Abstract
The ACI rat model of 17β-estradiol (E2)-induced mammary cancer is highly relevant for use in establishing the endocrine, genetic and environmental bases of breast cancer etiology and identifying novel agents and strategies for preventing breast cancer. E2 treatment rapidly induces mammary cancer in female ACI rats and simultaneously induces pituitary lactotroph hyperplasia and adenoma. The pituitary tumors can result in undesired morbidity which compromises long term studies focused on mammary cancer etiology and prevention. We have defined the genetic bases of susceptibility to E2-induced mammary cancers and pituitary tumors and have utilized the knowledge gained in these studies to develop a novel inbred rat strain, designated ACWi, that retains the high degree of susceptibility to E2-induced mammary cancer exhibited by ACI rats but lacks the treatment related morbidity associated with pituitary lactotroph hyperplasia/adenoma. When treated with E2, female ACWi rats developed palpable mammary cancer at a median latency of 116 days, an incidence of 100% by 161 days and exhibited an average of 15.6 mammary tumors per rat following 196 days of treatment. These parameters did not differ from that observed for contemporaneously treated ACI rats. None of the E2 treated ACWi rats were euthanized prior to the intended experimental end point due to any treatment related morbidity other than mammary cancer burden, whereas 20% of contemporaneously treated ACI rats exhibited treatment related morbidity that necessitated premature euthanasia. The ACWi rat strain is well suited for use by those in the research community focusing on breast cancer etiology and prevention.
Keywords: ACWi rat, ACI rat, Copenhagen rat, estradiol, breast cancer
Introduction
Both endogenous and exogenous estrogens have been strongly linked to the etiology of breast cancer (Yager and Davidson 2006). Although it is clear that estrogen receptor (ER) dependent pathways are essential for development of a large fraction of breast cancers, the molecular mechanisms through which estrogens contribute to breast cancer etiology remain poorly defined. The ACI rat model of 17β-estradiol (E2)-induced mammary cancer serves as a unique and physiologically relevant model for defining the mechanisms through which estrogens contribute to breast cancer development, identifying genetic variants that determine susceptibility to breast cancer, and identifying agents and strategies for use in preventing breast cancer. Female ACI rats rapidly develop mammary carcinoma when treated continuously with physiological levels of E2 normally encountered during pregnancy (Shull, et al. 1997). Concurrent treatment with tamoxifen dramatically diminishes the ability of E2 to induce mammary cancer in ACI rats, indicating an important role of ER mediated pathways in mammary tumorigenesis (Li, et al. 2002; Singh, et al. 2011). The mammary cancers that develop in E2 treated ACI rats express ERα and progesterone receptor (Pgr), are dependent upon estrogens for survival and growth, and exhibit non-random patterns of chromosome copy number alterations that mirror somatic copy number alterations frequently observed in breast cancers (Adamovic, et al. 2007; Harvell, et al. 2000; Ruhlen, et al. 2009). Together, these data illustrate multiple important similarities between the mammary cancers induced by E2 in ACI rats and luminal type breast cancers in humans.
The ACI rat model of E2-induced mammary cancer has been extensively characterized genetically. Whereas female ACI rats are uniquely susceptible to mammary cancer induction by E2, female Copenhagen (COP) and Brown Norway (BN) rats are resistant (Shull 2007; Shull, et al. 2001; Shull et al. 1997; Spady, et al. 1998). Multiple quantitative trait loci (QTLs), designated Emca1 (Estrogen-induced mammary cancer) through Emca9, that harbor genetic determinants of susceptibility to E2-induced mammary cancer have been mapped in intercrosses between susceptible ACI rats and resistant COP or BN rats (Gould, et al. 2004; Schaffer, et al. 2006; Shull 2007). The existence of these Emca loci has been confirmed by generation and characterization of congenic rat strains that harbor alleles from the resistant COP or BN strain on the genetic background of the susceptible ACI strain (Colletti, et al. 2014; Schaffer, et al. 2013). Moreover, each of the Emca loci is orthologous to genetic determinants of breast cancer risk mapped in genome wide association studies (Colletti et al. 2014; Schaffer et al. 2013). These data strongly suggest that these rat models of E2-induced mammary cancer share multiple genetic determinants of breast cancer risk with humans.
Continuous treatment with naturally occurring or synthetic estrogens induces pituitary lactotroph hyperplasia and/or adenoma in multiple inbred rat strains (Spady, et al. 1999a; Stone, et al. 1979; Wiklund, et al. 1981b). ACI rats are among the most highly sensitive of inbred strains in this regard, and morbidity resulting from these pituitary tumors can compromise long-term studies focused on mammary cancer etiology and prevention (Shull et al. 1997; Spady et al. 1999a). By contrast, COP rats are only moderately sensitive and BN rats are relatively insensitive to the actions of estrogens in the induction of lactotroph hyperplasia/adenoma (Kurz, et al. 2014; Kurz, et al. 2008; Shull, et al. 2007; Spady et al. 1998; Spady et al. 1999a; Spady, et al. 1999b; Strecker, et al. 2005; Wendell, et al. 2000; Wendell and Gorski 1997). Multiple QTLs, designated Ept1 (Estrogen-induced pituitary tumor) through Ept14 have been mapped that determine the sensitivity to estrogen-induced lactotroph hyperplasia/adenoma in intercrosses between ACI and COP or BN rats (Kurz et al. 2014; Kurz et al. 2008; Shull et al. 2007; Strecker et al. 2005). The data from these studies indicate that most of the QTLs that determine responsiveness of the pituitary lactotroph to estrogens segregate independently from the QTLs that determine susceptibility to E2-induced mammary cancer. The objective of this study was to utilize knowledge gained from genetic studies of estrogen action in the rat pituitary and mammary glands to develop a novel inbred rat strain that retains the high degree of susceptibility to E2-induced mammary cancer exhibited by ACI rats but lacks the treatment related morbidity associated with pituitary lactotroph hyperplasia/adenoma. The data presented herein illustrate the unique phenotypic characteristics of such an inbred rat strain, designated ACWi. The ACWi rat strain is well suited for use by those in the community focusing on breast cancer etiology and prevention.
Materials and Methods
Care, Treatment, and Phenotypic Characterization of Animals
All procedures involving live animals were approved by the Institutional Animal Care and Use Committee of the University of Wisconsin-Madison. ACI/SegHsd rats were obtained from Harlan Sprague Dawley, Inc. (Indianapolis, IN). Copenhagen (COP/CrCrl) rats were obtained from Charles River Laboratories (Wilmington, MA). The ACI.COP-Ept1 and ACI.COP-Ept2 congenic rat strains were generated in our laboratory as described previously (Kurz et al. 2008). The ACWi rat strain (official strain designation: ACI.COP-(D3Rat130-D3Rat114)(D6Rat80-D6Rat146)/Shul, RGD ID 9589088) was generated by intercrossing the ACI.COP-Ept1 and ACI.COP-Ept2 strains and selectively breeding the progeny to generate animals that were homozygous for COP alleles at both the Ept1 locus on rat chromosome 6 (RNO6) and the Ept2 locus on RNO3. The ACWi strain will be submitted to the Rat Resource and Research Center at the University of Missouri for cryopreservation and distribution. All rats were housed under controlled temperature, humidity, and 12-hr light/12-hr dark conditions in facilities that were accredited by the American Association for Accreditation of Laboratory Animal Care and operated in accordance with The Guide for the Care and Use of Laboratory Animals. All procedures related to the care, propagation, genotyping, treatment with E2, evaluation for presence of mammary cancer and assessment of pituitary hyperplasia/adenoma have been described (Gould et al. 2004; Harvell et al. 2000; Schaffer et al. 2006; Schaffer et al. 2013; Shull et al. 2001; Shull et al. 1997; Spady et al. 1998). The number of grossly discernible tumors, i.e., approximately 1 mm in diameter, was quantified at necropsy. Pituitary mass was measured as a quantitative phenotypic indicator of pituitary lactotroph hyperplasia/adenoma as discussed below. The animals were euthanized following 84 days of treatment to evaluate pituitary mass or following 196 (-4 to +1) days of treatment to evaluate mammary tumor burden together with pituitary mass. In the latter experiment, animals were euthanized prior to the desired experimental end point if necessary due to mammary tumor burden or another treatment related morbidity.
Sources of Genomic Data
All genome coordinates presented in this manuscript are relative to rat genome assembly version 5.0. Whole genome sequence data for ACI, COP and BN rat strains were accessed through the Rat Genome Database and Ensembl Database (Flicek, et al. 2014; Laulederkind, et al. 2013; Nigam, et al. 2013).
Statistical Analyses of Data
Latency was defined as the number of days separating initiation of E2 treatment and the first detection of a palpable mammary tumor. Phenotypes exhibited by ACWi rats were compared to those exhibited by contemporaneously treated ACI rats as well as to recent historic data from identically treated ACI and COP rats. Median latency was derived from Kaplan-Meier analyses. The log rank test was used to compare latencies between strains and to calculate the relative risk of each set of congenic rats in comparison to ACI rats. These analyses were carried out in MSTAT Version 5.5. Between strain differences in mammary tumor multiplicity and pituitary mass at necropsy were evaluated using the Kruskal-Wallis and Wilcoxon rank sum tests with the Holm-Bonferroni method to correct for multiple pairwise comparisons. These analyses were carried out in R (R Core Team, 2013). P values ≤ 0.05 were considered to be statistically significant for all tests.
Results
ACWi rats are highly susceptible to E2-induced mammary cancer
The susceptibility of ACWi rats to E2-induced mammary cancer was compared to that of contemporaneously treated ACI rats to determine whether or not ACWi rats retained the highly susceptible phenotype that is characteristic of the parental ACI rat strain. The median latency to appearance of palpable mammary cancer for E2 treated ACWi rats was 116 days and 100% of the treated rats developed mammary cancer by 161 days of treatment (Figure 1). Median latency for contemporaneously treated ACI rats was 100 days and 100% of the treated rats exhibited palpable mammary cancers by 134 days of treatment. The time course to appearance of palpable mammary cancer in ACWi rats did not differ significantly from that in ACI rats (P = 0.071). Because the mammary cancer phenotypes exhibited by ACI rats and different ACI derived congenic rats have been observed to be stable over time and between laboratories, the susceptibility of ACWi rats to E2 induced mammary cancer was also compared to historic data from identically treated ACI and COP rats evaluated in our laboratory. Median latency to appearance of palpable mammary cancer in this larger group of E2 treated ACI rats was 123 days and 94% of the animals at risk developed mammary cancer by 196 days of treatment. As expected, COP rats were resistant to induction of mammary cancer by E2, relative to ACI and ACWi rats. The first palpable cancer in this group of E2 treated COP rats was observed following 154 days of treatment and only 33% of the treated COP rats developed mammary cancer during the course of treatment. The time course to appearance of mammary cancer in E2 treated ACWi rats did not differ significantly from that exhibited by the group of ACI rats evaluated previously (P = 0.184), but did differ significantly from that exhibited by the E2 treated COP rats (P < 10-4). Sham treated control ACWi, ACI and COP rats did not develop mammary cancer when evaluated over a 196 day time course.
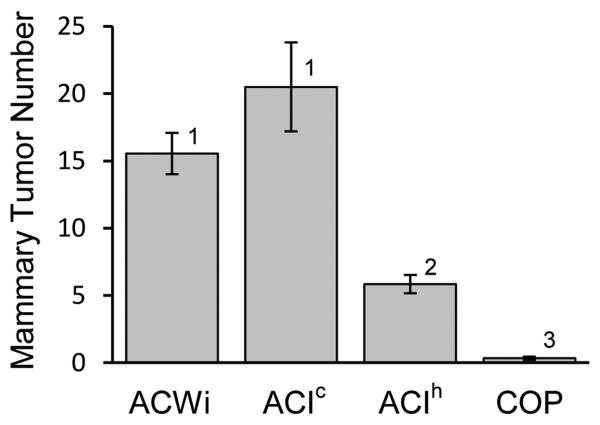
Figure 1. ACWi and ACI rats are highly susceptible to E2-induced mammary cancer; an examination of latency to appearance and incidence of mammary cancer.
Female ACWi (n = 11), ACI (n = 10 contemporaneous, ACIc; n = 63 historic, ACIh) and COP (n = 15) rats were treated with E2, released continuously from subcutaneous Silastic tubing implants, beginning at 9 weeks of age as described in Material and Methods. Thereafter, each rat was palpated once or twice weekly to detect mammary cancer. The data were subjected to Kaplan-Meier analyses. Each plot illustrates the number of days separating initiation of E2 treatment and appearance of palpable mammary cancer or grossly apparent mammary cancer at necropsy. Sham treated control ACWi, ACI and COP rats did not develop mammary cancer over the illustrated time course.
Grossly discernable mammary tumors were enumerated at necropsy. The mean numbers of mammary tumors observed in contemporaneously treated ACWi and ACI rats were 15.6 and 20.5, respective, and mammary tumor number did not differ between strains (P = 0.2822; Figure 2). Average mammary tumor number in the group of E2 treated ACI rats evaluated prior to the current study was 5.8 per rat, whereas average mammary tumor number in E2 treated COP rats was 0.3 per rat. Tumor number in ACWi rats differed significantly from that observed in these groups of ACI (P < 10-4) and COP rats (P < 10-4). Together, the data presented in Figures 1 and 2 clearly indicate that ACWi rats are as highly susceptible to E2-induced mammary cancer as ACI rats. The magnitude of the difference in average mammary tumor number observed between the contemporaneous and historic groups of ACI rats exceeded that normally observed between individual batches of ACI rats evaluated in our laboratory over the past several years. The bases for this observed difference between the contemporaneous and historic data are not known.
Figure 2. ACWi and ACI rats are highly susceptible to E2-induced mammary cancer; an examination of tumor multiplicity.
Female ACWi, ACI (contemporaneous, ACIc; historic, ACIh) and COP rats were treated with E2 as described in Figure 1 and Materials and Methods. Each data bar indicates the mean number of mammary tumors (± standard error of the mean) observed at necropsy for rats treated for at least 160 days; n = 8-57 animals per group. Data were evaluated using the Kruskal-Wallis and Wilcoxon rank sum tests with the Holm-Bonferroni method. Different numerals above the data bars indicate statistical significance (P < 0.05).
ACWi rats do not exhibit morbidity resulting from E2-induced pituitary lactotroph hyperplasia/adenoma
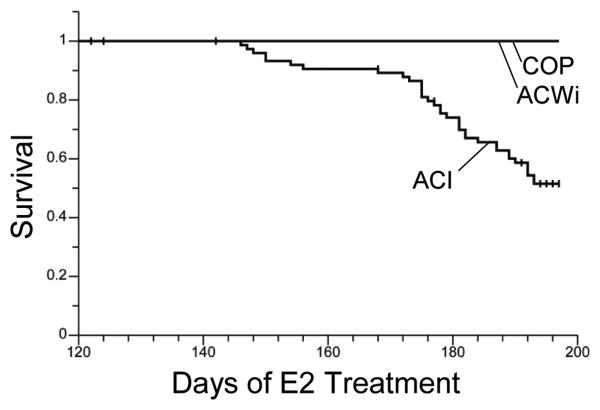
Continuous treatment of ACI rats with E2 or the synthetic estrogen diethylstilbestrol (DES) induces development of pituitary lactotroph hyperplasia/adenoma, and the resulting dramatic increase in pituitary gland mass and endocrine dysfunction (i.e., hyperprolactinemia) can result in morbidity that necessitates euthanasia of an experimental animal prior to the intended experimental end point (Spady et al. 1999a). Therefore, we evaluated the impact on survival of treatment related morbidity that resulted from any condition other than mammary cancer burden. No E2 treated ACWi or COP rats were euthanized due to any morbidity other than mammary cancer burden (Figure 3). By contrast, 20% of the contemporaneously treated ACI rats and 49% of the E2 treated ACI rats evaluated in our lab over the past 4 years were euthanized due to morbidity other than mammary cancer burden before the intended experimental end point. The survival curves for the two groups of E2 treated ACI rats did not differ significantly (P = 0.3453), and these groups were combined for comparison to treated ACWi and COP rats (Figure 3). Overall survival of E2 treated ACWi and COP rats was significantly higher relative to that observed in the combined population of E2 treated ACI rats (P = 0.0117 and P = 0.0019, respectively).
Figure 3. ACWi rats exhibit reduced treatment related morbidity relative to ACI rats.
Female ACWi, ACI and COP rats were treated with E2 as described in Figure 1 and Materials and Methods. The impact of any treatment related morbidity other than mammary tumor burden on survival was evaluated as a function of duration of E2 treatment and the data were subjected to Kaplan-Meier analyses. Each plot illustrates the number of days separating initiation of E2 treatment and euthanasia due to undesired treatment related morbidity.
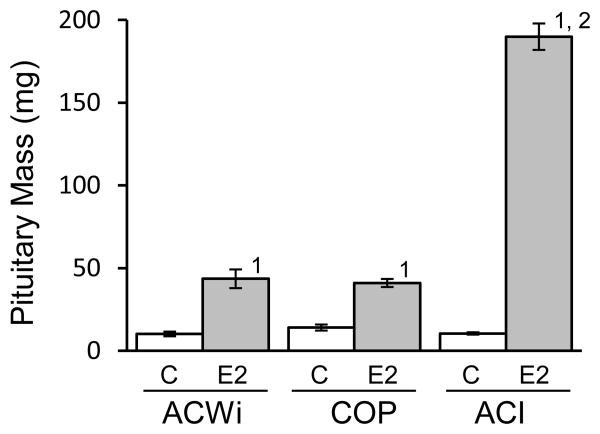
Pituitary gland mass was measured in all rats at necropsy. Pituitary mass in sham treated control ACWi, COP and ACI rats averaged 10.2, 14.0 and 10.5 mg, respectively, and no significant differences were observed between strains (Figure 4). E2 treatment increased pituitary mass 4.3-fold in ACWi rats, from an average of 10.2 mg in sham treated control ACWi rats to an average of 43.5 mg in E2 treated ACWi rats (P = 0.0420). Pituitary mass was similarly increased 2.9-fold in E2 treated COP rats, relative to sham treated control COP rats (P = 0.0013). By contrast, E2 treatment increased pituitary mass 15.5-fold (P = 0.0006) in contemporaneously treated ACI rats and 18.5-fold (P < 10-4) in the historic ACI population. Because pituitary mass did not differ significantly between these two groups of E2 treated ACI rats, they were combined to simplify between strain comparisons. Pituitary mass in E2 treated ACWi and COP rats differed significantly from that observed in E2 treated ACI rats (P < 10-4 for both comparisons), whereas pituitary mass in treated ACWi and COP rats did not differ (P = 1). As noted above, a significant fraction of E2 treated ACI rats exhibited treatment related morbidity and were euthanized prior to the desired experimental end point; i.e., 196 days of treatment. Average durations of E2 treatment in the ACWi, COP and ACI populations were 192, 196 and 185 days, respectively.
Figure 4. ACWi rats exhibit diminished pituitary growth response to estrogen relative to ACI rats.
ACWi, COP and ACI rats were treated as described in Figure 1 and Materials and Methods. Sham treated control rats (C) received empty implants. E2 treated animals received implants that release E2 continuously. Pituitary mass, a surrogate indicator of absolute lactotroph number, was evaluated at necropsy. Average durations of E2 treatment in the ACWi, COP and ACI populations were 192, 196 and 185 days, respectively. Each data bar indicates mean pituitary mass (± standard error of the mean). Data were evaluated using the Kruskal-Wallis and Wilcoxon rank sum tests with the Holm-Bonferroni method. Numerals above bars indicate statistical significance (P < 0.05): 1) within strain, relative to sham treated controls; 2) with the same treatment, relative to ACWi.
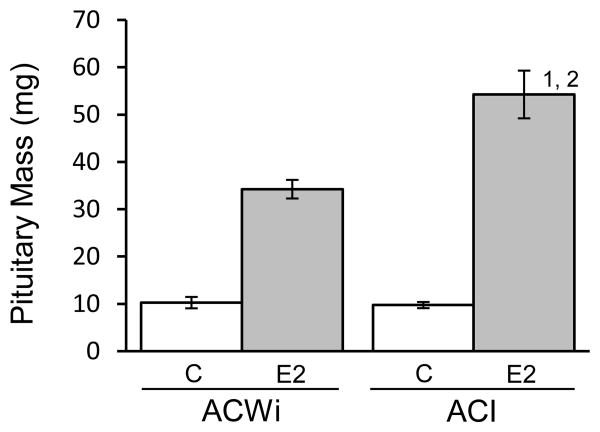
Ept1 and Ept2 were originally mapped in male F2 progeny that were generated in crosses between ACI and COP and treated with DES for 12 weeks, and the actions of these QTLs were subsequently confirmed in female congenic rats treated with E2 for 12 weeks (Kurz et al. 2008; Strecker et al. 2005). Therefore, we also evaluated pituitary mass in female ACWi and ACI rats that were treated with E2 for 12 weeks (Figure 5). Treatment of ACWi rats with E2 for 12 weeks increased pituitary mass 3.3 fold, from 10.3 to 34.2 mg (P = 0.0710). By contrast, 12 weeks of E2 treatment increased pituitary mass 5.5-fold in ACI rats (P = 0.0130). Average pituitary mass in treated ACWi rats was significantly less than in ACI rats following 12 weeks of treatment (P = 0.0350), further indicating that ACWi rats are less sensitive than ACI rats in regard to induction of pituitary lactotroph hyperplasia/adenoma by E2.
Figure 5. ACWi rats exhibit diminished pituitary growth response to estrogen relative to ACI rats; an examination following 12 weeks treatment.
ACWi and ACI rats were treated as described in Figure 1 and Materials and Methods. Sham treated control rats (C) received empty implants. E2 treated animals received implants that release E2 continuously. Pituitary mass was evaluated following 12 weeks of treatment. Each data bar indicates mean pituitary mass (± standard error of the mean). Data were evaluated using the Kruskal-Wallis and Wilcoxon rank sum tests with the Holm-Bonferroni method. Numerals above bars indicate statistical significance (P < 0.05): 1) within strain, relative to sham treated controls; 2) with the same treatment, relative to ACWi.
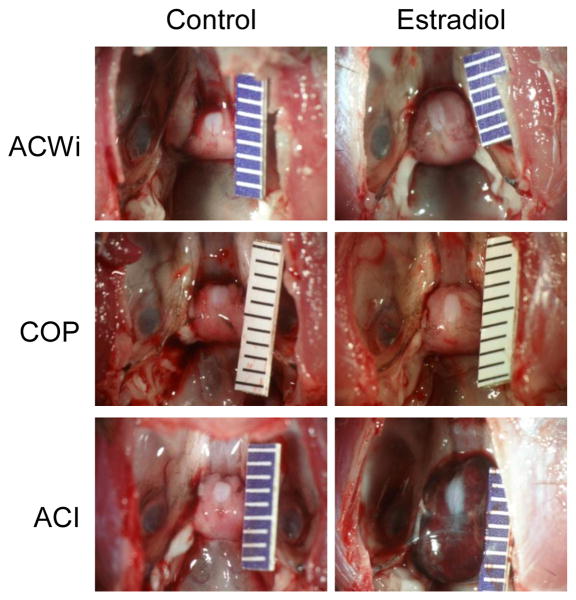
The gross appearance of the pituitary glands of E2 treated rats also differed upon comparison by rat strain. The glands of E2 treated ACWi and COP rats were enlarged, but were normal in shape and color (Figure 6). In addition, the posterior lobe of the pituitary gland remained apparent in E2 treated ACWi and COP rats. The pituitary glands of E2 treated ACI rats were enlarged and were usually abnormal in shape and red in color, which results from the presence of abundant dilated and congested vascular channels and focal regions of hemorrhage (Spady et al. 1999a; Spady, et al. 1999a).
Figure 6. Effects of Estrogen on Pituitary Gland Morphology.
Female ACWi, COP and ACI rats were treated with E2 as described in Figure 1 and Materials and Methods. Each image illustrates the appearance of the pituitary gland from a representative ACWi, COP or ACI rat that was either sham treated (Control) or treated with E2 (Estradiol) for 195-196 days. Each graduation on the scale ruler indicates 1 mm.
Discussion
Published genetic data from reciprocal intercrosses between ACI and COP rats indicate that the QTLs that determine susceptibility to E2-induced mammary cancer segregate independently of those QTLs that determine the sensitivity of the pituitary lactotroph population to estrogen-induced hyperplasia/adenoma (Gould et al. 2004; Strecker et al. 2005). Using this knowledge, we developed the ACWi rat strain as a novel rat mammary cancer model that retains the unique susceptibility of the ACI rat to E2-induced mammary cancer while virtually eliminating treatment related morbidity resulting from pituitary lactotroph hyperplasia/adenoma. The ACWi rat strain was generated by intercrossing previously described ACI.COP-Ept1 and ACI.COP-Ept2 congenic rats and selectively breeding to homozygosity for COP alleles at Ept1 on RNO6 and Ept2 on RNO3 (Kurz et al. 2008). This strategy was chosen because: 1) for those QTLs where COP alleles reduced the sensitivity of the pituitary lactotroph to estrogen, Ept1 and Ept2 exerted the greatest effects on phenotypic variance; and 2) pituitary lactotroph responsiveness in ACI.COP-Ept1 and ACI.COP-Ept2 congenic rats differed most from that of ACI rats (Kurz et al. 2008; Strecker et al. 2005). The responsiveness of the pituitary lactotroph population to E2 in ACWi rats, as indicated by quantification of pituitary mass, a surrogate indicator of absolute lactotroph number (discussed below), was dramatically reduced relative to ACI rats, and did not differ from that of COP rats. Comparison of the data from the current study to published data indicate that the combined actions of COP alleles at Ept1 and Ept2 on induction of pituitary lactotroph hyperplasia/adenoma in ACWi rats dramatically exceed the actions of COP alleles at either Ept1 or Ept2 alone (Kurz et al. 2008). Whereas pituitary mass in ACWi rats treated with E2 for 28 weeks averaged 43.4 mg, pituitary mass in identically treated ACI.COP-Ept1 and ACI.COP-Ept2 rats averaged 142.7 mg (P < 10-4 vs. ACWi) and 75.9 mg (P = 0.0033 vs. ACWi), respectively.
The sensitivity of the pituitary lactotroph population to induction of hyperplasia/adenoma by estrogen is rat strain-specific and highly heritable (Shull et al. 2007; Shull et al. 1997; Spady et al. 1999a; Spady et al. 1999b; Stone et al. 1979; Strecker et al. 2005; Wendell and Gorski 1997; Wendell, et al. 1996; Wiklund, et al. 1981a; Wiklund et al. 1981b). Pituitary mass is frequently used as a quantitative phenotype for estrogen-induced lactotroph hyperplasia/adenoma (Shull et al. 2007; Spady et al. 1999a; Spady et al. 1999b; Strecker et al. 2005; Wendell and Gorski 1997; Wendell et al. 1996; Wiklund et al. 1981a; Wiklund et al. 1981b). Because pituitary mass is directly correlated with pituitary DNA content and the level of prolactin in the systemic circulation, this phenotype serves as an accurate surrogate indicator of absolute lactotroph number (Kurz et al. 2008; Spady et al. 1999a; Spady et al. 1999b; Tachibana, et al. 2006; Wiklund et al. 1981b). Estrogen-induced lactotroph hyperplasia/adenoma results in sustained hyperprolactinemia (Spady et al. 1999a). Prolactin plays important roles in mammary gland development and lactation and has been implicated in the etiology of breast cancer (Clevenger, et al. 2003; Rose-Hellekant, et al. 2003). ACWi rats remain as highly susceptible as parental ACI rats to E2-induced mammary cancer while the sensitivity of the pituitary lactotroph population in ACWi rats to induction of hyperplasia/adenoma is significantly attenuated relative to ACI rats. These data further confirm that the molecular events that give rise to these two different estrogen-induced neoplasms are influenced by distinct genetic determinants. Moreover, these data further illustrate the lack of a direct association between pituitary tumor mass, hyperprolactinemia and susceptibility to E2-induced mammary cancer in the rat models.
The morbidity associated with pituitary lactotroph hyperplasia/adenoma in humans and estrogen-sensitive rat strains, such as ACI, results in large part from the effect of expanded pituitary mass on those tissues adjacent to the pituitary gland, primarily the hypothalamus and the optic nerves, as well as from endocrine imbalances, specifically hyperprolactinemia and/or deficiency of the other hormones normally produced by other pituitary cell types (Colao and Savastano 2011; Molitch 2002; Sam and Molitch 2005; Spady et al. 1999a). In this study, 46% of ACI rats treated with E2 to induce mammary cancer exhibited treatment related morbidity unrelated to mammary cancer burden and were euthanized prior to the desired experiment end point. By contrast, 0% of E2 treated ACWi rats were euthanized prior to the completion of the study due to morbidity other than mammary cancer burden. In an effort to reduce treatment related morbidity in E2 treated ACI rats, Ravoori et al. explored the use of a lower dose of E2 to induce mammary cancer, relative to that used in our studies. Although the lower E2 dose, achieved by reducing the length of the Silastic tubing implant, was effective in reducing treatment related morbidity, an extra 60 days of treatment was required to attain an equivalent mammary tumor multiplicity observed in ACI rats treated with the larger implants/higher dose of E2 (Ravoori, et al. 2007). Thus, ACWi rats offer clear advantages over ACI rats to those investigators who perform long-term studies focused on the etiology and prevention of E2-induced mammary cancer using these physiologically relevant rat models.
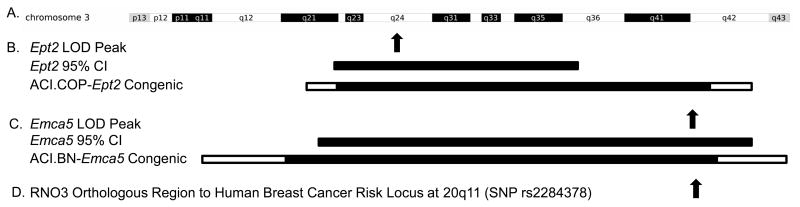
The ACWi rat strain was developed by intercrossing ACI.COP-Ept1 and ACI.COP-Ept2 rats followed by iterative inbreeding with selection for COP alleles at Ept1 and Ept2. It is noteworthy that the Ept2 congenic interval on RNO3 extends from D3Mgh21 (48.4 Mb) to D3Rat142 (171.5 Mb) and overlaps almost entirely with Emca5, a mammary cancer susceptibility QTL that was mapped in an intercross between susceptible ACI and resistant BN rats (Figure 7) (Colletti et al. 2014; Kurz et al. 2008; Schaffer et al. 2006; Strecker et al. 2005). Data presented herein as well as data published previously indicate that COP alleles across the Ept2 congenic interval do not impact susceptibility to E2-induced mammary cancer, whereas BN alleles across the Emca5 congenic interval dramatically reduce mammary cancer susceptibility (Colletti et al. 2014; Kurz et al. 2008). Together, these data suggest that the genetically related ACI and COP rat strains share alleles at Emca5. The peak LOD region of Emca5 is orthologous to a breast cancer risk locus mapped to human chromosome 20q11 that spans three genes, ASIP, RALY and EIF2S2 (Schaffer et al. 2006; Colletti et al. 2014; Siddiq, et al. 2012). ASIP encodes Agouti Signaling Protein, which regulates skin and hair pigmentation in humans and multiple other mammalian species, including rats (Bonilla, et al. 2005; Kanetsky, et al. 2002; Suzuki 2013). It is interesting to note that BN alleles at Emca5 confer upon ACI.BN-Emca5 congenic rats a black, non-agouti, coat color phenotype, indicating that ACI and BN rats harbor different alleles at Asip (unpublished data). By contrast, both ACI and ACI.COP-Ept2 congenic rats exhibit the agouti coat color, indicating that ACI and COP strains share alleles at Asip. An evaluation of available whole genome sequence data for the ACI, COP and BN rat strains further supports the assertion that ACI and COP rats share alleles across the peak LOD region of Emca5, including the Asip locus, whereas BN rats differ across this region. Together, these comparative genomic data indicate that BN rats harbor a genetic variant that inhibits production or function of Agouti Signaling Protein and suggest a possible functional association between this genetic variant and mammary cancer susceptibility.
Figure 7. Genetic Relationships between the Ept2 Pituitary Tumor Locus and the Emca5 Mammary Cancer Locus.
A. Rat chromosome 3 (RNO3) is illustrated as an ideogram that denotes defined cytogenetic bands. B. Ept2 was mapped to RNO3 in intercrosses between ACI and COP rats (Strecker, et al., 2005). The location of the Ept2 LOD peak is illustrated by the upward arrow above a horizontal black bar that indicates the 95% confidence interval for Ept2, which was defined by interval mapping analyses. Also illustrated is the Ept2 congenic interval; the horizontal black bar indicates the segment of RNO3 that is known to be derived from COP rats, the open termini of this horizontal bar indicate areas of recombination between the ACI and COP genomes, and the remainder of RNO3 is known to be derived from ACI rats (Kurz, et al., 2008). C. Emca5 was mapped to RNO3 in an intercross between BN and ACI rats (Schaffer, et al., 2006). The location of the Emca5 LOD peak, the Emca5 95% confidence interval, and the Emca5 congenic interval (Colletti, et al., 2014) are illustrated as described for Ept2. D. The upward arrow indicates the location of the region of RNO3 that is orthologous to the breast cancer risk locus in humans linked to single nucleotide polymorphism rs2284378 in a genome wide association study (Siddiq, et al., 2012). The overlap between the rat and human loci suggest these species share genetic determinants of breast cancer susceptibility (Colletti, et al., 2014).
Acknowledgments
We thank John Colletti for his contribution to this research. We also thank the staff of the Experimental Pathology Shared Service in the UW Carbone Cancer Center and staff of the Animal Care Core in the McArdle Laboratory for Cancer Research for their valuable contributions to this research.
Funding: This work was supported by grant R01-CA77876 from the National Institutes of Health (NIH). Shared research resources at the University of Wisconsin-Madison were supported by Cancer Center Support Grant P30-CA014520.
Footnotes
Conflict Of Interest Statement: The authors have no conflicts of interest to declare.
Author Contributions: KLD and NBS generated the ACWi rat strain. KLD, NBS, QEH, MPH, NLS and LD generated data. KLD and JDS evaluated data and prepared the manuscript. JDS conceived and directed the study and was the primary author of the manuscript.
References
- Adamovic T, Roshani L, Chen L, Schaffer BS, Helou K, Levan G, Olsson B, Shull JD. Nonrandom pattern of chromosome aberrations in 17beta-estradiol-induced rat mammary tumors: indications of distinct pathways for tumor development. Genes Chromosomes Cancer. 2007;46:459–469. doi: 10.1002/gcc.20428. [DOI] [PubMed] [Google Scholar]
- Bonilla C, Boxill LA, Donald SA, Williams T, Sylvester N, Parra EJ, Dios S, Norton HL, Shriver MD, Kittles RA. The 8818G allele of the agouti signaling protein (ASIP) gene is ancestral and is associated with darker skin color in African Americans. Hum Genet. 2005;116:402–406. doi: 10.1007/s00439-004-1251-2. [DOI] [PubMed] [Google Scholar]
- Clevenger CV, Furth PA, Hankinson SE, Schuler LA. The role of prolactin in mammary carcinoma. Endocr Rev. 2003;24:1–27. doi: 10.1210/er.2001-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colao A, Savastano S. Medical treatment of prolactinomas. Nat Rev Endocrinol. 2011;7:267–278. doi: 10.1038/nrendo.2011.37. [DOI] [PubMed] [Google Scholar]
- Colletti JA, 2nd, Leland-Wavrin KM, Kurz SG, Hickman MP, Seiler NL, Samanas NB, Eckert QA, Dennison KL, Ding L, Schaffer BS, et al. Validation of six genetic determinants of susceptibility to estrogen-induced mammary cancer in the rat and assessment of their relevance to breast cancer risk in humans. G3 (Bethesda) 2014;4:1385–1394. doi: 10.1534/g3.114.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinkwater NR. MSTAT Version 5.5.7. 2013 http://www.mcardle.wisc.edu/mstat/index.html.
- Flicek P, Amode MR, Barrell D, Beal K, Billis K, Brent S, Carvalho-Silva D, Clapham P, Coates G, Fitzgerald S, et al. Ensembl 2014. Nucleic Acids Res. 2014;42:D749–755. doi: 10.1093/nar/gkt1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould KA, Tochacek M, Schaffer BS, Reindl TM, Murrin CR, Lachel CM, VanderWoude EA, Pennington KL, Flood LA, Bynote KK, et al. Genetic determination of susceptibility to estrogen-induced mammary cancer in the ACI rat: mapping of Emca1 and Emca2 to chromosomes 5 and 18. Genetics. 2004;168:2113–2125. doi: 10.1534/genetics.104.033878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvell DM, Strecker TE, Tochacek M, Xie B, Pennington KL, McComb RD, Roy SK, Shull JD. Rat strain-specific actions of 17beta-estradiol in the mammary gland: correlation between estrogen-induced lobuloalveolar hyperplasia and susceptibility to estrogen-induced mammary cancers. Proc Natl Acad Sci U S A. 2000;97:2779–2784. doi: 10.1073/pnas.050569097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanetsky PA, Swoyer J, Panossian S, Holmes R, Guerry D, Rebbeck TR. A polymorphism in the agouti signaling protein gene is associated with human pigmentation. Am J Hum Genet. 2002;70:770–775. doi: 10.1086/339076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz SG, Dennison KL, Samanas NB, Hickman MP, Eckert QA, Walker TL, Cupp AS, Shull JD. Ept7 influences estrogen action in the pituitary gland and body weight of rats. Mamm Genome. 2014;25:244–252. doi: 10.1007/s00335-014-9504-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz SG, Hansen KK, McLaughlin MT, Shivaswamy V, Schaffer BS, Gould KA, McComb RD, Meza JL, Shull JD. Tissue-specific actions of the Ept1, Ept2, Ept6, and Ept9 genetic determinants of responsiveness to estrogens in the female rat. Endocrinology. 2008;149:3850–3859. doi: 10.1210/en.2008-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laulederkind SJ, Hayman GT, Wang SJ, Smith JR, Lowry TF, Nigam R, Petri V, de Pons J, Dwinell MR, Shimoyama M, et al. The Rat Genome Database 2013--data, tools and users. Brief Bioinform. 2013;14:520–526. doi: 10.1093/bib/bbt007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SA, Weroha SJ, Tawfik O, Li JJ. Prevention of solely estrogen-induced mammary tumors in female aci rats by tamoxifen: evidence for estrogen receptor mediation. J Endocrinol. 2002;175:297–305. doi: 10.1677/joe.0.1750297. [DOI] [PubMed] [Google Scholar]
- Molitch ME. Medical management of prolactin-secreting pituitary adenomas. Pituitary. 2002;5:55–65. doi: 10.1023/a:1022375429083. [DOI] [PubMed] [Google Scholar]
- Nigam R, Laulederkind SJ, Hayman GT, Smith JR, Wang SJ, Lowry TF, Petri V, De Pons J, Tutaj M, Liu W, et al. Rat Genome Database: a unique resource for rat, human, and mouse quantitative trait locus data. Physiol Genomics. 2013;45:809–816. doi: 10.1152/physiolgenomics.00065.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing 2013 [Google Scholar]
- Wendell DL, Daun SB, Stratton MB, Gorski J. Different functions of QTL for estrogen-dependent tumor growth of the rat pituitary. Mamm Genome. 2000;11:855–861. doi: 10.1007/s003350010168. [DOI] [PubMed] [Google Scholar]
- Ravoori S, Vadhanam MV, Sahoo S, Srinivasan C, Gupta RC. Mammary tumor induction in ACI rats exposed to low levels of 17beta-estradiol. Int J Oncol. 2007;31:113–120. [PubMed] [Google Scholar]
- Rose-Hellekant TA, Arendt LM, Schroeder MD, Gilchrist K, Sandgren EP, Schuler LA. Prolactin induces ERalpha-positive and ERalpha-negative mammary cancer in transgenic mice. Oncogene. 2003;22:4664–4674. doi: 10.1038/sj.onc.1206619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhlen RL, Willbrand DM, Besch-Williford CL, Ma L, Shull JD, Sauter ER. Tamoxifen induces regression of estradiol-induced mammary cancer in the ACI.COP-Ept2 rat model. Breast Cancer Res Treat. 2009;117:517–524. doi: 10.1007/s10549-008-0169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sam S, Molitch ME. The pituitary mass: diagnosis and management. Rev Endocr Metab Disord. 2005;6:55–62. doi: 10.1007/s11154-005-5225-z. [DOI] [PubMed] [Google Scholar]
- Schaffer BS, Lachel CM, Pennington KL, Murrin CR, Strecker TE, Tochacek M, Gould KA, Meza JL, McComb RD, Shull JD. Genetic bases of estrogen-induced tumorigenesis in the rat: mapping of loci controlling susceptibility to mammary cancer in a Brown Norway x ACI intercross. Cancer Res. 2006;66:7793–7800. doi: 10.1158/0008-5472.CAN-06-0143. [DOI] [PubMed] [Google Scholar]
- Schaffer BS, Leland-Wavrin KM, Kurz SG, Colletti JA, Seiler NL, Warren CL, Shull JD. Mapping of three genetic determinants of susceptibility to estrogen-induced mammary cancer within the Emca8 locus on rat chromosome 5. Cancer Prev Res (Phila) 2013;6:59–69. doi: 10.1158/1940-6207.CAPR-12-0346-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull JD. The rat oncogenome: comparative genetics and genomics of rat models of mammary carcinogenesis. Breast Dis. 2007;28:69–86. doi: 10.3233/bd-2007-28108. [DOI] [PubMed] [Google Scholar]
- Shull JD, Lachel CM, Murrin CR, Pennington KL, Schaffer BS, Strecker TE, Gould KA. Genetic control of estrogen action in the rat: mapping of QTLs that impact pituitary lactotroph hyperplasia in a BN x ACI intercross. Mamm Genome. 2007;18:657–669. doi: 10.1007/s00335-007-9052-2. [DOI] [PubMed] [Google Scholar]
- Shull JD, Pennington KL, Reindl TM, Snyder MC, Strecker TE, Spady TJ, Tochacek M, McComb RD. Susceptibility to estrogen-induced mammary cancer segregates as an incompletely dominant phenotype in reciprocal crosses between the ACI and Copenhagen rat strains. Endocrinology. 2001;142:5124–5130. doi: 10.1210/endo.142.12.8530. [DOI] [PubMed] [Google Scholar]
- Shull JD, Spady TJ, Snyder MC, Johansson SL, Pennington KL. Ovary-intact, but not ovariectomized female ACI rats treated with 17beta-estradiol rapidly develop mammary carcinoma. Carcinogenesis. 1997;18:1595–1601. doi: 10.1093/carcin/18.8.1595. [DOI] [PubMed] [Google Scholar]
- Siddiq A, Couch FJ, Chen GK, Lindstrom S, Eccles D, Millikan RC, Michailidou K, Stram DO, Beckmann L, Rhie SK, et al. A meta-analysis of genome-wide association studies of breast cancer identifies two novel susceptibility loci at 6q14 and 20q11. Hum Mol Genet. 2012;21:5373–5384. doi: 10.1093/hmg/dds381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Bhat NK, Bhat HK. Partial inhibition of estrogen-induced mammary carcinogenesis in rats by tamoxifen: balance between oxidant stress and estrogen responsiveness. PLoS One. 2011;6:e25125. doi: 10.1371/journal.pone.0025125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady TJ, Harvell DM, Snyder MC, Pennington KL, McComb RD, Shull JD. Estrogen-induced tumorigenesis in the Copenhagen rat: disparate susceptibilities to development of prolactin-producing pituitary tumors and mammary carcinomas. Cancer Lett. 1998;124:95–103. doi: 10.1016/s0304-3835(97)00455-2. [DOI] [PubMed] [Google Scholar]
- Spady TJ, McComb RD, Shull JD. Estrogen action in the regulation of cell proliferation, cell survival, and tumorigenesis in the rat anterior pituitary gland. Endocrine. 1999a;11:217–233. doi: 10.1385/ENDO:11:3:217. [DOI] [PubMed] [Google Scholar]
- Spady TJ, Pennington KL, McComb RD, Birt DF, Shull JD. Estrogen-induced pituitary tumor development in the ACI rat not inhibited by dietary energy restriction. Mol Carcinog. 1999a;26:239–253. [PubMed] [Google Scholar]
- Spady TJ, Pennington KL, McComb RD, Shull JD. Genetic bases of estrogen-induced pituitary growth in an intercross between the ACI and Copenhagen rat strains: dominant mendelian inheritance of the ACI phenotype. Endocrinology. 1999b;140:2828–2835. doi: 10.1210/endo.140.6.6757. [DOI] [PubMed] [Google Scholar]
- Stone JP, Holtzman S, Shellabarger CJ. Neoplastic responses and correlated plasma prolactin levels in diethylstilbestrol-treated ACI and Sprague-Dawley rats. Cancer Res. 1979;39:773–778. [PubMed] [Google Scholar]
- Strecker TE, Spady TJ, Schaffer BS, Gould KA, Kaufman AE, Shen F, McLaughlin MT, Pennington KL, Meza JL, Shull JD. Genetic bases of estrogen-induced pituitary tumorigenesis: identification of genetic loci determining estrogen-induced pituitary growth in reciprocal crosses between the ACI and Copenhagen rat strains. Genetics. 2005;169:2189–2197. doi: 10.1534/genetics.104.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H. Evolutionary and phylogeographic views on Mc1r and Asip variation in mammals. Genes Genet Syst. 2013;88:155–164. doi: 10.1266/ggs.88.155. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Lu L, Hiai H, Tamura A, Matsushima Y, Shisa H. Quantitative trait loci determining weight reduction of testes and pituitary by diethylstilbesterol in LEXF and FXLE recombinant inbred strain rats. Exp Anim. 2006;55:91–95. doi: 10.1538/expanim.55.91. [DOI] [PubMed] [Google Scholar]
- Wendell DL, Gorski J. Quantitative trait loci for estrogen-dependent pituitary tumor growth in the rat. Mamm Genome. 1997;8:823–829. doi: 10.1007/s003359900586. [DOI] [PubMed] [Google Scholar]
- Wendell DL, Herman A, Gorski J. Genetic separation of tumor growth and hemorrhagic phenotypes in an estrogen-induced tumor. Proc Natl Acad Sci U S A. 1996;93:8112–8116. doi: 10.1073/pnas.93.15.8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiklund J, Rutledge J, Gorski J. A genetic model for the inheritance of pituitary tumor susceptibility in F344 rats. Endocrinology. 1981a;109:1708–1714. doi: 10.1210/endo-109-5-1708. [DOI] [PubMed] [Google Scholar]
- Wiklund J, Wertz N, Gorski J. A comparison of estrogen effects on uterine and pituitary growth and prolactin synthesis in F344 and Holtzman rats. Endocrinology. 1981b;109:1700–1707. doi: 10.1210/endo-109-5-1700. [DOI] [PubMed] [Google Scholar]
- Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]