaghan et al. 2010; Hinzman et al. 2005, 2013; IPCC 2013). Warmer temperatures may stimulate increased release of carbon dioxide (CO2) and methane (CH4) from tundra ecosystems (Billings et al. 1982; von Fischer et al. 2010; Harazono et al. 2006; Oechel et al. 1995; Zona et al. 2009) which are largely temperature and moisture limited. The global warming potential (GWP100) of CH4 is 28.5 times greater than that of CO2, making it an important greenhouse gas (IPCC 2013). CH4 concentration increased in the Arctic by 31 % between 2003 and 2007 accounting for around 8–10 % of global CH4 emissions (Bloom et al. 2010; Dlugokencky et al. 2011). In addition to temperature, the hydrological status of the soil is a very important control on CH4 fluxes (Bubier et al. 1993; Moore and Roulet 1993; Zona et al. 2009). The predicted increase in rainfall at northern high latitudes (IPCC 2013) may increase CH4 loss by increasing the anoxic status of the soil (Bhullar et al. 2013b; Blodau et al. 2004; Moore and Roulet 1993; Sebacher et al. 1986). Finally, as vegetation has a significant role for both CH4 transport and for the provision of substrate for methanogens, vegetation changes might significantly affect the Arctic CH4 budget (Bhullar et al. 2013a; Joabsson and Christensen 2001; Shannon et al. 1996; Walter and Heimann 2000).
The processes controlling methanogenesis are tightly coupled to surrounding environmental conditions (von Fischer et al. 2010; Harazono et al. 2006; Harriss and Frolking 1992; Jones et al. 1987) and are holocoenotic (Billings 1952). Because of the complexity of arctic ecosystems, there are still large uncertainties in the impact that environmental changes will have on CH4 emissions from the Arctic, with different CH4 models disagreeing on both the direction and magnitude of future changes in CH4 emissions from northern high latitudes with warming and increased CO2 (Melton et al. 2013).
Production, oxidation and transport are the three most important processes in controlling the rate of arctic tundra CH4 emission (Brummell et al. 2012; Bubier et al. 1993; Cao et al. 1996; von Fischer et al. 2010; Harazono et al. 2006; Lai 2009). CH4 is transported from the soil to the atmosphere through four main pathways: it can diffuse directly across the surface of the soil, be transported by pressure changes and wind, released as bubbles of gas (ebullition) in standing water (Bubier et al. 1993; Klapstein et al. 2014; Walter et al. 2006) or it can diffuse through the aerenchyma of vascular plants (Joabsson et al. 1999; Whalen and Reeburgh 1992). Therefore changes in vegetation composition and density might also substantially impact CH4 emissions (Lai et al. 2014a,b; Sebacher et al. 1985; Shannon et al. 1996). Vegetation can have a key influence on CH4 fluxes (von Fischer and Hedin 2007; Harazono et al. 2006; Jones et al. 1987; Schimel 1995; Ström et al. 2003) through the supply of organic substrates for CH4 production and by increasing CH4 transport from the soil to the atmosphere (Bhullar et al. 2013a; Joabsson and Christensen 2001; Noyce et al. 2014; Schimel 1995; Shannon et al. 1996; Torn and Chapin 1993). Photosynthetically driven root exudation of organic compounds and the decomposition of dead plant matter provides the primary substrates for CH4 production (Joabsson et al. 1999; King et al. 1998; Lai 2009; Olefeldt et al. 2013; Shannon et al. 1996; Singh 2001; Ström et al. 2012). Post production, plants facilitate transport of CH4 by providing important conduits for CH4 flux between the soil and atmosphere (Bhullar et al. 2013a,b; Brummell et al. 2012; Joabsson et al. 1999; Ström et al. 2003; Whalen 2005), allowing CH4 to bypass oxic layers within the soil where it would otherwise be re-oxidised (Frenzel and Karofeld 2000; Heilman and Carlton 2001; Inubushi et al. 2001; Jespersen et al. 1998; Joabsson and Christensen 2001; Ström et al. 2005; Whalen and Reeburgh 1990; Wilson and Humphreys 2010). Structurally, the tissue of some vascular plants found in tundra, especially sedges, are comprised of soft aerenchyma and lacunae tissues which contain tiny airspaces that allow for this gaseous exchange between roots and shoots via molecular diffusion (Armstrong and Armstrong 1991; Le Mer and Roger 2001; Shannon et al. 1996; Torn and Chapin 1993). The importance of vascular plants in CH4 emission is particularly evident during the growing season when the increase in the plant productivity and plant biomass, by increasing both substrate availability and the CH4 transport, ultimately increases CH4 emissions (Couwenberg et al. 2011; von Fisher and Hedin 2007; Greenup et al. 2000; Grunfeld and Brix 1999; Joabsson et al. 1999; Joabsson and Christensen 2001; Shannon et al. 1996). On the other hand, vascular plants can aid the competing process of CH4 oxidation by transporting O2 to their roots which supports methanotrophy when it is released to the surrounding soil (Conrad 1996; Harazono et al. 2006; Sebacher et al. 1985). The net effect of these processes helps determine the CH4 emissions from arctic ecosystems (Harazono et al. 2006; Joabsson et al. 1999; Shannon et al. 1996). Increased CH4 emission has been found to correlate with higher abundances of more conductive vascular plant species such as graminoids (Bhullar et al. 2013a,b; Bubier et al. 1993; Dias et al. 2010; Ström et al. 2003, 2005).
The complexity and heterogeneous pattern of all these biotic and abiotic processes controlling CH4 fluxes leads to high variations in CH4 measurements across arctic landscapes, as measured by chamber flux and eddy covariance techniques (Budishchev et al. 2014; Kutzbach et al. 2004; Morrissey and Livingston 1992; Sebacher et al. 1986). For example, previously reported cumulative peak growing season rates (late July to August) range from 30 to 120 mg C CH4 m−2 d−1 with daily averages ranging from 4.5 to 9.6 mg C CH4 m−2 d−1 and can vary considerably, even across consecutive measurements within the same sites (Harazono et al. 2006; Sturtevant and Oechel 2013; Vourlitis and Oechel 1997; Whiting and Chanton 1993; Wille et al. 2008). Despite extensive research into the patterns and controls of CH4 emissions from the Arctic (Joabsson et al. 1999; Lai et al. 2014a,b; Morrissey and Livingston 1992; Schimel 1995; Sturtevant and Oechel 2013; Whalen and Reeburgh 1990; Zona et al. 2009) the most important limiting factors, their relative importance, and the role of vegetation in controlling CH4 emissions are still highly debated. Some studies have argued that methanogenesis (and overall CH4 emissions from the Arctic) is substrate limited (Dunfield et al. 1993; King et al. 2002; Rinnan et al. 2007; Ström et al. 2003; Yoshitake et al. 2007) while others identify transport as the key limitation for CH4 emission (Bhullar et al. 2013a; Joabsson et al. 1999; Joabsson and Christensen 2001; Schimel 1995; Sebacher et al. 1985). To add further complexity, vegetative and environmental controls driving CH4 exchange within the tundra ecosystem are not independent, but rather have a combined influence upon local CH4 flux. For example, differences in water table levels, soil temperatures, pH and nutrient content not only directly affect CH4 production within the soil, but also determine the growth rates, activities and compositions of vascular plants, thus indirectly influencing vegetation control of CH4 fluxes (Couwenberg et al. 2011; von Fischer et al. 2010; Harazono et al. 2006; Lai et al. 2014a,b; Schimel 1995).
To enhance our understanding of these complex controls on CH4 emission, we measured CH4 fluxes using portable chambers across four arctic tundra ecosystems, including wet-sedge tundra and tussock tundra ecosystems, with different degrees of polygonization. Portable chamber measurements of microrelief patterns in greenhouse gas fluxes are useful for disentangling the fine scale environmental and vegetation controls on CH4 emission and will provide a basis for upscalling to generate estimates of CH4 flux patterning at the ecosystem scale (Hill et al. 2009; Sachs et al. 2010).
In order to determine the relative importance of environmental controls on CH4 flux, an extensive range of environmental variables were measured alongside CH4 fluxes, together with a classification of vegetation types, in these four sites in Alaska. Net ecosystem exchange (NEE), ecosystem respiration (ER) and gross primary productivity (GPP) were also determined to assess the importance of plant productivity on CH4 emissions. We hypothesised that increased soil and air temperature, water table height, vascular plant cover, GPP and thaw depth would be associated with increased CH4 emissions. We also expected that interactions between these factors may be important in determining rates of CH4 flux.
Methods
Site description
This study was performed at four sites: three in the northern part of the Arctic Coastal Plain (Barrow) (BEN, 71°17ʹ11.80ʺN, 156°36ʹ12.23ʺW, BES, 71°16ʹ51.17ʺN, 156°35ʹ47.28″W and BEO, 71° 16ʹ 51.61ʺN, 156° 36ʹ 44.44ʺ W) (Zona et al. 2009, 2012) and one at the foothill of the Brooks Range (Ivotuk, 68.49° N, 155.74° W). The Barrow study sites (BEN, BEO, and BES) are located in the North Coast of Alaska, USA. The vegetation in these northern sites is classified as sedge-moss wetland (CAVM Team 2003; W2, Walker et al. 2005), and includes prostrate dwarf shrubs, lichen, grass, forbs, rushes and bryophytes (CAVM Team 2003; Raynolds et al. 2005; Walker et al. 2005); with substantial ice wedge polygon development (Billings and Peterson 1980; Britton 1957). The presence of permafrost and the substantial development of ice-wedge polygons results in large spatial heterogeneity with high and dry oxic rims and low anoxic centres, with high water tables for most of the growing season (Harazono et al. 2006; Kwon et al. 2006; Vourlitis and Oechel 1997; Zona et al. 2009, 2011). High environmental microtopographic variation allows colonisation by a wide variety of moss, lichen and vascular dwarf shrub vegetation (Billings and Peterson 1980). Among these three study sites, BEN and BEO have the more developed polygons (low-centre and high-centre polygons respectively), while the BES site presents fairly flat and homogenous terrain. The southern study location (Ivotuk), is classified as tussock-sedge, dwarf-shrub, moss tundra and has no substantial polygon development (Riedel et al. 2005; Romanovsky et al. 2003; G4, Walker et al. 2005).
The multiple sampling locations in Barrow included a variety of microhabitats with different local environmental conditions and vegetation types. In the BEO site at the beginning of the summer, eight colourless transparent acrylic soil collars (200 x 440 x 440 mm) were inserted into the moss layer with a serrated knife. These eight sampling plots were located across a 100 m transect including drier polygon rims (dry sites) and wetter polygons centres (wet sites), spaced approximately 5–10 m apart. Sites were classified as wet when the water table was at or above the soil surface level for the entire duration of the measuring period (soils were assumed to be mostly anaerobic for the entire summer); dry sites presented water tables below surface level (1 cm or deeper) for the entire measuring period (therefore containing an upper oxic soil layer, where CH4 oxidation can potentially occur). The cylindrical collars (radius 140 mm) used in the BES and BEN sites were inserted during a previous experiment in summer 2005 (Zona et al. 2009). Finally, in Ivotuk, cylindrical collars (radius 100 mm) were inserted, using the serrated knife method, in six wet sites and six dry sites (where the dry sites comprised of three tussock sites and three inter-tussock sites) (Fig. 1). Across all these four sites in both Barrow and Ivotuk, there were 15 sites with water table permanently below the surface (dry sites) and 16 sites flooded for the entire summer (wet sites) (Fig. 1). All soil collars were left for 24 h before measurements began to avoid soil disturbance effects on trace gas flux measurements.
Fig. 1.
Soil collar vegetation sites at Barrow; BEN (low-centre, developed polygons), BEO (high-centre, developed polygons) and BES (fairly flat, homogenous terrain) and Ivotuk (IVO) (no substantial polygon development); From top to bottom row: three dry and three wet sites in BEN; two dry and three wet sites in BES; four dry sites in BEO; four wet sites in BEO; three tussock and three intertussock dry sites in IVO; six wet sites in IVO
CH4 and CO2 flux measurements
All sites in Barrow were measured between the end of July and the beginning of September 2013. CH4 and CO2 fluxes were measured on a weekly basis for six weeks in Barrow (29th July to 15th September 2013) and once in Ivotuk (18th August 2013). The Barrow sites are within driving distance from a research station, which allowed multiple sampling during the season, while the remote location of Ivotuk, with no commercial airport, required chartering a plane and was accessed only once during the summer. CH4 and CO2 fluxes at each site were measured using an LGR™, Ultra-Portable Greenhouse Gas Analyser (Model 915–0011, Los Gatos Research, Palo Alto, CA, USA) with a 1 Hz sampling rate, connected to a transparent, colourless acrylic chamber. At BEO, the large clear acrylic chamber (638 × 440 × 440 mm) was connected via inlet and outlet tubing (3.5 m by 2 mm internal diameter of Bev-A-Line) to the LGR™ analyser. An elastic bungee rope was attached between the chamber and collar to ensure a gas tight seal (Moosavi and Crill 1997). At BES and BEN, smaller cylindrical chambers (140 mm height x 290 mm diameter) were used. Sampling at Ivotuk was performed using an opaque Licor (LI-8100A) automated soil CO2 Flux System (155 height x 188 mm diameter) clamped closed, and connected to the LGR™ to collect gas fluxes under respiratory conditions for 2.5 min. Similar sized chambers were used in previous studies at these sites (Oberbauer et al. 2007; Olivas et al. 2011; Vourlitis et al. 1993; Zona et al. 2011) and their fluxes were in close agreement with fluxes estimated by eddy covariance, despite the difference in size (Oechel et al. 1998; Zona et al. 2011).
Before each measurement, the chamber was carefully placed on each collar forming a gas tight seal. At BEO, the chamber was left on each soil collar for 4.5 min to achieve a stable increase in CH4 and CO2 concentration within the chamber headspace. The chamber was then lifted from the collar and waived in the air to expel any built up gas and to allow for ambient air levels to re-establish. The chamber was then covered with a black felt blanket and placed back on the collar for an additional 4.5 min to measure ER and estimate GPP (GPP = NEE + ER). As the CH4 fluxes did not differ between the dark and light measurements, means of these values were used to perform the statistical analysis. Because of the smaller size of the chamber used in BEN and BES, and the shorter time required to achieve a stable increase in CO2 and CH4 concentration, both light and dark measurements in these two sites were performed for 2.5 min each.
CH4 and CO2 fluxes were calculated from the linear increase in gas concentrations inside the chamber headspace as measured by the LGR™. Least squares linear regression was applied to the increase in CH4 after chamber closure. The obtained rate of concentration increase was then used with the following equation to obtain the CH4 and CO2 flux at each site.
Where:
- F0
Flux at the time of chamber closure (μg C CH4/CO2 m−2 h−1)
- S
Time derivative (slope) CH4 and CO2 concentration change over time (ppm s−1)
- V
Chamber volume (m3)
- A
Chamber area (m2)
- M
Molecular mass of CH4/CO2 (g mol−1)
- Vm
Ideal gas mole volume (0.0224 m3 mol−1)
Each regression plot was individually assessed and their R-squared values were used as a form of quality control for the selection of fluxes incorporated into the analysis; 94 % of all fluxes had a R-squared value of 0.7 or above (of which, 83 % had a R-squared value of 0.9 or above).
Environmental measurements
Measurement of environmental variables (thaw depth, water table height) and soil parameters (pH, temperature and moisture) were performed at the same time as flux sampling in each plot. Soil temperature was measured just below the soil surface (1–4 cm) and at depth (9–11 cm) using a portable type T thermocouple, volumetric soil moisture was measured within the top 20 cm of soil (TDR 300 Fieldscout, Spectrum technologies INC) and soil pH at 3–7 cm (Thermo Scientific Orion 3-Star Plus pH Meter). The pH probe was calibrated against standards (pH 3 and 7) before starting the field campaign, and regularly during the field season, as a quality control of the measurements. Thaw depth and water table height were measured using a graduated metal rod, as described in Zona et al. (2009). Ambient air temperatures were recorded by the LGR™ Ultra-Portable Greenhouse Gas Analyser. Percentage vascular plant and moss cover was estimated visually after the end of the field season using photographs collected from each plot, during each sampling week.
Statistical analysis
The importance of the variables explaining CH4 fluxes was determined using linear mixed models. CH4 fluxes were log transformed to meet the normality and homoscedasticity assumptions required for the analyses. All statistical analyses were carried out in R version 3.1.0 (R Core Team 2014). The following variables, their two-way interactions and squared terms were all tested as candidate explanatory variables; ER, NEE, GPP, thaw depth, water table depth, soil temperature at 9–11 cm, soil moisture, soil pH and percentage vascular plant cover. Initially, curvature in the relationship between explanatory and response variables was tested by fitting all explanatory variables and their squared terms, and only those statistically significant quadratic terms were retained. A series of models each containing main effects and a subset of all possible two way interactions were used to identify potentially significant interactions. A full model was then constructed using all main effect terms plus the quadratic and two way interactions already identified by the procedure described above. This model was simplified by the sequential removal of non-significant terms until removal of further terms caused an increase in AIC (Crawley 2012). For all mixed models the identity of the chamber (chamber ID) was included as a random intercept term to account for the repeated measurements taken at the same plots. Interactions were interpreted using the methods of Aiken and West (1991). Marginal R2 (R2 LMM(m)), which describes the proportion of the variance in the data explained by the fixed effects, and conditional R2 (R2 LMM(C)) which describes the proportion of the data explained by both fixed and random effects were calculated following Nakagawa and Schielzeth (2013). Model fits were checked visually to ensure that they conformed to model assumptions. Final p values were Bonferroni adjusted (multiplied by 54, the number of candidate explanatory variables) to mitigate the risk of type I error.
Because missing data for some variables (e.g., soil moisture data were missing due to power failure of the instrument) limited the number of observations available for the multiple regression, further mixed effect models were used to assess the importance of percent vascular plant cover and water table height on CH4 flux. Vascular plant cover and water table (above/below surface) were included as fixed effects and chamber was included as a random intercept. Initially three levels of the vascular plant cover were included (<10, 10–60 and >60 %) however this was reduced to two levels (<10 and >10 %) following model simplification. Further mixed effect models were fitted to test the impact of soil submergence on ER, NEE, GPP and CH4 flux. In each of these models, submergence (water table above/below soil surface) was fitted as a fixed effect while chamber ID was included as a random intercept. The dependant variable was transformed where necessary to meet the assumptions of homoscedasticity and homogeneity of variance.
As the sampling plots were stratified by wetness, we also tested the difference in NEE, ER, GPP, and CH4, between dry and wet sites by using a mixed model, again with chamber included as a random intercept. Wald test p values are presented.
Results
Environmental variables
During the course of the experiment, average air temperatures in Barrow and Ivotuk were 10.9 ° C ± 5.36 s.d. and 5.6 ° C ± 0.32 s.d. respectively, with peak temperatures in Barrow in early August (max. 21.8 ° C) decreasing steadily throughout August and September (min. 1.38 ° C). Thaw depths in Barrow ranged from 25 to 47 cm below the surface in wet sites (average of 34 cm ± 4.04 s.d., n = 62) and from 10 to 42 cm below surface in the dry sites (average of 34 cm ± 6.68 s.d., n = 48) and in Ivotuk from 44 to 53 cm below surface in wet sites (average of 48 cm ± 3.25 s.d., n = 7) and from 45 to 50 cm below surface in dry sites (average of 48 cm ± 2.11 s.d., n = 5). Water tables within wet plots ranged from surface to 16 cm above surface (average 7 cm ± 11.5 s.d., n = 62) at Barrow and from surface to 5 cm above surface (average 2 cm ± 6.4 s.d., n = 7) in Ivotuk (Fig. 2). Water tables within dry plots in Barrow ranged from 1 to 33 cm below surface (average 13 cm ± 11.6 s.d., n = 44) and from 8 to 15 cm below surface (average 9 cm ± 5.2 s.d., n = 5) in Ivotuk. Across all sites, surface soil temperature (1–4 cm) ranged from 0.2 ° C to 14.6 ° C (average 5.9 ° C ± 4.0 s.d., n–122) and deeper soil temperatures (9–11 cm) ranged from 0.3 to 9.1 ° C (average 3.8 ° C ± 2.5 s.d., n = 122). Soil pH was consistently acidic, ranging from 2.7 to 6.5 (average 4.4 ± 0.7 s.d., n = 94) throughout the measurement period. Thaw depth was weakly correlated to both soil temperature (R2 = 0.08) and water table (R2 = 0.06) within wet sites, where wetter and warmer soils tended to have deeper thaw.
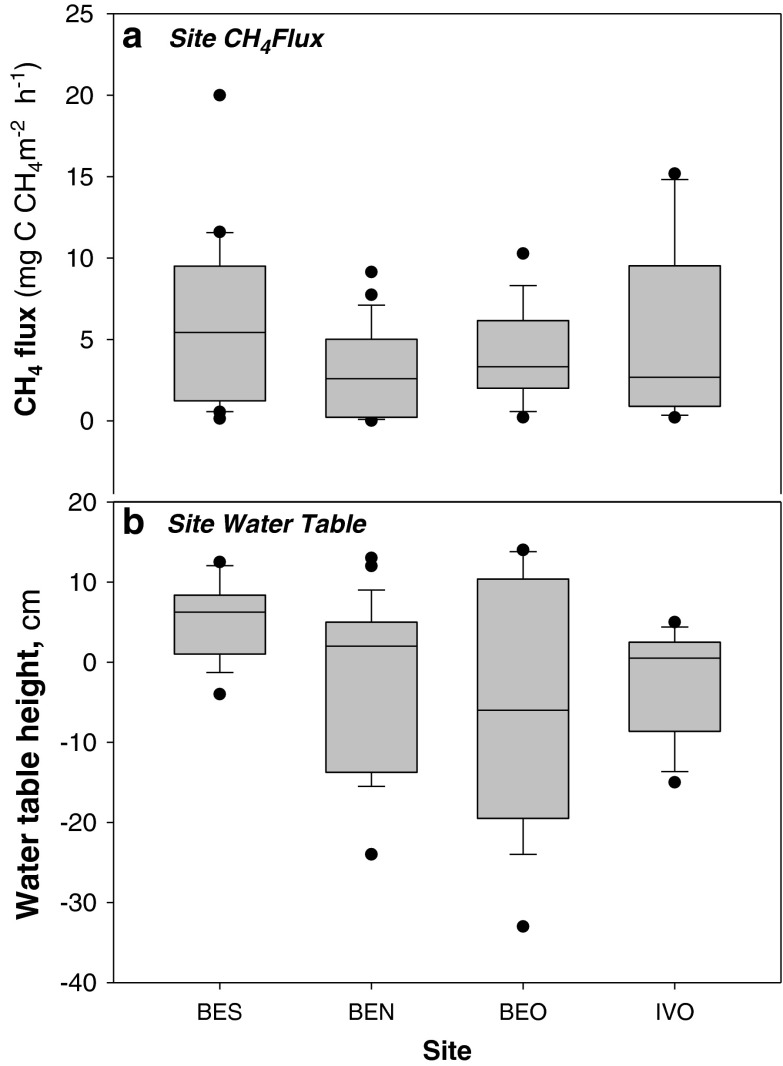
Fig. 2.
a) CH4 flux (mg C CH4 m−2 h−1) in August in BES (fairly flat, homogenous terrain) (n = 20, 1–25 August), BEN (low-centre, developed polygons) (n = 24, 1–25 August), BEO (high-centre, developed polygons) (n = 18, 6–26 August) in Barrow and Ivotuk (no substantial polygon development) (n = 11, on 18 August) and b) ground water table, cm, at sites BES (n = 18), BEN (n = 30), BEO (n = 20) in Barrow and Ivotuk (n = 12). Boxplots represent median (midline), quartiles (box), maximum and minimum (whisker) with outliers represented as black points
Spatial variability in and influence of water table depth on CH4 fluxes
CH4 emission was observed across all sites with no CH4 uptake recorded even in the driest of plots. High variability in CH4 emission was recorded, with rates ranging from 20 mg C CH4 m−2 h−1 (measured on the 10/08/2013 in Barrow) to 0.01 mg C CH4 m−2 h−1 (measured on the 11/09/2013 in Barrow) (Fig. 2), corresponding with decreasing air temperatures from 21.2 ° C (Barrow, 10/08/2013) to 7.7 ° C (Barrow, 11/09/2013). As expected, the wettest site (BES) showed the highest CH4 emissions (Fig. 2 and Fig. 3). The average of the entire measurement period indicated that CH4 emissions were significantly greater from wet sites (4.52 mg C CH4 m−2 h−1 ± 0.45 s.e., n = 64) compared to dry sites (2.17 mg C CH4 m−2 h−1 ± 0.55 s.e., n = 42) (Wald test, n = 106, F 1,75 = 8.2, p = 0.005) (Fig. 3d). The spatial variability in water table heights was more pronounced in the sites with more developed polygons (BEO: high centre polygons and BEN: low centre polygons; Fig. 2b). However, this variability in water table levels was not reflected in a similar variability in CH4 fluxes, which were more variable in the BES and Ivotuk sites despite their lower degrees of polygonization (Fig. 2a).
Fig. 3.
Average a) Ecosystem Respiration b) Net Ecosystem Exchange and c) Gross Primary Production fluxes (g C CO2 m−2 h−1) and d) CH4 flux (mg C CH4 m−2 h−1) at sample locations in Barrow (BES, BEO and BEN) and Ivotuk split by sites where water table height is below (-ve) or above (+ve) surface level (cm). Bars represent means with error bars shown as standard errors. ** denotes bars are significantly different at p < 0.01, ● denotes p < 0.1
The influence of water table depth on CO2 fluxes
There was a marginally significant trend for net ecosystem exchange (NEE) to be more negative (i.e., more net ecosystem CO2 uptake) in wet sites (-0.08 g C CO2 m−2 h−1 ± 0.1 s.e., n = 51) compared to dry sites (−0.05 g C CO2 m−2 h−1 ± 0.01 s.e., n = 36; Wald test, n = 87, F1,67 = 3.552, p = 0.0638; Fig. 3b). However ER (Wald test, n = 93, F1,61 = 0.628, p = 0.4309; Fig. 3a) and GPP (Wald test, n = 72, F1,52 = 0.972, p = 0.3287; Fig. 3c) did not differ significantly between the wet and dry sites.
Environmental and vegetation control on CH4 flux
Based on our multiple regression modelling, the most important variables explaining CH4 fluxes were GPP and water table depth, followed by the interaction between water table and soil temperature (Table 1). All these variables combined explained 60 % (R2 LMM(m) = 0.60) of the variability in CH4 fluxes across the four sites investigated (Table 1).
Table 1.
Parameter estimates for the fixed effects in a linear mixed model of the variables influencing CH4 flux; n = 51, R2 LMM(m) = 0.60, R2 LMM(c) = 0.77. Bonferroni adjusted p values are displayed
| Parameter | Estimate | SE | df | t | p |
|---|---|---|---|---|---|
| Intercept | 3.750942 | 0.525874 | 28 | 7.132776 | <0.001 |
| GPP | 19.017379 | 4.478884 | 28 | 4.246008 | 0.011 |
| Water table depth | 0.170248 | 0.043746 | 28 | 3.891735 | 0.032 |
| Soil temperature at 9–11 cm depth | 0.153864 | 0.165532 | 28 | 0.929509 | 1.000 |
| pH | −0.388229 | 0.781263 | 28 | −0.496925 | 1.000 |
| pH2 | −1.585871 | 0.588373 | 28 | −2.695347 | 0.637 |
| Water table depth* Soil temperature at 9–11 cm depth | 0.067599 | 0.015570 | 28 | 4.341595 | 0.011 |
Methane flux increased with increasing GPP (Table 1). GPP was significantly higher when vascular plant cover was >10 % in comparison to <10 %, and this relationship explains 18 % of the variation in GPP (mixed effect model, p = 0.005, R2 LLM(m) = 0.176, R2 LMM(c) = 0.176) while soil temperature (at 9–11 cm depth) explained 43 % of the variation in GPP (mixed effect model, p < 0.001, R2 LMM(m) = 0.431, R2 LMM(c) = 0.622)
There was a conditional effect of water table depth on CH4 emissions, with those sites with a deeper water table being more conducive to CH4 emission (Table 1, Fig. 3). This conditional effect was influenced by a significant interaction between water table depth and soil temperature at 9–11 cm (Table 1, Fig. 4). As the depth of the water table increased, the relationship between CH4 emission and soil temperature switched from negative to positive, with the sign of the slope of the relationship changing near the point where the water table is just above the soil surface (Fig. 4).
Fig. 4.
The influence of the interaction between soil temperature 9–11 cm below the surface and water table depth on CH4 flux. Points are mapped onto a colour scale to show the water table depth for each measurement. Regression lines show conditional influence of soil temperature on CH4 flux at the mean water table height (0.0 cm above the surface) and at 1 standard deviation above and below the mean (10.2 and −10.2 cm respectively) determined using the methods of Aiken and West (1991). For statistics see Table 1
Methane emmissions were influenced by a significant interaction between soil wetness (water table above ground surface vs. below ground suface) and percentage vascular plant cover (Table 2, Fig. 5). Importantly, within wet sites, CH4 emissions were less dependent on vascular plant cover (increasing from 3.3 to 4.69 mg C CH4 m−2 h−1), whereas in dry sites there was a much more substantial increase in CH4 emission (almost an order of magnitude) from 0.35 mg C CH4 m−2 h−1 (n = 29) to 2.45 mg C CH4 m−2 h−1 (n = 30) with increasing vascular plant cover (Fig. 5, Table 2). Dry sites with >10 % vascular coverage had an average CH4 emission (2.46 mg C CH4 m−2 h−1, n = 30) similar to that in wet sites with <10 % vascular cover (3.29 mg C CH4 m−2 h−1, n = 27) (Fig. 5). The combination of soil wetness and vegetation cover explained 56 % of the variation seen in CH4 emissions (R2 LMM(c) = 0.56, Table 2).
Table 2.
Parameter estimates for the fixed effects in the linear mixed effects model of the effect of waterlogging and degree of vascular plant cover on CH4 flux; n=108, R2 LMM(m) = 0.52, R2 LMM(c) = 0.56
| Parameter | Estimate | SE | df | t | p |
|---|---|---|---|---|---|
| Intercept | −1.89 | 0.258 | 74 | −7.34 | <0.001 |
| Vascular plant cover | 2.59 | 0.347 | 28 | 7.47 | <0.001 |
| Waterlogging | 2.81 | 0.396 | 74 | 7.09 | <0.001 |
| Vascular plant cover*waterlogging | −2.29 | 0.486 | 74 | −4.71 | <0.001 |
Fig. 5.
The influence of water table depth (above or below the soil surface) and vegetation cover on CH4 flux. Boxplots represent median (midline), quartiles (box), maximum and minimum (whisker) with outliers represented as black points. Grey points with error bars represent means with 95 % bootstrapped confidence intervals. For statistics see Table 2.
Discussion
All sites, representing a diversity of conditions given the high spatial heterogeneity, had positive CH4 flux across the entire experimental period, even the driest sites (water table about 24 cm below the surface) had relatively low emissions of <1.5 mg m-2 h−1. This is in contrast to some previous studies that have found CH4 uptake in dry soils due to oxic layers reducing CH4 production while promoting oxidation (Chen et al. 2014; Whalen and Reeburgh 1990). This was probably due to the substantial CH4 emission rates that occur during the growing season, in this nutrient rich, anaerobic environment, which is favourable to high rates of methanogenesis (Christensen et al. 2002; Grunfeld and Brix 1999; Harazono et al. 2006; Mastepanov et al. 2013; Morrissey and Livingston 1992; Sturtevant and Oechel 2013).
The most significant control on CH4 fluxes across all the sites was found to be GPP. This may suggest a dominant role of plant productivity on CH4 emissions, as higher plant productivity (i.e., higher GPP) is likely to stimulate CH4 emission by providing photosynthetically derived substrates for methanogenic processes (Harazono et al. 2006; Lai et al. 2014b). However those plots with the highest GPP also tended to have a greater percentage cover of vascular plants, meaning both substrate input and the provision of CH4 transport pathways may have increased simultaneously (Lai et al. 2014b; Shannon et al. 1996; Fig. 6). In comparison to mosses, vascular plants have a higher photosynthetic capacity and their substantial root exudation and litter input increase substrate availability for methane production (Olivas et al. 2011; Riutta et al. 2007). Furthermore, vascular plants play a critical role in the transport of CH4 from the soil (Joabsson et al. 1999; Noyce et al. 2014), which is a key limit on CH4 flux, where emissions can depend more on the transport than CH4 production itself (Born et al. 1990; Harazono et al. 2006). With an absence of vascular plants, within drier sites at the polygon rims, limitation of transport and/or substrate availability appeared to be of major relevance in suppressing CH4 emission to relatively low levels (Fig. 5 and Fig. 6). For this reason, very low CH4 emissions were observed with low vascular plant cover (<10 %) within dry oxic sites (Fig. 5) in comparison to wet sites at the polygon centre, where CH4 can diffuse directly from the surface water (Fig. 6). However, in sites with vascular plants present, CH4 was transported through plant stems, bypassing oxic soil layers where it would otherwise be re-oxidised by methanotrophs (Joabsson and Christensen 2001; Shannon et al. 1996; Fig. 6). Mechanistically, vascular plants act as a conduit for methanogenesis, connecting the CH4 produced at depth within the soil to the atmosphere, thereby enhancing the release of CH4 (von Fischer et al. 2010; Harazono et al. 2006; Joabsson and Christensen 2001; Sebacher et al. 1985; Shannon et al. 1996).
Fig 6.
CH4 exchange within arctic tundra. CH4 is transported to the atmosphere directly through diffusion from the soil and indirectly through the roots and stems of vascular plants. In opposition, CH4 oxidation is aided by O2 diffusion directly into the soil and root aeration
The ability of vascular plants to both transport CH4 and provide soil C for methanogenesis varies by species. For example, the presence of Eriophorum ssp (cotton grass) results in CH4 emissions between 1.4–2.2 and 3.7–5.5 times higher than the Maianthemum/Ledum and the shrub Chamaedaphne communities respectively (Lai et al. 2014a). The amount and extent of plant roots varies between vascular species, where deeper and wider root structures facilitate the increased production and release of CH4 from soil layers below the water table and closer to the permafrost layer (Harazono et al. 2006; Joabsson and Christensen 2001; Lai et al. 2014a; Shannon et al. 1996). However, we show in this study that the influence of vegetation on CH4 emissions is strongly dependent on the water level and this interaction must be taken into account when considering overall CH4 loss. With sparse vascular plant cover, wet sites tend to be higher CH4 emitters than dry sites (Fig. 5). On the other hand, in the presence of substantial vascular plant cover, both wet polygon troughs and dry oxic rims emitted substantial CH4 (Table 2, Fig. 5). This created local scale spatial variability within the ice wedge polygon landscape in relation to vascular plant community cover. It should be mentioned, however, that downward transportation of O2 into the soil by vascular plants can increase methane oxidation by methanotrophs, lowering CH4 emission (Frenzel and Rudolph 1998; Harazono et al. 2006; Heilman and Carlton 2001; Ström et al. 2005). This process, however, was likely to be less important in comparison to the enhancement of both CH4 transport and carbon (C) supply, and never resulted in an uptake of CH4, even within the driest sites during this study (Fig. 5). NEE was marginally significantly lower in the wetter sites, perhaps because plant productivity was promoted due to increased nutrient availability resulting from warmer temperatures in these soils (Nadelhoffer et al. 1991; Rustad et al. 2001). Hence the wet conditions which promote CH4 emission by causing anoxia may further promote CH4 emission by increasing GPP and vascular plant growth, which could promote both CH4 transport and substrate production (Joabsson et al. 1999; King et al. 2002; Rinnan et al. 2007; Ström et al. 2003).
Water table depth was the next most significant control on CH4 emission after GPP, with wet sites showing higher CH4 emission (Table 1, Fig. 3). This is consistent with other studies where site wetness has been found to be a strong driver of CH4 emission due to the high abundance of methanogens in anaerobic, waterlogged conditions (Bubier et al. 1993; von Fischer et al. 2010; Lai et al. 2014a; Moore and Roulet 1993; Roulet et al. 1992; Zona et al. 2009). Christensen et al. (2003) described water table as an ‘on-off switch’ controlling CH4 flux, while other factors control CH4 flux within water tables shallower than a certain threshold, above which site wetness governs CH4 emission. On the other hand, wet sites are not always found to be correlated with higher CH4 emission, for example Brown et al. (2014) found a critical zone for maximum rates of methanogenesis at 40 to 55 cm below the surface, which they speculated coincided with the maximum provision of fresh organic material and necessary redox potentials, in addition to facilitating the potential degassing of stored CH4. In our sites the water table was never below 33 cm, which may have explained the substantial CH4 losses in all of the sites measured here, including the driest (Fig. 5).
Interestingly, water table level determined the temperature dependence of CH4 emissions, as shown by the significant interaction of water table and soil temperatures on CH4 loss (Table 1, Fig. 4). Wetter peat soils tend to be warmer due to a higher heat capacity of water (Dunfield et al. 1993; Whiting and Chanton 1993). Generally, higher soil temperatures are expected to increase substrate availability and the abundance of methanogens in peat, and therefore CH4 emissions (Dunfield et al. 1993; Valentine et al. 1994). Increases in temperatures from 2 to 12 ° C have been correlated with an increase in CH4 emission by a factor of 6.7 (von Fischer et al. 2010; Svensson and Rosswall 1984). However, in the dry, oxic sites, CH4 oxidation occurs together with methanogenesis, and these two processes might cancel each other out resulting in the lack of a net increase in CH4 emissions with temperature increase (Lai et al. 2014a; Svensson and Rosswall 1984; Zhu et al. 2014). This result suggests the need to stratify the measurements in this highly polygonized tundra environment to be able to capture the different response of different microtopographic features, including both dry and wet sites.
In addition to water table, thaw depth has been found in other studies to be a key control on CH4 emission from tundra ecosystems (Nakano et al. 2000; Sturtevant and Oechel 2013; Verville et al. 1998; Zona et al. 2009). However, there was fairly low variability in thaw depths in this study (from 26 to 42 cm below surface level), partially because of the limited temporal range of sampling (from peak to late season) across sites, and this may have explained why it was not found to be significant in explaining CH4 fluxes. This contrasts with previous work within this region (Harazono et al. 2006; Morrissey and Livingston 1992; Torn and Chapin 1993; Zona et al. 2009, 2011) which showed thaw depth to be a critical control of fluxes over the growing season (but these studies included early as well as late season, resulting in a broader range of thaw depths). Within this acidic tundra, pH across the study sites presented a large variation (2.7–6.49) and yet did not significantly correlate with CH4 fluxes, however the highest CH4 emissions were observed at a pH of around 4.2. These unusually low pH values (2.7–3.4) were found in Ivotuk plot sites, where similar values (down to 2) have been previously recorded within a similar ecosystem (Lipson et al. 2012). Due to the particularly dry conditions, dry plot sites with low pH were probably more oxidised than usual (for example oxidation of Fe(II), S compounds and NH4 +) releasing protons and making these extreme soil pH values possible within localised areas of the tundra (Lipson, personal communication). In contrast, the few wet sites found with low pH had high proportions of peat accumulation and dense moss cover, mostly characterised by dwarf shrub and acidophilic mosses that further secrete organic acids during growth (Gornall et al. 2007; Hobbie and Gough 2004; Riedel et al. 2005). Variable responses of CH4 emissions on soil pH have been previously reported in field studies ranging from no correlation (Brummell et al. 2012; Ohtsuka et al. 2006), to positive correlations (Moore et al. 1990) and negative correlations (Kato et al. 2011; Walker et al. 1998).
In our study, CH4 fluxes ranged from 0.01 to 20 mg C CH4 across a spatially dynamic environment, with wetter sites with higher GPP having higher emission. This high spatial variability, where CH4 emissions can vary by an order of magnitude between different plots, has consistently been found across other studies where daily averages can range some 4.5 to 9.6 mg C CH4 m−2 d−1, across consecutive measurements within the same sites (Harazono et al. 2006; Schimel 1995; Shannon et al. 1996; Wille et al. 2008). The general scarcity of data on the plot scale from these arctic environments limits our understanding of the controls over this large variability in CH4 fluxes, where fine scale datasets are critical for increasing our understanding of the smaller scale landscape heterogeneity (Sachs et al. 2010). Fine-scale relationships between CH4 fluxes, vegetation and environmental conditions might be missed by eddy covariance measurements measuring C fluxes over a wider scale in these highly heterogeneous arctic ecosystems (Fox et al. 2008; Sachs et al. 2010; Wickland et al. 2006). Therefore our results, as measured by chambers, might prove very useful for identifying the detailed relationship between environmental and vegetation controls, namely GPP, water table depth and soil temperature, and for describing how fluxes relate to fine scale microtography.
Conclusion
In this study we showed that multiple complex processes driving CH4 flux emissions within the wet sedge and tussock tundra ecosystems interacted with each other in controlling CH4 flux. Crucially we have demonstrated the importance of vascular plant cover in determining CH4 flux and that increased vascular plant cover can promote CH4 production and release from both waterlogged and drier soils. The most important environmental control on CH4 emissions within our study locations was GPP. Vascular plant coverage seemed to be the factor most correlated with CH4 emissions within dry sites, highlighting the importance of CH4 oxidation and potentially labile C availability in controlling emissions from the high-centre polygons and rims. In these dry sites, greater vascular plant cover increased CH4 emission by almost an order of magnitude to levels equivalent of wet sites. Overall, given the importance of vascular plant cover on CH4 emissions, hydrological changes in the Arctic might affect CH4 emissions very differently depending on the plant communities present and how they develop under a changing climate.
Acknowledgements
This work was funded by the Office of Polar Programs of the National Science Foundation (NSF) (award no. 1204263) and would not have been possible without logistical support funded by the NSF Office of Polar Programs. Additional funding was provided by the COST Action ABBA, ES0804, Short Term Scientific Missions (STSM), SURE studentship, NERC Arctic Research Programme, CYCLOPS Grant (NE/K00025X/1), and a Righ Foundation Research Scholarship. Preparation of this paper was supported by the Carbon in Arctic Reservoirs Vulnerability Experiment (CARVE), an Earth Ventures (EV-1) investigation, under contract with the National Aeronautics and Space Administration and by NSF grant (award n. 1204263). This research was conducted on land owned by the Ukpeagvik Inupiat Corporation (UIC). We would like to thank Professor W. Oechel together with the Global Change Research Group at San Diego State University in particular Virginie Moreaux, Patrick Murphy, Salvatore Losacco, Eric Wilkman and Herbert Mbufong Njuabe for their invaluable instruction, guidance and support throughout the fieldwork and initial data analysis and Dr. Gareth Phoenix for helpful comments on an earlier version of this paper. We would also like to thank Michael Berger for his assistance in the field, and UMIAQ and UIC for logistical support.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Armstrong J, Armstrong W. A convective through-flow of gases in Phragmites australis (Cav) Trin ex Steud. Aquat Bot. 1991;39:75–88. doi: 10.1016/0304-3770(91)90023-X. [DOI] [Google Scholar]
- Bhullar GS, Edwards PJ, Olde Venterink H. Variation in the plant-mediated methane transport and its importance for methane emission from intact wetland peat mesocosms. J Plant Ecol. 2013;6:298–304. doi: 10.1093/jpe/rts045. [DOI] [Google Scholar]
- Bhullar GS, Irabani M, Edwards PJ, Olde Venterink H (2013b) Methane transport and emissions from soil as affected by water table and vascular plants. SMC Ecol 13 [DOI] [PMC free article] [PubMed]
- Billings WD. The environmental complex in relation to plant growth and distribution. Q Rev Biol. 1952;27:251–265. doi: 10.1086/399022. [DOI] [PubMed] [Google Scholar]
- Billings WD, Peterson KM. Vegetational change and ice-wedge polygons through the thaw-lake cycle in Arctic Alaska. Arctic Alpine Res. 1980;12:413–432. doi: 10.2307/1550492. [DOI] [Google Scholar]
- Billings WD, Luken JO, Mortensen DA, Peterson KM. Arctic tundra: A source or sink for atmospheric carbon dioxide in a changing environment? Oecologia. 1982;53:7–11. doi: 10.1007/BF00377129. [DOI] [PubMed] [Google Scholar]
- Blodau C, Basiliko N, Moore TR. Carbon turnover in peatland mesocosms exposed to different water table levels. Biogeochemistry. 2004;67:331–351. doi: 10.1023/B:BIOG.0000015788.30164.e2. [DOI] [Google Scholar]
- Bloom AA, Palmer PI, Fraser A, Reay DS, Frankenberg C. Large-scale controls of methanogenesis inferred from methane and gravity spaceborne data. Science. 2010;327:322–325. doi: 10.1126/science.1175176. [DOI] [PubMed] [Google Scholar]
- Born M, Doerr H, Levin I. Methane consumption in aerated soils of the temperate zone. Tellus B. 1990;42:2–8. doi: 10.1034/j.1600-0889.1990.00002.x. [DOI] [Google Scholar]
- Britton ME. Vegetation of the arctic tundra. In: Hansen HP, editor. Arctic Biology. Corvallis: Oregon State University Press; 1957. pp. 26–72. [Google Scholar]
- Brown MG, Humphreys ER, Moore TR, Roulet NT, Lafleur PM. Evidence for a nonmonotonic relationship between ecosystem-scale peatland methane emissions and water table depth. J Geophys Res-Biogeo. 2014;119:826–835. doi: 10.1002/2013JG002576. [DOI] [Google Scholar]
- Brummell ME, Farrell RE, Siciliano SD. Greenhouse gas soil production and surface fluxes at a high arctic polar oasis. Soil Biol Biochem. 2012;52:1–12. doi: 10.1016/j.soilbio.2012.03.019. [DOI] [Google Scholar]
- Bubier JL, Moore TR, Roulet NT. Methane Emissions from Wetlands in the Midboreal Region of Northern Ontario, Canada. Ecology. 1993;74:2240–225. doi: 10.2307/1939577. [DOI] [Google Scholar]
- Budishchev A, Mi Y, van Huissteden J, Belelli-Marchesini L, Schaepman-Strub G, Parmentier FJW, Fratini G, Gallagher A, Maximov TC, Dolman AJ. Evaluation of a plot scale methane emission model at the ecosystem scale using eddy covariance observations and footprint modelling. Biogeosciences Discuss. 2014;11:3927–3961. doi: 10.5194/bgd-11-3927-2014. [DOI] [Google Scholar]
- Callaghan TV, Bergholm F, Christensen TR, Jonasson C, Kokfelt U, Johansson M (2010) A new climate era in the sub‐Arctic: Accelerating climate changes and multiple impacts. Geophys Res Lett 37
- Cao M, Marshall S, Gregson K. Global carbon exchange and methane emissions from natural wetlands: Application of a process-based model. J. Geophys. Res. 1996;101:14,399–14,414. doi: 10.1029/96JD00219. [DOI] [Google Scholar]
- CAVM Team Mapping, Walker DA, Trahan GT (2003) Circumpolar Arctic Vegetation. US Fish and Wildlife Service
- Chen Q, Zhu R, Wang Q, Xu H. Methane and nitrous oxide fluxes from four tundra ecotopes in Ny-Alesund of the High Arctic. J Environ Sci-China. 2014;26:1403–1410. doi: 10.1016/j.jes.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Christensen TR, Prentice IC, Kaplan J, Haxeltine A, Sitch S. Methane flux from northern wetlands and tundra. Tellus B. 2002;48:652–661. doi: 10.1034/j.1600-0889.1996.t01-4-00004.x. [DOI] [Google Scholar]
- Christensen TR, Ekberg A, Ström L, Mastepanov M, Panikov N, Öquist M, Svensson BH, Nykänen H, Martikainen PJ, Oskarsson H. Factors controlling large scale variations in methane emissions from wetlands. Geophys. Res. Lett. 2003;30:1414. doi: 10.1029/2002GL016848. [DOI] [Google Scholar]
- Conrad R (1996) Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol Rev 60:609–640 [DOI] [PMC free article] [PubMed]
- Couwenberg J, Thiele A, Tanneberger F, Augustin J, Barisch S, Dubovik D, Liashchynskaya N, Michaelis D, Minke M, Skuratovich A, Joosten H. Assessing greenhouse gas emissions from peatlands using vegetation as a proxy. Hydrobiologia. 2011;674:67–89. doi: 10.1007/s10750-011-0729-x. [DOI] [Google Scholar]
- Crawley MJ. The R Book. Chichester: J Wiley & Sons; 2012. [Google Scholar]
- Dias ATC, Hoorens B, Van Logtestijn RSP, Vermaat JE, Aerts R. Plant species composition can be used as a proxy to predict methane emissions in peatland ecosystems after land-use changes. Ecosystems. 2010;13:526–538. doi: 10.1007/s10021-010-9338-1. [DOI] [Google Scholar]
- Dlugokencky EJ, Nisbet EG, Fisher R, Lowry D. Global atmospheric methane: budget, changes and dangers. Philos T Roy Soc A. 2011;369:2058–2072. doi: 10.1098/rsta.2010.0341. [DOI] [PubMed] [Google Scholar]
- Dunfield P, Dumont R, Moore T. Methane production and consumption in temperate and subarctic peat soils: response to temperature and pH. Soil Biol Biochem. 1993;25:321–326. doi: 10.1016/0038-0717(93)90130-4. [DOI] [Google Scholar]
- Fox AM, Huntley B, Lloyd CR, Williams M, Baxter R. Net ecosystem exchange over heterogeneous Arctic tundra: Scaling between chamber and eddy covariance measurements. Global Biogeochem Cy. 2008;22:GB2027. doi: 10.1029/2007GB003027. [DOI] [Google Scholar]
- Frenzel P, Karofeld E. CH4 emission from a hollow-ridge complex in a raised bog: The role of CH4 production and oxidation. Biogeochemistry. 2000;51:91–112. doi: 10.1023/A:1006351118347. [DOI] [Google Scholar]
- Frenzel P, Rudolph J (1998) Methane emission from a wetland plant: the role of CH4 oxidation in Eriophorum. Plant Soil 202:27–32
- Gornall JL, Jónsdóttir IS, Woodin SJ, Van der Wal R. Arctic mosses govern below-ground environment and ecosystem processes. Oecologia. 2007;153:931–941. doi: 10.1007/s00442-007-0785-0. [DOI] [PubMed] [Google Scholar]
- Greenup AL, Bradford MA, McNamara NP, Ineson P, Lee JA (2000) The role of Eriophorum vaginatum in CH4 flux from an ombrotrophic peatland. Plant Soil 227:265–272
- Grünfeld S, Brix H. Methanogenesis and methane emissions: effects of water table, substrate type and presence of Phragmites australis. Aquat Bot. 1999;64:63–75. doi: 10.1016/S0304-3770(99)00010-8. [DOI] [Google Scholar]
- Harazono Y, Mano M, Miyata A, Yoshimoto M, Zulueta RC, Vourlitis GL, Kwon H, Oechel W. Temporal and spatial differences of methane flux at arctic tundra in Alaska. Natl Inst. Polar Res, Spec. Issue. 2006;59:79–95. [Google Scholar]
- Harriss RC, Frolking SE. The sensitivity of methane emissions from northern freshwater wetlands to global warming. Springer New York: Global Climate Change and Freshwater Ecosystems; 1992. pp. 48–67. [Google Scholar]
- Heilman MA, Carlton RG. Methane oxidation associated with submersed vascular macrophytes and its impact on plant diffusive methane flux. Biogeochemistry. 2001;52:207–224. doi: 10.1023/A:1006427712846. [DOI] [Google Scholar]
- Hill TC, Stoy PC, Baxter R, Clement R, Disney M, Evans J, Fletcher B, Gornall J, Harding R, Hartley IP, Ineson P, Moncrieff J, Phoenix G, Sloan V, Poyatos R, Prieto-Blanco A, Subke J, Street L, Wade TJ, Wookey P, Williams MD (2009) The Sub-Arctic Carbon Cycle: Assimilating Multi-Scale Chamber, Tower and Aircraft Flux Observations into Ecological Models. American Geophysical Union, Fall Meeting 2009, abstract #B33B-0394
- Hinzman LD, Bettez ND, Bolton WR, Chapin FS, Dyurgerov MB, Fastie CL, Griffith B, Hollister RD, Hope A, Huntington HP, Jensen AM, Jia GJ, Jorgenson T, Kane DL, Klein DR, Kofinas G, Lynch AH, Lloyd AH, McGuire AD, Nelson FE, Oechel WC, Osterkamp TE, Racine CH, Romanovsky VE, Stone RS, Stow DA, Sturm M, Tweedie CE, Vourlitis GL, Walker MD, Walker DA, Webber PJ, Welker JM, Winker KS, Yoshikawa K. Evidence and implications of recent climate change in northern Alaska and other arctic regions. Climatic Change. 2005;72:251–298. doi: 10.1007/s10584-005-5352-2. [DOI] [Google Scholar]
- Hinzman LD, Deal CJ, McGuire AD, Mernild SH, Polyakov IV, Walsh JE. Trajectory of the Arctic as an integrated system. Ecol Appl. 2013;23:1837–1868. doi: 10.1890/11-1498.1. [DOI] [PubMed] [Google Scholar]
- Hobbie SE, Gough L. Litter decomposition in moist acidic and non-acidic tundra with different glacial histories. Oecologia. 2004;140:113–124. doi: 10.1007/s00442-004-1556-9. [DOI] [PubMed] [Google Scholar]
- Inubushi K, Sugii H, Nishino S, Nishino E. Effect of aquatic weeds on methane emission from submerged paddy soil. Am J Bot. 2001;88:975–9. doi: 10.2307/2657078. [DOI] [PubMed] [Google Scholar]
- IPCC . IPCC climate Change 2013: The Physical Science Basis. Cambridge, United Kingdom and New York, NY, USA: Cambridge University Press; 2013. [Google Scholar]
- Jespersen DN, Sorrell BK, Brix H. Growth and root oxygen release by Typha latifolia and its effects on sediment methanogenesis. Aquat Bot. 1998;61:165–80. doi: 10.1016/S0304-3770(98)00071-0. [DOI] [Google Scholar]
- Joabsson A, Christensen TR. Methane emissions from wetlands and their relationship with vascular plants: an Arctic example. Glob Change Biol. 2001;7:919–932. doi: 10.1046/j.1354-1013.2001.00044.x. [DOI] [Google Scholar]
- Joabsson A, Christensen TR, Walleén B. Vascular plant controls on methane emissions from northern peatforming wetlands. Trends Ecol Evol. 1999;14:385–388. doi: 10.1016/S0169-5347(99)01649-3. [DOI] [PubMed] [Google Scholar]
- Jones WJ, Nagle DP, Whitman WB. Methanogens and the diversity of archaebacteria. Microbiol Rev. 1987;51:135–177. doi: 10.1128/mr.51.1.135-177.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Hirota M, Tang Y, Wada E (2011) Spatial variability of CH4 and N2O fluxes in alpine ecosystems on the Qinghai-Tibetan Plateau. Atmos Environ 45:5632–5639
- King JY, Reeburgh WS, Regli SK. Methane emission and transport by arctic sedges in Alaska: Results of a vegetation removal experiment. J. Geophys. Res.-Atmos. 1998;103:29083–29092. doi: 10.1029/98JD00052. [DOI] [Google Scholar]
- King JY, Reeburgh WS, Thieler KK, Kling GW, Loya WM, Johnson LC, Nadelhoffer KJ. Pulse-labeling studies of carbon cycling in Arctic tundra ecosystems: the contribution of photosynthates to methane emission. Global Biogeochem Cy. 2002;16:10–1. doi: 10.1029/2001GB001456. [DOI] [Google Scholar]
- Klapstein SJ, Turetsky MR, McGuire AD, Harden JW, Czimczik CL, Xu X, Chanton JP, Waddington JM. Controls on methane released through ebullition in peatlands affected by permafrost degradation. J. Geophys. Res.- Biogeo. 2014;119:418–431. doi: 10.1002/2013JG002441. [DOI] [Google Scholar]
- Kutzbach L, Wagner D, Pfeiffer EM (2004) Effect of microrelief and vegetation on methane emission from wet polygonal tundra, Lena Delta, Northern Siberia. Biogeochemistry 69:341–362
- Kwon HJ, Oechel WC, Zulueta RC, Hastings SJ (2006) Effects of climate variability on carbon sequestration among adjacent wet sedge tundra and moist tussock tundra ecosystems. J Geophys Res-Biogeosciences 111
- Lai DYF. Methane dynamics in northern peatlands: a review. Pedosphere. 2009;19:409–421. doi: 10.1016/S1002-0160(09)00003-4. [DOI] [Google Scholar]
- Lai DYF, Moore TR, Roulet NT. Spatial and temporal variations of methane flux measured by autochambers in a temperate ombrotrophic peatland. J. Geophys. Res.-Biogeo. 2014;119:864–880. doi: 10.1002/2013JG002410. [DOI] [Google Scholar]
- Lai DYF, Roulet NT, Moore TR (2014b) The spatial and temporal relationships between CO2 and CH4 exchange in a temperate ombrotrophic bog. Atmos Environ 89:249–259
- Le Mer J, Roger P. Production, oxidation, emission and consumption of methane by soils: A review. Eur J Soil Biol. 2001;37:25–50. doi: 10.1016/S1164-5563(01)01067-6. [DOI] [Google Scholar]
- Lipson DA, Zona D, Raab TK, Bozzolo F, Mauritz M, Oechel WC. Water-table height and microtopography control biogeochemical cycling in an Arctic coastal tundra ecosystem. Biogeociences. 2012;9:577–591. doi: 10.5194/bg-9-577-2012. [DOI] [Google Scholar]
- Mastepanov M, Sigsgaard C, Tagesson T, Ström L, Tamstorf MP, Lund M, Christensen TR. Revisiting factors controlling methane emissions from high- Arctic tundra. Biogeosciences. 2013;10:5139–5158. doi: 10.5194/bg-10-5139-2013. [DOI] [Google Scholar]
- Melton JR, Wania R, Hodson EL, Poulter B, Ringeval B, Spahni R, Bohn T, Avis CA, Beerling DJ, Chen G, Eliseev AV, Denisov SN, Hopcroft PO, Lettenmaier DP, Riley WJ, Singarayer JS, Subin ZM, Tian H, Zürcher S, Brovkin V, van Bodegom PM, Kleinen T, Yu ZC, Kaplan JO. Present state of global wetland extent and wetland methane modelling: conclusions from a model inter-comparison project (WETCHIMP) Biogeosciences. 2013;10:753–788. doi: 10.5194/bg-10-753-2013. [DOI] [Google Scholar]
- Moore TR, Roulet NT. Methane flux: water table relations in northern wetlands. Geophys Res Lett. 1993;20:587–590. doi: 10.1029/93GL00208. [DOI] [Google Scholar]
- Moore T, Roulet N, Knowles R. Spatial and temporal variations of methane flux from subarctic/northern boreal fens. Global Biogeochem Cy. 1990;4:29–46. doi: 10.1029/GB004i001p00029. [DOI] [Google Scholar]
- Moosavi SC, Crill PM (1997) Controls on CH4 and CO2 emissions along two moisture gradients in the Canadian boreal zone. J Geophys Res-Atmos 102:29261–29277
- Morrissey LA, Livingston GP. Methane emissions from Alaska arctic tundra – assessment of local spatial variability. J Geophys Res-Atmos. 1992;97:16661–16670. doi: 10.1029/92JD00063. [DOI] [Google Scholar]
- Nadelhoffer KJ, Giblin AE, Shaver GR, Laundre JA. Effects of temperature and substrate quality on element mineralization in six Arctic soils. Ecol. 1991;72:242–253. doi: 10.2307/1938918. [DOI] [Google Scholar]
- Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Method Ecol Evol. 2013;4:133–142. doi: 10.1111/j.2041-210x.2012.00261.x. [DOI] [Google Scholar]
- Nakano T, Kuniyoshi S, Fukuda M. Temporal variation in methane emission from tundra wetlands in a permafrost area, northeastern Siberia. Atmos Environ. 2000;34:1205–1213. doi: 10.1016/S1352-2310(99)00373-8. [DOI] [Google Scholar]
- Noyce GL, Varner RK, Bubier JL, Frolking S. Effect of Carex rostrata on seasonal and interannual variability in peatland methane emissions. J Geophys Res-Biogeo. 2014;199:24–34. doi: 10.1002/2013JG002474. [DOI] [Google Scholar]
- Oberbauer SF, Tweedie CE, Welker JM, Fahnestock JT, Henry GHR, Webber PJ, Hollister RD, Walker MD, Kuchy A, Elmore E, Starr G (2007) Tundra CO2 fluxes in response to experimental warming across latitudinal and moisture gradients. Ecol Monogr 77:221–238
- Oechel WC, Vourlitis GL, Hastings SJ, Bochkarev SA (1995) Change in Arctic CO2 Flux Over Two Decades: Effects of Climate Change at Barrow, Alaska. Ecol Appl 5:846–855
- Oechel WC, Vourlitis GL, Brooks S, Crawford TL, Dumas E (1998) Intercomparison among chamber, tower and aircraft net CO2 and energy fluxes measured during the Arctic System Science Land-Atmosphere-Ice Interactions (ARCSS-LAII) flux study. J Geophys Res 103:28933–29003
- Ohtsuka T, Adachi M, Uchida M, Nakatsubo T. Relationships between vegetation types and soil properties along a topographical gradient on the northern coast of the Brøgger Peninsula, Svalbard. Pol Biosci. 2006;19:63–72. [Google Scholar]
- Olefeldt D, Turetsky MR, Crill PM, McGuire AD. Environmental and physical controls on northern terrestrial methane emissions across permafrost zones. Global Change Biol. 2013;19:589–603. doi: 10.1111/gcb.12071. [DOI] [PubMed] [Google Scholar]
- Olivas PC, Oberbauer SF, Tweedie C, Oechel WC, Lin D, Kuchy A. Effects of Fine-Scale Topography on CO2 Flux Components of Alaskan Coastal Plain Tundra: Response to Contracting Growing Seasons. Arct Antarct Alpine Res. 2011;43:256–266. doi: 10.1657/1938-4246-43.2.256. [DOI] [Google Scholar]
- R Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- Raynolds MK, Walker DA, Maier HA. Plant community-level mapping of arctic Alaska based on the Circumpolar Arctic Vegetation. Phytocoenologia. 2005;35:821–848. doi: 10.1127/0340-269X/2005/0035-0821. [DOI] [Google Scholar]
- Riedel SM, Epstein HE, Walker DA, Richardson DL, Calef MP, Edwards E, Moody A. Spatial and temporal heterogeneity of vegetation properties among four tundra plant communities at Ivotuk, Alaska, U.S.A. Arct Antarct Alp Res. 2005;37:25–33. doi: 10.1657/1523-0430(2005)037[0025:SATHOV]2.0.CO;2. [DOI] [Google Scholar]
- Rinnan R, Michelsen A, Bååth E, Jonasson S. Mineralization and carbon turnover in subarctic heath soil as affected by warming and additional litter. Soil Biol Biochem. 2007;39:2014–3023. doi: 10.1016/j.soilbio.2007.02.017. [DOI] [Google Scholar]
- Riutta T, Laine J, Tuittila ES. Sensitivity of CO2 exchange of fen ecosystem components to water level variation. Ecosy. 2007;10:718–733. doi: 10.1007/s10021-007-9046-7. [DOI] [Google Scholar]
- Romanovsky VE, Sergueev DO, Osterkamp TE (2003) Temporal variations in the active layer and near-surface permafrost temperatures at the long-term observatories in northern Alaska. Permafrost, Phillips, Springman & Arenson (eds) Swets & Zeitlinger, Lisse, ISBN 90 5809 582 7 1: 989-994
- Roulet NT, Ash R, Moore TR. Low boreal wetlands as a source of atmospheric methane. J Geophys Res-Atmos. 1992;97:3739–3749. doi: 10.1029/91JD03109. [DOI] [Google Scholar]
- Rustad LE, Campbell JL, Marion GM, Norby RJ, Mitchell MJ, Hartley AE, Cornelissen JHC, Gurevitch J, GCTE-NEWS A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Ocecologia. 2001;126:543–562. doi: 10.1007/s004420000544. [DOI] [PubMed] [Google Scholar]
- Sachs T, Giebel M, Boiker J, Kutzbach L. Environmental controls on CH4 emission from polygonal tundra on the microsite scale in the Lena river delta, Siberia. Glob Change Biol. 2010;16:3096–3110. [Google Scholar]
- Schimel JP. Plant-transport and methane production as controls on methane flux from arctic wet meadow tundra. Biogeochemistry. 1995;28:183–200. doi: 10.1007/BF02186458. [DOI] [Google Scholar]
- Sebacher DI, Harriss RC, Bartlett KB. Methane emissions to the atmosphere through aquatic plants. J Environ Qual. 1985;14:40–46. doi: 10.2134/jeq1985.00472425001400010008x. [DOI] [Google Scholar]
- Sebacher DI, Harriss RC, Bartlett KB, Sebacher SM, Grice SS. Atmospheric methane sources: Alaskan tundra bogs, an alpine fen and a subarctic boreal marsh. Tellus B. 1986;38:1–10. doi: 10.1111/j.1600-0889.1986.tb00083.x. [DOI] [Google Scholar]
- Shannon RD, White JR, Lawson JE, Gilmour BS. Methane efflux from emergent vegetation in peatlands. J Ecol. 1996;84:239–246. doi: 10.2307/2261359. [DOI] [Google Scholar]
- Singh SN. Exploring correlation between redox potential and other edaphic factors in field and laboratory conditions in relation to methane efflux. Environ Int. 2001;27:265–274. doi: 10.1016/S0160-4120(01)00055-1. [DOI] [PubMed] [Google Scholar]
- Ström L, Ekberg A, Mastepanov M, Christensen TR. The effect of vascular plants on carbon turnover and methane emissions from a tundra wetland. Global Change Biol. 2003;9:1185–1192. doi: 10.1046/j.1365-2486.2003.00655.x. [DOI] [Google Scholar]
- Ström L, Mastepanov M, Christensen TR. Species-specific effects of vascular plants on carbon turnover and methane emissions from wetlands. Biogeochemistry. 2005;75:65–82. doi: 10.1007/s10533-004-6124-1. [DOI] [Google Scholar]
- Ström L, Tagesson T, Mastepanov M, Christensen TR. Presence of Eriophorum scheuchzeri enhances substrate availability and methane emission in an Arctic wetland. Soil Biol Biochem. 2012;45:61–70. doi: 10.1016/j.soilbio.2011.09.005. [DOI] [Google Scholar]
- Sturtevant CS, Oechel WC (2013) Spatial variation in landscape-level CO2 and CH4 fluxes from arctic coastal tundra: influence from vegetation, wetness and the thaw lake cycle. Global Change Biol 19:2853–2866 [DOI] [PubMed]
- Svensson BH, Rosswall T. In situ methane production from acid peat in plant communities with different moisture regimes in a subarctic mire. Oikos. 1984;43:341–350. doi: 10.2307/3544151. [DOI] [Google Scholar]
- Torn MS, Chapin FS. Environmental and biotic controls over methane flux from arctic tundra. Chemosphere. 1993;26:357–68. doi: 10.1016/0045-6535(93)90431-4. [DOI] [Google Scholar]
- Valentine DW, Holland EA, Schimel DS. Ecosystem and physiological controls over methane production in northern wetlands. J Geophys Res. 1994;99:1563–1571. doi: 10.1029/93JD00391. [DOI] [Google Scholar]
- Verville JH, Hobbie SE, Chapin FS, Hooper DU (1998) Response of tundra CH4 and CO2 flux to manipulation of temperature and vegetation. Biogeochemistry 41:215–235
- von Fischer JC, Rhew RC, Ames GM, Fosdick BK, von Fischer PE (2010) Vegetation height and other controls of spatial variability in methane emissions from the Arctic coastal tundra at Barrow, Alaska. J. Geophys. Res.-Biogeos 115
- von Fischer JC, Hedin LO (2007) Controls on soil methane fluxes: Tests of biophysical mechanisms using stable isotope tracers. Global Biogeochem Cy 21
- Vourlitis GL, Oechel WC. The role of northern ecosystems in the global methane budget. Global Change and Arctic Terrestrial Ecosystems. Ecol Stud. 1997;124:266–289. doi: 10.1007/978-1-4612-2240-8_15. [DOI] [Google Scholar]
- Vourlitis GL, Oechel WC, Hastings SJ, Jenkins MA (1993) A System for Measuring in situ CO2 and CH4 Flux in Unmanaged Ecosystems: An Arctic Example. Funct Ecol 7:369–379
- Walker DA, Auerbach NA, Bockheim JG, Chapin FS, Eugster W, King JY, McFadden JP, Michaelson GJ, Nelson FE, Oechel WC, Ping CL, Reeburg WS, Regli S, Shiklomanov NI, Vourlitis GL. Energy and trace-gas fluxes across a soil pH boundary in the Arctic. Nature. 1998;394:469–472. doi: 10.1038/28839. [DOI] [Google Scholar]
- Walker DA, Raynolds MK, Daniëls FJA, Einarsson E, Elvebakk A, Gould WA, Katenin AE, Kholod SS, Markon CJ, Melnikov ES, Moskalenko NG, Talbot SS, Yurtsev BA, the other members of the CAVM Team The circumpolar Arctic vegetation map. J Veg Sci. 2005;16:267–282. doi: 10.1111/j.1654-1103.2005.tb02365.x. [DOI] [Google Scholar]
- Walter BP, Heimann M. A process-based climate-sensitive model to derive methane emissions from natural wetlands: Application to five wetland sites, sensitivity to model parameters, and climate. Global Biogeochem Cy. 2000;14:745–765. doi: 10.1029/1999GB001204. [DOI] [Google Scholar]
- Walter KM, Zimov SA, Chanton JP, Verbyla D, Chapin FS. Methane bubbling from Siberian thaw lakes as a positive feedback to climate warming. Nature. 2006;443:71–75. doi: 10.1038/nature05040. [DOI] [PubMed] [Google Scholar]
- Whalen SC (2005) Biogeochemistry of methane exchange between natural wetlands and the atmosphere. Environ Eng Sci 22
- Whalen SC, Reeburgh WS. Consumption of atmospheric methane by tundra soils. Nature. 1990;346:160–162. doi: 10.1038/346160a0. [DOI] [Google Scholar]
- Whalen SC, Reeburgh WS. Interannual variations in tundra methane emission a 4-year time series at fixed sites. Global Biogeochem Cy. 1992;6:139–159. doi: 10.1029/92GB00430. [DOI] [Google Scholar]
- Whiting GJ, Chanton JP. Primary production control methane emission from wetlands. Nature. 1993;364:794–795. doi: 10.1038/364794a0. [DOI] [Google Scholar]
- Wickland KP, Striegl RG, Neff JC, Sachs T (2006) Effects of permafrost melting on CO2 and CH4 exchange of a poorly drained black spruce lowland. J Geophys Res- Biogeosciences 111:G2
- Wille C, Kutzbach L, Sachs T, Wagner D, Pfeiffer E. Methane emission from Siberian arctic polygonal tundra: eddy covariance measurements and modeling. Global Change Biol. 2008;14:1395–1408. doi: 10.1111/j.1365-2486.2008.01586.x. [DOI] [Google Scholar]
- Wilson KS, Humphreys ER. Carbon dioxide and methane fluxes from Arctic mudboils. Can J Soil Sci. 2010;90:441–449. doi: 10.4141/CJSS09073. [DOI] [Google Scholar]
- Yoshitake S, Uchida M, Koizumi H, Nakatsubo T. Carbon and nitrogen limitation of the soil microbial respiration in a High Arctic successional glacier foreland near Ny-Ålesund, Svalbard. Polar Res. 2007;26:22–30. doi: 10.1111/j.1751-8369.2007.00001.x. [DOI] [Google Scholar]
- Zhu RB, Ma DW, Xu H (2014) Summertime N2O, CH4 and CO2 exchanges from a tundra marsh and an upland tundra in maritime Antarctica. Atmos Environ 83:269–281
- Zona D, Oechel WC, Kochendorfer J, Paw U, Salyuk AN, Olivas PC, Oberbauer SF, Lipson DA (2009) Methane fluxes during the initiation of a large-scale water table manipulation experiment in the Alaskan Arctic tundra. Global Biogeochem Cy 23
- Zona D, Lipson DA, Zulueta RC, Oberbauer SF, Oechel WC (2011) Micro-topographic controls on ecosystem functioning in the Arctic Coastal Plain. J Geophys Res 116
- Zona D, Lipson DA, Paw U, Kyaw T, Oberbauer SF, Olivas P, Gioli B, Oechel WC (2012) Increased CO2 loss from vegetated drained lake tundra ecosystems due to flooding. Global Biogeochem Cy 26