Abstract
Purpose
Arthralgia occurs in up to 50% of breast cancer survivors treated with aromatase inhibitors (AIs) and is the most common reason for poor AI adherence. We conducted, in 121 breast cancer survivors receiving an AI and reporting arthralgia, a yearlong randomized trial of the impact of exercise versus usual care on arthralgia severity.
Patients and Methods
Eligibility criteria included receiving an AI for at least 6 months, reporting ≥ 3 of 10 for worst joint pain on the Brief Pain Inventory (BPI), and reporting < 90 minutes per week of aerobic exercise and no strength training. Participants were randomly assigned to exercise (150 minutes per week of aerobic exercise and supervised strength training twice per week) or usual care. The BPI, Western Ontario and McMaster Universities Osteoarthritis (WOMAC) index, and Disabilities of the Arm, Shoulder and Hand (DASH) questionnaire were completed at baseline and at 3, 6, 9, and 12 months. Intervention effects were evaluated using mixed-model repeated measures analysis, with change at 12 months as the primary end point.
Results
Over 12 months, women randomly assigned to exercise (n = 61) attended 70% (± standard deviation [SD], 28%) of resistance training sessions and increased their exercise by 159 (± SD, 136) minutes per week. Worst joint pain scores decreased by 1.6 points (29%) at 12 months among women randomly assigned to exercise versus a 0.2-point increase (3%) among those receiving usual care (n = 60; P < .001). Pain severity and interference, as well as DASH and WOMAC pain scores, also decreased significantly at 12 months in women randomly assigned to exercise, compared with increases for those receiving usual care (all P < .001).
Conclusion
Exercise led to improvement in AI-induced arthralgia in previously inactive breast cancer survivors.
INTRODUCTION
Guidelines recommend that postmenopausal women with hormone receptor–positive breast cancer receive an aromatase inhibitor (AI) as part of their breast cancer treatment.1–4 However, adverse effects often result in poor AI adherence, with up to 50% of patients not taking AIs as prescribed and discontinuation rates of 20% within the first year of use.5–7 Both nonadherence and early discontinuation of AIs have been shown to be independent predictors of mortality.8
Arthralgia, defined as pain or stiffness in the joints, is the most common reason for poor AI adherence and drug discontinuation5–7 and is reported in up to 50% of patients with breast cancer within 6 months of starting AI therapy.9,10 There are few data regarding effective treatment of AI-induced arthralgia.
Exercise may improve AI-induced arthralgia, because it has been shown to be beneficial for osteoarthritis.11 Exercise may also have beneficial effects on disease-free survival and quality of life, which are also adversely affected by AI therapy.12,13 To our knowledge, no trial has examined the effect of exercise on AI-associated arthralgia in breast cancer survivors. The purpose of the HOPE (Hormones and Physical Exercise) study was to examine the effect of an exercise intervention on severity of AI-induced arthralgia in women receiving AIs and experiencing arthralgia.
PATIENTS AND METHODS
Our study was a randomized trial comparing the impact of a 12-month exercise intervention versus usual care (control) on AI-induced arthralgia. All procedures, including written informed consent, were approved by the Yale School of Medicine Human Investigation Committee and Connecticut Department of Public Health Human Investigation Committee.
Participants and Recruitment
Breast cancer survivors were recruited between June 1, 2010, and December 30, 2012, from five hospitals in Connecticut through the Rapid Case Ascertainment Shared Resource of the Yale Cancer Center, a field arm of the Connecticut Tumor Registry. Eligible participants were physically inactive (< 90 minutes per week of physical activity in past 6 months and no strength training in past year), postmenopausal women diagnosed 0.5 to 4.0 years before enrollment with hormone receptor–positive stage I to III breast cancer who had been receiving an AI for at least 6 months. Participants had to have been experiencing arthralgia for at least 2 months that were at least mild in severity (ie, score of ≥ 3 of 10 for worst pain item of Brief Pain Inventory [BPI]).14 Women were eligible if their arthralgia started after initiation of an AI or if they had preexisting joint pain that was exacerbated by AI use.
Primary Outcome Measures
Arthralgia.
We assessed arthralgia via three different questionnaires completed at baseline and at 3, 6, 9, and 12 months.
BPI.
The BPI is a 14-item questionnaire developed for use in patients with cancer that assesses worst pain, pain severity, and pain interference over the past week, reported on a scale of 0 to 10.14 Worst pain is categorized as mild (score of 3 to 4), moderate (score of 5 to 7), or severe pain (score of 8 to 10). Pain severity is measured as the average of responses to questions on worst pain, average pain, least pain, and pain right now. Pain interference is the average of seven interference items, such as walking, mood, and sleep. The BPI is the most common, valid, and reliable measure to assess joint pain in cancer survivors (Cronbach's α and test-retest reliability score > 0.80).14 The BPI was modified to capture joint pain and stiffness by adding the term “joint pain/stiffness” rather than just the word “pain” throughout the questionnaire.
Western Ontario and McMaster Universities Osteoarthritis index.
The Western Ontario and McMaster Universities Osteoarthritis (WOMAC) index measures lower-extremity joint symptoms in the past 7 days in three domains: pain, stiffness, and physical function.15 Scores are normalized into a 0- to 100-point scale, with higher scores indicating worse pain, stiffness, and functional limitations. Internal consistency is good (Cronbach's α > 0.85; test-retest reliability scores [ie, intraclass correlation coefficients] ranging from 0.58 to 0.92).15
Disabilities of the Arm, Shoulder and Hand questionnaire.
The Disabilities of the Arm, Shoulder and Hand (DASH) questionnaire is a 30-item questionnaire designed to measure physical function and symptoms in patients with musculoskeletal disorders of the upper limbs.16 It is a reliable and valid instrument, with high internal consistency (Cronbach's α, 0.91; test-retest reliability [intraclass correlation coefficient, 0.92]). A higher score indicates more upper-extremity disability.16
Grip strength.
We assessed grip strength using a baseline bulb dynamometer at baseline and at 6 and 12 months. Each participant underwent three trials of squeezing a rubber ball with the dominant hand, with the pressure (in psi) averaged over three trials.
Secondary Outcome and Covariate Measures
Demographics and medical history.
Medical record review and an interviewer-administered questionnaire were used to determine disease stage, surgery, adjuvant therapy, endocrine therapy, and comorbidities.
Pain medication.
At baseline and at 6 and 12 months, participants completed a medicine-supplement questionnaire that asked about current over-the-counter or prescription medication use. Current use of glucosamine and chondroitin was also captured. Participants also completed the following pain medication question on the BPI: Are you taking any oral medications for pain/stiffness (yes or no)?
AI adherence.
Participants recorded their daily AI use in a log reviewed monthly by telephone or in person with study staff. Reasons for missed doses were assessed by study staff.
Height and weight.
Height (stadiometer) and weight (digital scale; no shoes) were measured at baseline and at 6 and 12 months. All measurements were taken twice and averaged.
Physical activity.
At baseline (for screening purposes) and at 6 and 12 months, participants completed a physical activity questionnaire, assessing the past 6 months of activity, including the type, frequency, and duration of 20 activities.17
Cardiorespiratory fitness.
Cardiorespiratory fitness was measured at baseline and at 12 months with a standard maximal oxygen consumption (VO2 max) treadmill test.18
Exercise Intervention
The yearlong exercise intervention was a combination of a twice-per-week supervised resistance training program (under supervision of American College of Sports Medicine–certified cancer exercise trainer) at a local health club and a home-based aerobic exercise program of 150 minutes per week, in accordance with current exercise recommendations for cancer survivors.19 Participants wore heart-rate monitors during each workout. After each exercise session, participants recorded the type, duration, and average heart rate during exercise in physical activity logs as a measure of exercise adherence.20 Participants returned logs to the exercise trainers at the end of each week. Exercise trainers recorded attendance to the supervised sessions.
The aerobic exercise intervention consisted primarily of brisk walking (treadmill or outside), although participants could choose other aerobic exercise, such as stationary bicycling. Exercise started at 50% of maximal heart rate (determined from VO2 max testing) and increased over the first month to 60% to 80% of maximal heart rate for the study duration. The strength-training protocol consisted of six exercises (ie, bench press, latissimus pull down, seated row, leg press, leg extension, and leg curl) performed for eight to 12 repetitions for three sets. Participants progressed up to three sets per exercise over the first month. After two sessions during which a participant lifted the same weight 12 times during each set, the weight was increased by the smallest possible increment.
Usual-Care Group
Women were instructed to continue with their usual activities. Participants were not discouraged from exercising on their own but were not given any exercise instruction until the end of the study. Women were telephoned monthly by research staff to determine AI adherence.
Both the exercise and usual-care groups were provided with an educational booklet prepared for the HOPE study, which addressed breast cancer topics such as lymphedema and fatigue. Topics were discussed monthly over the telephone (usual-care group) or at an exercise session (exercise group).
Statistical Analyses
Sample size was estimated at the design stage to detect a difference in the primary end point (ie, change in BPI score at 12 months). We powered our study with 60 patients per group to detect a difference of 1.5 (standard deviation [SD], 2.5) in the BPI worst pain change score with 90% power at a two-sided significance level of .05 based on results of the study by Hershman et al.21 Participants were grouped according to the intention-to-treat principle. Permuted block randomization (at 1:1 ratio) with random block size was performed, stratified by joint pain before AI therapy and current bisphosphonate use (related to our secondary aim of bone mass). Intervention effects were evaluated by differences in mean changes at follow-up time points between exercise and usual-care groups using mixed-model repeated measures analysis. This approach is robust, because it includes all available data and accounts for correlations between repeated measures. Because the two study groups did not differ at baseline, analyses only adjusted for pain medication use (assessed via BPI) and baseline score for the respective arthralgia outcome measure. The inclusion of baseline arthralgia score as a covariate corresponds to the analysis of covariance approach, with efficiency to test group differences.22 Group-by-time interaction was also included as a fixed effect. Post hoc comparisons at each time point were conducted with Bonferroni correction for multiple comparisons (0.013 was used as significance cutoff). Sensitivity analyses using a random-effects pattern mixture model approach were performed to evaluate the potential influence of study completion status and AI adherence on primary analyses.23 Participants were divided into two groups on the basis of completion status at the 12-month visit. The corresponding dichotomous covariate, as well as its interaction with main effects of time, group, and group-by-time interaction, was included in the analysis. Completion status did not differ between groups (P = .26). No statistically significant joint effect was detected for any completion status–related term or by group-by-time interaction, indicating the estimated group effects were not dependent on completion status or AI adherence. Analyses were performed using SAS software (version 9.3; SAS Institute, Cary, NC). Statistical significance was set at P < .05 using two-sided tests.
RESULTS
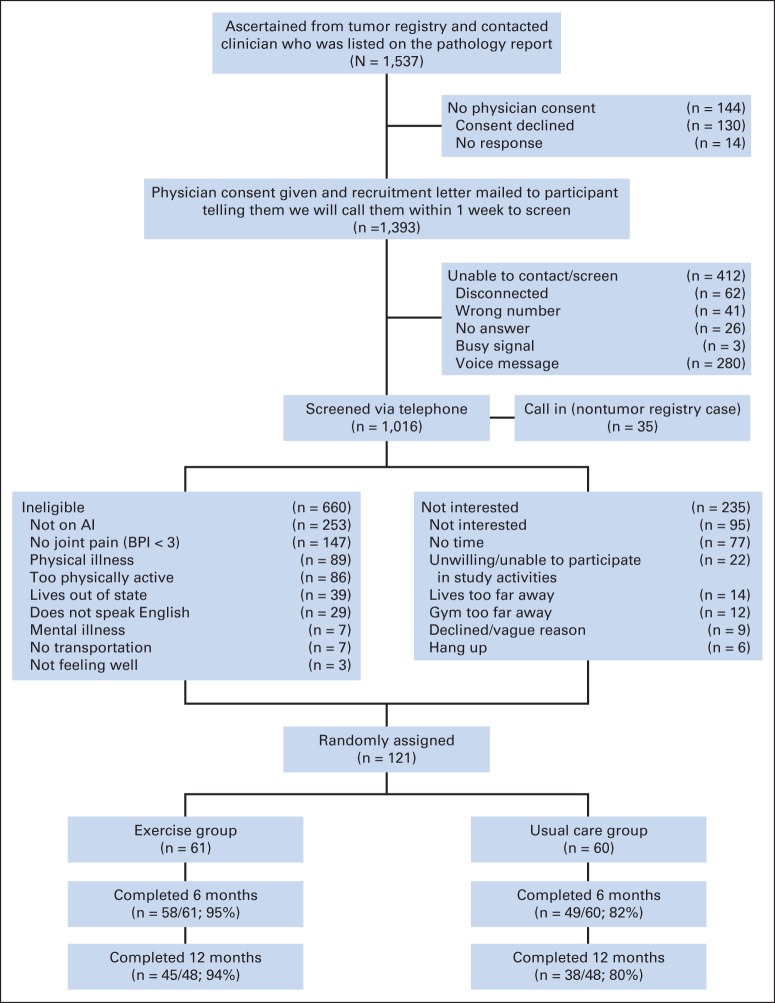
A total of 1,537 estrogen receptor–positive breast cancer survivors were identified through the Rapid Case Ascertainment Shared Resource of the Yale Cancer Center (Fig 1). Screening telephone calls were completed with 1,016 women (66% of patients with breast cancer treated at five Connecticut hospitals). Of these 1,016 women screened, 253 had already stopped taking an AI because of adverse effects or had chosen not to take an AI because of potential adverse effects. An additional 407 women were ineligible, leaving 356 eligible women, with 121 eligible women (34%) randomly assigned. Given funding cuts, the last 25 of the 121 women recruited were enrolled into a 6-month rather than 12-month trial. Thus, their study compliance was based on 6-month data (Fig 1).
Fig 1.
Flow of participants through Hormones and Physical Exercise study. AI, aromatase inhibitor; BPI, Brief Pain Index.
Baseline Characteristics
The average age of study participants was 61 years (Table 1). A majority of participants were white (88%) and had been diagnosed with stage I breast cancer (60%). Average time between diagnosis and enrollment was 3.0 years.
Table 1.
Baseline Characteristics of Randomly Assigned Participants in HOPE Study (N = 121)
| Characteristic | Exercise Group (%) |
Usual-Care Group (%) |
P | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age, years | 62.0 | 7.0 | 60.5 | 7.0 | .25 |
| Race/ethnicity | .85 | ||||
| Non-Hispanic white | 85 | 84 | |||
| Hispanic | 2 | 5 | |||
| African American | 10 | 7 | |||
| Asian/Pacific Islander | 2 | 2 | |||
| American Indian | 0 | 2 | |||
| Education | .25 | ||||
| High school graduate | 10 | 15 | |||
| Some school after high school | 33 | 42 | |||
| ≥ College graduate | 57 | 43 | |||
| Time since diagnosis, years | 2.7 | 3.1 | 3.3 | 3.9 | .30 |
| Time since initiating AI therapy, years | 1.9 | 2.9 | 1.8 | 1.3 | .89 |
| Disease stage | .70 | ||||
| 0 | 1 | 0 | |||
| I | 59 | 62 | |||
| II | 30 | 32 | |||
| III | 10 | 7 | |||
| Chemotherapy | .22 | ||||
| Yes | 54 | 43 | |||
| No | 46 | 57 | |||
| Radiation therapy | .65 | ||||
| Yes | 82 | 75 | |||
| No | 18 | 25 | |||
| BMI, kg/m2 | 30.0 | 6.8 | 28.7 | 5.5 | .27 |
| Taking pain medication | 52 | 42 | |||
| Physician-diagnosed arthritis | 49 | 32 | |||
| Current glucosamine and chondroitin use | 13 | 18 | |||
Abbreviations: BMI, body-mass index; HOPE, Hormones and Physical Exercise; SD, standard deviation.
Intervention Adherence
Women randomly assigned to exercise increased their physical activity by an average 159 minutes per week, compared with 49 minutes per week in the usual-care group (P < .001; Table 2). Women randomly assigned to exercise also reported their exercise prospectively in daily activity logs and reported an average 119 minutes per week of aerobic exercise, with an average of 70% of strength-training sessions completed, resulting in an average 62% and 42% increase in one-repetition maximum for leg press and bench press at 12 months, respectively. Cardiorespiratory fitness increased by 6.5% in women randomly assigned to exercise, versus a 1.8% decrease in those receiving usual care (P = .001). Body weight was reduced by 2.4% in women randomly assigned to exercise, versus no change in the usual-care group (P = .037). There were no adverse events associated with the exercise program. Attendance to monthly telephone calls for women randomly assigned to usual care was 53%.
Table 2.
Physical Activity, Cardiorespiratory Fitness, Muscular Strength, and Body Weight Changes and Adherence to Exercise in HOPE Study
| Measure | Exercise Group |
Usual-Care Group |
P | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Physical activity questionnaire, minutes per week | |||||
| Baseline | 54.8 | 93.0 | 60.7 | 99.0 | .74 |
| 12 months | 222.1 | 118.6 | 103.6 | 104.7 | < .001 |
| Change | 159 | 136 | 49 | 86 | < .001 |
| Percent reporting ≥ 150 | 70 | 6 | |||
| Percent reporting ≥ 120 | 74 | 15 | |||
| VO2 max, ml/kg per minute | |||||
| Baseline | 23.0 | 5.3 | 23.1 | 3.5 | .88 |
| 12 months | 24.6 | 5.5 | 23.0 | 4.7 | .17 |
| Change | 1.5 | 2.1 | −0.4 | 2.7 | < .001 |
| Percent change | 6.5 | 3.7 | −1.8 | 11.2 | .0013 |
| Body weight, kg | |||||
| Baseline | 78.5 | 18.1 | 75.5 | 14.5 | .32 |
| Change | −2.1 | 4.3 | 0.1 | 3.6 | .014 |
| Percent change | −2.4 | 5.4 | 0.0 | 4.8 | .037 |
| Daily activity log* | |||||
| Aerobic exercise, minutes per week | 119 | 78 | NA | ||
| Twice-per-week strength-training session attendance, % | 70 | 28 | NA | ||
| One-repetition maximum, lbs | |||||
| Leg press | NA | ||||
| Baseline | 156 | 58 | |||
| 12 months | 245 | 75 | |||
| Change | 82 | 61 | |||
| Percent change | 62 | 52 | |||
| Bench press | |||||
| Baseline | 43 | 17 | |||
| 12 months | 60 | 19 | |||
| Change | 16 | 11 | |||
| Percent change | 42 | 32 | |||
Abbreviations: HOPE, Hormones and Physical Exercise; NA, not applicable; SD, standard deviation; VO2 max, maximal oxygen consumption.
Daily activity logs were completed for each week of exercise intervention; if woman did not exercise that week, value of 0 was reported in her activity log.
Effect of Exercise on Arthralgia
At baseline, BPI-assessed worst joint pain scores averaged 5.6 (± SD, 2.1) and 5.9 (± SD, 1.9) for exercisers and those receiving usual care, respectively (P = .42; Table 3; Fig 2). Over 12 months, worst joint pain scores decreased by 1.6 points (29% decrease) in women randomly assigned to exercise, versus a 0.2-point increase (3% increase) in women randomly assigned to usual care (difference, 1.8; 95% CI, 0.9 to 2.6; P < .001). Statistically significant differences in pain severity and pain interference were also observed between exercisers versus the usual-care group (both P < .001).
Table 3.
Effect of Exercise Versus Usual Care on BPI-Assessed Pain at Baseline and Changes at 3, 6, 9, and 12 Months*
| Outcome† | Exercise Group |
Usual-Care Group |
Treatment Effect (control minus exercise) |
P | |||
|---|---|---|---|---|---|---|---|
| Change From Baseline | 95% CI | Change From Baseline | 95% CI | Change From Baseline | 95% CI | ||
| Worst pain | |||||||
| Baseline | 5.6 | 5.2 to 6.2 | 5.9 | 5.4 to 6.3 | .42 | ||
| 3 months | −1.2 | −1.9 to −0.5 | −0.1 | −0.8 to 0.7 | 1.2 | 0.1 to 2.2 | .03 |
| 6 months | −0.6 | −1.2 to 0.0 | −0.3 | −0.9 to 0.4 | 0.3 | −0.5 to 1.2 | .45 |
| 9 months | −1.4 | −2.2 to −0.6 | −0.1 | −0.9 to 0.7 | 1.3 | 0.2 to 2.5 | .03 |
| 12 months | −1.6 | −2.2 to −1.1 | 0.2 | −0.5 to 0.8 | 1.8 | 0.9 to 2.6 | < .001 |
| Severity | |||||||
| Baseline | 4.0 | 3.6 to 4.4 | 4.2 | 3.7 to 4.7 | .51 | ||
| 3 months | −1.0 | −1.4 to −0.5 | −0.2 | −0.7 to 0.3 | 0.8 | 0.1 to 1.5 | .03 |
| 6 months | −0.5 | −0.9 to 0.0 | −0.4 | −0.9 to 0.1 | 0.1 | −0.6 to 0.7 | .87 |
| 9 months | −0.8 | −1.4 to −0.2 | −0.4 | −0.9 to 0.2 | 0.4 | −0.4 to 1.2 | .31 |
| 12 months | −1.1 | −1.6 to −0.6 | 0.3 | −0.2 to 0.8 | 1.4 | 0.7 to 2.1 | < .001 |
| Interference | |||||||
| Baseline | 2.9 | 2.4 to 3.4 | 3.0 | 2.4 to 3.6 | .64 | ||
| 3 months | −0.9 | −1.5 to −0.3 | −0.1 | −0.8 to 0.6 | 0.8 | −0.1 to 1.7 | .09 |
| 6 months | −0.7 | −1.2 to −0.3 | −0.3 | −0.8 to 0.2 | 0.4 | −0.2 to 1.1 | .20 |
| 9 months | −0.8 | −1.4 to −0.2 | −0.3 | −0.9 to 0.3 | 0.5 | −0.3 to 1.4 | .22 |
| 12 months | −1.1 | −1.6 to −0.5 | 0.4 | −0.2 to 0.9 | 1.4 | 0.7 to 2.2 | < .001 |
Abbreviation: BPI, Brief Pain Inventory.
Adjusted for baseline value and pain medication use. Sample sizes for 3 and 6 months were 58 and 49 patients in exercise and usual-care groups, respectively; sample sizes for 9 and 12 months were 45 and 38 patients, respectively.
BPI-assessed pain on scale of 0 to 10.
Fig 2.
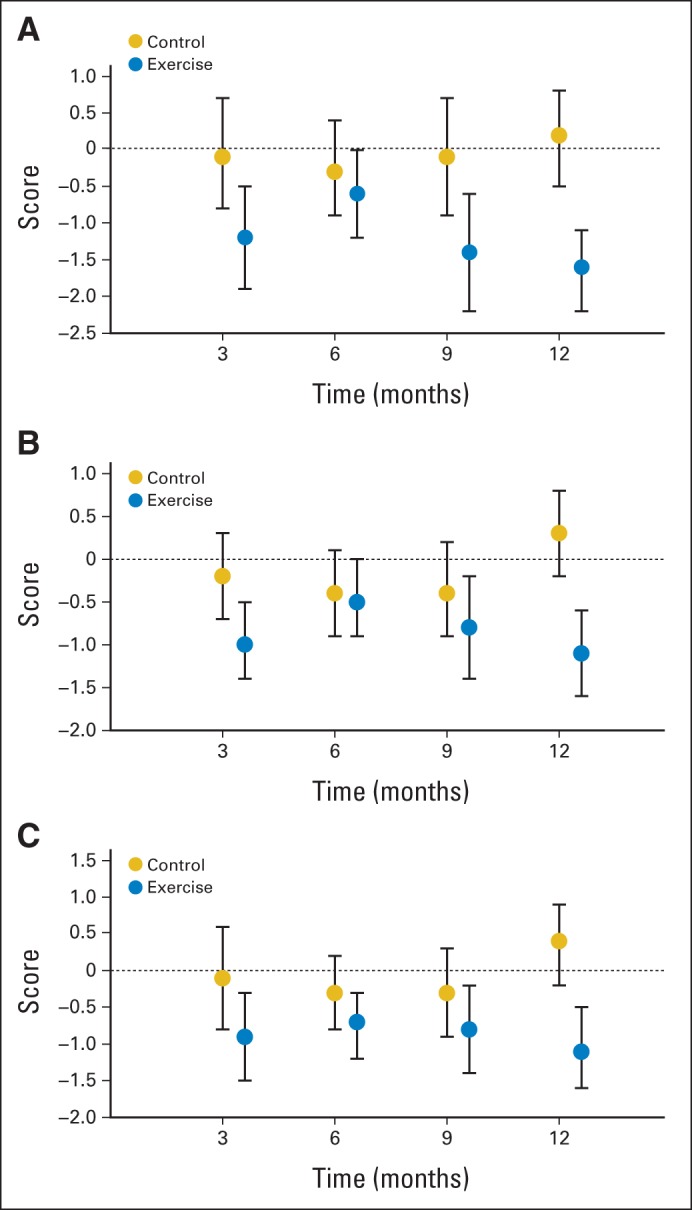
Changes in (A) worst pain, (B) severity, and (C) interference.
Similar findings were observed when measuring upper- and lower-body symptoms via the DASH and WOMAC questionnaires (Table 4; Fig 1). The WOMAC total score for lower extremities decreased by 9.4 points (37% decrease) in women randomly assigned to exercise at 12 months, compared with a 0.5-point increase (2% increase) in the usual-care group (difference, 9.9; 95% CI, 2.8 to 16.9; P < .001). The DASH upper-extremity score decreased by 6.7 points (33% decrease) in women randomly assigned to exercise at 12 months, compared with a 1.3-point increase (12% increase) in those receiving usual care (difference, 8.0; 95% CI, 3.1 to 12.9; P = .002). Adjusting for arthritis did not change the effect of exercise on arthralgia.
Table 4.
Effect of Exercise Versus Usual Care on WOMAC- and DASH-Assessed Symptoms at Baseline and Changes at 3, 6, 9, and 12 Months*
| Outcome | Exercise Group |
Usual-Care Group |
Treatment Effect (control minus exercise) |
P | |||
|---|---|---|---|---|---|---|---|
| Change From Baseline | 95% CI | Change From Baseline | 95% CI | Change From Baseline | 95% CI | ||
| DASH† | |||||||
| Baseline | 20.0 | 16.4 to 23.6 | 19.3 | 15.9 to 22.7 | .77 | ||
| 3 months | −7.6 | −10.9 to −4.3 | −1.0 | −4.6 to 2.6 | 6.6 | 1.7 to 11.5 | .03 |
| 6 months | −6.6 | −9.1 to −4.1 | −0.5 | −3.2 to 2.3 | 6.1 | 2.4 to 9.8 | .001 |
| 9 months | −5.4 | −9.5 to −1.4 | 1.1 | −2.9 to 5.1 | 6.5 | 0.9 to 12.2 | .02 |
| 12 months | −6.7 | −10.0 to −3.4 | 1.3 | −2.3 to 4.9 | 8.0 | 3.1 to 12.9 | .002 |
| WOMAC pain scale‡ | |||||||
| Baseline | 21.1 | 15.1 to 27.1 | 21.1 | 14.8 to 27.3 | .99 | ||
| 3 months | −8.7 | −13.0 to −4.3 | −6.1 | −10.9 to −1.3 | 2.6 | −3.9 to 9.1 | .43 |
| 6 months | −8.9 | −13.4 to −4.4 | −2.9 | −7.6 to 1.8 | 6.0 | −0.5 to 12.5 | .07 |
| 9 months | −4.3 | −12.5 to 3.8 | 1.4 | −6.8 to 9.6 | 5.7 | −5.8 to 17.3 | .32 |
| 12 months | −6.0 | −11.6 to −0.3 | 0.7 | −5.3 to 6.8 | 6.7 | −1.6 to 15.0 | .11 |
| WOMAC physical function scale† | |||||||
| Baseline | 26.8 | 21.7 to 32.0 | 24.8 | 19.6 to 30.0 | .58 | ||
| 3 months | −11.6 | −15.5 to −7.8 | −8.3 | −12.7 to −3.9 | 3.3 | −2.5 to 9.2 | .26 |
| 6 months | −9.3 | −13.4 to −5.2 | −3.5 | −7.9 to 0.9 | 5.8 | −0.2 to 11.8 | .06 |
| 9 months | −7.9 | −15.5 to −0.2 | −0.6 | −8.3 to 7.1 | 7.3 | −3.6 to 18.1 | .19 |
| 12 months | −10.4 | −15.0 to −5.8 | 1.1 | 4.0 to 6.1 | 11.5 | 4.7 to 18.3 | .001 |
| WOMAC total† | |||||||
| Baseline | 25.7 | 20.5 to 30.9 | 24.5 | 19.1 to 29.9 | .76 | ||
| 3 months | −11.1 | −15.0 to −7.3 | −7.9 | −12.2 to −3.5 | 3.3 | −2.5 to 9.1 | .27 |
| 6 months | −9.2 | −13.4 to −5.0 | −4.1 | −8.5 to 0.3 | 5.1 | −1.0 to 11.1 | .10 |
| 9 months | −7.9 | −15.5 to −0.3 | −0.4 | −8.1 to 7.3 | 7.5 | −3.4 to 18.3 | .18 |
| 12 months | −9.4 | −14.2 to −4.6 | 0.5 | −4.7 to 5.7 | 9.9 | 2.8 to 16.9 | < .001 |
| Grip strength, psi | |||||||
| Baseline | 10.6 | 10.0 to 1.2 | 10.6 | 10.0 to 1.1 | .88 | ||
| 6 months | 0.2 | −0.2 to 0.6 | −0.6 | −1.0 to −0.1 | −0.7 | −1.4 to −0.1 | .03 |
| 12 months | 0.4 | −0.2 to 0.9 | 0.1 | −0.5 to 0.7 | −0.3 | −1.1 to 0.5 | .47 |
Abbreviations: DASH, Disabilities of the Arm, Shoulder and Hand; WOMAC, Western Ontario and McMaster Universities Osteoarthritis.
Adjusted for baseline value and pain medication use. Sample sizes for 3 and 6 months were 58 and 49 patients in exercise and usual-care groups, respectively; sample sizes for 9 and 12 months were 45 and 38 patients, respectively.
DASH-assessed pain on scale of 0 to 25.
WOMAC-assessed pain on scale of 0 to 100.
There was no dose-response effect of exercise on arthralgia assessed via BPI, DASH, or WOMAC. Greater attendance to strength-training sessions, more minutes per week of aerobic exercise, and larger increases in VO2 max or one-repetition maximum were not associated with greater improvements in arthralgia (data not shown), implying that the average exercise adherence observed in our study of 2 hours per week of aerobic exercise and twice-per-week strength-training sessions, performed over 1 year, is optimal for improving AI-associated arthralgia.
Use of Pain Medications and AI Adherence
At baseline, 47% of women reported pain medication use, assessed via the BPI pain medication use question, whereas slightly fewer used pain medication at 12 months (39%), with no differences between exercisers or those receiving usual care. Specifically, 17% of women stopped taking pain medication at 12 months, 8% started taking pain medication at 12 months, and 75% had no change from baseline. Use of pain medication did not confound the effect of exercise versus usual care on arthralgia. Similar pain medication use was reported on the medicine-supplement questionnaire completed by participants.
Of the 121 women enrolled, four women (control, n = 2; exercise, n = 2) stopped taking AIs during the trial because of joint pain, GI distress, or cognitive function. Adherence to AIs was good, with 80% and 76% of exercisers and those receiving usual care, respectively, adhering to AI therapy daily at 12 months.
DISCUSSION
In this trial of women receiving AIs for breast cancer, we found AI-associated arthralgia symptoms worsened over time in women randomly assigned to usual care, whereas exercise reduced AI-associated arthralgia pain scores by approximately 30% or 1.5 points. On average, pain scores in women randomly assigned to exercise decreased from moderate at baseline to mild at the end of the intervention period (ie, BPI worst pain score of approximately 6 to 4 points). Women randomly assigned to exercise also experienced increases in cardiorespiratory fitness, upper- and lower-body strength, and losses in body weight.
AIs are recommended for postmenopausal women with hormone receptor–positive breast cancer, which represents almost 50% of all newly diagnosed cases of breast cancer.1,2 Despite the efficacy of AIs, adverse effects often result in poor AI adherence, which can reduce effectiveness and increase mortality.5–8 Arthralgia is the most common reason for AI discontinuation5–7; however, there are few data regarding effective treatments for AI-induced arthralgia. Studies have examined glucosamine, vitamin D, acupuncture, yoga, and tai chi as treatments for arthralgia, with promising results.21,24–29 However, most studies were small (ie, < 50 participants), uncontrolled, and of short duration. The decrease in AI-associated arthralgia observed in the HOPE study was larger in magnitude than that reported with these other arthralgia treatments. To our knowledge, no other randomized trial has examined the impact of exercise on AI-induced arthralgia in breast cancer survivors receiving an AI and experiencing arthralgia.
The etiology of AI-induced arthralgia is not completely understood; however, most hypotheses focus on estrogen deprivation, which is the intended outcome of AI therapy.30 The mechanisms through which exercise could improve AI-induced arthralgia are not entirely clear. Exercise improves blood flow to tissues and increases maximal oxygen consumption,31 which in turn could make activities of daily living easier to perform and therefore less painful. Exercise increases range of motion and may improve pain threshold.32,33 Exercise also improves cancer-related fatigue and overall quality of life and is associated with lowered rates of mortality in breast cancer survivors.12,19
Strengths of our study include the randomized design, high adherence to the exercise intervention, and a focus on women experiencing arthralgia resulting from AI use. However, our study also had some limitations. First, the questionnaires used to assess AI-associated arthralgia were not designed to specifically assess this adverse effect. Development of a questionnaire to specifically assess AI-associated arthralgia in breast cancer survivors is needed. Second, our results may only be generalizable to physically inactive breast cancer survivors who continue to take AIs despite adverse effects. Third, our intervention was supervised by exercise trainers; however, community-based exercise programs are increasingly available, such as LIVESTRONG at the YMCA, which offers free exercise programs to cancer survivors at various YMCA locations across the United States. Finally, our primary aim was to examine the impact of exercise on improving AI-associated arthralgia rather than AI adherence. Thus, eligible women had to be receiving an AI and planning to continue to take the medication for 1 year; women also had to be experiencing at least mild AI-associated arthralgia. These eligibility criteria allowed us to examine the effect of exercise on a common adverse effect of AIs (ie, arthralgia) without a potential confounding effect of AI adherence. Given that our results show a beneficial effect of exercise on treating arthralgia, additional work is needed to determine whether exercise can improve AI adherence.
In conclusion, given the efficacy of AIs in preventing breast cancer recurrence and the proportion of women who discontinue these drugs because of adverse events, interventions to improve adverse effects are important. The HOPE study demonstrates that exercise is effective in improving AI-induced arthralgia in previously inactive breast cancer survivors who adhere to their AI medication despite this common adverse effect. Although some benefit of exercise was observed after 3 months of exercise, the strongest benefit occurred after 12 months of exercise. Further research is needed to determine if exercise improves AI adherence and breast cancer survival.
Supplementary Material
Acknowledgment
We thank Mia Sorkin, Dan Root, Willie Moore, Liz Fraser, Michelle Baglia, Adrienne Viola, Yanchang Zhang, Bridget Winterhalter, Norbert Hootsmans, Celeste Wong, and Meghan Hughes for their assistance. We thank Rajni Mehta and the Rapid Case Ascertainment of Yale Cancer Center, Smilow Cancer Hospital at Yale–New Haven, St Raphael's Hospital, St Vincent's Medical Center, Bridgeport Hospital, and Greenwich Hospital and all the clinicians who consented or referred their patients to our study. Most importantly, we thank the participants for their dedication to and time spent on the HOPE (Hormones and Physical Exercise) study.
Glossary Terms
- aromatase inhibitors:
inhibitors used in treating breast cancer in postmenopausal women. Aromatase inhibitors inhibit the conversion of androgens to estrogens by the enzyme aromatase, thus depriving the tumor of estrogenic signals. Because of decreased production of estrogen, estrogen receptors, which are important in the progression of breast cancer, cannot be activated.
- estrogen receptor:
ligand-activated nuclear proteins, belonging to the class of nuclear receptors, present in many breast cancer cells that are important in the progression of hormone-dependent cancers. After binding, the receptor-ligand complex activates gene transcription. There are two types of estrogen receptors (ERα and ERβ). ERα is one of the most important proteins controlling breast cancer function. ERβ is present in much lower levels in breast cancer, and its function is uncertain. Estrogen receptor status guides therapeutic decisions in breast cancer.
Footnotes
Listen to the podcast by Dr Gralow at www.jco.org/podcasts
Processed as a Rapid Communication manuscript.
Supported by National Cancer Institute Grant No. R01 CA132931 and in part by a grant from the Breast Cancer Research Foundation (M.L.I.), Yale Cancer Center Support Grant No. P30 CA016359, and Clinical and Translational Science Award Grant No. UL1 TR000142 from the National Center for Advancing Translational Science, a component of the National Institutes of Health. Certain data used in this study were obtained from the Connecticut Tumor Registry, located in the Connecticut Department of Public Health. The authors assume full responsibility for analyses and interpretation of these data.
Clinical trial information: NCT02056067.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Melinda L. Irwin, Cary P. Gross, Marianna Rothbard, Kathryn Schmitz, Tuhina Neogi, Dawn Hershman, Jennifer Ligibel
Financial support: Melinda L. Irwin
Administrative support: Melinda L. Irwin, Marianna Rothbard
Provision of study materials or patients: Melinda L. Irwin, Brenda Cartmel, Cary P. Gross, Tara Sanft
Collection and assembly of data: Melinda L. Irwin, Brenda Cartmel, Elizabeth Ercolano, Martha Fiellin, Scott Capozza, Marianna Rothbard, Yang Zhou, Maura Harrigan, Tara Sanft
Data analysis and interpretation: Melinda L. Irwin, Brenda Cartmel, Cary P. Gross, Fangyong Li, Xiaopan Yao, Marianna Rothbard, Kathryn Schmitz, Tuhina Neogi, Dawn Hershman, Jennifer Ligibel
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Exercise Trial of Aromatase Inhibitor–Induced Arthralgia in Breast Cancer Survivors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Melinda L. Irwin
No relationship to disclose
Brenda Cartmel
Stock or Other Ownership: Pfizer (I)
Consulting or Advisory Role: Pfizer (I)
Cary P. Gross
Research Funding: Medtronic, Johnson & Johnson
Elizabeth Ercolano
No relationship to disclose
Fang-yong Li
No relationship to disclose
Xiaopan Yao
No relationship to disclose
Martha Fiellin
No relationship to disclose
Scott Capozza
No relationship to disclose
Marianna Rothbard
Employment: Molecular NeuroImaging
Stock or Other Ownership: Pfizer (I), Merck (I)
Yang Zhou
No relationship to disclose
Maura Harrigan
No relationship to disclose
Tara Sanft
Consulting or Advisory Role: bioTheranostics
Research Funding: bioTheranostics
Kathryn Schmitz
No relationship to disclose
Tuhina Neogi
No relationship to disclose
Dawn Hershman
No relationship to disclose
Jennifer Ligibel
No relationship to disclose
REFERENCES
- 1.Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after 5 years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–1802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 2.Clarke M. Meta-analyses of adjuvant therapies for women with early breast cancer: The Early Breast Cancer Trialists' Collaborative Group overview. Ann Oncol. 2006;17(suppl 10):x59–x62. doi: 10.1093/annonc/mdl238. [DOI] [PubMed] [Google Scholar]
- 3.Gnant M, Harbeck N, Thomssen C. St. Gallen 2011: Summary of the consensus discussion. Breast Care (Basel) 2011;6:136–141. doi: 10.1159/000328054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: Update on adjuvant endocrine therapy for women with hormone receptor–positive breast cancer. J Clin Oncol. 2010;28:3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy CC, Bartholomew LK, Carpentier MY, et al. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: A systematic review. Breast Cancer Res Treat. 2012;134:459–478. doi: 10.1007/s10549-012-2114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crew KD, Greenlee H, Capodice J, et al. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol. 2007;25:3877–3883. doi: 10.1200/JCO.2007.10.7573. [DOI] [PubMed] [Google Scholar]
- 7.Stestak I, Cuzick J, Sapunar F, et al. Risk factors for joint symptoms in patients enrolled in the ATAC trial: A retrospective, exploratory analysis. Lancet Oncol. 2008;9:866–872. doi: 10.1016/S1470-2045(08)70182-7. [DOI] [PubMed] [Google Scholar]
- 8.Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–538. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henry NL, Giles JT, Ang D, et al. Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat. 2008;111:365–372. doi: 10.1007/s10549-007-9774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mao JJ, Stricker C, Bruner D, et al. Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer. 2009;115:3631–3639. doi: 10.1002/cncr.24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas KS, Muir KR, Doherty M, et al. Home based exercise program for knee pain and knee osteoarthritis. BMJ. 2002;325:752. doi: 10.1136/bmj.325.7367.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ballard-Barbash R, Friedenreich CM, Courneya KS, et al. Physical activity, biomarkers and disease outcomes in cancer survivors. J Natl Cancer Inst. 2012;104:815–840. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fallowfield LJ, Bliss JM, Porter LS, et al. Quality of life in the Intergroup Exemestane study. J Clin Oncol. 2006;24:910–917. doi: 10.1200/JCO.2005.03.3654. [DOI] [PubMed] [Google Scholar]
- 14.Cleeland CS, Ryan KM. Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 15.Bellamy N, Buchanana WW, Goldsmith CH, et al. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to anti-rheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 16.Kennedy CA, Beaton DE, Solway S, et al. Disabilities of the Arm, Should and Hand (DASH): The DASH and QuickDASH Outcome Measure User's Manual (ed 3) Toronto, Ontario, Canada: Institute for Work and Health; 2011. [Google Scholar]
- 17.Kriska AM, Knowler WC, LaPorte RE, et al. Development of a questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13:401–411. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 18.Peel AB, Thomas SM, Dittus K, et al. Cardiorespiratory fitness in breast cancer patients: A call for normative values. J Am Heart Assoc. 2014;3:e000432. doi: 10.1161/JAHA.113.000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 20.Blair SN, Haskell WL, Ho P, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122:794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- 21.Crew KD, Capodice JL, Greenlee H, et al. Randomized, blinded, sham-controlled trial of acupuncture for the management of aromatase inhibitor-associated joint symptoms in women with early-stage breast cancer. J Clin Oncol. 2010;28:1154–1160. doi: 10.1200/JCO.2009.23.4708. [DOI] [PubMed] [Google Scholar]
- 22.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Wiley-Interscience, Hoboken: NJ; 2011. pp. 122–132. [Google Scholar]
- 23.Hedeker D, Gibbons RD. Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychol Methods. 1997;2:64–78. [Google Scholar]
- 24.Greenlee H, Crew KD, Shao T, et al. Phase II study of glucosamine with chondroitin on aromatase inhibitor-associated joint symptoms in women with breast cancer. Support Care Cancer. 2013;21:1077–1087. doi: 10.1007/s00520-012-1628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan QJ, Reddy PS, Kimler BF, et al. Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res Treat. 2010;119:111–118. doi: 10.1007/s10549-009-0495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mao JJ, Xie SX, Farrar JT, et al. A randomized trial of electro-acupuncture for arthralgia related to aromatase inhibitor use. Eur J Cancer. 2014;50:267–276. doi: 10.1016/j.ejca.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh B, Kimble B, Costa DS, et al. Acupuncture for treatment of arthralgia secondary to aromatase inhibitor therapy in women with early stage breast cancer: Pilot study. Acupunct Med. 2013;31:264–271. doi: 10.1136/acupmed-2012-010309. [DOI] [PubMed] [Google Scholar]
- 28.Galantino ML, Desai K, Greene L. Impact of yoga on functional outcomes in breast cancer survivors with aromatase inhibitor-associated arthralgias. Integr Cancer Ther. 2012;11:313–320. doi: 10.1177/1534735411413270. [DOI] [PubMed] [Google Scholar]
- 29.Galantino ML, Callens ML, Cardena GJ, et al. Tai chi for well-being of breast cancer survivors with aromatase inhibitor-associated arthralgias: A feasibility study. Altern Ther Health Med. 2013;19:38–44. [PubMed] [Google Scholar]
- 30.Chlebowski R. Aromatase inhibitor-associated arthralgias. J Clin Oncol. 2009;27:4932–4934. doi: 10.1200/JCO.2009.23.3270. [DOI] [PubMed] [Google Scholar]
- 31.Church TS, Earnest CP, Skinner JS, et al. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: A randomized controlled trial. JAMA. 2007;297:2081–2091. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- 32.Schmitz KH, Ahmed RL, Troxel A, et al. Weight lifting in women with breast-cancer-related lymphedema. N Engl J Med. 2009;361:664–673. doi: 10.1056/NEJMoa0810118. [DOI] [PubMed] [Google Scholar]
- 33.Jones MD, Booth J, Taylor JL, et al. Aerobic training increases pain tolerance in healthy individuals. Med Sci Sports Exerc. 2014;46:1640–1647. doi: 10.1249/MSS.0000000000000273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.