Abstract
We implemented a virtual reality system to quantify differences in the use of visual feedback to maintain balance during walking between healthy young (n = 12, mean age: 24 years) and healthy old (n = 11, 71 years) adults. Subjects walked on a treadmill while watching a speed-matched, virtual hallway with and without mediolateral visual perturbations. A motion capture system tracked center of mass (CoM) motion and foot kinematics. Spectral analysis, detrended fluctuation analysis, and local divergence exponents quantified old and young adults’ dynamic response to visual perturbations. Old and young adults walked normally with comparable CoM spectral characteristics, lateral step placement temporal persistence, and local divergence exponents. Perturbed visual flow induced significantly larger changes in mediolateral CoM motion in old vs. young adults. Moreover, visual perturbations disrupted the control of lateral step placement and compromised local dynamic stability more significantly in old than young adults. Advanced age induces a greater reliance on visual feedback to maintain balance during waking, an effect that may compensate for degradations in somatosensation. Our findings are relevant to the early diagnosis of sensory-induced balance impairments and also point to the potential use of virtual reality to evaluate sensory rehabilitation and balance training programs for old adults.
Keywords: Optical flow, Stability, Elderly, Sensorimotor, Virtual reality
1. Introduction
Lateral step placement is an important control variable in the maintenance of balance during walking (Bauby & Kuo, 2000; Donelan, Shipman, Kram, & Kuo, 2004). In contrast to step placement in the direction of movement, which benefits from some passive stability, lateral step placement is more highly dependent upon the integration of reliable visual, vestibular, and somatosensory feedback (Bauby & Kuo, 2000; Collins & Kuo, 2013; Donelan et al., 2004; O'Connor & Kuo, 2009; O'Connor, Xu, & Kuo, 2012). It is well recognized that the quality of sensory information declines considerably with advanced age (Patel, Magnusson, Kristinsdottir, & Fransson, 2009). These changes are functionally exacerbated by longer reflex latencies and slower maximum rates of muscle force development in old adults (Dorfman & Bosley, 1979; Thelen, Schultz, Alexander, & Ashton-Miller, 1996). Ultimately, age-related sensorimotor decline may compromise old adults’ effective use of lateral step placement to maintain balance during walking, thereby increasing their risk of falling. Indeed, one third of old adults (i.e., 65+ years) fall annually and most of these falls occur during locomotion (Berg, Alessio, Mills, & Tong, 1997; Niino, Tsuzuku, Ando, & Shimokata, 2000; Tinetti, Speechley, & Ginter, 1988).
Evidence from the postural control literature may provide indirect insight into how old adults differ from young adults in their use of sensory feedback to maintain balance during walking. For example, using a unique combination of visual and somatosensory perturbations, Eikema, Hatzitaki, Konstantakos, and Papaxanthis (2013) found that advanced age brought an increased sensitivity to visual feedback coupled with a reduced sensitivity to tendon vibration. A common interpretation of these findings is that a decline in somatosensory feedback with age brings a greater reliance on visual feedback for postural control (Bugnariu & Fung, 2007; Eikema et al., 2013; Jeka, Allison, & Kiemel, 2010; Sundermier, Woollacott, Jensen, & Moore, 1996; Yeh, Cluff, & Balasubramaniam, 2014). In addition, Yeh et al. (2014) found that visual reliance in old adults was direction-dependent, with greater sensitivity to visual perturbations in the mediolateral control of posture. The well documented age-related increase in visual reliance suggests that there may also be a unique role of visual feedback in old adults’ control of lateral step placement and balance during walking.
Removing or disrupting visual feedback impairs the control of lateral step placement during walking in young adults (Bauby & Kuo, 2000; McAndrew, Dingwell, & Wilken, 2010; O'Connor & Kuo, 2009; O'Connor et al., 2012). For example, compared to normal walking, Bauby and Kuo (2000) found that step width variability disproportionately increased when young adults walked with their eyes closed. More recently, O'Connor and Kuo (2009) and O'Connor et al. (2012) have used virtual reality to reveal that the control of lateral step placement in young adults is compromised more by mediolateral than anterior-posterior visual perturbations. If old adults rely more on visual feedback than young adults to maintain balance during walking, we would anticipate more pronounced effects of visual perturbations on their control of lateral step placement.
Dynamic analysis of center of mass (CoM) motion and step placement can provide insights into the sensorimotor control of balance during walking. For example, spectral analysis can quantify one's dynamic response to visual perturbations (Loughlin & Redfern, 2001), and the temporal dynamics of CoM motion can delineate old adults at risk of falls (Latt, Menz, Fung, & Lord, 2009). In addition, the emergence of lateral step placement as a balance control variable during walking suggests a high probability for step-to-step dependence. Detrended fluctuation analysis (DFA) quantifies step-to-step correlations and is commonly used to study walking in healthy adults and those with neurological impairment (Dingwell & Cusumano, 2010; Hausdorff et al., 1997). Finally, local dynamic stability quantified via maximum divergence exponents is strongly associated with falls risk in old adults and characterizes one's resilience to naturally occurring perturbations arising from both internal (e.g., age-related neuromuscular noise) and external (e.g., altered visual flow) factors (Kang & Dingwell, 2008; Toebes, Hoozemans, Furrer, Dekker, & van Dieen, 2012). Together, these analyses represent robust metrics to investigate the disparate role of visual feedback in old and young adults’ maintenance of balance during walking.
The purpose of this study was to investigate whether old adults rely more on visual feedback than young adults to actively control balance during walking. We implemented a virtual reality system to perturb visual flow during treadmill walking and quantified the effects on center of mass motion and step placement dynamics. We hypothesized that perturbed visual flow would: (1) amplify mediolateral CoM motion, (2) disrupt the step-to-step control of lateral step placement, and (3) compromise local dynamic stability in old significantly more than young adults.
2. Methods
2.1. Subjects
12 healthy young (mean ± standard deviation, age: 23.6 ± 3.8 years, mass: 70.7 ± 11.3 kg, leg length: 0.82 ± 0.05 m) and 11 healthy old adults (age: 71.2 ± 4.2 years, mass: 66.9 ± 9.6 kg, leg length: 0.75 ± 0.04 m) participated. Subjects provided written informed consent as per the University of Wisconsin Health Sciences Internal Review Board. All subjects completed a health questionnaire based on recommendations of the American College of Sports Medicine (ACSM., 2014). We excluded subjects based on the following: BMI ≥ 30, sedentary lifestyle, first degree family history of coronary artery disease, tobacco use, high blood pressure, high cholesterol, diabetes or prediabetes, orthopedic or neurological condition, taking medication that causes dizziness, or any unanticipated falls in the prior 6 months.
2.2. Experiment
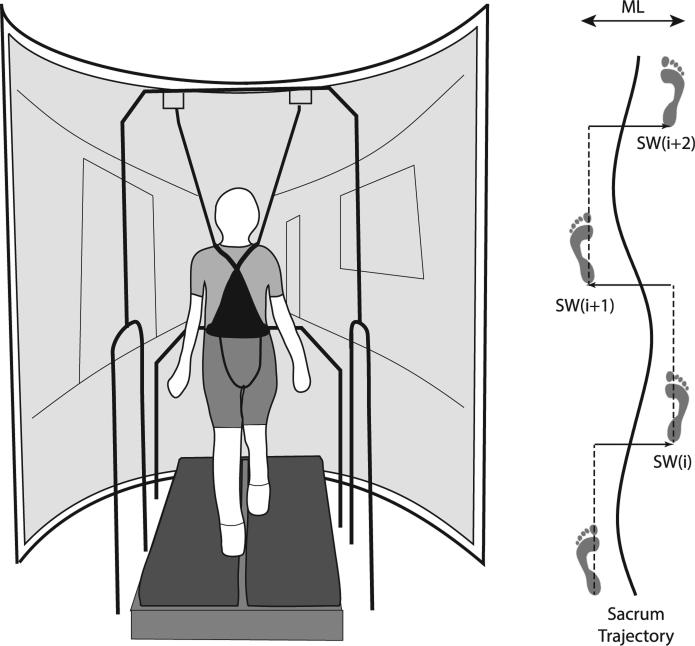
We first assessed subjects’ preferred overground walking speed from the average of two times taken to traverse the middle 6 m of a 10 m walkway when asked to walk at a normal, comfortable speed (old: 1.28 ± 0.10 m/s, young: 1.36 ± 0.12 m/s, p = .11). Subjects then completed all treadmill walking conditions at their preferred overground speed. Note that three old adults were unable to maintain their preferred overground speed on the treadmill without holding onto the handrails. To accommodate these subjects, we decreased their treadmill speed by 10% before collecting data. Subjects walked on a dual-belt, force-sensing treadmill (Bertec, Inc., Columbus, OH) while viewing a speed-matched virtual hallway (Fig. 1). The virtual hallway, designed to previously published specifications (O'Connor & Kuo, 2009; O'Connor et al., 2012), was rear-projected onto a semicircular screen approximately 2.75 m high and 2.25 m wide which surrounded the treadmill. To account for the screen's curvature, a custom Matlab (Mathworks, Inc., Natick, MA) routine corrected the geometry of the image, composed of a dark hallway with randomly placed white rectangles and doors and windows at regular intervals. We asked the subjects to look forward, but provided no further instructions to allow subjects to naturally adapt to the visual information. Old adults walked while wearing a support harness connected overhead.
Fig. 1.
We implemented a virtual reality system to perturb visual flow during treadmill walking and quantified the effects on sacrum motion (a surrogate for the center of mass) and step placement dynamics. We used heel marker data to construct time series of alternating left and right step widths.
In random order, subjects walked while watching the virtual hallway with and without continuous mediolateral (ML) visual perturbations. Perturbations consisted of a sum of two sinusoids (0.135 and 0.442 Hz) with 0.175 m amplitudes, applied as translational perturbations of the visual field in the frontal plane. The perturbations were consistent with the visual feedback associated with head movements within a hallway, such that the hallway's end moved very little compared to the hallway portion in the foreground (O'Connor et al., 2012). This ensured that perturbations induced balance and not heading corrections. These perturbations retained the smaller amplitude sinusoids from a perturbation previously found to significantly affect young adults’ lateral foot placement (O'Connor et al., 2012). After subjects became comfortable walking without using the handrails (~1 min), we recorded kinematic data for 3 min per trial. A motion capture system operating at 100 Hz recorded the three-dimensional positions of retroreflective markers placed on subjects’ sacrum (a surrogate for their CoM) and right and left posterior calcanei.
2.3. Data analysis
We low-pass filtered marker trajectories using a fourth-order Butterworth filter and a 12 Hz cut-off frequency. We identified the instant of right and left heel-strikes based on the anterior-posterior heel positions relative to the sacral marker (Zeni, Richards, & Higginson, 2008). Zeni et al. (2008) found that this technique identified heel-strikes during walking to within 0.017 s compared to those identified more conventionally using ground reaction forces. We analyzed at least 300 consecutive steps for each 3 min trial.
We quantified old and young adults’ dynamic response to visual perturbations via three analyses. First, we used spectral analysis to quantify the intensity of visual perturbation frequencies in ML sacrum kinematics. Matlab routines computed the fast Fourier transform (FFT) and power spectral density of the ML sacral marker trajectories during normal and visually perturbed walking. Because analyzing only absolute or relative spectral characteristics can obscure age-related differences (Loughlin & Redfern, 2001), we calculated for each condition both the average FFT (i.e., absolute sway intensity) and energy-normalized power spectral density (i.e., sway intensity relative to the total energy of sway) at each of the two visual perturbation frequencies.
Second, we assessed the statistical persistence of lateral step placement using detrended fluctuation analysis (DFA), which quantifies the likelihood that sequential steps are correlated over time. To do this, we constructed time series of step widths from the ML distance between calcaneus markers across successive steps, averaged during midstance prior to heel-rise (i.e., 12–25% of each stride) (Perry & Burnfield, 2010). These time series contained alternating left and right step widths, as illustrated in Fig. 1. We then quantified the temporal persistence of step width in these time series using DFA. Prior studies have most often used linear DFA to remove 1st order trends arising from nonstationarities (i.e., time-dependent signal drift) in step kinematics during walking (Dingwell & Cusumano, 2010; Hausdorff et al., 1997). However, due to the prevalence of nonlinear processes in neuromuscular control (Guastello, 2006; Ting et al., 2009), nonlinear DFA has the potential to reveal clinically relevant information not captured by linear DFA (Deffeyes et al., 2009). Therefore, we used 1st and 2nd order DFA to compute the root mean square of detrended residuals of step width over a range of steps between 4 and N/4, where N was the total number of steps (Dingwell & Cusumano, 2010). We interpreted the scaling exponents (α1D and α2D) of the relation between the root mean square (RMS) residuals of step width and the number of steps as follows: α = 0.5 indicates uncorrelated white noise; α < 0.5 indicates that deviations in one direction are likely to be followed by deviations in the opposite direction (i.e., anti-persistent); and α > 0.5 indicates that deviations in one direction are likely to be followed by deviations in the same direction (i.e., persistent).
Finally, we calculated maximum divergence (Lyapunov) exponents from time series of right and left heel positions relative to the sacrum position. Maximum divergence exponents quantify the sensitivity of a system to small, naturally occurring perturbations and serve as a metric of local dynamic stability (Kang & Dingwell, 2008). There is no consensus in the literature as to which variables to include in local dynamic stability analyses of walking. Therefore, we adopted time series most consistent with the purpose of this study – those corresponding to foot trajectories and CoM motion. Consistent with prior studies in young adults (McAndrew, Wilken, & Dingwell, 2011), visual perturbations introduced considerable nonstationarities in the ML marker positions. Therefore, we opted to exclude the ML kinematics from our local dynamic stability analysis (see Section 4 for alternative approaches and outcomes). For the embedding delay, we used one quarter of subjects’ average stride time for all conditions (Virgin, 2000) and determined the corresponding embedding dimension (dE = 5) using a 10% criterion in a false nearest neighbors analysis (Kennel, Brown, & Abarbanel, 1992). We then calculated the average maximum exponential rates of divergence of pairs of initially neighboring trajectories using procedures outlined in detail previously (Kang & Dingwell, 2008; Rosenstein, Collins, & De Luca, 1993; Toebes et al., 2012). We time normalized the divergence curves to account for differences in subjects’ stride period, and computed short-term (γs, 0–1 stride) and long-term (γL, 4–10 strides) divergence exponents for each subject, where larger positive values imply larger local instability (Kang & Dingwell, 2008).
2.4. Statistical analysis
An analysis of variance (ANOVA) for repeated measures tested for significant main effects of and interactions between age and condition on CoM spectral peak values, DFA scaling exponents, and short- and long-term divergence exponents using a p < .05 criterion. We further evaluated significant main effects of age and condition using independent-samples t-tests and post hoc pairwise comparisons, respectively.
3. Results
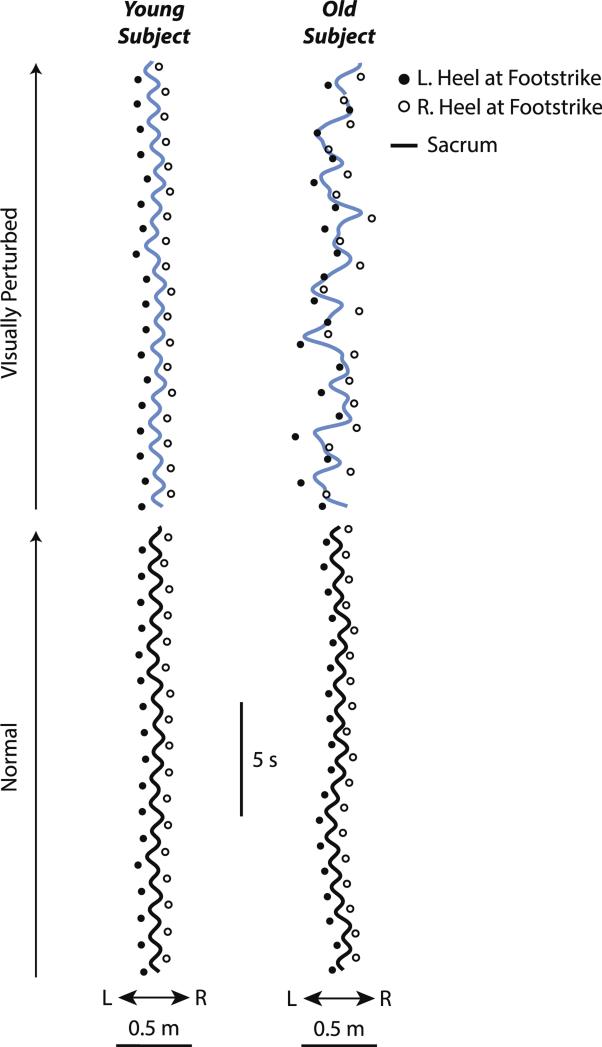
Visual perturbations uniquely affected the relation between ML sacrum motion and lateral step placement during walking in old adults. Lateral step placement delimited ML sacrum motion during both normal and visually perturbed walking in young adults, and during normal walking in old adults (Fig. 2). In contrast, visual perturbations disrupted this systematic behavior in old adults, frequently leading to lateral step placements that fell beneath or even contralateral to the instantaneous ML sacrum position.
Fig. 2.
Relation between ML sacrum motion and lateral step placement during normal (black line) and visually perturbed (blue line) waking for representative old and young subjects. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.1. Center of mass spectral characteristics
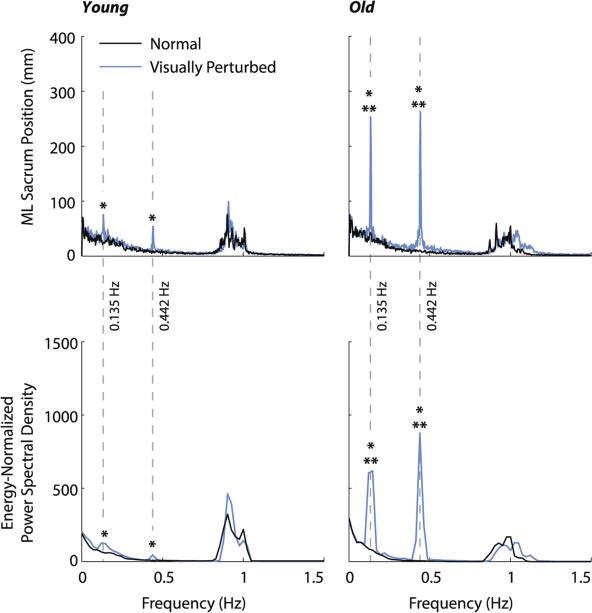
Relative to normal walking, ML sacrum kinematics in both age groups exhibited significantly greater signal intensity at the two visual perturbation frequencies (p < .01), indicating considerable ML sway during visually perturbed walking (Fig. 3). However, this effect was significantly larger for old adults (age × condition, p < .01). For example, although the spectral characteristics of old and young adults did not differ during normal walking, old adults averaged an order of magnitude larger change in peak power spectral density than young adults in response to visual perturbations (Fig. 3).
Fig. 3.
Group average spectral characteristics of the ML sacrum position in old and young adults. Gray dashed lines indicate the visual perturbation frequencies. Single asterisks (*) indicate significant difference between normal and visually perturbed walking, and double asterisks (**) indicate significant difference between old and young adults (p < .05).
3.2. Step-to-step control of lateral step placement
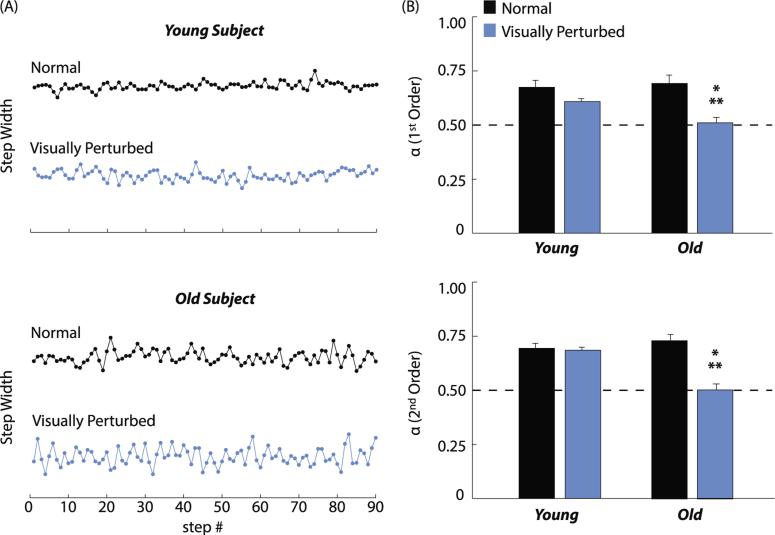
During normal walking, time series of step widths for both old and young adults exhibited prolonged phases over which consecutive steps became progressively wider or narrower (Fig. 4A). Indeed, lateral step placement was equally temporally persistent (i.e., α >> 0.5) in old and young adults during normal walking (p > .32) (Fig. 4B and Table 1). However, independent of whether we used a first or second order model, visual perturbations effectively eliminated the temporal persistence of step width in old but not young adults (old vs. young, α1D: 0.51 ± 0.09 vs. 0.61 ± 0.06, p < .01; α2D: 0.50 ± 0.09 vs. 0.68 ± 0.05, p < .01). The decorrelation in old adults’ lateral step placement during visually perturbed walking was qualitatively evidenced by indiscriminate step-to-step fluctuations in step width (Fig. 4A).
Fig. 4.
Detrended fluctuation analysis results. (A) Step width time series over 90 consecutive steps extracted from the 300 analyzed during normal (black line) and visually perturbed (blue line) walking for representative old and young subjects. Normal and visually perturbed conditions are offset vertically only for clarity, and do not represent differences in mean step width. (B) Mean (standard error) 1st and 2nd order scaling exponents of the relation between the root mean square (RMS) residual of step width and number of steps. We interpreted these scaling exponents as follows: α = 0.5 indicates uncorrelated white noise; α < 0.5 indicates that deviations in one direction are likely to be followed by deviations in the opposite direction (i.e., anti-persistent); and α > 0.5 indicates that deviations in one direction are likely to be followed by deviations in the same direction (i.e., persistent). Single asterisks (*) indicate significant difference between normal and visually perturbed walking, and double asterisks (**) indicate significant difference between old and young adults (p < .05). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Goodness of fit (R2) for estimates of DFA and maximum local divergence exponents.
| DFA, α (1st order) |
DFA, α (2nd order) |
λ
S
|
λ
L
|
|||||
|---|---|---|---|---|---|---|---|---|
| Old | Young | Old | Young | Old | Young | Old | Young | |
| Normal | 0.96 (0.02) | 0.96 (0.03) | 0.95 (0.02) | 0.94 (0.04) | 0.99 (~) | 0.99 (~) | 0.98 (0.01) | 0.98 (0.02) |
| Visually perturbed | 0.95 (0.03) | 0.97 (0.01) | 0.92 (0.03) | 0.94 (0.02) | 0.99 (~) | 0.99 (~) | 0.96 (0.05) | 0.96 (0.03) |
Values are mean (standard deviation). DFA: detrended fluctuation analysis. λS and λL refer to the maximum short- and long-term local divergence exponents. Tildes (~) indicate standard deviation <.005.
3.3. Local dynamic stability
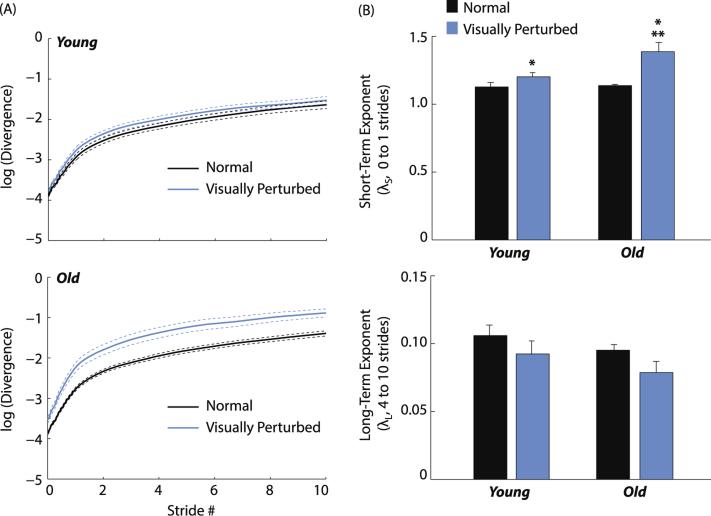
Old and young adults exhibited similar short-term (0–1 strides) and long-term (4–10 strides) local divergence exponents during normal walking (p = .73 and p = .17 for γS and γL, respectively) (Fig. 5 and Table 1). However, compared to normal walking, short-term divergence exponents increased significantly more for old than young adults in response to visual perturbations (old: 1.38 ± 0.20 vs. 1.14 ± 0.05; young: 1.20 ± 0.10 vs. 1.12 ± 0.12; age × condition, p = .01). Visual perturbations elicited a 226% larger increase in short-term divergence exponents for old vs. young adults, indicating significantly greater local instability. Visual perturbations did not significantly alter long-term divergence exponents in old or young adults (p = .08 and p = .12, respectively) (Fig. 5).
Fig. 5.
Local dynamic stability results. (A) Mean (standard error) divergence curves (Dingwell, 2006) for old and young adults during normal (black line) and visually perturbed (blue line) walking. (B) Mean (standard error) maximum short-term and long-term divergence exponents, where larger positive values are indicative of greater local dynamic instability. Single asterisks (*) indicate significant difference between normal and visually perturbed walking, and double asterisks (**) indicate significant difference between old and young adults (p < .05). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Our findings provide compelling evidence that old adults rely more on visual feedback than young adults to actively control balance during walking. As hypothesized, perturbed visual flow induced significantly larger changes in the spectral characteristics of CoM motion in old vs. young adults. Moreover, and also as hypothesized, we found that visual perturbations disrupted the step-to-step control of lateral step placement and compromised local dynamic stability in old significantly more than young adults. As we discuss in more detail below, these findings may be particularly relevant to the early diagnosis of sensory-induced balance impairments and also point to the potential use of virtual reality to evaluate the efficacy of sensory rehabilitation and balance training programs for old adults.
Studies of the postural control of standing have revealed that advanced age is accompanied by a reliance on visual feedback (Bugnariu & Fung, 2007; Jeka et al., 2010; Sundermier et al., 1996; Yeh et al., 2014) coupled with a reduced sensitivity to somatosensory perturbations (Eikema et al., 2013; Thelen, Brockmiller, Ashton-Miller, Schultz, & Alexander, 1998). These changes are attributed to age-related sensory reweighting, predicated on a decline in the quality of somatosensory signaling in old adults. Our findings extend these observations and imply that age-related sensorimotor adaptations manifest in old adults’ relative utilization of sensory feedback to maintain balance during walking. We arrived at this conclusion by interpreting the results of three dynamic analyses each shown previously to provide unique insights into age-related balance impairment (Dingwell & Cusumano, 2010; Hausdorff et al., 1997; Latt et al., 2009; Loughlin & Redfern, 2001).
When old and young adults walked normally, the spectrum of their ML sacrum motion was comparable and dominated by motion at their stride frequencies (~1 Hz). During visually perturbed walking, distinct peaks at both of the perturbation frequencies also appeared in old and young adults’ dynamic response, presumably through a complex series of sensorimotor pathways. However, compared to that in young adults, ML sacrum motion at the visual perturbation frequencies averaged 3.2 times larger in old adults. Conceptually, if we simplify the sensorimotor control of walking to that of a linear dynamic system, with visual information as an input and CoM motion as the output, our results imply that old adults exhibit a substantially larger visual feedback gain than young adults during walking. Prior studies have proposed a similar interpretation for the postural control of standing (Eikema et al., 2013; Jeka et al., 2010; Sundermier et al., 1996; Yeh et al., 2014). This age-related increase in visual feedback gain (or, alternatively, a decrease in visual inhibition) provides important clues regarding the sensorimotor control of balance during walking in old adults.
In contrast to the continuous control of posture, ML motion of the body's CoM during walking is believed to be primarily controlled on a step-to-step basis by adjusting lateral step placement (Bauby & Kuo, 2000; Collins & Kuo, 2013; O'Connor & Kuo, 2009; O'Connor et al., 2012). Walking normally, lateral step placement in old and young adults exhibited temporal persistence, such that a wide right step was likely to be followed by a wide left step (and vice versa). Intuitively, this step-to-step dependence effectively delimits fluctuations in ML CoM motion (Fig. 2), and is therefore an important characteristic of normal walking (Collins & Kuo, 2013; Hof, van Bockel, Schoppen, & Postema, 2007). However, as hypothesized, we found that visual perturbations effectively eliminated the step-to-step temporal persistence of lateral foot placement in old but not young adults. In other words, due to the heightened sensitivity of old adults to visual feedback, visual perturbations compromised the systematic use of lateral step placement to maintain balance during walking. Prior studies have shown that patients with various central nervous system disorders exhibit a similar decorrelation in step parameters that parallels their degree of functional impairment (Hausdorff et al., 1997). Hence, our findings point to a direct link between the CNS integration of visual information and its control of lateral step placement – a link that is enhanced with advanced age.
Finally, as hypothesized, visual perturbations compromised the local dynamic stability of old significantly more than young adults. On average, compared to young adults, short-term (0–1 stride) local divergence exponents of the feet and CoM trajectories increased 230% more for old adults in response to visual perturbations. The greater sensitivity of short-term local divergence exponents to visual perturbations is consistent with prior studies in young adults (McAndrew et al., 2011). In contrast, long-term local divergence exponents are generally insensitive to aging or perturbations because walking on a treadmill at a constant speed necessitates that subjects’ movements remain loosely bounded over the course of many strides (McAndrew et al., 2011; Toebes et al., 2012). Old adults’ short-term local dynamic instability is often interpreted in the context of their inability to recover from an unexpected trip or fall during walking (Kang & Dingwell, 2008; Toebes et al., 2012). Indeed, short-term local dynamic instability, in particular, is strongly associated with falls history in old adults (Toebes et al., 2012). Hence, our results imply that old adults may be less resilient to perturbations and more prone to falling during walking when visual information is poor, absent, or incorrect.
In this study, we opted to exclude the ML marker kinematics to compute local divergence exponents. We acknowledge that there are alternative approaches in local dynamic stability analyses to minimize the effect of nonstationarities in marker positions that arise during perturbed walking. For example, McAndrew et al. (2010) studied visual perturbations in young adults and minimized the effect of nonstationarities by using three-dimensional marker velocity time series to calculate local dynamic stability. We explored this alternative, recognizing that appropriately incorporating ML marker kinematics in our state space reconstruction may better unify these outcomes with the DFA analysis of lateral step placement. However, incorporating ML marker kinematics, either as position or velocity time series, yielded divergence curves with substantially nonlinear short-term regions and thus, no properly defined maximum divergence exponent (Dingwell, 2006). Nevertheless, a linear fit to these obviously nonlinear short-term regions yielded similar trends. Indeed, our primary findings were largely insensitive to the specific state space reconstruction. Alternative approaches that included ML marker kinematics similarly revealed that visual perturbations elicited significantly larger reductions in short-term local dynamic stability for old vs. young adults (110% and 123% for position and velocity time series, respectively, p < .01).
There are several important limitations of this study. First, we used heel marker trajectories that do not account for changes in foot rotation that could influence subjects’ effective step width during walking. Also, we interpret old adults’ enhanced sensitivity to visual perturbations during walking as implying an increased reliance on visual feedback compared to young adults. However, we did not investigate age-related changes in the relative sensitivity of subjects to visual, vestibular, and somatosensory perturbations. A possible alternative conclusion is that advanced age brings a greater sensitivity to sensory perturbations in general. However, Jeka et al. (2010) found visual reliance during the postural control of standing even after controlling for visual, vestibular, and somatosensory acuity. We also did not assess the relative quality of subjects’ sensory feedback systems, and thus cannot establish a causal link between somatosensory decline and the increased sensitivity to visual perturbations observed here for old vs. young adults. Finally, there are numerous methods available to characterize the control of balance during walking (Bruijn, Meijer, Beek, & van Dieen, 2013). Despite each measure having varying levels of validity, old adults’ unique sensitivity to visual perturbations distinctly emerged for each outcome measure we assessed. Regarding the reliability of these estimates, we note that our trial duration and sampling frequency provided an overall number of samples (~3 × 104) found in Rispens et al. (2014) to yield local divergence exponents within 10% of those for a few simple, analytical systems (Rispens et al., 2014).
5. Conclusions
Taken together, our findings suggest that advanced age alters the integration and relative contributions of visual feedback to actively control balance during walking. We propose that advanced age induces an increased reliance on visual feedback to actively control balance during waking, an effect that may compensate for degradations in somatosensory signaling. Consequently, visual perturbations exposed age-related changes relevant to the active control of balance that were not readily apparent during normal, unperturbed walking. Based on this observation, we envision novel clinical tests in which visual perturbations applied during walking make possible the early diagnosis of sensory-induced balance impairments in old adults. Our findings also forecast the promising potential of virtual reality to evaluate the efficacy of sensory rehabilitation and balance training programs for old adults.
Acknowledgments
Supported in part by grants from NIH (F31AG046945, F32AG044904). We thank Michael Schmidt and Holly Schoenberg for their help with data collection and processing. We also thank Richard Wiebe, Ph.D. for his helpful insights regarding the analysis of maximum divergence exponents.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to disclose.
References
- ACSM . Resource manual for guidelines for exercise testing and prescription. 7th ed. American college of sports medicine; Baltimore, MD.: 2014. [Google Scholar]
- Bauby CE, Kuo AD. Active control of lateral balance in human walking. Journal of Biomechanics. 2000;33:1433–1440. doi: 10.1016/s0021-9290(00)00101-9. [DOI] [PubMed] [Google Scholar]
- Berg WP, Alessio HM, Mills EM, Tong C. Circumstances and consequences of falls in independent community-dwelling older adults. Age and Ageing. 1997;26:261–268. doi: 10.1093/ageing/26.4.261. [DOI] [PubMed] [Google Scholar]
- Bruijn SM, Meijer OG, Beek PJ, van Dieen JH. Assessing the stability of human locomotion: A review of current measures. Journal of the Royal Society, Interface. 2013;10:20120999. doi: 10.1098/rsif.2012.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugnariu N, Fung J. Aging and selective sensorimotor strategies in the regulation of upright balance. Journal of NeuroEngineering and Rehabilitation. 2007;4:19. doi: 10.1186/1743-0003-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SH, Kuo AD. Two independent contributions to step variability during over-ground human walking. PLoS One. 2013;8:e73597. doi: 10.1371/journal.pone.0073597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deffeyes JE, Kochi N, Harbourne RT, Kyvelidou A, Stuberg WA, Stergiou N. Nonlinear detrended fluctuation analysis of sitting center-of-pressure data as an early measure of motor development pathology in infants. Nonlinear Dynamics, Psychology, and Life Science. 2009;13:351–368. [PubMed] [Google Scholar]
- Dingwell JB. Lyapunov exponents. In: Akay M, editor. The wiley encyclopedia of biomedical engineering. John Wily & Sons Inc.; New York, NY: 2006. p. 14. [Google Scholar]
- Dingwell JB, Cusumano JP. Re-interpreting detrended fluctuation analyses of stride-to-stride variability in human walking. Gait Posture. 2010;32:348–353. doi: 10.1016/j.gaitpost.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelan JM, Shipman DW, Kram R, Kuo AD. Mechanical and metabolic requirements for active lateral stabilization in human walking. Journal of Biomechanics. 2004;37:827–835. doi: 10.1016/j.jbiomech.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Dorfman LJ, Bosley TM. Age-related changes in peripheral and central nerve conduction in man. Neurology. 1979;29:38–44. doi: 10.1212/wnl.29.1.38. [DOI] [PubMed] [Google Scholar]
- Eikema DJ, Hatzitaki V, Konstantakos V, Papaxanthis C. Elderly adults delay proprioceptive reweighting during the anticipation of collision avoidance when standing. Neuroscience. 2013;234:22–30. doi: 10.1016/j.neuroscience.2012.12.053. [DOI] [PubMed] [Google Scholar]
- Guastello SJ. Motor control research requires nonlinear dynamics. American Psychologist. 2006;61:77–78. doi: 10.1037/0003-066X.61.1.77. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Mitchell SL, Firtion R, Peng CK, Cudkowicz ME, Wei JY, et al. Altered fractal dynamics of gait: Reduced stride-interval correlations with aging and Huntington's disease. Journal of Applied Physiology (1985) 1997;82:262–269. doi: 10.1152/jappl.1997.82.1.262. [DOI] [PubMed] [Google Scholar]
- Hof AL, van Bockel RM, Schoppen T, Postema K. Control of lateral balance in walking. Experimental findings in normal subjects and above-knee amputees. Gait Posture. 2007;25:250–258. doi: 10.1016/j.gaitpost.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Jeka JJ, Allison LK, Kiemel T. The dynamics of visual reweighting in healthy and fall-prone older adults. Journal of Motor Behavior. 2010;42:197–208. doi: 10.1080/00222895.2010.481693. [DOI] [PubMed] [Google Scholar]
- Kang HG, Dingwell JB. Effects of walking speed, strength and range of motion on gait stability in healthy older adults. Journal of Biomechanics. 2008;41:2899–2905. doi: 10.1016/j.jbiomech.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennel MB, Brown R, Abarbanel HD. Determining embedding dimension for phase-space reconstruction using a geometrical construction. Physical Review A. 1992;45:3403–3411. doi: 10.1103/physreva.45.3403. [DOI] [PubMed] [Google Scholar]
- Latt MD, Menz HB, Fung VS, Lord SR. Acceleration patterns of the head and pelvis during gait in older people with Parkinson's disease: A comparison of fallers and nonfallers. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2009;64:700–706. doi: 10.1093/gerona/glp009. [DOI] [PubMed] [Google Scholar]
- Loughlin PJ, Redfern MS. Spectral characteristics of visually induced postural sway in healthy elderly and healthy young subjects. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2001;9:24–30. doi: 10.1109/7333.918273. [DOI] [PubMed] [Google Scholar]
- McAndrew PM, Dingwell JB, Wilken JM. Walking variability during continuous pseudo-random oscillations of the support surface and visual field. Journal of Biomechanics. 2010;43:1470–1475. doi: 10.1016/j.jbiomech.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAndrew PM, Wilken JM, Dingwell JB. Dynamic stability of human walking in visually and mechanically destabilizing environments. Journal of Biomechanics. 2011;44:644–649. doi: 10.1016/j.jbiomech.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niino N, Tsuzuku S, Ando F, Shimokata H. Frequencies and circumstances of falls in the National Institute for Longevity Sciences, Longitudinal Study of Aging (NILS-LSA). Journal of Epidemiology. 2000;10:S90–S94. doi: 10.2188/jea.10.1sup_90. [DOI] [PubMed] [Google Scholar]
- O'Connor SM, Kuo AD. Direction-dependent control of balance during walking and standing. Journal of Neurophysiology. 2009;102:1411–1419. doi: 10.1152/jn.00131.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor SM, Xu HZ, Kuo AD. Energetic cost of walking with increased step variability. Gait Posture. 2012;36:102–107. doi: 10.1016/j.gaitpost.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M, Magnusson M, Kristinsdottir E, Fransson PA. The contribution of mechanoreceptive sensation on stability and adaptation in the young and elderly. European Journal of Applied Physiology. 2009;105:167–173. doi: 10.1007/s00421-008-0886-4. [DOI] [PubMed] [Google Scholar]
- Perry J, Burnfield JM. Gait analysis: Normal and pathological function. 2nd ed. SLACK Incorporated; Thorofare, NJ: 2010. [Google Scholar]
- Rispens SM, Pijnappels M, van Dieen JH, van Schooten KS, Beek PJ, Daffertshofer A. A benchmark test of accuracy and precision in estimating dynamical systems characteristics from a time series. Journal of Biomechanics. 2014;47:470–475. doi: 10.1016/j.jbiomech.2013.10.037. [DOI] [PubMed] [Google Scholar]
- Rosenstein MT, Collins JJ, De Luca CJ. A practical method for calculating largest Lyapunov exponents from small data sets. Physica D: Nonlinear Phenomena. 1993;65:117–134. [Google Scholar]
- Sundermier L, Woollacott MH, Jensen JL, Moore S. Postural sensitivity to visual flow in aging adults with and without balance problems. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 1996;51:M45–M52. doi: 10.1093/gerona/51a.2.m45. [DOI] [PubMed] [Google Scholar]
- Thelen DG, Brockmiller C, Ashton-Miller JA, Schultz AB, Alexander NB. Thresholds for sensing foot dorsi- and plantarflexion during upright stance: effects of age and velocity. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 1998;53:M33–M38. doi: 10.1093/gerona/53a.1.m33. [DOI] [PubMed] [Google Scholar]
- Thelen DG, Schultz AB, Alexander NB, Ashton-Miller JA. Effects of age on rapid ankle torque development. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 1996;51:M226–M232. doi: 10.1093/gerona/51a.5.m226. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. New England Journal of Medicine. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- Ting LH, van Antwerp KW, Scrivens JE, McKay JL, Welch TD, Bingham JT, et al. Neuromechanical tuning of nonlinear postural control dynamics. Chaos. 2009;19:026111. doi: 10.1063/1.3142245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toebes MJ, Hoozemans MJ, Furrer R, Dekker J, van Dieen JH. Local dynamic stability and variability of gait are associated with fall history in elderly subjects. Gait Posture. 2012;36:527–531. doi: 10.1016/j.gaitpost.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Virgin LN. Introduction to experimental nonlinear dynamics: A case study in mechanical vibration. Cambridge University Press; Cambridge, UK: 2000. [Google Scholar]
- Yeh TT, Cluff T, Balasubramaniam R. Visual reliance for balance control in older adults persists when visual information is disrupted by artificial feedback delays. PLoS One. 2014;9:e91554. doi: 10.1371/journal.pone.0091554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeni JA, Jr., Richards JG, Higginson JS. Two simple methods for determining gait events during treadmill and overground walking using kinematic data. Gait Posture. 2008;27:710–714. doi: 10.1016/j.gaitpost.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]