Abstract
Upper airway dilator muscles are phasically activated during respiration. We assessed the interaction between central respiratory drive and local (mechanoreceptive) influences upon genioglossal (GG) activity throughout inspiration. GGEMG and airway mechanics were measured in 16 awake subjects during baseline spontaneous breathing, increased central respiratory drive (inspiratory resistive loading; IRL), and decreased respiratory drive (hypocapnic negative pressure ventilation), both prior to and following dense upper airway topical anesthesia. Negative epiglottic pressure (Pepi) was significantly correlated with GGEMG across inspiration (i.e. within breaths). Both passive ventilation and IRL led to significant decreases in the sensitivity of the relationship between GGEMG and Pepi (slope GGEMG vs Pepi), but yielded no change in the relationship (correlation) between GGEMG and Pepi. During negative pressure ventilation, pharyngeal resistance increased modestly, but significantly. Anesthesia in all conditions led to decrements in phasic GGEMG, increases in pharyngeal resistance, and decrease in the relationship between Pepi and GGEMG. We conclude that both central output to the GG and local reflex mediated activation are important in maintaining upper airway patency.
Keywords: Control of breathing, central respiratory drive, Mammals, humans, Mechanics of breathing, upper airways, Muscles, genioglossus, Receptors, mechanoreceptors, upper airways, Sleep, apnea, Upper airways, dilator muscles
1. Introdution
There is substantial evidence in both animals and humans that the activity of the genioglossus muscle (GG) plays an important role in maintaining airway patency (Mathew et al., 1982a,b; Van Lunteran and Strohl, 1986; Mezzanotte et al., 1992; Horner, 1996; Kobayashi et al., 1996; Schwartz et al., 1996). Therefore, it is important to understand the stimuli that control the activation of this muscle. The GG can potentially be activated from at least four sources: (i) the brainstem respiratory pattern generator (Bianchi et al., 1995); (ii) voluntary contraction (Mezzanotte et al., 1996a,b; Corfield et al., 1998); (iii) chemoreceptive reflexes (Onal et al., 1981a,b; Gauda et al., 1994; Shea et al., 1999); and (iv) mechanoreceptive reflexes (Mathew et al., 1982a,b; Horner, 1996; Malhotra et al., 2000a,b). Each of the last three could exert its influence via effects on either the brainstem respiratory pattern generating neurons or via direct effects on the hypoglossal motorneurons (i.e. bypassing pattern generating neurons).
The interactions between these different pathways in the activation of the GG are currently incompletely understood. Under most conditions, the GG is phasically activated during inspiration. That central respiratory pattern generating neurons play a role is demonstrated by GG activation 50–150 ms prior to inspiratory airflow on each breath (i.e. ‘pre-activation’), thus preparing the upper airway for the negative pressure generated by the diaphragm (Strohl et al., 1980; Gottfried et al., 1983; Hudgel and Harasick, 1990; Hudgel et al., 1993). The various different influences on central pattern generator activity such as behavioral effects and other pre-motor inputs to the hypoglossal nucleus are also poorly characterized in humans. The source of the activation during inspiration is similarly uncertain, but could be principally driven by mechanoreceptive reflexes from the upper airway which function to preserve airway patency in the face of collapsing forces (Berry et al., 1997; Malhotra et al., 2000a,b). We have recently reported that mechanoreceptive reflexes importantly contribute to the inspiratory phasic activation of the GG, likely on a moment to moment basis throughout inspiration (Akahoshi et al., 2001). We observed increased GG activation in response to the relatively small negative pressure changes which occur during normal tidal breathing (White et al., 1998; Malhotra et al., 2000a,b; Akahoshi et al., 2001) and the large range of negative pressures which occur during inspiratory resistive loading (IRL) and negative pressure ventilation (Malhotra et al., 2000b). Additionally, we and others have reported that the genioglossal activation both during basal breathing and in response to negative pressure pulses can be substantially reduced by dense topical anesthesia, suggesting that mucosal receptors mediate the afferent limb of this reflex (Horner et al., 1991b; White et al., 1998; Fogel et al., 2000).
The aim of the present study was to assess the interaction between central respiratory drive and mechanoreceptive influences in controlling upper airway dilator muscle activity on a moment to moment basis throughout inspiration. To address this, we (a) increased central respiratory drive as well as the pharyngeal negative pressure stimulus using inspiratory resistive loading; (b) decreased central respiratory drive and increased the airway pressure stimulus using hypocapnic negative pressure mechanical ventilation; and (c) reduced the mechanoreceptive stimuli using topical airway anesthesia during spontaneous breathing in each of the previously described conditions. We hypothesized that mechanoreceptive reflexes are the principal influence on phasic GG activation, and thus the relationship between airway pressure and GG activation will be minimally affected by alterations in central respiratory drive. In other words, we expected that the slope of the relationship between airway pressure and GG activation across inspiration would be similar during spontaneous breathing, loaded breathing and hypocapnic mechanical ventilation. However, if central respiratory drive is the overriding influence on phasic GG activation, then the slope of the relationship between airway pressure and GG activation should decrease with hypocapnic mechanical ventilation and increase with loaded breathing. Finally, respiratory phasic activation of GG would be expected to be abolished by a combination of passive mechanical ventilation (reduces central respiratory GG activation) and dense upper airway anesthesia (reduces local mechanoreceptive mechanisms).
2. Methods
2.1. Subjects
Sixteen healthy volunteers (nine males) without sleep complaints were studied. The mean age was 25.7 ± 1.2 (SEM) years and mean body mass index 21.7 ± 0.42 kg/m2. Informed consent was obtained from each subject, with the protocol having the prior approval of the Human Subjects Committee of the Brigham and Women’s Hospital.
2.2. Equipment and techniques
All studies were performed during wakefulness (eyes open as confirmed by video camera) in the supine posture. Subjects lay within a negative pressure ventilator (Iron Lung, Series J; Emerson, MA), which was activated only for the mechanical ventilation condition (see below).
2.2.1. Airway mechanics
Subjects wore a nasal mask (Healthdyne Technologies, Marietta, GA) connected to a two-way valve partitioning inspiration and expiration. Inspiratory flow was determined with a pneumotachometer (Fleish, Inc., Lausanne, Switzerland) and differential pressure transducer (Validyne Corp., Northridge, CA), calibrated with a rotameter. Subjects were instructed to breathe exclusively through the nose and were carefully monitored by video camera to ensure that the mouth was completely closed. Mask leak was detected from a perforated catheter surrounding the mask-face interface which continuously sampled for CO2. In addition, end tidal PCO2 (PETCO2) was monitored from the mask using an infrared analyzer (Capnograph Monitor, BCI, Waukesha, WI).
Pressures were monitored in the mask with an open catheter attached to a pressure transducer (Validyne Corp.) and in the airway at the level of the choanae and the epiglottis using pressure-tipped catheters (MPC-500, Millar, Houston, Texas). One nostril was decongested (oxymetazoline HCl) and anesthetized (lidocaine HCl), and the Millar catheters were inserted through this nostril and localized at the choanae and epiglottis. Prior to insertion, all three pressure signals were calibrated simultaneously in a rigid cylinder using a standard water manometer. These three signals plus flow were demonstrated to be without amplitude or phase lags at up to 2 Hz.
2.2.2. Muscle activation
The GGEMG was measured with a pair of unipolar intramuscular electrodes referenced to a single ground, thus producing a bipolar recording. Two stainless steel Teflon-coated 36-gauge wire electrodes were inserted 15–20 mm into the body of the genioglossal muscle 3 mm lateral to the frenulum of the tongue on each side, using a 25-gauge needle, which was quickly removed, leaving the wires in place. This technique has been used previously in our laboratory (Wheatley et al., 1993).
The raw EMG was amplified (Grass Instruments, Quincy, MA), band pass filtered (between 30 and 10 000 Hz), rectified, and electronically integrated on a moving-time-average (MTA) basis with a time constant of 100 ms (CWE, Inc., Ardmore, PA). The EMG was quantified as a percent of maximal activation. To define maximal muscle EMG activity subjects performed three maneuvers: each subject maximally inspired against an occluded inspiratory line, maximally protruded the tongue against the back of the teeth, and swallowed. Each of these maneuvers was performed several times with the maximal value recorded (from any maneuver) being called 100%. Electrical zero was then determined, with subsequent muscle activity being quantified as a percentage of maximal activation for that individual.
2.2.3. Inspiratory resistive loading
Resistance was added to inspiration using a specially designed variable resistance device, placed distal to the inspiratory valve. Inspiration could be loaded to any desired level by varying the effective caliber of the inspiratory pathway. The baseline resistance of the system was 2.5 cmH2O/L/sec at a flow of 1 L/sec. The variable inspiratory resistance device consisted of a water-filled latex balloon with a wall thickness of 0.15 mm, mounted on a 6.0 mm outer diameter tube, which was centered within the inspiratory pathway tube (12.4 mm inner diameter). This balloon could be inflated using a graduated syringe. As the balloon was distended it filled more of the tube’s caliber and effectively reduced the cross-sectional area available for airflow (Pillar et al., 1997). In this experiment, one level of load was used (25 cm H2O/L/sec) and was applied for three consecutive breaths and was removed for a minimum of 30 sec prior to the next load application.
2.2.4. Mechanical ventilation (‘Iron lung’)
Subjects were studied while supine with the head outside and body within a negative pressure ventilator (Iron Lung; Series J; Emerson, MA). The ventilator was switched on only for specific parts of the experiment (see protocol below). This device was lightly sealed around the neck with a flexible twisted nylon sheet while an external piston created negative pressure around the chest and abdominal wall thus assisting ventilation. The iron lung could be adjusted to achieve the desired upper airway pressure and breathing frequency, so that passivity was achieved. All subjects required some initial coaching to enable passive mechanical ventilation. This involved asking the subjects to remain completely relaxed and providing feedback on a breath-by-breath basis to achieve consistent timing and shapes of the pressure and flow traces. Recordings were stopped when there was departure from this passive pattern until adequate passivity could be achieved, or the experiment was terminated. Data were collected only during steady-state conditions as judged by the investigator during data acquisition. In order to achieve the desired passive ventilation subjects were hyperventilated (PETCO2 10 mmHg below eucapnia).
2.2.5. Anesthesia
Nasopharyngeal anesthesia was accomplished with nebulized 4% lidocaine HCl applied to both nasal passages until a cotton-tipped swab could be inserted with minimal detection. The oropharynx was then anesthetized until there was both complete loss of the gag reflex and the subjective sensation of difficulty swallowing, demonstrating effective laryngeal and upper airway anesthesia. All studies performed under anesthesia were completed in less than 30 min, due to the short duration of action of lidocaine.
2.3. Protocol
Each subject reported to the laboratory having fasted for at least 4 h. The pressure catheters and intramuscular EMG wires were inserted first. Subjects then lay inside the ‘iron lung’ ventilator while a nasal mask was attached. Subjects then lay with their eyes open in the supine posture and acclimated to the equipment. Determination of maximal GGEMG was performed next. Thereafter, each subject was studied in three conditions prior to anesthesia. Initially basal breathing (at least 5 min) was recorded. Subsequently inspiratory resistive loading was performed, with the load being applied following at least five consecutive breaths without a swallow. The load remained in place for three breaths. If the subject swallowed during loading, this sequence was excluded from analysis. Thereafter, the inspiratory resistance balloon was deflated for 5–15 breaths (at least 30 sec and a return to basal breathing pattern) before applying another load. The inflation and deflation of the balloon took place during expiration. In each subject, the load was applied three times. Next, the ‘iron lung’ ventilator was turned on. Subjects in the iron lung were instructed to ‘relax and let the machine breathe for them’. Often, it required 5–10 min of breath-by-breath feedback until satisfactory passivity could be established as evidenced by the uniformity of the airway pressure and flow waveforms with complete synchronization with the ventilator cycle. An additional post-hoc test of passivity included the elimination of the pre-activation of the GG before the onset of airflow (see Section 3). When passive ventilation was achieved, all signals were recorded for 5 min. Finally subjects underwent dense topical nasal and pharyngeal anesthesia, as described above. When satisfactory anesthesia had been achieved (see above), the protocol outlined above was repeated
2.4. Data recordings and analyses
Signals (GGEMG [raw and moving time average], airway pressure [mask, choanal, epiglottic], and inspiratory flow) were recorded on a 16-channel Grass model 78 polygraph (Grass Instruments, Quincy, MA) and on computer (digitized at 125 Hz) using signal-averaging software (Spike 2; Cambridge Electronic Design, Ltd., Cambridge, UK).
For each condition, a single breath profile was generated for each signal by averaging all breaths which were aligned to the onset of inspiratory airflow. From each averaged breath the following variables were determined: peak negative pressure (at the levels of choanae and epiglottis), peak flow, tonic GGEMG (level of activation during expiration), and peak phasic GGEMG (peak activation during inspiration). Pharyngeal resistance (Rpha, choanae to epiglottis) was calculated at peak inspiratory flow.
2.5. Statistical analyses
All statistical analyses were performed with commercially available software (Excel 97, Microsoft; and SigmaStat + Sigmaplot, SPSS, Chicago). Standard linear regression analyses were performed to determine the slope and correlation between epiglottic negative pressure and GGEMG on a within breath basis from the signal averaged breath in each condition. Comparisons were performed between the three pre-anesthesia conditions and between pre- and post-anesthesia conditions using repeated measures ANOVA, or, ANOVA on ranks (Kruskal–Wallis) whenever the data were not normally distributed. Only if the ANOVA found significance (main effect) was this followed by post-hoc Tukey test to determine the groups with significant differences. When comparisons involved all six conditions (pre- and post-anesthesia plus spontaneous breathing vs IRL vs IL), two-way ANOVA (2 × 3) was used, whereas when three conditions were compared (e.g. pre-anesthesia BB vs IRL vs IL), one-way ANOVA was used. Thus the two-way ANOVA tested for anesthetic effects, breathing condition effects, and interaction effects. For all analyses, alpha was set at 0.05. Results are presented as means ± SEM.
3. Results
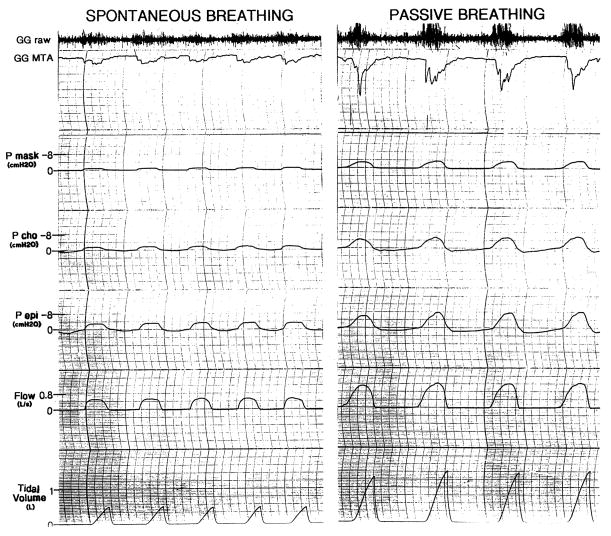
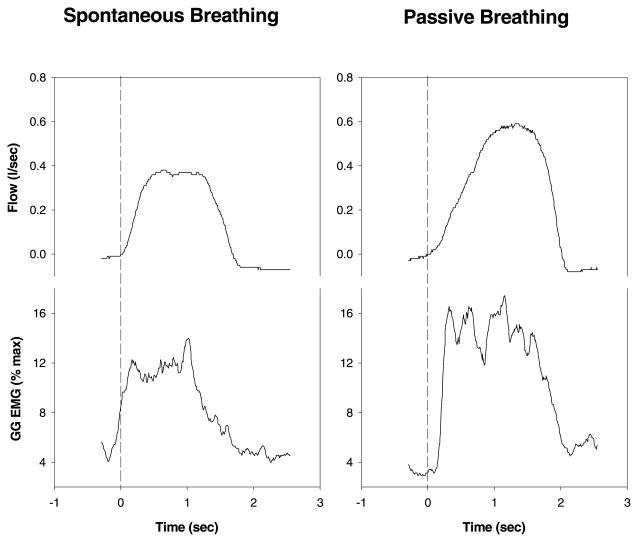
Complete data sets were obtained in all subjects. Raw data from two of the conditions are shown in Fig. 1. Airway mechanics and muscle activation in all three conditions pre- and post-anesthesia are shown in Table 1. Ventilation and tidal volume were preserved during IRL when compared to spontaneous breathing, indicating that central respiratory drive increased in this condition. In the negative pressure ventilation condition, to facilitate passive ventilation, the subjects were slightly hyperventilated. As a result, tidal volume and minute ventilation were significantly greater than during spontaneous breathing, and PETCO2 (not shown in table) decreased from 40.3 ± 0.7 to 29.7 ± 0.8 mmHg (P < 0.05). Fig. 2 demonstrates raw data from one subject, illustrating that during spontaneous breathing there was pre-activation of the GG (i.e. EMG increases prior to the onset of inspiratory flow), while during negative pressure ventilation the GGEMG increases minimally prior to flow. The peak GGEMG is greater during passive breathing as the negative pharyngeal pressure reached was greater than during spontaneous breathing. This attenuation of pre-activation provides indirect evidence of decreased output of the central respiratory pattern generator to this muscle, or at least a substantial modification of the relationship between central respiratory drive and GGEMG.
Fig. 1.
Depicted are raw data from the polygraphic recording. The passive breathing condition (on the right) is remarkable for the more negative epiglottic pressures, the greater genioglossus EMG activity and the loss of pre-activation when compared with spontaneous (on the left). The loss of pre-activation is more easily appreciated in Fig. 2. [raw electromyogram in arbitary units, GGMTA: moving time average genioglossus electromyogram in %maximum unit (an increase in activity is in a downward direction on this tracing), Pmask: mask pressure in cmH2O, Pcho: choanal pressure in cmH2O, Pepi: epiglottic pressure in cmH2O]. Only inspiratory values can be seen due to the one way valve used in the breathing apparatus.
Table 1.
Ventilation, airway mechanics, and muscle activation in all three conditions pre- and post-anesthesiaa
| Pre-Anesthesia
|
Significance
|
Anesthesia
|
Significance
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Spont | IL | IRL | Main effect | Comparison | Spont | IL | IRL | Main effect anesthesia | |
| Vt (l) | 0.52 ± 0.0 | 0.88 ± 0.1c | 0.59 ± 0.1 | P<0.05 | Spont<IL | 0.6 ± 0.0b | 0.94 ± 1.0 | 0.61 ± 0.1 | P<0.05 |
| Ve (l/min) | 7.83 ± 0.3 | 12.62 ± 0.8c | 7.28 ± 0.5 | P<0.05 | Spont<IL | 7.88 ± 0.6 | 13.11 ± 1.0 | 7.01 ± 0.5 | ns |
| Pcho | −1.38 ± 0.1 | −3.9 ± 0.3c | −7.04 ± 0.7d | P<0.05 | Spont<IL and IRL | −1.45 ± 0.1 | −4.01 ± 0.2 | −6.74 ± 0.6 | ns |
| Pepi (cmH2O) | −1.87 ± 0.2 | −5.28 ± 0.4c | −7.61 ± 0.7d | P<0.05 | Spont<IL and IRL | −2.03 ± 0.2 | −5.32 ± 0.4 | −7.55 ± 0.6 | ns |
| Rpha | 0.93 ± 0.3 | 1.52 ± 0.4c | 1.87 ± 0.7 | P<0.05 | Spont<IL | 1.38 ± 0.3b | 2.17 ± 0.4b | 2.71 ± 0.6b | P<0.05 |
| GGtonic | 4 ± 0.6 | 3.97 ± 0.7 | 4.55 ± 0.9 | ns | 4.45 ± 1.0 | 4.4 ± 1.2 | 4.98 ± 1.4 | ns | |
| GGpeak | 9.22 ± 1.6 | 11.9 ± 2.6c | 11.7 ± 2.4d | P<0.05 | Spont<IL and IRL | 6.91 ± 1.4b | 9.58 ± 2.4b | 9.43 ± 1.7b | P<0.05 |
Spont, spontaneous breathing; IL, iron lung; IRL, inspiratory resistive load (= 25 cmH2O/L/sec); VT Tidal Volume (l), Ve, Minute Ventilation (1 min); Pcho, Choanal Pressure (cmH2O); Pepi, Epiglottic Pressure(cmH2O); Rpha, Pharyngeal Resistance (cmH2O/L/sec); GG tonic, tonic activation of genioglossus (% of maximum); GGpeak, peak phasic activation of genioglossus (% of maximum). Main Effect refers to significance of ANOVA. Based on post-hoc Tukey test.
P<0.05 pre- vs post-anesthesia.
P<0.05 spontaneous vs negative pressure ventilation (IL).
P<0.05 spontaneous vs IRL.
Fig. 2.
Raw data example of the loss of pre-activation in changing from spontaneous breathing to iron lung passive breathing. Note the clear increase in GG activity prior to the onset of inspiratory flow in the spontaneous but not the passive breathing example. The peak GGEMG is higher in the passive example due to the more negative pharyngeal pressure generated by the ventilator as compared with spontaneous breathing.
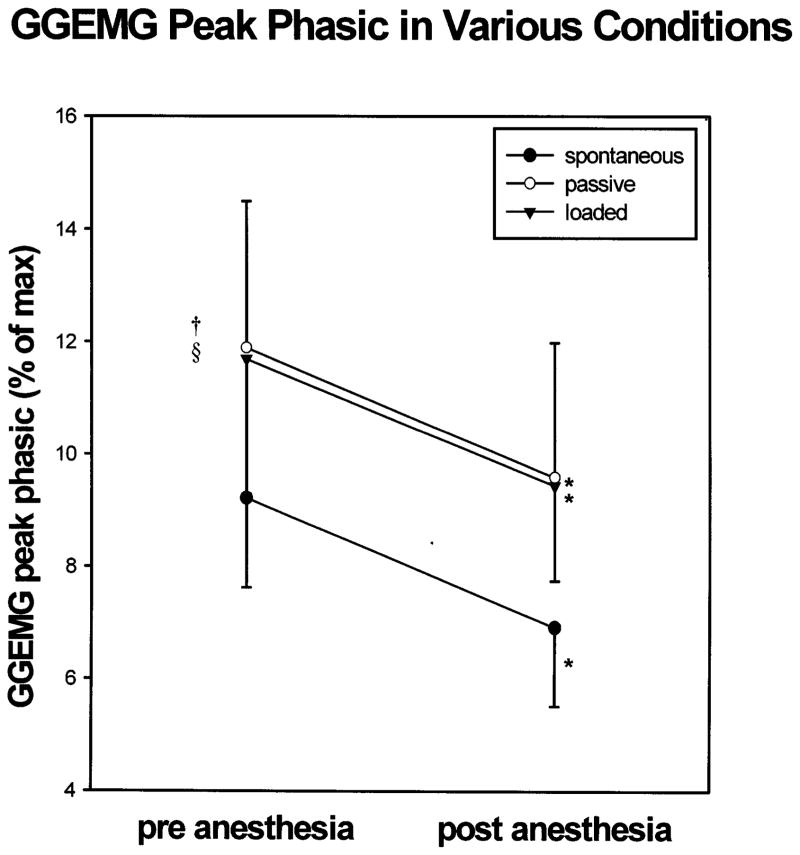
Peak phasic GGEMG increased significantly during both passive ventilation and IRL, in association with the increasingly negative epiglottic pressures (P < 0.05 for Tukey post-hoc test following significant ANOVA results, see Table 1). There were no changes in tonic GG activation between the three conditions pre-anesthesia (ANOVA, P = ns). In all three conditions, anesthesia was associated with a significant reduction in peak phasic GGEMG (Fig. 3, Table 1). However, even with passive ventilation and dense topical anesthesia, GG activity remained at least minimally phasic (Table 1).
Fig. 3.
Peak phasic GGEMG increased significantly with both mechanical ventilation and IRL as compared with spontaneous breathing. In all three conditions, anesthesia was associated with a significant reduction in peak phasic GGEMG. * = P < 0.05 pre- vs post-anesthesia; † = P < 0.05 spontaneous vs negative pressure ventilation; § = P < 0.05 spontaneous vs IRL.
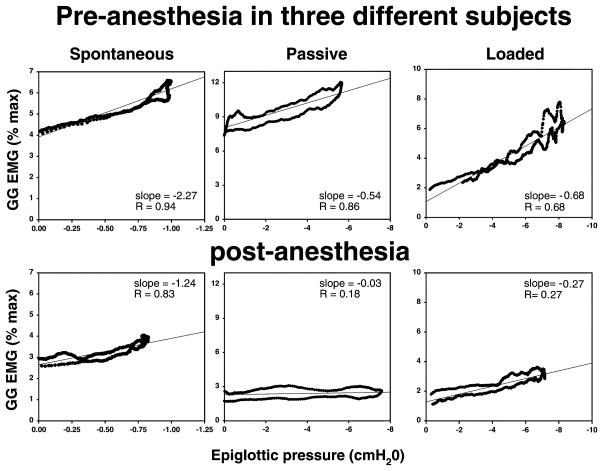
In all conditions pre-anesthesia, there was a tight and significant correlation between negative epiglottic pressure and GGEMG (P < 0.05, Table 2). In all conditions, anesthesia led to a decrement in the tightness of the correlation (coefficient) between Pepi and GGEMG, and a reduced sensitivity of GGEMG to increasingly negative Pepi (as assessed by the GGEMG/Pepi slope). Individual examples are shown in Fig. 4 (spontaneous breathing, passive ventilation, and breathing during loading). For the group as a whole (under all conditions), the average correlation coefficient (R) between GGEMG and Pepi decreased from 0.80 ± 0.03 pre-anesthesia to 0.71 ± 0.03 post-anesthesia (P < 0.05). Data for each condition are provided in Table 2. The slope of the GGEMG/Pepi relationship (Table 2) decreased significantly with both resistive loading and mechanical ventilation, from 1.78 ± 0.4%max/cmH2O during spontaneous breathing to 1.09 ± 0.3 during passive ventilation and to 0.59 ± 0.1%max/cmH2O during IRL (P < 0.05 for both, comparison with basal breathing pre-anesthesia by Tukey post-hoc test following significant ANOVA result). The y intercept of the GGEMG/Pepi plot represents the GGEMG just prior to inspiration (after pre-activation, if present). As can be seen, these values tended to be higher than those of tonic GGEMG (Table 1). As the method of determination for tonic GGEMG and y-intercept were different (i.e. signal averaging vs regression analysis), direct comparison of these values would be invalid and therefore was not attempted. However, none of the differences in the y intercept values across the various conditions was significant (ANOVA, P = ns, see Table 2).
Table 2.
Slope, R values and y-intercept values for GG/Pepi relationship pre-and post-anesthesia and under various conditions a
| Pre-anesthesia
|
Comparison | Anesthesia
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Spont | IL | IRL | Significance: main effect | Spont | IL | IRL | Main Effect Anesthesia | ||
| Slope | 1.78 ± 0.4 | 1.09 ± 0.3c | 0.59 ± 0.1d | P<0.05 | Sp ± IL and IRL | 0.95 ± 0.3b | 0.62 ± 0.3b | 0.36 ± 0.1b | P<0.05 |
| R value | 0.83 ± 0.1 | 0.81 ± 0.0 | 0.78 ± 0.0 | ns | 0.79 ± 0.0 | 0.69 ± 0.1b | 0.71 ± 0.1 | P<0.05 | |
| Y intercept | 5.83 ± 1.1 | 5.83 ± 1.5 | 6.99 ± 1.8 | ns | 4.89 ± 1.5 | 6.13 ± 1.8 | 6.5 ± 1.7 | ns | |
Spont, spontaneous breathing; IL, iron lung; IRL, inspiratory resistive load (= 25 cmH2O/L/sec), Main Effect refers to significance of ANOVA Based on post-hoc Tukey test.
P<0.05 pre- vs post-anesthesia.
P<0.05 spontaneous vs negative pressure ventilation (IL).
P<0.05 spontaneous vs IRL.
Fig. 4.
During spontaneous breathing, this individual showed a decline in both the slope and the R value for the GG/Pepi relationship following anesthesia. During passive ventilation, this example illustrates a loss of the GG/Pepi relationship (based on both R value and slope) following anesthesia. During loaded breathing, dense topical anesthesia led to a substantial decline in the slope of the GG/Pepi relationship although the R value was preserved in this individual.
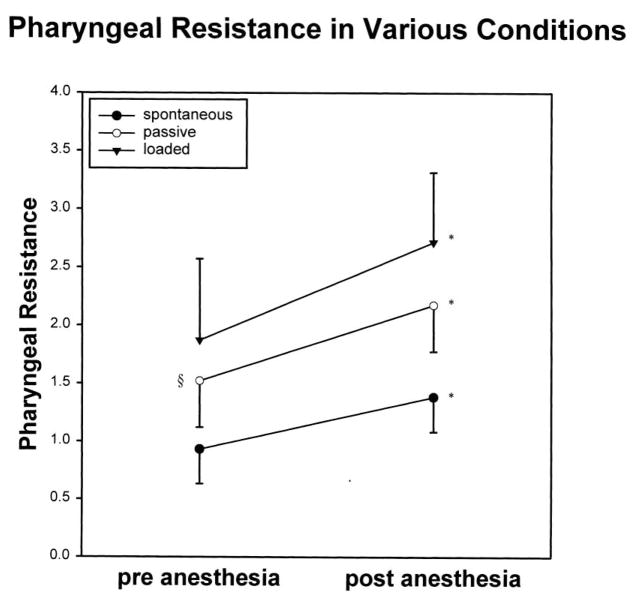
Pharyngeal resistance pre-anesthesia increased from spontaneous breathing to passive ventilation (0.93 ± 0.3–1.52 ± 0.4 cmH2O/L/sec, P < 0.05 for Tukey post-hoc test following significant ANOVA result), and further during loaded breathing, although this latter increment was of borderline statistical significance (1.87 ± 0.68, P = 0.06, Fig. 5). In addition, in all conditions, pharyngeal resistance increased significantly following anesthesia (Table 1, Fig. 5, P < 0.05). Finally, for all comparisons where two-way ANOVA was used, no important interaction between the anesthetic effects and the breathing condition effects (spontaneous, IL, IRL) were observed (P = ns for all).
Fig. 5.
In all three conditions, (spontaneous breathing, negative pressure ventilation and IRL), there was an increase in pharyngeal resistance following dense topical anesthesia. * = P < 0.05 pre- vs post-anesthesia; § = P < 0.05 spontaneous vs negative pressure (passive) ventilation.
4. Discussion
The findings of this study improve our understanding of the variables controlling the activation of the genioglossus muscle. First, we found a robust relationship between pharyngeal negative pressure and GGEMG across a breath in all pre-anesthesia conditions. GG activity also increased in conditions with increasing negative epiglottic pressure (i.e. passive ventilation and inspiratory resistive loading). These data support our previous observations that negative epiglottic pressure drives GG activation on a moment to moment basis within breaths. Second, the fact that GG activation as well as the correlations between GGEMG to negative pharyngeal pressure decreased in all conditions with topical anesthesia, suggests that the pharyngeal pressure-induced GG activation is mediated through a topical (mucosal) receptor. Third, pharyngeal resistance significantly increased with both a reduction of central output to the GG (passive ventilation) and local activation (topical anesthesia) suggesting that both mechanisms are important in maintaining UA patency. Fourth, the sensitivity of GG to changes in pharyngeal pressure decreased both when central output to the GG was diminished (during passive ventilation) and when it was augmented (during loading). We do not have a clear explanation for this, but speculate that genioglossal activity is carefully modulated to protect airway patency. When central drive is high (IRL), less local activation is required to protect the airway and thus the GGEMG/Pepi slope falls. During negative pressure ventilation, the peak GGEMG increased, but the slope fell yielding a small increment in airflow resistance. Thus the muscle activity was modulated to primarily protect pharyngeal patency in the face of falling central respiratory output. Finally, the observation that even with passive ventilation (low central output to the GG) and dense topical anesthesia (minimal local effect of Pepi), GG activity remained substantially phasic, suggests that additional yet unrecognized factors may control the activation of this muscle.
In all conditions prior to anesthesia we found a tight and significant correlation between negative epiglottic pressure and GGEMG. This is supportive of previous observations of the link between GGEMG and Pepi via a reflex response to rapid pulses of negative pressure (Mathew et al., 1982a,b; Horner et al., 1991a,b; Wheatley et al., 1993). This relationship has also been observed more recently also during slowly generated airway negative pressure during passive ventilation (Akahoshi et al., 2001). However, this previous study did not report a variable GGEMG/Pepi slope depending on the condition as was clearly observed in our protocol. This is likely a product of the fact that in the previous study most testing was completed in the iron lung (negative pressure ventilator) with no inspiratory resistive loading procedure. Thus this prior study did not test the range of conditions that were used in the present study. However, both studies indicate a strong association between negative pressure and muscle activation across a breath.
The three conditions (pre-anesthesia) we chose to study to further examine this relationship (GG/Pepi) included baseline spontaneous breathing, and two interventions which we believe have opposite effects on the output of the central respiratory pattern generator. Negative pressure ventilation of a passive subject, especially during hypocapnia, likely turns down the output of the central respiratory pattern generator. This is evidenced by both a reduction in diaphragmatic surface EMG (as we have reported recently) (Akahoshi et al., 2001), and by a reduction in pre-activation of the GG (Fig. 2). On the other hand, inspiratory resistive loading assumedly increased the central respiratory output to the pump muscles as ventilation was preserved despite breathing against an added load (Table 1). That diaphragmatic EMG increases with IRL during wakefulness has also been previously reported (Strohl et al., 1980; Hudgel et al., 1987). We do not know with certainty that this increased output of the central respiratory pattern generator to the diaphragm also increases the output to the GG muscle, although the output to these muscles are likely linked as part of a common central respiratory complex (Strohl et al., 1980; Oliven et al., 1989). With both increased and decreased central output we observed increased peak phasic GG activation (Fig. 3), and decreased GGEMG/Pepi slope. The overall increased activation appears to be a result of increasingly negative epiglottic pressure in these two conditions, as in both there was a tight and significant linear correlation between Pepi and GGEMG across inspiration, and in both the Pepi was significantly more negative than baseline (Table 1). However, the sensitivity of GG to Pepi decreased both when central output to the GG was diminished (during mechanical ventilation) and when it was augmented (during loading). We do not have a clear explanation for this variable slope. One potential explanation is that in the normal physiological range of central respiratory output, the largest number of hypoglossal motoneurons are close to their threshold for depolarization. When the central stimulus is smaller (mechanical ventilation), there is a need for a greater local stimulus to activate the GG i.e. the slope of GGEMG/Pepi is smaller. On the other hand, when the central respiratory output is large (i.e. with loading), it could be that many neurons have already depolarized such that GG sensitivity (i.e. slope GGEMG/Pepi) is reduced. However, this concept is highly speculative.
The tonic GGEMG (Table 1) and the y intercept of the GGEMG/Pepi line (Table 2) did not change significantly between the various conditions. This further emphasizes that the different GGPeak we observed results from differences in the sensitivity of GG to Pepi. The GGEMG just prior to inspiration (y-intercept of GGEMG/Pepi relationship) was somewhat higher than the tonic GGEMG, possibly due to pre-activation. One might expect diminished pre-activation during passive ventilation, leading to smaller differences between the y-intercept and the tonic GGEMG. However, neither the differences in the y-intercept values nor those in tonic GG in the various conditions reached statistical significance. The slight differences in these values (y intercept vs tonic GGEMG) may also in part be explained by methodological differences, since the y intercept values were obtained by statistical regression analysis, while the tonic GGEMG was determined by signal averaging. Finally, further work is clearly needed to understand the variables influencing this tonic activity.
Regardless of the explanation for the reduced sensitivity of GG to Pepi with altering central respiratory output, it seems that the consequence is increased pharyngeal resistance. The significant increase in pharyngeal resistance with passive ventilation implies that preactivation and possibly also ongoing central activation of the GG across inspiration are important in maintaining pharyngeal patency. The decreased GGEMG/Pepi slope in this condition implies inadequate compensation by local mechanisms in the absence of central ones, and therefore, pharyngeal resistance increased. Although one might predict that a load-induced increment in central pattern generator activity would increase genioglossus activation and therefore preserve pharyngeal patency, the concomitant increase in pump muscle activity also serves to substantially increase pharyngeal negative pressure [the lowest in this study (−7.6 ± 0.7, Table 1)]. With IRL, the central output is likely increased, but pharyngeal resistance tended to increase in response to the substantially increased pharyngeal collapsing pressure. Thus, a reduced GGEMG/Pepi slope and quite negative pharyngeal pressures in the IRL condition, resulted in increased pharyngeal resistance despite the high output from the central pattern generator.
An alternative argument could be made that the combined local and central genioglossal control mechanisms serve to protect airway patency. Under all conditions studied, there were modest changes in pharyngeal resistance despite substantial changes in airway negative pressure. As with any physiological system, one would not expect a perfect maintenance of the variable being examined when challenged by forces that would tend to compromise it. There has to be some error signal. In the case of pharyngeal resistance, this value rose slightly when central respiratory output was diminished (negative pressure ventilation), but was maintained within a physiologically acceptable range. With IRL, airway negative pressure increased substantially, but again, there was little change in resistance. Thus airway patency is largely protected despite substantial changes in the mechanisms controlling dilator muscle activity. Thus local and central mechanisms work in concert to yield adequate muscle activation to maintain patency.
Our observation that nasal and pharyngeal anesthesia leads to a decrement in both genioglossus activation as well as its responsiveness to negative pressure is consistent with prior literature (Horner et al., 1991b; White et al., 1998; Fogel et al., 2000). These data strongly suggest that local mucosal receptors in the upper airway importantly influence genioglossal activity in normal subjects. With anesthesia, both muscle activation (EMG/Pepi slope) and the correlation between GGEMG and Pepi significantly decreased. The functional importance of these local receptor mechanisms is quite clear, as pharyngeal airflow resistance increased significantly following local anesthesia (Fig. 5). Thus when one of the pharyngeal dilator muscle control mechanisms is reduced or eliminated, the ability to protect the airway deteriorates which speaks to the need for several potentially redundant control systems.
Finally, the observation that despite passive ventilation (decreased central respiratory neuronal output) and dense topical anesthesia (reduced local control), phasic GG activity was still observed, can be explained in one of three ways. First, it could be that ventilation was not completely passive with the iron lung. We believe this is an unlikely explanation as individuals with no pre-activation at all, still demonstrated phasic GG activity. Second, it could be that the topical anesthesia did not completely eliminate the local mechanoreceptors that mediate the GGEMG –Pepi activity. Although each subject, during anesthesia, completely lost his/her gag reflex as well as subjective sensation, incomplete anesthesia is a distinct possibility. Some combination of incomplete anesthesia and incompletely passive ventilation may also explain this finding. Third, the possibility exists that mechanisms other than negative epiglottic pressure and central respiratory pattern-generating neurons may drive GG activation. Subglottic receptors or muscle spindle-driven muscle activation represent two possibilities (Horner et al., 1991a,b).
Our study has several potential limitations. First, the use of dense topical pharyngeal anesthesia may not solely influence mucosal mechanoreceptors. As other receptors (flow, temperature, CO2) have been suggested in the upper airway, they would also presumably have been anesthetized. Furthermore, we cannot anatomically localize the region within the upper airway responsible for the observed results. However, others have reported that primarily nasal and laryngeal receptors are responsible for the negative pressure reflex (Horner et al., 1991b). As the goal of this study was to separate central from local mechanisms controlling phasic activation of the GG muscle and not receptor localization, we believe that total pharyngeal topical anesthesia was a reasonable approach. Second, although each subject during anesthesia completely lost his/her gag-reflex as well as topical sensation, incomplete anesthesia is likely. However, the level of anesthesia achieved was sufficient to yield interpretable results even if complete anesthesia was not accomplished. Third, we acknowledge that our definition of passive ventilation is not optimal, as we could not directly measure the output of the respiratory pattern generator, and we did not quantitatively assess diaphragmatic activity which can be difficult in humans. It is thus possible that some subjects were entrained to the iron lung breathing in total synchrony with it, but were not completely passive. However, the reduction in or elimination of GG pre-activation does suggest that ventilation was passive or at least that the phase relationship between the output of the central pattern generator and GG activation had changed substantially. In addition, we have previously reported the loss of surface diaphragm activity in this passive ventilation model (Akahoshi et al., 2001). As a result, our failure to accomplish complete pharyngeal anesthesia and/or completely eliminate premotor input to the genioglossus could make data interpretation difficult. However, quite consistent results were obtained in the three conditions, both before and after anesthesia. Therefore, we believe the complexity of our observations represents the reality of an intricately modulated motor control system and not data variability due to inadequate methodology.
Fourth, as the study was performed during wakefulness we could not eliminate some voluntary activation of the GG. We did, however, try to maximally eliminate voluntary effects by having the subjects relaxed in the bed. In addition, we did not distinguish between several possible sources of ‘central drive’ such as the primary pattern generator, chemoreceptors or vagal influences and thus lumped them all under the rubric of ‘central respiratory drive’. We recognize that this represents an oversimplification of ventilatory control. However, our desire was to dissociate mechanoreceptive (local) muscle activation from all central mechanisms. We did not try to separate the various sources of central drive. Fifth, it should be noted that we have determined wakefulness based on video-monitoring documenting that the subject has his/her eyes open. Due to the lack of EEG recordings, we cannot rule out that some brief periods of microsleep occurred. However, we visually inspected all of the data for unexplained variability and found no time periods where sleep or micro-sleep episodes were likely impacting the recorded variables. Finally we monitored only one upper airway muscle and can therefore not address how others might have behaved. It seems quite probable that these muscles do behave somewhat differently as we have previously observed no phasic activation of the tensor palatini (a tonic muscle) across a breath. However, we speculate that inspiratory phasic pharyngeal dilators likely behave relatively similarly to the genioglossus.
In conclusion, we believe our study confirms that epiglottic negative pressure drives GG activation via local reflex mechanisms, independent of central respiratory influences. However the sensitivity of the GG to this negative pressure appears to be highest during normal ventilation, and decreases when the output of the central respiratory pattern generator is either increased or decreased. This suggests that the combination of both pathways of GG activation are required to maintain UA patency. Finally, our results also indicate that additional yet unrecognized factors may be important in the control of upper airway muscles.
Acknowledgments
This work was supported by NIH NCRR GCRC MO1 RR02635, 1 P50 HL60292, RO1 HL48531 and T32 HL07633. In addition, Dr Pillar has received a Fulbright scholarship for his research. Dr Malhotra is supported by grants from the Medical Research Council of Canada and the American Heart Association. The authors wish to thank Dr Shelly Hurwitz for her advice on statistical methods.
References
- Akahoshi T, White DP, Edwards JK, Beauregard J, Shea SA. Phasic mechanoreceptor stimuli can induce phasic activation of upper airway muscles in humans. J Physiol London. 2001;531:677–691. doi: 10.1111/j.1469-7793.2001.0677h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry R, McNellis M, Kouchi K, Light R. Upper airway anesthesia reduces phasic genioglossus activity during sleep apnea. Am J Respir Crit Care Med. 1997;156:127–132. doi: 10.1164/ajrccm.156.1.9608037. [DOI] [PubMed] [Google Scholar]
- Bianchi A, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–31. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Corfield D, Murphy K, Guz A. Does the motor cortical control of the diaphragm ‘bypass’ the brain stem respiratory centres in man? Respir Physiol. 1998;114:109–117. doi: 10.1016/s0034-5687(98)00083-8. [DOI] [PubMed] [Google Scholar]
- Fogel R, Malhotra A, Edwards JK, Shea SA, White DP. Reduced genioglossal activity with upper airway anesthesia in awake patients with OSA. J Appl Physiol. 2000;88:1346–1354. doi: 10.1152/jappl.2000.88.4.1346. [DOI] [PubMed] [Google Scholar]
- Gauda EB, Carroll TP, Schwartz AR, Smith PL, Fitzgerald RS. Mechano- and chemoreceptor modulation of respiratory muscles in response to upper airway negative pressure. J Appl Physiol. 1994;76:2656–2662. doi: 10.1152/jappl.1994.76.6.2656. [DOI] [PubMed] [Google Scholar]
- Gottfried S, Strohl K, Van de Graaff W, Fouke J, DiMarco A. Effects of phrenic stimulation on upper airway resistance in anesthetized dogs. J Appl Physiol. 1983;55:419–55. 426. doi: 10.1152/jappl.1983.55.2.419. [DOI] [PubMed] [Google Scholar]
- Horner RL, Innes JA, Murphy K, Guz A. Evidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in man. J Physiol London. 1991a;436:15–29. doi: 10.1113/jphysiol.1991.sp018536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner RL, Innes JA, Holden HB, Guz A. Afferent pathway(s) for pharyngeal dilator reflex to negative pressure in man: a study using upper airway anaesthesia. J Physiol London. 1991b;436:31–44. doi: 10.1113/jphysiol.1991.sp018537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner RL. Motor control of the pharyngeal musculature and implications for the pathogenesis of obstructive sleep apnea. Sleep. 1996;19:827–853. doi: 10.1093/sleep/19.10.827. [DOI] [PubMed] [Google Scholar]
- Hudgel DW, Mulholland M, Hendricks C. Neuromuscular and mechanical responses to inspiratory resistive loading during sleep. J Appl Physiol. 1987;63:603–608. doi: 10.1152/jappl.1987.63.2.603. [DOI] [PubMed] [Google Scholar]
- Hudgel DW, Harasick T. Fluctuation in timing of upper airway and chest wall inspiratory muscle activity in obstructive sleep apnea. J Appl Physiol. 1990;69:443–450. doi: 10.1152/jappl.1990.69.2.443. [DOI] [PubMed] [Google Scholar]
- Hudgel DW, Devadatta P, Hamilton H. Pattern of breathing and upper airway mechanics during wakefulness and sleep in healthy elderly humans. J Appl Physiol. 1993;74:2198–2204. doi: 10.1152/jappl.1993.74.5.2198. [DOI] [PubMed] [Google Scholar]
- Kobayashi I, Perry A, Rhymer J, Wuyam B, Hughes P, Murphy K, Innes JA, McIvor J, Cheesman AD, Guz A. Inspiratory coactivation of the genioglossus enlarges retroglossal space in laryngectomized humans. J Appl Physiol. 1996;80:1595–1604. doi: 10.1152/jappl.1996.80.5.1595. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Fogel R, Edwards J, Shea S, White DP. Local Mechanisms Drive Genioglossus Muscle Activation in Obstructive Sleep Apnea. Am J Respir Crit Care Med. 2000a;161:1746–1749. doi: 10.1164/ajrccm.161.5.9907109. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Pillar G, Fogel RB, Beauregard J, Edwards JK, Slamowitz DI, Shea SA, White DP. Genioglossus but not palatal muscle activity relates closely to pharyngeal pressure. Am J Respir Crit Care Med. 2000b;162:1058–1062. doi: 10.1164/ajrccm.162.3.9912067. [DOI] [PubMed] [Google Scholar]
- Mathew OP, Abu-Osba YK, Thach BT. Genioglossus muscle response to upper airway pressure changes: afferent pathways. J Appl Physiol. 1982a;52:445. doi: 10.1152/jappl.1982.52.2.445. [DOI] [PubMed] [Google Scholar]
- Mathew OP, Abu-Osba YK, Thach BT. Influence of upper airway pressure changes on genioglossus and muscle respiratory activity. J Appl Physiol. 1982b;52:438. doi: 10.1152/jappl.1982.52.2.438. [DOI] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal EMG in sleep apnea patients vs normal controls (a neuromuscular compensatory mechanisms) J Clin Inves. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Influence of sleep onset on upper-airway muscle activity in apnea patients vs normal controls. Am J Resp Crit Care Med. 1996a;153:1880–1887. doi: 10.1164/ajrccm.153.6.8665050. [DOI] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Waking and sleeping upper airway muscle activity in apnea patients vs normal control. Am J Resp Crit Care Med. 1996b;153:1880–1887. doi: 10.1164/ajrccm.153.6.8665050. [DOI] [PubMed] [Google Scholar]
- Oliven A, Odeh M, Gavriely N. Effect of hypercapnia on upper airway resistance and collapsibility in anesthetized dogs. Respir Physiol. 1989;75:29–38. doi: 10.1016/0034-5687(89)90084-4. [DOI] [PubMed] [Google Scholar]
- Onal E, Lopata M, O’Connor T. Diaphragmatic and genioglossal electromyogram responses to isocapnic hypoxia in humans. Am Rev Resp Dis. 1981a;124:215–217. doi: 10.1164/arrd.1981.124.3.215. [DOI] [PubMed] [Google Scholar]
- Onal E, Lopata M, O’Connor TD. Diaphragmatic and genioglossal electromyogram responses to CO2 rebreathing in humans. J Appl Physiol. 1981b;50:1052–1055. doi: 10.1152/jappl.1981.50.5.1052. [DOI] [PubMed] [Google Scholar]
- Pillar G, Schnall RP, Peled N, Oliven A, Lavie P. Impaired respiratory response to resistive loading during sleep in healthy offspring of patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1997;155:1602–1608. doi: 10.1164/ajrccm.155.5.9154864. [DOI] [PubMed] [Google Scholar]
- Schwartz AR, Eisele DW, Hari A, Testerman R, Erickson D, Smith PL. Electrical stimulation of the lingual musculature in obstructive sleep apnea. J Appl Physiol. 1996;81:643–652. doi: 10.1152/jappl.1996.81.2.643. [DOI] [PubMed] [Google Scholar]
- Shea S, Akahoshi T, Edwards J, White DP. Blood gas effects on genioglossal reflex to upper airway negative pressure. Am J Respir Crit Care Med. 1999;159:A689. doi: 10.1164/ajrccm.162.2.9908111. [DOI] [PubMed] [Google Scholar]
- Strohl KP, Hensley MJ, Hallett M, Saunders NA, Ingram RH. Activation of upper airway muscles before onset of inspiration in normal humans. J Appl Physiol. 1980;49:638–642. doi: 10.1152/jappl.1980.49.4.638. [DOI] [PubMed] [Google Scholar]
- Van Lunteran E, Strohl KP. The muscles of the upper airways. Clin Chest Med. 1986;7:171. [PubMed] [Google Scholar]
- Wheatley J, Mezzanotte W, Tangel D, White DP. Influence of sleep on genioglossus muscle activation by negative pressure in normal men. Am Rev Respir Dis. 1993;148:597–605. doi: 10.1164/ajrccm/148.3.597. [DOI] [PubMed] [Google Scholar]
- White DP, Edwards JK, Shea SA. Local Reflex Mechanisms: Influence on Basal Genioglossal Muscle Activation in Normal Subjects. Sleep. 1998;21:719–728. doi: 10.1093/sleep/21.7.719. [DOI] [PubMed] [Google Scholar]