Abstract
Mu-opioids remain vastly important for the treatment of pain, and would represent ideal analgesics if their analgesic effects could be separated from their many side effects. A recently synthesized compound, iodobenzoylnaltrexamide (IBNtxA), acting at 6-transmembrane (6-TM) splice variants of the mu-opioid receptor gene, was shown to have potent analgesic actions against acute, thermal pain accompanied by a vastly improved side-effect profile compared to 7-TM-acting drugs such as morphine. Whether such analgesia can be seen in longer-lasting and non-thermal algesiometric assays is not known. The current study demonstrates potent and efficacious IBNtxA inhibition of a wide variety of assays, including inflammatory and neuropathic hypersensitivity and spontaneous pain. We further demonstrate the dependence of such analgesia on 6-TM mu-opioid receptor variants using isobolographic analysis and the testing of Oprm1 (the mu-opioid receptor gene) exon 11 null mutant mice. Finally, the effect of nerve damage (spared nerve injury) and inflammatory injury (complete Freund’s adjuvant) on expression of mu-opioid receptor variant genes in pain-relevant central nervous system loci was examined, revealing a downregulation of the mMOR-1D splice variant in the dorsal root ganglion after spared nerve injury. These findings are supportive of the potential value of 6-TM-acting drugs as novel analgesics.
1. Introduction
Opioids remain the gold standard for the treatment of moderate-to-severe pain, with an increase in opioid prescriptions for chronic pain in recent years [5]. Despite their high efficacy in the context of acute pain, opioids are associated with a number of troubling side-effects, including respiratory depression, reduced gastric motility, and dependence/addiction [2]. As a result, a major goal of pain research is to identify compounds with similar efficacy but fewer liabilities.
Three opioid receptor genes are known, and the vast majority of current opioid analgesics bind to and activate the mu-opioid receptor [12], a seven-transmembrane (7-TM) G-protein-coupled receptor. Cloning of the mu-opioid receptor gene (OPRM1) has demonstrated its high complexity, comprising a wide array of mRNA splice variants [18]. These splice variants can subsequently be grouped into those coding for full-length, 7-TM proteins, truncated six-transmembrane (6-TM) proteins, and finally single transmembrane (1-TM) proteins [21,28,30].
Recently, we demonstrated that 3-iodo-6β-benzoylnaltrexamide (IBNtxA), a naltrexone derivative, acting on 6-TM alternative splice variants of the mouse Oprm1 gene, produces analgesia against acute, thermal pain ~10 fold more potently than morphine, without evidence of respiratory depression, significant constipation, reward, aversion or dependence (as evidenced by precipitated withdrawal) [11]. Although these assays (i.e., hot-plate and tail-withdrawal tests) have historically been predictive of clinical opioid efficacy against somatic pain, they are highly limited in their prediction of efficacy against inflammatory and neuropathic, leading to the development of many new assays in modern pain research [13]. Thus, one purpose of the current work was to test the analgesic potential of IBNtxA in more clinically relevant chronic inflammatory (zymosan, complete Freund’s adjuvant) and neuropathic (spared nerve injury) assays, and using multiple measures of different pain modalities (thermal, mechanical, injury-induced mechanical hypersensitivity, and injury-induced spontaneous pain). To further understand its mechanism of action, isobolographic analysis was performed on the co-administration of IBNtxA and morphine. We assessed the dependence of IBNtxA analgesia against multiple pain types on exon 11-containing splice variants using transgenic mutant mice. Finally, we examined the effect of chronic inflammatory and neuropathic pain states on the expression of Oprm1 gene splice variants in the dorsal root ganglion and spinal cord dorsal horn.
2. Materials and Methods
2.1. Mice
Subjects of most studies were naïve, young adult (6–14 weeks old) outbred CD-1® (ICR:Crl) mice of both sexes (Charles River, Boucherville, QC); equal numbers of male and female mice were used in all behavioral experiments. Other studies employed wildtype (WT) or exon 11 null mutant mice (Exon 11 KO) previously described [17], both on a fully congenic C57BL/6 genetic background. All mice were housed in standard polycarbonate cages in groups of 2–5 same-sex littermates in a temperature-controlled (20 ± 1 °C) environment (14:10 h light/dark cycle; lights on at 07:00 h); tap water and food (Harlan Teklad 8604) were available ad lib. All procedures were consistent with appropriate national guidelines and approved by the McGill Downtown Animal Care and Use Committee or the Institutional Animal Care and Use Committee of the Memorial Sloan-Kettering Cancer Center. Testing was conducted approximately mid-photoperiod in the light phase. Mice were habituated to the testing environment for at least 60 min before testing commenced. All behavioral data were collected by the first author. Sample sizes ranged from n=5–8 mice/dose/genotype in all experiments.
2.2. Drugs
IBNtxA (0–1.0 mg/kg, s.c.) and/or morphine sulfate (1–20 mg/kg, s.c.) were used in all experiments. Both were dissolved in physiological saline and administered subcutaneously into the flank in a 10 ml/kg injection volume. Mice were randomized within-cage to drug dose, and the experimenter was blinded to dose during testing.
2.3. Nociceptive assays
2.3.1. Hot-Plate Test
Fifteen min after injection of IBNtxA, mice were placed within a transparent Plexiglas cylinder (15 cm diameter; 22.5 cm high) on a metal surface (Columbus Instruments Hotplate Analgesia Meter) maintained at 54.0 °C (±0.2 °C). The latency to respond with a hindpaw lick or shake/flutter, whichever came first, was measured to the nearest 0.1 s with a stopwatch. This test was only performed once, since repeated testing leads to systematic latency alterations [27].
2.3.2. Tail-Withdrawal Test
While lightly restrained in a cloth/cardboard holder, the distal half of the mouse’s tail was dipped into a bath of water thermostatically controlled at 49.0 °C (±0.1 °C). Latency to respond to the heat stimulus by vigorous flexion of the tail was measured. Mice were tested for baseline sensitivity, injected with IBNtxA, and then retested at 30, 60, and 90 min post-injection. At each time point, two latency determinations (separated by ≈20 s) were made and averaged.
2.3.3. Paw-Withdrawal Test
Mice were placed on a 3/16th-in. thick glass floor within small (9 × 5 × 5 cm high) Plexiglas cubicles, and a focused high-intensity projector lamp beam was shone from below onto the mid-plantar surface of the hindpaw [8]. The commercial device (IITC Model 336) was set to 20% active intensity. Latency to withdraw from the stimulus was measured to the nearest 0.1 s. Mice were tested for baseline sensitivity, injected with IBNtxA, and then retested at 30, 60, and 90 min post-injection. At each time point, two latency determinations for each hind paw (separated by ≈20 s) were made and averaged.
2.3.4. von Frey Test
Mice were placed on a metal mesh floor within small Plexiglas cubicles (9 × 5 × 5 cm high), above an UgoBasile Dynamic Plantar Aesthesiometer. A filament was aimed at the mid-plantar hind paw, and pressure gradually increased by the device until the hind paw was withdrawn. Mice were tested for baseline sensitivity, injected with IBNtxA, and then retested at 30, 60, and 90 min post-injection. At each time point, two withdrawal determinations for each hind paw (separated by ≈20 s) were made and averaged.
2.3.4. Formalin Test
Mice were habituated for at least 30 min to four individual Plexiglas cylinders placed atop a glass surface suspended over four high-resolution video cameras. Mice were then removed from their cylinder, injected with IBNtxA (or, in one study, morphine alone or in addition to IBNtxA), and placed back in the cylinder. Fifteen minutes later, all subjects were then given a subcutaneous injection of 5% formalin into the plantar right hind paw (20 μl volume), and videotaped digitally for 60 min. Videotape observations were later sampled for 10 s at 1-min intervals, and the presence or absence of right hindpaw licking/biting in that 10-s period was scored [6]. The early phase of the formalin test was defined as 0–10 min post-injection, and the late phase as 10–60 min post-injection. Data are presented as the percentage of samples in each phase in which licking/biting was detected.
2.3.5. Facial Grimacing After Ankle Zymosan
Zymosan A (5 mg/kg) was injected intra-articularly (20 μl injection volume; using a 30.5-gauge needle inserted at a right angle into the tibiotarsal joint space) into both ankles, under brief isoflurane-oxygen anaesthesia. Mice remained in their home cages for 4 h, were injected with IBNtxA, and then placed in Plexiglas observation cubicles (9 × 5 × 5 cm high) for 15 min before being videoed for the next 30 min using two high-resolution (1920 × 1080) digital video cameras (High-Definition Handycam Camcorder, model HDR-CX100, Sony). Still images were ‘grabbed’ from the video using Rodent Face Finder software [23], cropped and then copied into PowerPoint, and scored blindly for facial grimacing as previously described [9].
2.3.6. Complete Freund’s Adjuvant-Induced Mechanical Allodynia
After testing on two separate occasions (averaged) for baseline mechanical sensitivity using the automated von Frey test as described in 2.3.4. above, mice were injected with complete Freund’s adjuvant (CFA; 50%; Sigma) into the left hind paw. Mice were retested on day 4 post-CFA injection to confirm the existence of mechanical hypersensitivity. Immediately thereafter, they were injected with IBNtxA, returned to their cubicles, and retested on the von Frey test at 15, 30, 45, 60, 90 and 120 min post-injection. In one experiment, WT and KO mice were tested for mechanical sensitivity on days 3, 4, 5, 7 and 10 after CFA, with no drug injections.
2.3.7. Spared Nerve Injury-Induced Mechanical Allodynia
After testing on two separate occasions (averaged) for baseline mechanical sensitivity using the automated von Frey test as described in 2.3.4. above, mice underwent spared nerve injury (SNI) surgery [7,22]. We spared the sural nerve, and thus the von Frey filament was aimed at the lateral aspect of the hind paw. Mice were retested on day 7 (or, in other experiments, day 14) post-surgery to confirm the existence of mechanical hypersensitivity. Immediately thereafter, they were injected with IBNtxA, returned to their cubicles, and retested on the von Frey test at 15, 30, 45, 60, 90 and 120 min post-injection. In one experiment, WT and KO mice were tested for mechanical sensitivity on days 1, 4, 7, 10 and 14 after SNI, with no drug injections.
2.4. Data transformations
In an attempt to compare the relative potency of IBNtxA across assays, we converted all nociceptive data to % of the maximum possible effect (%MPE). For the hot-plate test, we imposed an arbitrary “maximum” of a two-fold increase over the saline group mean. For acute assays featuring multiple measures (tail-withdrawal test, paw-withdrawal test, von Frey test), the area under the curve (AUC) was calculated, and compared to the AUC of a mouse exhibiting two-fold increases over its own baseline at all post-injection time points. For the formalin and zymosan (facial grimacing) assays, the maximum effect was zero licking or grimacing, respectively. Percentage of maximum possible mechanical allodynia in CFA and SNI models was calculated by comparing the AUC of each mouse to that of a mouse exhibiting 0 g thresholds at all post-injection/surgery time points. Finally, to estimate the reversal of CFA- and SNI-induced mechanical allodynia (i.e., percentage anti-allodynia), the AUC of each mouse was compared to the AUC of a mouse with the same baseline and full recovery (i.e., baseline level thresholds) at every post-injection time point.
2.5. Tissue dissection, RNA extraction and RT-qPCR
Dorsal root ganglion (DRG), dorsal lumbar spinal cord (L4–L6) and periaqueductal gray (PAG) were dissected, collected in QiAzol Reagent (Qiagen) and immediately homogenized. The homogenates were stored at −80°C for further RNA isolation. Total RNAs from these tissues (n=4 pools, each pool obtained from 3 mice) were extracted using miRNeasy kit (Qiagen) following the manufacturer’s protocol. RNA concentration was determined by using Qubit 2.0 Fluorometer (Invitrogen). RNAs were first treated with Turbo-DNA free reagent (Ambion) following the manufacturer’s protocol to remove any potentially contaminated genomic DNA, and reverse transcribed with Superscript II reverse transcriptase (Invitrogen) and random hexamers. SYBR Green qPCR was performed using the first-strand cDNA from RT as template, appropriate primers and HotStart-IT SYBR green qPCR Master Mix (Affymetrix) in triplicate with Opticon 2 DNA Engine System (Bio-Rad), as previously described [29]. Table S1 lists all qPCR primers and conditions. Four reference genes—succinate dehydrogenase subunit A (SDHA), TATA box binding protein (TBP), β2-microglobulin (β2M) and 18S ribosomal RNA (18S)—were selected for normalization to obtain normalization factor (NF) using the following formula [19] [25]: NF = (C(t)SDHA × C(t)TBP × C(t)b2M × C(t)18S)1/4. Normalized expression (NE) for each variant was calculated using the formula [19]: NEvariant = E−(C(t)variant − C(t)NF), where E is efficiency determined by standard curves using serial dilutions of corresponding cDNAs as templates in qPCRs (Table S1).
2.6. Statistical analyses
In all cases a criterion α=0.05 was adopted. Transformed dose-response data were first analyzed by ANOVA, followed by a one-tailed Dunnett’s case-comparison posthoc test comparing to saline. Half-maximal analgesic doses (AD50s) and associated 95% confidence intervals (CIs) and potency ratios were calculated using the method of Tallarida and Murray [24], as implemented by the FlashCalc 40.1® macro (M.H. Ossipov, University of Arizona). Genotype differences were assessed using two-sample Student’s t-test; one-sample t-tests were used to compare to zero. Data from four mice were omitted from further analysis because they were identified as statistical outliers (Studentized residual > 3).
Isobolographic analysis was performed to test for possible synergism between IBNtxA and morphine. Equipotent doses were determined using AD50 values, yielding a 1:2.5 ratio between IBNtxA and morphine. Various IBNtxA and morphine cocktails were then mixed in this ratio and administered to mice as described above. To determine if the interaction between morphine and IBNtxA is synergistic, a theoretical additive value was determined using the AD50s of each drug separately and compared to the experimental AD50 of the cocktail. If the observed AD50 is significantly less (p<0.05) than the theoretical additive value, than the interaction is synergistic.
3. Results
All data were analyzed first by sex [14]. Significant main effects of sex were obtained in the tail-withdrawal test (p<0.05), CFA mechanical allodynia (p<0.05), and SNI mechanical allodynia (p<0.01) data sets. However, in no cases were statistically significant dose x sex or genotype x sex interactions observed, so data were collapsed by sex for all analyses reported below. Statistical data reported below for behavioral experiments are from parametric tests (ANOVAs); non-parametric Kruskal-Wallis tests were run in addition, and in no cases were significance levels reduced below p<0.05.
3.1. IBNtxA produces robust analgesia on acute nociceptive assays
We compiled full analgesic dose-response curves for IBNtxA on three assays of acute thermal nociception—differing in the stimulated body part, the presumed role of spinal versus supraspinal processing, and the nature of the nocifensive response—and one assay of acute mechanosensitivity. IBNtxA produced robust and dose-dependent analgesia on the 54 °C hot-plate test (F3,20 = 6.2, p<0.005; Fig. 1A, B) and the 49 °C hot water tail-withdrawal test (dose x repeated measures: F9,60 = 2.2, p<0.05; %MPE: F3,20 = 3.7, p<0.05; Fig. 1C, D). Drug AD50’s on these tests were calculated as 0.53 and 0.78 mg/kg, respectively (see Table 1), corresponding fairly well to previous estimates of 0.60 and 0.34 mg/kg on the 55 °C hot-plate and radiant heat tail-withdrawal test [11], despite the differences in assay parameters and data transformation procedures. IBNtxA also produced very robust analgesic effects in the radiant heat paw-withdrawal assay (dose x repeated measures: F9,57 = 2.4, p<0.05; %MPE: F3,19 = 12.7, p<0.001; Fig. 1E, F), and less robust analgesia on the von Frey test (dose x repeated measures: F9,69 = 1.7, p=0.12; %MPE: F3,23 = 3.1, p<0.05; Fig. 1G, H).
Fig. 1.
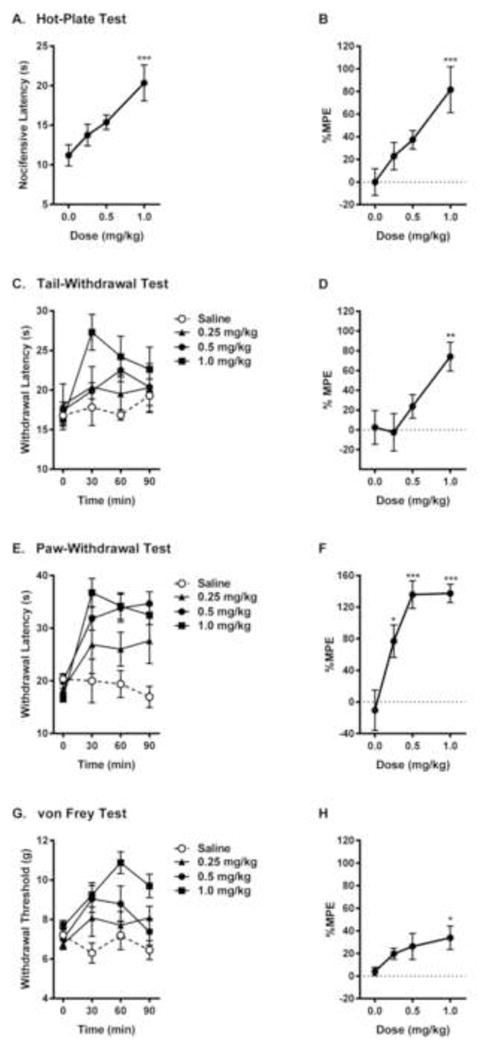
Dose-dependent analgesic effect of IBNtxA (0.25–1.0 mg/kg) in acute thermal (A, B, 54 °C hot-plate test; C, D, 49 °C tail-withdrawal test; E, F, radiant heat paw withdrawal test) and mechanical (G, H, von Frey test) nociceptive assays. Symbols in graphs on left side represent mean ± SEM latency to nocifensive behavior (A), withdrawal latency (C, E) or withdrawal threshold (G). Symbols in graphs on right side represent mean ± SEM maximum possible effect (%MPE; see Materials and Methods for calculation definitions). Sample sizes are n=6 mice/dose, except for n=5 in saline group in graphs E, F. The dotted lines indicate 0 %MPE.
Table 1.
IBNtxA analgesia across multiple nociceptive modalities.
| Assay | Measure | AD50 (95% CI) |
|---|---|---|
| Hot-Plate (54°C) | %MPE (2x saline latency) | 0.53 (0.35–0.81) |
| Tail-Withdrawal (49°C) | %MPE (2x saline AUC) | 0.78 (0.53–1.15) |
| Paw-Withdrawal (15%) | %MPE (2x saline AUC) | 0.10 (0.06–0.20) |
| von Frey (auto) | %MPE (2x saline AUC) | 8.5 (0.3–275) |
| Formalin (late phase) | %MPE (to zero licking) | 0.36 (0.28–0.48) |
| Ankle Zymosan (MGS) | %MPE (to zero grimacing) | 0.04 (0.02–0.83) |
| CFA (mechanical allodynia) | % Anti-allodynia | 0.35 (0.27–0.46) |
| SNI (mechanical allodynia) | % Anti-allodynia | 0.35 (0.24–0.50) |
Abbreviations: AD50: half-maximal analgesic dose; AUC, area under the curve; CFA, complete Freund’s adjuvant; MGS, Mouse Grimace Scale; SNI, spared nerve injury; 95% CI, 95% confidence interval; %MPE, percentage of the maximum possible effect. See Materials and Methods section for details.
3.2. IBNtxA produces robust analgesia on tonic/chronic nociceptive assays
We compiled full analgesic dose-response curves for IBNtxA on two measures of spontaneous inflammatory pain, and on reversal of mechanical allodynia after inflammatory or neuropathic injury. IBNtxA produced robust and dose-dependent analgesia on the late phase (F3,22 = 29.6, p<0.001) but not the early phase (F3,22 = 2.8, p=0.06) of the formalin test (Fig. 2A, B). Similar efficacy was evinced for the inhibition of spontaneous pain (facial grimacing) after ankle zymosan injection (F3,24 = 8.1, p<0.001; Fig. 2C, D). Finally, despite limited efficacy against acute mechanosensation (see above), IBNtxA was fully efficacious in reversing, for at least 120 min post-injection, fully developed mechanical allodynia 3 days after CFA injection (dose x repeated measures: F21,140 = 3.7, p<0.001; % anti-allodynia: F3,20 = 14.0, p<0.001; Fig. 2E, F) and 7 days after SNI surgery (dose x repeated measures: F21,133 = 3.1, p<0.001; % anti-allodynia: F3,19 = 9.8, p<0.001; Fig. 2G, H). No significant effects of IBNtxA on withdrawal thresholds of the contralateral paw were observed in either assay (data not shown), although higher doses of IBNtxA tended to raise withdrawal thresholds (also see Fig. 1G).
Fig. 2.
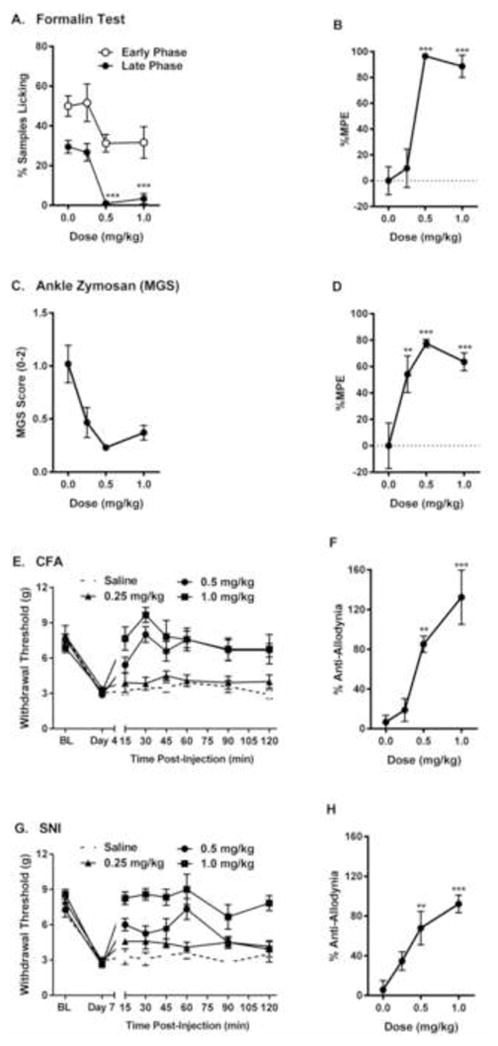
Dose-dependent analgesic effect of IBNtxA (0.25–1.0 mg/kg) in tonic (A, B, Formalin test; C, D, ankle zymosan test) and chronic pain (E, F, complete Freund’s adjuvant inflammatory pain test; G, H, spared nerve injury neuropathic pain test) assays. Symbols in graphs on left represent mean ± SEM percent samples licking (A), facial grimacing score (MGS) (B), or withdrawal threshold (E, G). Symbols in graphs on right side represent mean ± SEM maximum possible effect (%MPE; see Materials and Methods for calculation definitions; late phase data only for formalin test). Sample sizes are n=6–8 mice/dose, except for n=5 in saline group in graphs G, H. The dotted lines indicate 0 %MPE.
3.3. IBNtxA and morphine: analgesia
The late phase of the formalin test was used to compare IBNtxA and morphine potency directly. In a counterbalanced experiment, dose-response curves for both drugs were compiled. As shown in Fig. 3A, both drugs produced dose-dependent and fully efficacious analgesia (IBNtxA: F3,18 = 21.4, p<0.001; morphine: F3,21 = 4.3, p<0.05). AD50’s were calculated as 0.3 mg/kg (95% CI: 0.2–0.4 mg/kg) for IBNtxA and 6.4 mg/kg (95% CI: 3.7–11.2 mg/kg) for morphine, revealing that IBNtxA is 20.2-fold (95% CI: 11.0–37.3) more potent than morphine in this assay. Expressed in molar terms the AD50s (and 95% CIs) for IBNtxA and morphine are 48.5 (31.6–63.3) μM and 1684.2 (973.7–2947.4) μM, respectively, with a potency ratio of 34.7.
Fig. 3.
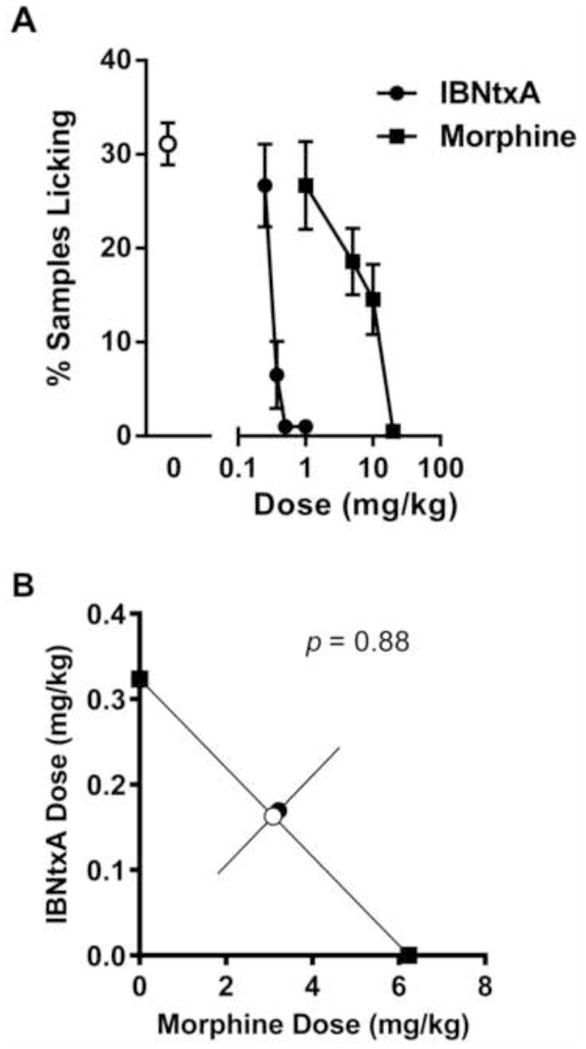
Additive analgesia between IBNtxA (0.25–1.0 mg/kg) and morphine (1–20 mg/kg) in the late phase formalin test. A) Dose-response curves for IBNtxA and morphine; symbols mean ± SEM percent samples featuring licking. B) Isobologram showing a perfectly additive relationship between the two analgesics in the late phase formalin test. Symbols represent individual drug AD50s (filled black squares) calculated from data in graph A, the theoretical AD50 of IBNtxA:morphine coadministration assuming additivity (open circle), and the experimentally determined AD50 of IBNtxA:morphine drug combinations (filled black circle). Sample sizes are n=4–10 mice/dose or dose combination.
Various combinations of IBNtxA and morphine given together were assessed in order to investigate possible synergistic interactions between the two drugs. As shown in Fig. 3B, their analgesia on the late-phase formalin test was perfectly additive.
3.4. IBNtxA anti-allodynia is dependent on exon 11 variant of Oprm1
Consistent with the notion that IBNtxA is producing its analgesic effects on a 6-TM receptor, IBNtxA analgesia on the radiant heat tail-withdrawal test was abolished in mice lacking exon 11 of the Oprm1 gene [11]. To see if IBNtxA’s efficacy against chronic pain was similarly mediated, we induced mechanical allodynia in WT and KO mice using CFA injections and SNI surgery. Both genotypes displayed expected time courses of allodynia (fully resolving by 10 days post-CFA; stable out to 14 days post-SNI); exon 11 KO mice exhibited marginally significantly higher levels of allodynia after both CFA (genotype x repeated measures: F5,50 = 2.5, p<0.05; % allodynia: t10 = 2.1, p=0.06) and SNI (genotype x repeated measures: F5,65 = 0.7, p=0.68; % allodynia: t13 = 1.5, p=0.15) (see Supplementary Figure 1 online). We tested IBNtxA at a dose very close to the calculated AD75 (i.e., three-quarter maximal analgesic dose) against both CFA and SNI allodynia: 0.5 mg/kg. IBNtxA at this dose was fully able to reverse established CFA-induced (genotype x repeated measures: F7,56 = 11.0, p<0.001; Fig. 4A, C) and SNI-induced (genotype x repeated measures: F7,56 = 5.2, p<0.001; Fig. 4B, C) mechanical allodynia for 60–120 min post-injection in WT (C57BL/6 strain) mice, replicating our previous observations in CD-1 mice (see Fig. 2E–H). IBNtxA at the same dose produced no antiallodynic effects whatsoever in KO mice either against CFA allodynia (KO compared to zero: t4 = 0.6, p=0.28; genotype comparison: t8 = 4.7, p<0.005) or SNI allodynia (KO compared to zero: t5 = 0.8, p=0.43; genotype comparison: t10 = 5.4, p<0.001).
Fig. 4.
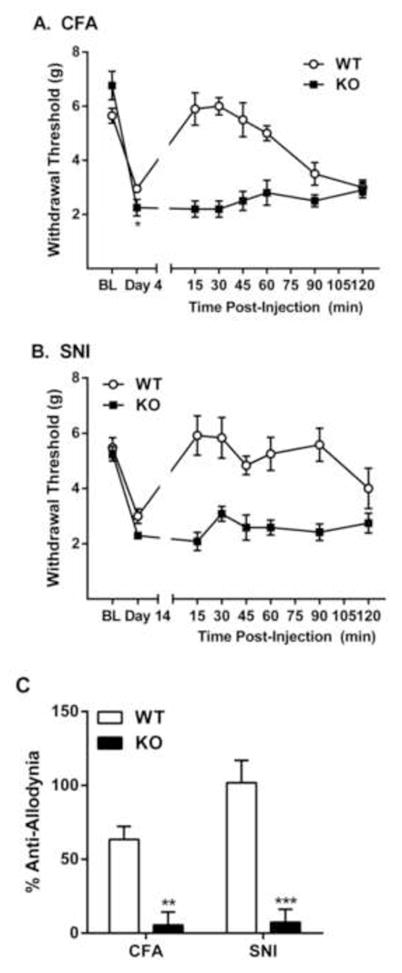
No IBNtxA reversal of inflammatory and neuropathic mechanical allodynia in Oprm1 exon 11 KO mice. A) CFA allodynia and its reversal by 0.5 mg/kg IBNtxA in WT and KO mice; symbols represent mean ± SEM hind paw withdrawal thresholds (g) of mice before CFA (BL), on day 4 post-CFA (Day 4), and 15–120 min post-IBNtxA administration. B) SNI allodynia and its reversal by 0.5 mg/kg IBNtxA in WT and KO mice; symbols represent mean ± SEM hind paw withdrawal thresholds (g) of mice before surgery (BL), on day 14 post-SNI (Day 14), and 15–120 min post-IBNtxA administration. C) Data from graphs A and B expressed as percentage of the maximum possible anti-allodynia (see Materials and Methods). *p<0.05, **p<0.01, ***p<0.001 compared to WT. Sample sizes are n=5–6 mice/genotype/assay.
3.5. Effects of chronic pain on expression of Oprm1 splice variants
The mu-opioid receptor undergoes extensive splicing, with the 6-TM variants responsible for IBNtxA analgesia in a range of tests, including SNI and CFA. Both assays are associated with extended periods of pain, raising the question of whether they may influence the expression of the various Oprm1 splice variants. To address this question, we carried out quantitative real-time PCR (qPCR) on twelve of the splice variants in the dorsal root ganglia (DRG), dorsal spinal cord and periaqueductal gray (PAG). Data were analyzed conservatively by individual one-sample (two-tailed; biological replicates only) t-tests comparing the ratio to 1.0, with Dunn-Sidak correction for multiple comparisons. These yielded equivalent results, shown in Figure 5. When compared to levels in sham controls, most of the variants failed to show any significant changes. The mRNA expression of all the full length 7-TM variants together, as measured by looking at expression of products from exon 1–2, were unchanged in all the conditions. However, one individual variant revealed statistically significant decreases in mRNA expression: mMOR-1D, which was significantly downregulated in the DRG following SNI (t3 = 6.3; adjusted p=0.02).
Fig. 5.
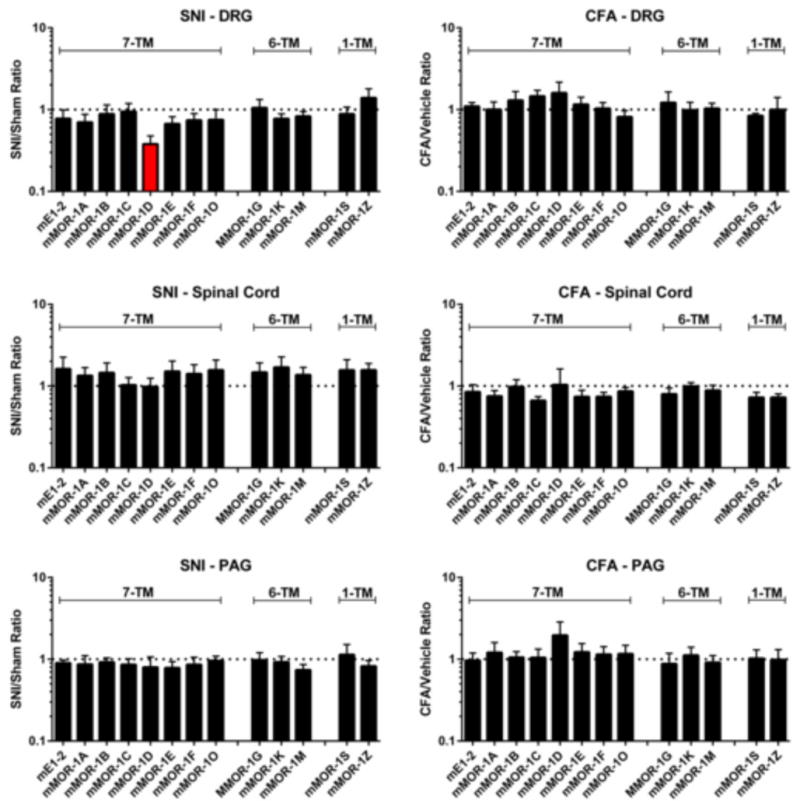
qPCR of Oprm1 splice variants following SNI (7 days post-surgery) or CFA (3 days post-injection) in the DRG, spinal cord, and PAG. Variants are placed into three groupings from left to right [11]: 7-TM variants (mE1–2, total exon 1–2-derived variants, plus individual variants), 6-TM variants, and 1-TM variants. Bars represent mean ± SEM ratios of the injury/sham groups with three technical replicates nested within n=4 pooled biological replicates. The variant displaying values statistically significantly different (p<0.05) from 1.0 (two-tailed; Dunn-Sidak corrected for multiple comparisons) is shown in red. The dotted lines indicate a ratio of 1.0.
4. Discussion
4.1. Generalized analgesic efficacy of IBNtxA
Here we document the robust and widespread analgesic efficacy of IBNtxA, which acts through the 6-TM alternative splice variants of the mouse Oprm1 gene. Dose-dependency and full efficacy was observed against acute nociceptive assays, tonic spontaneous pain, and mechanical allodynia produced by inflammation or nerve injury. That is, IBNtxA appears to be fully efficacious in preclinical pain assays spanning all four “waves of development” as described by Mogil [13]. Moreover, IBNtxA’s efficacy against chronic pain was confirmed in two separate mouse strains, outbred CD-1 and inbred C57BL/6. The relative paucity of novel analgesic compounds displaying clinical efficacy being developed in recent decades has led to much speculation that existing assays are insufficiently valid as models of human clinical pain [3,15,26]. One long-term trend is towards the use of preclinical assays in which the pain experience lasts for days, weeks and months (zymosan, CFA, and SNI, respectively) instead of seconds (hot-plate, tail-withdrawal, paw-withdrawal, and von Frey) or minutes (formalin). A more recent push is for the development of new dependent measures that tap into affective, cognitive and/or spontaneous pain domains instead of simply measuring reflexive withdrawal from evoking thermal or mechanical stimuli [see 13]. One such method was used here, the Mouse Grimace Scale [9], which scores spontaneously emitted facial grimacing, without any evoking stimulus, and may be measuring affective responses.
IBNtxA in the dose range used was fully efficacious (with %MPEs exceeding 75% at higher doses) in all assays except for the von Frey test. Although IBNtxA produced only modest increases in withdrawal thresholds to mechanical stimulation at baseline, possibly because threshold von Frey fibers applied to non-injured tissue may not actually represent a noxious stimulus [4], it was nonetheless fully able to reverse mechanical allodynia after both inflammatory and neuropathic injury, far more clinically relevant symptoms. We did not test the ability of IBNtxA to reverse thermal hypersensitivity, given the relatively low frequency and importance of this symptom compared to spontaneous pain and mechanical allodynia [1,10,20]. Save for the von Frey test (relatively insensitive) and the paw-withdrawal test (relatively sensitive), IBNtxA AD50’s were statistically equivalent on all assays, with a median value across assays of 0.355 mg/kg.
4.2. Mechanism of action of IBNtxA
Evidence for a non-traditional mu-opioid mechanism of IBNtxA analgesia was provided via the demonstration of IBNtxA’s analgesic profile in knockout mice. IBNtxA analgesia is abolished in mutants lacking exon 11 of the Oprm1 gene, the exon required for all known 6-TM splice variants (mMOR-1G, -1K, -1L, -1M, and -1N). These animals remain sensitive to morphine and methadone presumably due their continued expression of the full length 7-TM variants [11]. In contrast, IBNtxA retains activity in an exon 1 Oprm1 knockout that lacks all the full length 7-TM variants but still expresses 6-TM variants [11]. This residual analgesia is not due to cross-activation of delta- or kappa1-opioid receptors. IBNtxA analgesia also was preserved in triple mu/delta/kappa-opioid null mutant mice generated by crossing the exon 1 Oprm1 knockout with Oprd1 (delta-opioid) and Oprk1 (kappa1-opioid) knockout mice. The current study reinforces the notion that IBNtxA is producing pain inhibition across various modalities via 6-TM opioid receptors. First, the isobolographic analysis revealed full additivity between IBNtxA and morphine on the formalin test. Additivity strongly suggests that these compounds are activating separable and non-interacting biological mechanisms. Second, for CFA- and SNI-induced mechanical allodynia, IBNtxA was utterly without efficacy in exon 11 KO mice at doses producing full reversal of allodynia in wildtypes.
4.3. A role for 6-TM μ-opioid receptors in pain processing?
Although not reaching statistical significance, we were intrigued by the trend towards increased mechanical allodynia after CFA and SNI in the exon 11 KO mice (see Supplementary Fig. 1), especially since that trend reappeared in the separate IBNtxA anti-allodynia experiment (Fig. 4), showing statistical significance on day 4 after CFA. Thus, the possibility exists that 6-TM μ-opioid receptors might be affecting pain levels after injury, perhaps by exerting some sort of analgesic “opioid tone”. A fuller understanding of this possibility will require the identification of the endogenous ligand for 6-TM variants.
4.5. Downregulation of splice variant mMOR-1D by SNI
The SNI and the CFA pain models had very little effect on mRNA levels. The sole change achieving statistical significance after correction for multiple comparisons was the lowered expression of mMOR-1D in the DRG after SNI. The lack of changes of the other variants in these models is equally important, suggesting that the SNI and CFA pain models can modulate expression of a specific splice variant in a region-specific fashion through alternative splicing mechanisms, rather than promoter activities. The significance of the observed change in mMOR-1D is not clear at present, and will need to be explored further. It also is important to note that changes in mRNA may, or may not, correspond to changes in protein levels.
4.6. 6-TM and 7-TM opioid pain modulatory systems
Our results support the concept of two, independent mu-opioid pain modulatory systems. The traditional opioids, such as morphine and methadone, work through the traditional full length 7-TM variants, whereas IBNtxA works independently of these receptors, but is instead dependent upon the 6-TM variants. Evidence is accumulating that some opioids may have mixed actions. This is best seen with buprenorphine (S. Grinnell, M. Aronson, Y.-X. Pan, J. Pintar, and G.W. Pasternak, manuscript submitted). Buprenorphine analgesia is diminished in the exon 1 Oprm1 KO mouse. At the same time, buprenorphine analgesia is totally eliminated in the exon 11 KO, indicating the importance of both systems. Another compound with both traditional and 6-TM actions is levorphanol [11]. This dual classification for levorphanol may help explain earlier studies showing unidirectional cross-tolerance between morphine and levorphanol [16]. In these studies, rats tolerant to morphine remained sensitive to levorphanol while levorphanol-tolerant rats also were tolerant to morphine. Thus, there appear to be three separate categories of mu-opioids. One includes drugs like morphine that act through the full length 7-TM set of variants, a second includes compounds like IBNtxA that act through the 6-TM set, and a third group that works through a combination of both.
Supplementary Material
Acknowledgments
This research was supported by the Louise and Alan Edwards Foundation (J.S.M.) and the U.S. National Institutes of Health (G.W.P.). The first author was supported by a Vanier Canada Graduate Scholarship from the Canadian Institutes for Health Research.
Footnotes
None of the authors has a conflict of interest related to this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Backonja M-M, Stacey B. Neuropathic pain symptoms relation to overall pain rating. J Pain. 2004;5:491–497. doi: 10.1016/j.jpain.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Opioid complications and side effects. Pain Physician. 2008;11:S105–S120. [PubMed] [Google Scholar]
- 3.Berge O-G. Predictive validity of behavioural animal models for chronic pain. Br J Pharmacol. 2011;164:1195–1206. doi: 10.1111/j.1476-5381.2011.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bove G. Mechanical sensory threshold testing using nylon monofilaments: the pain field’s “Tin Standard”. Pain. 2006;124:13–17. doi: 10.1016/j.pain.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 5.Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain. 2004;109:514–519. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Chesler EJ, Ritchie J, Kokayeff A, Lariviere WR, Wilson SG, Mogil JS. Genotype-dependence of gabapentin and pregabalin sensitivity: the pharmacogenetic mediation of analgesia is specific to the type of pain being inhibited. Pain. 2003;106:325–335. doi: 10.1016/S0304-3959(03)00330-0. [DOI] [PubMed] [Google Scholar]
- 7.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 8.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 9.Langford DL, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, LaCroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SB, Tabaka JM, Wong D, van den Maagdenberg AMJM, Ferrari MD, Craig KD, Mogil JS. Coding of facial expressions of pain in the laboratory mouse. Nat Meth. 2010;7:447–449. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- 10.Maier C, Baron R, Tolle TR, Binder A, Birbaumer N, Birklein F, Gierthmuhlen J, Flor H, Geber C, Huge V, Krumova EK, Landwehrmeyer GB, Magerl W, Maihofner C, Richter H, Rolke R, Scherens A, Schwarz A, Sommer C, Tronnier V, Uceyler N, Valet M, Wasner G, Treede R-D. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150:439–450. doi: 10.1016/j.pain.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Majumdar S, Grinnell S, Le Rouzic V, Burgman M, Polikar L, Ansonoff M, Pintar J, Pan YX, Pasternak GW. Truncated G protein-coupled mu opioid receptor MOR-1 splice variants are targets for highly potent opioid analgesics lacking side effects. Proc Natl Acad Sci USA. 2011;108:19778–19783. doi: 10.1073/pnas.1115231108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthes HWD, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the μ-opioid-receptor gene. Nat. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 13.Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- 14.Mogil JS, Chanda ML. The case for the inclusion of female subjects in basic science studies of pain. Pain. 2005;117:1–5. doi: 10.1016/j.pain.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Mogil JS, Crager SE. What should we be measuring in behavioral studies of chronic pain in animals? Pain. 2004;112:12–15. doi: 10.1016/j.pain.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 16.Moulin DE, Ling GSF, Pasternak GW. Unidirectional cross-tolerance between morphine and levorphanol in the rat. Pain. 1988;33:233–239. doi: 10.1016/0304-3959(88)90095-4. [DOI] [PubMed] [Google Scholar]
- 17.Pan Y-X, Xu J, Xu M, Rossi GC, Matulonis JE, Pasternak GW. Involvement of exon 11-associated variants of the mu opioid receptor MOR-1 in heroin, but not morphine, actions. Proc Natl Acad Sci U S A. 2009;106:4917–4922. doi: 10.1073/pnas.0811586106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasternak GW, Pan YX. Mu opioids and their receptors: evolution of a concept. Pharmacol Rev. 2013;65:1257–1317. doi: 10.1124/pr.112.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scholz J, Mannion RJ, Hord DE, Griffin RS, Rawal B, Zheng H, Scoffings D, Phillips A, Guo J, Laing RJ, Abdi S, Decosterd I, Woolf CJ. A novel tool for the assessment of pain: validation in low back pain. PLoS Med. 2009;6:e1000047. doi: 10.1371/journal.pmed.1000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shabalina SA, Zaykin DV, Gris P, Ogurtsov AY, Gauthier J, Shibata K, Tchivileva IE, Belfer I, Mishra B, Kiselycznyk C, Wallace MR, Staud R, Spiridonov NA, Max MB, Goldman D, Fillingim RB, Maixner W, Diatchenko L. Expansion of the human μ-opioid receptor gene architecture: novel functional variants. Hum Mol Genet. 2009;18:1037–1051. doi: 10.1093/hmg/ddn439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shields SD, Eckert WA, III, Basbaum AI. Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. J Pain. 2003;4:465–470. doi: 10.1067/s1526-5900(03)00781-8. [DOI] [PubMed] [Google Scholar]
- 23.Sotocinal SG, Sorge RE, Zaloum A, Tuttle AH, Martin LJ, Wieskopf JS, Mapplebeck JCS, Wei P, Zhan S, Zhang S, McDougall JJ, King OD, Mogil JS. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain. 2011;7:55. doi: 10.1186/1744-8069-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tallarida RJ, Murray RB. Manual of Pharmacologic Calculation. New York: Springer-Verlag; 1981. [Google Scholar]
- 25.Vandesompele J, De PK, Pattyn F, Poppe B, Van RN, De PA, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:34. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vierck CJ, Hansson PT, Yezierski RP. Clinical and pre-clinical pain assessment: are we measuring the same thing? Pain. 2008;135:7–10. doi: 10.1016/j.pain.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Wilson SG, Mogil JS. Measuring pain in the (knockout) mouse: big challenges in a small mammal. Behav Brain Res. 2001;125:65–73. doi: 10.1016/s0166-4328(01)00281-9. [DOI] [PubMed] [Google Scholar]
- 28.Xu J, Xu M, Bolan E, Gilbert AK, Pasternak GW, Pan Y-X. Isolating and characterizing three alternatively spliced mu opioid receptor variants: mMOR-1A, mMOR-1O and mMOR-1P. Synapse. 2013 doi: 10.1002/syn.21727. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J, Xu M, rossi GC, Kest B, Pasternak GW, Pan Y-X. Differential expression of alternatively spliced variant mRNAs from the mu opioid receptor (OPRM1) gene in brain regions of four inbred mouse strains. Soc Neurosci Abstr. 2009;35:232.231. doi: 10.1371/journal.pone.0111267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu J, Xu M, Rossi GC, Pasternak GW, Pan Y-X. Identification and characterization of seven new exon 11-associated splice variants of the rat mu opioid receptor gene, OPRM1. Mol Pain. 2011;7:9. doi: 10.1186/1744-8069-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


